Abstract
Aging is a complex process that is accompanied with changes in both muscle mass and muscle function (strength and performance). Therefore, the current longitudinal study aimed to provide a better insight in 10-year aging-related changes in whole-body muscle mass and strength performance of the leg extensors during the adult life span. Data were gathered within the framework of the first- (2002–2004: baseline) and third-generation Flemish Policy Research Center Sport (2012–2014: follow-up). Results are based on muscle characteristics data of 591 Flemish Caucasian adults (19–73 years, 381 men). Skeletal muscle mass (SMM) was determined with bioelectrical impedance analysis. Biodex Medical System 3® dynamometer was used to measure isometric (PTstatic120°) and isokinetic (PTdynamic60° and PTdynamic240°) strength, ballistic movement speed (S20 %), and muscular endurance (work) of the knee extensors. Overall strength performance was higher at both evaluation moments in men compared to women (p < 0.01). But only S20 % declined significantly faster in men compared to women (p < 0.01). Age and baseline strength performance were negatively related with the change in strength performance, even when corrected for SMM, protein intake, and energy expenditure during sports (Esport). In conclusion, strength performance was not associated with Esport in this study, but protein intake was associated with isometric strength in men, and with ballistic and isokinetic strength in women. Changes in S20 % were significantly greater in men compared to women. Baseline values of strength performance and age were associated with changes in strength performance parameters, even after correction for SMM, protein intake, and Esport.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-016-9900-7) contains supplementary material, which is available to authorized users.
Keywords: Aging, Muscle mass, Muscle function, Ballistic movement speed, Protein intake, Energy expenditure during sports
Introduction
In Western countries, the population of people aged 60 years or more is increasing at a fast pace, with an expected increase of 45 % by 2050 (rising from 287 million in 2013 to 417 million in 2050). This underlines the importance for public health-care systems to better understand age-related syndromes and diseases, such as sarcopenia. This condition is characterized by a progressive loss of skeletal muscle mass and its action generating capacity—also termed muscle function—and implies a risk of adverse outcomes such as fall-related injuries and impaired quality of life (Campbell et al. 1989; Cruz-Jentoft et al. 2010; Rizzoli et al. 2013). In addition, it is estimated that the prevalence of sarcopenia is approximately 1–33 % in the community (both genders combined) and that this prevalence increases in older populations (Cruz-Jentoft et al. 2014). However, the onset of sarcopenia occurs at an earlier phase, so it is important to understand how muscle characteristics change over the adult life span. A cross-sectional study by (Charlier et al. 2015) described changes in muscle function over the adult life span (18–78 years) in Flemish Caucasians; nonetheless, a longitudinal design is more appropriate to examine aging-related changes. In this kind of design, data are collected in a time sequence that allows for documentation of the direction as well as the magnitude of change over time. This allows one to study the individual development of a certain outcome variable over time, and the individual development of a certain outcome variable can be related to the individual development of other variables (Twisk 2013). The present study is, therefore, a further continuation of the previous observations by Charlier et al. (2015).
There are currently a number of studies that have examined the longitudinal changes in muscle mass and muscle function (e.g., Baltimore Longitudinal Study of Aging, the InCHIANTI study, Health ABC Study), but only a limited number examined study populations with a large age range (Metter et al. 1997; Delmonico et al. 2009; Hicks et al. 2012). Most of them have found significant age-related declines in both muscle mass and muscle function. However, the majority of these studies examined either small sample sizes (n < 50) or Frontera et al. 2008 (<5 years.) (Goodpaster et al. 2006; Frontera et al. 2008; Dey et al. 2009; Marcell et al. 2014; Reid et al. 2014). It should also be noted that study populations often consisted of a homogenous group, mostly older adults, and although in most cases this is a strength, it allows for a limited interpretation of lifelong changes in these parameters (Goodpaster et al. 2006; Frontera et al. 2008; Delmonico et al. 2009; Dey et al. 2009; Reid et al. 2014). Nevertheless, results between studies are similar, pointing out losses in muscle mass of up to 12.9 and 5.3 % after a 9.7-year follow-up period in men and women, respectively, aged 45–75 years (Hughes et al. 2001). In addition, isometric strength decreased with ~0.87 % per year in men aged between 45 and 49 years, whereas declines in isokinetic strength were up to 2.56 % per year at 60°/s and 4.20 % per year at 240°/s in 62–81-year-old men and women (Frontera et al. 2008; Kennis et al. 2014).
In reporting age-related changes in muscle mass or muscle function, these previous studies did not account for lifestyle (e.g., dietary habits, physical activity). In 2012, Stenholm et al. reported that lifestyle (e.g., education, leisure time and work-related physical activity, smoking behavior, alcohol consumption), and physical health (e.g., chronic conditions such as hypertension, coronary heart disease, cardiovascular disease, or diabetes mellitus) earlier in life determine the rate of muscle strength decline (measured as handgrip strength) in old age (Stenholm et al. 2012). Furthermore, various studies have evidenced that nutritional supplementation—such as vitamin D or protein intake—and exercise interventions can have a beneficial effect on muscle strength and physical performance, even in older populations (Bonnefoy et al. 2003; Binder et al. 2005; Chale-Rush et al. 2010; Pahor et al. 2014; Cesari et al. 2015). In 2015, Sahni et al. found a significantly higher leg lean mass in both males (n = 1166, age 60.2 ± 9.3 years) and females (n = 1509, age 59.0 ± 9.3 years) in the highest quartile of total protein and animal protein intake compared with those in the lowest quartiles (Sahni et al. 2015). In addition, quadriceps strength was higher in participants in the highest quartile of plant protein intake, although this association was no longer significant after adjustment for fruit and vegetable intake (Sahni et al. 2015). Regular physical activity has also been shown to prevent both the age-associated loss in muscle strength and the increase in muscle fat infiltration in older adults (5 men and 17 women, 76.7 ± 1.0 year) (Goodpaster et al. 2008).
Consequently, the current study aims to provide a better insight in the comprehension of 10-year aging-related changes in muscle mass and muscle function of the leg extensors during the adult life span. Specifically, a longitudinal study in Flemish, Caucasian adults was performed to examine inter-individual differences in 10-year aging and predict age-related changes in muscle mass and muscle function, accounting for protein intake and energy expenditure during sports. Because there are large differences between subjects in aging, it is our hypothesis that both muscle mass and muscle function will show a moderate tracking over a 10-year follow-up period in both genders. Also, since there is a decrease in firing rate and motor unit recruitment with aging, it is assumed that the age-related loss in ballistic strength is higher compared to (slow) isokinetic and endurance strength, and isometric strength is expected to show the smallest decreases. Furthermore, based on previous longitudinal findings, it is suggested that these declines will be more precipitous in men compared to women.
Methods
Subjects
Data were collected in the framework of the first (2002–2004) and third generations (2012–2015) of the Flemish Policy Research Center on Sport. A total of 923 men (45.6 ± 12.0 years) and 646 women (43.9 ± 10.5 years) were tested for the first time between October 2002 and April 2004 (baseline), of which a total of 420 men (age at baseline 46.8 ± 10.3 years) and 232 women (age at baseline 44.7 ± 9.0 years) were tested again between April 2012 and January 2014 (follow-up). The selection of this sample is described elsewhere (Matton et al. 2007a; Wijndaele et al. 2007). The purpose of this longitudinal study was to examine the relationship between physical activity, physical fitness, and several health parameters in a randomly selected community sample of 18- to 80-year-old subjects in Flanders, Belgium (Wijndaele et al. 2007), and their changes over a decades time. In the current study, results are based on muscle characteristics data of 381 men and 210 women, aged 19 to 73 at baseline, of Flemish Caucasian origin that were not excluded for Biodex measurements at either baseline or follow-up. Exclusion criteria for Biodex assessment are further discussed in Wijndaele et al. (2007).
