Abstract
With adult aging, eccentric strength is maintained better than isometric strength leading to a higher ratio of eccentric/isometric force production (ECC/ISO) in older than younger adults. The purpose was to investigate the ECC/ISO during electrical activation of the adductor pollicis during lengthening (20–320° s−1) contractions in 24 young (n = 12, ∼24 years) and old (n = 12, ∼72 years) males across muscle temperatures (cold ∼19 °C; normal ∼30 °C; warm ∼35 °C). For isometric force, the old were 20–30 % weaker in the normal and cold conditions (P < 0.05) with no difference for the warm condition compared to young (P > 0.05). Half-relaxation time (HRT) did not differ across age for the normal and warm temperatures (P > 0.05), but it slowed significantly for old in the cold condition compared with young (∼15 %; P < 0.05), as well, there was a 20 and 40 % increase in muscle stiffness for the young and old, respectively. ECC/ISO was 50–60 % greater for the cold condition than the normal and warm conditions. There was no age difference in ECC/ISO across ages for the normal and warm conditions (P > 0.05), but for the cold, the old exhibited a 20–35 % higher ECC/ISO than did the young for velocities above 60° s−1 (P < 0.05). A contributing factor to the elevated ECC/ISO is an increased proportion of weakly compared to strongly bound crossbridges. These findings highlight the relationship (r = 0.70) between intrinsic muscle contractile speed (HRT) and eccentric strength in old age.
Keywords: Muscle, Lengthening, Residual force enhancement, Stiffness, Weakness, Sarcopenia
Introduction
Despite a clear age-related reduction in force during shortening muscle actions (Dalton et al. 2015; Power et al. 2013a; Power et al. 2014b), the ability to produce force during active lengthening contractions (i.e., eccentric) is well-maintained with natural adult aging (Roig et al. 2010; Vandervoort 2002). This maintenance of eccentric force is well-characterized across various muscle groups (Cannon et al. 2006; Hortobagyi et al. 1995; Porter et al. 1997; Poulin et al. 1992; Power et al. 2013b; Power et al. 2012). However, the mechanisms contributing to this phenomenon are unclear. In the present study, we utilized temperature changes to alter crossbridge kinetics and the ratio of weakly to strongly bound crossbridges in an attempt to study crossbridge-based mechanisms of age-related maintenance of eccentric strength in vivo.
Upon muscle activation, there exists an equilibrium ratio of weakly bound and strongly bound crossbridge states (Ranatunga et al. 2010). The strongly bound states are the force producing states while both the weakly and strongly bound states contribute to stiffness (Roots et al. 2008). When a muscle is experimentally cooled, it typically produces less force than a muscle at physiological and warm temperatures. However, the cooled muscle may have a similar stiffness as a muscle at a physiological and warmed temperature, which could be explained by a greater ratio of weakly to strongly bound crossbridges (Ranatunga et al. 2010) and a similar proportion of attached crossbridges in all transitional states (Roots et al. 2012). Conversely, isometric tetanic forces have been shown previously to increase with increasing temperatures, while instantaneous muscle stiffness has been reported to remain roughly the same across temperatures (Coupland et al. 2001; Piazzesi et al. 2003; Wang et al. 2001). From these observations, it can be assumed that there is an increase in the average force per crossbridge as temperature increases, potentially caused by a shift to a greater proportion of strongly compared to weakly bound crossbridges with increasing temperatures (Ranatunga et al. 2010). Most investigations on the temperature dependence of force production have been performed in isolated muscle or fiber preparations (Ranatunga et al. 2010), and little is known about the effects of cooling and warming on eccentric force production in muscles in vivo.
An unexplored mechanism potentially contributing to the age-related maintenance of eccentric strength is a slowing of muscle contractile properties and an increased proportion of weakly to strongly bound crossbridges. Slowed crossbridge cycling in old adults (i.e., longer crossbridge attachment times as compared with young) is suggested to contribute to the overall slowing of whole muscle contractile properties (Miller et al. 2013). Therefore, during lengthening contractions with increasing stretch velocity, there will presumably be a higher probability of a greater number of attached crossbridges, at a given moment, contributing to elevated force during stretch. We recently showed a velocity dependence of eccentric strength in old adults whereby old had similar absolute strength values, as compared with young, during “fast” lengthening velocities of the ankle dorsiflexors (Power et al. 2015). A second mechanism for the maintenance of eccentric strength in the elderly could be an increased proportion of weakly bound crossbridges. Indeed, old adults have a greater proportion of weakly compared to strongly bound crossbridges as compared with young (Lowe et al. 2001; Prochniewicz et al. 2007), and this increased proportion in old could be an underlying mechanism of the age-related maintenance of eccentric strength. During an isometric contraction, the muscle of an old adult will presumably produce a lower force per crossbridge and subsequently lower force than a muscle from a young individual since only the strongly bound crossbridges contribute to force production. However, during muscle lengthening, weakly and strongly bound crossbridges contribute to force (Linari et al. 2004; Linari et al. 2000) whereby old adults are able to produce a relatively greater force during lengthening than during isometric or shortening contractions. To investigate the effects of slowed contractile properties and an increased proportion of weakly bound crossbridge configurations in the context of the age-related maintenance of eccentric strength, muscle temperature can be changed, to alter the crossbridge kinetics and the proportion of weakly to strongly bound crossbridges (Ranatunga et al. 2010; Roots et al. 2012; Roots et al. 2008).
The purpose of this study was to investigate the effects of increased/decreased crossbridge cycling rates and weakly/strongly bound crossbridge states on the age-related maintenance of eccentric strength. Owing to a decreased isometric force, slowed contractile properties, and similar muscle stiffness in the cold condition, we hypothesized that eccentric to isometric force ratios will be greatest for the cold condition and the highest stretch speeds. Secondly, owing to a greater slowing of contractile properties in the muscles of older adults compared to young, we expect eccentric to isometric force ratios will be greater in the old compared to the young study participants.
Materials and methods
Participants
This study was approved by the local ethics committee (REB number 15,396) and the procedures conformed to the Declaration of Helsinki. All young (n = 12, 24 ± 4 years, 77.5 ± 6.5 kg, 182.1 ± 8.1 cm) and old (n = 12, 72.6 ± 4.1 years, 77.5 ± 14.5 kg, 176.3 ± 7.1 cm) males were asked to refrain from any unaccustomed or strenuous exercise for 24 h and caffeine consumption for 2 h prior to testing. All participants were recreationally active, free from any known neuromuscular or musculoskeletal disorders, and did not undertake any regular hand exercises. To assess activity levels, the Yale physical activity survey was completed by all subjects (Dipietro et al. 1993).
