Abstract
Intramuscular fat may mediate associations between obesity and physical disability. We examined the associations between muscle attenuation, a proxy for intramuscular fat, and physical function. Paraspinous muscle computed tomography attenuation was obtained on a Framingham Heart Study subgroup (n = 1152, 56 % women, mean age 66 years). Regressions modeled cross-sectional associations between muscle attenuation and mobility disability, grip strength, and walking speed with standard covariates; models additionally adjusted for body mass index (BMI) and visceral adipose tissue (VAT). Separate models investigated associations between VAT and subcutaneous adipose tissue (SAT) and physical function. Per 1 standard deviation decrement in muscle attenuation (i.e., more muscle fat), we observed 1.29 (95 % CI = 1.11, 1.50; p = 0.0009) increased odds of walking speed ≤1 m/s in women and men. This persisted after separate BMI and VAT adjustments (p < 0.02). In men, there was a 1.29 kg (95 % CI = 0.57, 2.01; p = 0.0005) decrement in grip strength, which persisted after BMI and VAT adjustments (p ≤ 0.0004). For VAT and SAT, similar associations were not observed. Intramuscular fat is associated with increased odds of walking speed ≤1 m/s in both sexes and lower grip strength in men. There were no similar associations for VAT and SAT, highlighting the specificity of intramuscular fat in association with physical function.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-016-9893-2) contains supplementary material, which is available to authorized users.
Keywords: Epidemiology, Muscle, Adipose tissue, Physical function
Introduction
The effects of greater obesity on health are wide-ranging and have been associated with both reduced disability-free life expectancy and longevity (Al et al. 2007). With an aging population, there is a particular interest in the association between obesity and physical disability (Sturm et al. 2004), which is important from both a health and an economic perspective (Ostbye et al. 2007; Al et al. 2007). Higher body mass index (BMI) is associated with poorer physical function (Hergenroeder et al. 2011; Alley and Chang 2007) and other markers of adiposity, including greater waist circumference (Angleman et al. 2006), greater visceral adipose tissue (VAT) volume (Murphy et al. 2014), lower VAT density (Murphy et al. 2014), and greater abdominal subcutaneous adipose tissue (SAT) volume (Murphy et al. 2014) are associated with an increased risk of physical disability. VAT in particular has been associated with worse physical function (Murphy et al. 2014) as well as detrimental metabolic changes (Fox et al. 2007).
The potential associations between muscle composition and disability have been less well-characterized than the associations between adipose tissue markers and disability. Intermuscular fat, defined as fat in between muscle fibers, is associated with increased mobility limitation (Murphy et al. 2014) and decreased walking speed (Beavers et al. 2013). This suggests that muscle fat, perhaps more so than visceral fat, may interfere with muscle function and ability. Intramuscular fat, fat within muscle fibers, is a form of localized muscle fat whose association with physical function and performance has previously been studied, but is not yet fully parsed (Reinders et al. 2015; Cawthon et al. 2009; Cesari et al. 2009; Visser et al. 2005; Goodpaster et al. 2001; Hicks et al. 2005a, b; Frank et al. 2015).
Thus, the goal of our study was to test the cross-sectional association between intramuscular fat and physical function and performance, including walking speed and grip strength. Slower walking speed and weaker grip strength are both associated with increased mobility limitation and increased mortality (Studenski et al. 2011; Cesari et al. 2005; Sallinen et al. 2010; Sasaki et al. 2007). Additionally, we examined the associations of VAT and SAT volumes with these physical performance measures to be used as a comparison. We hypothesized that lower muscle attenuation, an estimation of greater intramuscular fat, would be associated with greater mobility disability, weaker grip strength, and slower walking speed. We additionally hypothesized that these associations would be independent of BMI.
Methods
Study participants
The study sample was derived from the offspring of the original cohort in the Framingham Heart Study. The Framingham Heart Study is a community-based study founded in 1948 with 5209 first generation participants (Dawber et al. 1963). The study pool was expanded to the first generation’s children and their children’s spouses in 1971 (KANNEL et al. 1979). The Framingham Heart Study conducts regular exams on participants and routinely collects health information from their physicians.
A total of 1390 s generation participants in this study had undergone multidetector computed tomography (MDCT) scans between 2002 and 2005 (Parikh et al. 2007), and an exam including physical performance measures was performed afterward from 2005 to 2008. There was an average of 2.5 years between the physical examination and the MDCT scan. Ages of participants were restricted to 40 years or older for women and 35 years or older for men as the MDCT protocol was initially designed for a study to detect coronary and abdominal aorta calcification (Parikh et al. 2007). Due to weight restrictions of the MDCT machines, participants weighing over 160 kg were excluded. There were 1152 participants who met these criteria with no missing data. Compared to participants included in the study, those excluded, usually due to not having undergone MDCT scanning, tended to be older, have more self-reported mobility disability, weaker grip strength, and slower walking speeds (Supplemental Table 1).
Measurement of muscle attenuation and visceral and subcutaneous Fat
Our measurement of muscle attenuation has been described previously (Therkelsen et al. 2013). Briefly, after participants had undergone an eight-slice MDCT scan producing 25 contiguous slices of 5-mm thickness, two 1-cm regions of interest were selected, one over each paraspinous muscle at the mid-abdominal level. The muscle attenuation of each participant was the average of the muscle attenuation of these two regions of interest. The correlation between the reads of adjacent paraspinous muscles was 0.86 and inter-reader correlation was 0.88 (Therkelsen et al. 2013). The use of muscle attenuation on MDCT scan to estimate intramuscular fat has been validated via muscle biopsy study with a Pearson correlation coefficient of −0.43 (p = 0.01, n = 45) (Goodpaster et al. 2000).
