Abstract
Advanced aging is associated with the loss of structural and biomechanical properties in bones, which increases the risk for bone fracture. Aging is also associated with reductions in circulating levels of the anabolic signaling hormone, insulin-like growth factor (IGF)-1. While the role of IGF-1 in bone development has been well characterized, the impact of the age-related loss of IGF-1 on bone aging remains controversial. Here, we describe the effects of reducing IGF-1 at multiple time points in the mouse life span—early in postnatal development, early adulthood, or late adulthood on tibia bone aging in both male and female igff/f mice. Bone structure was analyzed at 27 months of age using microCT. We find that age-related reductions in cortical bone fraction, cortical thickness, and tissue mineral density were more pronounced when IGF-1 was reduced early in life and not in late adulthood. Three-point bone bending assays revealed that IGF-1 deficiency early in life resulted in reduced maximum force, maximum bending moment, and bone stiffness in aged males and females. The effects of IGF-1 on bone aging are microenvironment specific, as early-life loss of IGF-1 resulted in decreased cortical bone structure and strength along the diaphysis while significantly increasing trabecular bone fraction and trabecular number at the proximal metaphysis. The increases in trabecular bone were limited to males, as early-life loss of IGF-1 did not alter bone fraction or number in females. Together, our data suggest that the age-related loss of IGF-1 influences tibia bone aging in a sex-specific, microenvironment-specific, and time-dependent manner.
Keywords: IGF-1, MicroCT, Tibia, Three-point bone bending
Introduction
The loss of structural and biomechanical properties in aged bones leads to increased fragility and increased susceptibility to bone fracture. Decreased osteoblast activity, with a concomitant increase in osteoclast activity and adipogenesis, ultimately leads to increased bone resorption with the resulting loss of bone structure in advanced age. As important regulators of bone cell function, growth factors such as insulin-like growth factor-1 (IGF-1) likely influence the age-related changes observed in these cells. IGF-1 promotes the differentiation and maturation of chondrocytes, osteoblasts, and osteoclasts (Wang et al. 2006a, 2011, 2013; Xian et al. 2012). Thus, it is not surprising that reduced IGF-1 signaling has been associated with impaired bone development. It has previously been reported that animals with a homozygous knock-out of IGF-1, which die prematurely, exhibit significantly reduced bone and body growth (Baker et al. 1993; Bikle et al. 2001; Liu et al. 1993; Wang et al. 2006b; Woods et al. 1996). Decreased production of IGF-1 in the liver, where a majority of IGF-1 in circulation is derived, results in decreased bone length (Sjogren et al. 2002), bone mineral density (Courtland et al. 2011), and femoral strength and stiffness (Yakar et al. 2009) in adulthood. IGF-1 can also be produced locally within the chondrocytes and osteoblasts. Conditional knock-out of IGF-1 production within these cells also leads to decreased bone mineral density and reduced bone mass (Govoni et al. 2007a; Govoni et al. 2007b). Moreover, knock-out of the IGF receptor in osteoblasts leads to impairments in bone volume and mineralization (Kubota et al. 2013; Zhang et al. 2002). Together, these studies highlight the critical role for IGF-1 signaling in bone development.
Despite the importance for IGF-1 in bone development, very few studies have addressed the effects of IGF-1 on bone aging. In one study, cortical thickness in the aged femur was significantly increased when an important stabilizing protein for IGF-1, acid-labile subunit (ALS), was knocked out early in life (Courtland et al. 2013). Contrary to these data, a recent study found that a decrease in circulating IGF-1 during adulthood leads to reduced cortical and trabecular bone thickness in the femur during aging (Gong et al. 2014). Together, these disparate results suggest that the consequences of IGF-1 deficiency may be dependent on the stage of life that the deficiency is induced. In support of this concept, we recently reported that the age of onset of IGF-1 deficiency differentially influenced vertebral aging—early-life loss of IGF-1 was associated with increased trabecular structure in advanced age, while late-life loss of IGF-1 had no effect (Ashpole et al. 2015).
The differing effects of IGF-1 deficiency on bone structure may be the result of normal fluctuations in IGF-1 throughout the life span. There is a robust surge in circulating IGF-1 during adolescence, a time of rapid growth and bone maturation. The knock-out studies suggest that this surge in IGF-1 is important for bone development but detrimental to bone structure in advanced aging. Following puberty, IGF-1 levels steadily decline into advanced age (Bando et al. 1991; Corpas et al. 1993; Niblock et al. 1998; Smith et al. 1989). The conditional knock-out induced during adulthood suggests that this loss is detrimental to bone aging. Moreover, clinical studies indicate that reduced levels of IGF-1 are associated with decreased bone mineral density (Gillberg et al. 2002; Janssen et al. 1998; Kurland et al. 1997; Langlois et al. 1998; Mohamad and Khater 2015; Rhee et al. 2004; Sugimoto et al. 1997; Szulc et al. 2004) and increased risk of osteoporosis in advanced age (Kurland et al. 1997; Liu et al. 2008; Paccou et al. 2012; Sugimoto et al. 1997). Considering this, it is possible that early-life deficiency in IGF-1 may have differing effects on bone aging than deficiency induced later in life.
Here, we examine the effects of early-life, mid-life, and late-life IGF-1 deficiencies on tibia bone structure and strength in 27-month aged mice. In addition, our study includes male and female mice in order to examine sex-specific changes in bone aging. Moreover, we elucidate microenvironment-specific effects by assessing both cortical bone in the diaphysis and trabecular bone in the metaphysis. Our results provide evidence that IGF-1 influences tibia aging in a time-dependent, sex-specific, and microenvironment-specific manner.
Materials and methods
Animals
All procedures were approved by and followed the guidelines of the Institutional Animal Care and Use Committee and veterinarians at University of Oklahoma Health Sciences Center (OUHSC). Male and female igf1f/f mice were purchased from Jackson Laboratories (B6.129(FVB)-Igf1<tm1Dlr>/J) and backcrossed to C57Bl/6 for six generations in house. Cre recombination in this strain results in the excision of exon 4 of IGF-1, a region responsible for the binding of IGF-1 to the IGF receptor. All animals were housed (three to four per cage) in Allentown XJ cages with Anderson’s Enrich-o-cob bedding (Maumee, OH) on a 12-h light/12-h dark cycle at 21 ± 2 °C, as previously described (Ashpole et al. 2015). The animals were maintained in the specific pathogen-free Rodent Barrier Facility at OUHSC where all animals are free of helicobacter and parvovirus. Mice were given access to standard irradiated bacteria-free rodent chow (5053 Pico Lab, Purina Mills, Richmond, IN) and reverse osmosis water ad libitum.
