Abstract
Intermittent fasting (IF) reportedly increases resistance and intestinal IgA response to Salmonella typhimurium infection in mature mice. The aim of this study was to explore the effect of aging on the aforementioned improved immune response found with IF. Middle-aged male BALB/c mice were submitted to IF or ad libitum (AL) feeding for 40 weeks and then orally infected with S. typhimurium. Thereafter, infected animals were all fed AL (to maximize their viability) until sacrifice on day 7 or 14 post-infection. We evaluated body weight, bacterial load (in feces, Peyer’s patches, spleen and liver), total and specific intestinal IgA, lamina propria IgA+ plasma cells, plasma corticosterone, and messenger RNA (mRNA) expression of α-chain, J-chain, and the polymeric immunoglobulin receptor (pIgR) in liver and intestinal mucosa. In comparison with the infected AL counterpart, the infected IF group (long-term IF followed by post-infection AL feeding) generally had lower intestinal and systemic bacterial loads as well as higher total IgA on both post-infection days. Both infected groups showed no differences in corticosterone levels, body weight, or food and caloric intake. The increase in intestinal IgA was associated with enhanced pIgR mRNA expression in the intestine (day 7) and liver. Thus, to maintain body weight and caloric intake, IF elicited metabolic signals that possibly induced the increased hepatic and intestinal pIgR mRNA expression found. The increase in IgA probably resulted from intestinal IgA transcytosis via pIgR. This IgA response along with phagocyte-induced killing of bacteria in systemic organs (not measured) may explain the resolution of the S. typhimurium infection.
Keywords: Intermittent fasting, Middle-aged mice, Intestinal IgA, Salmonella typhimurium infection, Liver polymeric immunoglobulin receptor
Introduction
Intermittent fasting is a dietary restriction regimen that increases resistance to age-associated conditions such as obesity (Mattson et al. 2003). According to evidence from mouse and rat models, susceptibility to Salmonella typhimurium infection is age-dependent, being lower in young adults and higher in very early or late stages in life (Bradley and Kauffman 1990; Burns-Guydish et al. 2005; Emmerling et al. 1979; Ren et al. 2009; Rhee et al. 2005). S. typhimurium is used as a rodent model of human typhoid fever by Salmonella typhi. Therefore, the effect of intermittent fasting on susceptibility to bacterial infection at different life stages is of particular interest.
The experimental protocol for intermittent fasting in rodents consists of alternate days of ad libitum feeding and food deprivation for three or more months (Anson et al. 2003; Duan et al. 2001; Singh et al. 2012). As a result of this protocol, the cellular stress response reportedly triggers the production of proteins that protect against age-associated detrimental effects of oxidative damage (Li et al. 2013). Clinical studies have shown that fasting strengthens intestinal immunity by maintaining adequate levels of intestinal immunoglobulin A (IgA) in patients suffering from skeletal, muscular, and connective tissue diseases (Beer et al. 2001). In BALB/c mice, intermittent fasting increases both the intestinal level of IgA and resistance to S. typhimurium infection (Godinez-Victoria et al. 2014).
S. typhimurium is an invasive intracellular pathogen that causes both intestinal and extra-intestinal infection in susceptible mouse strains like BALB/c. After being orally ingested by BALB/c mice, S. typhimurium passes through the stomach, reaches the intestine, and colonizes the intestinal epithelium. The pathogen eventually traverses this tissue layer, usually via M cells that cover the luminal surface of Peyer’s patches. At the subepithelial level, the bacteria survive within macrophages and are transported to the liver and spleen via mesenteric lymph nodules, where they replicate and eventually cause systemic infection (Watson and Holden 2010).
Susceptibility to infections by enteropathogenic agents in older animals results from dysfunctions in antigen uptake by M cells, antigen presentation, B isotype switching, IgA+ B cell maturation and homing, IgA+ plasma cell secretion, and IgA transport via pIgR (Schmucker et al. 2003). A previous report showed an association between resistance to S. typhimurium infection and the intestinal generation of IgA (Godinez-Victoria et al. 2014). IgA is released in the intestinal lamina propria by IgA+ plasma cells, mainly as a dimer (or polymer) joined by the J-chain. Dimeric IgA (dIgA) is captured by polymeric immunoglobulin receptors (pIgRs) expressed on the basolateral membrane of the epithelial layer. The dIgA-pIgR complex is internalized and transcytosed to the apical membrane. There, the extracellular region of pIgR is proteolytically cleaved and released into the lumen along with dIgA to render secretory IgA (SIgA) (Asano and Komiyama 2011). SIgA and pIgR have an essential role to the control of an S. typhimurium infection at the intestinal level by blocking luminal colonization, thus enabling bacterial clearance (Michetti et al. 1992; Wijburg et al. 2006).
Experimental studies in animals have shown that aging decreases the frequency (expressed as a percentage) and number of intestinal IgA+ B lymphocytes and antibody-secreting cells (Ebersole et al. 1988; Van der Heijden et al. 1988), as well as increasing the total number of intestinal IgA+ plasma cells. Although aging does not alter the antibody secretion ability in animal models, it impairs the anamnestic capacity and specific antigen response (Ebersole and Steffen 1989; Thoreux et al. 2000). Therefore, intestinal levels of IgA may be unaffected or even augmented in aged animals, but the ability of IgA to bind to bacterial antigens is impaired (Ebersole et al. 1985; Santiago et al. 2008; Senda et al. 1988). IgA levels can be affected by pIgR expression. Whereas an in vitro study showed that pIgR expression is unchanged with aging (Daniels et al. 1988), according to an in vivo study (Yanagihara et al. 2004), this parameter is decreased by the aging process.
The aforementioned study evidencing that intermittent fasting increases both intestinal levels of IgA and resistance to S. typhimurium infection was carried out on mature BALB/c mice from the age of 19 to 30 weeks (Godinez-Victoria et al. 2014). The aim of the present study was to evaluate the effect of long-term intermittent fasting on the IgA response and on the outcome of an S. typhimurium infection in middle-aged BALB/c mice from 19 to 59 weeks of age (for a total of 40 weeks).
Material and methods
Animals
Eight-week-old male BALB/c mice (from Harlan Sprague-Dawley) were individually housed and handled in accordance with Mexican federal regulations for animal experimentation and care (NOM-062-ZOO-1999, Ministry of Agriculture, Mexico City, Mexico). The protocol was approved by the Institutional Animal Care and Use Committee and Institutional Ethics Committee of the Superior School of Medicine, National Polytechnic Institute.
For the purpose of this study, the life phases of BALB/c mice are considered as follows: maturity from 3 to 6 months of age, middle age from 10 to 15 months, and old age from 18 to 24 months (Anisimov et al. 2011; Flurkey and Harrison 2007). From 15 to 18 months of life, BALB/c mice show changes in senescence in some biomarkers of aging (Flurkey and Harrison 2007). Very old mice are highly vulnerable to illness because of the cumulative influence of aging (Yuan R et al. 2009). Thus, the present approach was carried out with middle-aged BALB/c mice that underwent intermittent fasting or ad libitum feeding for 40 weeks before being subjected to an S. typhimurium infection.
Experimental protocol
Forty-two mice were fed ad libitum with the NIH-31 (7017 Harlan Lab Madison WI, USA) diet from 8 to 18 weeks of life before being divided in two groups (n = 21 each) and subjected to distinct protocols from 19 to 59 weeks of life. Whereas one group was subjected to intermittent fasting, the other one continued with ad libitum feeding (Fig. 1). Food was always removed and replaced at 1 p.m. Body weight as well as food and caloric intake was assessed weekly (on the same day of the week and at the same time of day) from 19 to 62 weeks of life. Energy intake was calculated based on the fact that the energy density of each gram of NIH-31 is 3 kcal, an estimate of metabolizable energy based on the Atwater factors assigning 4 kcal/g to proteins, 9 kcal/g to fat, and 4 kcal/g to available carbohydrates (NIH-31, Harland Lab).
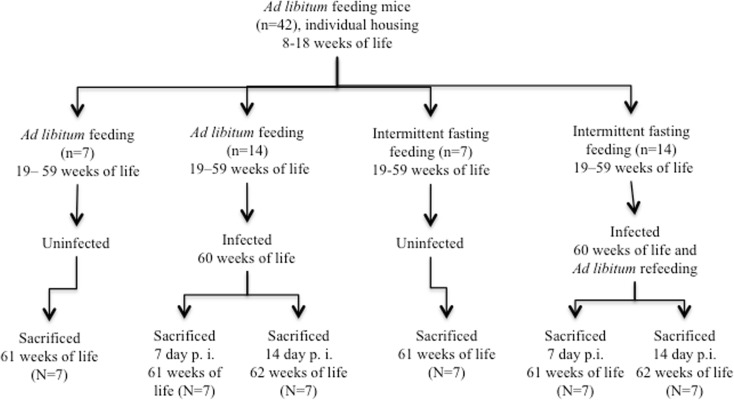
Fig. 1.
