Abstract
Ankle proprioceptive information is integrated by the central nervous system to generate and modulate muscle contractions for maintaining standing balance. This study evaluated the association of ankle joint proprioception with objective and self-report measures of balance, mobility, and physical function across the adult life span. Seven hundred and ninety participants (age range 24–97 years, 362 women) who completed ankle proprioception assessment between 2010 and 2014 were included in the present study from the population-based cohort of the Baltimore Longitudinal Study of Aging (BLSA), USA. Outcome measures included ankle joint proprioception measured as threshold for perception of passive movement (TPPM); single leg stance time; perceived difficulty for standing balance; usual, fastest, and narrow-path gait speed; walking index; short physical performance battery score; and self-reported activity restriction due to fear of falling. Descriptive variables included age, sex, body mass index, education, strength, and cognition. Analyses of covariance (ANCOVA) in general linear model (GLM) or multinomial logistic regression analyses were performed, as appropriate, to test the hypothesis that balance, mobility, and physical function were significantly different according to TPPM quintiles even after adjusting for relevant covariates. Those with TPPM >2.2° consistently demonstrated poor balance, mobility, and physical function. However, with increase in challenge (single leg stance, fastest walking speed, and SPPB), TPPM >1.4° was associated with significantly worse performance. In conclusion, ankle proprioceptive acuity has an overall graded relationship with objective and self-report measures of balance, mobility, and physical function. However, the cutoff proprioceptive acuity associated with substantial decline or inability to perform could depend on the challenge induced.
Keywords: Ankle proprioception, Threshold for perception of passive movement, Balance, Gait speed, Mobility, Aging
Introduction
Morphological studies have established that the neural structures pertinent to proprioception deteriorate with age (Swash and Fox 1972; Morisawa 1998) and psychophysical studies have demonstrated age-related decline in measured ankle proprioception (Verschueren et al. 2002; Deshpande et al. 2003; Westlake and Culham 2006). Ankle proprioceptive information is used to generate and modulate muscle contractions aimed at maintaining vertical standing balance (Fitzpatrick and McCloskey 1994; Colledge et al. 1994; Goble et al. 2011). During gait, the proprioceptive signal is integrated in a phasic and context-specific manner to precisely control movements of the swing limb and appropriately move the center of mass using the supporting limb (Sorensen et al. 2002; Pearson 2004). Consequently, impaired ankle proprioception has been associated with poor balance and falls in older persons (Lord et al. 1991; Goble et al. 2009, 2011). Due to its critical role in standing and dynamic postural control, deterioration in ankle proprioception may also contribute to decline in global mobility and overall physical function. Further, although subconscious integration of proprioceptive information at the cerebellar level is highly critical for precision of movements (Bhanpuri et al. 2013), this information is also integrated at the cortical level for conscious perception (Rosenkranz and Rothwell 2012). Therefore, it is possible that deficits in ankle proprioception may also contribute to perceived instability during standing and while performing mobility tasks.
Ankle proprioception can be quantified by measuring the threshold for perception of passive movement (TPPM) which is a reliable and sensitive measure of ankle proprioception (Deshpande et al. 2003; Westlake and Culham 2006; Ko et al. 2015). Therefore, this study aimed to examine the association of TPPM at the ankle joint with objective and subjective measures of balance, mobility, and physical function and to get an insight if TPPM values can distinguish the performance level.
Methods
Participants
The Baltimore Longitudinal Study of Aging (BLSA) is a prospective study of aging initiated in 1958 by the National Institute on Aging (NIA), USA. BLSA participants are community-residing adult volunteers aged 21 to 90+ years who are in good health and free of chronic diseases and physical and cognitive impairments at enrollment. Participants visit the NIA Clinical Research Unit in Baltimore, MD, for ~3 days of research testing. Details about recruitment and testing have been reported elsewhere (Shock et al. 1984). Seven hundred and ninety (428 men and 362 women, age range of 24–97 years) BLSA participants who completed ankle proprioception assessment between 2010 and 2014 were included in this study. For participants who had more than one visit in this period, data from the latest visit were used in the analysis. Participants who reported pain in the foot, paresis/paralysis of the right lower limb, or cognitive impairment identified as Blessed Mental score ≥8 (Katzman et al. 1983) were excluded from ankle proprioception measurement.