Experimental setup
The experimental timeline is outlined in Fig. 1 Thumb adduction force and carpometacarpal angular displacement were measured using a custom-designed dynamometer as explained in detail elsewhere (Fortuna et al. 2012; Lee et al. 2002; Seiberl et al. 2015) (Fig. 2). Briefly, the hand was immobilized with a reusable clinical cast lined with a cooling/heating pad extending to the dorsal and ventral aspect of the hand. Temperature was controlled by a Gaymar T/Pump localized temperature system (Model TP700 series, Gaymar Industries Inc., Orchard Park, NY, USA). A 0° reference angle was defined for each subject as the highest degree of thumb adduction possible before the dynamometer thumb piece came in contact with the cast. Thumb angles increased with abduction, ranging from 0 to 40°. A thermometer was secured with tape on the skin directly over the adductor pollicis.
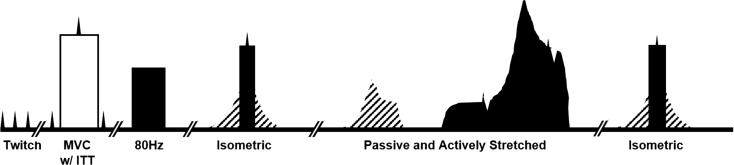
Fig. 1.
Experimental timeline. Contractions conducted within one temperature condition (normal, cold, and warm). Open boxes are voluntary contractions, hashed boxes are passive stretches, and black boxes are electrical stimulation. Maximum voluntary contraction; MVC. Interpolated twitch technique; ITT, was used to assess voluntary activation during the MVC
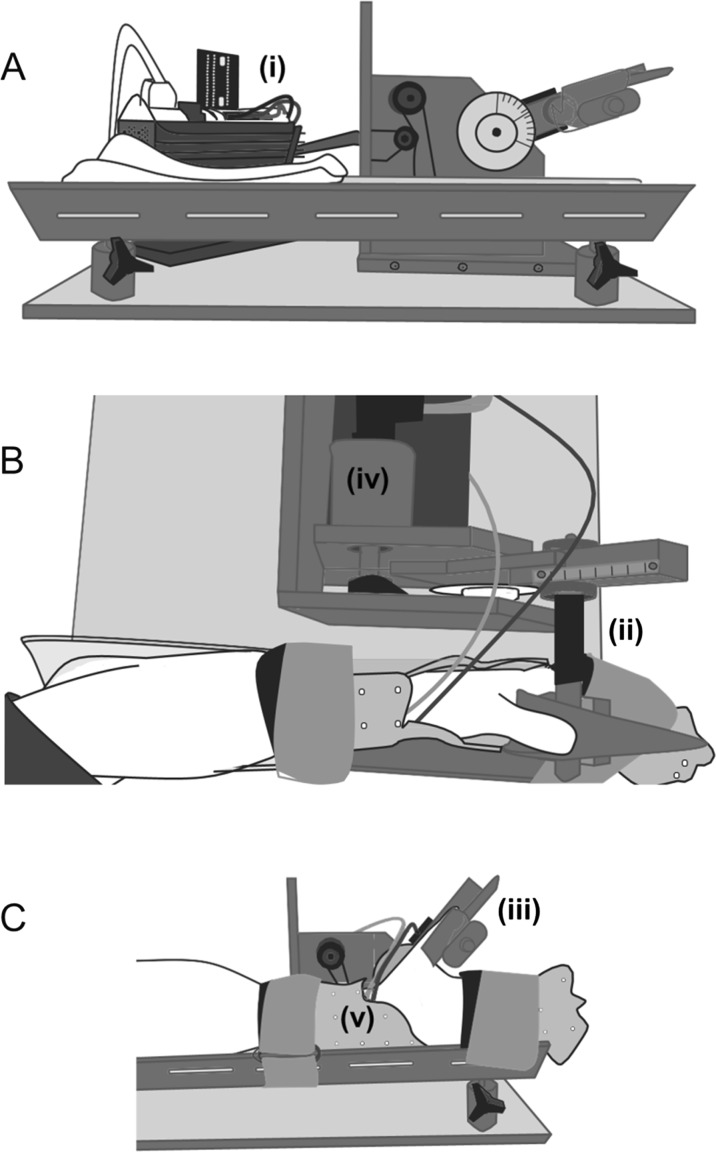
Fig. 2.
The apparatus used to conduct the study: A side view, B overhead view, C. side view with participants arm. i A rotary step motor with digital stepper controller. ii Represents the aluminum rod with pairs of strain gauges, iii the auxiliary thumb holder. The analog encoder is iv. The arm is fixed by the clinical cast v and secured with Velcro straps
Electrical stimulation
All experiments were performed using electrical stimulation of the ulnar nerve to activate the adductor pollicis muscle. Two self-adhering Ag-AgCl surface electrodes (2 × 3 cm) were placed over the ulnar nerve to electrically stimulate the adductor pollicis. The cathode was placed approximately 2 cm proximal to the pisiform bone on the medial wrist, and the anode was placed 2 cm proximal to the cathode. A computer-triggered stimulator (model DS7AH, Digitimer, Welwyn Garden City, Hertfordshire, UK) was used to increase the electrical current until further increases failed to produce an increase in twitch force (single 100-μs square wave pulses, 400 V). This current was then used to assess voluntary activation (VA) using the interpolated twitch technique for maximum voluntary isometric contractions (MVCs). Following determination of MVCs, all subsequent contractions were involuntary and electrically evoked, as described below.
Experimental procedures
Peak twitch force amplitude was determined as mentioned above. Participants then performed 1–2 MVCs, separated by 3-min rest, at a thumb angle of 40° to assess peak force and voluntary activation. Participants were encouraged verbally during all MVCs and visual feedback of the force produced was provided. Only subjects with VA >90 % completed the remainder of the protocol. Percent voluntary activation was calculated as
All testing across temperature conditions was performed using an identical electrical stimulation protocol. Current was increased (80 Hz; square wave pulses with 100-μs pulse width) until the evoked force reached a value between 50 and 60 % of the participant’s MVC peak force.
Participants performed six lengthening contractions at each speed and muscle temperature: (1) “normal” muscle temperature occurring at room temperature (24 °C), (2) “cold” muscle temperature was induced by cooling using a 15 °C water bath for 20 min, and (3) “warm” muscle temperature which was elicited using a 43 °C water bath for 20 min. Muscle temperature was derived from skin temperature and calculated using a previously established equation (de Ruiter et al. 1999) whereby muscle temperature = 1.02 (skin temperature) + 0.89 °C. This technique has a strong relationship between skin and muscle temperature (R2 = 0.98) (de Ruiter et al. 1999). The equation was established in young adults and for the purpose of the present study, we make the assumption that the relationship does not change with age. The angular velocities for thumb abduction were 0, −20, −40, −60, −80, −160, and −320° s−1 followed by a final isometric contraction. Given the muscle architecture of the adductor pollicis and joint angle excursion, a close approximation of fiber lengths per second as related to the angular velocities would be (assuming an overall muscle length of 4.5 cm and an average fiber length of 2.25 cm) about 0.2–3 fiber lengths∙s−1 for the slowest and fastest angular velocity, respectively. This approximation could be biased to even greater speeds in older adults owing to shorter fascicle lengths as compared with young.
