Abstract
To investigate various risk factors of cognitive decline in the very old, we studied 494 subjects over 85 years old without diagnosis of dementia at baseline from the Tokyo Oldest Old Survey on Total Health, an ongoing, community-based cohort in Japan. Cognitive function was assessed at baseline and at 3-year follow-up using Mini-Mental State Examination (MMSE). Plasma samples were assayed for levels of cytomegalovirus (CMV) immunoglobulin G (IgG) antibodies, tumor necrosis factor-alpha, interleukin-6, and blood chemistry. Carotid artery plaques were measured using an ultrasonography. In the cross-sectional analyses using Tobit regression, individuals with high carotid artery plaque score (≥5.0) had MMSE scores that were 1.08 points lower compared to those with no plaque (95 % confidence interval (CI) −1.95 to −0.20; p = 0.016), adjusted for age, sex, and education. Individuals with CMV IgG titers in the highest quartile had MMSE scores that were 1.47 points lower compared to individuals in the lowest quartile (95 % CI −2.44 to −0.50; p = 0.003). CMV and carotid atherosclerosis showed evidence of an interaction, where the association between CMV and MMSE was present only in subjects with carotid artery plaque. In the longitudinal analyses using linear regression, carotid atherosclerosis, smoking, low grip strength, and poor activities of daily living (ADL) status were associated with faster cognitive decline, adjusted for age, sex, education, and baseline cognitive function. Our findings suggest that carotid atherosclerosis is consistently associated with low cognitive function in the very old and modifies the association between latent CMV infection and cognition.
Keywords: Cognitive decline, Atherosclerosis, Cytomegalovirus, Very old, Inflammation
Introduction
With remarkable improvements in life expectancy over the past century, the number of individuals with dementia, estimated to be 24 million in 2001 globally, is expected to double every 20 years to 81 million in 2040 (Ferri et al. 2005). Population-based studies demonstrated an exponential increase in dementia incidence after the age of 65, with prevalence ranging from 18 to 38 % among those aged 85 and older (Gardner et al. 2013) and more than 50 % in centenarians (Yang et al. 2013). Identification of preventative strategies for maintenance of cognitive function well into advanced age is a key element in counteracting the dementia epidemic.
In observational studies, vascular risk factors, such as hypertension, obesity, and metabolic syndrome at midlife, have been associated with increased risk of dementia (Skoog et al. 1996; Yaffe et al. 2004). However, the associations between vascular factors and cognition have been inconsistent and even reversed (Van den Berg et al. 2007) in the very old, aged 85 and older. This is partly because age-related decline in vascular function, characterized by lowering cholesterol levels and body mass index (BMI), could mask the effect of lifetime exposures to atherogenic and neurodegenerative processes. To date, few studies have examined biomarkers of lifelong burden of atherosclerosis, such as carotid artery plaques and arterial stiffness in relation to cognitive decline in the very old (Van Exel et al. 2002).
Meanwhile, emerging epidemiological evidence suggests that inflammation triggered by viral infections such as cytomegalovirus (CMV) is a potent risk factor for not only atherosclerosis but vascular dementia and Alzheimer’s disease in old age (Katan et al. 2013; Strandberg et al. 2003). Given a greater risk of age-related deterioration of immune system, the effect of latent CMV infection on atherogenic and neurodegenerative process might be enhanced at advanced ages as compared to traditional vascular risk factors. However, only few epidemiological studies have shown the impact of CMV infection on the relationship between carotid atherosclerosis and cognitive function (Katan et al. 2013; Elkind et al. 2010).
We hypothesized that latent CMV infection and inflammatory burden could be associated with cognitive decline at advanced ages, and atherosclerosis is a possible modifier of this process. Therefore, we investigated both the cross-sectional and longitudinal association of carotid atherosclerosis, CMV IgG titers, and inflammation with cognitive function in comparison to traditional vascular risk factors within a population of the very old.
Methods
Subjects
Data are from the Tokyo Oldest Old Survey on Total Health (TOOTH), an ongoing, longitudinal research project at Keio University, which investigates the multidimensional structure of health in the very old (Arai et al. 2010). Briefly, 542 inhabitants of Tokyo, aged 85 years and older, were randomly selected between March 2008 and November 2009. Of those, 504 were nondemented based on self-report and anti-dementia drug use history. Four hundred and ninety-four had a Mini-Mental State Examination (MMSE) score at baseline and were included in the cross-sectional analyses. All participants were prospectively followed up for 3 years until November 2012 by annual telephone contact or mail survey. At 3 years, those who remained in the cohort were examined according to the same protocol as in the baseline survey. At the 3-year follow-up, 76 had died, and 18 had unknown survival status (4 had dropped out, and 14 had at least one vital status follow-up), 65 participated to telephone or mail survey only, and 5 declined for MMSE assessment; thus, 330 had an updated record of MMSE at follow-up. A total of 328 without severe cognitive impairment at baseline (MMSE score of 18 or above) comprised the longitudinal analyses to identify factors associated with cognitive decline.
Written informed consent to participate was obtained either from the participants or proxy when individuals lacked the capacity to consent. This research was approved by the ethics committees at Keio University School of Medicine (N0. 19-47, 2007). TOOTH is registered in the UMIN-Clinical Trial Registry (CTR) as UMIN-CTR (ID: UMIN000001842).
Assessment
Subjects were examined by trained geriatricians for medical conditions, medications, and physical functional status (Arai et al. 2010). BMI (kg/m2) was calculated from the direct height and weight measurements. High blood pressure was defined as a systolic blood pressure of 160 mm Hg and greater or taking hypertension medication. Nonfasting blood samples were obtained from all participants and were stored at −80 °C until subsequent assay. Biochemical measures, such as plasma concentrations of total and high-density lipoprotein cholesterol, were measured from nonfasting blood samples using standard assay procedures. Low-density lipoprotein cholesterol (LDL-C) was calculated using Friedwald’s formula when the triglyseride level was <400 mg/dl. CMV immunoglobulin G (IgG) antibody titers were measured by an enzyme immunoassay kit (Denka Seiken Co, Ltd., Tokyo Japan). Inter-assay coefficient of variation (CV) of CMV IgG titer was 5.66 %. Plasma levels of interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-alpha) were measured using enzyme-linked immunosorbent assay (ELISA) kits (Quantikine HS [Human IL-6] and Quantikine HS [Human TNF-alpha], respectively; R&D Systems, Minneapolis, MN). Inter-assay CVs of IL-6 and TNF-alpha were 9.43 and 8.72 %, respectively. Carotid artery plaques were measured using a B-mode ultrasonography with a 10-MHz linear transducer (Hitachi EUB-525, Hitachi Medical, Tokyo, Japan). Common carotid artery (CCA) and internal carotid artery (ICA) were examined thoroughly for the presence of atherosclerotic plaques, defined as a clearly identifiable area of increased focal thickness (≥1.2 mm) of the intima-media layer. To assess the burden of subclinical atherosclerosis, we calculated the plaque score (PS) by summing all plaque thicknesses in both carotid systems (Handa et al. 1995). Plaque scores were classified into three categories as following: no plaque (PS < 1.2), mild atherosclerosis (1.2 ≤ PS < 5.0), and moderate to severe atherosclerosis (PS ≥ 5.0). All examinations were performed by a single physician blinded to the subject’s clinical information.