Outcome measures
Threshold for perception of passive movement
TPPM was measured using a custom-made apparatus. The high reliability of this technique has been demonstrated before with ICC of 0.88 (Ko et al. 2015). Participants were blindfolded and seated on a high chair with their feet strapped onto two pedals. Knee flexion was maintained at 80° and the initial ankle position was 10° plantarflexion. They were instructed to focus on the right ankle. The right pedal was moved by a servomotor at the angular velocity of 0.3°/s. Participants were asked to press the given switch as soon as they perceived movement and the direction of the movement (up/down). The corresponding ankle angular rotation was recorded in degrees. A total of four trials were performed in a set sequence of plantarflexion, dorsiflexion, dorsiflexion, and plantarflexion. Up to two extra trials were performed if an error occurred in identifying the direction. The maximum and minimum values are excluded and the average of the remaining two trials was used as TPPM.
Standing balance
Objective performance
Single-leg stance time was recorded. Participants were instructed to stand on one leg for up to 30 s. They could select either the right or left leg, depending on what leg they felt more comfortable maintaining balance. The time was recorded using a stop watch which was stopped if they touched the other leg to the ground or grabbed for support. If the participant could not complete the first 30-s trial, a second trial was performed and the best performance was recorded. If the participant was unable to perform single-leg stance, the time was recorded as 0 s.
Subjective performance
Participants were asked about the frequency of perceived difficulty maintaining balance on a normal level surface with eyes open and with eyes closed. Responses were dichotomized as no difficulty vs. any difficulty.
Mobility
Objective performance
Gait speed was measured over a 6-m course under three walking conditions, usual speed, fastest speed, and walking on a narrow path. Participants started each walk with their toes behind, but touching the starting line and walked a few steps past the finishing line. For usual speed, they were asked to walk at their self-selected comfortable pace. To impose additional challenge, participants were asked to walk as fast as they could safely. The narrow-path walking was used to impose challenge specifically in the mediolateral direction. The path width was limited to 20 cm thus restricting mediolateral foot-placement. The time to complete each walk was converted into gait speed (m/s). Gait speed was averaged over two trials for usual speed. The best performance was used for the fastest walking speed and narrow-path walking speed. For the narrow-path condition, a third trial was performed if the participant failed on the first two trials where the failure was determined as more than two deviations from the path where path deviation was defined as stepping on, or going outside of the colored tape or touching the wall. Inability to successfully complete narrow-path walking was defined as failing all three trials with gait speed recorded as 0 m/s.
Subjective performance
Walking index represented perceived mobility ranging from 0 to 9, 0 indicating self-report of inability to walk 1/4 mile and 9 indicating self-report of “very easy” to walk 1 mile. Walking index is particularly sensitive to detect functional differences in high functioning older persons (Simonsick et al. 2009).
Physical performance
Objective performance
The short physical performance battery (SPPB) is a cumulative objective measure of strength, endurance, and balance (Guralnik et al. 1994). The SPPB score is derived from timed performance on standing balance, walking speed, and ability to rise from a chair. Since many of the BLSA participants report good physical function, additional components, such as standing on one leg and walking on a narrow path, were included to obtain extended SPPB scores (Simonsick et al. 2001) in addition to standard SPPB scores of 0–12. The strategy used for deriving extended SPPB scores results in a score range of 0–4, higher scores indicating better performance. The details of this scoring strategy are provided by Simonsick et al. (2001).
Subjective performance
Participants were asked if they restricted their physical activities due to fear of falling. The reply was recorded as a dichotomous variable (yes/no).
Demographic variables included age (years), sex, height (m), weight (kg), and years of education. Body mass index (BMI, kg/m2) was derived from the weight and height data. Descriptive variables included global cognition measured using Blessed Mental test (Katzman et al. 1983) and maximum grip strength (kg) represented strength.
Statistical analysis
Distribution of continuous variables was tested for normality and, log10 transformations of skewed variables were used in subsequent analyses as appropriate. The summary data are presented as means ± SD unless mentioned otherwise. Two preliminary analyses were performed to establish the relationship of TPPM with age and sex; a bivariate correlation analysis between TPPM and age and two-way analysis of variance (ANOVA, age group × sex) with TPPM as the dependent variable. For the ANOVA, the age groups were classified as <40, 40 to <60, 6 to <80, and ≥80 years.