The experiment always commenced with a passive lengthening of the muscle at one of the prescribed velocities until the thumb was 40° abducted. The thumb was then returned to 0°, an electrically evoked isometric pre-activation was induced lasting for 500 ms to establish a plateau in the force recording before active lengthening at a given speed. Testing of the angular velocities was conducted in a randomized order with 5-min rest between contractions. Once all the velocities for a given temperature condition were completed, a final electrically evoked isometric contraction was performed. At the end of each temperature condition, all electrodes were removed from the participant’s arm, the location was marked with indelible ink, and the hand was submerged in the cool or warm bath for 20 min. The order of temperatures was fixed from normal to cold to warm. The order of temperature exposure was fixed for practical reasons, as a pilot study (n = 3) showed that if the warm tests preceded the cold tests, the muscle temperature did not drop sufficiently during the 20-min cold bath (Table 1). Once the cooling or warming exposure was completed, the stimulating electrodes were placed at the same location as for the initial condition, and the thermometer was secured over the adductor pollicis. The stimulation current for full activation was then re-established as described above.
Table 1.
Isometric muscle properties across temperatures
| Condition | Young (n = 12) | Old (n = 12) | ||||
|---|---|---|---|---|---|---|
| Normal | Cold | Warm | Normal | Cold | Warm | |
| Temp (°C) | 30.6 ± 1.9 | 19.7 ± 1.8b | 35.7 ± 2.0b | 31.3 ± 1.6 | 21.3 ± 2.1b | 35.1 ± 2.0b |
| MVC (N) | 110.3 ± 21.5a | – | – | 75.3 ± 14.3 | – | – |
| VA (%) | 95 ± 4 | – | – | 94 ± 4 | – | – |
| 80 Hz (N) | 55.0 ± 9.5a | 27.6 ± 4.1a,b | 45.1 ± 5.6 | 40.0 ± 8.6 | 20.0 ± 8.5b | 39.0 ± 9.1 |
| HRT (ms) | 104.8 ± 16.4 | 236.1 ± 28.8a,b | 79.9 ± 5.3 | 114.7 ± 12.4 | 270.3 ± 44.0b | 84.6 ± 10.4 |
| %MVC | 50.8 ± 10.1 | 25.5 ± 4.5b | 41.6 ± 6.1 | 53.9 ± 11.1 | 27.8 ± 11.2b | 51.9 ± 12.1 |
Values are means ± SD
Temp muscle temperature, MVC maximal voluntary isometric contraction, VA voluntary activation, 80 Hz 80 Hz peak force, HRT 80 Hz half-relaxation time, and %MVC 80 Hz peak force relative to MVC amplitude.
aDenotes significant difference between age
bDenotes significant difference from normal temperature
Data analysis
Force and position data were sampled at 2000 Hz and digitized via an analog-to-digital converter (PowerLab System 16/35, ADInstruments, Bella Vista, Australia). All data were low pass-filtered (10 Hz). As described in detail previously (Lee et al. 2003), instantaneous muscle stiffness (Fstiff) was determined via a change in force during a 2° quick-stretch (performed at 500°s−2) that was imposed 1 s after the thumb position reached 40°. Twitch and 80-Hz forces were calculated as the peak force amplitude. The 80-Hz tetanic half-relaxation time (HRT) was calculated from the last pulse of the isometric contraction until force had dropped to 50 % of its peak value. Peak eccentric force was determined as the peak force observed during the active stretch. Passive force was determined as the steady-state force following passive stretching. Active eccentric force was determined by subtracting the passive force during stretch at a given velocity from the peak eccentric force at that same velocity (Power et al. 2013b). Peak isometric force was determined as the peak force at a thumb angle of 40°. Passive isometric force was calculated at 40°, which was subtracted from the peak isometric force taken at 40° to calculate active isometric force.
Statistical analysis
SPSS software (version 20, IBM, Chicago, IL) was used for all statistical analyses. An unpaired t test was performed to assess room temperature neuromuscular function of the young and old adults for the normal condition. A two-way ANOVA was performed to assess differences in peak tetanic force at 80-Hz stimulation, 80-Hz HRT, and passive and active eccentric force as a function of age and muscle temperature. A three way ANOVA was performed to assess changes in the eccentric to isometric ratio (ECC/ISO) and stiffness as a function of age, thumb adduction lengthening velocity and muscle temperature. When significance was observed, a post hoc analysis using t tests was performed with a Bonferroni correction to identify significant differences. The VA values were not normally distributed, and thus, a Mann-Whitney U test was employed for this particular variable. The level of significance for all tests was set at P ≤ 0.05. Tabulated and text data are presented as mean ± standard deviation (SD) and figure values as mean ± standard error (SE).
Results
Physical activity energy expenditure (kcal·week−1) as assessed with the Yale Physical activity survey was similar (P = 0.71) between the young and old adults, respectively (95 % CI 5551, 7125 and 4156, 6821, respectively). The old adults were 32 % weaker than were young for MVC force (P < 0.05) despite similar and high levels of voluntary activation (P > 0.05; Table 1). Electrically evoked force expressed relative to the MVC was similar for young and old (range 50–60 %) at room temperature and for the warm temperature condition (P > 0.05; Table 1). However, for both age groups, electrically evoked force expressed relative to the MVC was lower (20–25 % MVC) in the cold condition as compared with normal and warm. There was no significant difference between the first and last isometric contraction of the testing session for old (P = 0.65) or young (P = 0.43).
