Abstract
Stimulus over-selectivity describes a phenomenon where only a subset of the relevant stimuli present in the environment, control an individual’s behavior. The current experiment explored the degree to which over-selectivity increases in old age. The level of over-selectivity in a visual discrimination task in 60 individuals aged 60–89 years was assessed, as well as the degree to which this reflected attentional control. In addition, the intellectual functioning and cognitive flexibility of the participants were assessed. Results showed that, as age increased, three effects were revealed: levels of stimulus over-selectivity increased, IQ scores decreased, and cognitive flexibility decreased. However, over-selectivity was not related to IQ or cognitive flexibility, and appeared related most to attentional impairments. Thus, ageing is related to significant declines in effective stimulus control. These effects can have a serious impact on the physical and psychological health of old adults, as well as their quality of life, and, therefore, this area of research warrants further exploration. The results are discussed in relation to the attention-deficit and comparator theory of over-selectivity.
Keywords: Over-selectivity, Older individuals, Extinction, Attention, Cognitive flexibility, IQ
“Stimulus over-selectivity” describes a phenomenon where an individual fails to attend to all available components of a single stimulus or only attends to a subset of the available stimuli present in the environment, and, thus, may restrict learning regarding the range, breadth, or number of stimuli that are important in any given situation (Dube 2009; Ploog 2010). Instances of over-selective responding are found in many populations that experience some assault to their levels of cognitive function, including individuals with intellectual disabilities (Smeets et al. 1985), learning disabilities (Bailey 1981; Dube and McIlvane 1999), Rett’s Disorder (Fabio et al. 2009), acquired brain injury (Wayland and Taplin 1982), schizophrenia (Feeney 1972), and Autism Spectrum Disorders (ASD; Kelly et al. 2015; Leader et al. 2009; Reed et al. 2009; Frankel et al. 1984). Moreover, previous research investigating over-selectivity in typically developing adults has demonstrated that adding a cognitive load can induce higher levels of over-selective responding (Reed and Gibson 2005; McHugh and Reed 2007; Broomfield et al. 2010; Reynolds and Reed 2011a, b; Reed et al. 2012; Reynolds et al. 2012).
As might be predicted by the increasing problems with cognitive capacity and attention deficits that can be noted with age (Chao and Knight 1997), higher levels of over-selectivity have also been noted in older people (Gard et al. 2014; McHugh et al. 2010; McHugh and Reed 2007). An increased level of over-selective responding may well have a serious impact on the physical and psychological health of older adults, as well as their quality of life, and, therefore, this area of research certainly warrants further exploration. Although higher levels of over-selectivity have been noted in older individuals compared to younger populations, a number of important theoretical aspects of this empirical finding are unclear.
Firstly, it has not yet been established if there is a developmental trend toward over-selectivity as a function of age, and at what point this trend may commence. McHugh and Reed (2007) compared one group of individuals whose mean age was 73 years old, with groups of individuals whose mean age was 50 and 20 years old. The sixteen participants in the oldest age group were aged between 70 and 80 years. At present, there is no comparative data for individuals across this higher end of the age range.
Secondly, over-selectivity can be related to a number of aspects of cognitive function, such as levels of intellectual functioning (cf. Kelly et al. 2015; Ploog 2010; Wilhelm and Lovaas 1976), and levels of executive function, especially as indexed by cognitive flexibility (Gard et al. 2014; Reed and McCarthy 2012; Solomon et al. 2011). Both of these areas are impacted by age-induced assaults on cognitive function (Brayne et al. 1995; Finucane et al. 2000; Park 2000; Tales and Porter 2008; Tales et al. 2011; Traykov et al. 2007). Given this, another aim of the current investigation was to determine if over-selectivity was related to intellectual functioning and/or cognitive flexibility in an older population.
Beyond these important relationships, there are a number of theories regarding the mechanisms underlying over-selective responding, and these have not been widely explored for this particular age group. One well-researched theoretical perspective regarding stimulus over-selectivity is the attention deficit theory (see Dube 2009). This theory posits that over-selective responding is caused by an inability to attend to all component elements of a stimulus. Stimuli that are not attended to cannot be processed or learned about; therefore, only the elements of the stimulus that are attended to can subsequently control behavior (e.g., Dube and McIlvane 1999; Dube et al. 1999; Koegel and Wilhelm 1973; Lovaas et al. 1971).An alternative view is suggested by the comparator theory of stimulus over-selectivity, which suggests that a comparator mechanism is responsible for selecting between stimuli that potentially could be responded to, and triggers responses only to the most important stimuli available (see Reed 2011). This view suggests that over-selectivity is not an acquisition, but a performance problem – with some stimuli, although learned about, not controlling behavior because of their relative lack of importance. It has been noted that extinguishing the over-selected stimuli allows the previously under-selected stimuli to control behavior with no additional training, suggesting that the latter were acquired but did not control behavior while the over-selected cues were present (Leader et al. 2009; Matzel et al. 1985; Reed et al. 2009; Reynolds et al. 2012; Wilkie and Masson 1976).