Participants were then stratified according to the TPPM quintiles. Analysis of variance (ANOVA) or chi-square analysis was performed, as appropriate, to identify characteristic differences between TPPM quintiles. Next, analysis of covariance (ANCOVA) was performed in general linear model (GLM) to test the hypothesis that the continuous variables of balance (single-leg stance time), mobility (gait speed and walking index), and physical function (SPPB) were significantly different according to TPPM quintiles. Analogously, multinomial logistic regression analyses were used to test the hypotheses that the proportion of participants who reported perceived balance difficulty, those who reported activity restriction due to fear of falling, those with usual walking speed <0.8 m/s which is the cutoff point identifying mobility-related disability (Abellan van Kan et al. 2009), and those who failed the narrow-path walking test would be greater with increasingly worse TPPM quintile. All analyses were performed adjusting for age and sex. Additionally, analyses for fastest and narrow-path walking performance were adjusted for usual gait speed. A p < 0.05 was considered statistically significant. All statistical analyses were conducted using IBM SPSS version 22.
Results
The total number of participants in the four age categories was as follows: <40 years = 20, <60 = 128, <80 = 436, and ≥80 = 206. The prevalence of self-reported comorbidities was as follows: diabetes 10.8 %, peripheral arterial disease 0.4 %, peripheral neuropathy 2.9 %, cataract 13.8 %, osteoarthritis 38.8 %, osteoporosis 13.3 %, and Parkinson’s disease 0.6 %.
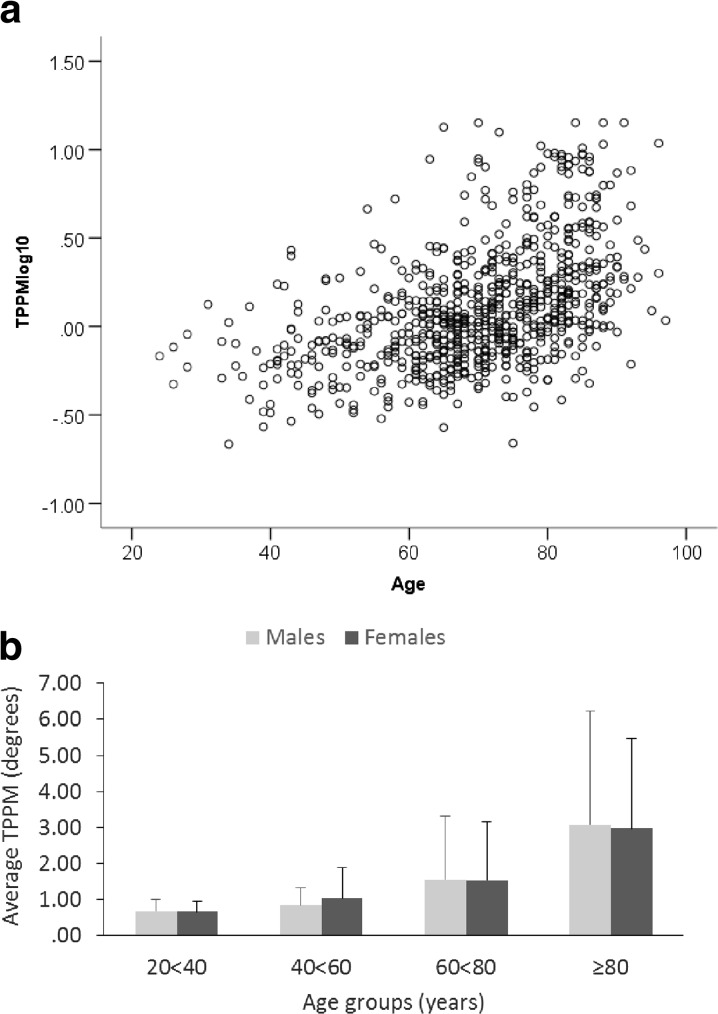
TPPM data were log10 transformed to approximate a normal distribution (TPPMlog10) (Fig. 1a, b). There was a significant correlation between age and TPPMlog10 (Fig. 2a, r2 linear = 0.227). A significant graded main effect of age group was found where older two age groups were significantly different from the two younger groups (p < 0.001–0.016) and from each other (<0.001). No main effect of sex (p = 0.953) or age group × sex interaction (p = 0.923) was found (Fig. 2b).
Fig. 1.
Threshold for perception of passive movement (TPPM) data was significantly skewed (a). Therefore, data were log10 transformed to achieve normal distribution (logTPPM) (b)
Fig. 2.