Effect of temperature on isometric properties
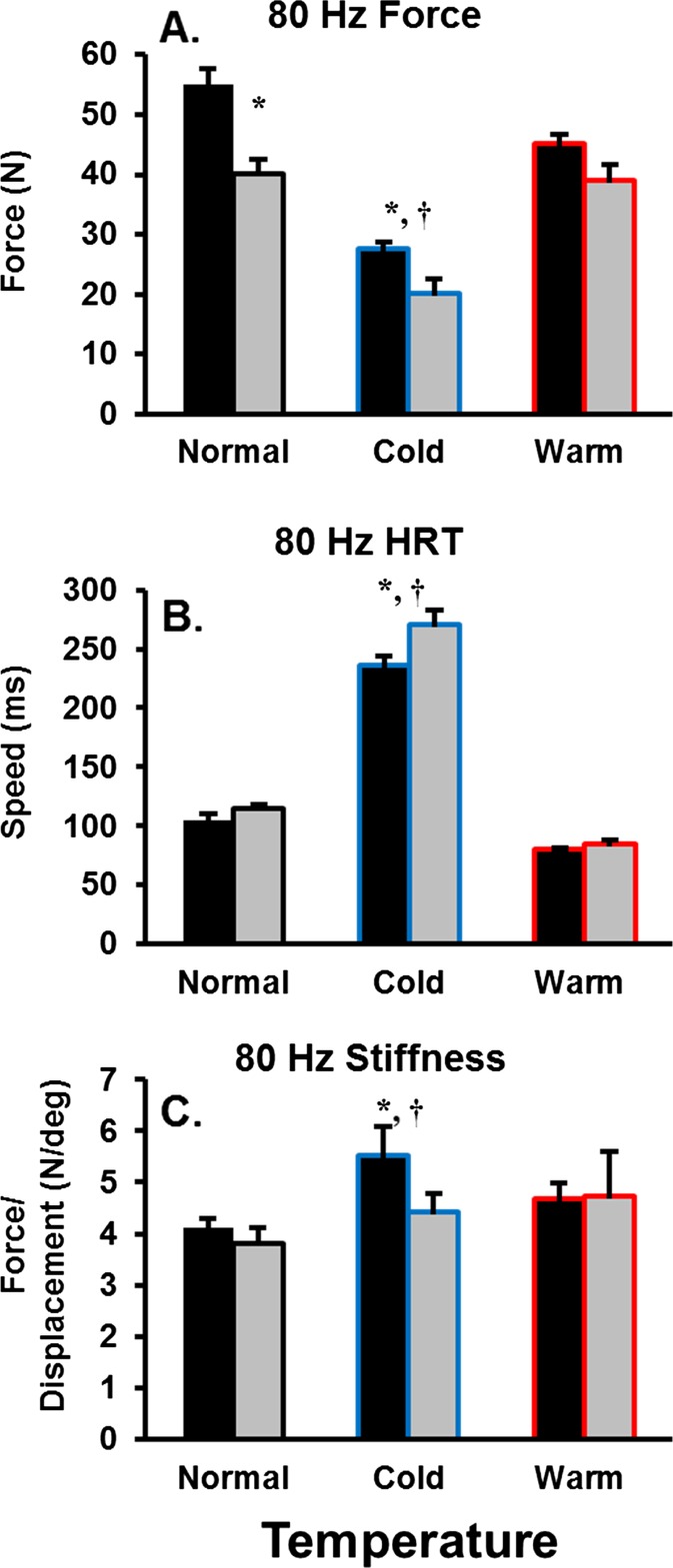
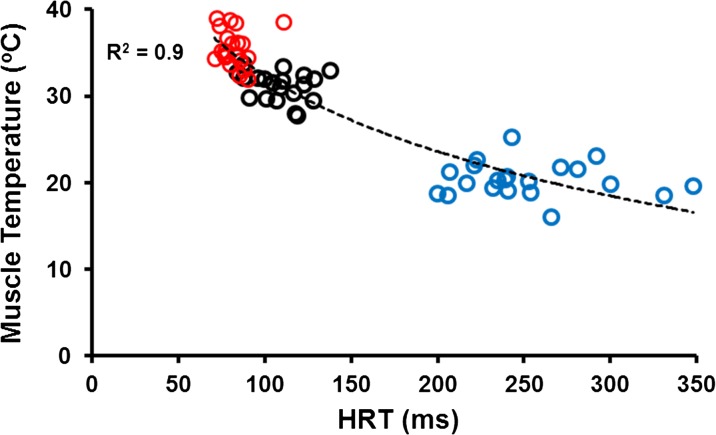
For electrically evoked 80 Hz isometric force, there were main effects for age (P < 0.001) and temperature (P < 0.001), but no interaction (P = 0.08). The old adults were ∼27 % weaker compared with young for the normal and cold conditions, with no difference for the warm condition. Isometric force was not different for the normal and warm temperature conditions in both young and old, while isometric force in the cold condition was 50 and 90 % of that measured during the normal and warm conditions for both groups, respectively (Fig. 3a). Altering muscle temperature had significant effects on muscle contractile speeds representing a logarithmic relationship (Fig. 4). For electrically evoked 80-Hz HRT, there were main effects for age (P < 0.05) and temperature (P < 0.001) and an age × temperature interaction (P < 0.05). The old adults were ∼15 % slower for HRT compared with young during the cold condition, with no difference for the normal and warm conditions. For both young and old, HRT was 70–135 % slower in the cold condition as compared with both the normal and warm conditions. There was no detectable difference between the normal and warm conditions (Fig. 3b). Instantaneous stiffness was determined during the plateau of the electrically evoked 80-Hz isometric force, and there was no main effect for age (P = 0.40). However, there was an effect for temperature (P < 0.05) but no interaction (P = 0.08). For both young and old, instantaneous stiffness was not different when comparing the normal and warm conditions; whereas, instantaneous stiffness was 20–40 % greater in the cold compared with the normal and warm conditions, respectively (Fig. 3c).
Fig. 3.
Isometric contractile properties for old and young adults across temperature conditions (normal; black, cold; blue, warm; red). Half-relaxation time (HRT). Instantaneous muscle stiffness was determined via a change in force during a 2° quick-stretch (performed at 500°s−2) assessed 1 s after the thumb position reached 40°. Asterisk denotes an age-related difference. Dagger denotes a temperature-related difference from the normal condition
Fig. 4.
