Abstract
We have reported telomere attrition in β and α cells of the pancreas in elderly patients with type 2 diabetes, but it has not been explored how the telomere lengths of these islet cells change according to age in normal subjects. To examine the telomere lengths of β and α cells in individuals without diabetes across a wide range of ages, we conducted measurement of the telomere lengths of human pancreatic β and α cells obtained from 104 autopsied subjects without diabetes ranging in age from 0 to 100 years. As an index of telomere lengths, the normalized telomere-centromere ratio (NTCR) was determined for β (NTCRβ) and α (NTCRα) cells by quantitative fluorescence in situ hybridization (Q-FISH). We found NTCRβ and NTCRα showed almost the same levels and both decreased according to age (p < 0.001 for both). NTCRs decreased more rapidly with age and were more widely distributed (p = 0.036 for NTCRβ, p < 0.001 for NTCRα) in subjects under 18 years of age than in subjects over 18 years. There was a positive correlation between NTCRβ and NTCRα only among adult subjects (p < 0.001). In conclusion, the telomeres of β and α cells become shortened with normal aging process.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-016-9923-0) contains supplementary material, which is available to authorized users.
Keywords: Telomere, Aging, Beta cell, Alpha cell, Quantitative fluorescence in situ hybridization (Q-FISH)
Introduction
In recent years, diabetes mellitus has shown an explosive increase worldwide, with an estimated 285 million cases (6.4 % of the total population) in 2010 (Nolan et al. 2011). Among diabetic patients, type 2 diabetes mellitus (T2DM) is the most prevalent, accounting for over 90 % of patients. Since a long disease duration or poor control is associated with an increased risk of various vascular complications, this not only compromises the quality of life of patients but also imposes a heavier burden on medical and social welfare costs, and therefore, prevention of the onset and progression of T2DM is an urgent priority.
In developed countries, the number of elderly patients with type 2 diabetes mellitus (T2DM) has been rising rapidly. Several mechanisms are believed to account for this increase. In the elderly, muscle mass generally decreases while fat mass increases, both of which induce insulin resistance. In addition, insulin secretion is decreased in many elderly T2DM patients accompanied by a decrease in the number of pancreatic islet β cells (Butler et al. 2003). Turnover of β cells in adults is quite characteristic. A rodent study reported that a single new β cell is supplied by replication of another mature β cell, rather than through differentiation from a precursor cell (Dor et al. 2004), whereas data on turnover of human islet β cells are very limited. However, several previous studies have shown that the rate of β cell proliferation decreases with age in the human pancreas (Maedler et al. 2006; Reers et al., 2009).
One of the key mechanisms that may be involved in susceptibility to T2DM in the elderly might be critical shortening of the telomeres in β cells. Telomeres are a repeating sequence of G-rich bases located at the ends of linear eukaryotic chromosomes, and it is thought that this sequence has various roles including protection of chromosomes against degeneration, reconstruction, fusion, and loss (Blackburn 1991). In most somatic cells, the telomere shortens with each cell division, and in cells with a telomere length below a certain threshold, cell division stops. This phenomenon, known as cellular senescence, involves p53, a tumor-suppressing gene. Activation of p53 also induces apoptosis, thus inhibiting tumorigenesis in aged cells (Deng and Chang, 2007). However, this series of reactions may lead to loss of aged pancreatic β cells when exposed to sustained stimulation with a high glucose concentration, and this might accelerate apoptosis (Donath et al. 1999), as aged β cells have lower replication potential.
Our group has reported that telomere attrition with age occurs in cells of various organs, except for cerebral cortex cells and myocardial cells (Takubo et al. 2002; Takubo et al. 2010a). However, no reported studies have examined changes in telomere length with age in pancreatic islet cells.