VAT and SAT volumes were also determined via MDCT scans described above (Fox et al. 2007). Abdominal slices were manually traced along the abdominal muscular wall. Fat within this tracing was marked as VAT and fat outside the tracing was marked as SAT. Fat was defined within the window of −195 to −45 HU with a center of −120 HU. Interreader and intrareader correlation for this method is >0.99 (Fox et al. 2007).
All muscle attenuation and VAT and SAT volume measurements were completed with Aquarius 3D Workstation software (TeraRecon Inc., San Mateo, CA, USA).
Measurement of mobility disability and physical performance measures
Mobility disability is defined as self-report of inability to walk half a mile or climb a flight of stairs. Hand grip strength was measured with a handheld adjustable hydraulic JAMAR dynamometer (Sammons Preston, Inc., Bolingbrook, IL) bilaterally. Three trials were done per hand with a 3-s squeeze. The highest of these six trials was defined as the participant’s grip strength. Walking speed was measured with a handheld stopwatch to the nearest 0.01 s over a 4-m course with participants instructed to walk at their usual pace. The test was completed twice with the faster of the two timed walks used for analysis. Walking aids were allowed if necessary, although other people were not permitted to assist. We additionally categorized participants’ walking speeds into ≤1 and >1 m/s, since walking speeds >1 m/s have been associated with higher rates of survival (Studenski et al. 2011).
Measurement of covariates
BMI was computed as weight (kg) divided by the square of the height (m). Smokers had smoked >1 cigarette per day in the year prior to their exam. As has been described previously (Kraigher-Krainer et al. 2013), physical activity was determined via a validated questionnaire that documents levels of physical activity during each hour of a participant’s day; the physical activity index (PAI) score was determined via calculation of daily physical activity strenuousness multiplied by an estimation of each activity’s oxygen consumption (Kannel and Sorlie 1979). Diabetes was defined by fasting blood glucose ≥126 mg/dL or self-reported use of insulin or oral hyperglycemic agents. Cardiovascular disease was determined via validated events.
Statistical analysis
Linear regression models were used to investigate the association between intramuscular fat modeled per one standard deviation (7 HU) decrement in muscle attenuation (i.e., more muscle fat) and grip strength and walking speed (adjusted for height). Similarly, logistic regression models were used to model per one standard deviation (7 HU) decrement in muscle attenuation the associated odds ratio of mobility disability and the odds ratio of walking speed ≤1 m/s (walking speed adjusted for height). Linear and logistic regression models were also used to investigate per 500 cm3 increment in VAT or SAT volume the change in physical performance measures, a unit consistent with prior studies (Abraham et al. 2015). For model 1 (muscle attenuation, VAT volume, or SAT volume as individual exposures), the model adjusted for age, smoking status, diabetes, prevalent CVD, and physical activity index score. Model 2 additionally adjusted for BMI. With muscle attenuation as the exposure, model 3 and model 4 adjusted for the covariates in model 1 and additionally adjusted for VAT volume and SAT volume, respectively, to account for regional adiposity. With VAT or SAT volume as the exposure, model 3 additionally adjusted model 1 for muscle attenuation. The overall model additionally accounted for sex. Sex interactions were calculated on model 1.
Analysis was completed with SAS v. 9.2. Significant associations were defined by p values <0.05.
Results
Study sample characteristics
Study sample characteristics can be found in Table 1. Women comprised slightly more than half of our sample and the average age of participants was 66.3 years. On average, participants were overweight. The average muscle attenuation in women was 51.1 HU and in men was 53.9 HU. Mobility disability was present in 10.8 % of women and 7.9 % of men. More than 20 % of women and nearly 15 % of men had a walking speed ≤1 m/s.
Table 1.
Study sample characteristics
| Women (n = 648) | Men (n = 504) | Overall (n = 1152) | |
|---|---|---|---|
| Age, years | 66.3 (8.7) | 66.1 (9.0) | 66.2 (8.8) |
| BMI, kg/m2 | 27.9 (5.9) | 28.8 (4.5) | 28.3 (5.3) |
| Waist circumference, cm | 99.0 (16.2) | 104.3 (11.3) | 101.3 (14.5) |
| Smoking, % | 6.8 (44) | 6.2 (31) | 6.5 (75) |
| Physical Activity, PAI Scorea | 35.2 (4.7) | 35.8 (6.0) | 35.4 (5.3) |
| Diabetes, % | 10.6 (69) | 15.9 (80) | 12.9 (149) |
| Prevalent cardiovascular disease, % | 12.2 (79) | 18.7 (94) | 15.0 (173) |
| Muscle attenuation, HUb | 51.1 (7.1) | 53.9 (7.6) | 52.4 (7.4) |
| VAT volume, cm3 | 1625 (867) | 2571 (1079) | 2039 (1073) |
| SAT volume, cm3 | 3291 (1406) | 2636 (1105) | 3004 (1323) |
| Mobility disability, % | 10.8 (70) | 7.9 (40) | 9.5 (110) |
| Walking speed, m/s | 1.21 (0.26) | 1.26 (0.28) | 1.23 (0.27) |
| Walking speed ≤1 m/s, % | 21.9 (142) | 14.7 (74) | 18.8 (216) |
| Grip strength, kg | 24.0 (5.9) | 41.9 (9.6) | 31.9 (11.8) |
Data presented as mean (standard deviation) for continuous characteristics and percentage (n) for categorical characteristics
aPhysical Activity Index Score
bHounsfield units
Multivariable-adjusted associations between muscle attenuation and physical performance
Men and women showed similar associations between muscle attenuation and physical function with a significant sex interaction only present for grip strength (p < 0.0001, all other p ≥ 0.06). As seen in Table 2, associations between lower muscle attenuation (i.e., more muscle fat) and slower walking speed as both a continuous and categorical variable were observed. For example, per one standard deviation decrement in muscle attenuation in men and women combined, there was a 1.29 (95 % CI = 1.11, 1.50; p = 0.0009) increase in odds of walking speed ≤1 m/s, which persisted after separate adjustments for VAT volume, SAT volume, and BMI (p < 0.02). No significant association was seen with mobility disability (p = 0.11). In men only, there was an associated decrement in grip strength (−1.29 kg, 95 % CI = −2.01, −0.57; p = 0.0005) that persisted after separate BMI, SAT, and VAT adjustments.