Relationship between 80-Hz half-relaxation time (HRT) and temperature across age. There was a strong negative logarithmic relationship between temperature and HRT (−12.64ln(x) + 90.56). As temperature increased above ∼32 °C, there was minimal increase in contractile speed
Active force during stretch
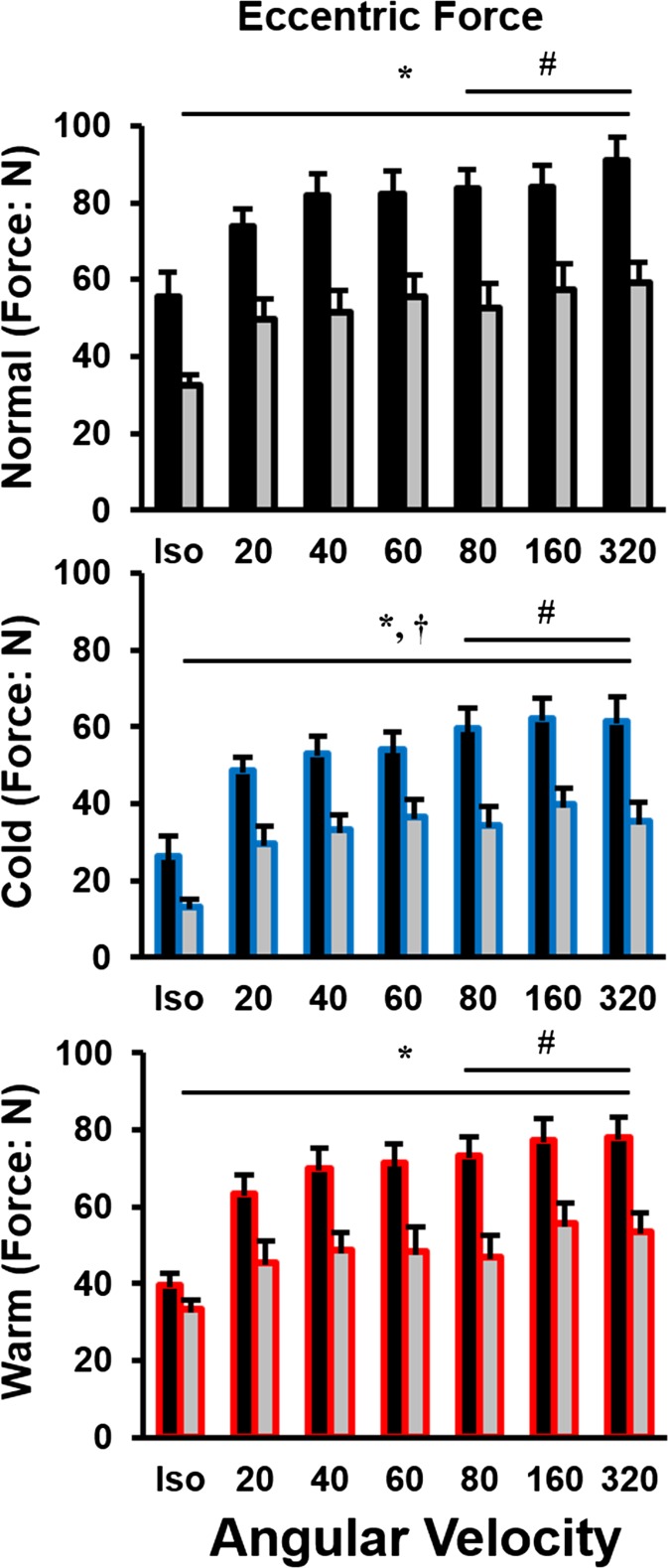
Active force during stretch was determined as the electrically evoked peak force amplitude at the end of stretch minus the corresponding passive force following a stretch of similar speed. There were main effects for age (P < 0.001), temperature (P < 0.001), and lengthening velocity (P < 0.001), with an age × temperature interaction (P < 0.001). Peak force occurred at a thumb angle of 40 and increased with an increasing velocity of lengthening. However, the old adults were ∼30–45 % weaker than were young for all temperatures independent of lengthening velocity (Fig. 5).
Fig. 5.
Eccentric force in all three conditions (normal; black, cold; blue, and warm; red) across an isometric contraction and six velocities (−20, −40, −60, −80, −160, −320° s−1). Force during lengthening in the cold condition was always less than normal and warm across all velocities. Number sign denotes velocity-dependent significance between ratios in comparison to 20° s−1. Asterisk denotes an age-related difference. Dagger denotes a temperature related difference from the normal condition
Age-related maintenance of eccentric strength
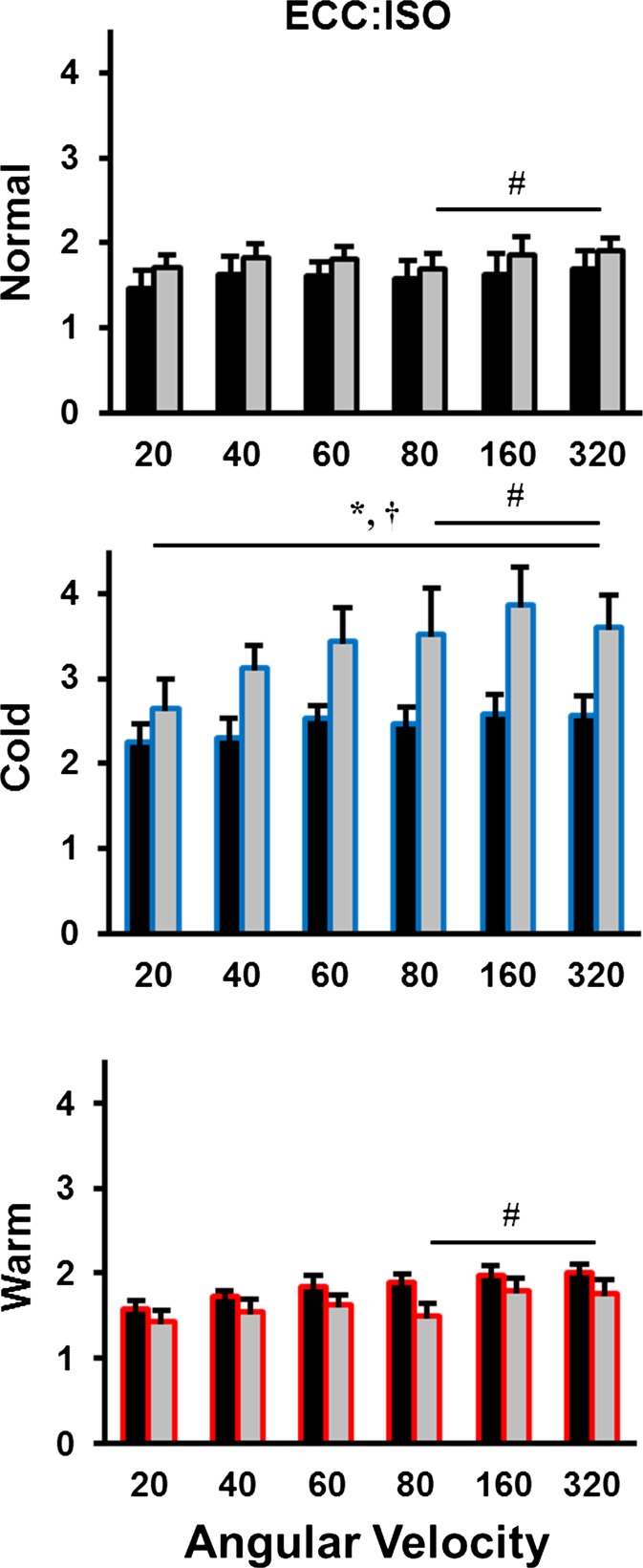
The ratio of active force during lengthening to isometric force exhibited main effects for age (P < 0.05), temperature (P < 0.001), and lengthening velocity (P < 0.05), with an age × temperature interaction (P < 0.001). The effect of age depended on the temperature of the muscle with the old having a 20–50 % higher ECC/ISO as compared with young during the cold, but no age-related differences were detected for the normal and warm conditions. In agreement with the peak force results, the ECC/ISO increased with increasing speed of lengthening (Fig. 6). For example, the ECC/ISO calculated from velocities >80° s−1 were ∼20–35 % larger than that same ratio at a speed of 20° s−1.
Fig. 6.
