Abstract
The present study examines whether non-active older adults are more dependent on visual information when executing aiming movements and whether age-related declines in proprioception play a mediating role herein. Young (N = 40) and older adults (N = 38) were divided into physically active and non-active subgroups based on self-reported sports participation levels. In experiment 1, participants executed wrist-aiming movements with and without visual feedback. In experiment 2, passive proprioceptive acuity was assessed using wrist motion detection and position matching tests. Results showed similar aiming accuracy across age groups both with and without visual feedback, but older adults exhibited longer movement times, prolonged homing-in phase, and made more corrective submovements. Passive proprioceptive acuity was significantly affected by physical activity level and age, with participants in the active group scoring better than their non-active peers. However, these declines did not predict performance changes on the aiming task. Taken together, our observations suggest that decline in proprioceptive acuity did not predict performance changes on the aiming task and older adults were able to compensate for their decreased motion and position sense when allowed sufficient time. In line with these observations, we proposed that older adults are able to compensate for their decline in proprioception by increasing their reliance on predictive models.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-016-9908-z) contains supplementary material, which is available to authorized users.
Keywords: Aging, Physical activity, Motor control, Visual feedback, Proprioception, Aiming performance
Introduction
According to the multiple-process model of limb control (Elliott et al. 2010), manual aiming movements generally consist of two consecutive phases: a primary movement and a homing-in phase. The primary movement corresponds to the initial pulse toward the vicinity of target. Although this preprogrammed movement phase is traditionally associated with open-loop control (Woodworth 1899), recent work has shown that vision is being used more continuously for the planning and control of limb movements (i.e., impulse control; see also Heath 2005; Khan et al. 2002, 2006; Saunders and Knill 2003). While corrections may occur very early in the movement, the main body of closed-loop control takes place during the homing-in phase. Herein, visual and proprioceptive feedback is used in the final movement phase to correct for any spatial discrepancy between hand and target positions (i.e., limb-target control; see Elliott et al. 2010). This final movement phase (homing-in) is characterized by one or more corrective submovements that guide the hand to the target’s position.
With respect to aging, both the duration and relative distance covered by the primary movement and the corrective (secondary) submovements appear to be affected. Older adults adapt their manual aiming behavior by making shorter amplitude primary movements (Lyons et al. 1996). This outcome is considered to reflect an increased dependence on limb-target control (Lyons et al. 1996; Seidler-Dobrin and Stelmach 1998) which possibly allows older adults to achieve the same level of endpoint accuracy as young adults (Boisseau et al. 2002; Ketcham et al. 2002; Lyons et al. 1996; Welsh et al. 2007). The increased dependence on limb-target control in older adults may perhaps be explained by a decline in proprioceptive acuity (Adamo et al. 2007; Adamo et al. 2009; Herter et al. 2014; Kokmen et al. 1978; Wright et al. 2011; see Goble et al. 2009, for a review), impaired efficiency of feedback processing (Rand and Stelmach 2011; Stelmach et al. 1988; Temprado et al. 2013; Van Halewyck et al. 2015b), or both. Indeed, well-documented evidence show that age-related declines in proprioceptive acuity has led many to suggest that visual feedback becomes increasingly important during accurate aiming in older age (e.g., Coats and Wann 2011; Rand and Stelmach 2011; Terrier et al. 2011). This view has been challenged, nonetheless, by evidence showing that older adults are not disadvantaged to a greater extent when online visual feedback is withdrawn (e.g., Chaput and Proteau 1996; Lyons et al. 1996; Seidler-Dobrin and Stelmach 1998). To explain this unexpected finding, Lyons et al. (1996) argued that older adults make better use of kinesthetic feedback for the control of goal-directed movement. They also suggested that a physically active lifestyle may be important in maintaining the performance of older adults.
The effects of age and physical activity level on manual aiming behavior and eye-hand coordination have been examined recently (Van Halewyck et al. 2014, 2015a). In these studies, both hand and eye movements were analyzed during performance of manual aiming in physically active and non-active older adults. Observations from these studies clearly showed that, compared to their younger peers, older adults produced slower and less forceful primary movements that undershot the target. They also made a higher number of hand trajectory corrections and corrective saccades in the homing-in phase. These observations were in line with findings from previous studies (Boisseau et al. 2002; Ketcham et al. 2002; Lyons et al. 1996; Pratt et al. 1994; Welsh et al. 2007) where similar adaptations of aiming behavior by older adults have been reported. The effects of physical activity level on aiming behavior appeared, nonetheless, to be inconsistent. On one hand, evidence from the study of Van Halewyck et al. (2015a) and others (Berchicci et al. 2014; Capranica et al. 2004; Cortis et al. 2009) indicated that, compared to active older adults, non-active older individuals tended to be slower and, generally, adapt their motor behavior during performance of the manual aiming task. On the other hand, findings from a second study by Van Halewyck et al. (2014) showed no obvious group differences in movement characteristics between active and non-active older adults, suggesting that the impact of physical activity level on aiming behavior was less distinct than the impact of age. However, an alternative explanation could be that older adults (in particular older adults with impaired proprioceptive acuity) adapt their control strategy to maintain endpoint accuracy in the absence of visual feedback. The question remains whether changes in manual aiming behavior and eye-hand coordination as a function physical activity level and age could be explained by differences in proprioceptive acuity between active and non-active older individuals.
To date, the impact of age and physical activity level on proprioception and manual aiming has been explored in relative isolation. To bridge this gap, two experiments have been conducted. In experiment 1, active and non-active young and older adults performed aiming movements with their preferred wrist, with and without visual feedback. In a second experiment (experiment 2), proprioceptive acuity was assessed in a position and motion sense task. To the best of our knowledge, this is the first time that the effects of both age and physical activity level on both aiming behavior and proprioceptive acuity have been explored in a single study. As previous studies have revealed an impact of physical activity on proprioceptive acuity (Adamo et al. 2009; Wright et al. 2011), we expected to observe an age-related decline in all proprioceptive acuity measures, particularly among the non-active older adults. In line with the prediction that an increased reliance on vision serves as a compensatory strategy for declines in proprioception (e.g., Ghez et al. 1995), the withdrawal of visual feedback was expected to increase aiming error. Accordingly, visual feedback may be particularly important for non-active older adults, as their proprioception may be reduced to an even greater extent compared to their physically active peers. Healthy active older adults were expected to preserve their proprioceptive acuity, suggesting that they may be less dependent on availability of visual feedback than non-active or sedentary older adults and thus will show fewer aiming errors in the absence of visual feedback (i.e., when visual information of the ongoing aiming movement and knowledge of results are removed). If the elimination of visual feedback results in older adults aiming with lower endpoint accuracy than young controls (hypothesis 1), our findings would be consistent with the theory of an increased dependence on vision in older age. Based on our previous research (Van Halewyck et al. 2014), we nevertheless expected all groups to achieve similar levels of endpoint accuracy with and without visual feedback (hypothesis 2). These competing hypotheses were addressed in experiment 1. As proprioception traditionally consists of motion (i.e., the ability to perceive limb movements) and position sense (i.e., the ability to identify a static limb position) (Herter et al. 2014; Proske and Gandevia 2012; Sherrington 1907), we anticipated that participants with poorer proprioceptive acuity (experiment 2) would also demonstrate poorer endpoint accuracy in the absence of visual feedback (experiment 1). Given the beneficial effect of physical activity on brain functions such as sensory processing, motor planning, and sensorimotor integration (Jacobs et al. 2011; Nagamatsu et al. 2012; Smith et al. 2011; Voelcker-Rehage et al. 2011; for a review, see Voelcker-Rehage and Niemann 2013), the association between declines in proprioceptive acuity and lower endpoint accuracy was expected to be more pronounced in non-active than active older adults (hypothesis 3).
Methods
Participants
Forty young adults (age range 20.0–26.7 years) and 40 older adults (60.0–72.3 years) were recruited for the study. Participants in both age groups were subdivided based on self-reported sports participation levels, using the Modified Baecke Questionnaire (Baecke et al. 1982; Voorrips et al. 1991). The active young group consisted of 20 young adults who reported at least 5 h per week of sports specifically training the upper limb during the past year (badminton, tennis, or squash) and at least 3 h of weekly upper limb training for the active older group. Participants in the non-active groups were 40 age- and gender-equated young (n = 20) and older (n = 20) volunteers who self-reported not to be involved in any sports over the past year. One active older adult was excluded from the study because she did not score 27 out of 30 on a mini-mental state examination (Folstein et al. 1975). Also, one non-active older adult was excluded because she did not achieve the maximum score on a 5.07/10-g Semmes-Weinstein monofilament test, indicating the start of peripheral neuropathy (Weinstein 1993). All remaining 78 participants included in the study reported to have normal or corrected-to-normal vision and were right-handed as they scored 50 or more on the Edinburgh Handedness Questionnaire (Oldfield 1971). They reported to be of average or better health on a 5-point scale. Working memory and fine motor skills were considered intact, as all participants scored within the expected boundaries on a digit symbol substitution test, a subtest from the Wechsler Adult Intelligence Scale (Wechsler 1997), and met the age- and gender-dependent criteria for the Nine-Hole Pegboard Test (Mathiowetz et al. 1985; Oxford Grice et al. 2003). Details of the participant’s demographic characteristics and scores of the aforementioned tests are provided in Supplementary Materials (Table S1). The study was approved by the Medical Ethics Committee of the KU Leuven and was conducted in accordance with the 1964 Declaration of Helsinki. Written informed consent was obtained from all participants prior to the experiment.