Table 2.
Multivariable regressions per 1 SD decrement in muscle attenuation
| Outcomea | Modelb | Women | Men | Overall | |||
|---|---|---|---|---|---|---|---|
| (95 % CI) | p value | (95 % CI) | p value | (95 % CI) | p value | ||
| Mobility disability, OR | MV | 1.25 (0.99, 1.58) | 0.06 | 1.03 (0.77, 1.37) | 0.86 | 1.15 (0.97, 1.37) | 0.11 |
| MV + BMI | 1.15 (0.90, 1.47) | 0.25 | 1.02 (0.75, 1.38) | 0.91 | 1.07 (0.89, 1.29) | 0.47 | |
| MV + VAT volume | 1.07 (0.83, 1.39) | 0.61 | 1.04 (0.77, 1.40) | 0.81 | 1.08 (0.89, 1.30) | 0.43 | |
| MV + SAT volume | 1.20 (0.94,1.53) | 0.14 | 1.02 (0.76,1.38) | 0.88 | 1.11 (0.93,1.33) | 0.24 | |
| Sex interaction | 0.23 | ||||||
| Grip strength, kg | MV | −0.07 (−0.49, 0.36) | 0.76 | −1.29 (−2.01, −0.57) | 0.0005 | −0.66 (−1.07, 0.25) | 0.002 |
| MV + BMI | −0.10 (−0.54, 0.34) | 0.66 | −1.41 (−2.17, −0.65) | 0.0003 | −0.72 (−1.14, −0.30) | 0.0009 | |
| MV + VAT volume | −0.06 (−0.53, 0.40) | 0.78 | −1.38 (−2.13, −0.62) | 0.0004 | −0.07 (−1.13, −0.27) | 0.002 | |
| MV + SAT volume | −0.08 (−0.51,0.36) | 0.74 | −1.25 (−2.00, −0.50) | 0.0012 | −0.65 (−1.07, −0.23) | 0.0023 | |
| Sex Interaction | <0.0001 | ||||||
| Walking speedc, m/s | MV | −0.04 (−0.06, −0.02) | <0.0001 | −0.03 (−0.05, −0.01) | 0.01 | −0.03 (−0.05, −0.02) | <0.0001 |
| MV + BMI | −0.03 (−0.05, −0.01) | 0.001 | −0.02 (−0.04, 0.01) | 0.14 | −0.03 (−0.04, −0.01) | 0.0008 | |
| MV + VAT volume | −0.03 (−0.05, −0.01) | 0.007 | −0.02 (−0.05, 0.00) | 0.077 | −0.03 (−0.04, −0.01) | 0.001 | |
| MV + SAT volume | −0.03 (−0.05, −0.01) | 0.0006 | −0.02 (−0.04, 0.01) | 0.13 | −0.03 (−0.04, −0.01) | 0.0005 | |
| Sex interaction | 0.06 | ||||||
| Walking speed ≤1 m/sc, OR | MV | 1.25 (1.02, 1.53) | 0.03 | 1.35 (1.07, 1.71) | 0.01 | 1.29 (1.11, 1.50) | 0.0009 |
| MV + BMI | 1.19 (0.97, 1.46) | 0.096 | 1.29 (1.01, 1.64) | 0.04 | 1.22 (1.05, 1.43) | 0.009 | |
| MV + VAT volume | 1.15 (0.92, 1.43) | 0.22 | 1.30 (1.02, 1.65) | 0.03 | 1.21 (1.03, 1.42) | 0.02 | |
| MV + SAT volume | 1.21 (0.99, 1.49) | 0.07 | 1.31 (1.03, 1.67) | 0.03 | 1.24 (1.07, 1.45) | 0.004 | |
| Sex interaction | 0.86 | ||||||
OR odds ratio
aOutcomes modeled per 1 standard deviation (7 unit decrement) in muscle attenuation (more intramuscular fat)
bMV Model adjusts for age, current smoking status, diabetes, CVD, and physical activity score
cWalking speed additionally adjusted for height
Multivariable-adjusted associations between VAT volume and physical performance
Table 3 summarizes the associations between VAT volume and physical performance. While VAT volume and physical performance measures showed significant associations, particularly in women, none persisted after adjustment for BMI. Significantly larger associations were seen in women than men between VAT volume and mobility disability (sex interaction p = 0.005) and continuous walking speed (sex interaction p = 0.03). Per 500 cm3 increment in VAT volume in women, there was a 1.32 (95 % CI = 1.13, 1.53; p = 0.0003) increase in odds of mobility disability and a 1.20 (95 % CI = 1.05, 1.36; p = 0.005) increase in odds of walking speed ≤1 m/s, both of which lost significance after adjustment for BMI (p ≥ 0.34). Similarly, no associations with VAT volume in men retained significance after adjustment for BMI. No significant associations were observed between VAT volume and grip strength in either sex (p > 0.62).
Table 3.