There was a significant correlation between age and TPPMlog10 (r 2 linear = 0.227) (a). A significant graded main effect of age group was found where older two age groups were significantly different from the two younger groups (p < 0.001–0.016) and from each other (<0.001). No main effect of sex (p = 0.953) or age group × sex interaction (p = 0.923) was found (b)
Table 1 describes participant characteristics according to TPPM quintiles (1st quintile, Q1 ≤0.7°; 2nd quintile, Q2 ≤1.0°; 3rd quintile, Q3 ≤1.3°; 4th quintile, Q4 ≤2.2°; 5th quintile, Q5 >2.2°). Participants in the higher quintiles were significantly older. After adjusting for age, there were no differences across TPPM quintiles for sex, height, BMI, or years of education. However, participants in the higher quintiles had significantly higher Blessed Mental test scores (p < 0.001) and lower grip strength (0.042) (Table 1).
Table 1.
Participant characteristics according to threshold for perception of passive movement (TPPM) quintiles
| Variable | 1st quintile n = 160 | 2nd quintile n = 154 | 3rd quintile n = 154 | 4th quintile n = 160 | 5th quintile n = 158 | F or χ 2 and p |
|---|---|---|---|---|---|---|
| Age (years) | 60.9 (18.6) | 65.0(12.5) | 70.4(10.9) | 74.4 (11.2) | 78.4(9.2) | 57.967, <0.001 |
| Sex (% women) | 50.6 | 53.2 | 57.1 | 56.7 | 53.2 | 1.901, 0.754 |
| Height (m) | 169.6(9.2) | 168.8(8.3) | 166.8(9.2) | 166.9(8.6) | 166.7(10.1) | 0.824, 0.510 |
| BMI (kg/m2) | 26.6(2.9) | 26.6(3.4) | 27.1(4.3) | 27.4(2.8) | 27.6(3.4) | 0.824, 0.510 |
| Grip strength (kg) | 35.3(11.2) | 33.4(10.8) | 30.6(9.9) | 28.5(10.0) | 27.4(9.3) | 2.491, 0.042 |
| Blessed Mental test | 1.1(1.3) | 1.2(1.2) | 1.5(1.5) | 1.6(1.6) | 2.0(1.7) | 6.159, <0.001 |
| Years completed in school | 16.9(2.5) | 17.3(2.4) | 16.8(2.5) | 17.1(2.4) | 17.2(3.1) | 0.970, 0.423 |
1st quintile ≤0.7°, 2nd quintile ≤1.0°, 3rd quintile ≤1.4°, 4th quintile ≤ 2.2°, and 5th quintile > 2.2°. Average age was significantly different in the quintiles; therefore, all other comparisons were adjusted for age
Q4 and Q5 had significantly shorter single-leg stance time than the three lower quintiles (p < 0.001–0.007) and Q5 had significantly shorter stance time than Q4 (p = 0.029). The proportion of participants who failed the single-leg stance test (p = 0.049) was also greater with increasing quintile. TPPM was significantly higher in those who failed single-leg stance test vs. those who did not (3.3 ± 2.9° vs. 1.6 ± 1.7°, F = 24.202, p < 0.001). The proportion of participants reporting balance difficulty was greater with increasing quintile for both, with eyes open (p = 0.043) and with eyes closed (p = 0.016) (Table 2).
Table 2.
Participants’ performance, (mean ± SD) or proportion, as appropriate, in threshold for perception of passive movement (TPPM) quintiles
| Variable | 1st quintile n = 160 | 2nd quintile n = 154 | 3rd quintile n = 154 | 4th quintile n = 160 | 5th quintile n = 158 | F or χ 2 and p | F or χ 2 and p Adjusted for grip strength and Blessed Mental test scores |
|---|---|---|---|---|---|---|---|
| Single-leg stand time (s) | 25.6 (8.6) | 24.6 (9.0) | 20.9 (11.5) | 16.6 (12.5) | 12.4 (12.1) | 8.810, <0.001 | 7.140, <0.001 |
| Failed single-leg stance% | 3.1 | 4.5 | 12.3 | 21.3 | 32.9 | 9.476, 0.049 | 7.164, 0.127 |
| No perceived difficulty on level surface (%) | 93.1 | 92.2 | 90.9 | 85.4 | 70.3 | 9.861, 0.043 | 8.968, 0.062 |
| No perceived difficulty eyes closed (%) | 86.9 | 88.3 | 87.0 | 78.0 | 66.5 | 12.195, 0.016 | 10.933, 0.027 |
| Usual gait speed (m/s) | 1.23 (0.21) | 1.19 (0.19) | 1.14 (0.21) | 1.09 (0.22) | 0.99 (0.22) | 8.462, <0.001 | 6.102, <0.001 |
| Usual gait speed <0.8 m/s (%) | 0.6 | 0.6 | 2.6 | 6.7 | 13.3 | 9.860, 0.043 | 7.309, 0.120 |
| Fastest gait speed (m/s) | 1.98 (0.39) | 1.94 (0.37) | 1.81 (0.34) | 1.68 (0.37) | 1.55 (0.38) | 3.221, 0.012 | 2.027, 0.089 |
| Narrow-path gait speed (m/s) | 1.20 (0.35) | 1.20 (0.38) | 1.17 (0.36) | 1.07 (0.40) | 0.88 (0.49) | 4.266, 0.002 | 4.147, 0.003 |
| Failed narrow-path test (%) | 1.9 | 2.6 | 3.9 | 8.5 | 20.9 | 11.911, 0.018 | 8.428, 0.037 |