Apparatus
Manual aiming task
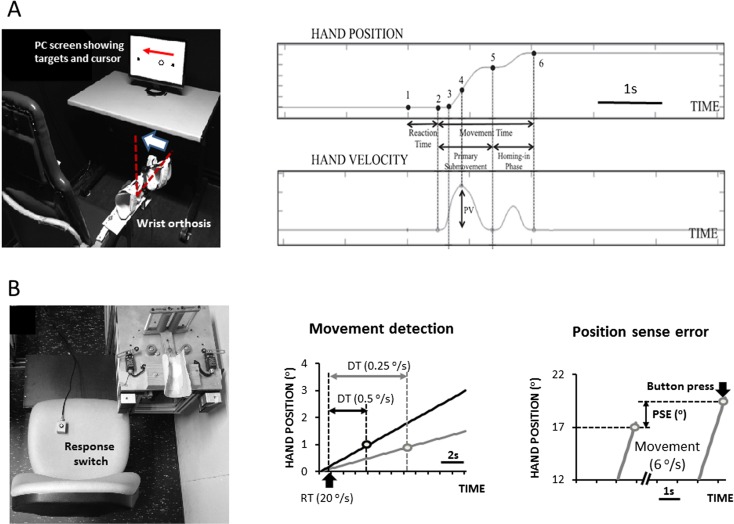
The apparatus used for the assessment of manual aiming (Fig. 1a) was identical to the one used in previous studies (Van Halewyck et al. 2014, 2015a, 2015b). Participants sat in a comfortable chair and wore a wrist hand orthosis on the preferred right forearm. The axis of the orthosis was aligned with the anatomical axis of the wrist joint and positioned in a way that the hand could only move in the horizontal plane. A high-precision shaft encoder (accuracy 0.006°) with sampling frequency of 250 Hz was attached onto the orthosis. Wrist angular position was presented as a 1.5-cm diameter circular cursor on a 60-cm computer monitor, which was located at a standardized distance of 125 cm in front of the participant at eye level. Two fixed square targets (width 1 cm, positioned 18 cm apart) appeared on the monitor in all blocks. The task consisted of moving the cursor from the right target to the left target. The aiming movement had an index of difficulty (ID) of 6.2 bits (ID = log2[2 × 18 / (1.5 − 1)]; Fitts 1954) in line with previous work from our group (Van Halewyck et al. 2014, 2015b).
Fig. 1.
a Test setup and schematic overview of the dependent variables in experiment 1, showing hand movement characteristics. b Test setup and schematic overview of the dependent variables in experiment 2, showing movement detection time (DT) and position sense error (PSE)
Proprioceptive acuity task
The apparatus used for the assessment of motion and position sense consisted of a motor-driven manipulandum (handpiece) and a forearm rest at the right-hand side and a press button at the left-hand side (Fig. 1b). Passive movements of the right wrist were induced by means of an AC servo motor (AMK DV764, Goedhard PMC, Helmond, NL) that was coupled to the rotating shaft of the manipulandum via a 1:10 redactor (Alpha Gearbox, Type LP120). The motor generated a continuous motion with programmable amplitude and duration to allow wrist rotation from −30° (flexion) to +30° (extension) relative to a 0° neutral position with the forearm and palmar hand surface aligned. A shaft encoder (accuracy 0.088°) was mounted underneath the manipulandum and was used to record the angular displacement of the wrist. The press button was held in the participant’s left hand. By pressing the button, participants could signal perceiving a wrist flexion movement (cfr. motion sense) or reaching a specific position (cfr. position sense). Both manipulandum and press button data were sampled at 1000 Hz (Signal software 4.0, Cambridge Electronic Design, Cambridge, UK) and stored for offline analysis.
Procedure
The aiming movement task (experiment 1) and the proprioceptive tasks (experiment 2) were performed on the same day. The order of the experiments was counterbalanced within groups: Half of the participants in each group performed the manual aiming task first (experiment 1), and the other half performed the proprioceptive acuity task first (experiment 2). Within subgroups of ten young participants (or nine older participants) in experiment 2, five started the motion detection task test whereas the remaining participants started with the position sense task. The participant’s maximum grip strength was assessed using a digital hand-grip dynamometer (Takei A5401, Tokyo, Japan) prior to the beginning of experiment 1 (or experiment 2 if conducted first). Grip strength was assessed while participants stood in an upright position with the right arm stretched downwards. They were instructed to make the strongest grip possible during three contractions, each separated by a 1-min break. The largest maximal voluntary contraction of each individual was used for further analysis (for group means, see Table S1 in Supplementary Materials).
Manual aiming task
All participants (40 young adults and 38 older adults) performed two familiarization blocks in which the aiming movement was practiced under two feedback conditions (VISION and NO VISION). In the VISION familiarization blocks, participants were instructed to position the cursor around the right (black) target at the start of each movement trial. They were instructed to move the cursor to the left by making a wrist flexion movement and surround the left target as fast and accurately as possible as soon as the target turned from black to red (GO stimulus). Participants were asked to return to the right target upon movement completion and prepare for the next GO stimulus. This sequence was repeated eight times (trials) per block. The interval between two consecutive GO stimuli varied randomly between 7000, 7500, 8000, and 8500 ms to avoid movement anticipation. The same procedure was repeated during the familiarization block of the NO VISION condition. In this case, the first two movement trials were conducted with the motion of cursor visible on the screen. For the remaining six trials, the cursor disappeared at the onset of the movement (i.e., cursor moved away from the right target edges) and reappeared when the cursor was returned to its original position (i.e., surrounding the right target) prior to the onset of the next trial. The participants were allowed to see the two target squares at all times and were instructed to start each aiming movement from the same home position.
Participants practiced the VISION familiarization block before practicing the NO VISION familiarization block. Thereafter, eight VISION and eight NO VISION experimental blocks were performed in a randomized and counterbalanced order. Participants were asked to complete eight test blocks with eight aiming movement trials per block. The first two aiming movement trials in each test block were not included in the analysis. To prevent participants from memorizing the final end position over blocks, the required amplitude of the wrist movement was alternated between blocks. Specifically, wrist movements were 17° flexion for “uneven” blocks and a 24° flexion for “even” blocks. We chose to test large amplitudes because they are more sensitive to age-related effects (Boisgontier and Swinnen 2015). As displacement of the curser was proportionally adjusted to cover the same distance between targets on the screen, the ID of each aiming movement remained identical throughout the experiment. In total, participants executed 64 aiming movements in each visual condition of which 48 were consistently used for data analysis.
Proprioceptive acuity task
Participants were the same as for experiment 1. Motion detection and the position sense task were presented in two separate blocks. For both motion detection and position sense trials, the manipulandum was positioned parallel to the sagittal axis at the start of each trial with the right wrist inserted in a neutral position (i.e., the forearm and the palmar surface of the hand were aligned). This position was defined as 0°.
In the motion detection task, the wrist was passively moved toward wrist flexion after receiving a verbal “GET READY” warning command. Movements were conducted at two different speeds that were repeated six times each: a 0.50°/s flexion movement and a 0.25°/s flexion movement, resulting in 12 test trials. Participants were instructed to press the button as soon as they perceived the wrist flexion. To prevent participants from pressing the button before perceiving the flexion movement, six extension and six sham trials were added. Here, the wrist made a 0.25°/s movement toward wrist extension or stayed in the neutral position respectively. Participants were instructed not to press the button should they not perceive wrist flexion. The order of the 24 trials was randomized. The motion detection task always ended with six trials of a simple reaction time task in which the motor induced an obvious right wrist flexion movement (20°/s). For the simple reaction time task, participants were informed beforehand that no extension or sham trials could occur and were instructed to press the button as soon as they felt the flexion movement.
In the position matching task, participants received verbal GET READY warning command after which the right wrist was passively moved toward a position of 10°, 17°, or 24° wrist flexion within 1 s (i.e., at speeds of 10°/s, 17°/s, or 24°/s, respectively). Participants were instructed to memorize this position. After standing still for 3 s, the manipulandum returned to the neutral position within 1 s and consequently started moving toward 30° wrist flexion at a speed of 6°/s. Participants were instructed to press the button when they reached the memorized position. The three different positions to be memorized (10°, 17°, and 24°) were provided six times in a randomized order to prevent participants from memorizing end positions over trials.
Dependent variables
Manual aiming task
In line with previous work (Van Halewyck et al. 2014, 2015a, 2015b), a first-order, low-pass Butterworth filter with a cutoff frequency of 20 Hz was applied on the hand position data prior to the calculation of the dependent variables (Lavrysen et al. 2007, 2008, 2012; Van Halewyck et al. 2015a). Filtered data were differentiated twice to obtain instantaneous velocity and acceleration profiles. Dependent variables of hand trajectory were hand reaction time (RT), hand movement time (MT), peak velocity (PV), relative distance of the primary movement end (PE), relative duration of homing-in (DHI), number of trajectory corrective submovements (nCS), and aiming error (AE). Schematic overview of the dependent variables is provided in Fig. 1a (right-hand panel). Reaction time was defined as the time interval between the onset of the GO stimulus and the movement initiation (first sample after the onset of the GO stimulus when the standard deviation of the velocity profile was superior to 0.75 mm/s for 80 ms (Van Halewyck et al. 2014, 2015a, 2015b). Movement time was defined as the time interval between movement initiation and termination (first sample after the movement initiation when the standard deviation of the velocity profile was inferior to 0.75 mm/s for 80 ms). The exact duration to reach peak velocity and the end of the primary movement were identified, and the magnitude of peak velocity and the end of the primary were calculated. The end of the primary movement was determined as the first zero-crossing after peak acceleration, based on the criterion of Khan et al. (1998). Next, we calculated the distance covered by the primary movement relative to the overall distance between both targets (relative distance primary movement) as well as the duration of the primary movement relative to the entire movement time (relative duration primary movement). The number of movement trajectory corrections was defined as the number of acceleration and deceleration pairs (two zero-line crossings) in the filtered acceleration profile in the homing-in phase of the movement (Ketcham et al. 2002). Finally, aiming error was defined as the exact unsigned distance between the middle of the left target and the middle of the cursor position at movement termination and was expressed as the percentage of the total distance between the two targets.