Multivariable regressions per 500-cm3 increment in VAT volume
| Outcomea | Modelb | Women | Men | Overall | |||
|---|---|---|---|---|---|---|---|
| (95 % CI) | p value | (95 % CI) | p value | (95 % CI) | p value | ||
| Mobility disability, OR | MV | 1.32 (1.13, 1.53) | 0.0003 | 0.98 (0.84, 1.15) | 0.83 | 1.15 (1.03, 1.28) | 0.01 |
| MV + BMI | 1.10 (0.89, 1.35) | 0.37 | 0.94 (0.76, 1.18) | 0.61 | 0.98 (0.85, 1.14) | 0.82 | |
| MV + Muscle Attenuation | 1.30 (1.10, 1.53) | 0.002 | 0.98 (0.83, 1.15) | 0.79 | 1.13 (1.01, 1.27) | 0.03 | |
| Sex interaction | 0.005 | ||||||
| Grip strength, kg | MV | −0.02 (−0.27, 0.24) | 0.91 | −0.05 (−0.42, 0.32) | 0.79 | −0.06 (−0.28, 0.17) | 0.62 |
| MV + BMI | −0.15 (−0.51, 0.21) | 0.42 | −0.07 (−0.57, 0.44) | 0.80 | −0.14 (−0.44, 0.16) | 0.36 | |
| MV + Muscle Attenuation | 0.00 (−0.28, 0.27) | 0.99 | 0.15 (−0.23, 0.53) | 0.43 | 0.07 (−0.17, 0.30) | 0.57 | |
| Sex interaction | 0.26 | ||||||
| Walking speedc, m/s | MV | −0.03 (−0.04, −0.01) | <0.0001 | −0.02 (−0.03, 0.00) | 0.008 | −0.02 (−0.03, −0.01) | <0.0001 |
| MV + BMI | −0.01 (−0.03, 0.00) | 0.13 | 0.00 (−0.02, 0.01) | 0.69 | −0.01 (−0.02, 0.00) | 0.18 | |
| MV + Muscle Attenuation | −0.02 (−0.03, −0.01) | 0.002 | −0.01 (−0.02, 0.00) | 0.04 | −0.02 (−0.02, −0.01) | 0.0003 | |
| Sex interaction | 0.03 | ||||||
| Walking speed ≤1 m/sc, OR | MV | 1.20 (1.05, 1.36) | 0.005 | 1.13 (1.00, 1.28) | 0.058 | 1.16 (1.06, 1.27) | 0.0009 |
| MV + BMI | 1.09 (0.91, 1.30) | 0.34 | 1.08 (0.90, 1.28) | 0.42 | 1.09 (0.96, 1.22) | 0.18 | |
| MV + Muscle attenuation | 1.16 (1.01, 1.33) | 0.03 | 1.08 (0.95, 1.24) | 0.24 | 1.12 (1.02, 1.23) | 0.02 | |
| Sex interaction | 0.49 | ||||||
OR odds ratio
aOutcomes modeled per 500-cm3 increase in VAT volume
bMV model adjusts for age, current smoking status, diabetes, CVD, and physical activity score
cWalking speed additionally adjusted for height
Multivariable-adjusted associations between SAT volume and physical performance
Similar associations between SAT volume and physical performance measures were observed for women and men with no significant sex interactions (all p ≥ 0.17). As seen in Table 4, associations between SAT volume and physical performance measures also largely did not retain significance after adjustment for BMI. For example, per 500-cm3 increment in SAT volume, there was an associated 1.10 (95 % CI = 1.02, 1.19; p = 0.02) increased odds of mobility disability, which was no longer significant after adjustment for BMI (p = 0.14). While in the overall model, an association between SAT volume and continuous walking speed persisted after adjustment for BMI (−0.01 m/s; 95 % CI = −0.02, 0.00; p = 0.05), this association did not persist in either sex separately (p ≥ 0.06).
Table 4.
Multivariable regressions per 500-cm3 increment in SAT volume
| Outcomea | Modelb | Women | Men | Overall | |||
|---|---|---|---|---|---|---|---|
| (95 % CI) | p value | (95 % CI) | p value | (95 % CI) | p value | ||
| Mobility disability, OR | MV | 1.13 (1.03, 1.24) | 0.008 | 1.01 (0.87, 1.18) | 0.90 | 1.10 (1.02, 1.19) | 0.02 |
| MV + BMI | 0.86 (0.72, 1.02) | 0.09 | 0.99 (0.77, 1.27) | 0.94 | 0.90 (0.78, 1.03) | 0.14 | |
| MV + Muscle Attenuation | 1.12 (1.02, 1.23) | 0.02 | 1.01 (0.86, 1.18) | 0.93 | 1.09 (1.01, 1.18) | 0.03 | |
| Sex interaction | 0.17 | ||||||
| Grip strength, kg | MV | 0.01 (−0.13, 0.16) | 0.85 | −0.23 (−0.57, 0.11) | 0.19 | −0.07 (−0.23, 0.09) | 0.39 |
| MV + BMI | −0.09 (−0.36, 0.18) | 0.51 | −0.57 (−1.12, −0.01) | 0.05 | −0.27 (−0.55, 0.01) | 0.06 | |
| MV + Muscle Attenuation | 0.02 (−0.13, 0.17) | 0.81 | −0.08 (−0.43, 0.28) | 0.68 | −0.02 (−0.18, 0.14) | 0.84 | |
| Sex interaction | 0.29 | ||||||
| Walking speedc, m/s | MV | −0.01 (−0.02, −0.01) | <0.0001 | −0.02 (−0.03, −0.01) | 0.0001 | −0.02 (−0.02, −0.01) | <0.0001 |
| MV + BMI | −0.01 (−0.02, 0.01) | 0.38 | −0.02 (−0.03, 0.00) | 0.060 | −0.01 (−0.02, 0.00) | 0.05 | |
| MV + Muscle Attenuation | −0.01 (−0.02, −0.01) | 0.0002 | −0.02 (−0.03, −0.01) | 0.0008 | −0.01 (−0.02, −0.01) | <0.0001 | |
| Sex interaction | 0.27 | ||||||
| Walking speed ≤1 m/sc, OR | MV | 1.11 (1.03, 1.20) | 0.009 | 1.10 (0.97, 1.25) | 0.13 | 1.11 (1.04, 1.18) | 0.003 |
| MV + BMI | 1.02 (0.88, 1.17) | 0.83 | 1.00 (0.81, 1.23) | 1.00 | 1.20 (0.91, 1.14) | 0.80 | |
| MV + Muscle Attenuation | 1.10 (1.02, 1.19) | 0.02 | 1.06 (0.93, 1.21) | 0.41 | 1.09 (1.02, 1.16) | 0.01 | |
| Sex interaction | 0.95 | ||||||
OR odds ratio
aOutcomes modeled per 500-cm3 increase in SAT volume
bMV Model adjusts for age, current smoking status, diabetes, CVD, and physical activity score
cWalking speed additionally adjusted for height
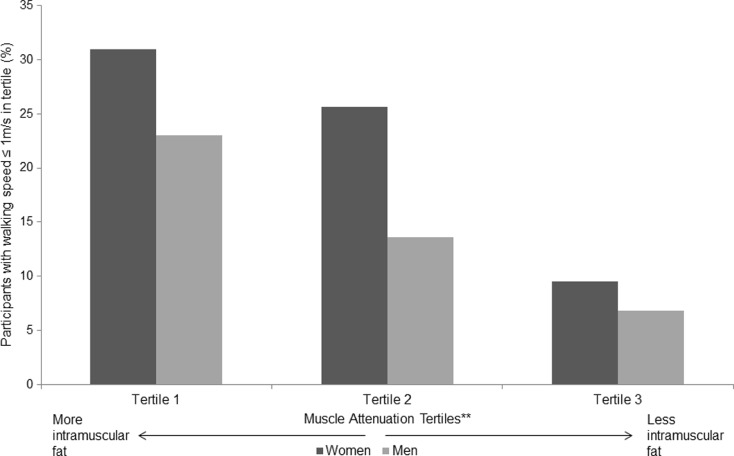