| Walking index | 8.5 (1.4) | 8.4 (1.4) | 8.0 (1.6) | 8.1 (1.6) | 7.4 (2.1) | 2.925, 0.020 | 2.268, 0.060 |
| Extended SPPB | 3.18 (0.29) | 3.11 (0.33) | 2.96 (0.47) | 2.81 (0.55) | 2.47 (0.76) | 15.524, <0.001 | 12.010, <0.001 |
| Standard SPPB | 11.90 (0.41) | 11.83 (0.05) | 11.56 (1.18) | 11.34 (1.3) | 10.81 (2.05) | 6.403, <0.001 | 4.886, 0.001 |
The analyses for single-leg stance, perceived balance difficulty, usual gait speed, walking Index, and SPPB were adjusted for age and sex, and the analyses for fastest gait speed, narrow-path gait speed, and failure on narrow-path test were adjusted for age, sex, and usual gait speed. Finally, as TPPM quintiles were significantly different in maximum grip strength and Blessed Mental test scores, all analyses were further adjusted for these two variables
The usual gait speed of persons in Q4 (p = 0.022) and Q5 (p < 0.001) was slower than those in Q1 and those in Q5 were slower than all other groups (p < 0.001–0.001). The distribution of those with usual gait speed <0.8 m/s increased with TPPM quintile (p = 0.043) (Table 2). Consequently, TPPM was higher in those with walking speed <0.8 m/s than those with usual gait speed faster than 0.8 m/s (3.5 ± 2.9 vs. 1.7 ± 2.0, F = 11.895, p = 0.001).
For the fastest gait speed, both Q4 and Q5 were significantly slower than the lowest two TPPM quintiles (p = 0.002–0.034). Narrow-path speed was also slower with increasing TPPM quintile (p < 0.001) with persons in Q5 slower than the remaining four groups (p < 0.001–0.004.). Sixty participants failed the narrow-path walking task. Their distribution increased across TPPM quintiles with a significantly higher proportion in Q5 (p = 0.01, Table 2). TPPM was higher (F = 6.817, p = 0.009) in those who failed the narrow-path walking task (3.5 ± 2.9) compared to those who did not (1.7 ± 1.9).
The walking index was lower with higher TPPM quintile (p = 0.021) where those in Q5 had lower scores than those in the other four quintiles (p = 0.002–0.045). Extended SPPB score was worse in Q4 and Q5 than the two lowest quintiles (p < 0.001–0.046). Q4 and Q5 were also significantly different from each other (p < 0.001). Overall, standard SPPB score was also lower with higher TPPM quintile (p < 0.001); those in Q5 had lower scores than those in the other four quintiles (p ≤ 0.001–0.002) (Table 2). Lastly, TPPM was higher in those who did (2.8 ± 3.2°) vs. those who did not (1.8 ± 2.0°) report self-imposed activity restriction related to fear of falling (F = 14.142, p < 0.001).
Discussion
This study measured ankle joint proprioception using TPPM in a population-based sample across the adult life span and demonstrated its relationship with objective as well as self-reported measures of standing balance, mobility, and physical function. Overall, those with TPPM >2.2° consistently demonstrated poor balance, mobility, and physical function.
In older persons, the single-leg stance time has been consistently shown to be associated with negative health events (Vellas et al. 1997; Jonsson et al. 2004; Lin et al. 2004). Further, the deterioration of balance in single-leg stance in young individuals with ankle sprain is proposed to be associated with ankle instability due to proprioceptive impairment (Akbari et al. 2006). Together, our results suggest that a significant decline in performance on this clinically important test in older adults could be associated with decline in ankle proprioception as detected by TPPM >1.4°. Additionally, average TPPM >3.3° was associated with inability to perform single-leg stance. When explored further, it was shown that adjusting for age and sex, those with <5 s single-leg stance time (the cutoff associated with injurious falls (Jonsson et al. 2004)) had significantly higher TPPM (2.8 ± 2.8) compared to those with ≥5 s (1.5 ± 1.3) (F = 26.617, p < 0.001). Overall, these findings possibly indicate physiological basis for poor performance in single-leg stance time test and a graded relationship of TPPM with the performance.