Proprioceptive acuity task
In line with previous work from our group (Alaerts et al. 2007; Boisgontier et al. 2014), the angular displacement signals of the right (passive moved) wrist were low-pass filtered (second-order Butterworth with a cutoff frequency of 8 Hz). Schematic overview of the dependent variables is provided in Fig. 1b (right-hand panel). For the motion sense task, the percentage of correct responses and the time needed to perceive wrist flexion movements (detection time) were calculated for each passive movement speed. To correct for the well-documented increases in older adults’ reaction times, the participant’s average reaction time (i.e., detection time obtained in the 20°/s flexion condition) was subtracted from the detection time in the 0.25°/s flexion and 0.50°/s flexion conditions. For the position sense task, position sense error was defined as the absolute difference between target wrist position at the end of the passive movement trial (i.e., 10°, 17°, or 24° wrist flexion) and the wrist position at the onset of button press. Individual differences in reaction time were corrected using the same method as described above for motion sense. The abovementioned dependent variables were then computed for each participant. Individual estimates of detection time from all corrected responses were aggregated across trials with the same movement speed. Individual estimates of position sense errors were obtained by averaging the absolute differences between the actual and target wrist position at end of the passive movement trial for all target positions.
Data analyses
A custom-written MATLAB script (MathWorks, Natick, MA) was used to compute the means and standard deviations of all dependent variables. Thirteen of 78 participants that initially participated in experiment 1 and experiment 2 were excluded from statistical analyses due to missing data in one or more sets of task conditions and/or dependent variables. Data for final analyses were obtained from 17 active young adults, 16 non-active young adults, 17 active older adults, and 15 non-active older adults. All statistical analyses were performed with STATISTICA (version 8.0, StatSoft Inc., Tulsa, OK).
Manual aiming task
Three-way 2 × 2 × 2 [AGE × PHYSICAL ACTIVITY LEVEL (PAL) × FEEDBACK] analyses of variance (ANOVA) were performed on manual aiming data from experiment 1. The results of the ANOVAs are summarized in Table 1. When ANOVAs revealed significant effects (i.e., main effect/interaction p < 0.05), false discovery rate (FDR) analysis was applied to test comparisons of interest (e.g., Boisgontier et al. 2014). In line with our research questions in experiment 1, we compared (1) performance differences as function of age and physical activity level between the four groups (i.e., young active, young non-active, older active, and older non-active) and (2) differences between VISION and NO VISION feedback conditions within each groups. The resulting p values were interpreted at the FDR-corrected threshold of significance. In contrast to the Bonferroni correction which controls the rate of false positives among all tests whether or not the null hypothesis is actually rejected, FDR controls for the proportion of incorrect rejections of the null hypothesis among those tests for which the null hypothesis is rejected (Benjamini and Hochberg 1995; Curran-Everett 2000). Moreover, FDR offers an objective way to select thresholds that is automatically adaptive across large data sets (e.g., Genovese et al. 2002). Results are displayed as group mean score ± standard error of the mean (SEM).
Table 1.
Results of the 2 × 2 × 2 ANOVAs showing F values for aiming errors (AEs) and hand movement characteristics in experiment 1
| Dependent variables | RT | MT | PV | PE | DHI | nCS | AE |
|---|---|---|---|---|---|---|---|
| AGE | 7.98** | 6.69* | <1 | 1.81 | 22.4*** | 11.8*** | <1 |
| PAL | 5.11* | <1 | 2.12 | <1 | <1 | <1 | <1 |
| FB | 83.4*** | 81.4*** | 199*** | 112*** | 7.80** | 56.5*** | 280*** |
| AGE × PAL | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| AGE × FB | 1.02 | <1 | <1 | <1 | 2.72 | <1 | <1 |
| PAL × FB | 3.69 | 1.07 | <1 | <1 | <1 | <1 | <1 |
| AGE × PAL × FB | <1 | <1 | 1.22 | 1.30 | <1 | <1 | <1 |
Level of significance was set at p < 0.05. Significant main effects and interactions are indicated in a bolded font. For all significant main effects and interactions: ηp2 (partial eta-squared) ≥ 0.08
AGE age group, PAL physical activity level, FB feedback conditions, RT hand reaction time, MT hand movement time, PV peak velocity, PE relative distance of primary movement end, DHI relative duration of homing-in, nCS number of corrective submovements
*p < 0.05
**p < 0.01
***p < 0.001
Proprioceptive acuity task
To compare the proportion of correct responses between age and physical activity level groups, chi-squared analyses were performed for each movement condition (i.e., 0.50°/s flexion, 0.25°/s flexion, 0.25°/s extension, and sham) separately. Next, group differences in detection times (0.25°/s flexion and 0.50°/s flexion conditions) and position sense errors were analyzed with a 2 × 2 ANOVA [AGE × PAL]. The results of the 2 × 2 ANOVA are summarized in Table 2. Resulting p values for multiple comparisons were interpreted at the FDR-corrected threshold of significance. Results are displayed as group mean score ± standard error of the mean (SEM).
Table 2.
Results of the 2 × 2 ANOVAs for proprioceptive acuity (experiment 2)
| Dependent variable | Detection time at 0.25°/s flexion | Detection time at 0.50°/s flexion | Motion sense error |
|---|---|---|---|
| AGE | 10.1** | 14.6*** | 6.99* |
| PAL | 7.59** | 6.53* | 8.69** |
| AGE × PAL | <1 | 3.20 | 2.01 |
F values are presented for detection times at 0.25°/s and 0.50°/s flexion conditions and motion sense errors. Level of significance was set at p < 0.05. Significant main effects and interactions are indicated in a bolded font. For all significant main effects and interactions: ηp2 (partial eta-squared) ≥ 0.10
AGE age group, PAL physical activity level
*p < 0.05
**p < 0.01
***p < 0.001
Correlations among dependent variables across groups and feedback conditions (regression analyses)
The relationships between aiming strategy, aiming errors, and proprioception as function of AGE and PAL and FEEDBACK conditions were studied, regarding (1) reciprocal interactions among hand movement characteristics, (2) interactions between hand movement characteristics and aiming errors, (3) relationships between motion/position sense and aiming errors, and (4) relationships between motion/position sense and hand movement characteristics. Correlation coefficients (Pearson’s R) for (1) and (2) were obtained by regressing manual aiming data (experiment 1) on each other. Correlation coefficients (Pearson’s R) for (3) and (4) were calculated by regressing manual aiming data (experiment 1) on movement detection and position sense data (i.e., detection time at the 0.25°/s flexion and 0.50°/s flexion conditions (experiment 2)). Regression analyses were performed on data from each AGE/PAL group and each of the feedback conditions, separately. For all regression analyses, level of significant was set at p < 0.01 (uncorrected p values).
Results
Effects of age and physical activity level on manual aiming
Aiming errors
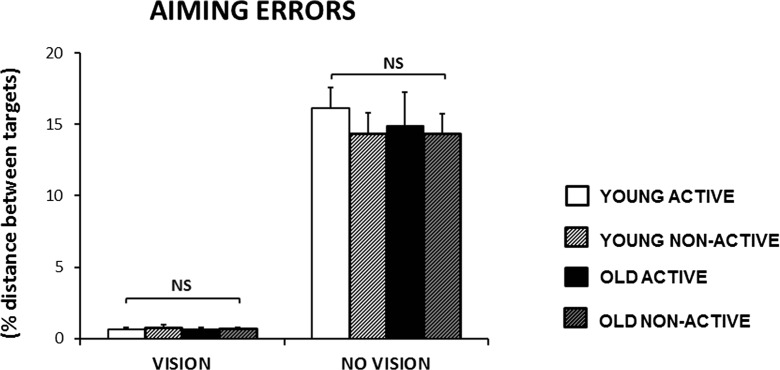
All groups reached similar levels of endpoint accuracy in the VISION and NO VISION condition (Fig. 2). Only the main effect for FEEDBACK was significant [F(1,61) = 280.00, p < 0.001], suggesting that aiming errors were significantly higher in the absence of visual feedback in all four groups [t tests, within group comparisons: all, p ≤ 0.001]. FDR threshold for significance: p = 0.013.
Fig. 2.