Both views have been shown to accommodate data in different populations (Reed et al. 2009, 2012; White and Ruske 2002)—with the attention-related view appearing most suitable for lower functioning individuals (Leader et al. 2009; Reed et al. 2012). This postulation is supported by the results reported by McHugh and Reed (2007) who noted that while the younger groups in their study did show extinction-induced recovery of responding to previously under-selected stimuli, the oldest group (70–80 years) did not show any such effect. The authors suggested that the reason for the absent emergence effect was that different processes may be involved in the oldest group of participants. A further aim of the current study is to determine the extent to which recovery of responding to previously under-selected stimuli can be seen in older individuals. If such recovery is noted it suggests that the mechanism is a performance-based one, but if it is not noted, then it suggests that the deficit is attentional in nature.
Method
Participants
Sixty healthy individuals participated; 20 in each of three age categories: 60–69 years (mean age = 64:5 ± 3:0); 70–79 years (mean = 75:1 ± 2:2); and 80–89 years (mean = 83:2 ± 2:2). All participants were volunteers recruited through personal acquaintances of the experimenters and none received payment for their participation. Each participant provided written informed consent prior to any testing. No participant reported any chronic health condition, and participants were excluded from the study if they did not obtain a score of 25 or more on the Mini-Mental State Examination (Folstein et al. 1975). The study employed a between-subjects design with 50 % of the participants in each age group randomly assigned to the experimental group (n = 30) and the other 50 % to the control group (n = 30). This group type manipulation was employed to analyze if over-selective responding is a result of an initial attentional processing deficit or a post-processing mechanism.
Materials
Mini-mental state examination
(MMSE; Folstein et al. 1975). The MMSE is a commonly used instrument for indicating the presence of cognitive impairment. The MMSE comprises simple questions concerning a number of areas: the time and place of the test, repeating lists of words, arithmetic (such as the serial sevens), language use and comprehension, and basic motor skills. A score greater than or equal to 25 (out of 30) is considered as indicating no cognitive problem. Below this level, scores can indicate severe (≤9), moderate (10–20), or mild (21–24), cognitive impairment.
Wechsler Adult Intelligence Scale—fourth edition
(WAIS-IV; Wechsler 2008). The WAIS-IV is a standardized test measuring intelligence in adults aged 16 to 90 years. It comprises 10 core subtests, and five supplementary subtests. Full Scale IQ scores were employed in the present study, and are based on the total combined performance of the Verbal Comprehension Index, Perceptual Reasoning Index, Working Memory Index, and Processing Speed Index. The WAIS-IV was standardized on a sample of 2200 people in the USA ranging in age from 16 to 90. The median FSIQ is centered at 100, with a standard deviation of 15. In a normal distribution, 68 % of adults would fall within one standard deviation above and below the mean IQ score (i.e., between 85 and 115).
Wisconsin Card Sorting Test
(WCST; Grant and Berg 1948). The WCST is a neuropsychological test of set-shifting or mental flexibility (Hill 2004). In the computerized version of the WCST, participants match response cards to four stimulus cards along one of three dimensions (color, form, or number) on the basis of verbal feedback (“correct” or “incorrect”). Participants are not given any other information about the dimensions. After receiving correct feedback for sorting a consecutive series of 10 cards according one category, participants receive correct feedback for sorting the cards in a different manner (but are not otherwise told about the change).Perseveration errors are defined as a failure to shift set to the new sorting criterion. The only dependent variable examined in the current experiment was percentage of perseverative errors, which is the most-commonly used measure of WCST performance (Hsieh et al. 2010).
Stimulus over-selectivity materials
Laminated cards (12 cm × 10 cm) consisted of one or two black stimuli on a white background. The elements were characters obtained from fonts available in Microsoft 2003. The fonts employed were symbol, wingdings, and wingdings 2. The cards either had two elements (e.g., AB) displayed for the training phase (see Fig. 1a for an example), or one stimulus element (e.g., A or B) displayed for the testing phase (see Fig. 1b for an example).