Experimental protocol. After being individually housed and fed ad libitum from the 8 to 18 weeks of life, two groups of BALB/c mice were formed, each subjected to a distinct dietary protocol from 19 to 59 weeks of life: (a) intermittent fasting and (b) ad libitum feeding. At 60 weeks of life, each of these two groups was divided into an uninfected and two infected subgroups. The uninfected subgroup (n = 7) from each dietary regimen was sacrificed at 61 weeks of life. The other mice from each dietary regimen (n = 14) were sublethally infected at 60 weeks of life by intragastric route with S. typhimurium. Afterward, all infected animals were fed ad libitum in order to maximize their viability. Half of the infected mice from each dietary regimen (n = 7) were sacrificed on day 7 and the other half (n = 7) on day 14 post-infection, corresponding to the 61st and 62nd week of life, respectively
At 60 weeks of life, within the ad libitum feeding and intermittent fasting groups, mice were subdivided into uninfected (n = 7) and infected (n = 14) groups. The uninfected animals were sacrificed at 61 weeks of life, thus representing basal groups under healthy conditions. The infected groups were administered a sublethal dose (104 colony-forming units (CFU)) of S. typhimurium (ATCC-14028, Manassas, VA, USA) by the intragastric route (Drago-Serrano et al. 2010) at 60 weeks of life. After infection, all mice regardless of the previous dietary regimen were fed ad libitum to prevent mortality. Infected mice were further divided into two subgroups (n = 6 or 7 each), to be sacrificed by a lethal dose of pentobarbital (180 mg/kg body weight) injected by intraperitoneal route at 7 or 14 days post-infection, corresponding to 61 or 62 weeks of life, respectively. For all mice, sacrifice was performed at 11:00 a.m. after having removed food 4 h previously in order to facilitate the collection of samples by decreasing the ingesta.
Sample collection
Following infection, freshly voided fecal pellets were collected daily from each mouse (at 8–9 a.m.). Pellets were weighed to relate their mass (g) to the number of bacteria and, in this way, compute the CFU per gram of sample in order to estimate the bacterial elimination. After sacrifice, animals were exsanguinated by cardiac puncture with a heparinized syringe. Blood samples were centrifuged at 650×g at room temperature, and the plasma samples were collected and stored at −70 °C to await corticosterone quantification.
Samples of Peyer’s patches from duodenum, spleen, and liver were excised from all mice and weighed in order to enumerate bacterial load by the plate count method. For comparative purposes, the duodenum was analyzed as in a previous study exploring the effect of intermittent fasting on the outcome of S. typhimurium infection in mature BALB/c mice at 30 weeks of life (Godinez-Victoria et al. 2014). Thus, duodenum samples were dissected and flushed with 5 mL of sterile phosphate-buffered saline (PBS) at pH 7.4, and intestinal contents were centrifuged at 10,000×g at 4 °C for 20 min. Intestinal supernatants were collected and mixed with a protease inhibitor cocktail (Cat. No. 11 836 153 001 Complete mini, Roche Diagnostics, Mannheim, Germany), then stored at −70 °C until the evaluation of the total and specific (to Salmonella surface proteins) IgA by immunoenzymatic assay (EIA).
After washing, intestinal segments were processed for the purification of lamina propria lymphocytes as described previously (Resendiz-Albor et al. 2010). In brief, intestinal samples (free of feces and Peyer’s patches) were turned outward by inserting an iron crochet needle. After that, intestinal segments were incubated with 1 mM EDTA diluted in Roswell Park Memorial Institute (RPMI) 1640 medium containing 1 % fetal calf serum (FCS), 50 μg/mL gentamicin, and 1 mM dithiothreitol (all from Sigma, St. Louis, MO, USA) and thereafter with 60 U/mL collagenase type IV (Sigma) diluted in RPMI with 1 % FCS and 50 μg/mL gentamicin. Single=cell suspensions of lamina propria lymphocytes were centrifuged in a discontinuous 40/70 % Percoll gradient (17-0891-01 GE Healthcare, Piscataway, NJ, USA). Lymphocytes from the interface were collected and washed with RPMI 1640 to determine the number of IgA+ plasma cells by flow cytometry (Resendiz-Albor et al. 2010). Additionally, intestinal samples were scraped with a coverslip to collect whole mucosa samples (containing submucosa and mucosa layers) that were stored at −70 °C. In whole mucosa and liver samples, the messenger RNA (mRNA) expression of α-chain, J-chain, and pIgR was analyzed by quantitative real-time reverse transcription polymerase chain reaction (RT-PCR) assays.
Bacterial plate count
Bacterial load from feces, liver, spleen, and Peyer’s patches was determined by plate count and expressed as CFU per gram, according to a previously reported procedure (Drago-Serrano et al. 2010).
Total and specific IgA concentration
Total protein concentration quantified by the Bradford assay was practically the same in all intestinal supernatants. For purposes of normalization, each sample was adjusted to the same protein concentration before proceeding with the estimation of total and specific IgA with the immunoenzymatic assay (EIA), according to a previously reported method (Drago-Serrano et al. 2010). Levels of total IgA were expressed in microgram per milliliter, while IgA specific to S. typhimurium was quantified with absorbance values at λ = 490 nm (A490 nm).
IgA+ plasma cells
The single-cell suspensions of lamina propria lymphocytes were adjusted to 1 × 106 cells per milliliter in PBS. Cells were labeled with monoclonal antibodies: anti-CD19-PE, CD138-APC, and IgA-FITC (all from Becton Dickinson Biosciences, San Jose, CA, USA). Plasma cells were fixed, permeabilized, and stained according to the manufacturer’s protocol for intracellular staining. The fluorescent signal intensity was recorded and analyzed in a FACSCalibur flow cytometer (Becton Dickinson Biosciences). For each sample, 20,000 events corresponding to the lymphocyte region were acquired and data were analyzed using Summit software version 4.3 (Dako, CO, USA). From these data, the frequency was expressed as the % of IgA+ CD19+/CD138+ cells using as denominator the total number of CD19+/CD138+ plasma cells (Resendiz-Albor et al. 2010). The latter in turn were gated from 20,000 events corresponding to the lymphocyte region.
mRNA levels of α-chain, J-chain, and pIgR
Samples of whole mucosa and liver were submitted to a process of mRNA extraction and cDNA synthesis by real-time RT-PCR assay, according to a previously reported method (Viloria et al. 2011). Specific oligonucleotide primers were originally generated by using the online assay design software (ProbeFinder: http://www.universal-probelibrary.com) and the primer sequence for each gene that is shown in Table 1. For α-chain, J-chain, and pIgR, mRNA levels were calculated with the comparative parameter threshold cycle (Ct) method and normalized to ribosomal RNA. Data are expressed as relative mRNA levels.
Table 1.
Forward and reverse primers for real-time PCR assays designed according to the ensemble transcript ID of the Mouse ProbeLibrary
| Gene | Ensemble transcript ID | Forward primer 5′– 3′ | Reverse primer 5′–3′ |
|---|---|---|---|
| α-Chain | 04692101001 | cgtccaagaattggatgtga | agtgacaggctgggatgg |
| J-chain | 04685105001 | gaactttgtataccatttgtcagacg | ctgggtggcagtaacaacct |
| pIgR | 04688635001 | agtaaccgaggcctgtcctt | gtcactcggcaactcagga |
Corticosterone assay
Plasma corticosterone levels were determined by using a commercially available ELISA kit for corticosterone assay (ADI-901-097 Enzo Life Sciences, Plymouth Meeting, PA, USA). The corticosterone concentration in plasma samples was calculated from a standard curve and expressed in nanogram per milliliter.
Statistical analysis
Three independent assays were carried out, and data are presented as the mean ± SD computed from one representative assay. For statistical analysis, sample size of uninfected and infected groups was 7 (or 6 for some parameters), except for days 1–7 in relation to bacterial count in feces for both infected groups (n = 14, including mice sacrificed at 7 and 14 days post-infection). Body weight, food consumption, and caloric intake were analyzed by one-way analysis of variance (ANOVA) and the Student’s t test. Bacterial load in feces was analyzed with one-way repeated measures (ANOVA) to look for differences between or within dietary regimens at 7 or 14 days. For the rest of the parameters, two-way ANOVA was performed to analyze differences between the two dietary regimens and the two post-infection days. If a significant main effect or association was identified, the means of the respective groups were compared using the Holm-Sidak method. For all tests, p ≤ 0.05 was considered significant. All analyses were performed using the SigmaPlot statistical software for Windows (version 2.03, SPSS, San Jose, CA, USA).
Results
Body weight, food consumption, and caloric intake assessment
From 19 to 62 weeks of life, evaluation of body weight (g/week), food consumption (g/week), and caloric intake (kcal/week) was made (data not shown). According to the statistical analysis (ANOVA), no significant differences were found within each group (intermittent fasting or ad libitum feeding) when comparing uninfected and infected mice in regard to body weight (F 2,15 = 2.4, p = 0.13 and F 2,15 = 0.216, p = 0.809, respectively), food consumption (F 2,18 = 2.5, p = 0.1 and F 2,15 = 3.3, p = 0.064, respectively), or caloric intake (F 2,18 = 2.47, p = 0.11 and F 2,15 = 3.3, p = 0.064, respectively). Likewise, no significant differences were found (Student’s t test) between the intermittent fasting and ad libitum feeding groups, either in uninfected (basal) mice or infected animals (on days 7 and 14 post-infection) in regard to body weight (Student’s t test: for the basal group, t = 0.213, df = 10, p = 0.084; for 7 days of infection, t = 2.28, df = 8, p = 0.052; for 14 days of infection, t = 0.287, df = 8, p = 0.781), food consumption (Student’s t test: for the basal group, t = 2.065, df = 11, p = 0.063; for 7 days of infection, t = 0.133, df = 11, p = 0.897; for 14 days of infection, t = 1.671, df = 11, p = 0.123), and caloric intake (Student’s t test: for the basal group, t = 1.846, df = 10, p = 0.092; for 7 days of infection, t = 0.162, df = 11, p = 0.874; for 14 days of infection, t = 1.981, df = 11, p = 0.073).