Walking speed ≤1 m/s by muscle attenuation tertile
In women, the percentage of participants with walking speed ≤1 m/s was greatest in tertile 1 (most intramuscular fat) (30.9 %) and least in tertile 3 (least intramuscular fat) (9.5 %) (Fig. 1). This trend was significant with p < 0.0001. A similar trend was seen in men (p < 0.0001).
Fig. 1.
Percentage of participants in each muscle attenuation tertile group (tertile 1—lowest muscle attenuation, i.e., greatest amount of intramuscular fat up to tertile 3—highest muscle attenuation, i.e., least amount of intramuscular fat) who have a walking speed of ≤1 m/s; n = 1152. *Test for trend in each case had p value < 0.0001 **Tertiles ranging from tertile 1 (lowest muscle attenuation, i.e., most intramuscular fat) to tertile 3 (highest muscle attenuation, i.e., least intramuscular fat)
Discussion
Principle findings
Our study demonstrates that lower muscle attenuation (i.e., more intramuscular fat) is associated with slower walking speed in both women and men and weaker grip strength in men only. These associations persisted after adjustment for both BMI and VAT volumes separately. Associations between VAT volume and SAT volume and measures of physical function did not persist after adjustment for BMI, suggesting that these associations are likely related to global adiposity.
In context of the current literature
Our study is consistent with the findings of a previous study from the Health, Aging and Body Composition Study which found that decreased thigh muscle attenuation is correlated with decreased walking speed and decreased grip strength (Cawthon et al. 2009). Similarly, an InCHIANTI study found that lower muscle density (i.e., more intramuscular fat) is correlated with slower walking speed (Cesari et al. 2009). We extend the current literature by stratifying our results by sex, which was not done previously for intramuscular fat with all of the physical function measures we examined (Cawthon et al. 2009; Cesari et al. 2009). By doing so, we found that while lower muscle attenuation was associated with slower walking speed in both sexes, weaker grip strength is an association unique to men. It is possible that the association between muscle attenuation and grip strength was not present in women due to additional medical conditions that are more prevalent in women. For example, both rheumatoid arthritis and osteoarthritis are more common in women and have been shown to be associated with weaker grip strength (Brorsson et al. 2014).
Our study adds to the current literature as it is the first to additionally adjust for BMI, SAT volume, and VAT volume separately. By showing the persistent associations of muscle attenuation with walking speed and grip strength after accounting for these global and regional adiposity measures, our results further support a local role of intramuscular fat in physical function.
Previous studies have investigated the association between mobility disability and intramuscular fat (Visser et al. 2005). In contrast to our results, this study found that increasing intramuscular fat is associated with increased risk of mobility disability in both men and women. Another study found that lower thigh muscle attenuation predicted increased mobility disability in women only (Reinders et al. 2015) and a second study of women found lower leg muscle attenuations in those who reported falls compared with those who did not (Frank et al. 2015). One possible reason for the conflicting results is that our study sample was younger. Since mobility disability is more common in older adults (22.3 % of women and 31.8 % of men in the former study), our sample may have been underpowered to observe this association (Visser et al. 2005). Another possible consideration for this discrepancy is that our study was cross-sectional whereas the previous report had repeated assessment of mobility disability over time (Visser et al. 2005).
Our study used paraspinous muscle attenuation to estimate intramuscular fat whereas most of previous studies have used thigh muscle attenuation (Visser et al. 2005; Goodpaster et al. 2001; Cawthon et al. 2009) or calf muscle density on peripheral quantitative computerized tomography (Cesari et al. 2009). However, our study agrees with previous findings regarding the association of increasing intramuscular fat and decreasing walking speed despite using a muscle that is not in the leg. This is likely because while paraspinous muscles are not the primary muscles for walking, they have an important secondary role in walking and balance by increasing pelvis stabilization and assisting hip extension (Kobetic et al. 1997).