In a recent study, we found that the telomeres of pancreatic islet β cells in elderly patients with T2DM were shorter than those in elderly individuals without diabetes (Tamura et al. 2014). Although the precise reason for this was unclear, we speculated that it might be attributable to a vicious cycle of diabetes and telomere attrition: hyperglycemia induces oxidative stress, which may damage telomeric ends in β cells, and this telomere attrition may induce accelerated cellular senescence and apoptotic cell death, thus in turn reducing the number of β cells and causing hyperglycemia. Since we studied only an elderly group (over 60 years) as the T2D patient control in the previous study, we did not find a negative correlation between age and the telomere length of β cells in patients without diabetes. In the present study, therefore, we included subjects covering a very wide age range from infants to centenarians (0–100 years) to help clarify the influence of age on the telomere length of β cells. We also examined the relationship between aging and telomere length in pancreatic α cells.
Materials and methods
Subjects
We analyzed all of the pancreas samples from Japanese patients consecutively autopsied at our hospitals between 1999 and 2010. As described previously, the telomeres of β and α cells in diabetic patients were significantly shorter than those of control patients (Tamura et al. 2014), and diabetes seems to be a potent factor impacting on telomere shortening in pancreatic cells. On the other hand, the main aim of this study was to elucidate the relationship between telomere length and aging in pancreatic cells. Therefore, we set two thresholds: first, we omitted diabetic patients from the subjects analyzed. The criteria for exclusion were (1) patients who had maximum HbA1c values exceeding 6.5 % and been diagnosed as having diabetes mellitus and (2) those who had been treated with oral hypoglycemic agents or insulin. Secondly, we disqualified samples showing autolysis. We analyzed all of the remaining samples (104 cases: 53 male and 51 female) without further selection. Approval for this study was obtained from the ethics committee of Tokyo Metropolitan Geriatric Hospital and Institute of Gerontology.
Tissue processing and measurement of telomere length
All tissue samples were fixed in formalin and then embedded in paraffin employing standard tissue processing methods. Before measurement of telomere length, 3-μm-thick tissue sections were first prepared from paraffin-embedded human pancreatic tail specimens and subjected to hematoxylin and eosin (H&E) staining. Any specimens showing areas of marked autolysis or inflammatory cell accumulation were excluded. Telomere lengths of cells in tissue sections were determined using a quantitative fluorescence in situ hybridization (Q-FISH) method reported previously (Aida et al., 2007, 2010). Briefly, 2-μm-thick tissue sections were hybridized with Cy3-labeled telomere and FITC-labeled centromere peptide nucleic acid probes. Images were captured by a fluorescence imaging microscope at ×40 magnification using the Image-Pro Plus software package (version 7.0; Media Cybernetics Co., Ltd., Silver Spring, MD, USA). Based on the fluorescence intensities, the telomere length index was determined from the telomere-centromere ratio (TCR) of each cell in the sample using our own analytical software package, Tissue-Telo Ver.3.1 (Takubo et al. 2010b; Tamura et al. 2014). To normalize for sample preparation, the TCR was also extracted from a control cell-block section placed on the same slide consisting of sectioned cells from a cultured cell strain, TIG-1, which is a human fetal lung-derived fibroblast-like cell strain established at our facility (Aida et al., 2008). TIG-1 is known to stop dividing after a certain number of subcultures (Ohashi et al., 1980), and its telomere length has been determined by Southern blotting. We used cells with a population doubling level (PDL) of 34, and the normalized TCR (NTCR) was calculated from the TCR of the pancreatic cells and the median (average 0.49) TCR of the TIG-1 cells.
Identification of α and β cells
Beta and α cells were distinguished using an anti-insulin antibody (no. N1542, Dako, Glostrup, Denmark) and an anti-glucagon antibody (no. M182, Takara Bio Inc., Shiga, Japan) and a secondary antibody labeled with Cy-5 (ab6567, Abcam plc, Cambridge, UK) after FISH. Identification of these cells was done by skilled pathologists (J.A. and K.T.). Between 118 and 290 β and α cells were analyzed in each subject. The median NTCR values, NTCRβ and NTCRα, were used as a representative telomere estimate for each subject’s β and αcells, respectively.