Outcome measurements
The current study is part of a longitudinal research project, and therefore, the measurements performed here have been previously described elsewhere (Wijndaele et al. 2007). A concise overview is presented below and supplemented where necessary.
Anthropometry
Anthropometric measurements were assessed by trained staff using standardized equipment and techniques. All subjects were barefoot and wore minimal clothing. Weight was measured to the nearest 0.1 kg using a digital scale (seca 841, seca GmbH, Hamburg, Germany), and height was measured to the nearest millimeter using a Holtain stadiometer (Holtain, Crymych, UK). Afterwards, body mass index (BMI) was calculated as [weight (kg)/(height (m))2].
Body composition
The percentage of body fat (%BF) was determined with bioelectrical impedance (BIA) according to the standardized procedures. Subsequently, fat mass (FM, kg) and fat free mass (FFM, kg) were calculated for each subject based on the %BF.
Skeletal muscle mass
Whole-body skeletal muscle mass (SMM) was calculated following the BIA equation of Janssen et al. (2000):
In this equation, height is in centimeters; BIA-resistance is in ohms; for gender, men = 1 and women = 0; and age is in years. This equation was established and cross-validated against magnetic resonance imaging measures of whole-body muscle mass by Janssen et al. (2000) in a total sample of 269 men and women, varying widely in adiposity (BMI = 16–48 kg/m2) and age (18–86 years.).
Strength performance
Handgrip strength
Handgrip strength (HGR) was determined using a hydraulic handgrip dynamometer (Jamar, Sammons Preston Rolyan, Bolingbrook, IL). The best performance of two maximal trials (in kg) was registered for data analysis.
Knee muscle strength
The force-velocity characteristics of the knee extensors were measured using the Biodex Medical System 3® dynamometer (Biodex Medical Systems, Shirley, New York, USA) using standardized positioning of the subjects (Wijndaele et al. 2007). Unless medically contraindicated, all measurements were performed unilaterally on the right side. Three standardized tests were performed, including isometric, speed of movement, and isokinetic tests. Each test was performed twice, and the best performance was used for further analysis.
Isometric tests
The static strength of the knee extensors was examined at knee joint angles of 120° and 90°. The subjects performed two maximal static knee extensions in each knee joint angle. Peak torque (Nm) was recorded. The highest score of the extension test at 120° (PTstatic120°) was retained for further analysis to assess maximal isometric knee extension strength.
Isotonic tests
Three maximal ballistic knee extensions were performed against a constant load of 20 % of the maximal isometric strength in a knee joint angle of 90°. The subjects were asked to extend their leg as quickly as possible from a knee joint angle of 90° to 160° and then passively return the leg to the starting position (90°). The best performance of three repetitions was defined as the maximum speed of movement (°/s) at 20 % (S20 %) and was used for data analysis.
Isokinetic tests
Dynamic knee extension strength was measured by conducting four maximal knee extension-flexion movements at a low velocity of 60°/s and six repetitions at a high velocity of 240°/s. Peak torque (Nm) of both knee extensions (PTdynamic60° and PTdynamic240°, respectively) was recorded and further analyzed.
Muscular endurance test
Finally, the subjects had to perform 25 knee extensions and flexions at a velocity of 180°/s. Total work (J) (work) was registered as a measure of resistance to fatigue of the knee extensor and flexor muscles.
Strength measures are presented at baseline and as a change over the entire follow-up period. Change is expressed as both an absolute (Δabsolute) and as a percent change per year (Δpercentage/year).
Lifestyle
Protein intake
Subjects were asked to record, in as much detail as possible, all food and drinks consumed during two weekdays and one weekend-day in a 3-day diet record (Deriemaeker et al. 2006). They had to weigh the amount of food and drinks or otherwise estimate the amounts they consumed by using standard household measures (e.g., spoon and cup). Afterwards, protein intake (g/kg body weight) was calculated per day through an analysis of the diet records using Becel Nutrition software (Unilever Co.; Rotterdam, The Netherlands).
Energy expenditure during sports
Energy expenditure during sports (Esport) was assessed using the Flemish Physical Activity Questionnaire (FPACQ) (Matton et al. 2007b). The subjects were asked to report the sports (max. three) they practiced during their leisure time and the amount of time they spent practicing them during an entire year. This way, the average metabolic equivalents of energy expenditure (MET) hours per week were estimated by multiplying together the number of hours per week a subject spent in these sports, the estimated MET-score for each specific sport based on the Compendium of Physical Activity, and the proportion of the year a subject spent in these specific sports. Esport in MET-hours per week was then used for further analysis (Ainsworth et al. 2000).
Statistical analysis
Data are presented as means ± standard deviations. A dropout analysis was performed using an unpaired t test to compare baseline values of the dropout group with the follow-up group for males and females separately. Subject characteristics were compared between genders and evaluation moments using a two-way ANOVA. Muscle function was compared between genders, evaluation moments, and age-categories (<40, 40–60, and 60+) using a mixed models ANOVA. Pearson partial correlation coefficients, with age as the controlling variable, were calculated to determine tracking between baseline and follow-up measures for muscle function parameters. Tracking is the study of the stability of a certain parameter (Do subjects that score high at baseline, score high at follow-up as well?) and is expressed with an inter-age correlation coefficient. Furthermore, full-entry linear regression models were used to predict absolute changes and percent changes per year in muscle function including baseline values and age (model 1) and additionally baseline values of SMM, protein intake, and Esport (model 2). Statistical analyses were conducted using the Statistical Analysis Systems statistical software package version 9.4 (SAS Institute, Cary, NC, USA). Statistical significance was set at p < 0.05 for all analyses.
Results
Dropout analysis
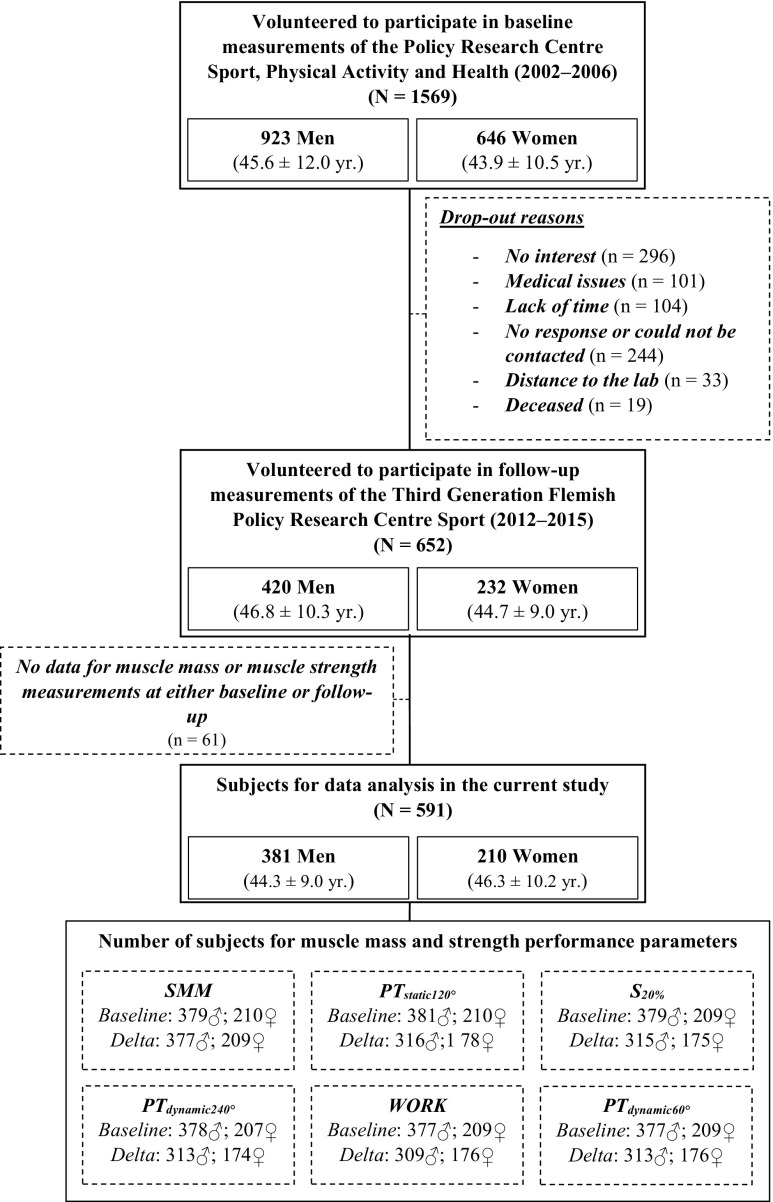
The overall response rate was 39.3 % (45.5 % in men and 35.9 % in women). The main reasons cited for dropping out were no interest (n = 296), medical issues (n = 101), lack of time (n = 104), and distance to the laboratory (n = 33). Nineteen subjects were deceased, and 244 subjects did not respond or could not be reached.
