Abstract
The N-methyl-d-aspartate receptor (NMDAr) is particularly vulnerable to aging. The GluN2B subunit of the NMDAr, compared to other NMDAr subunits, suffers the greatest losses of expression in the aging brain, especially in the frontal cortex. While expression levels of GluN2B mRNA and protein in the aged brain are well documented, there has been little investigation into age-related posttranslational modifications of the subunit. In this study, we explored some of the mechanisms that may promote differences in the NMDAr complex in the frontal cortex of aged animals. Two ages of mice, 3 and 24 months, were behaviorally tested in the Morris water maze. The frontal cortex and hippocampus from each mouse were subjected to differential centrifugation followed by solubilization in Triton X-100. Proteins from Triton-insoluble membranes, Triton-soluble membranes, and intracellular membranes/cytosol were examined by Western blot. Higher levels of GluN2B tyrosine 1472 phosphorylation in frontal cortex synaptic fractions of old mice were associated with better reference learning but poorer cognitive flexibility. Levels of GluN2B phosphotyrosine 1336 remained steady, but there were greater levels of the calpain-induced 115 kDa GluN2B cleavage product on extrasynaptic membranes in these old good learners. There was an age-related increase in calpain activity, but it was not associated with better learning. These data highlight a unique aging change for aged mice with good spatial learning that might be detrimental to cognitive flexibility. This study also suggests that higher levels of truncated GluN2B on extrasynaptic membranes are not deleterious to spatial memory in aged mice.
Keywords: Aging, Morris water maze, Learning and memory, NMDA receptor, Posttranslational modification, Calpain
Introduction
A vulnerability of the aging process is cognitive decline. Signs of memory decline may begin around 50 years of age, and some aspects of cognition begin to decline around 40 years of age (Scherr et al. 1988; Singh-Manoux et al. 2012). Spatial memory, the detailed recollection of one’s surroundings, is critical for navigating through everyday life and exhibits a significant decline with age (Gallagher et al. 1993). Age-related spatial memory impairments affect many mammalian species; therefore, rodents have served as an appropriate model for cognitive aging (Gallagher et al. 2011).
The N-methyl-d-aspartate receptor (NMDAr) is an ionotropic glutamate receptor that is essential for spatial memory tasks (Morris et al. 1986). The NMDAr is particularly vulnerable to change during aging (Magnusson et al. 2010). Protein expression levels of NMDAr subunits decline with age in the hippocampus and frontal cortex (Das and Magnusson 2011; Magnusson et al. 2002; Magnusson et al. 2007). GluN2B subunit expression declines significantly in most cellular fractions in the hippocampus of aged mice, but there is a more pronounced aging effect in the synaptic membrane, than in the tissue as a whole, in the frontal cortex (Zhao et al. 2009).
The NMDAr complex of proteins is around 2000 kDa and includes over 75 different proteins (Husi et al. 2000). The Src kinase, Fyn, phosphorylates tyrosines 1336 (p1335) and 1472 (p1472) on GluN2B subunits, leading to two different outcomes. Phosphorylation of tyrosine 1472 allows for enhanced binding of GluN2B with PSD95, concentrating and holding NMDAr on synaptic membranes, and increasing long-term potentiation (LTP) (Nakazawa et al. 2001; Roche et al. 2001; Xu 2011). Alternatively, p1336 provides the recognition for calpain-mediated cleavage of the C-terminal tail of GluN2B, resulting in a translocation of GluN2B-containing NMDAr from synaptic to extrasynaptic membranes (Bi et al. 2000; Goebel-Goody et al. 2009). Striatal-enriched protein tyrosine phosphatase (STEP) dephosphorylates tyrosines 1336 and 1472 on the GluN2B subunit, and inhibition of STEP enhances NMDAr current (Lee 2006; Pelkey et al. 2002). Calpain also influences members of the NMDAr complex, like PSD-95 and STEP, by calcium-induced cleavage, leading to deactivation and altered localization of these proteins (Guttmann et al. 2001; Lu et al. 2000; Nguyen et al. 1999).
Recent evidence indicates that there is an increased interaction between GluN2B subunits and postsynaptic density protein 95 (PSD-95), suggesting an additional effect of aging beyond subunit production (Zamzow et al. 2013). What is not currently known is if the increased interaction between PSD-95 and GluN2B is due to higher relative levels of p1472 and/or decreased p1336. In the current study, we assessed the spatial reference memory of C57BL/6 mice of two different ages, separated synaptic membranes by subcellular fractionation, and quantified protein expression and posttranslational modifications.
Materials and methods
Animals
A total of 24 male C57BL/6 mice from two age groups (3 and 24 months of age) were used for this study. The 12 mice in the older age group were obtained from the National Institute on Aging, NIH. Twelve young mice were purchased from JAX mice (Bar Harbor, MA), which stocks the NIH colony. They were fed ad libitum and housed with a 12/12-h light/dark cycle. The mice were fed a standard chow diet (LabDiet). After the behavioral testing, all animals were euthanized by exposure to CO2 and decapitated. The brains were harvested, frozen in dry ice, and stored at −80 °C.
Behavioral testing
Spatial reference memory, cognitive flexibility, and associative memory (cued control task) were tested with the use of the Morris water maze as previously described (Das et al. 2012). Briefly, for the first 2 days, mice were acclimated to the water maze, followed by 2 days of testing for spatial reference memory, 1 day of reversal training to test cognitive flexibility, and 1 day of associative memory testing (cued control task). Reference memory testing consisted of eight place trials (two four-trial sessions, 1 h inter-session interval), and a probe trial (1 h after the last place trial) each day. A naive probe trial was performed at the beginning of the first day of memory testing. The platform was kept in the same quadrant for each place trial. Place trials consisted of a maximum of 60 s in the water searching for the platform, 30 s on the platform, and 2 min of cage rest. If a mouse failed to find the platform within the designated 60 s swim time, it was led to the platform by the experimenter. Probe trials were performed to assess the animal’s ability to show a bias for the platform location. During the probe trial, the platform was removed and the mouse was allowed to search in the water for 30 s. After 2 days of reference memory testing, a reversal task was performed in order to assess cognitive flexibility. The platform was placed in the opposite quadrant in the tank, and place and probe trials were performed similar to the reference memory task.
Cued trials were designed to test motivation, visual acuity, and physical ability for the task. The mice performed six cued trials. The positions of entry and the platform positions varied between trials. The platform was kept submerged but was marked by a 20.3-cm support with a flag. The mice were allowed to search for the platform for 60 s. For all behavioral testing, the animal’s movements in the water maze were tracked and analyzed with the SMART tracking system (San Diego Instruments, San Diego, CA).
Tissue processing for cell subfractionation
Biochemical fractionation of the frontal cortex (rostral 4 mm of cortex) and hippocampus was performed as previously described (Dunah and Standaert 2001), with a few modifications. Briefly, tissue was homogenized on ice with a Dounce homogenizer in TE buffer (10 mM Tris-HCl, pH 7.4, 1 mM EDTA, 1 mM EGTA) plus 320 mM sucrose and protease/phosphatase inhibitor cocktail (Sigma). Homogenate was centrifuged at 4 °C 1000×g for 10 min and the resulting pellet (P1) was discarded. The supernatant (S1) was centrifuged at 4 °C 12000×g for 20 min in an Eppendorf centrifuge to produce the crude synaptosome pellet (P2) and the supernatant cytosolic and microsomal fraction (S2). The P2 fraction was then solubilized in Triton X-100 (Sigma) and fractionated as previously described (Milnerwood et al. 2010). Briefly, the P2 pellet was resuspended with 300 μl Triton buffer (10 mM Tris-HCl, 100 mM NaCl, 0.5 % Triton, pH 7.2) and rotated slowly (15 min, 4 °C) followed by centrifugation (12,000g, 20 min, 4 °C). The supernatant (Triton-soluble fraction) containing non-PSD membranes was retained. The resulting pellet was resuspended in 150 μl SDS buffer (10 mM Tris-HCl, 150 mM NaCl, 1 % Triton-X, 1 % deoxycholic acid, 1 % SDS, 1 mM DTT, pH 7.5) followed by gentle rotation (1 h, 4 °C) and centrifugation (10,000g, 15 min, 4 °C). The final pellet was discarded and the supernatant (Triton-insoluble PSD fraction) retained. Microsomal and cytosolic (S2), PSD (TxP), and non-PSD (TxS) samples were stored at −80 °C.
Western blot
Sodium dodecyl sulfate-poly acrylamide gel electrophoresis (10 %) was used for Western blotting as described previously (Zhao et al. 2009). Each gel contained four different microgram loads (2, 4, 8 and 16 μg/well) of standards, obtained from a homogenate prepared by combining caudal cortices from all young animals. Protein samples from representatives of each different age group were loaded on each gel and analyzed in triplicate. Proteins were transferred to PVDF membranes, blocked in Odyssey blocking buffer (LiCor, Lincoln, NE), Tris-buffered saline (TBS) (1:1, v/v), and incubated at 4 °C in primary antibodies; membranes were rinsed three times with TBS-T and incubated in infra-red fluorescence-based secondary antibody. Bands were visualized by scanning with a LI-COR Odyssey imager. See Table 1 for antibody dilutions and sources.