Statistical analyses
Statistical analyses were conducted using the R language (http://www.r-project.org) and the SPSS Statistics 20 software package (IBM, Armonk, NY, USA). Regression analysis of NTCRs and age was carried out using piecewise linear fits to the NTCRβ and NTCRα values with a break point at age 18 years, as described previously (Aubert et al. 2012). F tests were used to compare the fit of the model to the test data. ANOVA was applied to test whether the fits for NTCRβ and NTCRα were significantly different from one another. We next compared the homogeneity of variances of NTCRβ and NTCRα between subjects under 18 years and subjects over 18 years using the Levene’s test. Finally, correlations between age and NTCR in subjects aged 18 years and older and correlations between NTCRβ and NTCRα were analyzed with Spearman’s correlation test. In all comparisons, differences of p < 0.05 were considered to be significant.
Results
FISH images
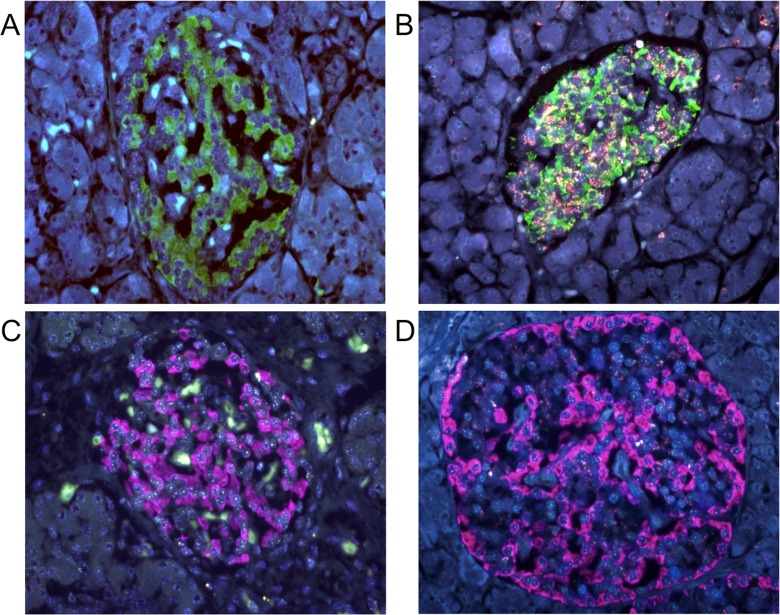
Representative FISH images of specimens from young and old subjects are shown in Fig. 1. Beta cells were identified by staining with anti-insulin antibody (cytoplasmic green), and α cells by staining with anti-glucagon antibody (cytoplasmic pink). Telomere (red) and centromere (green) signals were observed in the nuclei of the cells. In comparison with specimens from young subjects, red telomere signals were weaker in both islet cell types in samples from elderly subjects.
Fig. 1.
Representative FISH images showing immunofluorescence for insulin (a, b) and glucagon (c, d) in specimens from young (a, c) and old (b, d) subjects. Original magnification, ×40. In all slides, nuclear red, green, and blue signals are those for telomere-Cy3, centromere-FITC, and DAPI counterstaining for DNA, respectively. a, b Cytoplasmic green indicates the insulin-Cy-5 signal. a Image of a specimen from a 4-month-old infant. The NTCR for insulin-positive cells was 3.28. The reddish ivory color is not the telomere signal but non-specific fluorescence from the cytoplasm of islet cells. Red telomere and green centromere signals are always observed within the nuclei. b Images of a specimen from a 72-year-old woman. The NTCR for insulin-positive cells was 0.89. c, d Cytoplasmic pink indicates the glucagon-Cy-5 signal. c Image of a specimen from a 0-day-old neonate. The NTCR for glucagon-positive cells was 2.85 in this sample. d Image of a specimen from an 84-year-old woman. The NTCR for glucagon-positive cells was 0.88 in this sample