When baseline characteristics were compared between the dropout and follow-up groups in men, only age significantly differed (dropout 44.5 ± 13.2 years vs. follow-up 46.8 ± 10.3 years; p = 0.003). However, in women, a higher body weight (p = 0.004), BMI (p = 0.0003), and FM (p = 0.001) were observed in the dropouts (Table 1).
Table 1.
Dropout comparison
| Women | Men | |||
|---|---|---|---|---|
| Dropout | Follow-up | Dropout | Follow-up | |
| n (range) | 406–414 | 223–232 | 492–503 | 417–420 |
| Age (years) | 43.5 ± 11.3 | 44.7 ± 9.0 | 44.5 ± 13.2 | 46.8 ± 10.3* |
| Weight (kg) | 66.3 ± 11.5 | 63.9 ± 9.0* | 80.2 ± 11.7 | 79.5 ± 10.4 |
| Height (cm) | 164.3 ± 6.3 | 165.0 ± 5.8 | 176.8 ± 6.8 | 176.9 ± 6.5 |
| BMI (kg/m2) | 24.6 ± 4.2 | 23.5 ± 3.2* | 25.7 ± 3.4 | 25.4 ± 2.8 |
| FM (kg) | 22.1 ± 8.0 | 20.2 ± 6.0* | 17.6 ± 6.3 | 17.1 ± 5.1 |
| FFM (kg) | 44.3 ± 5.9 | 43.7 ± 4.9 | 62.8 ± 7.7 | 62.4 ± 7.2 |
| HGR (kg)a | 30.3 ± 8.0 | 31.4 ± 7.2 | 47.9 ± 10.1 | 48.8 ± 10.2 |
| SMM (kg) | 20.1 ± 2.7 | 20.2 ± 2.3 | 31.3 ± 3.9 | 31.2 ± 3.4 |
Data are means ± SD. Dropouts did not return for the follow-up measurements in 2012
n number of subjects
*Significant differences between dropouts and follow-ups for p < 0.01
aNumber of subjects for HGR are 395, 223, 485, and 412, respectively
Furthermore, of the 652 subjects that returned data, 61 participants were not included for statistical analysis because Biodex data was not available at baseline or at follow-up as a result of exclusion after medical examination or due to errors in the data. An overview is given in a flowchart of this study (Fig. 1).
Fig. 1.
Flowchart of the study
Subject characteristics
Subject characteristics are presented in Table 2 by gender and time of assessment.
Table 2.
Subject characteristics
| Women | Men | |||
|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | |
| n (range) | 203–210 | 205–210 | 369–381 | 375–381 |
| Age (years) | 44.3 ± 9.0 | 54.0 ± 8.7 | 46.3 ± 10.2 | 56.0 ± 10.1 |
| Weight (kg) | 63.4 ± 8.8 | 65.6 ± 10.1† | 79.3 ± 10.2$ | 80.5 ± 11.0†$ |
| Height (cm) | 165.0 ± 5.9 | 165.0 ± 5.8 | 176.9 ± 6.4$ | 176.9 ± 6.4$ |
| BMI (kg/m2) | 23.3 ± 3.0 | 24.1 ± 3.5† | 25.3 ± 2.8$ | 25.7 ± 3.1†$ |
| FM (kg) | 19.8 ± 5.7 | 22.3 ± 6.7† | 16.9 ± 5.1$ | 18.2 ± 5.4†$ |
| FFM (kg) | 43.6 ± 5.0 | 43.3 ± 5.4 | 62.4 ± 7.0$ | 62.2 ± 7.4$ |
| HGR (kg) | 31.2 ± 7.2 | 32.1 ± 6.3 | 48.9 ± 10.2$ | 51.2 ± 9.7$ |
| Proteins (g/kg)a | 1.28 ± 0.36 | 1.25 ± 0.36 | 1.24 ± 0.34 | 1.23 ± 0.36 |
| E sport (MET-h) | 17.9 ± 22.4 | 15.8 ± 25.5†† | 27.3 ± 31.3$ | 22.5 ± 32.0††$ |
| SMM (kg) | 20.2 ± 2.3 | 20.0 ± 3.4 | 31.3 ± 3.3$ | 31.8 ± 3.4$ |
Data are means ± SD. The exact number of subjects per variable is given in supplementary Table S1
n number of subjects
*Significant interaction effect at p <0.01; †significant main effect of evaluation moment at p < 0.01; ††trend for main effect of evaluation moment at p < 0.06; $significant main effect of gender at p < 0.01
aProtein data are available in 182 women and in 346 men in 2002 and in 180 women and in 341 men in 2012
Interaction effects
Although significant main effects of both evaluation moment and gender were observed, no significant interaction was found between these factors.
Gender differences
Men scored significantly better (p < 0.01) at both moments for all the muscle-related characteristics (FFM, SMM, and HGR), whereas women had a significantly higher FM compared to men (baseline 19.8 ± 5.7 kg vs. 16.9 ± 5.1 kg; follow-up 22.3 ± 6.7 kg vs. 18.2 ± 5.4 kg). Furthermore, a significant gender difference in energy expenditure during sports could be observed. Men had a greater energy expenditure during sports (p < 0.01) at both moments in comparison to women. No significant difference was observed between men and women for protein intake (g/kg).
Evaluation moment
In both men and women, weight, BMI, and FM (p < 0.01) significantly increased from baseline to follow-up. Furthermore, a trend (p < 0.06) for a decrease in Esport from baseline to follow-up was also observed.
Strength performance
Baseline values and 10-year changes (both absolute change over 10 years, and % change per year) in knee muscle function are presented in Table 3 by gender and in Table 4 by age category and gender, respectively.
Table 3.
Knee muscle function by gender
| Women (n = 174–210) | Men (n = 309–381) | |||||
|---|---|---|---|---|---|---|
| Baseline | Δ absolute | Δ percentage/year | Baseline | Δ absolute | Δ percentage/year | |
| PTstatic120° (Nm) | 120.8 ± 26.7 | −14.3 ± 23.6† | −1.07 ± 2.03† | 180.2 ± 41.0 | −18.9 ± 33.0†$ | −0.93 ± 1.92†$ |
| S 20 % (°/s) | 362.0 ± 42.2 | −37.2 ± 46.2* | −1.02 ± 1.36* | 404.0 ± 42.8 | −56.6 ± 51.0* | −1.43 ± 1.35* |
| PTdynamic60° (Nm) | 114.2 ± 24.1 | −11.2 ± 24.3† | −0.76 ± 2.29† | 172.3 ± 40.2 | −13.9 ± 39.0†$ | −0.46 ± 3.05†$ |
| PTdynamic240° (Nm) | 62.5 ± 13.9 | −4.80 ± 13.02† | −0.58 ± 2.26† | 97.8 ± 23.0 | −9.05 ± 20.7†$ | −0.65 ± 2.93†$ |
| Work (J) | 1844.0 ± 458.9 | −40.3 ± 374.7 | −0.024 ± 2.41 | 2988.5 ± 750.9 | −158.1 ± 663.6$ | −0.24 ± 3.37$ |
Data are means ± SD. Δ absolute = follow-up value − baseline value. Δ percentage/year = (follow-up value − baseline value)/(baseline value × follow-up period). The exact number of subjects per variable is given in supplementary Table S2
n number of subjects
*Significant interaction effect at p <0.01; †significant main effect of evaluation moment at p < 0.01; $significant main effect of gender at p < 0.01
Table 4.