Intermittent fasting reduced bacterial colonization at the intestinal and systemic levels
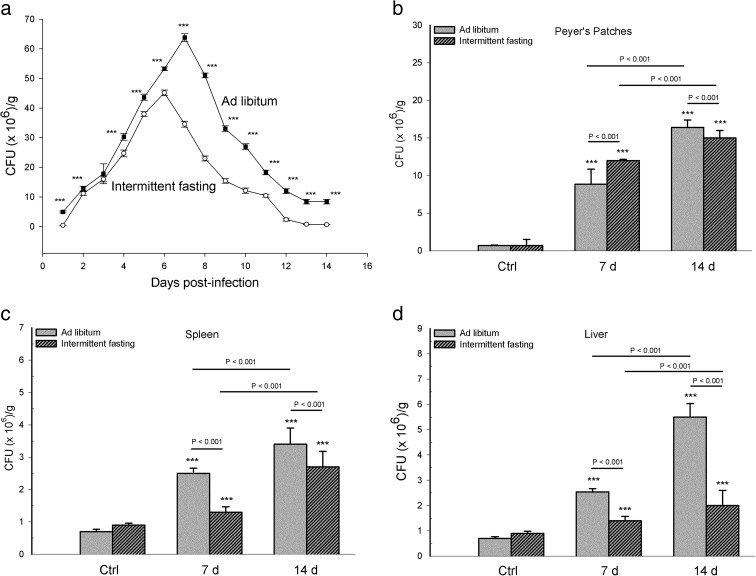
A significant effect of infection on fecal bacterial load was found during 14 days in the intermittent fasting group (F 13,120 = 3592.423, p < 0.001) and ad libitum group (F 13,120 = 2301.112, p < 0.001). Considering the former group, a significant difference was found in the fecal bacterial load between most of the 14 days in which this parameter was evaluated (p < 0.001). The exceptions were days 2 vs 11 (p = 0.088), 3 vs 9 (p = 0.182), 3 vs 1 (p = 0.270), 14 vs 1 (p = 0.421), and 13 vs 14 (p = 0.797). Likewise, considering the infected group of mice with ad libitum feeding, a significant difference was found in the fecal bacterial load between almost all of the 14 days of evaluation (p < 0.001). The exceptions were days 2 vs 12 (p = 152) and 11 vs 3 (p = 0.573). The fecal bacterial load reached a peak in the intermittent fasting group at 6 days and in the ad libitum group at 7 days post-infection. Comparing the fecal bacterial load between the infected mice of the two dietary regimens, the intermittent fasting group had a lower level than the ad libitum feeding group on almost all days of assessment (p < 0.001), with the exception of day 3 (t = 1.98, df = 26, p = 0.058) (Fig. 2a).
Fig. 2.
Bacterial load in samples from the groups with intermittent fasting and ad libitum feeding that were infected with S. typhimurium. Colony-forming units per gram of sample (CFU × 106/g) are expressed as the mean ± standard deviation (SD) from one representative assay of three independent assays carried out. a CFU × 106/g recorded daily in feces during 14 days post-infection expressed as the mean ± SD of 12 mice per group for days 1–7 and 6 mice per group for days 8–14. CFU per gram was expressed as the mean ± SD of six mice per group was recorded on days 7 and 14 post-infection in the following: b Peyer’s patches, c spleen, and d liver. Significant differences were found: (i) between the infected and uninfected (basal = Ctrl) groups within each dietary regimen (***p < 0.001), (ii) between infected mice with long-term intermittent fasting (followed by ad libitum feeding) and infected animals with long-term ad libitum feeding, at day 7 and day14 post-infection, and (iii) between the two infected subgroups of each dietary regimen, comparing day 7 with day 14 post-infection (p value above solid line)
Regarding bacterial load in the organs, there was a significant interaction between diet and infection. This difference was found in Peyer’s patches (F 2,30 = 74.58, p < 0.001), spleen (F 2,30 = 11.65, p < 0.001), and liver (F 2,30 = 101.511, p < 0.001). Comparing the organs of infected mice of the two dietary regimens, mice with intermittent fasting had a higher bacterial load in Peyer’s patches on day 7 but a lower level on day 14 and a lower bacterial load in spleen and liver on both of these days (p < 0.001). The organs of all infected mice had a higher bacterial load on day 14 than day 7 post-infection (p < 0.001). The infected groups of each dietary regimen had a higher bacterial load than their corresponding uninfected group (p < 0.001). Differences in CFU per gram were not found between the uninfected groups of the two dietary regimens (p = 1.00 all organs, Fig. 2b–d).
Intermittent fasting increased intestinal levels of total IgA
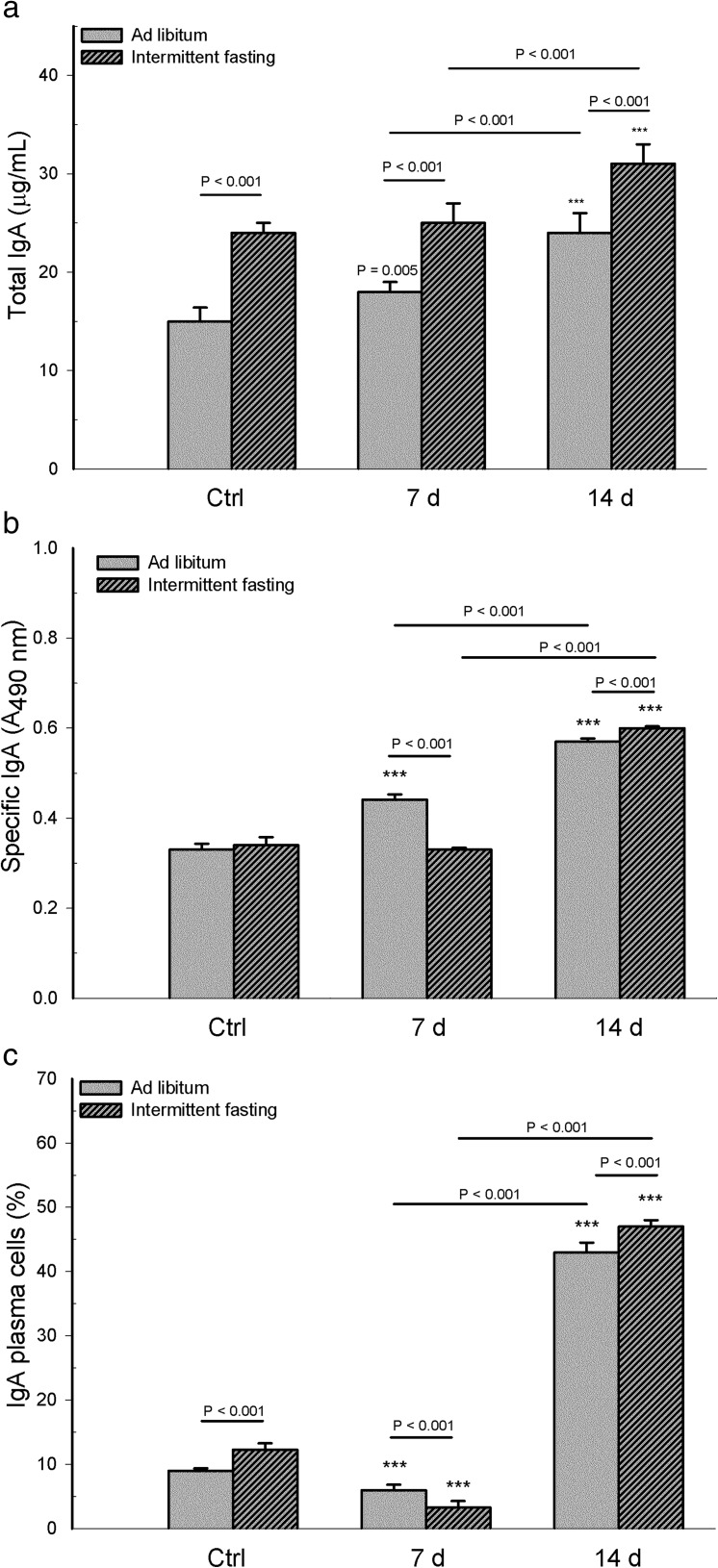
The analysis of IgA antibodies (specific and total) and IgA+ plasma cells indicates that the interaction between diet and infection was statistically significant for total IgA antibody (F 2,30 = 3.85, p = 0.029), specific IgA (F 2,30 = 127.45, p < 0.001), and IgA+ plasma cells (F 2,30 = 37.436, p < 0.001). A higher level of total IgA in intestinal supernatants was found in infected animals with intermittent fasting than those with ad libitum feeding, and this also was true also for uninfected mice (p < 0.001). Within the intermittent fasting and ad libitum feeding groups, there was a higher level of total IgA on day 14 than day 7 post-infection (p < 0.001, Fig. 3a). Within the intermittent fasting group, total IgA levels in infected versus uninfected animals were not different on day 7 (p = 0.273) but were higher on day 14 (p < 0.001). Within the ad libitum feeding group, this parameter was higher in infected versus uninfected animals on both days (p = 0.005 for day 7, p < 0.001 for day 14).