Table 1.
Antibody dilutions and sources for Western blots
| GluN2B | 1:1000 | Millipore | Fyn | 1:250 | Santa Cruz |
| p1472 | 1:1000 | Phosphosolutions | GIPC | 1:250 | Santa Cruz |
| p1336 | 1:1000 | Phosphosolutions | Calpain 1 | 1:250 | Santa Cruz |
| p1480 | 1:1000 | Phosphosolutions | Calpain 2 | 1:250 | Santa Cruz |
| PSD-95 | 1:1000 | Thermo Scientific | Actin | 1:250 | Santa Cruz |
| STEP | 1:1000 | Millipore | Secondary | 1:5000 | Rockland |
| GluN2A | 1:250 | Santa Cruz |
Data analysis
Data for behavioral testing were analyzed as previously described (Das et al. 2012). Cumulative proximity measures, which reflect search distance from the platform, were used for the place, reversal, and cued trials, and average proximity measures were used for probe trials (Gallagher et al. 1993). Proximity measures were corrected for start position (Das et al. 2012). Based on reference memory acquisition performance in the place trials, the old mice were divided into two categories for “Reference memory-Good” (RG; N = 7) and “Reference memory-Bad” (RB; N = 5) learners as previously described (Lee et al. 1994; Rowe et al. 2007; Yetimler et al. 2012) with some modifications. The criteria for RB old learners were established by selecting old mice with average 2-day reference scores that were 2 standard deviations above the mean of the young mice, while RG old learners were within the same 2 standard deviation window (Fig. 1a). One young mouse was also identified as a RB learner, using the same criteria. It was removed from further consideration because of the low N for RB young. The statistical comparisons between the young (good learners only; N = 11) and RG (old) and RB (old) will be referred to as reference memory status effects.
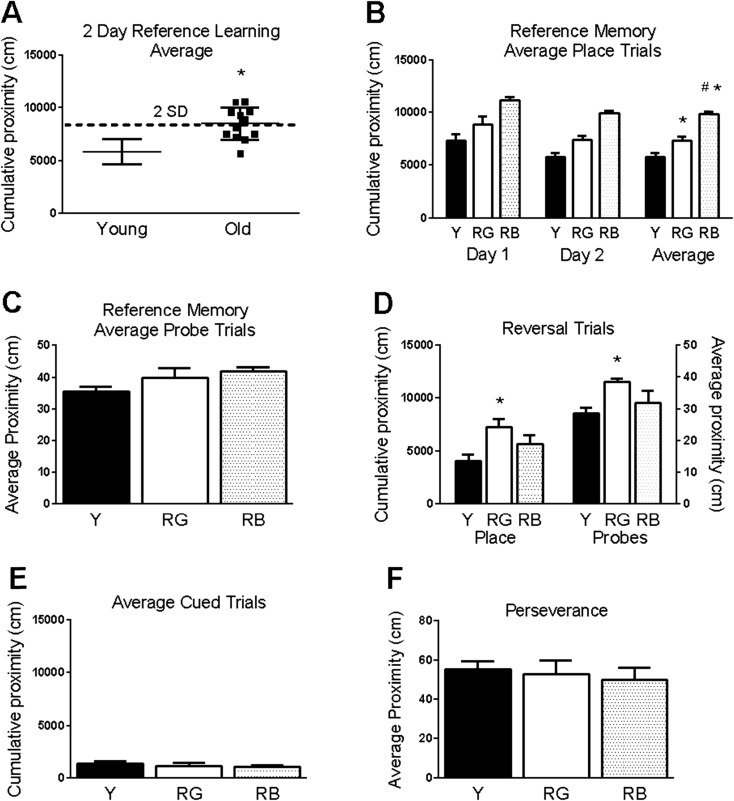
Fig. 1.
Behavioral testing. Aged mice have significantly poorer reference memory than young mice. a Old mice were separated based on acquisition of reference memory in place trials; bad old learners (RB) were >2 standard deviations (SD) above the young mean. b, c Significant differences in reference memory acquisition (b) but not in probe trials (c). d Significant differences were seen in averaged place and probe reversal trials. e–f No significance differences were seen in cued trials (e) or reversal perseverance for the old platform location (f). Asterisk indicates p < .05 for difference of from young. Number sign indicates p < .05 for difference of from RG (ANOVA and Fisher’s PLSD). Data = mean ± SEM. Y = young (N = 11). RG = old Reference memory-Good (N = 7). RB = old Reference memory-Bad (N = 5)
Protein blots were analyzed using Li-Cor Odyssey software. Integrated intensity measures were obtained using median background subtraction method. A standard curve was obtained using a linear fit with Excel (Microsoft) from integrated intensity values for known loads of caudal cortex. Sample values were interpolated from the standard curve as caudal cortex equivalents. Each protein was normalized to beta-actin within each sample of total protein. Statistical analyses for behavioral trials and protein expression using data from individual animals were done with analysis of variance (ANOVA) followed by Fisher’s protected least significant difference (PLSD),with the use of Statview software (SAS Institute) and a significance cutoff of p ≤ .05.
Results
Behavioral analysis
Performance for spatial learning and flexibility were evaluated for young and old mice in the Morris water maze. An examination of reference scores showed a significant overall effect of age (F(1,20) = 19.7, p = .0003; Fig. 1a). Based on acquisition in the reference memory task, the old mice were divided into the categories of Reference memory-Good (RG) and Reference memory-Bad (RB) learners (see “Materials and methods”). After dividing the old mice into the categories of RG and RB, the overall differences between the three reference memory status groups remained significant (F(2,20) = 17.87, p < .0001; Fig. 1b). Significantly higher proximity scores were seen in RB (p < .0001) and RG (p = .004) than the young and in RB than RG (p = .0005) in reference memory place trials across both days. Higher proximity scores are associated with searching further away from the platform position. There was an overall effect of reference memory status on reversal place trials (F(2,20) = 5.37, p = .01; Fig. 1d,) and the reversal probe trial (F(2,20) = 5.78, p = .01; Fig. 1d). RG had significantly higher proximity scores than the young in both averaged reversal place trials (p = .003) and the probe trial (p = .002). There was no significant overall effect of reference memory status for probe trials for reference memory (F(2,20) = 2.33, p = .12; Fig. 1c) or cued trials (F(2,20) = .48, p = .62; Fig. 1e).
Because the RG group performed better in reference memory trials than RB, but was the only group that performed worse than the young in reversal trials, we analyzed tracking data to see if the RG group was perseverating on the old location of the platform during reversal trials. The average distance from the old platform location was calculated for each mouse during the reversal trials, and no significant group differences were found (F(2,20) = 1.55, p = .23; Fig. 1f).
Age-related changes in GluN2 proteins
A previous study showed an age-related increase in the association of PSD-95 with GluN2B-containing NMDArs (Zamzow et al. 2013). PSD-95 is normally enriched on synaptic membranes, but it can also be found on extrasynaptic membranes in the mouse model of Huntington’s disease (Fan et al. 2012; Zheng et al. 2011). GluN2B-containing NMDArs are very mobile, moving easily between synaptic and extrasynaptic membranes (Groc et al. 2006; Parsons and Raymond 2014). We hypothesized that there may be an aging effect on the membrane localization of NMDArs and their effector proteins.
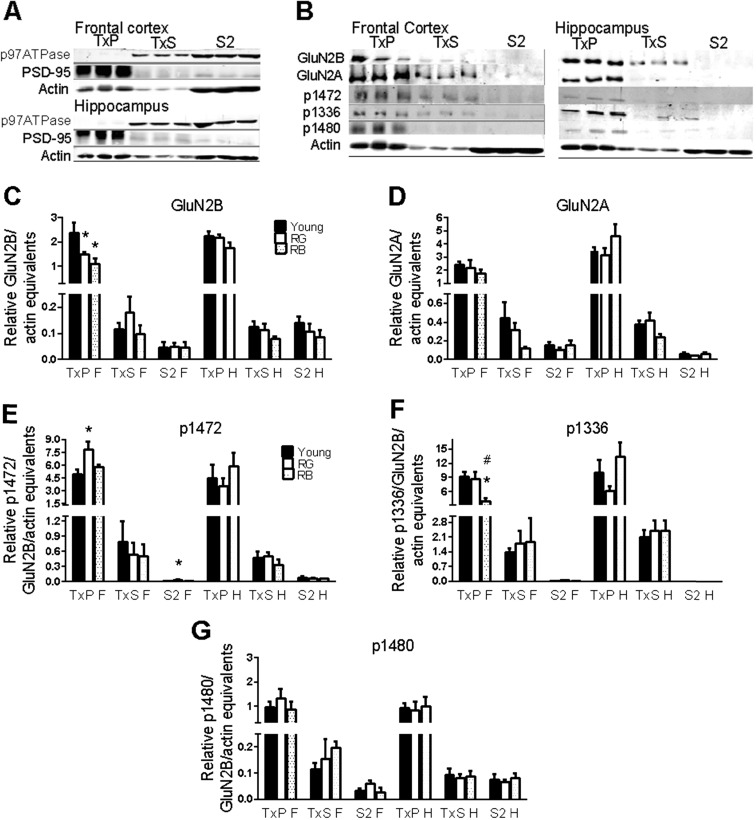
Differential centrifugation of brain homogenates, followed by membrane solubilization in 0.5 % Triton X-100, was used to test this theory. Because the synaptic membrane is insoluble in non-ionic detergents, exposure to a low concentration of Triton X-100 is an effective way to separate synaptic from peri- and extrasynaptic membranes (Goebel-Goody et al. 2009; Milnerwood et al. 2010). Proteins were examined from three synaptosomal fractions from the hippocampus and frontal cortex of young and aged mice: synaptic membranes (TxP), peri/extrasynaptic membranes (TxS), and cytosol/microsomes (S2). The latter two fractions will be referred to as extrasynaptic and cytosolic, respectively. To test for the proper separation of the fractions, a representative Western blot was probed for proteins known to reside on detergent-resistant synaptic membranes (PSD-95) or detergent-soluble membranes (p97ATPase), including the endoplasmic reticulum and Golgi apparatus (Jiang et al. 2011)(Fig. 2a).