Overview of group scores (mean ± SEM) for aiming errors in the VISION and NO VISION conditions
Reaction time
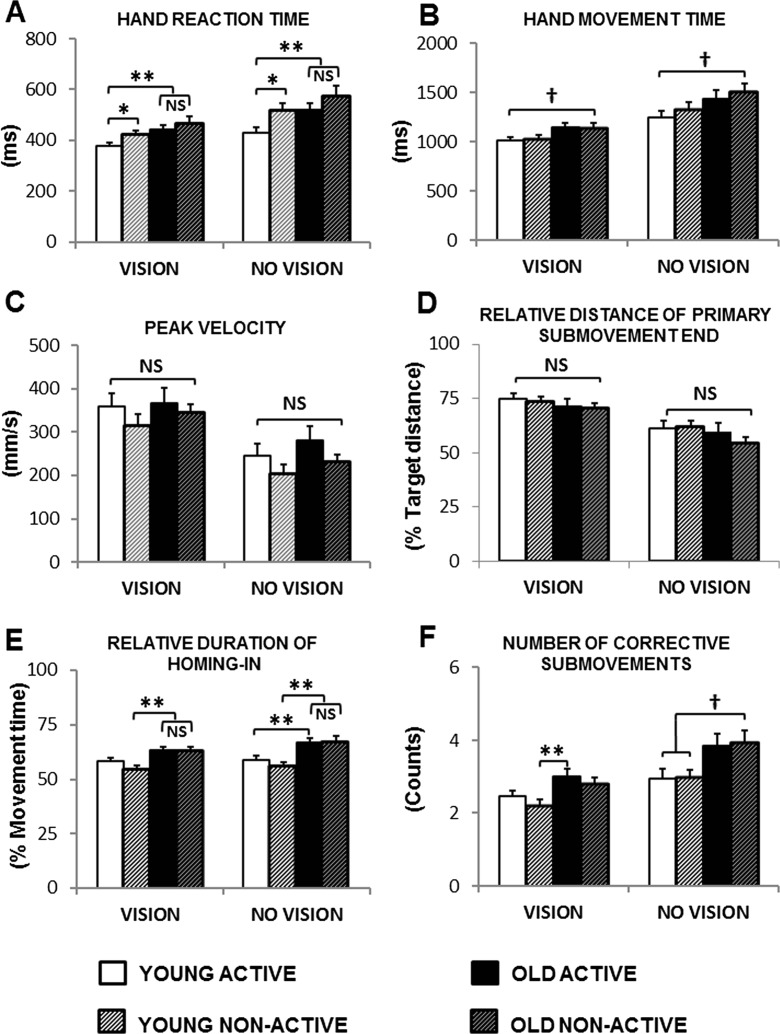
For all four groups, reaction times were significantly shorter in the VISION than in the NO VISION conditions [main effect for FEEDBACK: F(1,64) = 83.40, p < 0.001] irrespective of AGE and PAL [t tests, within group comparisons: all, p ≤ 0.001; false discovery rate threshold at p = 0.031]. The main effects of AGE [F(1,61) = 7.98, p < 0.01] and PAL [F(1,61) = 5.11, p < 0.05] were significant, but the AGE × PAL interaction was not [F(1,61) < 1]. The PAL × FEEDBACK interaction approached conventional levels of significance [F(1,61) = 3.69, p = 0.06]. For both feedback conditions, movement initiation was significantly fastest in young active and slowest in older non-active with participants in the young non-active and old active groups positioned in between (Fig. 3a). Hand reaction times in the VISION condition were 379 ± 12 ms (young active) vs. 423 ± 15 ms (young non-active), 444 ± 15 ms (older active), and 467 ± 26 ms (older non-active) [t tests, comparisons between young active group and the three remaining groups: all p ≤ 0.027]. Hand reaction times in the NO VISION condition were 432 ± 18 ms (young active) vs. 517 ± 27 ms (young non-active), 521 ± 27 ms (older active), and 572 ± 44 ms (older non-active) [t tests, comparisons between young active group and the three remaining groups: all p ≤ 0.013]. No significant differences were observed among RTs of the young non-active, older active, and older non-active: neither in the VISION nor in the NO VISION conditions [t test, between group comparisons: all, p > 0.1]. FDR threshold for significance: p = 0.031.
Fig. 3.
Overview of group scores (mean ± SEM) for hand movement characteristics in the VISION and NO VISION conditions. Significant main effects of age, physical activity level, and feedback conditions were observed only for reaction time (a). For the remaining hand movement characteristics, significant main effect for age were observed for movement time (b), relative duration of homing-in (e), and number of corrective submovements (f); see Table 1 for details. Significant differences are highlighted by an asterisk (if p < 0.05) or double asterisk (if p < 0.01). Corrections for multiple comparisons were made by using the false discovery rate procedure (Curran-Everett 2000). Marginal differences and/or trends (i.e., critical di ≤ p < 0.05) are highlighted by a dagger (see text for explanation)
Movement time
The main effect of AGE was significant [F(1,61) = 6.69, p < 0.05], indicating that older adults needed more time to perform the aiming movements than younger adults (Fig. 3b). Participants needed more time to perform the aiming movements in the NO VISION than in the VISION condition as indicated by the significant main effect of FEEDBACK [F(1,61) = 81.40, p < 0.001]. Significant differences between the two feedback conditions were observed for all four groups [t tests, within group comparisons: all, p ≤ 0.001], but no group differences were observed within each feedback condition [t tests, between group comparisons for VISION and NO VISION: all p ≥ 0.031]. FDR threshold for significance: p = 0.016.
Peak velocity and relative distance of primary movements
Only the main effect for FEEDBACK was significant [both, F(1,61) ≥ 112.00, p < 0.001]. Peak velocity was significantly higher in the VISION than in the NO VISION conditions in all four groups (Fig. 3c), and participants in all groups made shorter primary movements in the NO VISION as compared to the VISION conditions (Fig. 3d) [t tests, within group comparisons: all, p ≤ 0.001]. FDR threshold for significance: both, p = 0.013.
Relative duration of homing-in
The main effects of AGE was significant [F(1,61) = 22.50, p < 0.001], suggesting that older adults showed longer homing-in durations (i.e., spent more time homing-in) than young adults (Fig. 4e). The main effect for FEEDBACK was significant [F(1,61) = 7.60, p < 0.01], showing longer homing-in durations in the NO VISION as compared to the VISION conditions. However, significant differences in homing-in durations between the two feedback conditions were found only for the active older adults [t tests, within group comparisons: p = 0.013; for the remaining groups: all, p ≥ 0.044]. For the VISION condition, significant differences as function of age were found between non-active young (54.3 ± 1.8 % of total movement time) and active (63.4 ± 1.3 %) and non-active (62.9 ± 1.8 %) older adults [t tests, between group comparisons: both p ≤ 0.002]. For the NO VISION condition, significant differences as function of age were found between active young (58.6 ± 2.3 %) and active older adults (66.9 ± 1.8 %) and between non-active young (55.8 ± 2.1 %) and the active and non-active (67.1 ± 2.7 %) older adults [t tests, between group comparisons: all, p ≤ 0.008]. Interestingly, differences in homing-in duration between active young and active/non-active older adults (for VISION) or between active young and non-active older adults (for NO-VISION) did not reach the level of significance [t tests, between group comparisons: all, p ≥ 0.022]. FDR threshold for significance: p = 0.019.
Fig. 4.
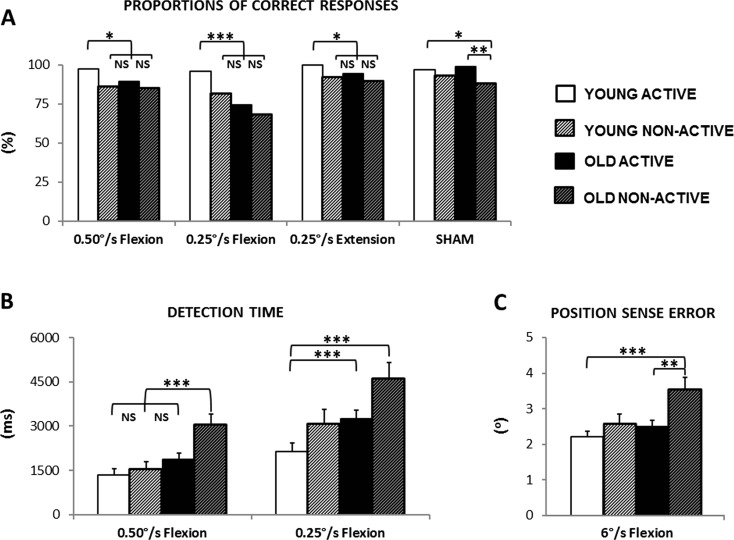
Proportions of correct responses (a) and overview of group scores (mean ± SEM) for motion sense characteristics (b) and position errors (c) in experiment 2. Significant main effects of age and/or physical activity level were observed for all dependent parameters; see Table 2 for details. Significant differences are highlighted by an asterisk (if p < 0.05) or double asterisk (if p < 0.01), triple asterisk (for p < 0.001). Corrections for multiple comparisons were made by using the false discovery rate procedure (Curran-Everett 2000). Marginal differences and/or trends (i.e., critical di ≤ p < 0.05) are highlighted by a dagger (see text for explanation)
Number of corrective submovements
Older adults made more corrective submovements than young adults [main effects of AGE: F(1,61) = 11.80, p < 0.001], and the number of trajectory corrections in the VISION condition was significantly lower than those observed in the NO VISION condition [main effect of FEEDBACK: F(1,61) = 56.50, p < 0.001] (Fig. 3f). Significant differences in the number of trajectory corrections as function of feedback condition were found for young non-active [2.21 ± 0.16 (VISION) vs. 2.96 ± 0.23 (NO VISION)], older active [3.01 ± 0.19 (VISION) vs. 3.85 ± 0.34 (NO-VISION)], and older non-active [2.78 ± 0.18 (VISION) vs. 3.92 ± 0.34 (NO VISION); t tests, within group comparisons: all p ≤ 0.002] but not for young active [2.46 ± 0.15 (VISION) vs. 2.96 ± 0.27 (NO VISION): t tests, within group comparisons: p = 0.019]. Significant differences in the number of trajectory corrections as function of age were observed only between young non-active (2.21 ± 0.16) and older active (3.01 ± 0.19) in the VISION condition [t tests, between group comparisons: p = 0.003 (otherwise, all p ≥ 0.025)]. FDR threshold for significance: p = 0.013.
Effects of age and physical activity level on proprioceptive acuity
Percentage of correct responses
Results are illustrated in Fig. 4a. In all test conditions where passive movement was applied (i.e., 0.25°/s flexion, 0.50°/s flexion, and 0.25°/s extension), participants of active young group demonstrated significantly higher proportions of correct responses than participants of the remaining three groups [chi-squared: all p ≤ 0.011]. Differences among the three remaining groups did not reach significance [chi-squared: all p ≥ 0.039]. In the sham condition, significant differences were observed only between active and non-active groups with the former demonstrating higher proportions of correct responses than the latter [chi-squared: young active vs. non-active (p = 0.020), older active vs. non-active (p = 0.003)]. FDR threshold for significance: p = 0.025.