Fig. 3.
From intestinal supernatants in mice with long-term intermittent fasting (followed by 2 weeks of ad libitum feeding post-infection) and from animals with long-term ad libitum feeding, levels were determined of a total IgA in microgram per milliliter and b specific IgA to Salmonella typhimurium in absorbance values at λ = 490 nm (A 490 nm). From intestinal lamina propia, determination was made of c the frequency of IgA+ plasma cells (expressed as percentage of IgA+ relative to the total CD19+ CD138+ plasma cells). Values are expressed as the mean ± SD of six mice per group from one representative assay. Significant differences existed (i) between the infected vs uninfected (Ctrl) subgroups within each dietary regimen (***p < 0.001 or p = 0.005 for total IgA of infected ad libitum group day 7), (ii) between infected mice with intermittent fasting and infected animals with ad libitum feeding, at day 7 and day 14 post-infection, and (iii) between the two infected subgroups of each dietary regimen, comparing day 7 with day 14 post-infection (p value above solid line)
Comparing the infected mice of the two dietary regimens, the specific anti-Salmonella IgA response found in intestinal supernatants was lower on day 7 and higher on day 14 for animals with intermittent fasting (p < 0.001). Within each dietary regimen, specific IgA levels were higher on day 14 than day 7 post-infection (p < 0.001). Considering the mice with intermittent fasting, specific IgA levels of the infected animals were not different on day 7 (p = 0.065) but higher on day 14 (p < 0.001) compared to those uninfected. Considering the mice with ad libitum feeding, specific IgA levels were higher on both post-infection days compared to the uninfected animals (p < 0.001, Fig. 3b). No differences were found in this parameter between the uninfected groups of the two dietary regimens (p = 0.591).
Comparing the frequency of lamina propria IgA+ plasma cells between the infected mice of the two dietary regimens, animals with intermittent fasting had a lower percentage on day 7 and a higher percentage on day 14 (p < 0.001) than those with ad libitum feeding. Within both dietary regimens, this parameter was higher on day 14 than day 7 post-infection (p < 0.001). Compared to the corresponding uninfected group, the infected mice of each dietary protocol showed a lower frequency of IgA+ plasma cells on day 7 and a higher percentage on day 14 (p < 0.001). Considering the uninfected groups, the frequency of IgA+ plasma cells was higher in the animals with intermittent fasting than those with ad libitum feeding (p < 0.001, Fig. 3c).
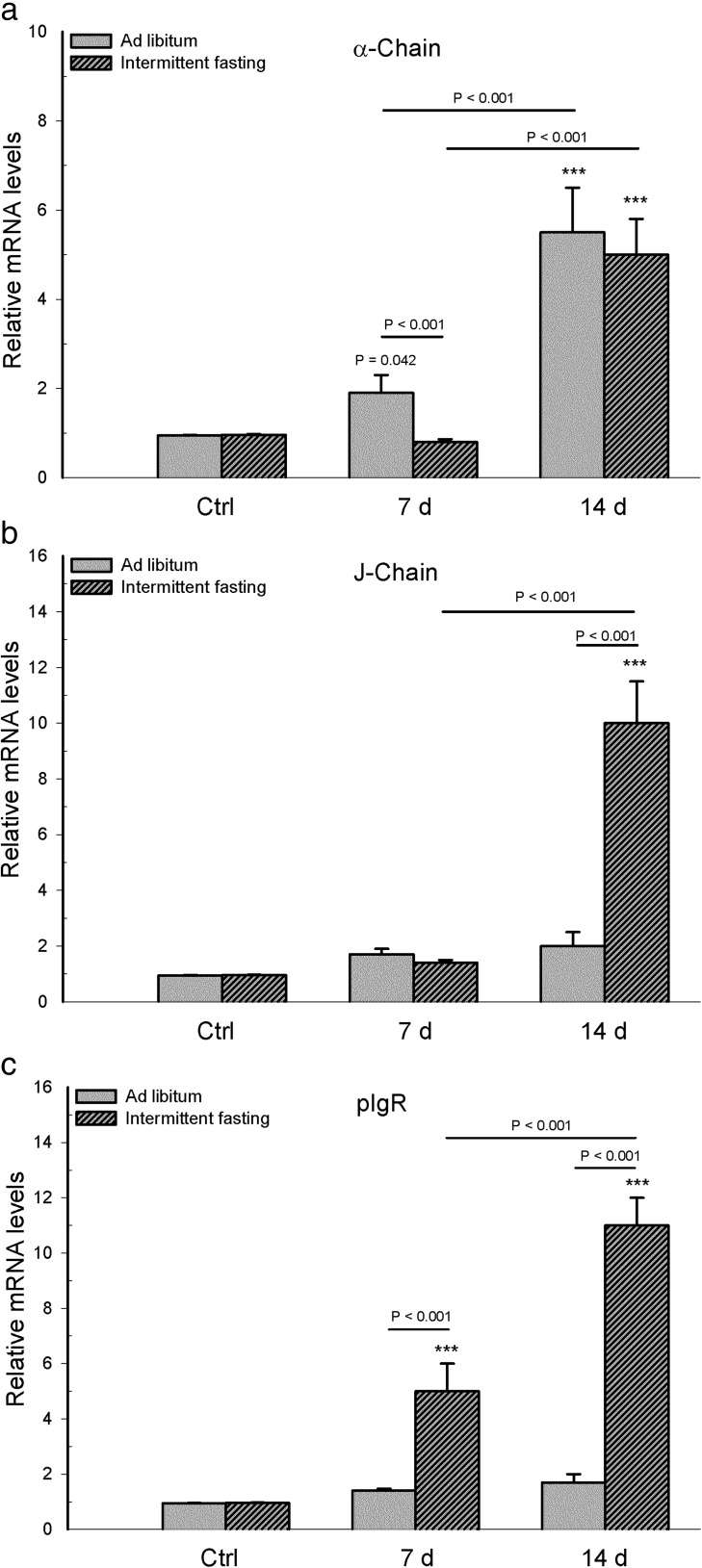
At the intestinal level, intermittent fasting decreased α-chain and J-chain mRNA on day 7 post-infection, while increasing pIgR mRNA on day 7 and decreasing it on day 14
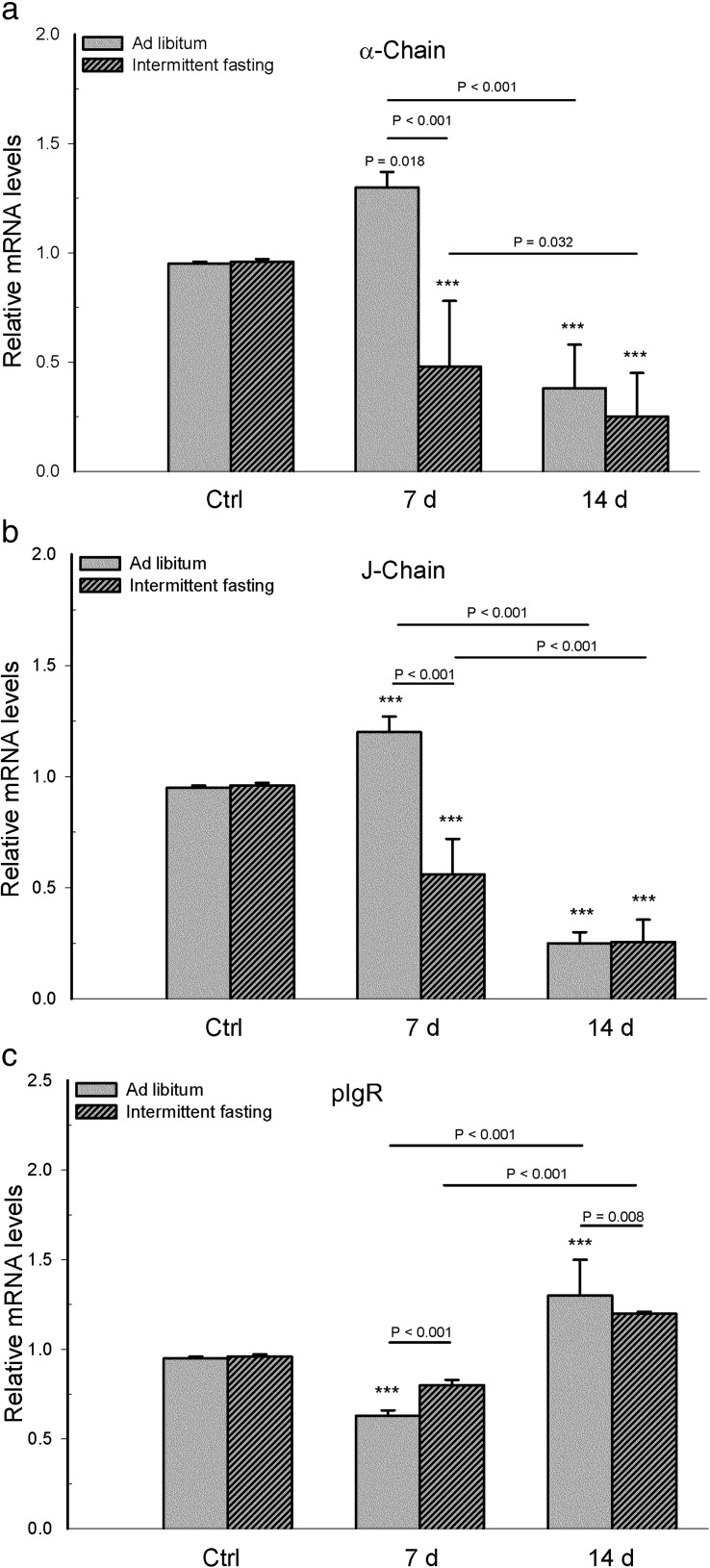
Analysis of mRNA parameters associated with intestinal IgA showed a statistically significant interaction between diet and infection for α-chain mRNA (F 2,30 = 18.66, p < 0.001), J-chain mRNA (F 2,30 = 48.551, p < 0.001), and pIgR mRNA (F 2,30 = 11.356, p < 0.001).