Outcome
The outcome of interest was cognitive function, measured by MMSE. MMSE consists of 30 items which assess orientation, memory, attention, calculation, language, and written and visual construction (Folstein et al. 1975). Scores range from 0 to 30. Higher scores represent higher cognitive function. Although MMSE has been widely used in studies of the oldest old, validation of the appropriate cutoff score is limited in this age group. Thus, we referred to the classification used in the Newcastle 85+ Study (Collerton et al. 2009), taking into account the similarity in study design. Scores above 26 represent normal cognitive function, while scores of 22–25 and 18–21 represent mild and moderate cognitive impairment, respectively. Scores below 17 indicate severe cognitive impairment. In the longitudinal analyses, we set the outcome as the annual change in MMSE over 3 years, where the difference in score at baseline and at 3-year follow-up was divided by follow-up time length for each individual.
Statistical analyses
Continuous variables were expressed as mean and standard deviation, while categorical variables were expressed as percentage. For comparisons between groups, we used two-sided t test for continuous data and chi-squared test for categorical data. Subject characteristics included sociodemographic factors (age, sex, education status, smoking, drinking, living alone, and BMI), vascular factors (CMV IgG antibody titer, carotid artery plaque score, TNF-alpha, IL-6, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol), medical history (hypertension, cardiovascular disease, diabetes mellitus, and chronic kidney disease (estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2)), and frailty (activities of daily living (ADL), Instrumental Activities of Daily Living (IADL) and grip strength).
For the cross-sectional analyses, multivariable Tobit regression models assessed the association of baseline characteristics and cognitive function. The Tobit approach has long been used to model data where there is a floor and/or ceiling effect (censoring) in the outcome (Tobin 1958). In the presence of these effects, an ordinary regression would give misleading results due to the attenuation of observations which have reached the minimum or maximum value when in fact, the value changes as the independent variable changes. The general idea of Tobit regression is that it models both the probability of reaching the floor or ceiling as well as the development over time between the floor and ceiling (Austin et al. 2000). Both the lower and upper limits (0 and 30, respectively) were specified in the models. Characteristics were assessed one by one, unadjusted and adjusted for age, sex, and education.
For the longitudinal analyses, multivariable linear regression models were used to investigate the association between baseline characteristics and annual MMSE change over the 3-year follow-up. The analyses were restricted to individuals with an MMSE score of 18 or above to exclude those with signs of severe cognitive impairment already at baseline. Characteristics were assessed one by one, unadjusted and adjusted for age, sex, education, and baseline MMSE score (categorized into three groups: ≥26, 22–25, 18–21).
In both the cross-sectional and longitudinal analyses, CMV IgG titers, inflammatory markers (TNF-alpha and IL-6), low- and high-density lipoprotein cholesterol, and grip strength were categorized into quartiles to detect nonlinear associations with the outcome. The lowest category was used as reference (coefficient = 0 or odds ratio = 1). Rationale for choice of interactions was based on literature and biological plausibility and was examined statistically using test for interaction. Results of the cross-sectional Tobit regression analyses were compared to that of normal linear regression. To compare fit, residual plots as well as Akaike information criterion (AIC) and Bayesian information criterion (BIC) measures were used. A value of p < 0.05 (two-tailed) was considered statistically significant in all models. Analyses were conducted using STATA software, version 10.1 (StataCorp LP, College Station, TX, USA).
Results
Table 1 outlines the baseline characteristics of 494 subjects included in the cross-sectional analyses, stratified by baseline cognitive function. Median age was 87.2 years, 55 % were women, and 37 % had attended high school. The majority of the population was noncurrent smokers (93 %) and noncurrent drinkers (64 %). Approximately 72 % had hypertension, 21 % had a history of cardiovascular disease, 12 % had diabetes mellitus, and 44 % had chronic kidney disease. At baseline, 71 % of the subjects had normal cognitive function (MMSE 26-30), 23 % had mild cognitive impairment (MMSE 22-25), 5 % had moderate cognitive impairment (MMSE 18-21), and 2 % had severe cognitive impairment (MMSE 0-17).
Table 1.
Subject characteristics according to baseline cognitive function (n = 494)
| Characteristic | Total sample (n = 494) | Cognitive impairment | |||
|---|---|---|---|---|---|
| Normal (26–30) | Mild (22–25) | Moderate (18–21) | Severe (0–17) | ||
| (n = 349) (71%) | (n = 113) (23%) | (n = 24) (5%) | (n = 8) (2%) | ||
| Age in years, median (IQR) | 87.2 (86.1–88.7) | 86.9 (85.9–88.4) | 87.9 (86.6–89.3) | 88.4 (87.5–89.1) | 87.6 (87.1–91.3) |
| Sex Female, n (%) | 272 (55) | 180 (52) | 66 (58) | 20 (83) | 6 (75) |
| Attended high school, n (%) | 180 (37) | 145 (43) | 31 (27) | 2 (9) | 2 (29) |
| Current Smoker, n (%) | 34 (7) | 26 (8) | 6 (5) | 2 (9) | 0 (0) |
| Current Alcohol Drinker, n (%) | 173 (36) | 129 (38) | 37 (33) | 5 (22) | 2 (29) |
| Living alone, n (%) | 168 (35) | 125 (37) | 36 (32) | 6 (25) | 1 (14) |
| Body Mass Index (kg/m2), mean ± SD a | 21.4 ± 3.2 | 21.6 ± 3.3 | 21.2 ± 2.9 | 20.1 ± 2.9 | 18.6 ± 3.2 |
| Carotid Artery Plaque Score, median (IQR) a | 1.7 (0.0–3.9) | 1.6 (0.0–3.8) | 1.8 (0.0–3.9) | 3.4 (0.0–4.0) | 2.0 (0.7–3.0) |
| No plaque (0), n (%) | 161 (33) | 125 (36) | 30 (27) | 5 (21) | 1 (12) |
| Mild atherosclerosis (1.2–4.9), n (%) | 199 (40) | 141 (40) | 48 (42) | 7 (29) | 3 (38) |
| Moderate to severe atherosclerosis (more than 5.0), n (%) | 134 (27) | 83 (24) | 35 (31) | 12 (50) | 4 (50) |
| Cytomegalovirus Immunoglobulin G Antibody, median (IQR) a | 18 (12–29) | 17 (11–28) | 20 (13–30) | 21 (13–37) | 35 (25–39) |
| Tumor Necrosis Factor-Alpha (pg/mL), median (IQR) a | 2.2 (1.9–2.8) | 2.2 (1.8–2.7) | 2.2 (1.9–2.9) | 2.4 (2.0–2.8) | 2.1 (2.3–2.5) |
| Interleukin-6 (pg/mL), median (IQR) a | 1.7 (1.3–2.5) | 1.7 (1.3–2.5) | 1.7 (1.3–2.5) | 1.6 (1.4–2.2) | 2.6 (2.9–3.3) |
| Low-Density Lipoprotein (LDL) Cholesterol (mg/dL), mean ± SD | 114.2 ± 27.1 | 114.7 ± 27.2 | 113.6 ± 26.6 | 111.4 ± 27.3 | 108.4 ± 32.4 |
| High-Density Lipoprotein (HDL) Cholesterol (mg/dL), mean ± SD | 59.2 ± 14.9 | 59.3 ± 14.9 | 59.6 ± 14.7 | 56.1 ± 16.1 | 57.1 ± 12.0 |
| Medical history | |||||
| Hypertension, n (%) | 354 (72) | 247 (71) | 86 (76) | 17 (71) | 4 (50) |
| Cardiovascular disease, n (%) | 102 (21) | 75 (21) | 20 (18) | 6 (25) | 1 (13) |
| Diabetes mellitus, n (%) | 61 (12) | 46 (13) | 12 (11) | 2 (8) | 1 (13) |
| Chronic kidney disease, n (%) | 218 (44) | 155 (44) | 46 (41) | 14 (58) | 3 (38) |
| Activities of Daily Living, median (IQR)a | 100 (100–100) | 100 (100–100) | 100 (95–100) | 95 (85–100) | 80 (72.5–95) |
| Instrumental Activities of Daily Living, median (IQR) a | 5 (4–5) | 5 (5–5) | 5 (4–5) | 4 (2–5) | 2.5 (0.5–3) |
| Grip strength (kg), mean ± SD | 20.3 ± 8.7 | 20.8 ± 5.6 | 18.4 ± 5.2 | 15.1 ± 6.1 | 12.5 ± 3.1 |
Continuous variables approximately normal were summarized as mean ± SD while skewed variables were summarized as median (IQR). Categorical variables are summarized as n (%).