Correlation between NTCRβ, NTCRα, and age
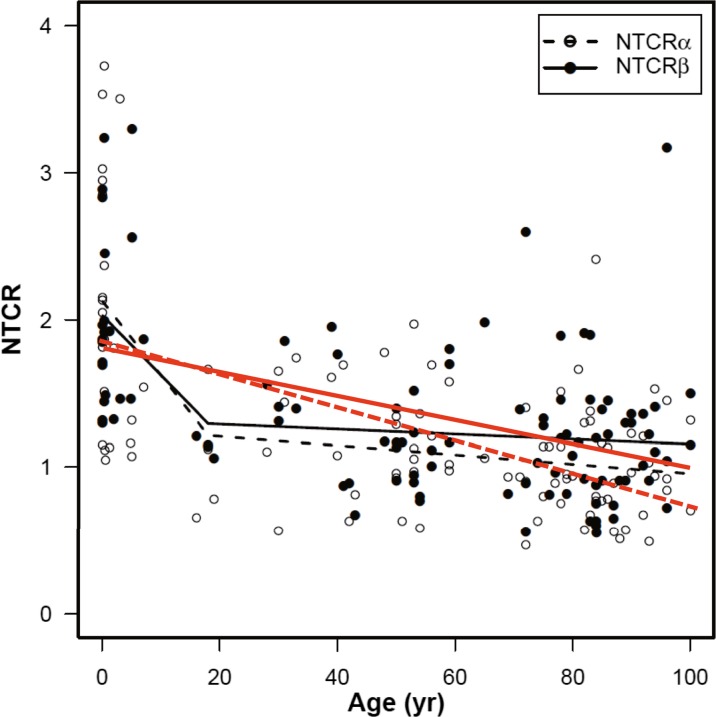
Figure 2 shows the correlation between NTCR and subject age for islet β- (NTCRβ) and α- (NTCRα) cells of all 104 tested subjects, using a simple linear regression model and a piecewise linear regression model with a breakpoint at 18 years of age. Scatter plot analyses of the whole sample provided robust inverse correlations between telomere length and age in both β and α cells (p < 0.001) (shown as red lines in Fig. 2). The regression models of NTCR to age for both β and α cells were significant (p < 0.001). Moreover, the negative correlation curves being steeper for subjects aged under 18 years. NTCRβ and NTCRα showed almost the same levels. The model fits to NTCRβ and NTCRα were not significantly different from one another (p = 0.229). However, whereas NTCRα was significantly and negatively correlated with age in subjects over 18 years, there was no significant correlation between NTCRβ and age in those in the same age range (Supplementary Fig. 1). Both NTCRβ and NTCRα were more widely distributed in subjects under 18 years than in subjects over 18 years (p = 0.036 for NTCRβ, p < 0.001 for NTCRα).
Fig. 2.
Telomere length dynamics in pancreatic α and β cells relevant to age. scatter plot analyses of the whole sample by a simple regression model, statistically significant regression lines are shown in red with decreasing slopes (p < 0.001, Solid line for NTCRβ: y = −0.0081x + 1.81, and dotted line for NTCRα: y = −0.011x + 1.86). In piecewise linear regression fits with a breakpoint at 18 years of age , regression lines are shown in black for each cell type (solid line for NTCRβ −0.0408X + 2.029 (0–18 years), −0.0017X + 1.326 (18–100 years), and dotted line for NTCRα −0.0506X + 2.126 (0–18 years), −0.0032X + 1.273 (18–100 years)). The regression models of NTCR to age for both β and α cells were significant (p < 0.001). The data distributions for NTCRα and NTCRβ were not significantly different from one another (p = 0.229)
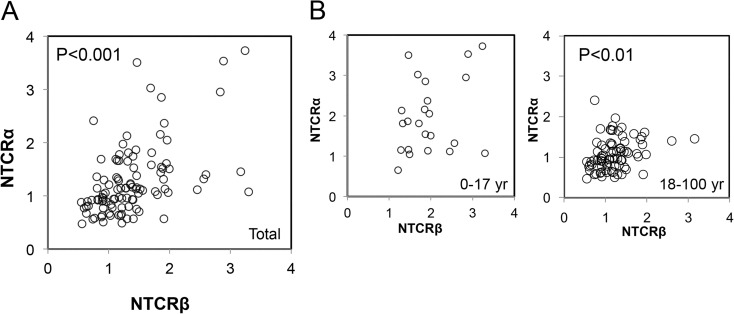
Correlation between NTCRβ and NTCRα
Finally, we investigated the correlation between NTCRβ and NTCRα. NTCRβ was significantly and positively correlated with NTCRα (p < 0.001) (Fig. 3a). When correlations were examined separately for the age groups 0–17 and 18–100 years, the correlation between NTCRβ and NTCRα was significant only in the older group (r = 0.352, p < 0.01) (Fig. 3b).