The numbers of animals per group as well as the baseline information for each group (weight, % body fat, age, IGF-1 levels) are outlined in Table 1. To target IGF-1 production early in development, igf1f/f mice were crossed with mice expressing Cre recombinase under an albumin promoter, as previously described (Yakar et al. 1999). The albumin gene is induced in the liver between post-natal day 10 and 15, thereby decreasing effective IGF-1 production early after birth (mice termed liver IGF-1-deficient (LID10d)). To target IGF-1 production in adulthood, adeno-associated virus (AAV8) containing Cre recombinase under the hepatocyte-specific thyroxine binding globin (TBG) promoter was purchased from the University of Pennsylvania Viral Vector Core (Toth et al. 2014). At 5 or 15 months of age, igf1f/f mice were randomly assigned to treatment groups, anesthetized via intraperitoneal injection of 20 % ketamine/3 % xylazine, and given a retro-orbital injection of approximately 1.3 × 1010 viral particles of AAV8-TBG-Cre or AAV8-TBG-eGFP diluted in 100 μl of physiological saline (Toth et al. 2014). Mice were monitored in their home cage until they were fully recovered from anesthesia. No mice exhibited adverse side effects of viral treatment. Mice that received AAV8-TBG-Cre at 5 months of age were termed LID5m, and mice that received AAV8-TBG-Cre at 15 months of age were termed LID15m. Aged studies were performed when animals were 27 months of age. Wild-type C57/Bl6 male and female mice (3–4 months of age) were purchased from Jackson Laboratories to serve as young reference controls in the structural studies (n = 3–5 per group). At the time of analysis, the order in which the mice were harvested was randomized using a block design.
Table 1.
Reductions in IGF-1 influence body weight and trabecular bone late in life
| Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | LID10d | LID5m | LID15m | Control | LID10d | LID5m | LID15m | |
| n | 19 | 7 | 10 | 7 | 20 | 10 | 9 | 7 |
| Age (days) | 822.7 ± 0.9 | 823.7 ± 1.1 | 820.6 ± 1.9 | 826.3 ± 0.6 | 821.9 ± 1.1 | 823.6 ± 1.2 | 826.0 ± 0.4 | 825.7 ± 0.9 |
| Body weight (g) | 26.68 ± 0.35 | 23.44 ± 0.64* | 23.48 ± 0.64* | 24.20 ± 0.69* | 23.45 ± 0.33 | 18.77 ± 0.45* | 18.80 ± 0.46* | 17.47 ± 0.54* |
| % Body fat | 10.16 ± 0.58 | 8.56 ± 0.38 | 8.45 ± 0.37 | 9.89 ± .87 | 14.13 ± 0.58 | 12.97 ± 0.54 | 13.37 ± 0.56 | 13.24 ± 0.62 |
| IGF-1 levels (ng/ml) | 304.39 ± 6.38 | 46.23 ± 11.81* | 56.21 ± 10.41* | 53.55 ± 12.75* | 293.17 ± 8.03 | 56.09 ± 11.07* | 38.32 ± 11.67* | 44.64 ± 13.23* |
| Tibia length (cm) | 1.89 ± 0.04 | 1.75 ± 0.08 | 1.86 ± 0.04 | 2.02 ± 0.12 | 1.89 ± 0.04 | 1.74 ± 0.08 | 1.86 ± 0.04 | 2.00 ± 0.10 |
| Trabecular BV/TV | 0.05 ± 0.004 | 0.09 ± 0.008* | 0.07 ± 0.008 | 0.06 ± 0.009 | 0.04 ± 0.006 | 0.05 ± 0.010 | 0.06 ± 0.010 | 0.09 ± 0.098* |
| Tb N (1/mm) | 2.47 ± 0.09 | 3.37 ± 0.15* | 2.72 ± 0.16 | 2.86 ± 0.18 | 2.28 ± 0.25 | 2.23 ± 0.23 | 2.29 ± 0.23 | 3.10 ± 0.35 |
| Tb Th (mm) | 0.047 ± 0.001 | 0.044 ± 0.002 | 0.045 ± 0.002 | 0.041 ± 0.002 | 0.056 ± 0.002 | 0.046 ± 0.003* | 0.045 ± 0.003* | 0.052 ± 0.005 |
| Tb Sp (mm) | 0.42 ± 0.01 | 0.30 ± 0.02* | 0.39 ± 0.03 | 0.35 ± 0.03 | 0.57 ± 0.03 | 0.48 ± 0.05 | 0.47 ± 0.05 | 0.38 ± 0.08 |
Average age, body weight, percent body fat, IGF-1 levels, and trabecular bone measured in the tibia at 27 months of age
Sera analysis: Whole blood was isolated from the submandibular vein and sera were separated via low-speed centrifugation (2500×g for 20 min at 4 °C). IGF-1 concentrations in the sera were quantified using the Mouse IGF-1 Quantikine ELISA kit (R&D Systems, Minneapolis, MN) in duplicate following the manufacturer’s recommendation.
Bone microCT
As previously described (Mocsai et al. 2004; Torchia et al. 2001), the tibia was fixed in 4 % formaldehyde and 70 % ethanol and scanned using a high-resolution Scanco vivaCT 40μCT scanner (Scanco Medical, Bassersdorf, Switzerland) with a resolution of 10.5 μm. A total of 100 consecutive 10.5-μm-thick sections of cancellous bone were analyzed. The segmentation values were set at 0.8/1/210. Cortical bone analysis was done in the proximal tibiofibular junction, with a total of 30 consecutive 10.5-μm-thick sections semi-automatically evaluated. The segmentation values were set at 0.8/1/260. Three-dimensional reconstruction and structural parameter quantification were calculated using Scanco Medical software by a blinded researcher. Nomenclature for the microCT studies followed the previously established guidelines (Bouxsein et al. 2010).
Bone bending
Three-point-bend tests were performed on an MTS 858 Mini Bionix system (MTS Systems, Eden Prairie, MN). The tibiae were cleaned, wrapped in saline-moistened gauze, and frozen at −80 °C until shipment to the University of Alabama at Birmingham (UAB) Biomechanics Core. Bone length was quantified, and the bones were mounted such that primary loading occurred in the mediolateral direction. The length between the bottom two supports was recorded prior to each test. The rate of loading was set to 0.5 mm/s with a sampling rate of 100 Hz. Data were recorded using MTS Basic TestWare with relevant parameters being time, axial force, and deflection. The data were important into Excel for analysis, and the max force, stiffness, and max bending moment were calculated.
Statistics
Data were analyzed, and figures were generated using SigmaPlot version 11 (Systat Software, San Jose, CA). Each animal was a unit of analysis. One-way ANOVA with post hoc Bonferroni test was used to identify differences between the multiple treatment groups compared to aged and/or young controls. For ease of comparison, the three control groups (wild type, 5-month green fluorescent protein (GFP), and 15-month GFP) were pooled and labeled wild type (WT). No differences between the individual control groups were observed. A p value less than 0.05 was considered statistically significant. Data are expressed as mean ± SEM.
Results
General
In this study, a deficiency in circulating IGF-1 was induced early in life (LID10d), at 5 months of age (LID5m), or at 15 months of age (LID15m) as previously described (Ashpole et al. 2015). In these models, IGF-1 production in the liver is knocked down using Cre recombinase driven by a liver-specific promoter. The reduced levels of circulating IGF-1 are maintained throughout the life span, and levels at 27 months of age remain 30 % of that observed in the aged control mice (Table 1). Consistent with previous studies highlighting the anabolic effects of IGF-1 on body weight, the IGF-1-deficient male and female mice exhibited a significant reduction in body weight late in life (Table 1). The IGF-1-deficient mice had no significant change in the percent body fat, although there was a trending decrease in the early-life LID10d males (p = 0.2) and females (p = 0.16) (Table 1). Despite the reductions in body weight, there were no differences in tibia length compared to control male or female mice (Table 1).