Comparing the α-chain and J-chain mRNA expression in whole mucosa samples between the infected mice of the two dietary regimens, a lower expression was found for animals with intermittent fasting on day 7 (p < 0.001), and no differences were found on day 14 (p = 0.197 for α-chain mRNA, p = 0.921 for J-chain mRNA). Within each dietary regimen, the infected mice had a higher mRNA level of α-chain and J-chain on day 7 than day 14 post-infection (p < 0.001, except p = 0.032 for α-chain of the intermittent fasting group). Compared to their corresponding uninfected group, the infected mice with intermittent fasting had lower levels of α-chain and J-chain mRNA on both post-infection days (p < 0.001), while the infected animals fed ad libitum had higher levels of these two parameters on day 7 and lower levels on day 14 (p < 0.001, except p = 0.018 for α-chain on day 7). No differences in these parameters were found between the uninfected groups of the two dietary regimens (p = 1.0 α-chain; p = 0.712 J-chain, Fig. 4a, b).
Fig. 4.
In mice with long-term intermittent fasting (followed by 2 weeks of ad libitum feeding post-infection) and animals with long-term ad libitum feeding, determinations were made of intestinal mRNA expression of a α-chain, b J-chain, and c pIgR. Values are expressed as the mean ± SD of six mice per group. Significant differences existed (i) between the infected and uninfected (Ctrl) groups within each dietary regimen (***p < 0.001 or p = 0.018 for α-chain mRNA of infected ad libitum group day 7), (ii) between infected mice with intermittent fasting and infected animals with ad libitum feeding, at day 7 and day 14 post-infection, and (iii) between the two infected subgroups of each dietary regimen, comparing day 7 with day 14 post-infection (p value above solid line)
Compared to the infected mice with ad libitum feeding, infected animals with intermittent fasting showed a higher level of pIgR mRNA expression on day 7 (p < 0.001) and a lower level on day 14 (p = 0.008). Regarding the infected groups of each dietary regimen, there was a higher level of pIgR mRNA expression on day 14 than day 7 (p < 0.001). Considering the intermittent fasting groups, there were no differences in pIgR mRNA expression between the uninfected group and each of the infected groups (p = 0.039 for day 7, p = 0.098 for day 14). Considering the ad libitum feeding groups, the level of pIgR mRNA in infected mice was lower on 7 days and higher on 14 day compared to the uninfected group (p < 0.001). No difference in this parameter was found between the uninfected groups of the two dietary regimens (p = 1, Fig. 4c).
Intermittent fasting decreased IgA mRNA and increased pIgR mRNA in the liver of infected mice
Analysis of parameters associated with hepatic IgA mRNA showed that the interaction between diet and infection was not significant for α-chain mRNA (F 2,30 = 1.569 p < 0.229) but was indeed significant for J-chain mRNA (F 2,30 = 63.24, p < 0.001) and pIgR mRNA (F 2,30 = 67.714, p < 0.001).
Transcriptional analysis of IgA was also performed in liver, since, in the proximal intestine of rodents, this antibody derives mostly from hepatobiliary transport from the blood to bile via pIgR transcytosis (Brown and Kloppel 1989). Compared to infected ad libitum mice, infected intermittent fasting animals had lower mRNA levels of α-chain on 7 days (p < 0.001) but similar levels on day 14 (p = 0.178) and higher levels of J-chain mRNA on day 14 (p < 0.001) but similar levels on day 7 (p = 0.511). Considering the infected intermittent fasting groups, mRNA expression of α-chain and J-chain was higher on day 14 versus day 7 (p < 0.001). Considering the infected ad libitum feeding groups, α-chain mRNA expression was also higher on day 14 versus day 7 (p < 0.001), but J-chain mRNA expression was similar on both post-infection days (p = 0.615). Compared to the corresponding uninfected group, infected mice with intermittent fasting showed higher mRNA expression of α-chain and J-chain on day 14 (p < 0.001) and similar expression of these parameters on day 7 (p = 0.459 for α-chain, p = 0.250 for J-chain), while infected animals with ad libitum feeding had higher mRNA expression of α-chain on days 7 (p = 0.042) and 14 (p < 0.001) but similar levels of J-chain mRNA on both post-infection days (p = 0.172 for day 7, p = 0.067 for day 14). Between the uninfected groups of the two dietary regimens, no differences were found in the mRNA levels of α-chain (p = 0.951) or J-chain (p = 0.067) (Fig. 5a, b).
Fig. 5.
In mice with long-term intermittent fasting (followed by 2 weeks of ad libitum feeding post-infection) and animals with long-term ad libitum feeding, determinations were made of hepatic mRNA expression of a α-chain, b J-chain, and c pIgR. Values are expressed as the mean ± SD of six mice per group. Significant differences existed (i) between the infected and uninfected (Ctrl) groups within each dietary regimen (***p < 0.001 or p = 0.042 for α-chain mRNA of the infected ad libitum group at day 7), (ii) between infected mice with intermittent fasting and infected animals with ad libitum feeding, at day 7 and day 14 post-infection, and (iii) between the two infected subgroups of each dietary regimen, comparing day 7 with day 14 post-infection (p value above solid line)
Compared to the infected mice with ad libitum feeding, infected animals with intermittent fasting had higher pIgR mRNA expression in liver on days 7 and 14 (p < 0.001). Considering the infected mice with intermittent fasting, there was a higher pIgR mRNA expression on day 14 than day 7 (p < 0.001). Considering the infected animals with ad libitum feeding, no differences were found between these two days (p = 0.637). Compared to the corresponding uninfected group, the infected mice with intermittent fasting had higher pIgR mRNA expression on days 7 and 14 (p < 0.001), while the infected animals with ad libitum feeding showed no difference in this parameter on either of these days (p = 0.489 for day 7, p = 0.249 for day14). No significant differences were found between the uninfected groups of the two dietary regimens (p = 1.0) (Fig. 5c).
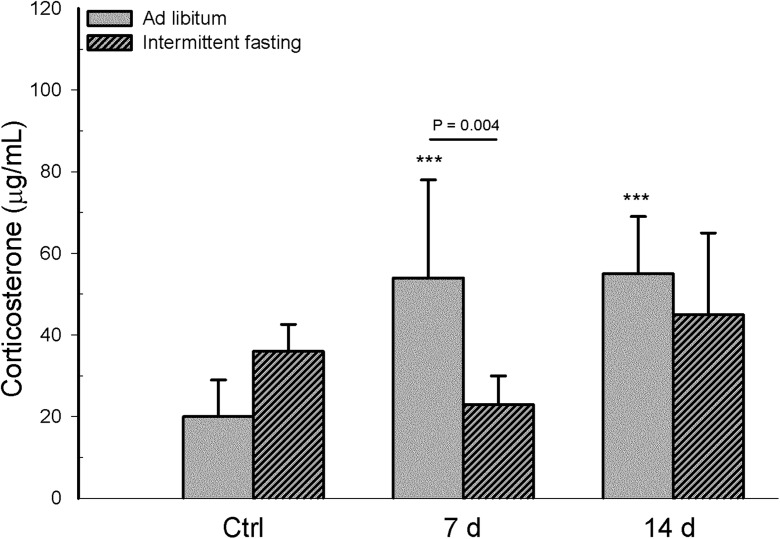
Intermittent fasting produced no effects on corticosterone levels in infected mice
Corticosterone was chosen as a biomarker to assess the stress response elicited by intermittent fasting (Wan et al. 2003). Interaction between diet and infection was statistically significant for corticosterone levels (F 2,30 = 6.313, p = 0.005). Comparing the infected mice of the two dietary regimens, corticosterone levels were lower in the intermittent fasting group on day 7 (p = 0.004) but similar in these two groups on day 14 (p = 0.226). Considering the infected mice within each dietary regimen, no differences were found in this parameter between day 7 and day 14 for either the intermittent fasting (p = 0.050) or ad libitum group (p = 0.878). In comparison with the corresponding uninfected group, infected mice with intermittent fasting showed no significant difference in corticosterone level on either day of assessment (p = 0.184 for day 7, p = 0.497 for day 14), while infected animals with ad libitum feeding had a higher level of this parameter on days 7 and 14 (p < 0.001). On the other hand, there were no differences in corticosterone levels between the uninfected groups of the two dietary regimens (p < 0.074) (Fig. 6).