The association of TPPM with mobility appears task dependent. For example, TPPM >2.2° was associated with significantly slower usual gait speed compared to ≤2.2°. It is possible that even for walking without an additional self-imposed, sensory, or environmental challenge, a certain level of proprioceptive integrity is required. However, the average usual speed of Q5 was >0.8 m/s which is an indicator of mobility-related disability (Abellan van Kan et al. 2009). When the participants were classified using usual walking speed 0.8 m/s as a cutoff, the average TPPM of those with usual walking speed <0.8 m/s was 3.5°. These results suggest that a certain level of deterioration in ankle proprioception is possibly tolerated and/or could be compensated before reaching mobility-related disability as determined by usual gait speed.
In contrast, when the neuromuscular challenge was increased by asking the participants to walk as fast as they could, a lower threshold was identified (TPPM >1.4°) that was associated with significantly slower fastest walking speed. These results indicate that a higher proprioceptive acuity may be required as the mobility task challenge increases. As the gait speed is increased, the single support phase duration increases (Tulchin et al. 2009). A significantly worse single-leg stance test performance of those with TPPM >1.4° in this study indicates that worse performance on fast walking speed in the higher TPPM quintiles could be related to worse postural control in the single-support phase as a consequence of poor ankle proprioception. Conversely, the results of the narrow-path walking that imposes significantly higher challenges in the mediolateral direction were more compatible to those of usual gait speed. It is possible that the specific nature of this challenge that increase postural control demands in the mediolateral direction necessitates stronger integration of hip proprioceptive and vestibular information rather than the ankle proprioception. Nonetheless, more than half of those who failed the narrow-path walking task belonged to Q5 and the average TPPM of those who failed narrow-path test was almost double compared to those who completed the test suggesting that a threshold ankle proprioceptive acuity is possibly critical for successfully completing this task.
The questions included in the walking index tool pertain to the challenge with respect to walking distance rather than a temporal constraint. Therefore, the performance could apparently relate to muscle and cardiovascular system endurance rather than the proprioception. However, it is possible that the participants’ judgment in their ability to walk at least a quarter mile was associated with walking outdoors in a natural environment that is more challenging rather than the controlled and inert indoor environment. Thus, the adaptation of gait required for walking in natural environment (Patla 1991) is probably associated with ankle proprioception.
The present study specifically demonstrated that those with poor ankle proprioception measured as TPPM >1.4° have significantly worse extended SPPB performance and those with >2.2° have significantly worse standard SPPB performance. These results further corroborate our findings that higher acuity in ankle proprioception is required for more challenging tasks. These results are crucial because performance on SPPB is a robust marker of lower extremity functional limitations and a strong predictor of disability in older persons (Guralnik et al. 1994). Within the standard SPPB (adjusting for age, sex, grip strength, and cognition), it was found that the scores of 5-times sit-to-stand and gait speed components were significantly different between TPPM quintiles (p = 0.001 and <0.001, respectively). Conversely, there was no difference in the TPPM quintiles for standing balance component scores (p = 0.200). Tandem standing is the most challenging standing positon used in the standard SPPB. It is possible that, unlike single-leg stand, ankle proprioception that is particularly measured in the sagittal plane may not play a vital role.
To our knowledge, this is the first study that investigated association of ankle TPPM to self-reported activity restriction due to fear of falling. Previous studies that included foot sole vibrotactile sensitivity did not show robust relationship either with fear of falling or with fear-induced activity restriction (Deshpande et al. 2008). Considering the consistent relationship of TPPM with perceived standing balance and mobility, it is possible that ankle proprioception plays a more crucial role in fear of falling-related activity restriction. Future studies should explore the independent role of ankle proprioception in fear of falling and consequent activity restriction.
The TPPM quintiles were significantly different in strength (grip strength) and global cognition (Blessed Mental test scores). Adjusting for these variables, however, did not substantially alter the results thus indicating the robustness in these relationships. Two variables in this study use cutoffs at the lowest end of the performance spectrum (failed single-leg stand and usual gait speed <0.8 m/s). There were no significant differences in the TPPM quintiles for these two variables. Global cognition was a significant covariate for failed single-leg stand. For usual gait speed <0.8 m/s, the overall p value became non-significant after adjusting for global cognition and strength; however, neither global cognition nor strength was a significant covariate.