Knee muscle function in women (top) and men (bottom)
| <40 year | 40–60 year | 60+ year | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Δ absolute | Δ percentage/year | Baseline | Δ absolute | Δ percentage/year | Baseline | Δ absolute | Δ percentage/year | MM | |
| Women | ||||||||||
| PTstatic120° (Nm) | 123.3 ± 27.4 | −11.6 ± 23.8 | −0.82 ± 1.81 | 122.2 ± 25.8 | −14.8 ± 24.1 | −1.09 ± 2.11 | 92.8 ± 20.8 | −19.2 ± 15.4 | −1.92 ± 1.74 | *†£ |
| S 20 % (°/s) | 380.7 ± 38.1 | −29.9 ± 41.5 | −0.73 ± 1.11 | 358.6 ± 40.6 | −38.6 ± 47.3 | −1.07 ± 1.40 | 323.8 ± 45.6 | −52.4 ± 52.1 | −1.57 ± 1.72 | *†£¤§ |
| PTdynamic60° (Nm) | 119.9 ± 22.1 | −7.4 ± 24.5 | −0.43 ± 2.20 | 114.4 ± 23.3 | −12.2 ± 24.6 | −0.81 ± 2.29 | 87.6 ± 25.8 | −16.6 ± 20.6 | −1.53 ± 2.67 | *†£ |
| PTdynamic240° (Nm) | 68.6 ± 12.9 | −3.5 ± 13.9 | −0.28 ± 2.12 | 61.9 ± 13.0 | −4.9 ± 12.9 | −0.60 ± 2.22 | 45.6 ± 13.0 | −8.8 ± 11.3 | −1.69 ± 3.21 | *†£ |
| Work (J) | 2093.7 ± 449.4 | 32.5 ± 381.5 | 0.34 ± 2.14 | 1801.4 ± 418.4 | −50.4 ± 377.8 | −0.013 ± 2.51 | 1301.1 ± 347.9 | −237.1 ± 213.4 | −1.75 ± 1.64 | *†£ |
| Men | ||||||||||
| PTstatic120° (Nm) | 186.3 ± 41.5 | −9.9 ± 33.5 | −0.38 ± 1.97 | 180.7 ± 40.3 | −20.8 ± 32.3 | −1.04 ± 1.86 | 163.8 ± 41.4 | −35.3 ± 29.3 | −2.05 ± 1.69 | *†£ |
| S 20 % (°/s) | 417.3 ± 39.0 | −40.1 ± 54.0 | −0.93 ± 1.35 | 403.8 ± 42.2 | −62.3 ± 47.4 | −1.60 ± 1.25 | 374.9 ± 40.5 | −65.4 ± 59.1 | −1.75 ± 1.76 | *†£¤§ |
| PTdynamic60° (Nm) | 182.2 ± 37.0 | −10.2 ± 40.1 | −0.31 ± 2.22 | 172.0 ± 40.7 | −14.5 ± 39.2 | −0.46 ± 3.40 | 151.1 ± 36.9 | −22.1 ± 32.0 | −1.06 ± 2.27 | *†£ |
| PTdynamic240° (Nm) | 106.7 ± 21.9 | −7.5 ± 22.0 | −0.54 ± 2.13 | 97.3 ± 22.2 | −9.48 ± 20.5 | −0.66 ± 3.26 | 80.5 ± 19.5 | −10.9 ± 18.2 | −1.04 ± 2.36 | *†£ |
| Work (J) | 3306.7 ± 686.8 | −7.2 ± 610.9 | 0.14 ± 2.06 | 2998.4 ± 698.9 | −189.8 ± 666.2 | −0.26 ± 3.78 | 2188.5 ± 617.7 | −458.5 ± 724.2 | −1.61 ± 3.36 | *†£ |
Data are means ± SD.
MM mixed model, n number of subjects,
*Main effect of gender at p < 0.01; †main effect of evaluation moment at p < 0.02; £main effect of age category at p < 0.01; ¤significant gender by evaluation interaction at p < 0.05; §significant evaluation moment by age category interaction at p < 0.05
Interaction effects
A significant evaluation moment by gender interaction effect (p < 0.05) was observed for S20 %. A larger decrease over time was observed in men compared to women (−1.43 ± 1.35 % per year vs. −1.02 ± 1.36 % per year, respectively). Furthermore, a significant evaluation moment by age category interaction effect (p < 0.05) was found in S20 % as well. In both genders, declines in strength performance are greater in elder age categories (“<40 years” < “40–60 years” < “+60 years”). No other interaction effects were observed.
Gender differences
A main effect of gender was observed for all muscle function parameters. At both evaluation moments, men scored significantly higher (p < 0.01) compared to women.
Evaluation moment
A significant main effect of the evaluation moment (p < 0.01) was observed for each strength performance parameter. Both men and women scored significantly lower at follow-up compared to baseline values. In both genders, the decrease in PTstatic120° was greatest in all age-categories (except for <40 years in men). A large decrease in PTdynamic240° (women), in S20 % (men), and in work (both genders) could also be observed in the eldest age category.
Age category
A main effect of the age category was observed for all the strength performance parameters. Baseline strength performance and changes in strength performance were lower and greater, respectively, in the higher age-categories compared to the youngest. In the eldest age category, percent changes per year in the muscle performance parameters were (more than) twice as large as compared to the youngest age category.
Tracking of strength performance parameters
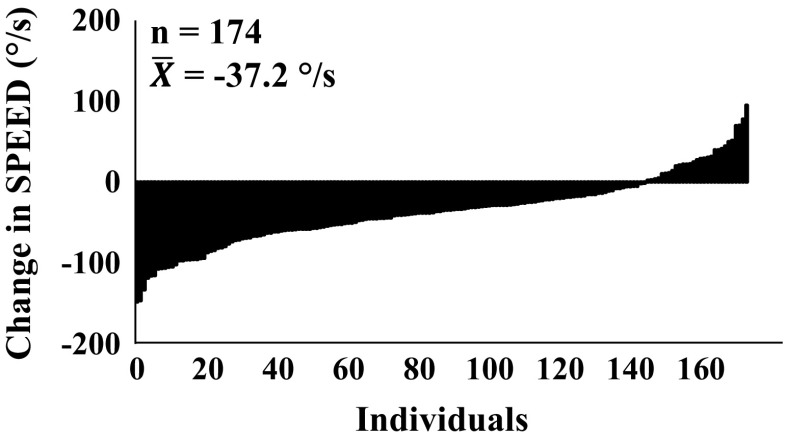
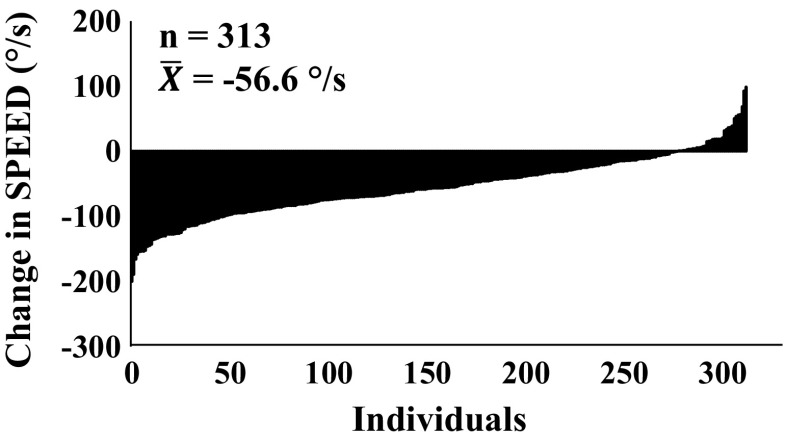
In women, SMM (r = 0.79), PTstatic120° (r = 0.58), and work (r = 0.55) show the highest tracking over the 10-year follow-up, whereas in men, SMM (r = 0.79), PTstatic120° (r = 0.63), PTdynamic240° (r = 0.50), and work (r = 0.52) show the highest partial correlations between baseline and follow-up. The lowest correlations were found for S20 % and PTdynamic60° in both genders (Table 5). The large heterogeneity in absolute changes in S20 % is depicted in Fig. 2 (women) and Fig. 3 (men).