Cognitive function was measured using Mini-Mental State Examination (MMSE) score. Scores range from 0 to 30, with lower scores indicating worse cognitive function. Log-transformed values were used in statistical analyses
SD standard deviation, IQR interquartile range
aVariable was categorized in statistical analyses
Table 2 shows the cross-sectional association between baseline subject characteristics and cognitive function using Tobit regression in all 494 subjects. Elevated levels of CMV IgG titer, TNF-alpha, and carotid artery plaques were associated with worse cognitive function, after adjusting for age, sex, and education. Individuals with CMV IgG titers in the highest quartile had MMSE scores that were 1.47 points lower compared to individuals in the lowest quartile (95 % confidence interval (CI) −2.44 to −0.50; p = 0.003). Meanwhile, individuals with TNF-alpha in the highest quartile had MMSE scores that were 1.54 points lower compared to individuals in the lowest quartile (95 % CI −2.50 to −0.58; p = 0.002), and individuals with moderate to severe atherosclerosis, characterized by high carotid PS, had MMSE scores that were 1.08 points lower compared to individuals with no plaque (95 % CI −1.95 to −0.20; p = 0.016).
Table 2.
Cross-sectional association between subject characteristics and baseline cognitive function using Tobit regression (N = 494)
| Characteristic | Unadjusted | p value | Adjusted for sociodemographicsa | p value | ||
|---|---|---|---|---|---|---|
| Age in years | −0.32 (−0.49 to −0.16) | <0.001 | – | −0.26 (−0.43 to −0.10) | 0.002 | – |
| Sex Female | −1.25 (−1.94 to −0.55) | <0.001 | – | −0.71 (−1.43 to 0.00) | 0.051 | – |
| Attended high school | 1.72 (1.00 to 2.43) | <0.001 | – | 1.37 (0.63 to 2.11) | <0.001 | – |
| Current Smoking | 0.89 (−0.48 to 2.27) | 0.200 | – | 0.29 (−1.04 to 1.63) | 0.667 | – |
| Current Alcohol Drinking | 0.70 (−0.02 to 1.41) | 0.060 | – | 0.31 (−0.42 to 1.04) | 0.402 | – |
| Living Alone | 0.56 (−0.18 to 1.30) | 0.137 | – | 1.02 (0.27 to 1.77) | 0.008 | – |
| Body Mass Index (kg/m2) | ||||||
| Underweight (−18.5) | −0.28 (−1.19 to 0.62) | 0.540 | 0.01 (−0.88 to 0.89) | 0.990 | ||
| Normal (18.5–25.0) | Reference | 0.192 | Reference | 0.190 | ||
| Overweight (25.0–) | 0.82 (−0.21 to 1.86) | 0.120 | 0.92 (−0.09 to 1.92) | 0.074 | ||
| Carotid Artery Plaque Score | ||||||
| No plaque (0) | Reference | 0.021 | Reference | 0.054 | ||
| Mild atherosclerosis (1.2–4.9) | −0.57 (−1.39 to 0.25) | 0.170 | −0.54 (−1.34 to 0.26) | 0.185 | ||
| Moderate to severe atherosclerosis (5.0) | −1.28 (−2.18 to –0.38) | 0.005 | −1.08 (−1.95 to −0.20) | 0.016 | ||
| Cytomegalovirus Immunoglobulin G Antibody | ||||||
| Q1 (2.0–12.3) | Reference | 0.003 | Reference | 0.028 | ||
| Q2 (12.4–18.3) | −1.00 (−1.97 to −0.02) | 0.045 | −0.96 (−1.90 to −0.02) | 0.046 | ||
| Q3 (18.4–29.3) | −1.06 (−2.03 to −0.09) | 0.032 | −0.82 (−1.77 to 0.13) | 0.089 | ||
| Q4 (29.4–) | −1.90 (−2.88 to −0.91) | <0.001 | −1.47 (−2.44 to −0.50) | 0.003 | ||
| Tumor Necrosis Factor-Alpha (pg/mL) | ||||||
| Q1 (1.12–1.86) | Reference | 0.082 | Reference | 0.008 | ||
| Q2 (1.87–2.174) | −1.07 (−2.07 to −0.07) | 0.036 | −1.40 (−2.36 to −0.44) | 0.005 | ||
| Q3 (2.175–2.77) | −0.95 (−1.95 to 0.05) | 0.063 | −1.05 (−2.02 to −0.08) | 0.034 | ||
| Q4 (2.78–) | −1.18 (−2.18 to −0.19) | 0.020 | −1.54 (−2.50 to −0.58) | 0.002 | ||
| Interleukin-6 (pg/mL) | ||||||
| Q1 (0.43–1.28) | Reference | 0.358 | Reference | 0.387 | ||
| Q2 (1.29–1.68) | −0.73 (−1.72 to 0.26) | 0.149 | −0.73 (−1.68 to 0.22) | 0.129 | ||
| Q3 (1.69–2.46) | −0.78 (−1.78 to 0.22) | 0.127 | −0.69 (−1.67 to 0.30) | 0.172 | ||
| Q4 (2.47–) | −0.73 (−1.72 to 0.27) | 0.152 | −0.67 (−1.64 to 0.29) | 0.171 | ||
| Low-density Lipoprotein (LDL) Cholesterol (mg/dL) | ||||||
| Q1 (47–96) | Reference | 0.515 | Reference | 0.291 | ||
| Q2 (97–114) | 0.69 (−0.31 to 1.70) | 0.177 | 0.74 (−0.24 to 1.72) | 0.141 | ||
| Q3 (115–130) | 0.06 (−0.93 to 1.06) | 0.904 | −0.07 (−1.04 to 0.91) | 0.896 | ||
| Q4 (131–239) | 0.19 (−0.80 to 1.17) | 0.711 | −0.03 (−1.01 to 0.94) | 0.946 | ||
| High-density lipoprotein (HDL) cholesterol (mg/dL) | ||||||
| Q1 (25–48) | Reference | 0.519 | Reference | 0.246 | ||
| Q2 (49–58) | −0.14 (−1.14 to 0.86) | 0.786 | 0.13 (−0.84 to 1.11) | 0.791 | ||
| Q3 (59–68) | 0.29 (−0.72 to 1.30) | 0.571 | 0.46 (−0.53 to 1.45) | 0.360 | ||
| Q4 (69–113) | 0.54 (−0.46 to 1.54) | 0.287 | 0.93 (−0.07 to 1.93) | 0.069 | ||
| Medical history | ||||||
| Hypertension | 0.07 (−0.71 to 0.85) | 0.865 | – | 0.01 (−0.74 to 0.76) | 0.977 | – |
| Cardiovascular disease | 0.64 (−0.07 to 1.35) | 0.079 | – | 0.55 (−0.14 to 1.24) | 0.118 | – |
| Diabetes mellitus | 0.59 (−0.48 to 1.66) | 0.280 | – | 0.24 (−0.80 to 1.27) | 0.655 | – |
| Chronic kidney disease | −0.12 (−0.83 to 0.58) | 0.729 | – | −0.10 (−0.78 to 0.58) | 0.777 | – |
| Activities of Daily Living | ||||||
| 1 (10–94) | Reference | <0.001 | Reference | <0.001 | ||
| 2 (95–99) | 0.59 (−0.81 to 1.98) | 0.407 | 0.15 (−1.24 to 1.53) | 0.835 | ||
| 3 (100) | 2.41 (1.40 to 3.41) | <0.001 | 1.78 (0.77 to 2.80) | 0.001 | ||
| Instrumental Activities of Daily Living Score | ||||||
| 1–3 | Reference | Reference | ||||
| 4 | 2.93 (1.66 to 4.21) | <0.001 | <0.001 | 2.27 (0.99 to 3.55) | 0.001 | <0.001 |