Fig. 3.
Correlation between NTCRβ and NTCRα. (p < 0.001). a Plots for the subjects as a whole. NTCRβ and NTCRα were positively correlated with each other (p < 0.001). b Plots for the subjects divided into two age categories, under 18 and 18 years and over. A significant positive correlation was found only for the adult group (p = 0.01)
Discussion
In a previous study, we found no statistically significant association between telomere length and age in pancreatic islet β and α cells in elderly subjects (Tamura et al. 2014). In fact, scatter plot analysis of this group yielded regression lines with almost flat slopes. In the present study, we examined the telomere lengths of these cells throughout the lifespan of non-diabetic patients, and found that telomere shortening progressed with aging in both cell types (Fig. 2), as has been the case for many other somatic cells reported previously (Takubo et al. 2010b).
Frenck and colleagues demonstrated that telomere attrition rate in human blood leukocytes vary remarkably at different ages, a rapid decline of telomere from birth through about age 4, followed by an apparent stable phase until early adulthood, and a gradual decline (final phase) later in life (Frenck et al. 1998). Recently, Lansdorp and colleagues provided further confirmative data by flow FISH analysis, demonstrating that individuals showed a broad range in average telomere length in granulocytes and lymphocytes at any given age and the average telomere length declined with age at a rate that differed between age-specific breakpoints and between cell types. (Aubert et al. 2012).
In our previous study using Southern blotting, we had demonstrated that telomeres shortened with age in the pancreas, including all cell types (Ishii et al. 2006). In that study, we used samples from the pancreatic head, which consists mainly of pancreatic excretory cells and few islet cells; the telomere lengths might reflect mainly those of pancreatic excretory cells. Intriguingly, the difference between young and middle age was statistically significant, but the difference between middle age and elderly was not significant. Most recently, we have analyzed the telomere dynamics of human pancreatic ductal epithelial cell by tissue Q-FISH (same method of present study) and found that the profile of the telomere attrition was essentially similar to that obtained by previous Southern blotting analysis (Matsuda et al., 2015).
However, the profiles of the regression lines for ductal cells and islet cells seem slightly different in some respects. First, the means of the initial NTCR values are diverse for ductal cells (acinar < centroacinar < ductal cells), whereas the means for NTCRβ and NTCRα were almost the same (Fig. 2). Second, the slopes of the regression lines for islet cells during the middle age to centenarian period were almost flat (α showing a lower and steeper decline line than β) (Fig. 2), whereas the plot for total ductal cells provided a robust significant declining slope during the middle age (over 50 years) to elderly period. (The slope for the duct sample without pancreatic intraepithelial neoplasia (PanIN) changes showed a more gentle decline without statistical significance, whereas that for ductal epithelia with PanIN changes showed a steeper decline than the total sample, with robust significance.) These distinctive profiles of telomere decline among islet cells and ductal cells may reflect the lineage-specific proliferation patterns (Kopp et al., 2016), and may differentially affect the pathophysiology of T2DM and pancreatic carcinoma, respectively.