Table 5.
Tracking in muscle mass and strength parameters between 2002 and 2012 muscle parameters
| Women | Men | |||
|---|---|---|---|---|
| r | n | r | n | |
| SMM, kg | 0.789* | 210 | 0.785* | 378 |
| PTstatic120°, Nm | 0.582* | 178 | 0.628* | 316 |
| S 20 %,°/s | 0.436* | 175 | 0.378* | 315 |
| PTdynamic60°, Nm | 0.434* | 176 | 0.443* | 313 |
| PTdynamic240°, Nm | 0.485* | 174 | 0.504* | 313 |
| Work, J | 0.553* | 176 | 0.523* | 309 |
Data are partial correlations between baseline and follow-up measurements with age as controlling variable
n number of subjects
*Significant at p < 0.01
Fig. 2.
Ranked individual change scores in S 20 % for women
Fig. 3.
Ranked individual change scores in S 20 % for men
Regression models
Model 1
The full entry regression model with age and the baseline parameter was able to significantly (p < 0.01) predict changes in strength performance (Table 6). In women, the models were able to predict 20–36 % of the variance in the change. Similar results were found in men, explaining 21–39 % of the variance. In both genders, baseline muscle function and age were negatively correlated with changes in strength performance. Furthermore, the baseline value was a better predictor for each change parameter as compared to age.
Table 6.
Prediction of changes in strength performance with model 1
| Women | Men | |||||
|---|---|---|---|---|---|---|
| Age | Baseline parameter | r 2 | Age | Baseline parameter | r 2 | |
| ISOM120 | ||||||
| Δ absolute (Nm) | −0.31 (0.1664) | −0.55 (0.0548) | 0.33 | −0.29 (0.1587) | −0.57 (0.0380) | 0.34 |
| Δ percentage/year (%) | −0.33 (0.0002) | −0.44 (0.0001) | 0.25 | −0.29 (0.0001) | −0.47 (0.00002) | 0.25 |
| Speed | ||||||
| Δ absolute (°/s) | −0.27 (0.3653) | −0.47 (0.0771) | 0.22 | −0.33 (0.2631) | −0.47 (0.0607) | 0.25 |
| Δ percentage/year (%) | −0.30 (0.0001) | −0.42 (0.00002) | 0.20 | −0.35 (0.0001) | −0.40 (0.00001) | 0.21 |
| ISOK60° | ||||||
| Δ absolute (Nm) | −0.27 (0.1722) | −0.61 (0.0624) | 0.36 | −0.25 (0.1824) | −0.63 (0.0445) | 0.39 |
| Δ percentage/year (%) | −0.28 (0.0002) | −0.54 (0.0001) | 0.29 | −0.20 (0.0002) | −0.55 (0.00004) | 0.29 |
| ISOK240° | ||||||
| Δ absolute (Nm) | −0.34 (0.0997) | −0.60 (0.0629) | 0.31 | −0.26 (0.1051) | −0.59 (0.0450) | 0.32 |
| Δ percentage/year (%) | −0.36 (0.0002) | −0.51 (0.0001) | 0.24 | −0.22 (0.0002) | −0.51 (0.0001) | 0.23 |
| ENDUR | ||||||
| Δ absolute (J) | −0.42 (3.1622) | −0.55 (0.0624) | 0.25 | −0.39 (3.6616) | −0.52 (0.0491) | 0.26 |
| Δ percentage/year (%) | −0.43 (0.0002) | −0.55 (0.00000) | 0.25 | −0.32 (0.0002) | −0.49 (0.00000) | 0.22 |
Data are beta-coefficients (standard errors). Regression models are significant at p < 0.001. Furthermore, all beta-coefficients are significant at p < 0.001
Model 2
Age and baseline value remained negatively related to the change in strength performance after adjustment for baseline values in SMM, protein intake, and sport-related energy expenditure, explaining up to 44 % of the variance in strength performance changes in women and up to 43 % of the variance in muscle function changes in men (Table 7). A higher SMM was positively correlated with changes in all muscle function parameters, with the exception of changes in S20 %. In women, protein intake was a negative predictor (p < 0.05) of the annual changes in S20 % and Δabsolute in PTdynamic60°, although trends (p < 0.07) could be observed for the Δabsolute in S20 % and Δpercentage/year in PTdynamic60°. However, in men, protein intake only predicted annual changes in PTstatic120° (p < 0.05).
Table 7.
Prediction of changes in muscle function with model 2
| Age | Baseline parameter | SMM | Protein | E sport | R 2 | ||
|---|---|---|---|---|---|---|---|
| Women | |||||||
| PTstatic120° | Δ absolute (Nm) | −0.24** | −0.71** | 0.29** | −0.04 | −0.07 | 0.44 |
| Δ percentage/year (%) | −0.26** | −0.61** | 0.31** | −0.05 | −0.04 | 0.35 | |
| S 20 % | Δ absolute (°/s) | −0.27** | −0.49** | 0.08 | −0.14a | 0.0004 | 0.25 |
| Δ percentage/year (%) | −0.28** | −0.46** | 0.08 | −0.15* | 0.0003 | 0.23 | |
| PTdynamic60° | Δ absolute (Nm) | −0.21** | −0.74** | 0.27** | −0.13* | 0.03 | 0.50 |
| Δ percentage/year (%) | −0.24** | −0.67** | 0.25** | −0.12a | 0.02 | 0.43 | |
| PTdynamic240° | Δ absolute (Nm) | −0.30** | −0.68** | 0.27** | −0.11 | −0.08 | 0.44 |
| Δ percentage/year (%) | −0.34** | −0.61** | 0.26** | −0.09 | −0.08 | 0.39 | |
| Work | Δ absolute (J) | −0.36** | −0.68** | 0.28** | −0.10 | 0.07 | 0.35 |
| Δ percentage/year (%) | −0.40** | −0.70** | 0.29** | −0.11 | 0.08 | 0.40 | |
| Men | |||||||
| PTstatic120° | Δ absolute (Nm) | −0.22** | −0.66** | 0.19** | −0.08 | 0.06 | 0.37 |
| Δ percentage/year (%) | −0.22** | −0.57** | 0.21** | −0.12* | 0.07 | 0.29 | |
| S 20 % | Δ absolute (°/s) | −0.33** | −0.49** | −0.05 | −0.009 | −0.001 | 0.28 |
| Δ percentage/year (%) | −0.35** | −0.42** | −0.04 | 0.01 | 0.01 | 0.23 | |
| PTdynamic60° | Δ absolute (Nm) | −0.24** | −0.71** | 0.14** | −0.07 | 0.008 | 0.43 |
| Δ percentage/year (%) | −0.17** | −0.65** | 0.21** | −0.04 | 0.02 | 0.34 | |
| PTdynamic240° | Δ absolute (Nm) | −0.21** | −0.67** | 0.17** | −0.002 | −0.009 | 0.34 |
| Δ percentage/year (%) | −0.18** | −0.60** | 0.21** | 0.03 | 0.005 | 0.28 | |
| Work | Δ absolute (J) | −0.34** | −0.63** | 0.21** | −0.06 | 0.03 | 0.32 |
| Δ percentage/year (%) | −0.28** | −0.63** | 0.26** | −0.01 | 0.06 | 0.30 | |
Data are beta-coefficients. Regression models are significant at p < 0.001. Standard errors are given in supplementary Table S3
*Significant beta-coefficient at p < 0.05; **significant beta-coefficient at p < 0.01
aTrend for a significant beta-coefficient
Discussion
This study was the first to examine changes in ballistic contraction speed and muscular endurance strength over the adult age span. A significant decline in S20 % was found in both genders, but it proved to be significantly larger in men compared to women. Work did not significantly decline between both measuring moments in the entire group, but it did decline when age categories were considered. In the older age category, the decline in work was larger compared to younger age categories in both genders. Also, age-corrected tracking was moderate for all muscle function parameters, but tracking of skeletal muscle mass was high in both genders (r = 0.79). This implies a large inter-individual variation in the changes in muscle function in both men and women. Furthermore, age and baseline muscle function were negatively related with the change in muscle function, even when corrected for SMM, protein intake, and Esport. Finally, higher skeletal muscle mass was found to be related with smaller decreases in muscle function.