Average eccentric to isometric force ratios (ECC/ISO) in all three conditions (normal; black, cold; blue, warm; red) across six lengthening velocities (−20, −40, −60, −80, −160, −320° s−1). The ECC/ISO in the cold condition was always greater than normal and warm across all velocities. Number sign denotes velocity-dependent significance between ratios in comparison to 20° s−1. Asterisk denotes an age-related difference. Dagger denotes a temperature-related difference from the normal condition
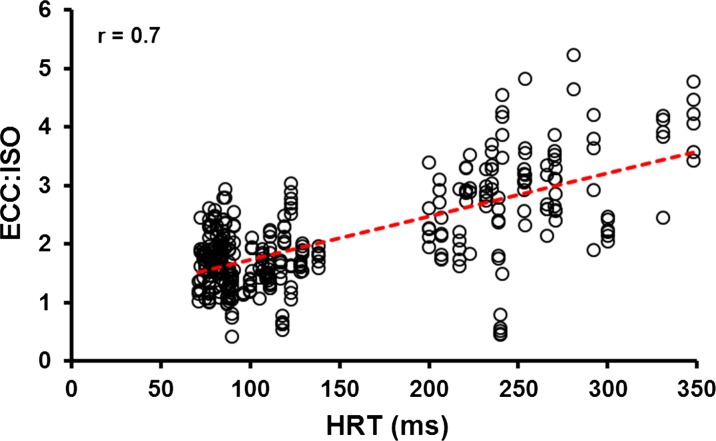
When HRTs were correlated with the ECC/ISO, a strong, positive relationship (r = 0.7; P < 0.05) was revealed. With slowed contractile speed for muscles that were “cooled” or muscles from the old subjects, there is a concomitant increase in the ECC/ISO (Fig. 7).
Fig. 7.
Relationship between 80-Hz half-relaxation time (HRT) and eccentric to isometric ratio (ECC/ISO) across velocity and temperature
Discussion
Natural adult aging is associated with structural and functional alterations of the human neuromuscular system which contributes to impaired performance (Power et al. 2014a). However, numerous studies report an age-related maintenance of force during lengthening (i.e., eccentric) (Phillips et al. 1991; Roig et al. 2010) as compared with isometric and shortening contractions. In this investigation, we attempted to elucidate the mechanisms responsible for the age-related maintenance of eccentric strength. The hypothesis that old adults will experience the typical age-related maintenance of eccentric strength across all temperature conditions was not fully supported in the model used in the present study (adductor pollicis). However, there was a temperature and velocity-dependent influence on force production during lengthening. The effect of age was dependent on the temperature of the muscle with the old adults exhibiting a 20–50 % greater ECC/ISO compared with young for the cold condition. There were no detectable age-related differences for the normal and warm conditions, which had similar contractile speeds (i.e., HRT) across age. Furthermore, the ECC/ISO increased with increasing velocity of lengthening. It appears that biasing the crossbridge distribution to a primarily weakly bound configuration (i.e., greater stiffness and less force in the cold condition compared with warm and normal) and the resulting slowing of contractile speed (i.e., slowing of 80 Hz HRT in the cold condition) leads to an elevated ECC/ISO in both young and old. Therefore, an active crossbridge-based mechanism of an increased proportion of weakly bound crossbridges, or a decreased detachment rate, enhances force during active lengthening as compared with other contraction modes, and this is particularly the case for old adults.
Muscle model used
The experimental muscle model used in the present study was the human adductor pollicis muscle activated via electrical stimulation of the ulnar nerve. The relatively small and flat fan-like adductor pollicis muscle was investigated because it can be maximally activated via electrical nerve stimulation, thereby minimizing influences from the central nervous system. Additionally, the adductor pollicis is located superficially and its temperature can be regulated easily by cooling or warming the hand externally. However, the similar physical activity levels between young and old, the unique combination of a high Type I (80 %) muscle fiber composition and fast muscle contractile properties (Round et al. 1984), may have contributed to the lack of age-related differences in the normal “room temperature” condition in the present study. Similarly, other groups have reported no change in HRT across age for this particular muscle (Ditor et al. 2000). However, in those individuals greater than 75 years of age, a slowing of HRT is evident (see Fig. 3 in (Narici et al. 1991). In the present study, a similar HRT for both age-groups for the normal condition may have been beneficial, in that elucidating the mechanisms of ECC/ISO for old adults required a cold temperature-induced slowing of HRT to expose the typical age-related maintenance of eccentric strength.
Age-related maintenance of eccentric strength
The maintenance of eccentric strength for old adults can be attributed to neural or non-neural mechanisms, or both (Roig et al. 2010). It has been suggested that old adults have increased antagonist co-activation and decreased central drive to muscles compared with young, therefore decreasing force preferentially during isometric and shortening, while maintaining strength during eccentric contractions (Roig et al. 2010). We reported previously that old participants were capable of near full voluntary activation during maximal lengthening contractions. Furthermore, the amplitude of the electromyogram (EMGRMS) of agonist and antagonist muscles did not differ across age or velocity (Power et al. 2015). These findings indicate that neural activation (i.e., as assessed with surface EMG) is not a limiting factor during isometric or lengthening contractions. Similar observations were made in tibialis anterior activation (EMG) for old and young across a series of lengthening velocities (Klass et al. 2005). Correspondingly, multiple reports suggest that old adults are fully capable of complete voluntary activation of muscles, if they are well-familiarized with the task and are highly motivated (Jakobi et al. 2002; Klass et al. 2005; Phillips et al. 1992). Thus, decreased activation or increased co-activation may serve to modify force during lengthening contractions, but is not the fundamental mechanism responsible for the elevated ECC:ISO in old adults as compared with young. The mechanism contributing to the age-related maintenance of eccentric strength is likely intrinsic to the contractile properties of the muscle.
Temperature dependence: support for an intrinsic muscle property leading to maintained eccentric strength
The temperature dependence of the ECC/ISO force ratio may be explained in part by the altered contractile kinetics in cold compared with the normal and warm muscle conditions. Evidence from skinned single muscle fiber segments biopsied from old adults suggests that an increase in weakly bound crossbridges and elevated instantaneous stiffness contribute to force during lengthening contractions (Ochala et al. 2006; Ochala et al. 2007). In the present study, there was an increase in the ECC/ISO force and instantaneous stiffness for both age groups for the cold condition compared with the normal and warm. Further, there was a decrease in isometric force and a concomitant increase in stiffness, thus a major contributing factor to the elevated ECC/ISO would presumably be an increased proportion of weakly compared to strongly-bound crossbridges that contribute to force during active lengthening. In our study, the ECC/ISO force ratio and the absolute stiffness were the same for the warm and normal temperature conditions and greater in the cold condition for the young and old. This finding suggests that the force per crossbridge (not number of attached crossbridges) increased with increasing muscle temperature. There was similar stiffness and force for the warm and normal muscle temperature conditions, which is indicative of an unchanged proportion of attached crossbridges and force per crossbridge for these two temperatures in old and young adults. However, due to the sigmoidal isometric force and temperature relationship, as highlighted in Fig. 4, we suspect that there is a temperature-dependent theoretical maximum to the equilibrium proportion of strongly to weakly bound crossbridges which was reached in the present study and appears to be ∼32 °C.