Several limitations of this study should be identified. The single-leg stance time was recorded for the leg that the participants felt comfortable with. However, the TPPM was tested for the right ankle due to the technical limitations of the equipment. Therefore, a direct inference for the role of TPPM in single-leg stance should be drawn with caution. Further, although fast walking and narrow-path walking conditions were included in this study, gait adaptations directly relevant to falls, e.g., due to trips and slips, were not included. To understand the mediatory role of ankle proprioception in falls of older persons, future studies should investigate its relationship with relevant walking tasks. Finally, it is possible that impaired ankle proprioception per se may not be a sole causal mechanism but an indicator of overall decline in sensory functions. Therefore, future longitudinal studies should investigate the risk associated with impaired ankle proprioception independent of vestibular or other somatosensory functions.
Conclusions
Ankle proprioception as measured by TPPM was significantly associated with age. More importantly, participants in higher TPPM quintiles demonstrated worse performance on objective as well as self-report measures of balance, mobility, and physical function. Overall, those with TPPM >2.2° consistently demonstrated poor balance, mobility, and physical function. However, with increase in challenge, TPPM >1.4° could be associated with significantly worse performance. TPPM >3° was associated with substantial decrement identified as failure to hold a single leg stance, walk within a narrow path and having usual gait speed <0.8 m/s. It is known that proprioceptive training improves balance and proprioception in young athletes with a history of ankle sprain (Berk 2010). Future intervention studies should explore the impact of such training on mobility and physical function of older persons.
Acknowledgments
The Baltimore Longitudinal Study of Aging was conducted as a component of the Intramural Research Program of the National Institute on Aging. This study was funded by National Institute on Aging’s Intramural Research Funds.
Abbreviations
- TPPM
Threshold for perception of passive movement
- BLSA
Baltimore Longitudinal Study of Aging
- SPPB
The short physical performance battery
- ANOVA
Analysis of variance
- Q1–Q5
1st quintile–5th quintile
References
- Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10):881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- Akbari M, Karimi H, Farahini H, Faghihzadeh S. Balance problems after unilateral lateral ankle sprains. J Rehabil Res Dev. 2006;43(7):819–824. doi: 10.1682/JRRD.2006.01.0001. [DOI] [PubMed] [Google Scholar]
- Berk KA. Is proprioceptive training effective in reducing the recurrence of ankle sprains among athletes? Philadelphia, Pennsylvania: Philadelphia College of Osteopathic Medicine; 2010. [Google Scholar]
- Bhanpuri NH, Okamura AM, Bastian AJ. Predictive modeling by the cerebellum improves proprioception. J Neurosci. 2013;33(36):14301–14306. doi: 10.1523/JNEUROSCI.0784-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colledge NR, Cantley P, Peaston I, Brash H, Lewis S, Wilson JA. Ageing and balance: the measurement of spontaneous sway by posturography. Gerontology. 1994;40(5):273–278. doi: 10.1159/000213596. [DOI] [PubMed] [Google Scholar]
- Deshpande N, Connelly DM, Culham EG, Costigan PA. Reliability and validity of ankle proprioceptive measures. Arch Phys Med Rehabil. 2003;84(6):883–889. doi: 10.1016/S0003-9993(03)00016-9. [DOI] [PubMed] [Google Scholar]
- Deshpande N, Metter EJ, Bandinelli S, Lauretani F, Windham BG, Ferrucci L. Psychological, physical, and sensory correlates of fear of falling and consequent activity restriction in the elderly: the InCHIANTI study. Am J Phys Med Rehabil. 2008;87(5):354–362. doi: 10.1097/PHM.0b013e31815e6e9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick R, McCloskey DI. Proprioceptive, visual and vestibular thresholds for the perception of sway during standing in humans. J Physiol. 1994;478:173–186. doi: 10.1113/jphysiol.1994.sp020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goble DJ, Coxon JP, Wenderoth N, Van Impe A, Swinnen SP. Proprioceptive sensibility in the elderly: degeneration, functional consequences and plastic-adaptive processes. Neurosci Biobehav Rev. 2009;33:271–278. doi: 10.1016/j.neubiorev.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Coxon JP, Van Impe A, Geurts M, Doumas M, Wenderoth N, Swinnen SP. Brain activity during ankle proprioceptive stimulation predicts balance performance in young and older adults. J Neurosci. 2011;31:16344–16352. doi: 10.1523/JNEUROSCI.4159-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.M85. [DOI] [PubMed] [Google Scholar]