Fig. 6.
In mice with long-term intermittent fasting (followed by 2 weeks of ad libitum feeding post-infection) and animals with long-term ad libitum feeding, determinations were made of plasma corticosterone levels (ng/mL). Values are expressed as the mean ± SD of six mice per group. Significant differences existed (i) between the infected and uninfected (Ctrl) groups within each dietary regimen (***p < 0.001), (ii) between infected mice with intermittent fasting and infected animals with ad libitum feeding, at day 7 and day 14 post-infection, and (iii) between the two infected subgroups of each dietary regimen, comparing day 7 with day 14 post-infection (p value above solid line)
Discussion
The intermittent fasting protocol is known to lengthen the life span of laboratory animals and provide health benefits (Goodrick et al. 1982; Johnson et al. 2006). A late-onset intermittent fasting protocol was chosen for this study because it provides greater benefits than an early-onset protocol in regard to retarding the age-associated detrimental effects ascribed to oxidative damage (Singh et al. 2012).
Some methodological considerations must be mentioned in regard to the decision to refer to the uninfected groups as “basal” rather than “control.” The uninfected group with intermittent fasting was not a control group in the strict sense, given that infected mice but not uninfected animals under this dietary regimen were switched back to ad libitum feeding at the beginning of the 60th week. Additionally, the age of the uninfected groups matched that of their infected counterparts only on day 7, not on day 14 post-infection.
The mice with intermittent fasting were fed ad libitum after infection in order to maximize their viability, given that the vulnerability to salmonella infection in susceptible animals like BALB/c and C57BL/6 mice (Simon et al. 2011) increases with age, as has been described for the C56BL/6 strain (Ren et al. 2009). Compared to ad libitum feeding, intermittent fasting had no significant impact on body weight, food consumption, and caloric intake of mice prior to infection, as was found in mature BALB/c mice under this dietary regimen in a previous study (Godinez-Victoria et al. 2014). The underlying mechanism of energy maintenance under conditions of intermittent fasting in middle-aged mice is unknown, but gorging on non-fasting days triggers β-hydroxybutyrate, a fat derivative used as an alternative energy supply under conditions of limited availability of glucose and insulin-like growth factor 1(IGF-1). Interestingly, the latter is associated with longevity (Anson et al. 2003).
Compared to the group with long-term ad libitum feeding, long-term intermittent fasting (weeks 19 to 59) followed by post-infection ad libitum feeding (weeks 60–61 or 60–62) had no significant influence on body weight, food consumption, or caloric intake. Regarding long-term intermittent fasting, the influence on the maintenance of body weight and caloric intake has been described in assays based on the paradigmatic model of alternate cycles of intermittent fasting and ad libitum feeding (Hill et al. 1987). Considering the present protocol of intermittent fasting, the underlying mechanism of reducing energy expenditure is unknown. However, assays using repeated cycles of intermittent fasting and ad libitum feeding suggest that this mechanism may involve reduced thermogenesis in brown adipose tissue (Desautels et al. 1988), greater lipoprotein lipase activity (Hill et al. 1987), and/or higher Fsp27 gene expression involved in lipid storage (Karbowska et al. 2012).
Assays in rat and mouse models of infection have documented that there is an age-related increase in post-infection weight loss and susceptibility to S. typhimurium infection (Bradley and Kauffman 1990; Emmerling et al. 1979; Ren et al. 2009). According to the current results with middle-aged mice, the impact of intermittent fasting on the maintenance of caloric intake prior to infection may have established the homeostatic conditions that enhanced resistance to subsequent S. typhimurium infection.
Regarding susceptibility to S. typhimurium infection, it must be pointed out why the IgA response was herein analyzed in the duodenum. Most studies have analyzed the bacterial load in Peyer’s patches from the distal small intestine, regarded as the preferred sites of colonization and invasion of S. typhimurium (Watson and Holden 2010). Nevertheless, this pathogen transits through the proximal region to arrive to Peyer’s patches of the distal intestinal segment of the small intestine (Griffin and McSorley 2011). The luminal surface of the duodenum has a high content of food-digestive enzymes and bile salts, providing erosive conditions that are overcome by S. typhimurium. This pathogen is endowed with virulence factors that enable it to survive harsh environment conditions (Rychlik and Barrow 2005). Thus, the duodenum is a critical point that tests the endurance of S. typhimurium on its way to the distal small intestine, where it traverses Peyer’s patches to eventually invade systemic organs (Rychlik and Barrow 2005).
Given the pivotal role of the duodenum in the capacity of the host immune response to counteract an S. typhimurium infection (Godínez-Victoria et al. 2014), in the present study, the IgA responses were analyzed in this segment of the small intestine. Adding further importance to this intestinal segment, transport of S. typhimurium from the intestinal tract to systemic organs via mesenteric lymph nodes occurs at the intracellular level by CD11c+ lamina propria cells (Uematsu et al. 2006). CD11c+ is a dendritic cell population that includes CD103+ CD11b+ subpopulation distributed preferentially in the duodenum (Mowat and Agace 2014; Pearson et al. 2013).
As aforementioned, evidence of increased susceptibility of older mice to S. typhimurium infection was reported as greater bacterial colonization of Peyer’s patches, spleen, and liver (Ren et al. 2009). Compared to the mice fed ad libitum in the present study, animals with long-term intermittent fasting had decreased bacterial colonization of Peyer’s patches, spleen, and liver as well as an increased total IgA antibody response. Intestinal IgA is known to contribute to resistance against S. typhimurium infection by hampering gut bacterial colonization of the lumen and enhancing clearance. These effects in turn decrease bacterial translocation to the spleen and liver (Griffin and McSorley 2011; Mastroeni 2002; Michetti et al. 1992; Wijburg et al. 2006).
In addition to the essential role of IgA in luminal bacterial clearance, other components not measured in this study may have contributed to the enhancement of bacterial resistance induced by intermittent fasting. For example, it is known that phagocytes have an important role in the systemic control of Salmonella infection by killing bacteria translocated to the spleen and liver (Mastroeni 2002).
Both in a previous study with mature mice (Godinez-Victoria et al. 2014) and the present study with middle-aged mice, long-term intermittent fasting (followed by 2 weeks of post-infection ad libitum feeding) decreased the bacterial load in Peyer’s patches (day 14) and feces during all days post-infection except day 3 at the intestinal level and up-modulated total IgA, specific IgA, and the number of IgA+ plasma cells (the latter two only on day 14). The present protocol of long-term intermittent fasting had a metabolic impact, evidenced by the maintenance of body weight and avoidance of energy expenditure. This was probably critical for resistance during the salmonella infection. Additionally, switching to ad libitum feeding after intermittent fasting may have also contributed to the higher responses seen on day 14 than day 7 post-infection, referring to IgA, IgA plasma cells, and hepatic pIgR mRNA expression. That is, switching to ad libitum feeding would reset circadian rhythms involved in the attenuation of aging, leading to a synchrony of metabolic and physiological events (Froy and Miskin 2010). The latter may underlie metabolic pathways for counteracting the deleterious effects of age on intestinal immunity, thus improving the IgA responses for bacterial resolution (Schmucker et al. 2003; Michetti et al. 1992; Wijburg et al. 2006).
Unlike in the previous study with mature mice (Godinez-Victoria et al. 2014), in the present study, intermittent fasting (followed by 2 weeks of post-infection ad libitum feeding) down-modulated intestinal α- and J-chain mRNA expression on day 7. This suggests that S. typhimurium infection following intermittent fasting (1) did not counteract the effects of age on the down-modulation of α- and J-chain transcripts for specific IgA synthesis, probably because of decreasing the maturation of IgA immunoblasts (Schmucker et al. 2003; Thoreux et al. 2000), and (2) increased the level of total IgA. The latter increase probably resulted from a greater number of IgA+ plasma cells derived from an existing pool of IgA+ B cell clones, which would come from IgM+ B cell precursors that underwent IgA class switch recombination (Suzuki and Fagarasan 2009).
Hepatic expression of α- and J-chain transcripts was herein analyzed in order to address the impact of the present protocol of long-term intermittent fasting on the contribution of the liver to the intestinal IgA response (Brown and Kloppel 1989). Regarding the infected ad libitum mice in the intermittent fasting group, the hepatic level of α-chain mRNA was lower on day 7 and similar on day 14, while that of J-chain RNA was higher on day 14 and similar on day 7. Contrarily, both parameters were previously reported to decrease in mature mice undergoing intermittent fasting followed by infection with S. typhimurium (Godinez-Victoria et al. 2014). Under conditions of infection, therefore, the effect of intermittent fasting on the increased α-chain and J-chain mRNA expression is modulated by age.
In this study, a presumable contribution of the liver to intestinal IgA levels was related to the increase in pIgR mRNA levels. Most IgA in the proximal intestine of rodents results from the hepatobiliary transport of this antibody from blood to bile via pIgR transcytosis (Asano and Komiyama 2011; Brown and Kloppel 1989). The current results indicate that the present protocol of intermittent fasting (followed by 2 weeks of ad libitum feeding) enhanced hepatic pIgR mRNA levels to a greater extent on day 14 than day 7 post-infection, while increasing this parameter at the intestinal level on day 7. It has been documented that age leads to a decline in pIgR expression (Yanagihara et al. 2004). However, in the middle-aged mice used presently, the present protocol of intermittent fasting followed by bacterial infection resulted in greater up-modulation of pIgR mRNA in liver than previously reported in mature mice (Godinez-Victoria et al. 2014).