Trabecular bone
The architecture of the trabecular bone was assessed just distal to the epiphyseal growth plate in the proximal tibial metaphysis using micro-CT. In male mice, early-life deficiency of IGF-1 (LID10d) resulted in a significant increase in the trabecular bone fraction with an accompanying increase in trabecular number and decrease in the spacing between trabeculae (Table 1). This effect was only observed in response to early-life deficiency of IGF-1 (LID10d); trabecular bone structure was not altered in LID5m or LID15m in males. The effects of IGF-1 deficiency on the trabecular bone in females differed from what was observed in the males. Within the females, early-life IGF-1 deficiency (LID10d) did not lead to changes in the trabecular bone fraction or trabecular number (Table 1). The LID10d females exhibited a significant decrease in trabecular thickness; however, the net effect on trabecular spacing was not significant. LID5m females also exhibited a significant decrease in thickness compared to aged control females with no change in trabecular spacing. In contrast, LID15m females exhibited significantly increased bone fraction and trabecular number with a corresponding decrease in trabecular spacing (Table 1). Together, these data are consistent with our previous findings that the effect of IGF-1 on trabecular bone structure is sex-specific and dependent on the age of onset of IGF-1 deficiency (Ashpole et al. 2015).
Cortical bone
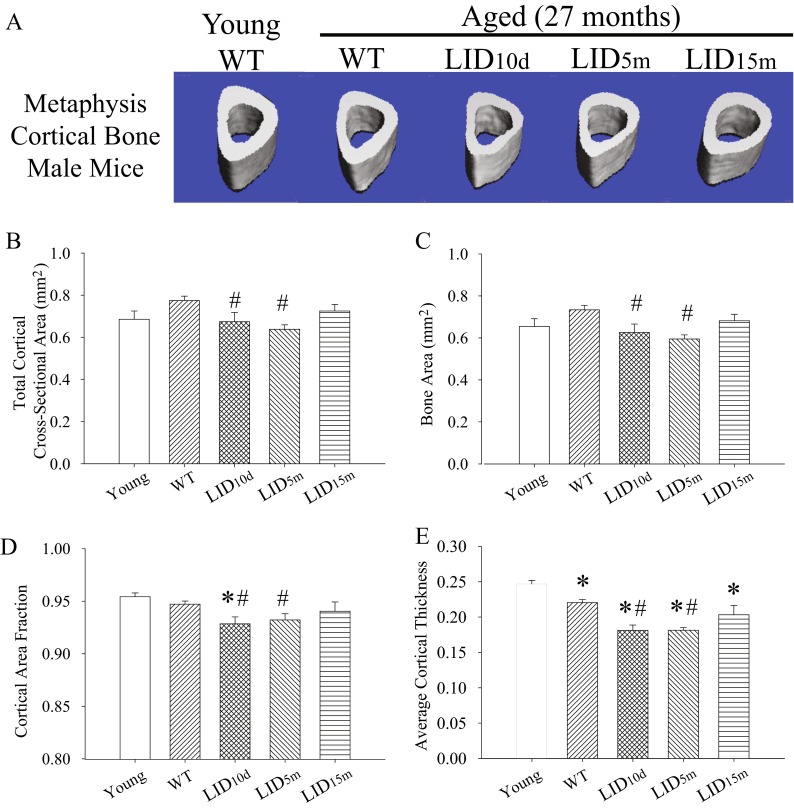
The structure of the cortical bone was quantified along the metaphysis distal to the tibiofibular junction using μCT. Young mice were included in this analysis to assess general age-related changes in structure. There was no effect of age in the cross-sectional area, bone area, and cortical bone fraction of control mice (Fig. 1a–d). However, aged male control animals did demonstrate a decrease in cortical thickness (Fig. 1e), which was even more pronounced in the IGF-1-deficient LID10d and LID5m groups (Fig. 1e). Decreased circulating IGF-1 in the male LID10d and LID5m groups also resulted in a significant reduction in cross-sectional area, bone area, and cortical bone fraction when compared to age-matched controls (Fig. 1a–d). IGF-1 deficiency induced later in adulthood (LID15m) did not alter cortical bone structure (Fig. 1a–e). These results indicate that levels of circulating IGF-1 during early and adult life are necessary for the maintenance of bone integrity later during the life span in male mice.
Fig. 1.
Early-life reductions in IGF-1 lead to decreased cortical bone structure in aged males. a Representative images of the cortical bone in the metaphysis of the tibia in young (n = 3), aged (n = 19), and aged IGF-1-deficient male mice (n = 6–7 per group). Average b cross-sectional area, c bone area, d cortical area fraction (BV/TV), and e cortical thickness in the metaphysis of the tibia in young, aged, and aged IGF-1-deficient male mice. The pound sign indicates a significant difference compared to wild-type controls at that time point, while the asterisk indicates a significant difference in the wild-type mice compared to the young 2-month time point, *#p < 0.05, mean ± SEM
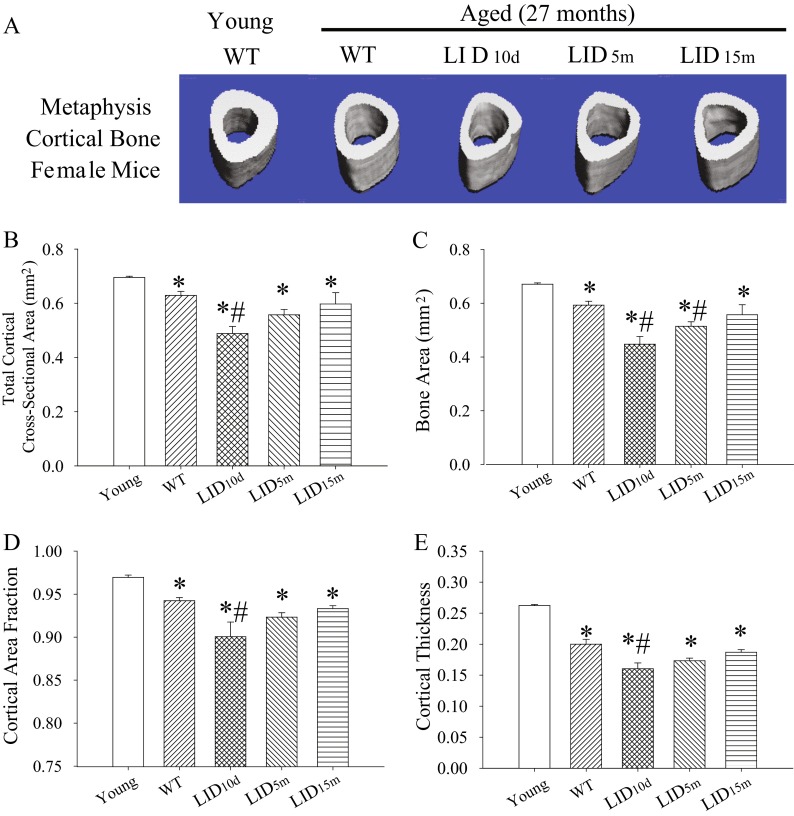
Aged female control mice exhibited a significant age-related reduction in the total cross-sectional area, bone area, cortical bone fraction, and cortical thickness compared to young animals (Fig. 2a–e). These decreases were further exacerbated in LID10d females (Fig. 2a–e). The LID5m females also exhibited a significant reduction in cortical bone area compared to aged controls (Fig. 2c); however, the differences in cortical bone fraction and cortical thickness in this group did not reach statistical significance (Fig. 2d, e). Consistent with the findings in male mice, reduced circulating levels of IGF-1 beginning at 15 months of age had no effect on aging cortical bone structure (Fig. 2a–e).
Fig. 2.