Fig. 2.
GluN2 subunits. a–b Representative blots for proof of fractionation from Triton X-100 membrane solubilization (a) and GluN2 proteins (b). Order of lanes within each fraction from left to right is young, RG, and RB. c–g Significant differences in reference memory status were seen for GluN2B (c), p1472 (e), and p1336 (f), but not for GluN2A (d) or p1480 (g). Asterisk indicates p < .05 for difference from young. Number sign indicates p < .05 for difference from RG (ANOVA and Fisher’s PLSD). Data = mean ± SEM. RG = old Reference memory-Good. RB = old Reference memory-Bad. TxP = Triton-insoluble pellet, synaptic membranes. TxS = Triton-soluble membranes, extrasynaptic membranes. S2 = cytosolic membranes. F = frontal cortex. H = hippocampus. N = 3–11. Sample sizes for each group with significant differences was Young (N = 11), RG (7), and RB (5), with the following exceptions: p1472-F(S2): Y(10), RG (6). p1336-F(TxP): RB(4)
There was a significant reference memory status effect on the GluN2B subunits in the synaptic membranes of frontal cortex synaptosomes (F(2,20) = 5.5, p = .01; Fig. 2b, c). Significant decreases in GluN2B subunit expression were found between the young and both RG (p = .025) and RB (p = .007). There were no significant age-related differences in GluN2B subunit expression in the extrasynaptic and cytosolic fractions from the frontal cortex or any of the fractions from the hippocampus (p = .17–.99; Fig. 2b, c). The GluN2A subunit did not show any significant age-related differences in any fraction or brain region (p = .16–.62; Fig. 2b, d).
Phosphorylation by Fyn kinase of tyrosines 1336 and 1472 on the C-terminus of the GluN2B subunit has profound effects on the localization of the subunit. Phosphotyrosine 1472 (p1472) inhibits binding of the GluN2B subunit with the clathrin adaptor protein AP-2, allowing PSD-95 to bind, keeping the GluN2B subunit at the synapse (Prybylowski et al. 2005). Conversely, phosphotyrosine 1336 (p1336) can be found on the GluN2B subunit localized to extrasynaptic membranes (Goebel-Goody et al. 2009; Jiang et al. 2011).
All synaptosomal fractions were probed with antibodies specific to p1472 and p1336. There was an overall effect of reference memory status on p1472 levels in the synaptic fraction from the frontal cortex (F(2,20) = 5.7, p = .01; Fig. 2b, e), with a significant increase between the young and RG (p = .003). There were no significant overall effects of reference memory status on p1472 in the extrasynaptic fraction of the frontal cortex or in any of the fractions from the hippocampus (p = .10–.84; Fig. 2b, e). There was a significant increase in p1472 in the cytosolic fraction of the frontal cortex in RG, as compared to the young (p = .04; Fig. 2b, e). We next examined the levels of p1336 in all fractions. There was an overall effect of reference memory status in the synaptic fraction of the frontal cortex (F(2,19) = 3.87, p = .039; Fig. 2b, f), with a significant decrease between the young and RB (p = .01) and between RG and RB (p = .036). Examination of the remaining fractions from both brain regions revealed no significant reference memory status differences in p1336 (p = .23–.83).
The GluN2B subunit can also be phosphorylated on serine 1480. Activation of the NMDAr causes casein kinase 2 to phosphorylate serine 1480 (p1480), which disrupts the binding of GluN2B with PSD-95 (Sanz-Clemente et al. 2013). p1480 was used as a proxy to ask whether the increased level of p1472 in the frontal cortex synaptic membranes was due to the decreased activation of the NMDAr. There were no significant differences in p1480 with age, brain region, or synaptosomal fraction (p = .23–.95; Fig. 2b, g), suggesting that the increased p1472 signal may not be entirely due to the decreased activation of the NMDAr.
Scaffolding proteins and GluN2B phosphorylation
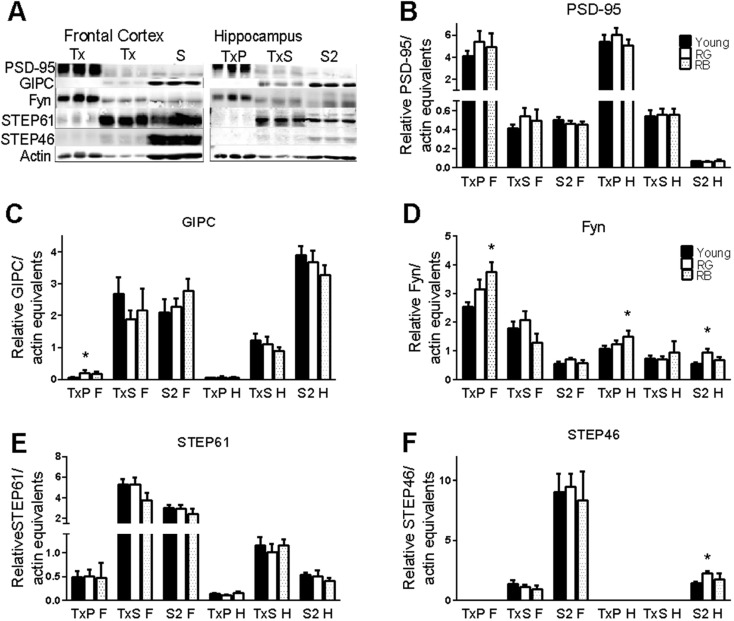
The GluN2B subunit shows an age-related increase in association with PSD-95, and there is an association between the binding of GluN2B to PSD-95 and to GAIP interacting protein, C-terminus (GIPC), and poor memory in aged animals (Zamzow et al. 2013). In young mice, Fyn phosphorylates tyrosines on GluN2 subunits of NMDArs, which is mediated by PSD-95 (Tezuka et al. 1999; Trepanier et al. 2012). Striatal-enriched protein phosphatase (STEP61) is also a part of the NMDAr complex and is involved with dephosphorylating p1472 (Braithwaite et al. 2006). We probed the cellular fractions with antibodies to PSD-95, GIPC, Fyn, and STEP to ascertain if there were any changes in expression or cellular localization associated with reference memory status.
Expression levels of PSD-95 did not change with reference memory status in any of the cellular fractions from the frontal cortex or the hippocampus (p = .33–.89; Fig. 3a, b). The synaptic fraction in the frontal cortex had a significant reference memory status-related increase in GIPC (F(2,16) = 4.56, p = .026; Fig. 3c), with a significant increase between the young and RG (p = .011). The remaining fractions of the frontal cortex, as well as all fractions of the hippocampus, saw no significant differences in GIPC, based on reference memory status (p = .44–.66; Fig. 3a, c).
Fig. 3.
Scaffolding, kinase, and phosphatase. a Representative blots of proteins. Order of lanes within each fraction from left to right is young, RG, and RB. b–f No changes were seen in PSD-95 (b) or STEP61 (e). Significant increases were seen for GIPC in the synaptic membrane of frontal cortex from old good reference learners (c). Fyn was increased in old bad reference memory mice in the synaptic membrane of both brain regions (d). Significant increases were also seen in Fyn expression in the hippocampal cytosol of old good reference learners (d). STEP46 expression was increased in hippocampal cytosol from old good reference learners. (f). Asterisk indicates p < .05 difference from young (ANOVA and Fisher’s PLSD). Data = mean ± SEM. RG = old Reference memory-Good. RB = old Reference memory-Bad. TxP = Triton-insoluble pellet, synaptic membranes. TxS = Triton-soluble membranes, extrasynaptic membranes. S2 = cytosolic membranes. F = frontal cortex. H = hippocampus. N = 3–11. Sample sizes for each group with significant differences was Young (N = 11), RG (7), and RB (5), with the following exceptions: GIPC-F(TxP): Y(8), RG(6)
There was a significant reference memory status-related increase in synaptic Fyn in the frontal cortex (F(2,20) = 5.36, p = .013) but no overall effect in the hippocampus (F(2,20) = 3.2, p = .06; Fig. 3a, d). The increase in synaptic Fyn between the young and RB was significant in both the frontal cortex (p = .005) and hippocampus (p = .02). There was also a significant reference memory status effect in the cytosolic fraction from the hippocampus (F(2,20) = 4.88, p = .02; Fig. 3a, d), with a significant increase between the young and the RG (p = .006). There were no other significant overall effects in any of the other fractions (p = .23–.33).