| 5 | 3.53 (2.46 to 4.59) | <0.001 | 2.87 (1.78 to 3.96) | <0.001 | ||
| Grip strength (kg) | ||||||
| Q1 (M: 11.0–21.0, F: 4.0–12.4) | Reference | <0.001 | Reference | <0.001 | ||
| Q2 (M: 21.1–24.4, F: 13.5–15.5) | 1.53 (0.56 to 2.51) | 0.002 | 1.49 (0.54 to 2.45) | 0.002 | ||
| Q3 (M: 24.5–27.2, F: 15.6–18.8) | 1.29 (0.33 to 2.25) | 0.009 | 1.06 (0.11 to 2.00) | 0.028 | ||
| Q4 (M: 27.3–41.5, F: 18.9–24.5) | 2.62 (1.65 to 3.61) | <0.001 | 2.38 (1.40 to 3.36) | <0.001 | ||
Values are β coefficients with corresponding 95 % confidence interval. Lower and upper limit was specified as 0 and 30, respectively, using Tobit regression. p values for categorical variables include p value for each level of category (left column) and its joint p value (right column). Cognitive function was measured using Mini-Mental State Examination (MMSE) score. Scores range from 0 to 30, with lower scores indicating worse cognitive function
aAdjusted for age, sex, and education
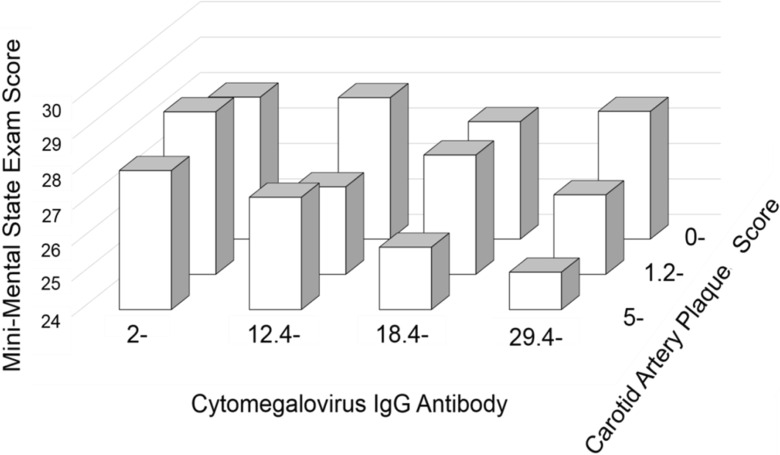
There was evidence for an interaction between CMV IgG titer and subclinical atherosclerosis, where the association between CMV and MMSE amplified with the degree of atherosclerosis. Table 3 shows the association between CMV IgG titer and MMSE stratified by the presence of carotid artery plaques. Within individuals with carotid artery plaque, individuals in the highest CMV IgG quartile had MMSE scores that were 2.22 points lower compared to individuals in the lowest quartile (95 % Cl −3.44 to −1.01; p < 0.001). In contrast, when we restricted the analyses to individuals with no plaque, there was no evidence for an association between CMV IgG titer and MMSE (p = 0.88). Adjusted MMSE scores in the model with an interaction term for CMV IgG titer and carotid artery plaque score (interaction term, joint p = 0.058) are shown in Fig. 1. There was no interaction between TNF-alpha and carotid artery plaques (test for interaction, p = 0.3), or between CMV IgG titer and TNF-alpha (test for interaction, p = 0.5).
Table 3.
Cross-sectional association between cytomegalovirus (CMV) immunoglobulin G (IgG) antibody titer and cognitive function stratified by atherosclerosis status using Tobit regression
| Any plaques (carotid artery plaque score ≥1.2) | |||||
|---|---|---|---|---|---|
| Coefficient | Confidence interval | p value | |||
| CMV IgG antibody titer | Q1 (2.0–12.3) | Reference (MMSE 28.15) | 0.004 | ||
| Q2 (12.4–18.3) | −1.52 | −2.70 to −0.34 | 0.012 | ||
| Q3 (18.4–29.3) | −1.16 | −2.37 to 0.06 | 0.063 | ||
| Q4 (29.4–) | −2.22 | −3.44 to −1.01 | <0.001 | ||
| No plaque (carotid artery plaque score <1.2) | |||||
| CMV IgG antibody titer | Q1 (2.0–) | Reference (MMSE 27.73) | 0.878 | ||
| Q2 (12.4–) | 0.25 | −1.23 to 1.74 | 0.738 | ||
| Q3 (18.4–) | −0.19 | −1.61 to 1.24 | 0.795 | ||
| Q4 (29.4–) | 0.36 | −1.20 to 1.92 | 0.652 | ||
Values are β coefficients with corresponding 95 % confidence interval. Lower and upper limit was specified as 0 and 30, respectively, using Tobit regression. p values for categorical variables include p value for each level of category (left column) and its joint p value (right column). Cognitive function was measured using Mini-Mental State Examination (MMSE) score. Scores range from 0 to 30, with lower scores indicating worse cognitive function. Adjusted for age, sex, and education
Fig. 1.
Adjusted Mini-Mental State Exam (MMSE) Score Stratified by Levels of Cytomegalovirus (CMV) IgG Antibody Titer and Carotid Artery Plaque Score. For the first, second, third, and fourth quartile of CMV, multivariable adjusted means of MMSE were 27.87 (standard error 0.16), 27.23 (0.16), 25.76 (0.21), and 25.06 (0.15) among individuals with the moderate to severe atherosclerosis (carotid artery plaque score ≥5.0), 28.63 (0.16), 26.47 (0.14), 27.33 (0.14), 26.24 (0.16) among individuals with mild atherosclerosis (1.2-5.0), and 28.00 (0.15), 27.94 (0.16), 27.36 (0.13), 27.60 (0.17) among individuals with no plaque (0–1.2)). Cognitive function was measured using Mini-Mental State Examination (MMSE) score. Scores range from 0 to 30, with lower scores indicating worse cognitive function. Model adjusted for age, sex, and education and included the interaction term for CMV IgG Antibody titer and carotid artery plaque score
Table 4 shows the longitudinal association between baseline subject characteristics and annual change in MMSE over the 3-year follow-up. Analyses were restricted to 328 subjects without severe cognitive function at baseline (MMSE≥18). Mean annual MMSE change was −0.27. Individuals with moderate to severe carotid atherosclerosis at baseline experienced faster cognitive decline, with mean annual difference of −0.35 compared to individuals with no plaque (95 % CI −0.69 to −0.02, p = 0.037). There was no interaction between carotid atherosclerosis and CMV IgG titer on the change in MMSE score over 3 years (p for interaction = 0.140). Current smoking (p = 0.006), poor ADL status (p = 0.018), and IADL status (p = 0.026) were also associated with faster cognitive decline. Traditional vascular risk factors including high BMI, high cholesterol, and diabetes were not associated with cognitive function in the cross-sectional or the longitudinal analyses.