Decreased temperature resulted in a slowing of muscle contractile speed, which may have contributed to the velocity dependence of eccentric force. Increased force with increasing lengthening velocity can be explained partially by the slowed crossbridge cycle during the active stretch (i.e., longer or similar time attached with a decreased detachment rate) allowing for longer duration of crossbridge attachment (myosin head remains attached for a longer period) during stretch and subsequently greater strain (Miller et al. 2013). The crossbridge strain associated with the active stretch is greater, thus producing a higher force than that during the isometric conditions. Typically, aging is associated with a slowing of the contractile speed (Dalton et al. 2014). However in the present study, there were no age-related differences in HRT for the normal and warm condition. The similar HRT may have been due to the similar activity levels of the participants or the unique fast contractile properties of the predominately Type I fiber composition adductor pollicis. In this study, there was a greater slowing of HRT in the old as compared with the young for the cold condition. The age-related slowing of crossbridge kinetics, specifically the slowed detachment rate of myosin (Miller et al. 2013), may allow for more attached crossbridges at a given time and thus provide greater stiffness of the muscle during stretch which would allow for old adults to develop relatively higher forces during lengthening muscle actions compared with the young and compared to isometric contractions. On average, when the velocity of the stretch is increased, the crossbridge strain increases as crossbridges are pulled to greater distances from their equilibrium condition (Ranatunga et al. 2010), which is supported by our result of an increasing peak force with increasing lengthening velocity.
Conclusion
We confirm here that the eccentric to isometric force production ratio is maintained with adult aging in an electrically activated muscle in vivo. We contest that this maintenance is not entirely owing to the adult aging process, but that changes in the ECC/ISO ratio can be explained, in part, by a strong positive relationship between ECC/ISO and muscle contractile speed (i.e., HRT). We suggest that the slowing of muscle contractile properties and a greater proportion of weakly bound crossbridges (i.e., increased stiffness and lower force in the cold condition) may be key contributors to an elevated ECC/ISO, particularly for old adults.
Acknowledgments
The authors would like to thank all those who participated in the study. These data were collected at the University of Calgary and was supported by funding from Alberta Innovates-Health Solutions, the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada, The Killam Foundation, and the Canada Research Chair Program. B.H. Dalton is supported by a New Investigator Grant from the Medical Research Foundation, Oregon Health and Science University Foundation. G.A. Power was supported by a Banting postdoctoral fellowship (Canadian Institutes for Health Research; CIHR) and Alberta Innovates Health Solutions.
Compliance with ethical standards
This study was approved by the local ethics committee (REB number 15,396) and the procedures conformed to the Declaration of Helsinki.
Conflict of interest disclosure
The authors declare that they have no conflicts of interest to disclose.
References
- Cannon J, Kay D, Tarpenning KM, Marino FE. Normalized lengthening peak torque is associated with temporal twitch characteristics in elderly women but not young women. Acta Physiol (Oxf) 2006;188:53–62. doi: 10.1111/j.1748-1716.2006.01606.x. [DOI] [PubMed] [Google Scholar]
- Coupland ME, Puchert E, Ranatunga KW. Temperature dependence of active tension in mammalian (rabbit psoas) muscle fibres: effect of inorganic phosphate. J Physiol. 2001;536:879–891. doi: 10.1111/j.1469-7793.2001.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton BH, Allen MD, Power GA, Vandervoort AA, Rice CL. The effect of knee joint angle on plantar flexor power in young and old men. Exp Gerontol. 2014;52:70–76. doi: 10.1016/j.exger.2014.01.011. [DOI] [PubMed] [Google Scholar]
- Dalton BH, Power GA, Paturel JR, Rice CL. Older men are more fatigable than young when matched for maximal power and knee extension angular velocity is unconstrained. Age (Dordr) 2015;37:9790. doi: 10.1007/s11357-015-9790-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter CJ, Jones DA, Sargeant AJ, de Haan A. Temperature effect on the rates of isometric force development and relaxation in the fresh and fatigued human adductor pollicis muscle. Exp Physiol. 1999;84:1137–1150. doi: 10.1111/j.1469-445X.1999.01895.x. [DOI] [PubMed] [Google Scholar]
- Dipietro L, Caspersen CJ, Ostfeld AM, Nadel ER. A survey for assessing physical activity among older adults. Med Sci Sports Exierc. 1993;25:628–642. [PubMed] [Google Scholar]
- Ditor DS, Hicks AL. The effect of age and gender on the relative fatigability of the human adductor pollicis muscle. Can J Physiol Pharmacol. 2000;78:781–790. doi: 10.1139/y00-061. [DOI] [PubMed] [Google Scholar]
- Fortuna R, Vaz MA, Herzog W. Catchlike property in human adductor pollicis muscle. J Electromyogr Kinesiol. 2012;22:228–233. doi: 10.1016/j.jelekin.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Hortobagyi T, Zheng D, Weidner M, Lambert NJ, Westbrook S, Houmard JA. The influence of aging on muscle strength and muscle fiber characteristics with special reference to eccentric strength. J Gerontol A Biol Sci Med Sci. 1995;50:B399–B406. doi: 10.1093/gerona/50A.6.B399. [DOI] [PubMed] [Google Scholar]
- Jakobi JM, Rice CL. Voluntary muscle activation varies with age and muscle group. J Appl Physiol. 2002;93:457–462. doi: 10.1152/japplphysiol.00012.2002. [DOI] [PubMed] [Google Scholar]
- Klass M, Baudry S, Duchateau J. Aging does not affect voluntary activation of the ankle dorsiflexors during isometric, concentric, and eccentric contractions. J Appl Physiol. 2005;99:31–38. doi: 10.1152/japplphysiol.01426.2004. [DOI] [PubMed] [Google Scholar]