Early-life reductions in IGF-1 lead to decreased cortical bone structure in aged females. a Representative images of the cortical bone in the metaphysis of the tibia in young (n = 3), aged (n = 18), and aged IGF-1-deficient female mice (n = 5–7). Average b cross-sectional area, c bone area, d cortical area fraction (BV/TV), and e cortical thickness in the metaphysis of the tibia in young, aged, and aged IGF-1-deficient female mice. The pound sign indicates a significant difference compared to wild-type controls at that time point, while the asterisk indicates a significant difference in the wild-type mice compared to the young 2-month time point, *#p < 0.05, mean ± SEM
Mineral density
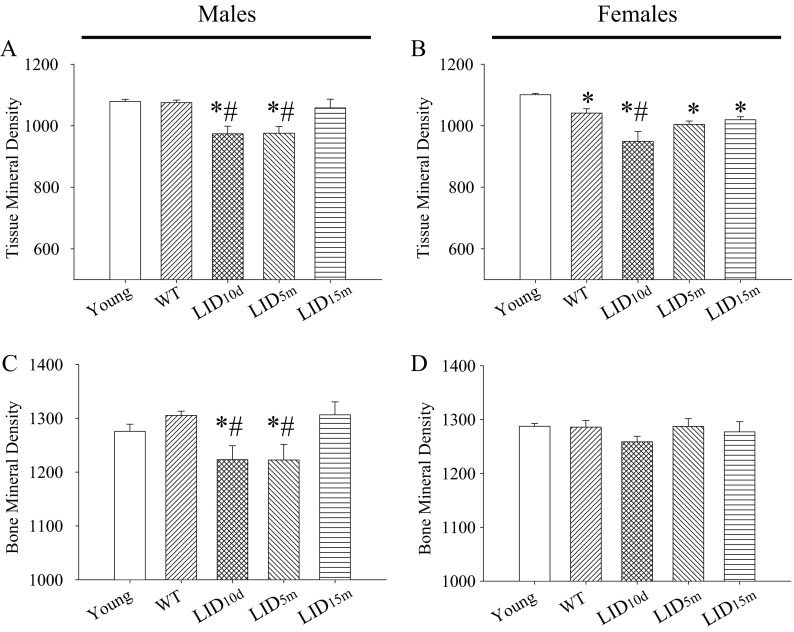
Tissue and bone mineral densities in the cortical bone were significantly decreased in aged LID10d and LID5m males (Fig. 3a, b). No differences were observed in the LID15m group (Fig. 3a, b), suggesting that early levels of circulating IGF-1 are important for establishing and/or maintaining mineral density in males. In females, tissue mineral density was decreased in all of the aged groups and mineral density was decreased further when IGF-1 deficiency was induced at 10 days of age (LID10d, Fig. 3c). No changes were observed in bone mineral density in any of the female treatment groups (Fig. 3d).
Fig. 3.
Early-life reductions in IGF-1 lead to decreased mineral density in aged mice. Average tissue mineral density in young (n = 3), aged (n = 18–19), and aged IGF-1-deficient a male and b female mice (n = 5–7). Average tissue mineral density in young, aged, and aged IGF-1-deficient c male and d female mice. The pound sign indicates a significant difference compared to wild-type controls at that time point, while the asterisk indicates a significant difference in the wild-type mice compared to the young 2-month time point, *#p < 0.05, mean ± SEM
Bone integrity
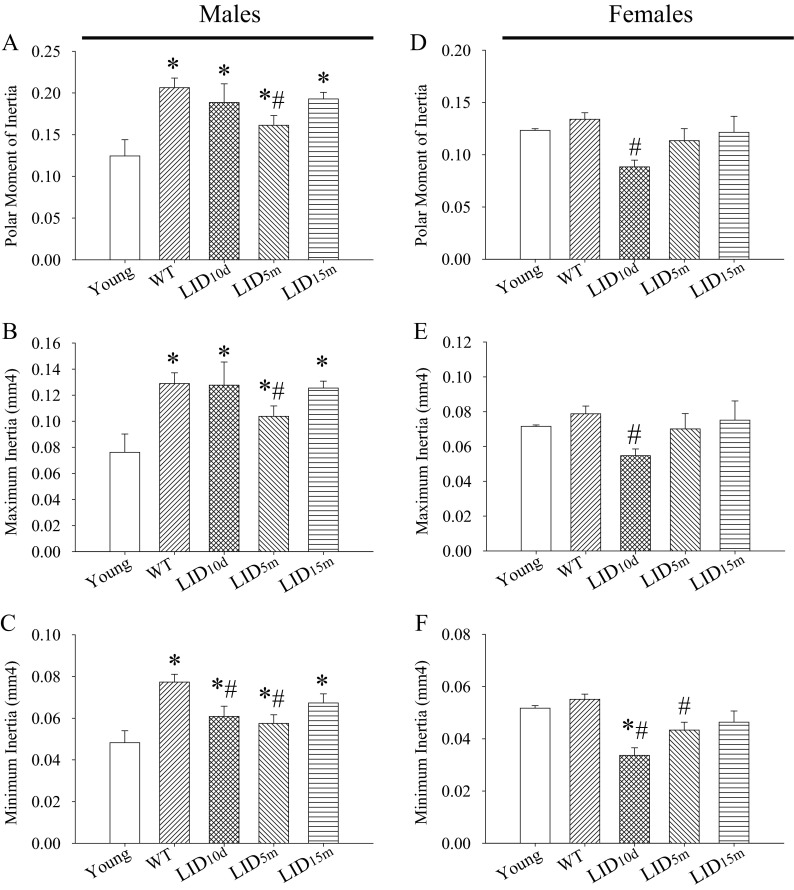
Alterations in cortical bone structure often underscore changes in geometric resistance to loading and torsion. Therefore, we estimated the geometric-based structural strength of these bones by calculating the moments of inertia from the μCT data. Male mice exhibited an age-related increase in polar moment of inertia as well as maximum and minimum moments of inertia with age (Fig. 4a–c). This finding is consistent with previous data showing that moments of inertia continue to develop past early adulthood in humans and mice (Brodt et al. 1999; Ruff and Hayes 1982; Willinghamm et al. 2010). The LID5m males exhibited a significant decrease in polar moment of inertia (Fig. 4a) as well as maximum moment of inertia (Fig. 4b). Both the LID10d and LID5m mice exhibited significant decreases in the minimum moments of inertia (Fig. 4c). No differences in these measures were observed in response to a reduction in IGF-1 at 15 months of age (LID15m).
Fig. 4.
IGF-1 influences moments of inertia in the tibia of aged mice. Average a polar moment of inertia, b maximum inertia, and c minimum inertia in the tibia of young, aged, and aged IGF-1-deficient male mice. Average d polar moment of inertia, e maximum inertia, and f minimum inertia in the tibia of young, aged, and aged IGF-1-deficient male mice. The pound sign indicates a significant difference compared to wild-type controls at that time point, while the asterisk indicates a significant difference in the wild-type mice compared to the young 2-month time point, *#p < 0.05, mean ± SEM
Aged female control mice showed no statistical differences in moments of inertia compared to young mice (Fig. 4d–f). However, the early-life LID10d mice exhibited a significant decrease in the polar moment of inertia (Fig. 4d) and maximum moment of inertia (Fig. 4e), consistent with the decrease in cortical bone structure and area observed in these mice (Fig. 2). No differences were observed in the LID15m female group (Fig. 4d, e). Similar to the males, female LID10d and LID5m groups exhibited a significant decline in minimum moment of inertia (Fig. 4f).