Cnop et al. measured the lipofuscin content of islet cells and reported that the number of islet cells in humans is largely established by age 20 years and then remains constant with a low turnover rate (Cnop et al. 2010, 2011). More recently, Mizukami et al. reported that Ki67—a marker for cell proliferation—positive rate of β and non-β islet cells is prominent in childhood but significantly decreased over 20 years of age (Mizukami et al., 2014). The pattern of telomere shortening in islet cells is compatible with the results of Cnop’s and Mizukami’s, suggesting that rapid islet cell telomere reduction in childhood and a slow reduction in adulthood. We also found greater variation in telomere length distribution of islet cells in younger than in older individuals (Fig. 2). One possible explanation for this observation is that β cell replication and mass expansion occur mainly in infancy and through to adulthood, and the growth rate in childhood is assumed to be highly variable among individuals (Meier et al. 2008), thus explaining the marked range of islet cell telomere length in childhood. In contrast, since the islet cell complement in adults is largely complete, and—as described above—hardly any cell replication or death occurs, the range of telomere length in adult islet cells is not wide. However, since the same distribution pattern is seen also in ductal cells (data not shown), the precise mechanism is yet to be clarified.
Recently accumulated evidence has suggested that, even in adult (pancreatic) tissues, each lineage of differentiated cells sustains tissue homeostasis by limited self-renewal (Kopp et al., 2016). Our present data on lineage-specific telomere attrition dynamics support this. On the other hand, various stimuli can trigger tissue injury-activated multiple signal pathways; for example, hyperglycemia may trigger the reactive oxidative stress—cytokine—transcription factor system, or Wnt signaling system, in self-renewal adult stem cells (Karin and Clevers, 2016).
In the present study, we also found that the telomere lengths of β and α cells were positively correlated with each other (Fig. 3a). This finding is compatible with our former observation that telomere length appears unique to each individual and correlated across many tissues in the body (Takubo et al. 2002). Another noteworthy finding of our present study was that the correlation of NTCR between β and α cells was significant only in adults and not in younger subjects (Fig. 3b). Although the biology of α cells in childhood is poorly understood, if the growth characteristics of α cells during this period are similar to those of β cells, and if the timing of maximum cell replication capacity varies not only between individuals but also between types of islet cells in any given individual, then this would explain the wide range seen in childhood. On the other hand, as telomere shortening in islet cells of each adult individual has largely been completed, the degree of telomere shortening in β and α cells could be similar in that individual. Further study will be needed to clarify the mechanism of aging in each type of islet cell.
In a previous study, we showed that the NTCRβ was significantly lower in elderly patients with T2DM than in those without (Tamura et al. 2014). Telomere shortening in β cells was also observed in patients who had been diagnosed as having T2DM only recently (unpublished data), suggesting that telomere attrition could already have been advanced before disease onset. To date, the time course of telomere attrition in β cells before the onset of T2DM is unclear, several hypotheses can be suggested that (1) short telomere may be determined congenitally, (2) telomeres may undergo accelerated shortening in childhood due to accelerated cellular replication during this period, and (3) telomere attrition may occur later, mainly in adulthood through unknown causes. Model animals showing spontaneous onset of diabetes might provide some clues to the mechanisms involved, but since the life history of β cells differs considerably between rodents and humans, this could present a considerable challenge. Some ground-breaking methods for determination of telomere length in human β cells in situ would be strongly desirable.
In conclusion, we have demonstrated that telomere attrition occurs with aging in both pancreatic β and α cells in individuals without diabetes. Since dysfunction of β cells due to a reduction in their number is supposed to be the major step responsible for onset of T2DM in the elderly, telomere attrition could play a key role in this process. Our findings may be of help for considering various forms of intervention during adolescence to middle age that could prevent the onset or progression of T2DM in the elderly through maintenance of β cell telomere length.
Electronic supplementary material
Correlation between age and NTCR in subjects aged 18 yr. and older. A significant negative correlation was found only for NTCRα (p = 0.02), but not for NTCRβ (p = 0.34). (GIF 45 kb)
Acknowledgment
This work was supported by Japan Society for the Promotion of Science (JSPS) Grant-in Aid for Scientific Research (C) Grant Number 23590440. We thank Dr. Donald K. Ingram for his constructive comments.