Because the amount of Fyn increased with age only in the RB mice and increased similarly in both the frontal cortex and the hippocampus of those mice, it seemed that Fyn expression could not explain the brain region differences in GluN2B phosphorylation states in old mice. Since STEP61 is known to dephosphorylate phosphotyrosine residues on GluN2B, the expression levels of STEP61 in the cellular fractions were examined. There were no significant overall reference memory status-related effects on any of the cellular fractions in either brain region for STEP61 (p = .23–.78; Fig. 3a, e). An alternatively spliced isoform, STEP46, is able to bind to the GluN2B subunit and affect phosphorylation of tyrosine 1472 (Snyder et al. 2005); therefore, we analyzed the relative expression levels of STEP46 in all cellular fractions. There were no significant effects of reference memory status in the cellular fractions from the frontal cortex (p = .56–.71); however, there was a slight, but significant, effect on STEP46 in the cytosolic fraction from the hippocampus (F(2,20) = 3.5, p = .05; Fig. 3a, f). A significant increase in STEP46 was seen between the young and RG (p = .016). These data suggest that the increase in p1472 in the frontal cortex of RG learners may not be due to the expression levels of Fyn, STEP, or PSD-95.
Calpain cleavage
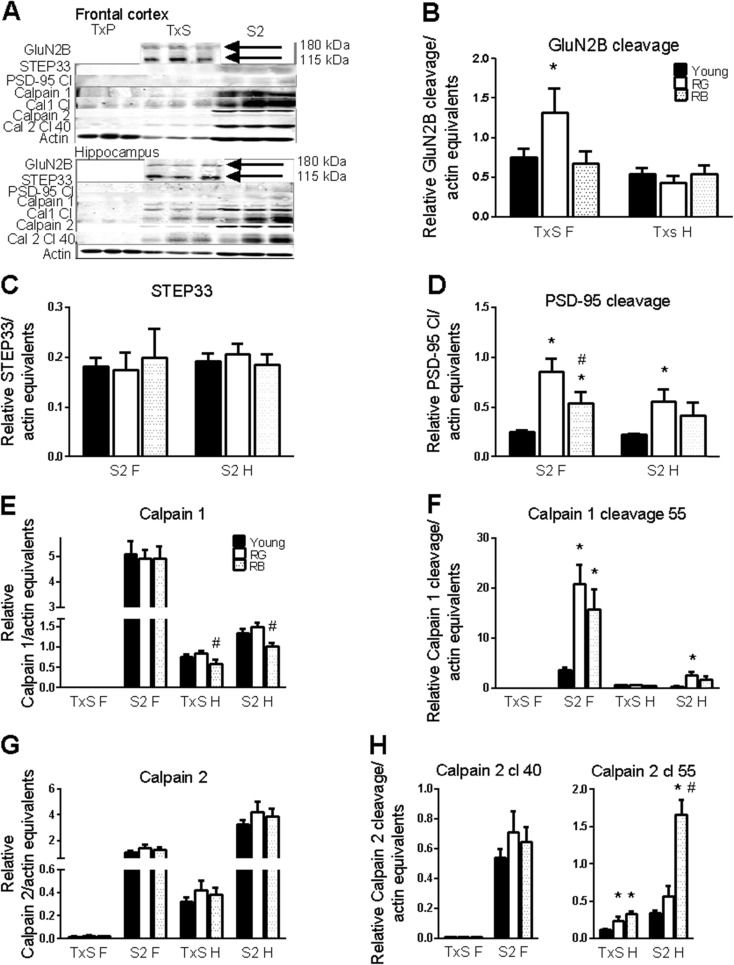
The GluN2 subunits are proteolytically cleaved by calpain in a calcium-dependent manner (Guttmann et al. 2001). The cleavage event on GluN2B is triggered by p1336, leading to a population of truncated GluN2B subunits residing on extrasynaptic membranes (Wu et al. 2007). The role that calpain cleavage may have in localizing NMDArs to extrasynaptic membranes was assessed. There was no significant overall effect of reference memory status (F(2,20) = 2.96, p = .074; Fig. 4a, b) on cleavage of the GluN2B subunit in the frontal cortex extrasynaptic fraction, but RG exhibited significantly more cleavage of GluN2B than the young (p = .04). There was no effect of reference memory status in the extrasynaptic fraction from the hippocampus (p = .60). Because both PSD-95 and STEP61 also undergo cleavage by calpain (Lu et al. 2000; Nguyen et al. 1999), calpain cleavage in aged animals was examined to see if it was limited to only the frontal cortex for other proteins. There was no significant effect of reference memory status on the cleavage product of STEP61, STEP33, in either the frontal cortex (p = .89; Fig. 4a, c) or the hippocampus (p = .78) in the cytosolic fractions. There was a significant effect of reference memory status on the 50-kDa calpain cleavage product of PSD-95 in the cytosolic fraction of the frontal cortex (F(2,20) = 15.46, p < .0001; Fig. 4a, d) and in the hippocampus (F(2,20) = 5.05, p = .017). RG exhibited more PSD-95 cleavage than both the young (p < .0001) and RB (p = .027), and RB had more than the young (p = .028) in the frontal cortex (Fig. 4a, d). RG also had significantly more PSD-95 cleavage than the young in the hippocampus cytosolic fraction (Fig. 4a, d).
Fig. 4.
Calpain activity. Calpain activity is increased in both the hippocampus and frontal cortex. a Representative blot of proteins. Order of lanes within each fraction from left to right is young, RG, and RB. b–h Calpain cleavage increased with age for GluN2B (b) and PSD-95 (d) but not STEP (c). Calpain 1 decreased slightly in the hippocampus of old mice with poor reference memory (e), but calpain 2 was unchanged (g). Increased autolysis activity of calpain 1 (f) and calpain 2 (h) in the aged brain. Asterisk indicates p < .05 for difference from young; number sign indicates p < .05 for difference from RG (ANOVA and Fisher’s PLSD). Data = mean ± SEM. RG = old Reference memory-Good. RB = old Reference memory-Bad. TxS = Triton-soluble membranes, extrasynaptic membranes. S2 = cytosolic membranes. F = frontal cortex. H = hippocampus. N = 3–11. Sample sizes for each group with significant differences was Young (N = 11), RG (7), and RB (5), with the following exceptions: calpain 1 cleavage 55-H(S2): Y(8)
Two isoforms of calpain predominate in the brain, μ-calpain (calpain 1) and m-calpain (calpain 2) (Wu and Lynch 2006). Both proteins undergo autolysis, with calpain 1 producing a 55-kDa fragment, while calpain 2 produces three fragments of 55, 40, and 30 kDa (Huang et al. 2010; Li et al. 2004; Nath et al. 1996). All cellular fractions were probed to test whether the increased calpain-mediated cleavage of the GluN2B subunits in the frontal cortex might be due to the higher expression and/or higher activation of calpains.
There was no reference memory status-mediated difference in calpain 1 expression in the cytosolic fraction of the frontal cortex (p = .97). There was no significant overall main effect of reference memory status in the extrasynaptic (F(2,20) = 3.35, p = .056) and cytosolic (F(2,20) = 3.45, p = .051; Fig. 4a, e) fractions from the hippocampus; however, the RB showed significantly less calpain 1 than RG in both extrasynaptic (p = .02) and cytosolic (p = .02) hippocampal fractions (Fig. 4a, e). There was no significant effect of reference memory status on calpain 2 expression in any cellular fraction of either the frontal cortex or hippocampus (p = .34–.45; Fig. 4a, g). There were significant reference memory status effects on the 55-kDa autolysis cleavage product of calpain 1 in the cytosolic fractions from the frontal cortex (F(2,20) = 13.83, p = .0002) and the hippocampus (F(2,20) = 6.8, p = .006). RG had significantly higher calpain 1 cleavage product than the young in both the frontal cortex (p = <.0001) and hippocampus (p = .002), and RB had a higher expression than the young in the frontal cortex in cytosolic fractions (p = .005; Fig. 4a, f).
A look at autolysis cleavage products of calpain 2 revealed an interesting distinction between the frontal cortex and the hippocampus. The 40-kDa cleavage product was found primarily in cellular fractions from the frontal cortex, while the 55-kDa cleavage product was found in the hippocampus (Fig. 4a, h). There was no reference memory status effect on the levels of the 40-kDa cleavage product in the cytosolic fraction from the frontal cortex (p = .42). A significant reference memory status effect was present in the extrasynaptic (F(2,20) = 8.61, p = .002) and the cytosolic (F(2,20) = 33.82, p < .0001; Fig. 4a, h) fractions for the 55-kDa cleavage product from the hippocampus. Both RG (p = .02) and RB (p = .0007) had significantly higher levels of calpain 2 cleavage 55 products in hippocampal extrasynaptic fractions (Fig. 4a, h). Hippocampal cytosolic fractions showed increases in RB from both the young and RG (p < .0001) for the 55-kDa cleavage of calpain 2 (Fig. 4a, h).