Fig. 1.
a and b Example of complex stimulus (AB) and example of single element stimulus (A)
Distractor task
A 4x4 grid containing four different shapes, one in each of four of the 16 squares, was used as the distractor task (see Fig. 2), which is employed to generate over-selective responding in non-clinical populations. This is the same distractor task used by Reed and Gibson (2005) and McHugh and Reed (2007). Previous research investigating over-selectivity in typically developing adults has demonstrated that adding a distractor task can induce higher levels of over-selective responding (Reed and Gibson 2005; Broomfield et al. 2010; Reed et al. 2012; Reynolds et al. 2012; Reynolds and Reed 2011a, b).
Fig. 2.
Example of a memory grid used as a distractor task to increase cognitive strain across participants in each age group
Procedure
Ethical approval was obtained for this research from the ethics research committee in the university of the first and second authors.
The participants were tested individually in a quiet laboratory room. The MMSE, WCST, and WAIS-IV, tests were conducted prior to the test of stimulus over-selectivity. If a participant scored lower than 25 out of 30 in the MMSE, testing was terminated at this point.
Distractor test
The participants were shown the distractor 4 × 4 grid (see Fig. 2) for 20 s, and were instructed to remember the shapes and the locations of the stimulus shapes in the grid, as they would be asked to replicate it by drawing it at the end of the experiment. The distractor task for an individual participant was randomly chosen from four different distractor cards available.
Training phase
At the start of this phase, participants were instructed as follows: “You will be shown two cards containing two symbols on each. Please select a card by pointing to that card. Point to the card rather than to an individual symbol. You will be given feedback of “yes” for some cards and “no” for others. Your choices will be recorded”. The participants had the chance to ask any questions about the procedure at that point.
Following this, on each trial, two two-element stimulus cards (e.g., AB and CD; see Fig. 1a) were placed next to each other in the center of the table, half-way between the participant and the experimenter. If the participant pointed to the predetermined reinforced compound (e.g., AB), they received positive feedback from the experimenter, who said “yes” enthusiastically with a smile. If the participant pointed to the other card (e.g., CD), participants received negative feedback from the experimenter, who said “no” in a flat tone without a smile. Each trial lasted until a response was made, and each inter-trial interval was approximately 5 s.
The positions of the cards were randomized, so that 50 % of the time the correct card (AB) was presented on the right, and 50 % of the time on the left. During the training phase, the reinforced compound (AB) was always paired with the same non-reinforced compound (CD) for an individual participant. However, the actual stimulus elements used in the compounds were randomly chosen from the fonts, and were different for each participant, in order to prevent an intrinsically more salient stimulus from always having the same role. Participants reached criterion in the training phase once they chose the reinforced compound 10 times consecutively.
Test phase
Participants were presented with two cards simultaneously, each comprising of just one element from the complex stimuli from the training phase. The pictures were paired so that the participants had a choice of a previously reinforced stimulus element (A or B; see Fig. 1b) and a previously non-reinforced stimulus element (C or D). There were five trials for each combination of previously positively reinforced and non-reinforced components of the compound stimuli (i.e., A vs. C, A vs. D, B vs. C, B vs. D), giving a total of 20 trials. No feedback was provided to the participant during test trials. To ensure maintenance of trained discriminations of complex stimuli, ten probe trials were presented with no feedback throughout the test phase, giving a total of 30 test trials. The presentation order of the complex stimulus and single stimulus cards was randomized over the total trials (20 test trials and 10 probe trials).
Extinction phase
The researcher calculated how many times the two single stimuli from the previously reinforced stimulus (i.e., “A” or “B”) were chosen within the ten trials. If “A” was chosen more times than “B” for example, “A” was determined to be the over-selected stimulus and “B” the under-selected stimulus. For the experimental group, the over-selected stimulus element from was determined (i.e., A or B; see Fig. 1b), and was paired with one of four novel stimuli (E, F, G, or H) in a series of trials. On each trial, the previously over-selected stimulus and one of the novel stimuli (selected at random on each trial) were presented side by side (as described above). Participants were reinforced, as described above, for choosing the novel stimuli, and not the previously over-selected stimulus element. Criterion was reached when the participants chose the novel stimuli 10 times consecutively.
Participants in the control group were shown two novel stimuli paired together. Selection of one of the stimuli was given feedback of “yes”, while the other was given the feedback “no”. No card was extinguished. Training continued for an individual participant for the same number of trails as a participant in the experimental group required to reach criteria.
Re-test phase
The same test procedure was used as the first testing phase, with no feedback provided to the participants.