Bone strength
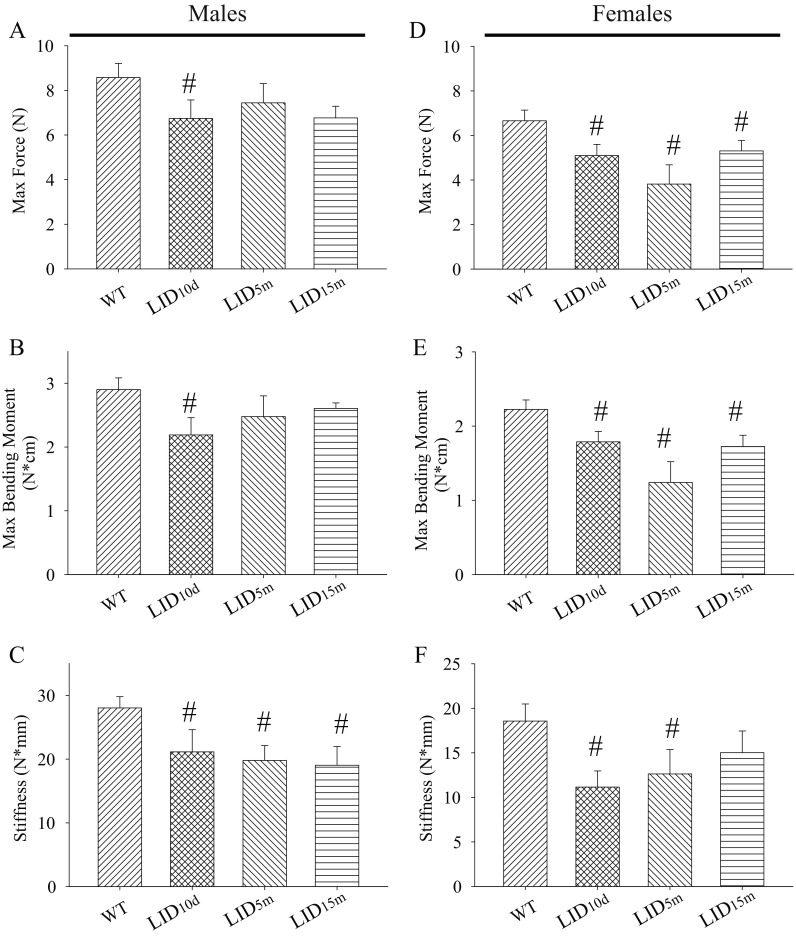
Decreases in the polar moment of inertia and bone mineral density within our IGF-1-deficient male mice predict a decrease in bone strength. Thus, we next assessed the biomechanical properties in these mice using a three-point bone-bending assay. The maximum force (N) applied before bone breaking and the maximum bending moment (N*cm) were significantly reduced in the LID10d male mice (Fig. 5a, b) indicating reduced bone strength in this group. There was no significant difference in the maximum force before breaking or the maximum bending moment in the male LID5m mice. These data diverge from the moment of inertia study that showed that LID5m mice exhibited the greatest deficiencies in geometric-based strength (Fig. 4), although in both cases, the mice deficient in IGF-1 before 15 months of age show trending decreases in geometric structure and strength. Interestingly, bone stiffness was reduced in all of the IGF-1-deficient male mice (Fig. 5c). Taken together, these data suggest that the loss of IGF-1 in male mice leads to inferior whole-bone mechanical properties in advanced age. Moreover, these detrimental effects on bone aging in males are most pronounced when IGF-1 is reduced early in life span.
Fig. 5.
Early-life reductions in IGF-1 result in decreased bone bending properties late in life. Average a force applied to the tibia before bone breaking, b maximum bending moment, and c stiffness in the tibia of wild-type (n = 12) and IGF-1-deficient male mice (n = 4–6 per group), as measured by three-point bending test. Average d force applied to the tibia before bone breaking, e maximum bending moment, and f stiffness in the tibia of wild-type (n = 10) and IGF-1-deficient female mice (n = 4–5 per group). The pound sign indicates a significant difference compared to wild-type controls at that time point, #p < 0.05, mean ± SEM
IGF-1 deficiency also led to alterations in bone bending characteristics in the tibias of female mice. The maximum force applied before bone breaking and the maximum bending moment were significantly reduced in all IGF-1-deficient groups (Fig. 5d, e), suggesting that a loss of IGF-1 at any point in female life span was detrimental to bone strength late in life. These data are consistent with the structural studies examining the moments of inertia in these bones (Fig. 4). Bone stiffness was also reduced in LID10d and LID5m animals (Fig. 5d). Together, these data indicate that circulating IGF-1 impacts tibia bone strength in advanced age and a loss of IGF-1 throughout life in females is detrimental to bone structure and strength.
Discussion
Advanced aging is associated with decreases in bone structure in humans, rats, and mice. The decrease in structure leads to increased risk of osteoporotic fractures—a complication faced by 8.9 million adults annually (50). Thus, understanding the mechanisms of bone aging is of critical importance. Our current study examined the effects of IGF-1 deficiency on age-related changes in the tibia. IGF-1 levels increase during adolescence and steadily decline in adulthood. While the age-associated decrease in IGF-1 has been reported to be beneficial for overall life span of several rodent species, it is closely associated with the loss of muscle and bone structure in humans (as reviewed by Sonntag et al. 2012). We recently reported that 27-month aged mice exhibit a reduction in circulating IGF-1 and a corresponding decrease in vertebral bone volume (Ashpole et al. 2015). We now report that in the tibia, aged WT mice exhibit significant reductions in several structural parameters including cortical bone fraction. Importantly, early-life reductions in IGF-1 exacerbated the age-related reductions in many of the observed bone phenotypes including a loss of geometric structure and reduced strength in advanced age. Together, our data suggest that reduced levels of circulating IGF-1 early in life are detrimental to the overall health of tibia bones in advanced age.
Previous studies have shown differential effects of IGF-1 on bone aging. In one study, early-life loss of IGF-1 (along with an important stabilizing protein) resulted in increased cortical thickness in advanced age (Courtland et al. 2013). In another, IGF-1 deficiency beginning in early adulthood resulted in reduced cortical thickness (Gong et al. 2014). The discrepancies in these studies could be associated with the use of different animal models and/or the different ages at which IGF-1 deficiency began. In our study, there were pronounced deficits in cortical bone fraction, tissue mineral density, and maximum force before bone breaking when IGF-1 deficiency began early in postnatal development (around 10 days) in both males and females. However, deficiencies beginning in late adulthood (15 months) induced far fewer alterations in bone structure and strength. Together, these data suggest that the time at which IGF-1 deficiency began had a dramatic influence on the phenotype of bone at 28 months of age. Mechanistically, the age-of-onset dependence could be related to the continued acquisition of peak bone mass into adulthood in murine models (as reviewed by Jilka 2013). Unlike humans, mice exhibit significant increases in bone growth following sexual maturity. Thus, growth factors like IGF-1 may continue influencing bone acquisition and remodeling after post-natal development. Future studies should examine whether these changes in cortical bone structure and strength in aged IGF-1-deficient mice are due to a failure to achieve equal peak bone mass or a differential loss of bone with age.