These data indicate that while calpain expression does not change dramatically in the old mice, the activities of the enzymes increased greatly in older mice. This amplified calpain activity does not, however, explain a lack of difference between the young and old in the levels of cleaved GluN2B subunits in the extrasynaptic fraction from the hippocampus.
Discussion
In this study, there was a significant decline in spatial memory with age, with the old mice subdivided based on good and bad acquisition of reference memory. The better spatial learning, however, appeared to be at a cost to cognitive flexibility. Expression of GluN2B declined with age in the synaptic fraction from frontal cortices, but p1472 in the synaptic fraction and calpain cleavage products of GluN2B in the extrasynaptic fraction from frontal cortex increased only in old mice with good reference memory. Expression of Fyn increased in the synaptic fraction from frontal cortices and hippocampi primarily in old mice with the worst memory. Finally, calpain activity was greater in old mice versus young mice.
In this study, all of the old mice as a group performed significantly worse than the young mice in the acquisition trials for reference memory, but there was a subset of old mice that showed reference memory acquisition more on a par with the younger mice. However, this aged group with good reference learning suffered from poor cognitive flexibility. All age groups performed equally well on reference memory probe trials in this study. This is in contrast with larger-scale studies that found age-related deficits were consistent for reference place and probe trials (Gallagher et al. 2015; Zamzow et al. 2013). The results from this study suggest that group differences in reference learning and cognitive flexibility were primarily due to deficits in prefrontal cortical synaptic function, but not hippocampal. Even though all aspects of spatial memory are highly integrated, probe trials are generally more hippocampal driven, while the prefrontal cortex can be involved in acquisition of spatial memory and is essential for cognitive flexibility (D’Hooge and De Deyn 2001). In rats, lesions of the lateral prefrontal cortex (insular and orbitofrontal cortices) impair spatial reference memory (Kolb et al. 1983) and reversal learning (Ragozzino 2007). NMDA receptor antagonists administered to the insular cortex impair memory formation, including spatial reference memory (Gutierrez et al. 1999). Enhancing GluN2B expression in the orbitofrontal cortex of aged mice improves spatial memory acquisition and retention (Brim et al. 2013). This suggests that alterations in the NMDA receptor in the prefrontal cortex can impact spatial reference memory.
The medial prefrontal cortex (mPFC) might be a factor in mice with cognitive flexibility deficits but intact reference learning. Rodents with mPFC lesions or rodents raised in social isolation had intact reference learning but a delay in reversal learning (de Bruin et al. 1994; Han et al. 2011). Interestingly, the rodents raised in social isolation also had increased expression of BDNF in the mPFC. This study examined combined prefrontal and frontal regions. It remains to be seen whether the same or different brain regions were responsible for the reference learning enhancements and cognitive flexibility problems.
Another factor from this study was a lack of perseverance in old mice with poor cognitive flexibility. Mice exposed to stress had reversal learning deficits without perseverance (Francis et al. 1995). Conversely, 5HT1B knockout mice exhibited reduced anxiety and improved reversal learning with no perseverance (Malleret et al. 1999). The lack of perseverance in the RG mice with poor ability to learn the new location rules out simply better learning of the old position than RB. Rather, it suggests a general confusion or lack of trust in the stability of the platform location occurred in the good learners after the situation changed.
The localization of GluN2B on synaptic membranes declined with age and reference memory status in the frontal cortex but not the hippocampus. Previous work from this lab suggests that this may be due to an increase in triheteromeric receptors (GluN1/2 A/2B), rather than a loss of GluN2B-containing receptors (Fig. 5) (Zamzow et al. 2013). At the same time, the levels of p1472 increased in the synaptic membrane from the frontal cortex of old mice with good reference memory, while levels in the hippocampus remained steady with age and reference memory status. The phosphorylation of tyrosine 1472 appears to remain steady with age in the hippocampus of rats as well (Coultrap et al. 2008). Phosphorylation of tyrosine 1472 enhances synaptic membrane localization by preventing the clathrin adaptor protein AP-2 from binding to GluN2B and internalizing the NMDAr (Lavezzari et al. 2003). There is evidence that triheteromeric NMDArs recycle back to the synaptic membrane as well as GluN2B/GluN1-containing NMDArs (Tang et al. 2010). Given the decline in GluN2B expression in the synaptic membrane, the increased levels of p1472 in the frontal cortex of aged mice who were better learners may have exerted an influence over the localization of triheteromeric GluN2A/GluN2B-containing NMDArs. A scenario exists whereby an increased number of triheteromeric receptors would contain GluN2B subunits with p1472, thereby preventing their movement off of the synaptic membrane, enhancing the NMDAr signal (Fig. 5). The triheteromeric receptors lacking p1472 GluN2B subunits in the bad learners could have their signal diminished during the recycling process. Therefore, it seems that even though an overall increase in triheteromeric NMDAr in aged mice shows no relationship to memory (Zamzow et al. 2013), modulation of the GluN2B subunit with p1472 could enhance acquisition of spatial memory in a subset of old animals. It is also possible that a stabilization of receptors with p1472 in the synaptic membrane may reduce cognitive flexibility.
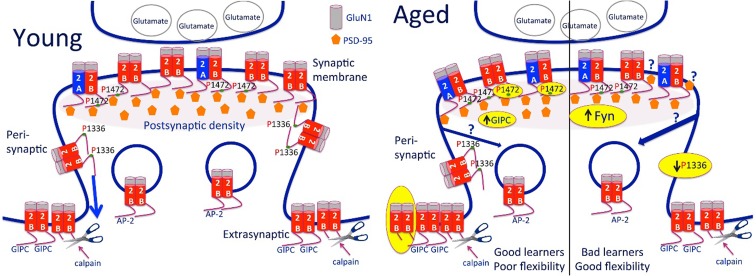
Fig. 5.
Summary diagram of changes related to GluN2B-containing NMDA receptors based on age and reference learning status. There was a decrease in GluN2B in the synaptic membrane of the old mice. Previous work suggests that this is due to an increase in triheteromeric (GluN1/2A/2B) NMDA receptors (Zamzow et al. 2013). The old good learners with poor cognitive flexibility showed increased phosphorylation (P) at amino acid 1472 of GluN2B and an increase in GIPC scaffolding protein in the synaptic membrane fraction and an increase in the 115-kDa GluN2B cleavage product in the extrasynaptic membranes compared to young. The old bad learners, with flexibility more similar to the young, showed a decrease in phosphorylation (P) at amino acid 1336 of GluN2B, despite an increase in Fyn kinase. The increased p1472 may be due to a better ability to respond to kinases and/or less recycling in the good learners than the bad. The previous finding of greater PSD-95 per GluN2B in animals with poorer memory (Zamzow et al. 2013) could be due to PSD-95 binding to more sites on the GluN2B tail or diminished trafficking extrasynaptically due to decreased P1336 in the bad learners or increased association between GluN2B and GIPC in the synaptic membrane of the good learners compared to bad. Note: Only GluN2B-containing NMDA receptors are shown. The receptors are shown as tetramers with two GluN1 and two GluN2 subunits. Yellow highlights indicate findings from the present study
The results of this study found no age-related differences in PSD-95 expression in any of the subcellular fractions investigated. Our laboratory was the first to report any relationship between PSD-95 in the frontal cortex and aging in the context of spatial memory (Zamzow et al. 2013). There was, however, a study that found decreased levels of PSD-95 in the PFC of elderly humans who had suffered from depression (Zhao et al. 2012). In rodents, there have been conflicting reports on the aging effects of PSD-95 expression. One study found an age-related loss of PSD-95 expression in the hippocampi of rats, but no assessment of spatial memory was performed (VanGuilder et al. 2010). Another study subdivided aged rats into impaired and intact groups based on cognitive status and examined PSD-95 expression in the hippocampus. The authors found that performance in probe trials for spatial memory retention correlated positively with PSD-95 expression in the aged rats (VanGuilder et al. 2011). Conversely, in a similar experimental design, reference memory acquisition was negatively correlated with PSD-95 in the hippocampus of rats in all age groups (Nyffeler et al. 2007). The present study found only age-related differences in acquisition of reference memory and in cognitive flexibility in aged mice, but not memory retention, and there was no relationship between memory and PSD-95 expression in the synaptic membrane.