Distractor test
Each participant was instructed to replicate the memory grid by drawing the four shapes in the correct four positions onto the empty 4x4 grid provided by the experimenter.
Results
Training phase: trials to criterion
All participants successfully completed the training phase. The mean numbers of trials to criterion (including the 10 consecutive correct trials) for the three age groups were: 24.80 (±4.36) for the 60–69-year-olds; 26.40 (±4.78) for the 70–79-year-olds; and 28.45 (±0.87) for the 80–89-year-olds. A one-way analysis of variance (ANOVA) was conducted on these data with age group as a between-subjects factor. The analysis revealed a statistically significant effect for age, F(2,57) = 3.53, p < 0.05, partial eta2 = 0.11 Tukey’s honestly significant difference (HSD) tests indicated that the mean score for the 60–69-year-old group was significantly lower than that of the 80–89-year-old group, p < 0.05, but that no other pairwise comparison was significant, p > 0.05.
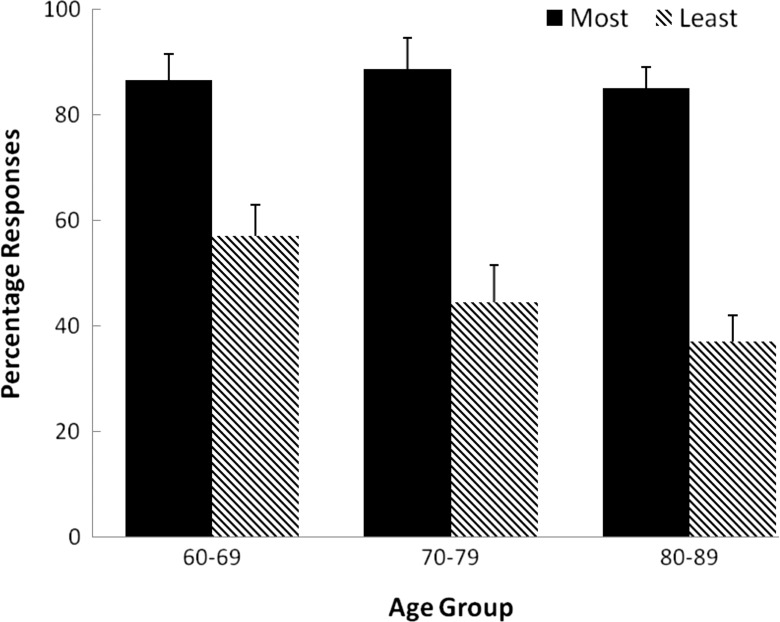
Test phase: most often and least often selected elements
The mean number of times that each of the elements from the previously reinforced stimulus (AB) were selected during the test phase was calculated. The element that was selected most often and least often for each participant during the test phase was determined. The group-mean percentages for the most and least-selected stimulus are shown in Fig. 3. Inspection of these data reveals a larger difference between the percentage times that the elements of the previously reinforced compound were selected as the age of the groups increased, with this being reflected in a decrease in the number of times that the least-selected stimulus was chosen.
Fig. 3.
Mean percentage scores for the most and least-selected elements for each age group in the test phase. Error bars = 95 % confidence intervals
A two-factor, mixed-model ANOVA was performed on these data, with stimulus type (most versus least-selected) as a within-subjects factor, and age group (60–69, 70–79, and 80–8) as the between-subjects factor. This analysis showed significant main effects of stimulus type, F(1,57) = 352.92, p < 0.001, partial eta2 = 0.86, and age group, F(2,57) = 8.04, p < 0.001, partial eta2 = 0.22, and a significant interaction between the two factors, F(2,57) = 6.80, p < 0.01, partial eta2 = 0.18. Simple effect analyses (using the pooled error term) conducted on the most-selected stimulus across the three age groups revealed no difference between the groups, F < 1, partial eta2 = 0.02. However, there was a significant simple effect of age group on the least-selected stimulus, F(2,57) = 14.41, p < 0.001, partial eta2 = 0.34. There was significant linear trend in these data, F(1,57) = 20.85, p < 0.01, partial eta2 = 0.27, but no significant quadratic trend,F < 1, partial eta2 = 0.01.
Test phase: correlations and predictors
Inspection of the data in Table 1 reveals that there were significant relationships between IQ and age, and IQ and WCST scores, and also between the age of the participant and the level of over-selective responding. A multiple regression was conducted with the difference between the stimuli as the target, and the participants’ age, IQ, and WCST scores as predictors. This revealed a statistically significant model, F(3,56) = 3.76, p < 0.05, R2 = 0.168, in which age acted as the only significant independent predictor (β = 0.077, t = 3.14 , p < 0.01); IQ (β = 0.228, t = 1.10 , p > 0.20); WCST (β = 0.432, t = 1.22 , p > 0.20).