Table 4.
Longitudinal association between subject characteristics and annual change in Mini-Mental State Examination (MMSE) score over the 3-year follow-up using linear regression (N = 328)
| Unadjusted | p value | Adjusted for sociodemographicsa | p value | |||
|---|---|---|---|---|---|---|
| Age in years | – | – | ||||
| Sex female | −0.12 (−0.37 to 0.12) | 0.316 | – | −0.11 (−0.37 to 0.14) | 0.386 | – |
| Attended high school | 0.10 (−0.15 to 0.35) | 0.414 | – | 0.18 (−0.08 to 0.44) | 0.181 | – |
| Current smoking | −0.55 (−1.03 to −0.08) | 0.022 | – | −0.66 (−1.13 to −0.19) | 0.006 | – |
| Current alcohol drinking | −0.05 (−0.30 to 0.21) | 0.726 | – | −0.08 (−0.34 to 0.18) | 0.538 | – |
| Living alone | 0.10 (−0.15 to 0.36) | 0.430 | – | 0.22 (−0.05 to 0.50) | 0.110 | – |
| Body mass index (kg/m2) | ||||||
| Underweight (−18.5) | −0.06 (−0.39 to 0.28) | 0.743 | 0.761 | −0.04 (−0.37 to 0.30) | 0.818 | 0.923 |
| Normal (18.5–25.0) | Reference | Reference | ||||
| Overweight (25.0–) | −0.12 (−0.47 to 0.22) | 0.476 | −0.06 (−0.40 to 0.27) | 0.715 | ||
| Carotid Artery Plaque Score | ||||||
| No plaque (0) | Reference | 0.369 | 0.097 | |||
| Mild atherosclerosis (1.2–4.9) | 0.00 (−0.27 to 0.28) | 0.983 | −0.06 (−0.33 to 0.21) | 0.646 | ||
| Moderate to severe atherosclerosis (5.0–) | −0.21 (−0.55 to 0.12) | 0.208 | −0.35 (−0.69 to −0.02) | 0.037 | ||
| Cytomegalovirus Immunoglobulin G antibody | ||||||
| Q1 (2.0–12.3) | Reference | 0.246 | Reference | 0.255 | ||
| Q2 (12.4–18.3) | 0.16 (−0.18 to 0.49) | 0.357 | 0.12 (−0.21 to 0.45) | 0.472 | ||
| Q3 (18.4–29.3) | −0.21 (−0.54 to 0.13) | 0.225 | −0.22 (−0.55 to 0.11) | 0.195 | ||
| Q4 (29.4–) | 0.05 (−0.30 to 0.40) | 0.791 | −0.00 (−0.35 to 0.35) | 0.991 | ||
| Tumor necrosis factor-alpha (pg/mL) | ||||||
| Q1 (1.12–1.86) | Reference | 0.197 | Reference | 0.088 | ||
| Q2 (1.87–2.174) | −0.13 (−0.46 to 0.19) | 0.426 | −0.26 (−0.58 to 0.07) | 0.124 | ||
| Q3 (2.175–2.77) | −0.07 (−0.40 to 0.27) | 0.703 | −0.09 (−0.43 to 0.25) | 0.606 | ||
| Q4 (2.78–) | 0.29 (−0.05 to 0.62) | 0.090 | 0.17 (−0.17 to 0.51) | 0.327 | ||
| Interleukin-6 (pg/mL) | ||||||
| Q1 (0.43–1.28) | Reference | 0.548 | Reference | 0.727 | ||
| Q2 (1.29–1.68) | 0.11 (−0.22 to 0.43) | 0.513 | 0.04 (−0.28 to 0.36) | 0.421 | ||
| Q3 (1.69–2.46) | 0.13 (−0.21 to 0.47) | 0.449 | 0.04 (−0.31 to 0.39) | 0.811 | ||
| Q4 (2.47–) | −0.10 (−0.44 to 0.24) | 0.575 | −0.13 (−0.47 to 0.21) | 0.447 | ||
| Low-density lipoprotein (LDL) cholesterol (mg/dL) | ||||||
| Q1 (47–96) | Reference | 0.900 | Reference | 0.842 | ||
| Q2 (97–114) | 0.01 (−0.34 to 0.37) | 0.937 | 0.07 (−0.28 to 0.42) | 0.690 | ||
| Q3 (115–130) | 0.12 (−0.23 to 0.47) | 0.494 | 0.15 (−0.19 to 0.50) | 0.379 | ||
| Q4 (131–239) | 0.03 (−0.31 to 0.37) | 0.866 | 0.05 (−0.30 to 0.40) | 0.778 | ||
| High-density lipoprotein (HDL) cholesterol (mg/dL) | ||||||
| Q1 (25–48) | Reference | 0.860 | Reference | 0.978 | ||
| Q2 (49–58) | 0.03 (−0.31 to 0.37) | 0.870 | 0.07 (−0.28 to 0.41) | 0.694 | ||
| Q3 (59–68) | −0.04 (−0.39 to 0.30) | 0.802 | 0.005 (−0.34 to 0.35) | 0.979 | ||
| Q4 (69–113) | −0.11 (−0.46 to 0.23) | 0.518 | 0.02 (−0.34 to 0.39) | 0.894 | ||
| Medical history | ||||||
| Hypertension | 0.13 (−0.14 to 0.41) | 0.337 | – | 0.13 (−0.14 to 0.40) | 0.340 | – |
| Cardiovascular disease | 0.01 (−0.24 to 0.25) | 0.958 | – | −0.003 (−0.25 to 0.24) | 0.982 | – |
| Diabetes mellitus | 0.04 (−0.34 to 0.41) | 0.850 | – | 0.03 (−0.34 to 0.41) | 0.854 | – |
| Chronic kidney disease | −0.01 (−0.26 to 0.23) | 0.926 | – | −0.02 (−0.26 to 0.22) | 0.862 | – |
| Activities of daily living | ||||||
| 1 (10–94) | Reference | 0.140 | Reference | 0.018 | ||
| 2 (95–99) | 0.18 (−0.38 to 0.74) | 0.530 | 0.19 (−0.36 to 0.75) | 0.497 | ||
| 3 (100–) | 0.39 (−0.03 to 0.81) | 0.069 | 0.54 (0.12 to 0.96) | 0.012 | ||
| Instrumental activities of daily living | ||||||
| 1–3 | Reference | 0.108 | Reference | 0.026 | ||
| 4 | 0.59 (0.03 to 1.15) | 0.040 | 0.67 (0.12 to 1.22) | 0.016 | ||
| 5 | 0.48 (−0.01 to 0.97) | 0.054 | 0.66 (0.17 to 1.14) | 0.008 | ||
| Grip strength (kg) | ||||||
| Q1 (M 11.0–21.0, F 4.0–12.4) | Reference | Reference | ||||
| Q2 (M 21.1–24.4, F 13.5–15.5) | 0.08 (−0.30 to 0.46) | 0.686 | 0.745 | 0.22 (−0.16 to 0.59) | 0.251 | 0.214 |
| Q3 (M 24.5–27.2, F 15.6–18.8) | 0.21 (−0.17 to 0.58) | 0.286 | 0.39 (0.01 to 0.77) | 0.045 | ||
| Q4 (M 27.3–41.5, F 18.9–24.5) | 0.11 (−0.26 to 0.47) | 0.562 | 0.33 (−0.05 to 0.70) | 0.085 | ||
Values are β coefficients with corresponding 95 % confidence interval. p values for categorical variables include p value for each level of category (left column) and its joint p value (right column). Cognitive function was measured using Mini-Mental State Examination (MMSE) score. Scores range from 0 to 30, with lower scores indicating worse cognitive function. The analyses were restricted to individuals with an MMSE score of 18 or above to exclude those with signs of severe cognitive impairment already at baseline
aAdjusted for age, sex, education, and baseline MMSE score (categorized into three groups: ≥26, 22–25, and 18–21)
Discussion
In this community-based cohort of the very old, we found high levels of CMV IgG antibody, TNF-alpha, and carotid artery plaques to be associated with cognitive impairment in the cross-sectional analyses, independent of age, sex, and education. CMV IgG titers and carotid artery plaques showed evidence of an interaction, where the association between CMV IgG titers and cognitive function was present only in subjects with carotid artery plaques. Moreover, in the longitudinal analyses, carotid artery plaques, but neither CMV IgG titers nor TNF-alpha, were significantly associated with faster cognitive decline over the 3-year follow-up. Collectively, these results suggest that subclinical atherosclerosis is consistently associated with cognitive decline at advanced ages and it modifies association between latent CMV infection and cognition.