Abbreviations
- T2DM
Type 2 diabetes mellitus
- HbA1c
Glycated hemoglobin
- Q-FISH
Quantitative fluorescence in situ hybridization
- FITC
Fluorescein isothiocyanate
- TCR
Telomere-centromere ratio
- NTCR
Normalized TCR
- NTCRα
NTCR values for α cells
- NTCRβ
NTCR values for β cells
- CA/DT
Centroacinar/terminal duct
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Contributor Information
Yoshiaki Tamura, Phone: +81-3-3964-3241, FAX: +81-3-3579-4776, Email: tamurayo@tmghig.jp.
Naoshi Ishikawa, Phone: +81-3-3964-3241, FAX: +81-3-3579-4776, Email: naoshi@tmig.or.jp.
References
- Aida J, Izumiyama-Shimomura N, Nakamura K, Ishii A, Ishikawa N, Honma N, Kurabayashi R, Kammori M, Poon SS, Arai T, Takubo K. Telomere length variations in 6 mucosal cell types of gastric tissue observed using a novel quantitative fluorescence in situ hybridization method. Hum Pathol. 2007;38:1192–1200. doi: 10.1016/j.humpath.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Aida J, Izumiyama-Shimomura N, Nakamura K, Ishikawa N, Poon SS, Kammori M, Sawabe M, Arai T, Matsuura M, Fujiwara M, Kishimoto H, Takubo K. Basal cells have longest telomeres measured by tissue Q-FISH method in lingual epithelium. Exp Gerontol. 2008;43:833–839. doi: 10.1016/j.exger.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Aida J, Izumo T, Shimomura N, Nakamura K, Ishikawa N, Matsuura M, Poon SS, Fujiwara M, Sawabe M, Arai T, Takubo K. Telomere lengths in the oral epithelia with and without carcinoma. Eur J Cancer. 2010;46:430–438. doi: 10.1016/j.ejca.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Aubert G, Baerlocher GM, Vulto I, Poon SS, Lansdorp PM. Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genes. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn EH. Structure and function of telomeres. Nature. 1991;350:569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- Cnop M, Hughes SJ, Igoillo-Esteve M, Hoppa MB, Sayyed F, van de Laar L, Gunter JH, de Koning EJ, Walls GV, Gray DW, Johnson PR, Hansen BC, Morris JF, Pipeleers-Marichal M, Cnop I, Clark A. The long lifespan and low turnover of human islet beta cells estimated by mathematical modelling of lipofuscin accumulation. Diabetologia. 2010;53:321–330. doi: 10.1007/s00125-009-1562-x. [DOI] [PubMed] [Google Scholar]
- Cnop M, Igoillo-Esteve M, Hughes SJ, Walker JN, Cnop I, Clark A. Longevity of human islet α- and β-cells. Diabetes Obes Metab. 2011;13(Suppl 1):39–46. doi: 10.1111/j.1463-1326.2011.01443.x. [DOI] [PubMed] [Google Scholar]
- Deng Y, Chang S. Role of telomeres and telomerase in genomic instability, senescence and cancer. Lab Investig. 2007;87:1071–1076. doi: 10.1038/labinvest.3700673. [DOI] [PubMed] [Google Scholar]
- Donath MY, Gross DJ, Cerasi E, Kaiser N. Hyperglycemia- induced beta-cell apoptosis in pancreatic islets of Psammomys obesus during development of diabetes. Diabetes. 1999;48:738–744. doi: 10.2337/diabetes.48.4.738. [DOI] [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- Frenck RW, Jr, Blackburn EH, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci U S A. 1998;95(10):5607–5610. doi: 10.1073/pnas.95.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A, Nakamura K, Kishimoto H, Honma N, Aida J, Sawabe M, Arai T, Fujiwara M, Takeuchi F, Kato M, Oshimura M, Izumiyama N, Takubo K. Telomere shortening with aging in the human pancreas. Exp Gerontol. 2006;41:882–886. doi: 10.1016/j.exger.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Karin M, Clevers H. Reparative inflammation takes charge of tissue regeneration. Nature. 2016;529:307–315. doi: 10.1038/nature17039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp JL, Grompe M, Sander M (2016) Stem cells versus plasticity in liver and pancreas regeneration. Nat Cell Biol:238–245. doi:10.1038/ncb3309 [DOI] [PubMed]