- Jonsson E, Seiger Å, Hirschfeld H. One-leg stance in healthy young and elderly adults: a measure of postural steadiness? Clin Biomech. 2004;19:688–694. doi: 10.1016/j.clinbiomech.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short orientation-memory-concentration test of cognitive impairment. Am J Psychiatrv. 1983;140(6):734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- Ko SU, Simonsick E, Deshpande N, Ferrucci L. Sex-specific age associations of ankle proprioception test performance in older adults: results from the Baltimore Longitudinal Study of Aging. Age Ageing. 2015;44(3):485–490. doi: 10.1093/ageing/afv005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MR, Hwang HF, Hu MH, Wu HD, Wang YW, Huang FC. Psychometric comparisons of the timed up and go, one-leg stand, functional reach, and Tinetti balance measures in community dwelling older people. J Am Geriatr Soc. 2004;52:1343–1348. doi: 10.1111/j.1532-5415.2004.52366.x. [DOI] [PubMed] [Google Scholar]
- Lord SR, Clark RD, Webster IW. Postural stability and associated physiological factors in a population of aged persons. J Gerontol. 1991;46:M69–M76. doi: 10.1093/geronj/46.3.M69. [DOI] [PubMed] [Google Scholar]
- Morisawa Y. Morphological study of mechanoreceptors on the coracoacromial ligament. J Orthop Sci. 1998;3(2):102–110. doi: 10.1007/s007760050029. [DOI] [PubMed] [Google Scholar]
- Patla A. Adaptability of human gait: Implications for the control of locomotion. Amsterdam: Pub North Holland; 1991. [Google Scholar]
- Pearson KG. Generating the walking gait: role of sensory feedback. Prog Brain Res. 2004;143:123–129. doi: 10.1016/S0079-6123(03)43012-4. [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Rothwell JC. Modulation of proprioceptive integration in the motor cortex shapes human motor learning. J Neurosci. 2012;27:9000–9006. doi: 10.1523/JNEUROSCI.0120-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shock NWGR, Andres RA, Arenberg D, et al. Design and operation of the Baltimore longitudinal study of aging. Bethesda, MD: NIH; 1984. [Google Scholar]
- Simonsick EM, Newman AB, Nevitt MC, Kritchevsky SB, Ferrucci L, Guralnik JM, Harris T, and for the Health ABC group Measuring higher level physical function in well-functioning older adults expanding familiar approaches in the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2001;56(10):M644–M649. doi: 10.1093/gerona/56.10.M644. [DOI] [PubMed] [Google Scholar]
- Simonsick EM, Newman AB, Ferrucci L, Satterfield S, Harris TB, Rodondi N, Bauer DC, Health ABC Study Subclinical hypothyroidism and functional mobility in older adults. Arch Intern Med. 2009;169(21):2011–2017. doi: 10.1001/archinternmed.2009.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen KL, Hollands MA, Patla E. The effects of human ankle muscle vibration on posture and balance during adaptive locomotion. Exp Brain Res. 2002;143(1):24–34. doi: 10.1007/s00221-001-0962-z. [DOI] [PubMed] [Google Scholar]
- Swash M, Fox KP. The effect of age on human skeletal muscle. Studies of the morphology and innervation of muscle spindles. J Neurol Sci. 1972;16(4):417–432. doi: 10.1016/0022-510X(72)90048-2. [DOI] [PubMed] [Google Scholar]
- Tulchin KOM, Adolfsen S, Karol L. The effects of walking speed on multisegment foot kinematics in adults. J Appl Biomech. 2009;25:377–386. doi: 10.1123/jab.25.4.377. [DOI] [PubMed] [Google Scholar]
- Vellas B, Rubenstein L, Ousset P, et al. One-leg standing balance and functional status in a population of 512 community-living elderly persons. Aging. 1997;9:95–98. doi: 10.1007/BF03340133. [DOI] [PubMed] [Google Scholar]
- Verschueren SM, Brumagne S, Swinnen SP, Cordo PJ. The effect of aging on dynamic position sense at the ankle. Behav Brain Res. 2002;15;136(2):593–603. doi: 10.1016/S0166-4328(02)00224-3. [DOI] [PubMed] [Google Scholar]
- Westlake KP, Culham EG. Influence of testing position and age on measures of ankle proprioception. Adv Physiother. 2006;8:41–48. doi: 10.1080/14038190600589226. [DOI] [Google Scholar]