In contrast to ample epidemiological evidence in the general population, the association between vascular risk factors and cognitive decline is nebulous or even reversed in the very old (Van den Berg et al. 2007). In this study, we did not detect associations between traditional vascular risk factors including high BMI, hyperlipidemia, hypertension, and diabetes with cognitive decline, which support these past findings. On the other hand, our study provides a unique finding that carotid artery plaque score, an index of lifelong atherosclerosis burden, is associated with cognitive decline over the 3-year follow-up beyond age 85, suggesting that the prevention of atherosclerosis could be a promising intervention to maintain cognitive function through later life. Randomized trials will be needed to assess the efficacy of vascular risk management in decelerating cognitive decline in this population.
CMV is a common herpes virus which infects most of the world’s population, from 40 to 100 % depending on sociodemographic factors (Staras et al. 2006). In a study of Japanese blood donors, CMV seropositivity became saturated by their 60s (Furui et al. 2013). High levels of CMV antibodies are assumed to indicate subclinical reactivation of the virus and are associated with increased proinflammatory responses as well as accumulation of CMV-specific, late-differentiated CD8+ T cells, a hallmark of immunosenescence (Pawelec 2013). Many, but not all epidemiological studies, have documented the association of CMV seropositivity, atherosclerosis, and low cognitive function. In 383 home-dwelling elderly with cardiovascular disease (mean age of 80 years), cumulative seropositivities of CMV and herpes simplex virus (HSV) 1 and 2 were associated with faster rate of cognitive decline over the 1-year follow-up based on MMSE measurements and clinical dementia rating (Strandberg et al. 2003). In 1204 subjects aged 60–101 years (mean age of 70.3 years) of the Sacramento Area Latino Study on Aging (SALSA), high CMV antibody levels were associated with faster rate of cognitive decline over the 4-year follow-up (Aiello et al. 2006) as well as with all-cause and cardiovascular mortality (Roberts et al. 2010). In the Northern Manhattan Study (NOMAS) aimed at the multiethnic, stroke-free subjects (mean age of 68.5 years old), composite viral burden of CMV and HSVs was cross-sectionally associated with poor MMSE score at baseline (Katan et al. 2013) and with carotid artery plaques (Elkind et al. 2010). Our results are compatible with previous studies reporting that the implication of CMV infection on cognition depends on degree of carotid atherosclerosis.
There are several possible interpretations regarding interaction between latent CMV infection and atherosclerosis on cognitive impairment observed in the present study. First, as previously demonstrated in immunosuppressed patients (Grattan et al. 1989), persistent CMV infection may accelerate atherogenic process in the very old, which is a strong risk factor of both vascular dementia and Alzheimer’s disease. Indeed, CMV antigen and nucleic acid were detected in carotid artery plaques obtained from patients with atherosclerosis (Melnick et al. 1994) and in the brains of vascular dementia patients (Lin et al. 2002). In an experimental model, CMV infection of endothelial cells increased the expression of adhesion molecules and promoted angiogenic response, known to trigger atherosclerotic plaque formation (Bentz and Yurochko 2008). All together, these epidemiological and experimental evidences suggest that atherosclerosis is one mechanistic link between CMV infection and cognitive impairment of the very old, who are at high risk for immunosenescence.
Second, there is substantial inconsistency over epidemiological associations between CMV serology and cognition (Mathei et al. 2011). Indeed, when analyses were restricted to those with no carotid artery plaque, we failed to demonstrate association between CMV antibody levels and cognition. These results raise a caveat that CMV antibody levels may not be a sensitive biomarker which distinguish individuals with true persistent CMV infection from those with resolved or past infections (Leng et al. 2011). In a study of 16 CMV-seropositive elderly, Leng et al. reported that only 9 (56 %) had detectable CMV DNA in their peripheral monocyte. Those with detectable CMV DNA had significantly higher percentages of CMV-specific CD8+ T cells than those without; nevertheless, levels of CMV IgG antibody were comparable among the two groups (Leng et al. 2011). More recently, Enrique et al. reported that in CMV seropositive patients, proportion of natural killer receptor NKG2C-positive cells, but not CMV antibody levels, were associated with higher carotid plaque burden, plaque instability, and high CRP levels but correlated with lower CD4/CD8 ratio (Martinez-Rodriguez et al. 2013). Collectively, these results suggest that CMV antibody level may only reflect part of the complex host-pathogen interaction, and coexisting atherosclerosis could be a surrogate marker for persistent reactivation of this virus. Future studies incorporating cellular immune parameters of CMV infection, such as CMV-specific CD8+ T cells and CMV viral DNA in peripheral monocyte, warrant the assessment of this notion.
In the longitudinal analyses, levels of cytomegalovirus IgG antibody were not associated with cognitive decline over 3 years. One potential reason is survival effect, where the very old participants may represent specific phenotypes less susceptible to long-term deleterious effects of CMV infection (Mathei et al. 2011). Indeed, our subjects with the highest quartiles of CMV IgG titer had both a significantly lower cognitive function at baseline and a higher hazard risk for all-cause mortality for 3 years (HR; 1.63, 95 % confidence interval; 1.00–2.64, as compared to the rest of the cohort, unpublished data). The exclusion of subjects with higher risk profiles may have led to the underestimation of longitudinal associations in the present study.