Our findings are also consistent with reports from the Health Aging and Body Composition study that found that an increment in average trunk muscle attenuation, a measure that included paraspinal muscles, was associated with a decrement in physical performance battery score (Hicks et al. 2005a). The physical performance battery included a 6-m walk, but did not include grip strength. Physical performance measure associations were not evaluated separately as they were in our study, so our individual results cannot be compared. Of note, a longitudinal study from the Health Aging and Body Composition study also found that lower trunk muscle attenuation predicted lower functional capacity over a 3 year period of time (Hicks et al. 2005b). Efforts to avoid mobility limitations need to focus not only on lower extremity muscles but also on trunk muscles to improve balance and maintain mobility.
There is a body of prior work that has examined the associations between intermuscular fat and physical function, which have overall found associations between greater amounts of intermuscular fat and decrements in physical function (Beavers et al. 2013; Murphy et al. 2014). However, intermuscular fat is in between muscle fibers, unlike intramuscular fat, which is within muscle fibers. Since these two types of fat may affect muscle function differently, though these mechanisms have not been made clear, we compared only to other studies examining proxies of intramuscular fat.
Potential mechanisms
There are several possible mechanisms to explain the unique associations that were observed between lower muscle attenuation (i.e., more intramuscular fat) and poorer physical function. First, more intramuscular fat may be associated with different ratios of muscle fiber types. More intramuscular fat, as estimated by muscle attenuation, is associated with lower muscle strength (Goodpaster et al. 2001). The reason for this has not been fully characterized, but may be due to both an infiltration of fat within muscle fibers affecting function and a higher proportion of type I muscle fibers (Goodpaster et al. 2001). Type I muscle fibers undergo less atrophy with age than type II fibers and also have higher lipid content (Essen et al. 1975; Larsson 1983). Type I muscle fibers generate less force than type II muscle fibers (Linssen et al. 1991). Therefore, a decrease in type II muscle fiber proportion would lead to decreased muscle strength which is in turn is associated with impaired physical function (Cawthon et al. 2009; Volpato et al. 2012).
Second, intramuscular fat and skeletal muscle itself both release cytokines that may affect muscle function. Intramuscular fat secretes adipokines, many of which are associated with inflammation. For example, increased intramuscular fat has been found to be associated with increased inflammatory adipokine interleukin-6 (IL-6) even after adjusting for BMI (Addison et al. 2014). Increased IL-6, possibly due to its inflammatory properties, is associated with poorer physical performance (Addison et al. 2014), including decrements in walking speed and grip strength (Cesari et al. 2004). Similarly, skeletal muscle secretes multiple myokines and their secretion is often linked to muscle contraction and exercise (Raschke and Eckel 2013). Myokines have many functions, but it is believed that overall they assist in metabolic functions necessary for muscle function (Raschke and Eckel 2013). Although speculative at this point, it is possible that greater amounts of intramuscular fat could impair muscle fiber contraction and myokine secretion, thereby further decreasing muscle function; this could be an additional systemic effect of intramuscular fat.
Finally, aging has been associated with a loss of both muscle mass and muscle strength (Goodpaster et al. 2006). However, the loss of muscle strength is greater than that which would be expected from loss of muscle mass alone (Goodpaster et al. 2006); this may be related to age-related changes in muscle quality (Moore et al. 2014). Multiple mechanisms likely contribute to these changes, including inflammation, oxidative stress, and impaired muscle repair (Kalyani et al. 2014). As intramuscular fat is associated with inflammatory marker IL-6 (Addison et al. 2014), it may have some association with these changes and subsequent muscle repair. One form of impaired muscle repair, muscle fibrosis, has been shown in animal studies to increase with aging as muscle stem cells undergo a change to fibrogenic progenitors (Brack et al. 2007). It is possible that muscle repair and fibrosis could affect attenuation.
Implications
This study emphasizes the associations between intramuscular fat and physical function that are potentially specific to intramuscular fat versus global adiposity or other fat depots. In particular, there were unique associations with walking speed and grip strength. These are important healthy aging markers due to their associations with loss of independence (Judge et al. 1996) and incident mortality (Sasaki et al. 2007; Studenski et al. 2011). Physical activity interventions have been associated with decreased muscle fat infiltration and increased muscle strength further supporting a relationship between muscle function and intramuscular fat (Goodpaster et al. 2008). However, a recent study found that physical activity interventions with or without weight loss were not associated with changes in thigh muscle attenuation (Santanasto et al. 2015). Further studies should focus on investigating the mechanisms behind these associations that could potentially lead to muscle-specific drug targets to improve physical function.
Strengths and limitations
The main strengths of our study include a community-based sample that was not enriched for adiposity, which may allow our results to be more generalizable. Our sample also had a wide range of ages and physical ability again allowing potentially improved generalizability of our results. Additionally, our results were adjusted for multiple well-documented covariates. There were a few limitations that should be noted. First, we used muscle attenuation as an estimation of intramuscular fat. While it has shown significant correlation with intramuscular fat in past studies (Goodpaster et al. 2000), it is not a direct measure of intramuscular fat. We also did not have a measurement of intermuscular fat. These limitations potentially may have biased our results towards the null hypothesis, but would not explain our significant findings. Second, using paraspinous muscles may have reduced the significance of associations for walking speed and grip strength, as they are not the primary muscle group for these actions and muscle attenuation varies between different muscles (Anderson et al. 2013; Goodpaster et al. 2001). We did not have data available on Framingham participants on other muscle groups such as lower extremity muscle attenuation for comparison or analysis. However, this again does not explain the positive findings. Third, our sample is predominately Caucasian, so these results may not apply to other ethnic groups. Fourth, we were unable to include participants in our study who did not elect to undergo a MDCT scan, which tended to be a group that was older with more self-reported mobility limitations and poorer physical function. This healthy selection bias may have decreased the size and strength of the associations we observed between muscle attenuation and physical function. Fifth, we did not have data available to adjust for total fat mass. Additionally, we did not have data on arthritis in participants that could be used as an adjustment on grip strength. Finally, as this was a cross-sectional and observational study, we cannot conclude causation or temporality.