- Maedler K, Schumann DM, Schulthess F, Oberholzer J, Bosco D, Berney T, Donath MY. Aging correlates with decreased beta-cell proliferative capacity and enhanced sensitivity to apoptosis: a potential role for Fas and pancreatic duodenal homeobox-1. Diabetes. 2006;55:2455–2462. doi: 10.2337/db05-1586. [DOI] [PubMed] [Google Scholar]
- Matsuda Y, Ishiwata T, Izumiyama-Shimomura N, Hamayasu H, Fujiwara M, Tomita K, Hiraishi N, Nakamura K, Ishikawa N, Aida J, Takubo K, Arai T. Gradual telomere shortening and increasing chromosomal instability among PanIN grades and normal ductal epithelia with and without cancer in the pancreas. PLoS One. 2015;10 doi: 10.1371/journal.pone.0117575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 2008;57:1584–1594. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami H, Takahashi K, Inaba W, Osonoi S, Kamata K, Tsuboi K, Yagihashi S. Age-associated changes of islet endocrine cells and the effects of body mass index in Japanese. J Diabetes Investig. 2014;12:38–47. doi: 10.1111/jdi.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet. 2011;378:169–181. doi: 10.1016/S0140-6736(11)60614-4. [DOI] [PubMed] [Google Scholar]
- Ohashi M, Aizawa S, Ooka H, Ohsawa T, Kaji K, Kondo H, Kobayashi T, Noumura T, Matsuo M, Mitsui Y, Murota S, Yamamoto K, Ito H, Shimada H, Utakoji T. A new human diploid cell strain, TIG-1, for the research on cellular aging. Exp Gerontol. 1980;15:121–133. doi: 10.1016/0531-5565(80)90083-2. [DOI] [PubMed] [Google Scholar]
- Reers C, Erbel S, Esposito I, Schmied B, Büchler MW, Nawroth PP, Ritzel RA. Impaired islet turnover in human donor pancreata with aging. Eur J Endocrinol. 2009;160:185–191. doi: 10.1530/EJE-08-0596. [DOI] [PubMed] [Google Scholar]
- Takubo K, Izumiyama-Shimomura N, Honma N, Sawabe M, Arai T, Kato M, Oshimura M, Nakamura K. Telomere lengths are characteristic in each human individual. Exp Gerontol. 2002;37:523–531. doi: 10.1016/S0531-5565(01)00218-2. [DOI] [PubMed] [Google Scholar]
- Takubo K, Aida J, Izumiyama-Shimomura N, Ishikawa N, Sawabe M, Kurabayashi R, Shiraishi H, Arai T, Nakamura K. Changes of telomere length with aging. Geriatr Gerontol Int. 2010;10(Suppl 1):S197–S206. doi: 10.1111/j.1447-0594.2010.00605.x. [DOI] [PubMed] [Google Scholar]
- Takubo K, Fujita M, Izumiyama N, Nakamura K, Ishikawa N, Poon SS, Fujiwara M, Sawabe M, Matsuura M, Grabsch H, Arai T, Aida J. Q-FISH analysis of telomere and chromosome instability in the oesophagus with and without squamous cell carcinoma in situ. J Pathol. 2010;221:201–209. doi: 10.1002/path.2704. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Izumiyama-Shimomura N, Kimbara Y, Nakamura K, Ishikawa N, Aida J, Chiba Y, Mori S, Arai T, Aizawa T, Araki A, Takubo K, Ito H. β-cell telomere attrition in diabetes: inverse correlation between HbA1c and telomere length. J Clin Endocrinol Metab. 2014;99:2771–2777. doi: 10.1210/jc.2014-1222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlation between age and NTCR in subjects aged 18 yr. and older. A significant negative correlation was found only for NTCRα (p = 0.02), but not for NTCRβ (p = 0.34). (GIF 45 kb)