A previous study from this laboratory did find an age-related negative correlation between PSD-95/GluN2B interaction in crude synaptosomes and spatial memory retention; increased PSD-95 per GluN2B was associated with poor memory (Zamzow et al. 2013). Although an increase in triheteromeric receptors would increase the PSD-95/GluN2B ratio, since GluN2A also binds PSD-95 (Xu 2011), there was no correlation between GluN2A and PSD-95 or spatial memory in the previous study (Zamzow et al. 2013). It was speculated that the negative correlation between the PSD-95/GluN2B ratio and spatial memory might be due to non-triheteromeric GluN2B-containing receptors interacting with PSD-95 and/or a possible shift of PSD-95 binding to the PSDP motif on GluN2B (Cousins and Stephenson 2012). The current data does not contradict this proposition. It is also possible that reduction in p1336 in the old bad learners might alter PSD-95 interactions or the increase in GIPC in the synaptic membrane could reduce PSD-95 binding in the old good learners (Fig. 5). Another possibility may lie in the posttranslational modification of PSD-95. Cyclin-dependent kinase 5 (Cdk5) phosphorylation of PSD-95 decreases p1472 and subsequent binding of PSD-95 to GluN2B (Zhang et al. 2008). What is not known is if aging has an effect on PSD-95 phosphorylation and protein interaction.
There were significant increases in Fyn levels in synaptic membranes from the frontal cortex (Fig. 5) and hippocampus and in hippocampal extrasynaptic fractions from bad learners. The pattern of Fyn expression levels in the hippocampus had no significant effect on p1472 and p1336 expression in synaptic membranes. The story is more complicated in the frontal cortex. The highest levels of Fyn in the synaptic membrane fraction were in the RB group, but the highest levels of p1472 were in the RG group and the RB group had the lowest levels of p1336. This indicates that increased expression of Fyn cannot be the sole contributor to differential phosphorylation of the GluN2B subunit. Transgenic mice that overexpress Fyn have higher levels of p1472 but not p1336 (Knox et al. 2013). However, Fyn also has its activity governed by phosphorylation, which is essential for its function (Ingley 2008). Stimulation of dopaminergic D1 receptors (D1R) will activate Fyn, leading to the phosphorylation of GluN2B tyrosines (Mao and Wang 2015; Dunah et al. 2004; Hu et al. 2010). Stimulation of D1R increases BDNF expression, and, as previously discussed, BDNF expression is increased in the mPFC of mice with good reference memory and poor cognitive flexibility (Han et al. 2011; Weinreb et al. 2015). The old good learners may have benefited from greater D1R stimulation than the old bad learners. The trade-off was a deficit in cognitive flexibility.
Phosphatases, which can influence p1472 and p1336 expression by removing the phosphorylation (Braithwaite et al. 2006), may also play a role in the phosphorylation differences seen in the aged mice; however, there were no significant group differences in levels of the phosphatase STEP61 in any fractions from the frontal cortex or hippocampus. There was a small, but significant, increase in expression of STEP46, an alternatively spliced isoform, in the cytosolic fraction from the hippocampus. STEP46 can bind to GluN2B, possibly to dephosphorylate p1472 (Snyder et al. 2005). STEP46 may have had an effect on the phosphorylation of GluN2B in the hippocampus, but we were unable to detect any significant differences. As with Fyn, STEP61 function can be affected by D1R (Braithwaite et al. 2006). Phosphorylation of STEP61 through D1R stimulation inhibits phosphatase activity, allowing Fyn to remain phosphorylated and active and phosphorylate GluN2B (Paul et al. 2000). STEP61 can be deactivated by dimerization and oxidative stress will lead to increased dimerization (Deb et al. 2011). Because aged brains suffer from increased oxidative stress and imbalanced redox state (Foster 2006; Parihar et al. 2008), STEP61 may be deactivated in the brains of aged mice. The increased levels of p1472 found in frontal cortex synaptic membranes of old mice with good reference memory may be a result of deactivated phosphatases. Whether phosphatases are overactive in the aged poor learners remains to be determined.
The levels of p1336 declined significantly in the synaptic fraction from the frontal cortex of old bad learners (Fig. 5). At the same time, levels of p1472 in the synaptic fraction from frontal cortex of old mice with bad reference memory remained similar to young mice. Recent evidence suggests that activation of the NMDAr does not affect p1472 and p1336 similarly. Activation of NMDAr causes a decrease in synaptic p1472, while p1336 remains unchanged (Ai et al. 2013). What is not known is if a decrease in NMDAr activation would lead to a change in p1336. It may be that the old bad learners suffer from a lack of NMDAr activation, keeping p1472 levels similar to young mice, while p1336 levels are decreased.
The C-terminus of the GluN2B subunit undergoes calpain-mediated cleavage initiated by p1336 (Wu et al. 2007). Calpain is also involved in the cleavage of PSD-95 (Lu et al. 2000). We found that the 115-kDa GluN2B cleavage product was located in the extrasynaptic fraction of the frontal cortex in significantly greater amounts in the aged good learners (Fig. 5). Similarly, the 55-kDa cleavage product of PSD-95 was significantly higher in the same fraction and group of mice. Evidence shows that PSD-95 will block the cleavage of GluN2 subunits (Dong et al. 2004). This suggests that one mechanism that induced an increase of the GluN2B cleavage product was the cleavage of PSD-95, allowing access of calpain to the C-terminus of GluN2B. Since GluN2B is found on extrasynaptic membranes more than GluN2A, the proteolytically cleaved GluN2B found in the extrasynaptic membrane fraction most probably represent the dimeric GluN2B-containing NMDAr (Groc et al. 2006).
One study found that calpain 1, but not calpain 2, expression was greater in the hippocampus of aged rats, leading to greater glutamate sensitivity and calcium dysregulation (Hajieva et al. 2009). Our results did not concur with their findings. We found a moderate, but significant, loss of calpain 1 expression in the hippocampus of old poor learner mice. Expression of calpain 1 and 2 remained steady with age in the frontal cortex, and there was no age-related loss of calpain 2 in the hippocampus of mice. There was, however, evidence of increased activity of calpain in aged mice. As mentioned previously, GluN2B and PSD-95 have increased levels of calpain-mediated proteolytic products. However, not all targets of calpain were affected by age. The phosphatase STEP61 can be cleaved to a 33-kDa product in a calpain-mediated fashion (Nguyen et al. 1999), but no age-related differences in the cleavage products were found in the present study. The GluN2A subunit is also a target of calpain, but no cleavage products were detected. This is most probably due to the rapid degradation of GluN2A after proteolytic cleavage by calpain (Guttmann et al. 2002). There was a robust age-related increase in the autolysis products of both calpain 1 and 2. We report here a unique 55-kDa cleavage product for calpain 1. This cleavage product has not been reported in vivo, but in vitro analysis seems to indicate that it is inactive (Li et al. 2004). The 55- and 40-kDa cleavage autolysis products of calpain 2 are similarly inactive. Calpain 1 and calpain 2 are differentially activated depending on the localization of NMDAr. Calpain 1 is activated via synaptic NMDAr and calpain 2 is activated via extrasynaptic NMDAr (Wang et al. 2013). Interestingly, activation of calpain 2 through extrasynaptic NMDAr caused a rapid rise in the 33-kDa cleavage product of STEP61, STEP33. No such accumulation of STEP33 was found in these mice nor was there any age-related difference in calpain 2 autolytic products. This suggests that there was minimal downstream effect from extrasynaptic NMDAr. Instead, the increased activation of calpains in the frontal cortex and hippocampus of aged mice might be due to increased oxidative stress (Paramo et al. 2013).
In this study, we found that posttranslational modifications of GluN2B subunits were perturbed with age and the changes were more prominent in the frontal cortex than the hippocampus. There were declines in GluN2B synaptic expression and calpain activity across aging. Increased phosphorylation and calpain-mediated cleavage of GluN2B were evident in the frontal cortex of aged mice that were good spatial learners. The old poor learners appeared to have problems with phosphorylation related to Fyn. These results suggest that, although age-related declines in GluN2B expression in the frontal cortex were related to spatial reference learning deficits, the variability in learning ability among older animals was related to phosphorylation and calpain cleavage. The compensations that helped learning, however, may have come at a cost to cognitive flexibility.