In this study, muscle mass characteristics (FFM and SMM) were higher in men compared to women. There were significant gains in weight, BMI, and FM in both genders from baseline to follow-up. However, there was no significant increase in FFM, where FM increased with 1.3 and 2.5 kg on average in men and women, respectively. Regarding muscle function, handgrip was significantly higher in men compared to women, but no significant difference was observed between evaluation moments. Also, men performed significantly better at both time points compared to women for all knee muscle function parameters. Nevertheless, only S20 % declined more precipitously in men compared to women. Significant main effects of time were observed in the entire group for all parameters, except for work. This might reflect a similar fatigue resistance between younger and older subjects due to a shift toward slower contractile properties in the older population (Mcphee et al. 2014). However, this was not confirmed when comparisons were made by age category (Table 4). Aging-related declines were greater in the oldest age category compared to the younger age categories. Also, the finding of an aging-related decrease in strength performance, but not in handgrip strength, is consistent with the findings of a differential aging of upper and lower extremities as noted by Lynch et al. (1999) and Newman et al. (2003).
A study by Frontera et al. (2008) has shown different percent changes per year compared to our results. They found percent changes per year of −2.56 ± 1.1 % in isokinetic strength of the knee extensors at 60°/s and −4.2 ± 1.2 % at 240°/s, but this study only considered 12 subjects (five men) (Frontera et al. 2008). Hughes et al. (2001) found declines in isokinetic speed at 60°/s of −1.18 ± 1.55 % in women and −1.45 ± 1.56 % in men, which is more in line with our results (Hughes et al. 2001). Their reported percent change per year is still larger, but it is probably explained by the older age range of their sample (46 to 78 years), whereas we considered subjects between 19 and 73 years old (baseline) (Hughes et al. 2001). However, if we only considered subjects above 46 years of age, Δpercentage/year was −0.96 ± 2.16 % in women and −0.77 ± 2.28 % in men. This is in agreement with the findings of Kennis et al. (2014): they observed a decrease of 0.71 ± 0.24 % per year in isokinetic strength at 60°/s in a subsample of this study (n = 105 men, age = 46.93 ± 0.06 years.). If we considered the oldest age category (+60 years), Δpercentage/year was −1.53 ± 2.67 % in women and −1.06 ± 2.27 % in men (Table 4).
To the best of our knowledge, there are currently no longitudinal studies reporting percent changes in ballistic contraction speeds or resistance to fatigue measures over the entire age span. A cross-sectional study by Mcphee et al. (2014) reported a superior fatigue resistance during sustained isometric contractions in older adults. In our study, we observed no significant difference between baseline and follow-up in the amount of work delivered during 25 repeated maximal contractions (Δpercentage/year women −0.024 ± 2.41 %; Δpercentage/year men −0.24 ± 3.37 %). However, the endurance protocol used in the study by Mcphee et al. (2014) is different from the protocol used in the current study. The largest decreases were observed for the knee extension contraction velocity (S20 %) (women −1.02 ± 1.36 % per year; men −1.43 ± 1.35 % per year) (Table 3). When age categories were considered, a main effect of the age category was found in work. The percent change per year in S20 % was significantly higher in men, indicating a gender difference in the rate of decline in contraction velocity. This is in line with the cross-sectional findings of Edwen et al. (2014). They found that velocity at peak power declined at a higher rate in men compared to women (p < 0.002) (Edwen et al. 2014). Also, a significant interaction effect of gender by evaluation moment was found in S20 %, pointing out a larger decrease in men compared to women after a 10-year follow-up period (Table 4).
These differences between men and women are proposed to be the consequence of a difference in the proportional area of “slow” type I fibers. There is some evidence that the proportional area of type I fibers is significantly greater in women compared to men and that men have larger fibers across most of the fiber types (Simoneau and Bouchard 1989; Staron et al. 2000; Hunter 2014). Moreover, a decline in fiber size, of type II fibers in particular, has been suggested to be the cause of age-related muscle atrophy (Lexell et al. 1988). A shift in fiber type from type II to type I with aging has also been stated, resulting in a larger proportion of type I fibers in the contractile mass (Russ et al. 2012). These findings might give a possible explanation of the results found in the current study since speed of contraction is largely determined by type II fibers, whereas type I fibers are more important for resistance to fatigue.
Declines in lower extremity muscle function were negatively associated with baseline muscle function and age for all muscle parameters. Higher age and higher baseline muscle function resulted in larger decreases in muscle function parameters. In 2006, Goodpaster et al. also found that baseline strength and age were negatively related with changes in strength after a 3-year follow-up period in 1880 older adults (Goodpaster et al. 2006). In addition, baseline muscle function was a stronger determinant in both genders of all the age-associated changes (both absolute and percentage) as compared to age in our study, which was also the case in the study by (Goodpaster et al. 2008). This is further underlined by the moderate-to-high (0.38 – 0.79) tracking that was found between the baseline and follow-up values of muscle function (Table 4), pointing out that higher baseline values were associated with higher follow-up values. It should be noted that, with the exception of SMM, correlations were not higher than 0.63, suggesting a considerably large inter-individual difference in the age-related declines.
It is likely that strength performance is influenced by both genetics and environmental factors, such as nutrition and exercise. As regard to lifestyle, there is currently much uncertainty on what the combined effects of diet and exercise are for muscle characteristics, especially in the long term (Paddon-Jones and Rasmussen 2009). Stenholm et al. (2012) found that strenuous work-related physical activity was significantly associated with a faster decline in handgrip strength compared to light work-related physical activity. They therefore concluded that lifestyle earlier in life was associated with the rate of muscle strength decline in old age. In the current study, no gender differences were observed in protein intake (g/kg body weight), but a higher energy expenditure during sports was observed in men. This is in accordance with previous reports (Baker et al. 2013). In addition, a trend (p < 0.06) was observed for a decrease in Esport after a 10-year follow-up in both men and women. Furthermore, mean protein intake was higher in both genders than the recommended amounts of 0.8 g kg bodyweight−1 day−1.
Age and baseline muscle function remained significant predictors when skeletal muscle mass, protein intake, and Esport were included in the regression models. Skeletal muscle mass showed a positive relationship with changes in muscle function and had comparable beta-coefficients to age. However, it was not related to changes in S20 % in both genders. This is probably due to the fact that movement speed is determined to a large extent by a neural firing rate and a fiber length rather than by skeletal muscle mass or fiber diameter alone. In the adjusted regression model, mixed results were observed for lifestyle factors. Energy expenditure during sports was not found to be a significant predictor of the age-related declines in muscle function parameters for either of the parameters, whereas protein intake did show some notable results. In men, it was a significant predictor of the percent changes per year in isometric strength. A negative relationship was found between protein intake and percent change per year in S20 % and change in PTdynamic60°, and a negative trend could also be observed for change in S20 % and percent change per year in PTdynamic60° in women (Table 6). This is not in line with previous findings of intervention studies, showing a positive relationship between muscle function, protein intake, and physical activity, even in older adults (Bonnefoy et al. 2003; Goodpaster et al. 2008). It should be noted that the current study did not account for the type of sport although previous studies have shown that resistance exercise is most effective in improving muscle function and that sports practice is also age-related. Finally, protein intake was not monitored during the entire follow-up period, which might account for the lack of a—or even negative—relationship.