Several studies have highlighted sex-specific differences in bone aging, with females often showing enhanced bone loss compared to males (Riggs et al. 2004). Our data are consistent with these previous reports with aged WT females exhibiting significant decreases in cortical bone area, cortical bone fraction, and tissue mineral density, while males showed minimal alterations. Unlike WT mice, both male and female IGF-1-deficient mice exhibited a loss in cortical bone structure as well as tissue and bone mineral densities, suggesting that adequate levels of IGF-1 are necessary to maintain bone health with age in both sexes. Interestingly, early-life loss of IGF-1 resulted in increased trabecular bone in aged males, with no effect observed in females. We recently reported sex-specific effects on trabecular bone aging in the vertebrae (Ashpole et al. 2015), where we observed significant increased trabecular bone in the aged females, with no effect in the males. While the mechanistic differences between the effects of IGF-1 in the vertebrae and tibia are not understood, site-specific and compartment-specific changes in bone have been reported (Courtland et al. 2011; Eckstein et al. 2007; Glatt et al. 2007; Willinghamm et al. 2010); thus, it is likely that microenvironments within different bones may be differentially affected by IGF-1 in advanced age.
As a key growth signal in the body, IGF-1 levels are tightly regulated. Growth hormone (GH), released from the pituitary gland, activates the GH receptor within the liver, which responds by elevating the production and release of IGF-1. In addition, tissues like the bone are also able to produce and release IGF-1 locally in a paracrine/autocrine manner (Guntur and Rosen 2013). In the current study, we reduced IGF-1 production specifically in the liver (which accounts for ∼70 % of circulating IGF-1). It is possible that this robust reduction of circulating IGF-1 is compensated for by increased IGF-1 production in the bone itself. Moreover, due to neuroendocrine feedback mechanisms, IGF-1-deficient mice also exhibit significantly increased GH levels in circulation (Yakar et al. 2001). Since GH also regulates bone structure and strength (as reviewed by Yakar and Isaksson 2015), we cannot rule out the possibility that the observed changes within the structure of the bone are not influenced by alterations in GH or local IGF-1. While this is important to note, previous studies on the effects of local GH/IGF-1 signaling in the bone do not support the idea that increased GH/IGF-1 would result in decreased bone structure or function (Brennan-Speranza et al. 2011; Wu et al. 2013; Zhao et al. 2000).
It is possible that reductions in circulating IGF-1 alter the cellular populations that regulate bone turnover within cortical and trabecular bone, contributing to the loss of structure and function. As mentioned, developmental studies have shown that IGF-1 is important for the differentiation and maturation of several cell types in the bone, including chondrocytes and osteoblasts (Wang et al. 2006a, 2011, 2013; Xian et al. 2012). Mesenchymal stem cells, known to give rise to chondrocytes and osteoblasts (Krebsbach et al. 1999; Moerman et al. 2004; Pittenger et al. 1999), have the capacity to influence bone turnover and regeneration after embryonic development. For example, knock-out of the IGFR in the mesenchymal stem cells of adolescent mice (weeks 3–7) leads to a reduction in osteoblast number along the bone perimeter and an accompanying decrease in peak bone mass (Crane et al. 2013). This study suggests that IGF-1 signaling within the stem cell population remains an important regulator of bone cell maturation after birth. Considering this, reduced IGF-1 could influence bone structure throughout life span by altering the differentiation and maturation of stem cells.
One of the potential explanations for the loss of tibia structure and strength in the IGF-1-deficient mice is differential loading of these bones. Our study, along with several previous reports, highlights that mice with reduced circulating IGF-1 weigh significantly less than their control counterparts (Baker et al. 1993; Bikle et al. 2001; Liu et al. 1993; Wang et al. 2006b; Woods et al. 1996). Analysis in our current study indicates a strong correlation between body weight and IGF-1 levels at 28 months of age (R2 = 0.68). Considering that loading positively regulates bone structure and strength, IGF-1-deficient mice may have reduced biomechanical properties in bones because of the reduced loading over time. Our data indicates that the bones of the IGF-1-deficient mice are less capable of resisting force, potentially increasing the risk of fracture; however, it is important to consider that there is less weight-bearing strain placed on these bones throughout the life span. If maximum bending force and stiffness were normalized to body weight, the effect of IGF-1 deficiency would be diminished. While we recognize that the weight-bearing load may influence structure and strength, we are not able to accurately estimate the bone strain during activities of daily living that these mice encounter throughout their life span. Consequently, the influence of the reduced bone structure within the IGF-1-deficient mice on their overall risk of fracture could be debated.
It was recently reported that caloric restriction induces similar age-related effects within weight-bearing bones; cortical and trabecular bone aging is regulated by caloric restriction in a site-specific and length-of-restriction-dependent manner (Behrendt et al. 2015). These findings were similar to a previous report indicating that calorie restriction decreases cortical bone but not trabecular bone (Hamrick et al. 2008). Interestingly, caloric restriction results in decreased body weight and, in several models, decreased circulating IGF-1 (Behrendt et al. 2015; Dunn et al. 1997; Fontana et al. 2008; Hadem and Sharma 2015; Sonntag et al. 1999). It is of great interest that caloric restriction and IGF-1 deficiency induce similar phenotypes in the bone, as both treatments have been associated with increased life span in several studies (Carter et al. 2002; Ikeno et al. 2006; Richardson et al. 2004; Van Remmen et al. 2001). Together, these studies may suggest that increased life span may come at the expense of bone health. Nevertheless, the data presented here indicate that the effects of IGF-1 on tibia bone aging appear to be temporally regulated, microenvironment-dependent, and sex-specific. Along with the well-characterized effects of IGF-1 on bone development, these data highlight the complex relationship between IGF-1 signaling and bone.
Acknowledgments
The authors would like to thank Julie Farley and Joanna Hudson for their assistance isolating tissues and Dr. Timothy Griffin for his critical comments on the manuscript. This work was supported by NIH R01AG038747 to WES and the Donald W. Reynolds Foundation.
Author contributions
NMA, JCH, PNE, and ELH performed experiments. NA, JCH, SL, AY, MBH, and WES analyzed data and interpreted results. NMA prepared figures and drafted the manuscript. NMA, JCH, PNE, MBH, and WES edited/revised manuscript. NMA, JCH, PNE, MBH, and WES designed research.
Compliance with ethical standards
All procedures were approved by and followed the guidelines of the Institutional Animal Care and Use Committee and veterinarians at University of Oklahoma Health Sciences Center (OUHSC).