References
- Ai H, Lu W, Ye M, Yang W. Synaptic non-GluN2B-containing NMDA receptors regulate tyrosine phosphorylation of GluN2B 1472 tyrosine site in rat brain slices. Neurosci Bull. 2013;29:614–620. doi: 10.1007/s12264-013-1337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi R, Rong Y, Bernard A, Khrestchatisky M, Baudry M. Src-mediated tyrosine phosphorylation of NR2 subunits of N-methyl-D-aspartate receptors protects from calpain-mediated truncation of their C-terminal domains. J Biol Chem. 2000;275:26477–26483. doi: 10.1074/jbc.M003763200. [DOI] [PubMed] [Google Scholar]
- Braithwaite SP, Paul S, Nairn AC, Lombroso PJ. Synaptic plasticity: one STEP at a time. Trends Neurosci. 2006;29:452–458. doi: 10.1016/j.tins.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brim BL, Haskell R, Awedikian R, Ellinwood NM, Jin L, Kumar A, Foster TC, Magnusson KR. Memory in aged mice is rescued by enhanced expression of the GluN2B subunit of the NMDA receptor. Behav Brain Res. 2013;238:211–226. doi: 10.1016/j.bbr.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin JP, Sanchez-Santed F, Heinsbroek RP, Donker A, Postmes P. A behavioural analysis of rats with damage to the medial prefrontal cortex using the Morris water maze: evidence for behavioural flexibility, but not for impaired spatial navigation. Brain Res. 1994;652:323–333. doi: 10.1016/0006-8993(94)90243-7. [DOI] [PubMed] [Google Scholar]
- Coultrap SJ, Bickford PC, Browning MD. Blueberry-enriched diet ameliorates age-related declines in NMDA receptor-dependent LTP. Age (Dordr) 2008;30:263–272. doi: 10.1007/s11357-008-9067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins SL, Stephenson FA. Identification of N-methyl-D-aspartic acid (NMDA) receptor subtype-specific binding sites that mediate direct interactions with scaffold protein PSD-95. J Biol Chem. 2012;287:13465–13476. doi: 10.1074/jbc.M111.292862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/S0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Das SR, Magnusson KR. Changes in expression of splice cassettes of NMDA receptor GluN1 subunits within the frontal lobe and memory in mice during aging. Behav Brain Res. 2011;222:122–133. doi: 10.1016/j.bbr.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SR, et al. Reducing expression of GluN1(0XX) subunit splice variants of the NMDA receptor interferes with spatial reference memory. Behav Brain Res. 2012;230:317–324. doi: 10.1016/j.bbr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb I, Poddar R, Paul S. Oxidative stress-induced oligomerization inhibits the activity of the non-receptor tyrosine phosphatase STEP61. J Neurochem. 2011;116:1097–1111. doi: 10.1111/j.1471-4159.2010.07165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong YN, Waxman EA, Lynch DR. Interactions of postsynaptic density-95 and the NMDA receptor 2 subunit control calpain-mediated cleavage of the NMDA receptor. J Neurosci. 2004;24:11035–11045. doi: 10.1523/JNEUROSCI.3722-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah AW, Standaert DG. Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane. J Neurosci. 2001;21:5546–5558. doi: 10.1523/JNEUROSCI.21-15-05546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah AW, Sirianni AC, Fienberg AA, Bastia E, Schwarzschild MA, Standaert DG. Dopamine D1-dependent trafficking of striatal N-methyl-D-aspartate glutamate receptors requires Fyn protein tyrosine kinase but not DARPP-32. Mol Pharmacol. 2004;65:121–129. doi: 10.1124/mol.65.1.121. [DOI] [PubMed] [Google Scholar]
- Fan J, Gladding CM, Wang L, Zhang LY, Kaufman AM, Milnerwood AJ, Raymond LA. P38 MAPK is involved in enhanced NMDA receptor-dependent excitotoxicity in YAC transgenic mouse model of Huntington disease. Neurobiol Dis. 2012;45:999–1009. doi: 10.1016/j.nbd.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Foster TC. Biological markers of age-related memory deficits: treatment of senescent physiology. CNS Drugs. 2006;20:153–166. doi: 10.2165/00023210-200620020-00006. [DOI] [PubMed] [Google Scholar]
- Francis DD, Zaharia MD, Shanks N, Anisman H. Stress-induced disturbances in Morris water-maze performance: interstrain variability. Physiol Behav. 1995;58:57–65. doi: 10.1016/0031-9384(95)00009-8. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–626. doi: 10.1037/0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 2015;129:540–548. doi: 10.1037/bne0000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Stocker AM, Koh MT. Mindspan: lessons from rat models of neurocognitive aging. ILAR J. 2011;52:32–40. doi: 10.1093/ilar.52.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel-Goody SM, Davies KD, Alvestad Linger RM, Freund RK, Browning MD. Phospho-regulation of synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult hippocampal slices. Neuroscience. 2009;158:1446–1459. doi: 10.1016/j.neuroscience.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Groc L, Heine M, Cousins SL, Stephenson FA, Lounis B, Cognet L, Choquet D. NMDA receptor surface mobility depends on NR2A-2B subunits. Proc Natl Acad Sci U S A. 2006;103:18769–18774. doi: 10.1073/pnas.0605238103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez H, Hernandez-Echeagaray E, Ramirez-Amaya V, Bermudez-Rattoni F. Blockade of N-methyl-D-aspartate receptors in the insular cortex disrupts taste aversion and spatial memory formation. Neuroscience. 1999;89:751–758. doi: 10.1016/S0306-4522(98)00360-1. [DOI] [PubMed] [Google Scholar]
- Guttmann RP, Baker DL, Seifert KM, Cohen AS, Coulter DA, Lynch DR. Specific proteolysis of the NR2 subunit at multiple sites by calpain. J Neurochem. 2001;78:1083–1093. doi: 10.1046/j.1471-4159.2001.00493.x. [DOI] [PubMed] [Google Scholar]
- Guttmann RP, Sokol S, Baker DL, Simpkins KL, Dong Y, Lynch DR. Proteolysis of the N-methyl-d-aspartate receptor by calpain in situ. J Pharmacol Exp Ther. 2002;302:1023–1030. doi: 10.1124/jpet.102.036962. [DOI] [PubMed] [Google Scholar]
- Hajieva P, Kuhlmann C, Luhmann HJ, Behl C. Impaired calcium homeostasis in aged hippocampal neurons. Neurosci Lett. 2009;451:119–123. doi: 10.1016/j.neulet.2008.11.068. [DOI] [PubMed] [Google Scholar]
- Han X, Wang W, Xue X, Shao F, Li N. Brief social isolation in early adolescence affects reversal learning and forebrain BDNF expression in adult rats. Brain Res Bull. 2011;86:173–178. doi: 10.1016/j.brainresbull.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Hu JL, Liu G, Li YC, Gao WJ, Huang YQ. Dopamine D1 receptor-mediated NMDA receptor insertion depends on Fyn but not Src kinase pathway in prefrontal cortical neurons. Mol Brain. 2010;3:20. doi: 10.1186/1756-6606-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Fileta J, Rawe I, Qu J, Grosskreutz CL. Calpain activation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2010;51:3049–3054. doi: 10.1167/iovs.09-4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci. 2000;3:661–669. doi: 10.1038/76615. [DOI] [PubMed] [Google Scholar]
- Ingley E. Src family kinases: regulation of their activities, levels and identification of new pathways. Biochim Biophys Acta. 2008;1784:56–65. doi: 10.1016/j.bbapap.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Jiang X, Knox R, Pathipati P, Ferriero D. Developmental localization of NMDA receptors, Src and MAP kinases in mouse brain. Neurosci Lett. 2011;503:215–219. doi: 10.1016/j.neulet.2011.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox R, Zhao C, Miguel-Perez D, Wang S, Yuan J, Ferriero D, Jiang X. Enhanced NMDA receptor tyrosine phosphorylation and increased brain injury following neonatal hypoxia-ischemia in mice with neuronal Fyn overexpression. Neurobiol Dis. 2013;51:113–119. doi: 10.1016/j.nbd.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Sutherland RJ, Whishaw IQ. A comparison of the contributions of the frontal and parietal association cortex to spatial localization in rats. Behav Neurosci. 1983;97:13–27. doi: 10.1037/0735-7044.97.1.13. [DOI] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Lee R, Roche KW. Differential binding of the AP-2 adaptor complex and PSD-95 to the C-terminus of the NMDA receptor subunit NR2B regulates surface expression. Neuropharmacology. 2003;45:729–737. doi: 10.1016/S0028-3908(03)00308-3. [DOI] [PubMed] [Google Scholar]
- Lee HK. Synaptic plasticity and phosphorylation. Pharmacol Ther. 2006;112:810–832. doi: 10.1016/j.pharmthera.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Ross ER, Gower A, Paris JM, Martensson R, Lorens SA. Spatial learning deficits in the aged rat: neuroanatomical and neurochemical correlates. Brain Res Bull. 1994;33:489–500. doi: 10.1016/0361-9230(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Li H, Thompson VF, Goll DE. Effects of autolysis on properties of mu- and m-calpain. Biochim Biophys Acta. 2004;1691:91–103. doi: 10.1016/j.bbamcr.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Lu X, Rong Y, Baudry M. Calpain-mediated degradation of PSD-95 in developing and adult rat brain. Neurosci Lett. 2000;286:149–153. doi: 10.1016/S0304-3940(00)01101-0. [DOI] [PubMed] [Google Scholar]