It should be acknowledged that the current study has some limitations. At first, a considerable number of subjects (60.7 %) did not complete the follow-up measurements. Although there was only a small age difference (44.5 ± 13.2 years vs. 46.8 ± 10.3 years) in men, a fitter group (lower weight, BMI, and FM) returned for the follow-up measurements in women, which might have led to an underestimation of the true age-related changes in muscle mass and muscle function in this group. It is therefore advised to take some caution when interpreting and generalizing the results to other populations. Second, only two time points were considered in this study. Therefore, data on additional time points could have helped to better interpret the results. The study of two-wave changes has some limitations. It cannot tell something about the shape of a person’s individual growth trajectory, since two-wave analyses assume linear change. Furthermore, it cannot distinguish true change from measurement error (Singer and Willett 2003). Test-retest reliability for Biodex measurements has been previously reported to be high (Lund et al. 2005). Also, no learning effects were observed for the Biodex measurements. Measurement error was 11.75 Nm for the isokinetic strength at 60°/s based on the reported findings in Lund et al. (2005). Interpretation of the change in PTdynamic60° should therefore be made taking these results into account, as should the changes in other strength performance parameters. Regression to the mean effects was explored by visual inspection of Galton squeeze plots and was interpreted to be minimal. Since the RTM effect at the lower end of the baseline distribution will decrease time-induced losses, while those at the upper end of the baseline distribution will rather increase time-induced losses, it can be argued that both RTM effects will counteract each other. Finally, lifestyle was determined based on a diet record and a physical activity questionnaire, which are subjective methods. Although a diet record is considered a gold standard to measure dietary habits, it has limitations mainly due to the tendency of subjects to report food consumption that is socially desirable. Furthermore, the FPACQ only addressed a subject’s physical activity of the past year and is also subject to reporting errors.
In the future, studies should aim to examine the interrelationship between muscle function and lifestyle in more controlled settings, although further longitudinal study is also recommended to better understand long-term effects of both protein intake and physical activity. In addition, it has already been shown that genetic sequence variation has a larger influence on both muscle mass and muscle function (~65 %) than lifestyle (Garatachea and Lucia 2013). Therefore, genetic effects on changes in muscle characteristics should also be further examined to fully understand this process. Nonetheless, only when the combined influence of both lifestyle and genetics is fully understood can researchers start to develop strategies to detect subjects at risk for sarcopenia in early stages and adapt training interventions in an appropriate manner to achieve optimal results.
In the current study, age-related declines in muscle function parameters were examined after a 10-year follow-up period in Flemish Caucasians aged between 19 and 73 years old. Based on the findings, the present study could not provide evidence for an association between muscle function and energy expenditure during sports, although protein intake was associated with isometric strength in men and with ballistic and isokinetic strength in women. Percent change per year in S20 % was significantly greater in men compared to women, possibly due to gender differences in sensitivity to aging-induced type II fiber atrophy. Furthermore, baseline values of muscle function and age were associated with changes in muscle function parameters, even after correction for skeletal muscle mass, protein intake, and energy expenditure during sports.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(XLSX 11 kb)
(XLSX 10 kb)
(XLSX 12 kb)
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O’Brien WL, Bassett DR, Jr, Schmitz KH, Emplaincourt PO, Jacobs DR, Jr, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- Baker JF, Davis M, Alexander R, Zemel BS, Mostoufi-Moab S, Shults J, Sulik M, Schiferl DJ, Leonard MB. Associations between body composition and bone density and structure in men and women across the adult age spectrum. Bone. 2013;53:34–41. doi: 10.1016/j.bone.2012.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EF, Yarasheski KE, Steger-May K, Sinacore DR, Brown M, Schechtman KB, Holloszy JO. Effects of progressive resistance training on body composition in frail older adults: results of a randomized, controlled trial. J Gerontol A Biol Sci Med Sci. 2005;60:1425–1431. doi: 10.1093/gerona/60.11.1425. [DOI] [PubMed] [Google Scholar]
- Bonnefoy M, Cornu C, Normand S, Boutitie F, Bugnard F, Rahmani A, Lacour JR, Laville M. The effects of exercise and protein-energy supplements on body composition and muscle function in frail elderly individuals: a long-term controlled randomised study. Br J Nutr. 2003;89:731–739. doi: 10.1079/BJN2003836. [DOI] [PubMed] [Google Scholar]
- Campbell AJ, Borrie MJ, Spears GF. Risk factors for falls in a community-based prospective study of people 70 years and older. J Gerontol. 1989;44:M112–M117. doi: 10.1093/geronj/44.5.M112. [DOI] [PubMed] [Google Scholar]
- Cesari M, Vellas B, Hsu FC, Newman AB, Doss H, King AC, Manini TM, Church T, Gill TM, Miller ME, Pahor M. A physical activity intervention to treat the frailty syndrome in older persons-results from the LIFE-P study. J Gerontol A Biol Sci Med Sci. 2015;70:216–222. doi: 10.1093/gerona/glu099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chale-Rush A, Guralnik JM, Walkup MP, Miller ME, Rejeski WJ, Katula JA, King AC, Glynn NW, Manini TM, Blair SN, Fielding RA. Relationship between physical functioning and physical activity in the lifestyle interventions and independence for elders pilot. J Am Geriatr Soc. 2010;58:1918–1924. doi: 10.1111/j.1532-5415.2010.03008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier R, Mertens E, Lefevre J, Thomis M. Muscle mass and muscle function over the adult life span: a cross-sectional study in Flemish adults. Arch Gerontol Geriatr. 2015;61:161–167. doi: 10.1016/j.archger.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Jentoft AJ, Landi F, Schneider SM, Zuniga C, Arai H, Boirie Y, Chen LK, Fielding RA, Martin FC, Michel JP, Sieber C, Stout JR, Studenski SA, Vellas B, Woo J, Zamboni M, Cederholm T. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS) Age Ageing. 2014;43:748–759. doi: 10.1093/ageing/afu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, Boudreau R, Manini TM, Nevitt M, Newman AB, Goodpaster BH. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deriemaeker P, Aerenhouts D, Hebbelinck M, Clarys P. Validation of a 3-day diet diary: comparison with a 7-day diet diary and a FFQ. Med Sci Sports Exerc. 2006;38:S328. doi: 10.1249/00005768-200605001-01407. [DOI] [Google Scholar]