Detection time
Young adults needed less time to detect wrist flexion movements as compared to older adults whereas active participants showed shorter detection time than non-active participants (Fig. 4b). Significant main effect of AGE [F(1,61) ≥ 6.03] and PAL [F(1,61) ≥ 7.59] were found for both the 0.25°/s flexion condition and the 0.50°/s flexion condition (all p ≤ 0.016), but the AGE × PAL interaction was not significant [both 0.25°/s and 0.50°/s conditions: F(1,61) < 0.3, p > 0.6]. For the 0.50°/s condition, non-active older showed significantly longer detection time (3036 ± 380 ms) than active young (1334 ± 215 ms), non-active young (1538 ± 253 ms), and active older (1881 ± 207 ms). For the 0.25°/s condition, significant differences in detection times were found between the active young (2138 ± 297 ms) and the two older groups: active (3251 ± 297) and non-active (4598 ± 549 ms) [t tests, between group comparisons for 0.50°/s and 0.25°/s conditions: all p ≤ 0.012 (otherwise, all p ≥ 0.033)]. FDR threshold for significance: p = 0.025.
Position sense
The main effects of AGE [F(1,61) = 6.99] and PAL [F(1,61) = 8.69] were significant (both p ≤ 0.01), indicating that young and active adults made smaller matching errors than older and non-active adults, respectively (Fig. 4c). No significant AGE × PAL interaction was observed [F(1,61) = 2.01, p > 0.1]. Significant differences in position sense errors were found between non-active older (3.54 ± 0.34°) and the two active groups: young (2.22 ± 0.15°) and older (2.51 ± 0.18°) [t tests, between group comparisons: both p ≤ 0.009 (otherwise, all p ≥ 0.032)]. FDR threshold for significance: p = 0.017.
Correlations among dependent variables across groups and feedback conditions
Reciprocal interactions among hand movement characteristics resulted in multiple significant correlations between the variables examined (Table 3). Otherwise, significant correlations among the remaining dependent variables were scarce. Therefore, results of the remaining regression analyses are presented in Supplementary Materials: Table S2 (correlations between hand movement characteristics and aiming errors), Table S3 (correlations between motion/position sense and aiming errors), and Table S4 (correlations between motion/position sense and hand movement characteristics).
Table 3.
Results of regression analyses for reciprocal correlations between hand movement characteristics in VISION (L-side) and NO VISION (R-side)
| VISION | NO VISION | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RT | MT | PV | PE | DHI | RT | MT | PV | PE | DHI | |
| Young active (n = 17) | ||||||||||
| RT | ||||||||||
| MT | 0.32 | 0.30 | ||||||||
| PV | −0.56* | −0.75 | −0.52 | −0.77 | ||||||
| PE | −0.30 | −0.85 | 0.83 | −0.27 | −0.88 | 0.80 | ||||
| DHI | −0.49 | 0.21 | 0.33 | −0.23 | −0.26 | 0.46 | 0.04 | −0.52 | ||
| nCS | −0.27 | 0.48 | 0.10 | −0.37 | 0.91 | 0.10 | 0.87 | −0.37 | −0.72 | 0.79 |
| Young non-active (n = 16) | ||||||||||
| RT | ||||||||||
| MT | 0.06 | 0.47 | ||||||||
| PV | −0.34 | −0.65 | −0.54 | −0.85 | ||||||
| PE | 0.01 | −0.78 | 0.68 | −0.38 | −0.83 | 0.74 | ||||
| DHI | −0.33 | 0.30 | 0.41 | −0.32 | 0.10 | 0.54* | −0.32 | −0.78 | ||
| nCS | −0.13 | 0.54* | 0.14 | −0.49 | 0.90 | 0.30 | 0.79 | −0.54* | −0.85 | 0.78 |
| Older active (n = 17) | ||||||||||
| RT | ||||||||||
| MT | 0.61 | 0.65 | ||||||||
| PV | −030 | −0.78 | −0.58 | −0.83 | ||||||
| PE | −0.36 | −0.80 | 0.90 | −0.50 | −0.89 | 0.83 | ||||
| DHI | 0.46 | 0.50 | −0.02 | −0.35 | 0.43 | 0.69 | −0.35 | −0.66 | ||
| nCS | 0.50 | 0.87 | −0.50 | −0.62 | 0.72 | 0.54 | 0.88 | −0.60* | −0.83 | 0.68 |
| Older non-active (n = 15) | ||||||||||
| RT | ||||||||||
| MT | −0.09 | 0.24 | ||||||||
| PV | −0.27 | −0.57 | −0.63* | −0.50 | ||||||
| PE | −0.05 | −0.19 | 0.49 | 0.07 | −0.76 | 0.40 | ||||
| DHI | −0.36 | 0.41 | 0.11 | −0.52 | −0.29 | 0.66 | 0.00 | −0.88 | ||
| nCS | −0.36 | 0.73 | −0.17 | −0.44 | 0.87 | −0.06 | 0.84 | −0.18 | −0.90 | 0.91 |
Correlation coefficients (Pearson’s R) at p ≤ 0.01 are indicated in a bolded font and underlined
RT hand reaction time, MT hand movement time, PV peak velocity, PE relative distance of primary submovement end, DHI relative duration of homing-in, nCS number of corrective submovements, n group size
*p > 0.01
Reciprocal relationships among hand movement characteristics
Correlation coefficients (Pearson’s R values) are summarized in Table 3. In general, participants in the young active, young non-active, and older active groups showed similar patterns of reciprocal relationships between hand movement characteristics of the primary submovement during VISION and NO VISION conditions: (1) significant negative correlations between MT and PV and MT and relative distance of PE [R ≤ −0.65; all, p ≤ 0.007 (uncorrected)] and (2) significant positive correlations between PV and PE [R ≥ 0.68; all p ≤ 0.006 (uncorrected)] were observed in all three groups. This was not the case for the older non-active group. Specifically, significant negative correlation was found only between MT and PE in the NO VISION condition [R = −0.76, p < 0.001 (uncorrected)]. Otherwise, correlations between PV and PE (as well as PV and MT) were not significant [both VISION and NO VISION: all |R| ≤ 0.57, p ≥ 0.027 (uncorrected)], suggesting suboptimal ability to control peak velocity in older non-active.
With respect to limb-target regulation (i.e., during homing-in), significant positive correlations were found between DHI and nCS in the VISION as well as in the NO VISION conditions for all four groups [all R ≥ 0.68, p ≤ 0.003 (uncorrected)], suggesting that the duration of the homing-in phase was proportional to the number of trajectory corrections. Significant positive correlations were found between MT and nCS for both older active and non-active subgroups in the VISION condition [both R ≥ 0.73, p ≤ 0.002 (uncorrected)] and for all four groups in the NO VISION condition [all ≥0.78, p < 0.001 (uncorrected)]. Interestingly, significant positive correlations between MT and DHI were observed only for the two older subgroups (i.e., active and non-active) in the NO VISION condition [both R ≥ 0.66, p ≤ 0.008 (uncorrected)]; otherwise, all four groups in VISION and both young active and non-active subgroups in NO-VISION [all |R| ≤ 0.54, p ≥ 0.032 (uncorrected)]. Interestingly, positive associations between RT and MT durations were observed in older active adults during both VISION and NO VISION [R ≥ 0.61, p ≤ 0.01 (uncorrected)], suggesting that participants in this subgroup tended to delay movement initiation and increase movement time. Taken together, the aforementioned observations suggest that all participants increased movement time to allow more corrective submovements in the absence of visual feedback, and this expression of limb-target regulation was also evident in older adults at the presence of visual feedback.
Finally, active older adults appeared to apply a “play-it-safe” strategy in the VISION condition by making shorter primary movements and increasing the number of corrective submovements as indicated by a significant negative correlation between PE and nCS [R = −0.62, p = 0.008 (uncorrected)]. In the NO VISION condition, significant negative correlation between PE and DHI were observed for the non-active young adults and the two older adult groups [all, R ≤ −0.66, p ≤ 0.004 (uncorrected)]. These observations suggest that participants adapted a play-it-safe strategy in the NO VISION condition by making shorter primary movements to allow more time for homing-in. The aforementioned relationships were not observed for the young active group [R = −0.52, p = 0.034 (uncorrected)] nor during VISION for all groups [|R| ≤ 0.52, p ≥ 0.034 (uncorrected)].
Relationships between hand movement characteristics and aiming errors
Significant correlations between aiming errors and hand movement characteristics were found only in the VISION condition (see Table S2 in Supplementary Materials). For non-active older adults, longer homing-in durations predicted lower aiming errors [R = −0.69, p = 0.005 (uncorrected)], suggesting that playing-it-safe in the VISION condition help participants in this subgroup to reduce aiming errors. For active young adults, (1) longer movement times were associated with greater aiming errors in [R = 0.64, p = 0.006 (uncorrected)] and (2) a trend toward negative correlations between aiming errors and peak velocity [R = −0.56, p = 0.018 (uncorrected)]. While the two latter observations may look peculiar at first sight, it suggests that poor performers in the young active group were slower to complete the aiming movement. Correlations between aiming errors and the remaining hand movement characteristics did not reach the critical p value for significance [all |R| ≤ 0.52, p ≥ 0.038 (uncorrected); both VISION and NO VISION].
Relationships between motion/position sense and aiming errors
No significant correlations between aiming errors (experiment 1) on one hand and motion sense (i.e., 0.25°/s and 0.50°/s flexion detection times) or position sense (experiment 2) on the other hand were observed [all |R| ≤ 0.47, p ≥ 0.070 (uncorrected)] (see Table S3 in Supplementary Materials). Therefore, decline in proprioceptive acuity was not associated with larger aiming errors.
Relationships between motion/position sense and hand movement characteristics
For the active young and the two older adult subgroups, correlations between motion/position sense characteristics and aiming movement characteristics did not reach the critical p value for significance [all p ≥ 0.017 (uncorrected)]; see Table S4 in Supplementary Materials. For non-active young adults, significant positive correlations were found: (1) in the VISION condition between detection time at 0.25°/s flexion and PE and (2) in the NO VISION condition between position sense error and RT [both R = 0.66, p = 0.006 (uncorrected)]. No other significant correlations between motion or position sense and aiming movement characteristics were found for this group [all p ≥ 0.031 (uncorrected)]. These observations suggest that age-related decline in proprioceptive acuity did not predict increased movement times, prolonged homing-in phase, and/or increased corrective submovements in older adults.