These findings suggest that the up-modulating influence of intermittent fasting (followed by ad libitum feeding) on pIgR mRNA may explain, in part, the increased level of IgA by favoring hepatic (and intestinal) transcytosis of this antibody derived from an existing pool of IgA+ plasma cells. When comparing older and younger BALB/c mice, it has also been found that with age, there is greater bactericidal activity following salmonella uptake by macrophages (Smallwood et al. 2011).
With long-term intermittent fasting (followed by 2 weeks of ad libitum feeding), the mechanism underlying the elicitation of pIgR mRNA is unknown but may result from metabolic signals that promote transcriptional expression of the tumor necrosis factor (TNF)-α, as reported for mice under short-term caloric restriction followed by a return to ad libitum feeding (Kliewer et al. 2015). Although expression of pIgR is constitutive, it also can be modulated by pro-inflammatory cytokines such as TNF-α (Asano and Komiyama 2011).
Intermittent fasting is known to elicit a hypothalamus-pituitary-adrenal (HPA) axis stress response, causing corticosterone release into the blood stream (Wan et al. 2003). Moreover, corticosterone modulates the expression of IgA and pIgR under conditions of stress (Jarillo-Luna et al. 2007; Reyna-Garfias et al. 2010).
However, the present protocol of long-term intermittent fasting did not influence corticosterone levels in middle-aged mice. This result is in contrast to the up- and down-modulation found previously with the same protocol used for mature mice (Godinez-Victoria et al. 2014). Corticosterone levels found in this study may reflect an adaptation to stress signals elicited by long-term intermittent fasting followed by bacterial infection and 2 weeks of ad libitum feeding. Corticosterone has a role in adaptive changes that avoid energy expenditure and facilitate the replenishment of stored fat, as evidenced by a previous report of low caloric intake followed by a return to ad libitum feeding (Dulloo et al. 1990).
As described in mature mice without infection, intermittent fasting triggered the total IgA and IgA plasma cell responses whereas up- or down-modulated the α-chain, J-chain, and pIgR mRNA expression at intestinal and hepatic levels, respectively (Godinez-Victoria et al. 2014). In the current study, the only significant changes found in uninfected mice under intermittent fasting were an increase in total levels of IgA and a greater frequency of IgA+ plasma cells. This indicates that the modulatory role of intermittent fasting on IgA and IgA-associated parameters depends not only on age but also on health status.
The high anti-salmonella IgA levels in the uninfected groups of both dietary regimens may be explained by the cumulative increase in the luminal IgA response caused by aging (Ebersole et al. 1985). Compared to the ileum and colon, colonization of the duodenum by commensal microorganisms is lower (Mowat and Agace 2014). However, the ability of microbiota to induce anti-salmonella IgA production in an antigen-unspecific manner (Wijburg et al. 2006) cannot be ruled out in this intestinal region. Furthermore, aging substantially decreases the ability of IgA to bind to bacterial antigens (Senda et al. 1988).
An obvious limitation of the present study is that some parameters were analyzed only at the transcriptional (not protein) level and were not evaluated in the spleen, one of the main systemic targets of S. typhimurium. Nevertheless, we herein provide experimental evidence of the role of intermittent fasting in enhancing resistance to S. typhimurium infection in middle-aged mice. Future studies are needed to address the possible impact of intermittent fasting on typhoid infection in middle-aged humans. Although in this study, long-term intermittent fasting enhanced resistance to salmonella infection, future studies should address its potential impact on cumulative metabolic abnormalities related, for example, to insulin resistance, as described with short-term intermittent fasting followed by a return to ad libitum feeding (Kliewer et al. 2015).
In conclusion, long-term intermittent fasting (followed by 2 weeks of ad libitum feeding post-infection) in middle-aged mice led to lower intestinal and systemic bacterial loads after S. typhimurium infection as well as higher total intestinal IgA (with or without infection), compared to animals with long-term ad libitum feeding. This increase in IgA was associated with enhanced pIgR mRNA expression in the intestine on day 7 and in the liver on day 7 and 14 post-infection. On the other hand, there were no differences between the animals of these two dietary regimens in corticosterone levels, body weight, or food and caloric intake. Hence, it is possible that the present protocol of intermittent fasting elicited metabolic signals for the maintenance of body weight and caloric intake, which in turn may have induced greater hepatic and intestinal pIgR mRNA. The increase found presently in intestinal IgA levels was probably due to augmented intestinal IgA transcytosis via pIgR. It is likely that there was a synergistic effect between the relatively high level of intestinal IgA and the killing of bacteria in systemic organs by phagocytes (not measured herein) to resolve the S. typhimurium infection.
References
- Anisimov VN, Egorov MV, Krasilshchikova MS, et al. Effects of the mitochondria-targeted antioxidant SkQ1 on lifespan of rodents. Aging. 2011;3:1110–1119. doi: 10.18632/aging.100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anson RM, Guo Z, de R C, et al. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A. 2003;100:6216–6220. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano M, Komiyama K. Polymeric immunoglobulin receptor. J Oral Sci. 2011;53:147–156. doi: 10.2334/josnusd.53.147. [DOI] [PubMed] [Google Scholar]
- Beer AM, Ruffer A, Balles J, Ostermann T. Progression of intestinal secretory immunoglobulin A and the condition of the patients during naturopathic therapy and fasting therapy. Forsch Komplementarmed Klass Naturheilkd. 2001;8:346–53. doi: 10.1159/000057251. [DOI] [PubMed] [Google Scholar]
- Bradley SF, Kauffman CA. Aging and the response to Salmonella infection. Exp Gerontol. 1990;25:75–80. doi: 10.1016/0531-5565(90)90012-Q. [DOI] [PubMed] [Google Scholar]
- Brown WR, Kloppel TM. The role of the liver in translocation of IgA into the gastrointestinal tract. Immunol Investig. 1989;18:269–285. doi: 10.3109/08820138909112242. [DOI] [PubMed] [Google Scholar]
- Burns-Guydish SM, Olomu IN, Zhao H, Wong RJ, Stevenson DK, Contag CH. Monitoring age-related susceptibility of young mice to oral Salmonella enterica serovar Typhimurium infection using an in vivo murine model. Pediatr Res. 2005;58:153–158. doi: 10.1203/01.PDR.0000157725.44213.C4. [DOI] [PubMed] [Google Scholar]
- Daniels CK, Schmucker DL, Bazin H, Jones AL. Immunoglobulin A receptor of rat small intestinal enterocytes is unaffected by aging. Gastroenterology. 1988;94:1432–1440. doi: 10.1016/0016-5085(88)90683-x. [DOI] [PubMed] [Google Scholar]
- Desautels M, Dulos RA. Effects of repeated cycles of fasting-refeeding on brown adipose tissue composition in mice. Am J Physiol. 1988;255:E120–E128. doi: 10.1152/ajpendo.1988.255.2.E120. [DOI] [PubMed] [Google Scholar]
- Drago-Serrano ME, Rivera-Aguilar V, Resendiz-Albor AA, Campos-Rodriguez R. Lactoferrin increases both resistance to Salmonella typhimurium infection and the production of antibodies in mice. Immunol Lett. 2010;134:35–46. doi: 10.1016/j.imlet.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Duan W, Guo Z, Mattson MP. Brain-derived neurotrophic factor mediates an excitoprotective effect of dietary restriction in mice. J Neurochem. 2001;76:619–626. doi: 10.1046/j.1471-4159.2001.00071.x. [DOI] [PubMed] [Google Scholar]
- Dulloo AG, Seydoux J, Girardier L. Role of corticosterone in adaptive changes in energy expenditure during refeeding after low calorie intake. Am J Physiol. 1990;259:E658–E664. doi: 10.1152/ajpendo.1990.259.5.E658. [DOI] [PubMed] [Google Scholar]
- Ebersole JL, Steffen MJ. Aging effects on secretory IgA immune responses. Immunol Investig. 1989;18:59–68. doi: 10.3109/08820138909112227. [DOI] [PubMed] [Google Scholar]
- Ebersole JL, Smith DJ, Taubman MA. Secretory immune responses in ageing rats. I. Immunoglobulin levels. Immunology. 1985;56:345–350. [PMC free article] [PubMed] [Google Scholar]
- Ebersole JL, Steffen MJ, Pappo J. Secretory immune responses in ageing rats. II. Phenotype distribution of lymphocytes in secretory and lymphoid tissues. Immunology. 1988;64:289–294. [PMC free article] [PubMed] [Google Scholar]
- Emmerling P, Hof H, Finger H. Age-related defense against infection with intracellular pathogens. Gerontology. 1979;25:327–336. doi: 10.1159/000212361. [DOI] [PubMed] [Google Scholar]