- Dey DK, Bosaeus I, Lissner L, Steen B. Changes in body composition and its relation to muscle strength in 75-year-old men and women: a 5-year prospective follow-up study of the NORA cohort in Goteborg, Sweden. Nutrition. 2009;25:613–619. doi: 10.1016/j.nut.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Edwen CE, Thorlund JB, Magnusson SP, Slinde F, Svantesson U, Hulthen L, Aagaard P. Stretch-shortening cycle muscle power in women and men aged 18–81 years: influence of age and gender. Scand J Med Sci Sports. 2014;24:717–726. doi: 10.1111/sms.12066. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Reid KF, Phillips EM, Krivickas LS, Hughes VA, Roubenoff R, Fielding RA. Muscle fiber size and function in elderly humans: a longitudinal study. J Appl Physiol (1985) 2008;105:637–642. doi: 10.1152/japplphysiol.90332.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garatachea N, Lucia A. Genes, physical fitness and ageing. Ageing Res Rev. 2013;12:90–102. doi: 10.1016/j.arr.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Chomentowski P, Ward BK, Rossi A, Glynn NW, Delmonico MJ, Kritchevsky SB, Pahor M, Newman AB. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol (1985) 2008;105:1498–1503. doi: 10.1152/japplphysiol.90425.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks GE, Shardell M, Alley DE, Miller RR, Bandinelli S, Guralnik J, Lauretani F, Simonsick EM, Ferrucci L. Absolute strength and loss of strength as predictors of mobility decline in older adults: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2012;67:66–73. doi: 10.1093/gerona/glr055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, Fiatarone Singh MA. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci. 2001;56:B209–B217. doi: 10.1093/gerona/56.5.B209. [DOI] [PubMed] [Google Scholar]
- Hunter SK. Sex differences in human fatigability: mechanisms and insight to physiological responses. Acta Physiol (Oxf) 2014;210:768–789. doi: 10.1111/apha.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol (1985) 2000;89:465–471. doi: 10.1152/jappl.2000.89.2.465. [DOI] [PubMed] [Google Scholar]
- Kennis E, Verschueren S, Van RE, Thomis M, Lefevre J, Delecluse C. Longitudinal impact of aging on muscle quality in middle-aged men. Age (Dordr) 2014;36:9689. doi: 10.1007/s11357-014-9689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294. doi: 10.1016/0022-510X(88)90132-3. [DOI] [PubMed] [Google Scholar]
- Lund H, Sondergaard K, Zachariassen T, Christensen R, Bulow P, Henriksen M, Bartels EM, Danneskiold-Samsoe B, Bliddal H. Learning effect of isokinetic measurements in healthy subjects, and reliability and comparability of Biodex and Lido dynamometers. Clin Physiol Funct Imaging. 2005;25:75–82. doi: 10.1111/j.1475-097X.2004.00593.x. [DOI] [PubMed] [Google Scholar]
- Lynch NA, Metter EJ, Lindle RS, Fozard JL, Tobin JD, Roy TA, Fleg JL, Hurley BF. Muscle quality. I. Age-associated differences between arm and leg muscle groups. J Appl Physiol (1985) 1999;86:188–194. doi: 10.1152/jappl.1999.86.1.188. [DOI] [PubMed] [Google Scholar]
- Marcell TJ, Hawkins SA, Wiswell RA. Leg strength declines with advancing age despite habitual endurance exercise in active older adults. J Strength Cond Res. 2014;28:504–513. doi: 10.1519/JSC.0b013e3182a952cc. [DOI] [PubMed] [Google Scholar]
- Matton L, Beunen G, Duvigneaud N, Wijndaele K, Philippaerts R, Claessens A, Vanreusel B, Thomis M, Lefevre J. Methodological issues associated with longitudinal research: findings from the Leuven longitudinal study on lifestyle, fitness and health (1969–2004) J Sports Sci. 2007;25:1011–1024. doi: 10.1080/02640410600951563. [DOI] [PubMed] [Google Scholar]
- Matton L, Wijndaele K, Duvigneaud N, Duquet W, Philippaerts R, Thomis M, Lefevre J. Reliability and validity of the Flemish physical activity computerized questionnaire in adults. Res Q Exerc Sport. 2007;78:293–306. doi: 10.1080/02701367.2007.10599427. [DOI] [PubMed] [Google Scholar]
- Mcphee JS, Maden-Wilkinson TM, Narici MV, Jones DA, Degens H. Knee extensor fatigue resistance of young and older men and women performing sustained and brief intermittent isometric contractions. Muscle Nerve. 2014;50:393–400. doi: 10.1002/mus.24174. [DOI] [PubMed] [Google Scholar]
- Metter EJ, Conwit R, Tobin J, Fozard JL. Age-associated loss of power and strength in the upper extremities in women and men. J Gerontol A Biol Sci Med Sci. 1997;52:B267–B276. doi: 10.1093/gerona/52A.5.B267. [DOI] [PubMed] [Google Scholar]
- Newman AB, Haggerty CL, Goodpaster B, Harris T, Kritchevsky S, Nevitt M, Miles TP, Visser M. Strength and muscle quality in a well-functioning cohort of older adults: the health, aging and body composition study. J Am Geriatr Soc. 2003;51:323–330. doi: 10.1046/j.1532-5415.2003.51105.x. [DOI] [PubMed] [Google Scholar]
- Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009;12:86–90. doi: 10.1097/MCO.0b013e32831cef8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, Espeland MA, Fielding RA, Gill TM, Groessl EJ, King AC, Kritchevsky SB, Manini TM, McDermott MM, Miller ME, Newman AB, Rejeski WJ, Sink KM, Williamson JD. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid KF, Pasha E, Doros G, Clark DJ, Patten C, Phillips EM, Frontera WR, Fielding RA. Longitudinal decline of lower extremity muscle power in healthy and mobility-limited older adults: influence of muscle mass, strength, composition, neuromuscular activation and single fiber contractile properties. Eur J Appl Physiol. 2014;114:29–39. doi: 10.1007/s00421-013-2728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli R, Reginster JY, Arnal JF, Bautmans I, Beaudart C, Bischoff-Ferrari H, Biver E, Boonen S, Brandi ML, Chines A, Cooper C, Epstein S, Fielding RA, Goodpaster B, Kanis JA, Kaufman JM, Laslop A, Malafarina V, Manas LR, Mitlak BH, Oreffo RO, Petermans J, Reid K, Rolland Y, Sayer AA, Tsouderos Y, Visser M, Bruyere O. Quality of life in sarcopenia and frailty. Calcif Tissue Int. 2013;93:101–120. doi: 10.1007/s00223-013-9758-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ DW, Gregg-Cornell K, Conaway MJ, Clark BC. Evolving concepts on the age-related changes in “muscle quality”. J Cachex Sarcopenia Muscle. 2012;3:95–109. doi: 10.1007/s13539-011-0054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahni S, Mangano KM, Hannan MT, Kiel DP, McLean RR. Higher protein intake is associated with higher lean mass and quadriceps muscle strength in adult men and women. J Nutr. 2015;145(7):1569–1575. doi: 10.3945/jn.114.204925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoneau JA, Bouchard C. Human variation in skeletal muscle fiber-type proportion and enzyme activities. Am J Physiol. 1989;257:E567–E572. doi: 10.1152/ajpendo.1989.257.4.E567. [DOI] [PubMed] [Google Scholar]
- Singer JD and Willett JB (2003) Applied longitudinal data analysis: modeling change and event occurrence.1:1–672
- Staron RS, Hagerman FC, Hikida RS, Murray TF, Hostler DP, Crill MT, Ragg KE, Toma K. Fiber type composition of the vastus lateralis muscle of young men and women. J Histochem Cytochem. 2000;48:623–629. doi: 10.1177/002215540004800506. [DOI] [PubMed] [Google Scholar]
- Stenholm S, Tiainen K, Rantanen T, Sainio P, Heliovaara M, Impivaara O, Koskinen S. Long-term determinants of muscle strength decline: prospective evidence from the 22-year mini-Finland follow-up survey. J Am Geriatr Soc. 2012;60:77–85. doi: 10.1111/j.1532-5415.2011.03779.x. [DOI] [PubMed] [Google Scholar]
- Twisk JWR. Applied longitudinal data analysis for epidemiology. 2. Cambridge: Cambridge University Press; 2013. [Google Scholar]
- Wijndaele K, Duvigneaud N, Matton L, Duquet W, Thomis M, Beunen G, Lefevre J, Philippaerts RM. Muscular strength, aerobic fitness, and metabolic syndrome risk in Flemish adults. Med Sci Sports Exerc. 2007;39:233–240. doi: 10.1249/01.mss.0000247003.32589.a6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX 11 kb)
(XLSX 10 kb)
(XLSX 12 kb)