Discussion
A first major finding of experiment 1 was that non-active older individuals were not affected by the removal of visual feedback to a greater extent than their active peers or younger adults (largely negating hypotheses 1 and 2). A second major finding of experiment 1 was that young and older adults used different aiming strategies to accomplish their aiming goals. Here, age-related movement adaptations were clearly observed as function of feedback conditions and physical activity level. For the most part, differences in aiming strategy across groups were observed during homing-in (i.e., in the limb-target regulation), and differences became more pronounced when visual feedback was not provided. Finally, a third major finding (of experiment 2) was that individuals with higher levels of physical activity showed superior motion sense and position sense on the proprioceptive tasks. This means that better proprioceptive acuity is to be expected in physically active young and older adults than in their non-active peers. Physically active young showed the best proprioceptive ability, and non-active older adults showed the worst proprioceptive ability with the two remaining groups positioned in between. However, all age groups achieved similar levels of aiming accuracy irrespective of their age and physical activity level. The collective evidence from experiment 1 and experiment 2 demonstrates that changes in proprioceptive ability as function of age and physical activity level do not predict changes in aiming behavior. Specifically, there was no evidence to show that the withdrawal of visual feedback increased aiming errors in the non-active older adults more than in the other three groups, even though the proprioceptive ability of this group was, generally, inferior (negating hypothesis 3). Those seemingly contradicting outcomes require further attention because main body of findings from both experiments are consisted with a vast body of literature on effects of physical activity and aging on manual aiming (e.g., Chaput and Proteau 1996; Lyons et al. 1996; Seidler-Dobrin and Stelmach 1998; Van Halewyck et al. 2014) and proprioception (e.g., Adamo et al. 2007; Adamo et al. 2009; Herter et al. 2014; Wright et al. 2011). The specific effects of age and physical activity level on manual aiming and proprioception as emerged in findings of experiment 1 and experiment 2 are discussed in more details next.
The effects of age and physical activity level on aiming behavior
All in all, our findings from experiment 1 are consistent with the large body of research on manual aiming and aging, showing that older adults can achieve similar levels of endpoint accuracy as their younger peers (e.g., Lyons et al. 1996; Van Halewyck et al. 2014). For the most part, older adults exhibited longer homing-in durations, increased number of corrective submovements, and, generally, longer movement times (e.g., Boisseau et al. 2002; Ketcham et al. 2002; Lyons et al. 1996). Although these movement adaptations are usually interpreted as an increased reliance on visual feedback in older age (Coats and Wann 2011; Rand and Stelmach 2011; Terrier et al. 2011), our data are inconsistent with this hypothesis again (see also Van Halewyck et al. 2014). The finding that older adults were not affected to a greater extent by the removal of online as well as offline visual feedback suggests that they were not more dependent on vision during the goal-directed aiming task as compared to their younger peers. Moreover, the results of the correlation analyses between hand movement characteristics indicated that participants in all four groups adapted their movement strategy to cope with the removal of visual feedback by adapting the play-it-safe strategy. All participants increased movement time and decreased the distance covered by the primary movement to allow longer homing-in duration and larger number of corrective submovements irrespective of their age and physical activity levels. Interestingly, non-active older adults showing longer homing-in duration showed lower aiming errors when online feedback was available but not when online feedback was removed. As movement trajectory corrections during homing-in phase in the absence of online visual feedback could solely be based on proprioceptive feedback, it has been assumed that proprioception acuity (and particularly position sense) was preserved in those older adults who showed lower aiming errors. However, no significant correlations between proprioception acuity and aiming errors were observed. The absence of significant correlations between proprioceptive acuity and aiming movement characteristics (in general) and aiming errors (in particular) suggests that poor proprioceptive acuity should not be considered as a limiting factor in the context of manual aiming.
Older adults exhibited longer movement times, suggesting that they spent a larger relative (and absolute) amount of time homing-in on the target (e.g., Boisseau et al. 2002; Ketcham et al. 2002; Lyons et al. 1996). These age-related movement adaptations have been argued to reflect an increased reliance of older adults on visual input for feedback control of movement (Coats and Wann 2011; Rand and Stelmach 2011; Terrier et al. 2011). Indeed, longer homing-in durations were found in non-active older adults who showed lower aiming errors during performance of the aiming task with visual feedback, whereas longer homing-in durations had no effect on the performance of this group during withdrawal of visual feedback. However, careful inspection of our data seems to negate this argument. First, when visual feedback was withdrawn, young and older adults achieved similar levels of endpoint accuracy (see also Van Halewyck et al. 2014). Moreover, aiming strategy adapted upon the removal of online visual feedback did not change dramatically across the four groups, suggesting that the same strategy was applied irrespective of their age and physical activity level. Instead, the movement adaptations traditionally observed in older adults’ aiming behavior (e.g., increasing movement time to allow larger number of corrective submovements) was also adopted by younger adults. In older adults, these alterations are considered to reflect decreased efficiency in the processing of visual feedback or adoption of a play-it-safe strategy. Evidence for these two changes in behavior has been found in other work from our lab (Van Halewyck et al. 2015b) and elsewhere (Welsh et al. 2007).
Referring to the multiple processes model (Elliott et al. 2010), the play-it-safe strategy could be implemented by formation of internal representations of longer movement time, shorter movement displacements, and lower movement speed during both movement initiation and homing-in phase. The fact that increased movement time and homing-in durations were accompanied by increased number of corrective submovements in all four groups suggests that the play-it-safe strategy was adopted as a default strategy when visual feedback was withdrawn. Specifically, significant positive correlations were found between (i) movement time, (ii) homing-in duration, and (iii) the number of corrective submovements, whereas significant negative correlations were found between the distance covered by the primary movement and (ii) and (iii). Taken together, these observations indicate that movement characteristics of both the primary movement and homing-in duration were adjusted simultaneously, presumably through generation of predictive models. In other words, we propose that predictive models were used to provide afferent information throughout the aiming movement when visual feedback was not available. This may have allowed both active and non-active older adults to successfully compensate for age-related deficiencies in detection and correction of movement errors through online sensory feedback in the homing-in phase. The fact that the active older adults showed the same correlation patterns during aiming with and without visual feedback suggests that they may have relied on use of predictive models to conduct the aiming movements more than their younger peers. Thus, it was concluded that the generation of predictions of the expected sensory consequences in the younger adults group was used only in the execution of the primary movement.
Besides the impact of age, we also investigated the potentially mediating role of physical activity level on aiming performance. In line with our previous work, though, the effect of physical activity levels on aiming behavior appeared rather limited (Van Halewyck et al. 2014). Only reaction times were shown to be generally longer in non-active adults, an outcome which is commonly interpreted as more elaborate planning processes (e.g., Khan et al. 2006). However, slightly prolonging reaction time was not considered meaningful, especially since none of the other variables of interest differed significantly between active and non-active adults. In line with this limited effect of physical activity level, no significant interactions emerged in either feedback condition. The lack of main and interaction effects involving PA when investigating endpoint accuracy suggests that position sense at wrist level may be preserved in non-active older adults. However, this interpretation clearly contradicts the findings of Adamo et al. (2009) and Wright et al. (2011) as well as the findings of experiment 2. Alternatively, the fact that both active and on-active older adults showed similar aiming strategies in the homing-in phase suggests that older individuals may partly compensate for impaired sensory acuity simply by increasing movement time and homing-in duration to allow sufficient time for sensory processing. We specifically address this issue in the next section.
The effects of age and physical activity level on proprioceptive acuity
In line with our expectations, clear age effects were observed when comparing the proprioceptive features between young and older adults in experiment 2. The latter group generally needed more time to evaluate passive wrist movements correctly and made greater matching errors. Overall, these results are strongly in line with the current field of knowledge on proprioceptive acuity in older age. Specifically, several other investigators have reported an age-related decline in motion (Kokmen et al. 1978; Wright et al. 2011) and position sense of the upper limbs (Adamo et al. 2007; Adamo et al. 2009; Herter et al. 2014). These deteriorations may partially be caused by physiological changes in the structure and function of muscle spindles throughout late adulthood (Herter et al. 2014; Kalisch et al. 2012; Proske and Gandevia 2012). Besides their gradual decline in number, aged muscle spindles generally show an increased capsular thickness, decreased diameter size, and lower sensitivity. Moreover, the aging process is associated with an overall denervation of muscle spindles, further impeding their functioning and signaling capacity. Clearly, physical activity levels played a considerable role in participants’ proprioceptive acuity. Here, young active adults generally demonstrated enhanced motion and position senses compared to their non-active and older counterparts (Fig. 4). Physical activity has been suggested to partially compensate for the abovementioned age-related declines in muscle functioning by entailing muscle fiber hypertrophy in older adults (Proske and Gandevia 2012). Thus, active older adults may delay their natural decline in proprioception by enhancing the signaling capacity of muscle spindles and joint movement receptors. Likewise, muscle hypertrophy may optimize the signaling capacity of active young adults.