Chronic inflammation, as indicated by high circulation levels of proinflammatory cytokines such as TNF-alpha and IL-6, is reportedly associated with cognitive impairment of the elderly (Rafnsson et al. 2007; Yaffe et al. 2003; Van Exel et al. 2003). In particular, TNF-alpha initiates the inflammatory cascade and is involved in the regulation of cytokine release, oxidative stress, recruitment of immune cell, and apoptosis, thereby affecting neuro-modulatory functions such as the regulation of microglia and astrocytes activation in the brain (Terrando et al. 2010). More importantly, TNF-alpha is shown to mediate a range of CMV-related health outcomes including carotid atherosclerosis (Jeong et al. 2015), cardiovascular mortality (Roberts et al. 2010), and frailty (Schmaltz et al. 2005) suggesting that this cytokine may be one molecular pathway linking CMV reactivation and cognitive impairment. In the present study, although elevated levels of TNF-alpha showed strong association with cognitive impairment, it was independent of CMV IgG titer or subclinical atherosclerosis, and there was no interaction between TNF-α, CMV IgG, and atherosclerosis. At advanced ages, inflammation underlies a wide variety of physiological and pathological processes; thus, further studies investigating the source of upregulated TNF-alpha in dementia are warranted.
Associations of physical and cognitive function have long been reported (Binder et al. 1999; Rosano et al. 2005), and recent research suggests its association to be bidirectional (Krall et al. 2014). In our study, all indicators of physical function, including ADL, IADL, and grip strength, were positively associated with both the cognitive function at baseline and decelerated cognitive decline over the 3-year follow-up even after adjusting for potential confounders, signifying the role of physical function in the preservation of cognition in late life.
Our study has limitations. First, neuropsychological measurements were restricted to MMSE scores, and we currently do not have detailed brain imaging data to confirm Alzheimer’s disease or vascular dementia. While MMSE does not reflect all memory disturbances and better if combined with other memory or word learning tests, it is a well-established tool to assess cognitive performance in large cohorts. Second, because of the very old age, substantial part of our subjects have died (n = 76), dropped out (n = 18), or declined for examination at 3-year follow-up (n = 65), representing a potential bias for longitudinal survey. As compared to those who completed MMSE at 3-year follow-up, those who died or did not complete the follow-up survey were more likely to be old (88.3 ± 2.7 vs. 87.5 ± 1.7, p < 0.001), have lower MMSE score (25.8 ± 3.8 vs. 26.8 ± 3.2, p = 0.005), and a higher prevalence of moderate to severe atherosclerosis (24.0 vs. 11.8 %, p = 0.005), but comparable levels of CMV IgG and TNF-alpha (CMV IgG 2.1 ± 0.8 vs. 1.9 ± 0.8, p = 0.193; TNF-α 2.8 ± 1.7 vs. 2.7 ± 2.3 pg/mL, p = 0.145, respectively) at baseline. This could have introduced a bias against longitudinal association between atherosclerosis and cognitive decline; nevertheless, our study demonstrated a robust association between the two measurements, supporting our hypothesis. Third, while we adjusted for potential confounders, including age, sex, and education, potential confounding remains possible. For example, we did not have information on APOE genotype (Barnes et al. 2015), which may have modified the association between CMV infection and cognitive function. In addition, we did not consider the presence of depressive state (Jaremka et al. 2013) a possible initiator of the reactivation of CMV infection and linked to cognitive function.
This study has several strengths. First, a population-based sample, designed to be representative of community-dwelling elderly over 85 years old in Tokyo, was used. Second, data on a wide range of biomarkers and risk factors were collected to capture a broad view of health profiles in the very old, including measurements of carotid artery plaque. Third, attrition of subjects was minimized through provision of transportation to study centers and in-home examination by geriatricians. Finally, Tobit regression was used to model MMSE in the cross-sectional analyses, which took into account the ceiling and floor effect of the MMSE index and led to better fit of the models.
In summary, this study assessed a wide range of risk factors of cognitive decline in a community-based sample of the Japanese elderly, aged 85 years and older. Carotid artery plaque score was associated with cognitive function in both the cross-sectional and longitudinal analyses. High levels of CMV antibody and TNF-alpha were associated to low cognitive function in the cross-sectional analyses. CMV and carotid atherosclerosis showed evidence of an interaction, where the association between CMV and MMSE was present only in subjects with subclinical atherosclerosis. While our results need to be validated in independent populations before they can be generalized, prevention of atherosclerosis may be an important factor in retaining cognitive function throughout late life. Replication of these findings through longer follow-up data and larger studies may lead to new preventative strategies for successful brain aging and healthy life expectancy.
Acknowledgments
We thank the participants and their families for their time and personal information and Ms. Miho Shimura for her kind assistance.
Author contributions
Study design: Arai, Mimura, and Hirose. Data collection: Arai, Takayama Michiyo, Takayama Midori, Abe, and Hirose. Data analyses and interpretation: Kawasaki, Arai, Takayama Michiyo, Takayama Midori, Hirata, Abe, Niimura, and Takebayashi. Final statistical analyses: Kawasaki. Data preparation: Abe. Critical revision of the draft: Mimura, Takebayashi, Niimura, and Hirata. Draft of the report: Kawasaki, Arai, Hirata, and Niimura. All authors approved the final version of the report.
The corresponding authors had full access to all data in the study and had final responsibility for the decision in submitting for publication.
Compliance with ethical standard
Conflict of interest
Dr. Arai received research grant from DAIICHI SANKYO COMPANY, LIMITED and Takeda Pharmaceutical Company Limited. Dr. Hirose received research grant from MSD K.K. All other authors have nothing to disclose.
Sponsor’s role
The funding agencies had no direct role in the design or conduct of the study; the collection, management, analyses, or interpretation of the data; or the preparation or approval of the manuscript.
Funded by the Grant-in-Aid for Scientific Research (B) (No. 21390245), (C) (No 20590706, 21590775) from Japan Society for the Promotion of Science, the medical-welfare-food-agriculture collaborating consortium project from Japan Ministry of Agriculture, Forestry and Fisheries, by the grant from Novartis Foundation for Gerontological Research, by the grant from Foundation for Total Health Promotion, and by the Chiyoda Mutual Life Foundation.