Conclusions
Lower muscle attenuation, an estimation of increasing intramuscular fat, is associated with poorer physical function. Specifically, it was associated with slower walking speed in both sexes and weaker grip strength in men. These associations persist after adjustment for BMI and VAT volume separately, suggesting that the association is due to a unique local quality of intramuscular fat. Further research into these associations could clarify the role of intramuscular fat in obesity and disability.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOCX 14 kb)
Compliance with ethical standards
Funding sources
The Framingham Heart Study of the National Heart, Lung and Blood Institute is supported by contract N01-HC-25195 and HHSN268201500001I. Dr Joanne Murabito is supported by R0129451.
Conflicts of interest
Pedley, A. Employment: Merck and Co, Inc. All other authors are without conflicts of interest or disclosures.
References
- Abraham TM, Pedley A, Massaro JM, Hoffmann U, Fox CS. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation. 2015;132:1639–1647. doi: 10.1161/CIRCULATIONAHA.114.015000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addison O, Drummond MJ, LaStayo PC, Dibble LE, Wende AR, McClain DA, et al. Intramuscular fat and inflammation differ in older adults: the impact of frailty and inactivity. J Nutr Health Aging. 2014;18:532–538. doi: 10.1007/s12603-014-0019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al SS, Ottenbacher KJ, Markides KS, Kuo YF, Eschbach K, Goodwin JS. The effect of obesity on disability vs mortality in older Americans. Arch Intern Med. 2007;167:774–780. doi: 10.1001/archinte.167.8.774. [DOI] [PubMed] [Google Scholar]
- Alley DE, Chang VW. The changing relationship of obesity and disability, 1988–2004. JAMA. 2007;298:2020–2027. doi: 10.1001/jama.298.17.2020. [DOI] [PubMed] [Google Scholar]
- Anderson DE, D’Agostino JM, Bruno AG, Demissie S, Kiel DP, Bouxsein ML. Variations of CT-based trunk muscle attenuation by age, sex, and specific muscle. J. Gerontol.A Biol. Sci Med Sci. 2013;68:317–323. doi: 10.1093/gerona/gls168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angleman SB, Harris TB, Melzer D. The role of waist circumference in predicting disability in periretirement age adults. Int J Obes (Lond) 2006;30:364–373. doi: 10.1038/sj.ijo.0803130. [DOI] [PubMed] [Google Scholar]
- Beavers KM, Beavers DP, Houston DK, Harris TB, Hue TF, Koster A, et al. Associations between body composition and gait-speed decline: results from the Health, Aging, and Body Composition study. Am J Clin Nutr. 2013;97:552–560. doi: 10.3945/ajcn.112.047860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Brorsson S, Nilsdotter A, Thorstensson C, Bremander A. Differences in muscle activity during hand-dexterity tasks between women with arthritis and a healthy reference group. BMC Musculoskelet Disord. 2014;15:154. doi: 10.1186/1471-2474-15-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon PM, Fox KM, Gandra SR, Delmonico MJ, Chiou CF, Anthony MS, et al. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc. 2009;57:1411–1419. doi: 10.1111/j.1532-5415.2009.02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M, Penninx BW, Pahor M, Lauretani F, Corsi AM, Rhys WG, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J. Gerontol.A Biol. Sci Med Sci. 2004;59:242–248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- Cesari M, Kritchevsky SB, Penninx BW, Nicklas BJ, Simonsick EM, Newman AB, et al. Prognostic value of usual gait speed in well-functioning older people—results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- Cesari M, Pahor M, Lauretani F, Zamboni V, Bandinelli S, Bernabei R, et al. Skeletal muscle and mortality results from the InCHIANTI Study. J. Gerontol.A Biol. Sci Med Sci. 2009;64:377–384. doi: 10.1093/gerona/gln031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawber TR, Kannel WB, LYELL LP. An approach to longitudinal studies in a community: the Framingham Study. Ann.N.Y.Acad.Sci. 1963;107:539–556. doi: 10.1111/j.1749-6632.1963.tb13299.x. [DOI] [PubMed] [Google Scholar]
- Essen B, Jansson E, Henriksson J, Taylor AW, Saltin B. Metabolic characteristics of fibre types in human skeletal muscle. Acta Physiol Scand. 1975;95:153–165. doi: 10.1111/j.1748-1716.1975.tb10038.x. [DOI] [PubMed] [Google Scholar]
- Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- Frank AW, Farthing JP, Chilibeck PD, Arnold CM, Olszynski WP, Kontulainen SA. Community-dwelling female fallers have lower muscle density in their lower legs than non-fallers: evidence from the Saskatoon Canadian Multicentre Osteoporosis Study (CaMos) cohort. J Nutr Health Aging. 2015;19:113–120. doi: 10.1007/s12603-014-0476-6. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, et al. Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol. 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J ApplPhysiol (1985) 2000;89:104–110. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J. Gerontol.A Biol. Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Chomentowski P, Ward BK, Rossi A, Glynn NW, Delmonico MJ, et al. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol (1985) 2008;105:1498–1503. doi: 10.1152/japplphysiol.90425.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergenroeder AL, Brach JS, Otto AD, Sparto PJ, Jakicic JM. The Influence of Body Mass Index on Self-report and Performance-based Measures of Physical Function in Adult Women. Cardiopulm Phys Ther J. 2011;22:11–20. [PMC free article] [PubMed] [Google Scholar]
- Hicks GE, Simonsick EM, Harris TB, Newman AB, Weiner DK, Nevitt MA, et al. Cross-sectional associations between trunk muscle composition, back pain, and physical function in the health, aging and body composition study. J. Gerontol.A Biol Sci Med Sci. 2005;60:882–887. doi: 10.1093/gerona/60.7.882. [DOI] [PubMed] [Google Scholar]