The positive effect of physical activity on proprioceptive acuity may be explained by preserved sensory processing capacity in active as compared to non-active older individuals. Specifically, findings from experiment 2 showed that active older adults performed the motion sense and position sense tests better than their non-active peers, supporting the view that active lifestyle has a positive effect on information processing and processing speed (e.g., Muions and Ballesteros 2014; Muions et al. 2015; Voelcker-Rehage et al. 2011). Noteworthy, significant differences in performance of the sensory tests between active and non-active older adults were observed under situations where those individuals had to evaluate motion/position in shorter time windows (i.e., for the 0.5°/s speed condition in the motion sense task and the 6°/s speed condition in the position sense task) or had to distinguish between false and true wrist movements during the motion sense task (i.e., in the sham condition). Taken together, observations from experiment 2 suggest that active older adults may delay their natural decline in proprioception by more effective activation of brain networks associated with sensory processing and cognitive control (Nagamatsu et al. 2012; Smith et al. 2011; Voelcker-Rehage et al. 2011; see review Voelcker-Rehage and Niemann 2013). This hypothesis is consistent with observations from recent studies showing that age-related declines in proprioception of the lower limb may particularly be visible in challenging conditions that demand more attention (Boisgontier et al. 2012; Boisgontier and Nougier 2013; see review Boisgontier et al. 2013). Future research may investigate the hypothesis by adding conditions without visual feedback in which aiming movements with high accuracy and/or attentional demands are executed under a strict time constraint. If these conditions result in non-active older adults making greater aiming errors than their active peers, it would provide additional evidence for the hypothesis that physically active older adults have a greater ability to recruit brain resources as a means to compensate for central and peripheral neurodegenerative processes that occur in older age.
Classification of physical activity level as a study limitation
It should be recognized that classification of active and non-active participants in this study was based on the results of questionnaires, which are in general subjective. The Modified Baecke Questionnaire has been reported to provide fair-to-moderate assessment of physical activity level in older adults when compared with assessments obtained from direct physical measures such as energy expenditure (Hertogh et al. 2008) or cardiorespiratory fitness (Mustelin et al. 2011). However, both Baecke Questionnaire (Baecke et al. 1982) and the Modified Baecke Questionnaire (Voorrips et al. 1991) focus on physical activity levels over the past year and does not take into account the participant’s earlier history of sport participation that could impact the aiming performance, for example, participation in competitive sports such as basketball or tennis. This shortcoming may provide an alternative explanation as to why differences between active and non-active older adults did not emerge consistently throughout the two experiments. The limitation was not addressed as it is extremely difficult to accurately quantify one’s lifelong history of physical activity. As a result, dividing participants based on the results of a physical activity questionnaire remains a common practice in motor control research (e.g., Adamo et al. 2007; Adamo et al. 2009; McGregor et al. 2011; Petrella et al. 1997; Pickard et al. 2003; Wright et al. 2011).
Conclusions
It appears that the impact of aging on the performance of goal-directed movements is not necessarily associated with an increased reliance on visual feedback that would compensate for the observed declines in proprioceptive acuity. Indeed, our results suggest that even when aiming without visual feedback, older adults are able to compensate for their decline in proprioception presumably by increasing their reliance on predictive models in both primary (open-loop) and secondary (closed-loop) phases of the movement. We propose that an ongoing reliance on predictive model improves proprioception through an integration of the copy of the motor commands (i.e., efference copy) that is sent directly to the cerebellum for feedback processing of the expected hand position (Boisgontier and Swinnen 2014), thus compensating for a lack of actual sensory input. The ongoing use of predictive models was accompanied by shorter primary movements and an increased number of corrective submovements, which are typical features of the play-it-safe strategy. These movement adaptations were also observed in young adults when visual feedback was not available. Taken together, our observations suggest that (1) when there was an increase in task demands, participants of all groups increased their reliance on predictive models and (2) used the play-it-safe strategy irrespective of age and physical condition. Lastly, a physically active lifestyle was clearly associated with an enhanced preservation of motion and position sense. This outcome seems to suggest that remaining physically active in older age may mitigate the age-related decline in feedback processing and sensorimotor integration.
Electronic supplementary material
(DOCX 61 kb)
Acknowledgments
Werner F. Helsen and Florian Van Halewyck would like to acknowledge the KU Leuven Research Council for financially supporting this research project. Matthieu P. Boisgontier is supported by a research grant (1504015N) and a post-doctoral fellowship of the Research Foundation—Flanders (FWO). The authors also wish to thank Ig. Marc Beirinckx and Ig. Paul Meugens for providing invaluable guidance in designing the research equipment.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Adamo DE, Alexander NB, Brown SH. The influence of age and physical activity on upper limb proprioceptive ability. J Aging Phys Activ. 2009;17:272–293. doi: 10.1123/japa.17.3.272. [DOI] [PubMed] [Google Scholar]
- Adamo DE, Martin BJ, Brown SH. Age-related differences in upper limb proprioceptive acuity. Percept Mot Skills. 2007;104:1297–1309. doi: 10.2466/pms.104.4.1297-1309. [DOI] [PubMed] [Google Scholar]
- Alaerts K, Levin O, Swinnen SP. Whether feeling or seeing is more accurate depends on tracking direction within the perception-action cycle. Behav Brain Res. 2007;178:229–234. doi: 10.1016/j.bbr.2006.12.024. [DOI] [PubMed] [Google Scholar]
- Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Berchicci M, Lucci G, Perri RL, Spinelli D, Di Russo F. Benefits of physical exercise on basic visuo-motor functions across age. Front Aging Neurosci. 2014;6:48. doi: 10.3389/fnagi.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisgontier MP, Nougier V. Ageing of internal models: from a continuous to an intermittent proprioceptive control of movement. Age. 2013;35:1339–1355. doi: 10.1007/s11357-012-9436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisgontier MP, Swinnen SP. Proprioception in the cerebellum. Front Hum Neurosci. 2014;8:212. doi: 10.3389/fnhum.2014.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisgontier MP, Swinnen SP. Age-related deficit in a bimanual joint position matching task is amplitude dependent. Front Aging Neurosci. 2015;7:162. doi: 10.3389/fnagi.2015.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisgontier MP, Beets IAM, Duysens I, Nieuwboer A, Krampe RT, Swinnen SP. Age-related differences in attentional cost associated with postural dual tasks: increased recruitment of generic cognitive resources in older adults. Neurosci Biobehav Rev. 2013;37:1824–1837. doi: 10.1016/j.neubiorev.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Boisgontier MP, Olivier I, Chenu O, Nougier V. Presbypropria: the effects of physiological ageing on proprioceptive control. Age. 2012;34:1179–1194. doi: 10.1007/s11357-011-9300-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisgontier MP, Van Halewyck F, Corporaal S, Willacker L, Van den Bergh V, Beets IAM, et al. Vision of the active limb impairs bimanual motor tracking in young and older adults. Front Aging Neurosci. 2014;6:320. doi: 10.3389/fnagi.2014.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisseau E, Scherzer P, Cohen H. Eye–hand coordination in aging and in Parkinson’s disease. Aging Neuropsychol Cogn. 2002;9:266–275. doi: 10.1076/anec.9.4.266.8769. [DOI] [Google Scholar]
- Capranica L, Tessitore A, Olivieri B, Minganti C, Pesce C. Field evaluation of cycled coupled movements of hand and foot in older individuals. Gerontology. 2004;50:399–406. doi: 10.1159/000080178. [DOI] [PubMed] [Google Scholar]
- Chaput S, Proteau L. Modifications with aging in the role played by vision and proprioception for movement control. Exp Aging Res. 1996;22:1–21. doi: 10.1080/03610739608253994. [DOI] [PubMed] [Google Scholar]
- Coats RO, Wann JP. The reliance on visual feedback control by older adults is highlighted in tasks requiring precise endpoint placement and precision grip. Exp Brain Res. 2011;214:139–150. doi: 10.1007/s00221-011-2813-x. [DOI] [PubMed] [Google Scholar]
- Cortis C, Tessitore A, Perroni F, Lupo C, Pesce C, Ammendolia A, Capranica L. Interlimb coordination, strength, and power in soccer players across the lifespan. J Strength Cond Res. 2009;23:2458–2466. doi: 10.1519/JSC.0b013e3181bc1b39. [DOI] [PubMed] [Google Scholar]
- Curran-Everett D. Multiple comparisons: philosophies and illustrations. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1–R8. doi: 10.1152/ajpregu.2000.279.1.R1. [DOI] [PubMed] [Google Scholar]
- Elliott D, Hansen S, Grierson LE, Lyons J, Bennett SJ, Hayes SJ. Goal-directed aiming: two components but multiple processes. Psychol Bull. 2010;136:1023–1044. doi: 10.1037/a0020958. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A Practical Method for Grading the Cognitive State of Patients for the. Clinician J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Ghez C, Gordon J, Ghilardi MF. Impairments of reaching movements in patients without proprioception: II Effects of Visual Information on Accuracy. J Neurophysiol. 1995;73:361–372. doi: 10.1152/jn.1995.73.1.361. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Coxon JP, Wenderoth N, Van Impe A, Swinnen SP. Proprioceptive sensibility in the elderly: Degeneration, functional consequences and plastic-adaptive processes. Neurosci Biobehav Rev. 2009;33:271–278. doi: 10.1016/j.neubiorev.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Heath M. Role of limb and target vision in the online control of memory-guided reaches. Mot Control. 2005;9:281–311. doi: 10.1123/mcj.9.3.281. [DOI] [PubMed] [Google Scholar]