References
- Ashpole NM, et al. IGF-1 regulates vertebral bone aging through sex-specific and time-dependent mechanisms. J Bone Mineral Res: Off J Am Soc Bone Miner Res. 2015 doi: 10.1002/jbmr.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. doi: 10.1016/S0092-8674(05)80085-6. [DOI] [PubMed] [Google Scholar]
- Bando H, Zhang C, Takada Y, Yamasaki R, Saito S. Impaired secretion of growth hormone-releasing hormone, growth hormone and IGF-I in elderly men. Acta Endocrinol. 1991;124:31–36. doi: 10.1530/acta.0.1240031. [DOI] [PubMed] [Google Scholar]
- Behrendt AK, et al. Dietary restriction-induced alterations in bone phenotype: effects of lifelong versus short-term caloric restriction on femoral and vertebral bone in C57BL/6 mice. J Bone Miner Res: Off J Am Soc Bone Miner Res. 2015 doi: 10.1002/jbmr.2745. [DOI] [PubMed] [Google Scholar]
- Bikle D, et al. The skeletal structure of insulin-like growth factor I-deficient mice. J Bone Miner Res off J Am Soc Bone Miner Res. 2001;16:2320–2329. doi: 10.1359/jbmr.2001.16.12.2320. [DOI] [PubMed] [Google Scholar]
- Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res: Off J Am Soc Bone Miner Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- Brennan-Speranza TC, Rizzoli R, Kream BE, Rosen C, Ammann P. Selective osteoblast overexpression of IGF-I in mice prevents low protein-induced deterioration of bone strength and material level properties. Bone. 2011;49:1073–1079. doi: 10.1016/j.bone.2011.07.039. [DOI] [PubMed] [Google Scholar]
- Brodt MD, Ellis CB, Silva MJ. Growing C57Bl/6 mice increase whole bone mechanical properties by increasing geometric and material properties. J Bone Miner Res off J Am Soc Bone Miner Res. 1999;14:2159–2166. doi: 10.1359/jbmr.1999.14.12.2159. [DOI] [PubMed] [Google Scholar]
- Carter CS, Ramsey MM, Sonntag WE. A critical analysis of the role of growth hormone and IGF-1 in aging and lifespan. Trends Genet: TIG. 2002;18:295–301. doi: 10.1016/S0168-9525(02)02696-3. [DOI] [PubMed] [Google Scholar]
- Corpas E, Harman SM, Pineyro MA, Roberson R, Blackman MR. Continuous subcutaneous infusions of growth hormone (GH) releasing hormone 1-44 for 14 days increase GH and insulin-like growth factor-I levels in old men. J Clin Endocrinol Metabo. 1993;76:134–138. doi: 10.1210/jcem.76.1.8421077. [DOI] [PubMed] [Google Scholar]
- Courtland HW, Elis S, Wu Y, Sun H, Rosen CJ, Jepsen KJ, Yakar S. Serum IGF-1 affects skeletal acquisition in a temporal and compartment-specific manner. PLoS one. 2011;6:e14762. doi: 10.1371/journal.pone.0014762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtland HW, Kennedy OD, Wu Y, Gao Y, Sun H, Schaffler MB, Yakar S. Low levels of plasma IGF-1 inhibit intracortical bone remodeling during aging. Age. 2013;35:1691–1703. doi: 10.1007/s11357-012-9469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JL, Zhao L, Frye JS, Xian L, Qiu T, Cao X. IGF-1 Signaling is essential for differentiation of mesenchymal stem cells for peak bone mass. Bone Res. 2013;1:186–194. doi: 10.4248/BR201302007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn SE, Kari FW, French J, Leininger JR, Travlos G, Wilson R, Barrett JC. Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer Res. 1997;57:4667–4672. [PubMed] [Google Scholar]
- Eckstein F, Matsuura M, Kuhn V, Priemel M, Muller R, Link TM, Lochmuller EM. Sex differences of human trabecular bone microstructure in aging are site-dependent. J Bone Miner Res: the Off J Am Soc Bone Miner Res. 2007;22:817–824. doi: 10.1359/jbmr.070301. [DOI] [PubMed] [Google Scholar]
- Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008;7:681–687. doi: 10.1111/j.1474-9726.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillberg P, Olofsson H, Mallmin H, Blum WF, Ljunghall S, Nilsson AG. Bone mineral density in femoral neck is positively correlated to circulating insulin-like growth factor (IGF)-I and IGF-binding protein (IGFBP)-3 in Swedish men. Calcif Tissue Int. 2002;70:22–29. doi: 10.1007/s002230020048. [DOI] [PubMed] [Google Scholar]
- Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J Bone Miner Res: Off J Am Soc Bone Miner Res. 2007;22:1197–1207. doi: 10.1359/jbmr.070507. [DOI] [PubMed] [Google Scholar]
- Gong Z, et al. Reductions in serum IGF-1 during aging impair health span. Aging Cell. 2014;13:408–418. doi: 10.1111/acel.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govoni KE, Lee SK, Chung YS, Behringer RR, Wergedal JE, Baylink DJ, Mohan S. Disruption of insulin-like growth factor-I expression in type IIalphaI collagen-expressing cells reduces bone length and width in mice. Physiol Genom. 2007;30:354–362. doi: 10.1152/physiolgenomics.00022.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govoni KE, Wergedal JE, Florin L, Angel P, Baylink DJ, Mohan S. Conditional deletion of insulin-like growth factor-I in collagen type 1alpha2-expressing cells results in postnatal lethality and a dramatic reduction in bone accretion. Endocrinology. 2007;148:5706–5715. doi: 10.1210/en.2007-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntur AR, Rosen CJ (2013) IGF-1 regulation of key signaling pathways in bone. Bonekey Rep 2:437. doi:10.1038/bonekey.2013.171 [DOI] [PMC free article] [PubMed]
- Hadem IK, Sharma R (2015) Age- and tissue-dependent modulation of IGF-1/PI3K/Akt protein expression by dietary restriction in mice. Horm Metab Res = Hormon- und Stoffwechselforschung = Hormones et metabolisme. doi:10.1055/s-0035-1559770 [DOI] [PubMed]
- Hamrick MW, Ding KH, Ponnala S, Ferrari SL, Isales CM. Caloric restriction decreases cortical bone mass but spares trabecular bone in the mouse skeleton: implications for the regulation of bone mass by body weight. J Bone Miner Res: Off J Am Soc Bone Miner Res. 2008;23:870–878. doi: 10.1359/jbmr.080213. [DOI] [PubMed] [Google Scholar]
- Ikeno Y, Lew CM, Cortez LA, Webb CR, Lee S, Hubbard GB. Do long-lived mutant and calorie-restricted mice share common anti-aging mechanisms?—a pathological point of view. Age. 2006;28:163–171. doi: 10.1007/s11357-006-9007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen JA, Burger H, Stolk RP, Grobbee DE, de Jong FH, Lamberts SW, Pols HA. Gender-specific relationship between serum free and total IGF-I and bone mineral density in elderly men and women. Eur J Endocrinol/Eur Fed Endocr Soc. 1998;138:627–632. doi: 10.1530/eje.0.1380627. [DOI] [PubMed] [Google Scholar]
- Jilka RL. The relevance of mouse models for investigating age-related bone loss in humans. J Gerontol A Biol Sci Med Sci. 2013;68:1209–1217. doi: 10.1093/gerona/glt046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebsbach PH, Kuznetsov SA, Bianco P, Robey PG (1999) Bone marrow stromal cells: characterization and clinical application. Crit Rev Oral Biol Med: Off Publ Am Assoc Oral Biol 10:165–181 [DOI] [PubMed]
- Kubota T, et al. Insulin-like growth factor-1 receptor in mature osteoblasts is required for periosteal bone formation induced by reloading. Acta Astronaut. 2013;92:73–78. doi: 10.1016/j.actaastro.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurland ES, et al. Insulin-like growth factor-I in men with idiopathic osteoporosis. J Clin Endocrinol Metab. 1997;82:2799–2805. doi: 10.1210/jcem.82.9.4253. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rosen CJ, Visser M, Hannan MT, Harris T, Wilson PW, Kiel DP. Association between insulin-like growth factor I and bone mineral density in older women and men: the Framingham Heart Study. J Clin Endocrinol Metab. 1998;83:4257–4262. doi: 10.1210/jcem.83.12.5308. [DOI] [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- Liu JM, et al. IGF-1 as an early marker for low bone mass or osteoporosis in premenopausal and postmenopausal women. J Bone Miner Metab. 2008;26:159–164. doi: 10.1007/s00774-007-0799-z. [DOI] [PubMed] [Google Scholar]