- Flurkey KCJ, Harrison DE. The mouse in aging research. In: Fox JG, editor. The mouse in biomedical research. 2. Elsevier: Burlington; 2007. pp. 637–624. [Google Scholar]
- Froy O, Miskin R. Effect of feeding regimens on circadian rhythms: implications for aging and longevity. Aging (Albany NY) 2010;2:7–27. doi: 10.18632/aging.100116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinez-Victoria M, Campos-Rodriguez R, Rivera-Aguilar V, et al. Intermittent fasting promotes bacterial clearance and intestinal IgA production in Salmonella typhimurium-infected mice. Scand J Immunol. 2014;79:315–324. doi: 10.1111/sji.12163. [DOI] [PubMed] [Google Scholar]
- Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider NL. Effects of intermittent feeding upon growth and life span in rats. Gerontology. 1982;28:233–241. doi: 10.1159/000212538. [DOI] [PubMed] [Google Scholar]
- Griffin AJ, McSorley SJ. Development of protective immunity to Salmonella, a mucosal pathogen with a systemic agenda. Mucosal Immunol. 2011;4:371–382. doi: 10.1038/mi.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JO, Thacker S, Newby D, Nickel M, Digirolamo M. A comparison of constant feeding with bouts of fasting-refeeding at three levels of nutrition in the rat. Int J Obes. 1987;11:251–262. [PubMed] [Google Scholar]
- Jarillo-Luna A, Rivera-Aguilar V, Garfias HR, Lara-Padilla E, Kormanovsky A, Campos-Rodríguez R. Effect of repeated restraint stress on the levels of intestinal IgA in mice. Psychoneuroendocrinology. 2007;32:681–692. doi: 10.1016/j.psyneuen.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Johnson JB, Laub DR, John S. The effect on health of alternate day calorie restriction: eating less and more than needed on alternate days prolongs life. Med Hypotheses. 2006;67:209–211. doi: 10.1016/j.mehy.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Karbowska J, Kochan Z. Intermittent fasting up-regulates Fsp27/Cidec gene expression in white adipose tissue. Nutrition. 2012;28:294–299. doi: 10.1016/j.nut.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Kliewer KL, Ke JY, Lee HY, Stout MB, Cole RM, Samuel VT, Shulman GI, Belury MA. Short-term food restriction followed by controlled refeeding promotes gorging behavior, enhances fat deposition, and diminishes insulin sensitivity in mice. J Nutr Biochem. 2015;26:721–728. doi: 10.1016/j.jnutbio.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wang Z, Zuo Z. Chronic intermittent fasting improves cognitive functions and brain structures in mice. PLoS One. 2013;8:e66069. doi: 10.1371/journal.pone.0066069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroeni P. Immunity to systemic Salmonella infections. Curr Mol Med. 2002;2:393–406. doi: 10.2174/1566524023362492. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Duan W, Guo Z. Meal size and frequency affect neuronal plasticity and vulnerability to disease: cellular and molecular mechanisms. J Neurochem. 2003;84:417–431. doi: 10.1046/j.1471-4159.2003.01586.x. [DOI] [PubMed] [Google Scholar]
- Michetti P, Mahan MJ, Slauch JM, Mekalanos JJ, Neutra MR. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect Immun. 1992;60:1786–1792. doi: 10.1128/iai.60.5.1786-1792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014;14:667–685. doi: 10.1038/nri3738. [DOI] [PubMed] [Google Scholar]
- Persson EK, Scott CL, Mowat AM, Agace WW. Dendritic cell subsets in the intestinal lamina propria: ontogeny and function. Eur J Immunol. 2013;43:3098–3107. doi: 10.1002/eji.201343740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z, Gay R, Thomas A, Pae M, Wu D, Logsdon L, Mecsas J, Meydani SN. Effect of age on susceptibility to Salmonella Typhimurium infection in C57BL/6 mice. J Med Microbiol. 2009;58:1559–1567. doi: 10.1099/jmm.0.013250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reséndiz-Albor AA, Reina-Garfias H, Rojas-Hernández S, Jarillo-Luna A, Rivera-Aguilar V, Miliar-García A, Campos-Rodríguez R. Regionalization of pIgR expression in the mucosa of mouse small intestine. Immunol Lett. 2010;128:59–67. doi: 10.1016/j.imlet.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Reyna-Garfias H, Miliar A, Jarillo-Luna A, Rivera-Aguilar V, Pacheco-Yepez J, Baeza I, Campos-Rodríguez R. Repeated restraint stress increases IgA concentration in rat small intestine. Brain Behav Immun. 2010;24:110–118. doi: 10.1016/j.bbi.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Rhee SJ, Walker WA, Cherayil BJ. Developmentally regulated intestinal expression of IFN-gamma and its target genes and the age-specific response to enteric Salmonella infection. J Immunol. 2005;175:1127–1136. doi: 10.4049/jimmunol.175.2.1127. [DOI] [PubMed] [Google Scholar]
- Rychlik I, Barrow PA. Salmonella stress management and its relevance to behaviour during intestinal colonisation and infection. FEMS Microbiol Rev. 2005;29:1021–1040. doi: 10.1016/j.femsre.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Santiago AF, Fernandes RM, Santos BP, Assis FA, Oliveira RP, Carvalho CR, Faria AM. Role of mesenteric lymph nodes and aging in secretory IgA production in mice. Cell Immunol. 2008;253:5–10. doi: 10.1016/j.cellimm.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Schmucker DL, Owen RL, Outenreath R, Thoreux K. Basis for the age-related decline in intestinal mucosal immunity. Clin Dev Immunol. 2003;10:167–172. doi: 10.1080/10446670310001642168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senda S, Cheng E, Kawanishi H. Aging-associated changes in murine intestinal immunoglobulin A and M secretions. Scand J Immunol. 1988;27:157–164. doi: 10.1111/j.1365-3083.1988.tb02334.x. [DOI] [PubMed] [Google Scholar]
- Simon R, Tennant SM, Galen JE, Levine MM. Mouse models to assess the efficacy of non-typhoidal Salmonella vaccines: revisiting the role of host innate susceptibility and routes of challenge. Vaccine. 2011;29:5094–5106. doi: 10.1016/j.vaccine.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Lakhanpal D, Kumar S, Sharma S, Kataria H, Kaur M, Kaur G. Late-onset intermittent fasting dietary restriction as a potential intervention to retard age-associated brain function impairments in male rats. Age (Dordr) 2012;34:917–933. doi: 10.1007/s11357-011-9289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood HS, Lopez-Ferrer D, Squier TC. Aging enhances the production of reactive oxygen species and bactericidal activity in peritoneal macrophages by upregulating classical activation pathways. Biochemistry. 2011;50:9911–9922. doi: 10.1021/bi2011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Fagarasan S. Diverse regulatory pathways for IgA synthesis in the gut. Mucosal Immunol. 2009;2:468–471. doi: 10.1038/mi.2009.107. [DOI] [PubMed] [Google Scholar]
- Thoreux K, Owen RL, Schmucker DL. Intestinal lymphocyte number, migration and antibody secretion in young and old rats. Immunology. 2000;101:161–167. doi: 10.1046/j.1365-2567.2000.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu S, Jang MH, Chevrier N, Guo Z, Kumagai Y, Yamamoto M, Kato H, Sougawa N, Matsui H, Kuwata H, Hemmi H, Coban C, Kawai T, Ishii KJ, Takeuchi O, Miyasaka M, Takeda K, Akira S. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol. 2006;7:868–874. doi: 10.1038/ni1362. [DOI] [PubMed] [Google Scholar]
- Van der Heijden PJ, Bianchi AT, Stok W, Bokhout BA. Background (spontaneous) immunoglobulin production in the murine small intestine as a function of age. Immunology. 1988;65:243–248. [PMC free article] [PubMed] [Google Scholar]
- Viloria M, Lara-Padilla E, Campos-Rodríguez R, et al. Effect of moderate exercise on IgA levels and lymphocyte count in mouse intestine. Immunol Investig. 2011;40:640–656. doi: 10.3109/08820139.2011.575425. [DOI] [PubMed] [Google Scholar]
- Wan R, Camandola S, Mattson MP. Intermittent fasting and dietary supplementation with 2-deoxy-D-glucose improve functional and metabolic cardiovascular risk factors in rats. FASEB J. 2003;17:1133–1134. doi: 10.1096/fj.02-0996fje. [DOI] [PubMed] [Google Scholar]
- Watson KG, Holden DW. Dynamics of growth and dissemination of Salmonella in vivo. Cell Microbiol. 2010;12:1389–1397. doi: 10.1111/j.1462-5822.2010.01511.x. [DOI] [PubMed] [Google Scholar]
- Wijburg OL, Uren TK, Simpfendorfer K, Johansen FE, Brandtzaeg P, Strugnell RA. Innate secretory antibodies protect against natural Salmonella typhimurium infection. J Exp Med. 2006;203:21–26. doi: 10.1084/jem.20052093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara T, Kumagai Y, Norose Y, Moro I, Nanno M, Murakami M, Takahashi H. Age-dependent decrease of polymeric Ig receptor expression and IgA elevation in ddY mice: a possible cause of IgA nephropathy. Lab Investig. 2004;84:63–70. doi: 10.1038/labinvest.3700012. [DOI] [PubMed] [Google Scholar]
- Yuan R, Tsaih SW, Petkova SB et al (2009) Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell 8:277–287. doi:10.1111/j.1474-9726.2009.00478.x [DOI] [PMC free article] [PubMed]