- Herter TM, Scott SH, Dukelow SP. Systematic changes in position sense accompany normal aging across adulthood. J Neuroeng Rehabil. 2014;11:1–12. doi: 10.1186/1743-0003-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertogh EM, Monninkhof EM, Schouten EG, Peeters PH, Schuit AJ. Validity of the modified Baecke questionnaire: comparison with energy expenditure according to the doubly labeled water method. Int J Behav Nutr Phys Act. 2008;5:30. doi: 10.1186/1479-5868-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs HI, Leritz EC, Williams VJ, Van Boxtel MP, Elst WV, Jolles J, et al. Association betweenwhite matter microstructure, executive functions, and processing speed inolder adults: the impact of vascular health. Hum Brain Mapp. 2011;34:77–95. doi: 10.1002/hbm.21412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch T, Kattenstroth JC, Kowalewski R, Tegenthoff M, Dinse HR. Age-related changes in the joint position sense of the human hand. Clin Interv Aging. 2012;7:499–507. doi: 10.2147/CIA.S37573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketcham CJ, Seidler RD, Van Gemmert AW, Stelmach GE. Age-related kinematic differences as influenced by task difficulty, target size, and movement amplitude. J Gerontol B Psychol Sci Soc Sci. 2002;57:54–64. doi: 10.1093/geronb/57.1.P54. [DOI] [PubMed] [Google Scholar]
- Khan MA, Elliott D, Coull J, Chua R, Lyons J. Optimal control strategies under different feedback schedules: kinematic evidence. J Mot Behav. 2002;34:45–57. doi: 10.1080/00222890209601930. [DOI] [PubMed] [Google Scholar]
- Khan MA, Franks IM, Elliott D, Lawrence GP, Chua R, Bernier PM, et al. Inferring online and offline processing of visual feedback in target-directed movements from kinematic data. Neurosci Biobehav Rev. 2006;30:1106–1121. doi: 10.1016/j.neubiorev.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Khan MA, Franks IM, Goodman D. The effect of practice on the control of rapid aiming movements: evidence for an independency between programming and feedback processing. Q J Exp Psychol. 1998;51:425–444. doi: 10.1080/713755756. [DOI] [Google Scholar]
- Kokmen E, Bossemeyer RWJ, Williams WJ. Quantitative evaluation of joint motion sensation in an aging population. Gerontology. 1978;33:62–67. doi: 10.1093/geronj/33.1.62. [DOI] [PubMed] [Google Scholar]
- Lavrysen A, Elliott D, Buekers MJ, Feys P, Helsen WF. Eye–hand coordination asymmetries in manual aiming. J Mot Behav. 2007;39:9–18. doi: 10.3200/JMBR.39.1.9-18. [DOI] [PubMed] [Google Scholar]
- Lavrysen A, Heremans E, Peeters R, Wenderoth N, Feys P, Swinnen SP, et al. Hemispheric asymmetries in goal-directed hand movements are independent of hand preference. NeuroImage. 2012;62:1815–1824. doi: 10.1016/j.neuroimage.2012.05.033. [DOI] [PubMed] [Google Scholar]
- Lavrysen A, Heremans E, Peeters R, Wenderoth N, Helsen WF, Feys P, et al. Hemispheric asymmetries in eye–hand coordination. NeuroImage. 2008;39:1938–1949. doi: 10.1016/j.neuroimage.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Lyons J, Elliott D, Swanson LR, Chua R. Use of vision in manual aiming by young and older adults. J Aging Phys Act. 1996;4:165–178. [Google Scholar]
- Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the Box and block test of manual dexterity. Am J Occup Ther. 1985;39:386–391. doi: 10.5014/ajot.39.6.386. [DOI] [PubMed] [Google Scholar]
- McGregor KM, Zlatar Z, Kleim E, Sudhyadhom A, Bauer A, Phan S, et al. Physical activity and neural correlates of aging: a combined TMS/fMRI study. Behav Brain Res. 2011;222:158–168. doi: 10.1016/j.bbr.2011.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muions M, Ballesteros S. Peripheral vision and perceptual asymmetries in young and older martial arts athletes and nonathletes. Atten Percept Psychophys. 2014;76:2465–2476. doi: 10.3758/s13414-014-0719-y. [DOI] [PubMed] [Google Scholar]
- Muions M, Ballesteros M, Ballesteros S. Sports can protect dynamic visual acuity from aging: a study with young and older judo and karate martial arts athletes. Atten Percept Psychophys. 2015;77:2061–2073. doi: 10.3758/s13414-015-0901-x. [DOI] [PubMed] [Google Scholar]
- Mustelin L, Latvala A, Pietiläinen KH, Piirilä P, Sovijärvi AR, Kujala UM, et al. Associations between sports participation, cardiorespiratory fitness, and adiposity in young adult twins. J Appl Physiol. 2011;110:681–686. doi: 10.1152/japplphysiol.00753.2010. [DOI] [PubMed] [Google Scholar]
- Nagamatsu LS, Handy TC, Hsu CL, Voss M, Liu-Ambrose T. Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Arch Intern Med. 2012;172:666–668. doi: 10.1001/archinternmed.2012.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oxford Grice K, Vogel KA, Le V, Mitchell A, Muniz S, Vollmer MA. Adult norms for a commercially available Nine Hole Peg test for finger dexterity. Am J Occup Ther. 2003;57:570–573. doi: 10.5014/ajot.57.5.570. [DOI] [PubMed] [Google Scholar]
- Petrella RJ, Lattanzio PJ, Nelson MG. Effect of age and activity on knee proprioception. Am J Phys Med Rehabil. 1997;76:235–241. doi: 10.1097/00002060-199705000-00015. [DOI] [PubMed] [Google Scholar]
- Pickard CM, Sullivan PE, Allison GT, Singer KP. Is there a difference in hip joint position sense between young and older groups? J Gerontol A Biol Sci Med Sci. 2003;58:631–635. doi: 10.1093/gerona/58.7.M631. [DOI] [PubMed] [Google Scholar]
- Pratt J, Chasteen AL, Abrams RA. Rapid aimed limb movements: age differences and practice effects in component submovements. Psychol Aging. 1994;9:325–334. doi: 10.1037/0882-7974.9.2.325. [DOI] [PubMed] [Google Scholar]
- Proske U, Gandevia SC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev. 2012;92:1651–1697. doi: 10.1152/physrev.00048.2011. [DOI] [PubMed] [Google Scholar]
- Rand MK, Stelmach GE. Adaptation of gaze anchoring through practice in young and older adults. Neurosci Lett. 2011;492:47–51. doi: 10.1016/j.neulet.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JA, Knill DC. Humans use continuous visual feedback from the hand to control fast reaching movements. Exp Brain Res. 2003;152:341–352. doi: 10.1007/s00221-003-1525-2. [DOI] [PubMed] [Google Scholar]
- Seidler-Dobrin RD, Stelmach GE. Persistence in visual feedback control by the elderly. Exp Brain Res. 1998;119:467–474. doi: 10.1007/s002210050362. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. On the proprioceptive system, especially in its reflex aspect. Brain. 1907;29:467–482. doi: 10.1093/brain/29.4.467. [DOI] [Google Scholar]
- Smith JC, Nielson KA, Woodard JL, Seidenberg M, Durgerian S, Antuono P, et al. Interactive effects ofphysical activity and APOE-epsilon 4 on BOLD semantic memory activation inhealthy elders. NeuroImage. 2011;54:635–644. doi: 10.1016/j.neuroimage.2010.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelmach GE, Goggin NL, Amrhein PC. Aging and the restructuring of precued movements. Psychol Aging. 1988;3:151–157. doi: 10.1037/0882-7974.3.2.151. [DOI] [PubMed] [Google Scholar]
- Temprado JJ, Sleimen-Malkoun R, Lemaire P, Rey-Robert B, Retornaz F, Berton E. Aging of sensorimotor processes: asystematic study in Fitts’ task. Exp Brain Res. 2013;228:105–116. doi: 10.1007/s00221-013-3542-0. [DOI] [PubMed] [Google Scholar]
- Terrier R, Forestier N, Berrigan F, Germain-Robitaille M, Lavalliere M, Teasdale N. Effect of terminal accuracy requirements on temporal gaze-hand coordination during fast discrete and reciprocal pointings. J Neuroeng Rehabil. 2011;8:10. doi: 10.1186/1743-0003-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Halewyck F, Lavrysen A, Levin O, Boisgontier MP, Elliott D, Helsen WF. Factors underlying age-related changes in discrete aiming. Expl Brain Res. 2015;233:1733–1744. doi: 10.1007/s00221-015-4247-3. [DOI] [PubMed] [Google Scholar]
- Van Halewyck F, Lavrysen A, Levin O, Elliott D, Helsen WF. The impact of age and physical activity level on manual aiming performance. J Aging Phys Act. 2015;23:169–179. doi: 10.1123/japa.2013-0104. [DOI] [PubMed] [Google Scholar]
- Van Halewyck F, Lavrysen A, Levin O, Boisgontier MP, Elliott D, Helsen WF. Both age and physical activity level impact on eye-hand coordination. Hum Mov Sci. 2014;36:80–96. doi: 10.1016/j.humov.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Voelcker-Rehage C, Niemann C. Structural and functional brain changes related to different types of physicalactivity across the life span. Neurosci Biobehav Rev. 2013;37:2268–2295. doi: 10.1016/j.neubiorev.2013.01.028. [DOI] [PubMed] [Google Scholar]
- Voelcker-Rehage C, Godde B, Staudinger UM. Cardiovascular and coordination training differentially improve cognitive performance and neuralprocessing in older adults. Front Hum Neurosci. 2011;5:26. doi: 10.3389/fnhum.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorrips LE, Ravelli AC, Dongelmans PC, Deurenberg P, Van Staveren WA. A physical activity questionnaire for the elderly. Med Sci Sports Exerc. 1991;23:974–979. doi: 10.1249/00005768-199108000-00015. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Technical manual for the Wechsler adult Intelligence test –. Third. San Antonio: San Antonio Psychological Corporation; 1997. [Google Scholar]
- Weinstein S. Fifty years of somatosensory research: from the semmes-weinstein monofilaments to weinstein enhanced sensory test. J Hand Ther. 1993;6:11–22. doi: 10.1016/S0894-1130(12)80176-1. [DOI] [PubMed] [Google Scholar]
- Welsh TN, Higgins L, Elliott D. Are there age-related differences in learning to optimize speed, accuracy, and energy expenditure? Hum Mov Sci. 2007;26:892–912. doi: 10.1016/j.humov.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Woodworth RS. The accuracy of voluntary movement. Psychol Rev. 1899;3:1–119. [Google Scholar]
- Wright ML, Adamo DE, Brown SH. Age-related declines in the detection of passive wrist movement. Neurosci Lett. 2011;500:108–112. doi: 10.1016/j.neulet.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 61 kb)