- Lee HD, Herzog W. Force enhancement following muscle stretch of electrically stimulated and voluntarily activated human adductor pollicis. J Physiol. 2002;545:321–330. doi: 10.1113/jphysiol.2002.018010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HD, Herzog W. Force depression following muscle shortening of voluntarily activated and electrically stimulated human adductor pollicis. J Physiol. 2003;551:993–1003. doi: 10.1113/jphysiol.2002.037333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linari M, Bottinelli R, Pellegrino MA, Reconditi M, Reggiani C, Lombardi V. The mechanism of the force response to stretch in human skinned muscle fibres with different myosin isoforms. J Physiol. 2004;554:335–352. doi: 10.1113/jphysiol.2003.051748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linari M, Lucii L, Reconditi M, Casoni ME, Amenitsch H, Bernstorff S, Piazzesi G, Lombardi V. A combined mechanical and X-ray diffraction study of stretch potentiation in single frog muscle fibres. J Physiol. 2000;526(Pt 3):589–596. doi: 10.1111/j.1469-7793.2000.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe DA, Surek JT, Thomas DD, Thompson LV. Electron paramagnetic resonance reveals age-related myosin structural changes in rat skeletal muscle fibers. Am J Physiol Cell Physiol. 2001;280:C540–C547. doi: 10.1152/ajpcell.2001.280.3.C540. [DOI] [PubMed] [Google Scholar]
- Miller MS, Bedrin NG, Callahan DM, Previs MJ, Jennings ME, 2nd, Ades PA, Maughan DW, Palmer BM, Toth MJ. Age-related slowing of myosin actin cross-bridge kinetics is sex specific and predicts decrements in whole skeletal muscle performance in humans. J Appl Physiol (1985) 2013;115:1004–1014. doi: 10.1152/japplphysiol.00563.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narici MV, Bordini M, Cerretelli P. Effect of aging on human adductor pollicis muscle function. J Appl Physiol (1985) 1991;71:1277–1281. doi: 10.1152/jappl.1991.71.4.1277. [DOI] [PubMed] [Google Scholar]
- Ochala J, Dorer DJ, Frontera WR, Krivickas LS. Single skeletal muscle fiber behavior after a quick stretch in young and older men: a possible explanation of the relative preservation of eccentric force in old age. Pflugers Arch. 2006;452:464–470. doi: 10.1007/s00424-006-0065-6. [DOI] [PubMed] [Google Scholar]
- Ochala J, Frontera WR, Dorer DJ, Van Hoecke J, Krivickas LS. Single skeletal muscle fiber elastic and contractile characteristics in young and older men. J Gerontol A Biol Sci Med Sci. 2007;62:375–381. doi: 10.1093/gerona/62.4.375. [DOI] [PubMed] [Google Scholar]
- Phillips SK, Bruce SA, Newton D, Woledge RC. The weakness of old age is not due to failure of muscle activation. J Gerontol. 1992;47:M45–M49. doi: 10.1093/geronj/47.2.M45. [DOI] [PubMed] [Google Scholar]
- Phillips SK, Bruce SA, Woledge RC. In mice, the muscle weakness due to age is absent during stretching. J Physiol. 1991;437:63–70. doi: 10.1113/jphysiol.1991.sp018583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazzesi G, Reconditi M, Koubassova N, Decostre V, Linari M, Lucii L, Lombardi V. Temperature dependence of the force-generating process in single fibres from frog skeletal muscle. J Physiol. 2003;549:93–106. doi: 10.1113/jphysiol.2002.038703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter MM, Vandervoort AA, Kramer JF. Eccentric peak torque of the plantar and dorsiflexors is maintained in older women. J Gerontol A Biol Sci Med Sci. 1997;52:B125–B131. doi: 10.1093/gerona/52A.2.B125. [DOI] [PubMed] [Google Scholar]
- Poulin MJ, Vandervoort AA, Paterson DH, Kramer JF, Cunningham DA. Eccentric and concentric torques of knee and elbow extension in young and older men. Can J Sport Sci. 1992;17:3–7. [PubMed] [Google Scholar]
- Power GA, Allen MD, Booth WJ, Thompson RT, Marsh GD, Rice CL. The influence on sarcopenia of muscle quality and quantity derived from magnetic resonance imaging and neuromuscular properties. Age (Dordr) 2014;36:9642. doi: 10.1007/s11357-014-9642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power GA, Dalton BH, Rice CL. Human neuromuscular structure and function in old age: A brief review. J Sport Health Sci. 2013;2:215–226. doi: 10.1016/j.jshs.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power GA, Makrakos DP, Rice CL, Vandervoort AA. Enhanced force production in old age is not a far stretch: an investigation of residual force enhancement and muscle architecture. Physiol Rep. 2013;1:e00004. doi: 10.1002/phy2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power GA, Makrakos DP, Stevens DE, Herzog W, Rice CL, Vandervoort AA. Shortening-induced torque depression in old men: implications for age-related power loss. Exp Gerontol. 2014;57:75–80. doi: 10.1016/j.exger.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Power GA, Makrakos DP, Stevens DE, Rice CL, Vandervoort AA. Velocity dependence of eccentric strength in young and old men: the need for speed! Appl Physiol Nutr Metab. 2015;40:703–710. doi: 10.1139/apnm-2014-0543. [DOI] [PubMed] [Google Scholar]
- Power GA, Rice CL, Vandervoort AA. Increased residual force enhancement in older adults is associated with a maintenance of eccentric strength. PLoS one. 2012;7:e48044. doi: 10.1371/journal.pone.0048044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochniewicz E, Thompson LV, Thomas DD. Age-related decline in actomyosin structure and function. Exp Gerontol. 2007;42:931–938. doi: 10.1016/j.exger.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranatunga KW, Coupland ME. Crossbridge mechanism(s) examined by temperature perturbation studies on muscle. Adv Exp Med Biol. 2010;682:247–266. doi: 10.1007/978-1-4419-6366-6_14. [DOI] [PubMed] [Google Scholar]
- Roig M, Macintyre DL, Eng JJ, Narici MV, Maganaris CN, Reid WD. Preservation of eccentric strength in older adults: evidence, mechanisms and implications for training and rehabilitation. Exp Gerontol. 2010;45:400–409. doi: 10.1016/j.exger.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roots H, Pinniger GJ, Offer GW, Ranatunga KW. Mechanism of force enhancement during and after lengthening of active muscle: a temperature dependence study. J Muscle Res Cell Motil. 2012;33:313–325. doi: 10.1007/s10974-012-9307-8. [DOI] [PubMed] [Google Scholar]
- Roots H, Ranatunga KW. An analysis of the temperature dependence of force, during steady shortening at different velocities, in (mammalian) fast muscle fibres. J Muscle Res Cell Motil. 2008;29:9–24. doi: 10.1007/s10974-008-9138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JM, Jones DA, Chapman SJ, Edwards RH, Ward PS, Fodden DL. The anatomy and fibre type composition of the human adductor pollicis in relation to its contractile properties. J Neurol Sci. 1984;66:263–272. doi: 10.1016/0022-510X(84)90015-7. [DOI] [PubMed] [Google Scholar]
- Seiberl W, Power GA, Herzog W, Hahn D (2015). The stretch-shortening cycle (SSC) revisited: residual force enhancement contributes to increased performance during fast SSCs of human m. adductor pollicis. Physiol Rep 3. doi:10.14814/phy2.12401 [DOI] [PMC free article] [PubMed]
- Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve. 2002;25:17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- Wang G, Kawai M. Effect of temperature on elementary steps of the cross-bridge cycle in rabbit soleus slow-twitch muscle fibres. J Physiol. 2001;531:219–234. doi: 10.1111/j.1469-7793.2001.0219j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]