- Magnusson KR, Brim BL, Das SR. Selective vulnerabilities of N-methyl-D-aspartate (NMDA) receptors during brain aging. Front Aging Neurosci. 2010;2:11. doi: 10.3389/fnagi.2010.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson KR, Nelson SE, Young AB. Age-related changes in the protein expression of subunits of the NMDA receptor. Brain Res Mol Brain Res. 2002;99:40–45. doi: 10.1016/S0169-328X(01)00344-8. [DOI] [PubMed] [Google Scholar]
- Magnusson KR, Scruggs B, Zhao X, Hammersmark R. Age-related declines in a two-day reference memory task are associated with changes in NMDA receptor subunits in mice. BMC Neurosci. 2007;8:43. doi: 10.1186/1471-2202-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malleret G, Hen R, Guillou JL, Segu L, Buhot MC. 5-HT1B receptor knock-out mice exhibit increased exploratory activity and enhanced spatial memory performance in the Morris water maze. J Neurosci. 1999;19:6157–6168. doi: 10.1523/JNEUROSCI.19-14-06157.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao LM, Wang JQ. Dopaminergic and cholinergic regulation of Fyn tyrosine kinase phosphorylation in the rat striatum in vivo. Neuropharmacology. 2015;99:491–499. doi: 10.1016/j.neuropharm.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milnerwood AJ, et al. Early increase in extrasynaptic NMDA receptor signaling and expression contributes to phenotype onset in Huntington’s disease mice. Neuron. 2010;65:178–190. doi: 10.1016/j.neuron.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, et al. Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 2001;276:693–699. doi: 10.1074/jbc.M008085200. [DOI] [PubMed] [Google Scholar]
- Nath R, et al. Non-erythroid alpha-spectrin breakdown by calpain and interleukin 1 beta-converting-enzyme-like protease(s) in apoptotic cells: contributory roles of both protease families in neuronal apoptosis. Biochem J. 1996;319(Pt 3):683–690. doi: 10.1042/bj3190683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TH, Paul S, Xu Y, Gurd JW, Lombroso PJ. Calcium-dependent cleavage of striatal enriched tyrosine phosphatase (STEP) J Neurochem. 1999;73:1995–2001. [PubMed] [Google Scholar]
- Nyffeler M, Zhang WN, Feldon J, Knuesel I. Differential expression of PSD proteins in age-related spatial learning impairments. Neurobiol Aging. 2007;28:143–155. doi: 10.1016/j.neurobiolaging.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Paramo B, Montiel T, Hernandez-Espinosa DR, Rivera-Martinez M, Moran J, Massieu L. Calpain activation induced by glucose deprivation is mediated by oxidative stress and contributes to neuronal damage. Int J Biochem Cell Biol. 2013;45:2596–2604. doi: 10.1016/j.biocel.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Parihar MS, Kunz EA, Brewer GJ. Age-related decreases in NAD(P)H and glutathione cause redox declines before ATP loss during glutamate treatment of hippocampal neurons. J Neurosci Res. 2008;86:2339–2352. doi: 10.1002/jnr.21679. [DOI] [PubMed] [Google Scholar]
- Parsons MP, Raymond LA. Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron. 2014;82:279–293. doi: 10.1016/j.neuron.2014.03.030. [DOI] [PubMed] [Google Scholar]
- Paul S, Snyder GL, Yokakura H, Picciotto MR, Nairn AC, Lombroso PJ. The dopamine/D1 receptor mediates the phosphorylation and inactivation of the protein tyrosine phosphatase STEP via a PKA-dependent pathway. J Neurosci. 2000;20:5630–5638. doi: 10.1523/JNEUROSCI.20-15-05630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey KA, et al. Tyrosine phosphatase STEP is a tonic brake on induction of long-term potentiation. Neuron. 2002;34:127–138. doi: 10.1016/S0896-6273(02)00633-5. [DOI] [PubMed] [Google Scholar]
- Prybylowski K, Chang K, Sans N, Kan L, Vicini S, Wenthold RJ. The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron. 2005;47:845–857. doi: 10.1016/j.neuron.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann N Y Acad Sci. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- Roche KW, Standley S, McCallum J, Dune Ly C, Ehlers MD, Wenthold RJ. Molecular determinants of NMDA receptor internalization. Nat Neurosci. 2001;4:794–802. doi: 10.1038/90498. [DOI] [PubMed] [Google Scholar]
- Rowe WB, et al. Hippocampal expression analyses reveal selective association of immediate-early, neuroenergetic, and myelinogenic pathways with cognitive impairment in aged rats. J Neurosci. 2007;27:3098–3110. doi: 10.1523/JNEUROSCI.4163-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Clemente A, Gray JA, Ogilvie KA, Nicoll RA, Roche KW. Activated CaMKII couples GluN2B and casein kinase 2 to control synaptic NMDA receptors. Cell Rep. 2013;3:607–614. doi: 10.1016/j.celrep.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherr PA, et al. Correlates of cognitive function in an elderly community population. Am J Epidemiol. 1988;128:1084–1101. doi: 10.1093/oxfordjournals.aje.a115051. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, et al. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ. 2012;344:d7622. doi: 10.1136/bmj.d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Tang TT, Badger JD, 2nd, Roche PA, Roche KW. Novel approach to probe subunit-specific contributions to N-methyl-D-aspartate (NMDA) receptor trafficking reveals a dominant role for NR2B in receptor recycling. J Biol Chem. 2010;285:20975–20981. doi: 10.1074/jbc.M110.102210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezuka T, Umemori H, Akiyama T, Nakanishi S, Yamamoto T. PSD-95 promotes Fyn-mediated tyrosine phosphorylation of the N-methyl-D-aspartate receptor subunit NR2A. Proc Natl Acad Sci U S A. 1999;96:435–440. doi: 10.1073/pnas.96.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepanier CH, Jackson MF, MacDonald JF. Regulation of NMDA receptors by the tyrosine kinase Fyn. FEBS J. 2012;279:12–19. doi: 10.1111/j.1742-4658.2011.08391.x. [DOI] [PubMed] [Google Scholar]
- VanGuilder HD, Farley JA, Yan H, Van Kirk CA, Mitschelen M, Sonntag WE, Freeman WM. Hippocampal dysregulation of synaptic plasticity-associated proteins with age-related cognitive decline. Neurobiol Dis. 2011;43:201–212. doi: 10.1016/j.nbd.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanGuilder HD, Yan H, Farley JA, Sonntag WE, Freeman WM. Aging alters the expression of neurotransmission-regulating proteins in the hippocampal synaptoproteome. J Neurochem. 2010;113:1577–1588. doi: 10.1111/j.1471-4159.2010.06719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Briz V, Chishti A, Bi X, Baudry M. Distinct roles for mu-calpain and m-calpain in synaptic NMDAR-mediated neuroprotection and extrasynaptic NMDAR-mediated neurodegeneration. J Neurosci. 2013;33:18880–18892. doi: 10.1523/JNEUROSCI.3293-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb O, Badinter F, Amit T, Bar-Am O, Youdim MB. Effect of long-term treatment with rasagiline on cognitive deficits and related molecular cascades in aged mice. Neurobiol Aging. 2015;36:2628–2636. doi: 10.1016/j.neurobiolaging.2015.05.009. [DOI] [PubMed] [Google Scholar]
- Wu HY, Lynch DR. Calpain and synaptic function. Mol Neurobiol. 2006;33:215–236. doi: 10.1385/MN:33:3:215. [DOI] [PubMed] [Google Scholar]
- Wu HY, Hsu FC, Gleichman AJ, Baconguis I, Coulter DA, Lynch DR. Fyn-mediated phosphorylation of NR2B Tyr-1336 controls calpain-mediated NR2B cleavage in neurons and heterologous systems. J Biol Chem. 2007;282:20075–20087. doi: 10.1074/jbc.M700624200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W. PSD-95-like membrane associated guanylate kinases (PSD-MAGUKs) and synaptic plasticity. Curr Opin Neurobiol. 2011;21:306–312. doi: 10.1016/j.conb.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetimler B, Ulusoy G, Celik T, Jakubowska-Dogru E. Differential effect of age on the brain fatty acid levels and their correlation with animal cognitive status in mice. Pharmacol Biochem Behav. 2012;103:53–59. doi: 10.1016/j.pbb.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Zamzow DR, Elias V, Shumaker M, Larson C, Magnusson KR. An increase in the association of GluN2B containing NMDA receptors with membrane scaffolding proteins was related to memory declines during aging. J Neurosci. 2013;33:12300–12305. doi: 10.1523/JNEUROSCI.0312-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Edelmann L, Liu J, Crandall JE, Morabito MA. Cdk5 regulates the phosphorylation of tyrosine 1472 NR2B and the surface expression of NMDA receptors. J Neurosci. 2008;28:415–424. doi: 10.1523/JNEUROSCI.1900-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Bao AM, Qi XR, Kamphuis W, Luchetti S, Lou JS, Swaab DF. Gene expression of GABA and glutamate pathway markers in the prefrontal cortex of non-suicidal elderly depressed patients. J Affect Disord. 2012;138:494–502. doi: 10.1016/j.jad.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Zhao X, Rosenke R, Kronemann D, Brim B, Das SR, Dunah AW, Magnusson KR. The effects of aging on N-methyl-D-aspartate receptor subunits in the synaptic membrane and relationships to long-term spatial memory. Neuroscience. 2009;162:933–945. doi: 10.1016/j.neuroscience.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng CY, Seabold GK, Horak M, Petralia RS. MAGUKs, synaptic development, and synaptic plasticity. Neuroscientist. 2011;17:493–512. doi: 10.1177/1073858410386384. [DOI] [PMC free article] [PubMed] [Google Scholar]