References
- Aiello AE, Haan MN, Blythe L, et al. The influence of latent viral infection on rate of cognitive decline over 4 years. J Am Geriatr Soc. 2006;54:1046–1054. doi: 10.1111/j.1532-5415.2006.00796.x. [DOI] [PubMed] [Google Scholar]
- Arai Y, Iinuma T, Takayama M, et al. The Tokyo Oldest Old survey on Total Health (TOOTH): a longitudinal cohort study of multidimensional components of health and well-being. BMC Geriatr. 2010;10:35. doi: 10.1186/1471-2318-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin PC, Escobar M, Kopec JA. The use of the Tobit model for analyzing measures of health status. Qual Life Res. 2000;9:901–910. doi: 10.1023/A:1008938326604. [DOI] [PubMed] [Google Scholar]
- Barnes LL, Capuano AW, Aiello AE, et al. Cytomegalovirus infection and risk of Alzheimer disease in older black and white individuals. J Infect Dis. 2015;211(2):230–237. doi: 10.1093/infdis/jiu437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentz GL, Yurochko AD. Human CMV infection of endothelial cells induces an angiogenic response through viral binding to EGF receptor and beta1 and beta3 integrins. Proc Natl Acad Sci USA. 2008;105:5531–5536. doi: 10.1073/pnas.0800037105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EF, Storandt M, Birge SJ. The relation between psychometric test performance and physical performance in older adults. J Gerontol A Biol Sci Med Sci. 1999;54:428–432. doi: 10.1093/gerona/54.8.M428. [DOI] [PubMed] [Google Scholar]
- Collerton J, Davies K, Jagger C, et al. Health and disease in 85 year olds: baseline findings from the Newcastle 85+ cohort study. BMJ. 2009;339:b4904. doi: 10.1136/bmj.b4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkind MSV, Luna JM, Moon YP, et al. Infectious burden and carotid plaque thickness: the Northern Manhattan Study. Stroke. 2010;41:e117–e122. doi: 10.1161/STROKEAHA.109.571299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri CP, Prince M, Brayne C et al (2005) Global prevalence of dementia: a Delphi consensus study. Lancet 366:2112–2117 [DOI] [PMC free article] [PubMed]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Furui Y, Satake M, Hoshi Y. Cytomegalovirus (CMV) seroprevalence in Japanese blood donors and high detection frequency of CMV DNA in elderly donors. Transfusion. 2013;53:2190–2197. doi: 10.1111/trf.12390. [DOI] [PubMed] [Google Scholar]
- Gardner RC, Valcour V, Yaffe K. Dementia in the oldest old: a multi-factorial and growing public health issue. Alzheimers Res Ther. 2013;5:27. doi: 10.1186/alzrt181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grattan MT, Moreno-Cabral CE, Starnes VA, et al. Cytomegalovirus infection is associated with cardiac allograft rejection and atherosclerosis. JAMA. 1989;261:3561–3566. doi: 10.1001/jama.1989.03420240075030. [DOI] [PubMed] [Google Scholar]
- Handa N, Matsumoto M, Maeda H, et al. Ischemic stroke events and carotid atherosclerosis. Results of the Osaka Follow-up Study for Ultrasonographic Assessment of Carotid Atherosclerosis (the OSACA Study) Stroke. 1995;26:1781–1786. doi: 10.1161/01.STR.26.10.1781. [DOI] [PubMed] [Google Scholar]
- Jaremka LM, Fagundes CP, Glaser R, Bennett JM, Malarkey WB, Kiecolt-Glaser JK. Loneliness predicts pain, depression, and fatigue: understanding the role of immune dysregulation. Psychoneuroendocrinology. 2013;38(8):1310–1317. doi: 10.1016/j.psyneuen.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong SJ, Ku NS, Han SH, et al. Anti-cytomegalovirus antibody levels are associated with carotid atherosclerosis and inflammatory cytokine production in elderly Koreans. Clin Chim Acta. 2015;445:65–69. doi: 10.1016/j.cca.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Katan M, Moon YP, Paik MC, et al. Infectious burden and cognitive function: the Northern Manhattan Study. Neurology. 2013;80:1209–1215. doi: 10.1212/WNL.0b013e3182896e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall JR, Carlson MC, Fried LP, et al. Examining the dynamic, bidirectional associations between cognitive and physical functioning in older adults. Am J Epidemiol. 2014;180:838–846. doi: 10.1093/aje/kwu198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng SX, Qu T, Semba RD, et al. Relationship between cytomegalovirus (CMV) IgG serology, detectable CMV DNA in peripheral monocytes, and CMV pp 65 495–503-specific CD8 + T cells in older adults. Age. 2011;33:607–614. doi: 10.1007/s11357-011-9205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WR, Wozniak MA, Wilcock GK, et al. Cytomegalovirus is present in a very high proportion of brains from vascular dementia patients. Neurobiol Dis. 2002;9:82–87. doi: 10.1006/nbdi.2001.0465. [DOI] [PubMed] [Google Scholar]
- Martinez-Rodriguez JE, Munne-Collado J, Rasal R, et al. Expansion of the NKG2C+ natural killer-cell subset is associated with high-risk carotid atherosclerotic plaques in seropositive patients for human cytomegalovirus. Arterioscler Thromb Vasc Biol. 2013;33:2653–2659. doi: 10.1161/ATVBAHA.113.302163. [DOI] [PubMed] [Google Scholar]
- Mathei C, Vaes B, Wallemacq P, et al. Associations between cytomegalovirus infection and functional impairment and frailty in the BELFRAIL cohort. J Am Geriatr Soc. 2011;59:2201–2208. doi: 10.1111/j.1532-5415.2011.03719.x. [DOI] [PubMed] [Google Scholar]
- Melnick JL, Hu C, Burek J, et al. Cytomegalovirus DNA in arterial walls of patients with atherosclerosis. J Med Virol. 1994;42:170–174. doi: 10.1002/jmv.1890420213. [DOI] [PubMed] [Google Scholar]
- Pawelec G. Immunosenenescence: role of cytomegalovirus. Exp Gerontol. 2013;54:1–5. doi: 10.1016/j.exger.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Rafnsson SB, Deary IJ, Smith FB, et al. Cognitive decline and markers of inflammation and hemostasis: the Edinburgh artery study. J Am Geriatr Soc. 2007;55:700–707. doi: 10.1111/j.1532-5415.2007.01158.x. [DOI] [PubMed] [Google Scholar]
- Roberts ET, Haan MN, Dowd JB, et al. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am J Epidemiol. 2010;172:363–371. doi: 10.1093/aje/kwq177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C, Simonsick EM, Harris TB, et al. Association between physical and cognitive function in healthy elderly: the health, aging and body composition study. Neuroepidemiology. 2005;2:8–14. doi: 10.1159/000081043. [DOI] [PubMed] [Google Scholar]
- Schmaltz HN, Fried LP, Xue QL, et al. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005;53:747–754. doi: 10.1111/j.1532-5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]
- Skoog I, Nilsson L, Persson G, et al. 15-Year Longitudinal Study of Blood Pressure and Dementia. Lancet. 1996;347:1141–1145. doi: 10.1016/S0140-6736(96)90608-X. [DOI] [PubMed] [Google Scholar]
- Staras SA, Dollard SC, Radford KW et al (2006) Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America 43:1143–1151 [DOI] [PubMed]
- Strandberg TE, Pitkala KH, Linnavuori KH, et al. Impact of viral and bacterial burden on cognitive impairment in elderly persons with cardiovascular diseases. Stroke. 2003;34:2126–2131. doi: 10.1161/01.STR.0000086754.32238.DA. [DOI] [PubMed] [Google Scholar]
- Terrando N, Ma D, Foxwell BMJ, et al. Tumor necrosis factor- α triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci USA. 2010;107:20518–20522. doi: 10.1073/pnas.1014557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin BYJ. Estimation of Relationships for Limited Dependent. The Econometric Society of relationships for limited dependent variables. 1958;26:24–36. [Google Scholar]
- Van den Berg E, Biessels GJ, De Craen AJ, et al. The metabolic syndrome is associated with decelerated cognitive decline in the oldest old. Neurology. 2007;69:979–985. doi: 10.1212/01.wnl.0000271381.30143.75. [DOI] [PubMed] [Google Scholar]
- Van Exel E, Gussekloo J, Houx P, et al. Atherosclerosis and cognitive impairment are linked in the elderly. The Leiden 85-plus Study. Atherosclerosis. 2002;165:353–359. doi: 10.1016/S0021-9150(02)00253-8. [DOI] [PubMed] [Google Scholar]
- Van Exel E, De Craen AJM, Remarque EJ, et al. Interaction of atherosclerosis and inflammation in elderly subjects with poor cognitive function. Neurology. 2003;61:1695–1701. doi: 10.1212/01.WNL.0000098877.07653.7C. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Penninx BW, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61:76–80. doi: 10.1212/01.WNL.0000073620.42047.D7. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- Yang Z, Slavin JM, Sachdev PS. Dementia in the oldest old. Nature Neurology. 2013;9:382–393. doi: 10.1038/nrneurol.2013.105. [DOI] [PubMed] [Google Scholar]