- Mocsai A, et al. The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc NatL Acad Sci U S A. 2004;101:6158–6163. doi: 10.1073/pnas.0401602101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004;3:379–389. doi: 10.1111/j.1474-9728.2004.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamad MI, Khater MS. Evaluation of insulin like growth factor-1 (IGF-1) level and its impact on muscle and bone mineral density in frail elderly male. Arch Gerontol Geriatr. 2015;60:124–127. doi: 10.1016/j.archger.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Niblock MM, Brunso-Bechtold JK, Lynch CD, Ingram RL, McShane T, Sonntag WE. Distribution and levels of insulin-like growth factor I mRNA across the life span in the Brown Norway x Fischer 344 rat brain. Brain Res. 1998;804:79–86. doi: 10.1016/S0006-8993(98)00645-3. [DOI] [PubMed] [Google Scholar]
- Paccou J, Dewailly J, Cortet B. Reduced levels of serum IGF-1 is related to the presence of osteoporotic fractures in male idiopathic osteoporosis. Joint, Bone, Spine: Revue Du Rhumatisme. 2012;79:78–82. doi: 10.1016/j.jbspin.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Rhee EJ, et al. Age, body mass index, current smoking history, and serum insulin-like growth factor-I levels associated with bone mineral density in middle-aged Korean men. J Bone Miner Metab. 2004;22:392–398. doi: 10.1007/s00774-003-0500-0. [DOI] [PubMed] [Google Scholar]
- Richardson A, Liu F, Adamo ML, Van Remmen H, Nelson JF. The role of insulin and insulin-like growth factor-I in mammalian ageing. Best Pract Res Clin Endocrinol Metab. 2004;18:393–406. doi: 10.1016/j.beem.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Riggs BL, et al. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res: Off J Am Soc Bone Miner Res. 2004;19:1945–1954. doi: 10.1359/jbmr.040916. [DOI] [PubMed] [Google Scholar]
- Ruff CB, Hayes WC. Subperiosteal expansion and cortical remodeling of the human femur and tibia with aging. Science. 1982;217:945–948. doi: 10.1126/science.7112107. [DOI] [PubMed] [Google Scholar]
- Sjogren K, et al. Effects of liver-derived insulin-like growth factor I on bone metabolism in mice. J Bone Miner Res: Off J Am Soc Bone Miner Res. 2002;17:1977–1987. doi: 10.1359/jbmr.2002.17.11.1977. [DOI] [PubMed] [Google Scholar]
- Smith CP, et al. Relationship between insulin, insulin-like growth factor I, and dehydroepiandrosterone sulfate concentrations during childhood, puberty, and adult life. J Clin Endocrinol Metab. 1989;68:932–937. doi: 10.1210/jcem-68-5-932. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Cefalu WT, Ingram RL, Bennett SA, Thornton PL, Khan AS. Pleiotropic effects of growth hormone and insulin-like growth factor (IGF)-1 on biological aging: inferences from moderate caloric-restricted animals. J Gerontol A Biol Sci Med Sci. 1999;54:B521–B538. doi: 10.1093/gerona/54.12.B521. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Csiszar A, de Cabo R, Ferrucci L, Ungvari Z. Diverse roles of growth hormone and insulin-like growth factor-1 in mammalian aging: progress and controversies. J Gerontol A Biol Sci Med Sci. 2012;67:587–598. doi: 10.1093/gerona/gls115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto T, Nishiyama K, Kuribayashi F, Chihara K. Serum levels of insulin-like growth factor (IGF) I, IGF-binding protein (IGFBP)-2, and IGFBP-3 in osteoporotic patients with and without spinal fractures. J Bone Miner Res: Off J Am Soc Bone Miner Res. 1997;12:1272–1279. doi: 10.1359/jbmr.1997.12.8.1272. [DOI] [PubMed] [Google Scholar]
- Szulc P, Joly-Pharaboz MO, Marchand F, Delmas PD. Insulin-like growth factor I is a determinant of hip bone mineral density in men less than 60 years of age: MINOS study. Calcif Tissue Int. 2004;74:322–329. doi: 10.1007/s00223-003-0090-9. [DOI] [PubMed] [Google Scholar]
- Torchia EC, Stolz A, Agellon LB. Differential modulation of cellular death and survival pathways by conjugated bile acids. BMC Biochem. 2001;2:11. doi: 10.1186/1471-2091-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, et al. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab. 2014;34:1887–1897. doi: 10.1038/jcbfm.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Remmen H, Guo Z, Richardson, A (2001) The anti-ageing action of dietary restriction. Novartis Foundation Symposium 235:221–230; discussion 230-223 [DOI] [PubMed]
- Wang Y, Nishida S, Elalieh HZ, Long RK, Halloran BP, Bikle DD. Role of IGF-I signaling in regulating osteoclastogenesis. J Bone Miner Res: the Off J Am Soc Bone Miner Res. 2006;21:1350–1358. doi: 10.1359/jbmr.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, et al. Insulin-like growth factor-I is essential for embryonic bone development. Endocrinology. 2006;147:4753–4761. doi: 10.1210/en.2006-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, et al. IGF-1R signaling in chondrocytes modulates growth plate development by interacting with the PTHrP/Ihh pathway. J Bone Miner Res off J Am Soc Bone Miner Res. 2011;26:1437–1446. doi: 10.1002/jbmr.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Bikle DD, Chang W. Autocrine and paracrine actions of IGF-I signaling in skeletal development. Bone Res. 2013;1:249–259. doi: 10.4248/BR201303003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willinghamm MD, Brodt MD, Lee KL, Stephens AL, Ye J, Silva MJ. Age-related changes in bone structure and strength in female and male BALB/c mice. Calcif Tissue Int. 2010;86:470–483. doi: 10.1007/s00223-010-9359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods KA, Camacho-Hubner C, Savage MO, Clark AJ. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene the New England. J Med. 1996;335:1363–1367. doi: 10.1056/NEJM199610313351805. [DOI] [PubMed] [Google Scholar]
- Wu Y, et al. Serum IGF-1 is insufficient to restore skeletal size in the total absence of the growth hormone receptor. J Bone Miner Res off J Am Soc Bone Miner Res. 2013;28:1575–1586. doi: 10.1002/jbmr.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian L, et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med. 2012;18:1095–1101. doi: 10.1038/nm.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Isaksson O. Regulation of skeletal growth and mineral acquisition by the GH/IGF-1 axis: lessons from mouse models. Growth Horm IGF Res: Off J Growth Horm Res Soc Int IGF Res Soc. 2015 doi: 10.1016/j.ghir.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, et al. Liver-specific igf-1 gene deletion leads to muscle insulin insensitivity. Diabetes. 2001;50:1110–1118. doi: 10.2337/diabetes.50.5.1110. [DOI] [PubMed] [Google Scholar]
- Yakar S, et al. Serum IGF-1 determines skeletal strength by regulating subperiosteal expansion and trait interactions. J Bone Miner Res off J Am Soc Bone Miner Res. 2009;24:1481–1492. doi: 10.1359/jbmr.090226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, et al. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277:44005–44012. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- Zhao G, et al. Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: increased trabecular bone volume without increased osteoblast proliferation. Endocrinol. 2000;141:2674–2682. doi: 10.1210/endo.141.7.7585. [DOI] [PubMed] [Google Scholar]