- Hicks GE, Simonsick EM, Harris TB, Newman AB, Weiner DK, Nevitt MA, et al. Trunk muscle composition as a predictor of reduced functional capacity in the health, aging and body composition study: the moderating role of back pain. J. Gerontol.A Biol. Sci Med Sci. 2005;60:1420–1424. doi: 10.1093/gerona/60.11.1420. [DOI] [PubMed] [Google Scholar]
- Judge JO, Schechtman K, Cress E. The relationship between physical performance measures and independence in instrumental activities of daily living The FICSIT Group. Frailty and Injury: Cooperative Studies of Intervention Trials. J Am Geriatr Soc. 1996;44:1332–1341. doi: 10.1111/j.1532-5415.1996.tb01404.x. [DOI] [PubMed] [Google Scholar]
- Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014;2:819–829. doi: 10.1016/S2213-8587(14)70034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel WB, Sorlie P. Some health benefits of physical activity The Framingham. Study Arch Intern Med. 1979;139:857–861. doi: 10.1001/archinte.1979.03630450011006. [DOI] [PubMed] [Google Scholar]
- KANNEL WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- Kobetic R, Triolo RJ, Marsolais EB. Muscle selection and walking performance of multichannel FES systems for ambulation in paraplegia. IEEE Trans Rehabil Eng. 1997;5:23–29. doi: 10.1109/86.559346. [DOI] [PubMed] [Google Scholar]
- Kraigher-Krainer E, Lyass A, Massaro JM, Lee DS, Ho JE, Levy D, et al. Association of physical activity and heart failure with preserved vs. reduced ejection fraction in the elderly: the Framingham Heart Study. EurJHeart Fail. 2013;15:742–746. doi: 10.1093/eurjhf/hft025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L. Histochemical characteristics of human skeletal muscle during aging. Acta Physiol Scand. 1983;117:469–471. doi: 10.1111/j.1748-1716.1983.tb00024.x. [DOI] [PubMed] [Google Scholar]
- Linssen WH, Stegeman DF, Joosten EM, Binkhorst RA, Merks MJ, ter Laak HJ, et al. Fatigue in type I fiber predominance: a muscle force and surface EMG study on the relative role of type I and type II muscle fibers. Muscle Nerve. 1991;14:829–837. doi: 10.1002/mus.880140906. [DOI] [PubMed] [Google Scholar]
- Moore AZ, Caturegli G, Metter EJ, Makrogiannis S, Resnick SM, Harris TB, et al. Difference in muscle quality over the adult life span and biological correlates in the Baltimore Longitudinal Study of Aging. J Am Geriatr Soc. 2014;62:230–236. doi: 10.1111/jgs.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy RA, Reinders I, Register TC, Ayonayon HN, Newman AB, Satterfield S, et al. Associations of BMI and adipose tissue area and density with incident mobility limitation and poor performance in older adults. Am J Clin Nutr. 2014;99:1059–1065. doi: 10.3945/ajcn.113.080796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostbye T, Dement JM, Krause KM. Obesity and workers’ compensation: results from the Duke Health and Safety Surveillance System. Arch Intern Med. 2007;167:766–773. doi: 10.1001/archinte.167.8.766. [DOI] [PubMed] [Google Scholar]
- Parikh NI, Hwang SJ, Larson MG, Cupples LA, Fox CS, Manders ES, et al. Parental occurrence of premature cardiovascular disease predicts increased coronary artery and abdominal aortic calcification in the Framingham Offspring and Third Generation cohorts. Circulation. 2007;116:1473–1481. doi: 10.1161/CIRCULATIONAHA.107.705202. [DOI] [PubMed] [Google Scholar]
- Raschke S, Eckel J. Adipo-myokines: two sides of the same coin—mediators of inflammation and mediators of exercise. Mediators Inflamm. 2013;2013:320724. doi: 10.1155/2013/320724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinders I, Murphy RA, Koster A, Brouwer IA, Visser M, Garcia ME, et al. Muscle Quality and Muscle Fat Infiltration in Relation to Incident Mobility Disability and Gait Speed Decline: the Age, Gene/Environment Susceptibility-Reykjavik Study. J. Gerontol.A Biol. Sci Med Sci. 2015;70:1030–1036. doi: 10.1093/gerona/glv016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallinen J, Stenholm S, Rantanen T, Heliovaara M, Sainio P, Koskinen S. Hand-grip strength cut points to screen older persons at risk for mobility limitation. J Am Geriatr Soc. 2010;58:1721–1726. doi: 10.1111/j.1532-5415.2010.03035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santanasto, A. J., Newman, A. B., Strotmeyer, E. S., Boudreau, R. M., Goodpaster, B. H., & Glynn, N. (2015). Effects of changes in regional body composition on physical function in older adults: a pilot randomized controlled trial. J Nutr Health Aging 1–9. [DOI] [PMC free article] [PubMed]
- Sasaki H, Kasagi F, Yamada M, Fujita S. Grip strength predicts cause-specific mortality in middle-aged and elderly persons. Am J Med. 2007;120:337–342. doi: 10.1016/j.amjmed.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm R, Ringel JS, Andreyeva T. Increasing obesity rates and disability trends. Health Aff (Millwood) 2004;23:199–205. doi: 10.1377/hlthaff.23.2.199. [DOI] [PubMed] [Google Scholar]
- Therkelsen KE, Pedley A, Speliotes EK, Massaro JM, Murabito J, Hoffmann U, et al. Intramuscular fat and associations with metabolic risk factors in the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2013;33:863–870. doi: 10.1161/ATVBAHA.112.301009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J. Gerontol.A Biol. Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- Volpato S, Bianchi L, Lauretani F, Lauretani F, Bandinelli S, Guralnik JM, et al. Role of muscle mass and muscle quality in the association between diabetes and gait speed. Diabetes Care. 2012;35:1672–1679. doi: 10.2337/dc11-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 14 kb)