Table 1.
Means (standard deviations) for the difference between the most and least stimulus during Phase 1, the age of the participants (months), IQ scores, and Wisconsin Card Sorting Task (WCST) scores, as well as the Pearson correlations between these scores
| Age | IQ | WCST | ||
|---|---|---|---|---|
| Stimulus difference | 40.17 (18.56) | 0.380** | −0.056 | 0.143 |
| Age (months) | 891.08 (97.73) | −0.330** | 0.124 | |
| IQ | 103.87 (13.02) | −0.479*** | ||
| WCST | 24.33 (7.22) | |||
*p < .05; **p < .01; ***p < .001
Extinction phase: trials to criterion
All 30 participants in the experimental group reached criterion in the extinction phase. The mean trials to extinction criterion (including the 10 consecutive trials not responding to the previously over-selected stimulus) for the three age groups were: 15.25 (±3.84) for the 60–69-year-olds; 15.90 (±2.75) for the 70–79-year-olds; and 18.15 (±4.60) for the 80–89-year-olds. A one-way ANOVA with age group as a between-subject factor revealed a statistically significant difference, F(2,57) = 3.20, p < 0.05, partial eta2 = 0.10. Tukey’s HSD test indicated that the mean score for the 60–69 year old group was significantly different from the 80–89 year old group, p < 0.05, but no other pairwise group difference was significant, p > 0.05.
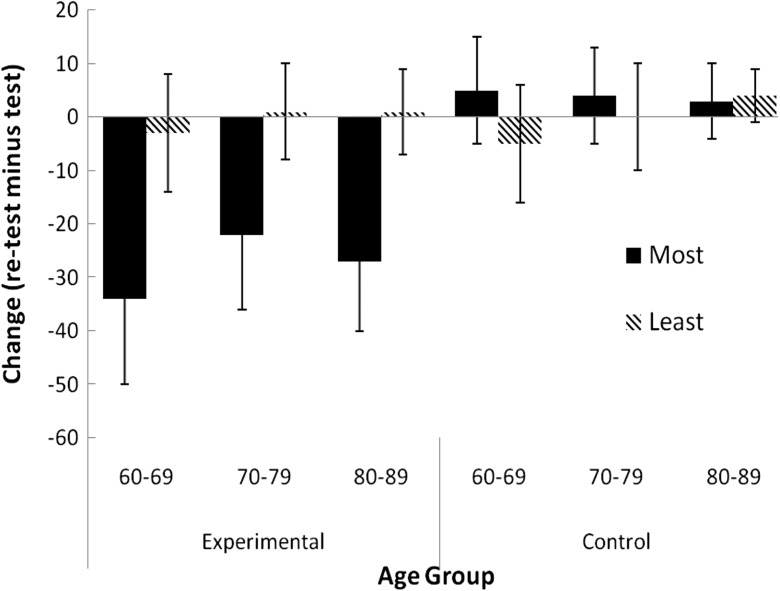
Re-test phase: percentage change from test to re-test
Inspection of Fig. 4 reveals that for all three groups who had received extinction (i.e., the experimental groups), the most-selected stimuli were chosen less often in the re-test phase than it had been in the initial test phase. However, there was very little increase in control exerted by the previously under-selected stimuli in any group. In the control group, the most-selected stimuli were chosen more times in the re-test phase across all three age groups. There was little change in the control exerted by the previously under-selected stimuli in any group.
Fig. 4.
Mean percentage change from the test to the re-test phase for the most and least-selected stimuli in the experimental and control groups across the three age groups. Error bars = 95 % confidence intervals
A three-factor, mixed-model ANOVA was conducted on these data, with stimulus type (most versus least-selected) as a within-subject variables, and group type (experimental versus control) and age group (60–69, 70–79, and 80–89) as between-subject variables. This analysis revealed significant main effects of stimulus type, F(1,54) = 17.99, p < 0.001, partial eta2 = 0.25, and group type, F(1,54) = 25.17, p < 0.001, partial eta2 = 0.32, but no main effect for age group, F(2,54) = 1.09, p > 0.30, partial eta2 = 0.04. There was a significant interaction between stimulus type and group type, F(1,54) = 33.83, p < 0.001, partial eta2 = 0.39, but no interaction effects for stimulus type and age group, F < 1, partial eta2 = 0.02, age group and group type, F < 1, partial eta2 = 0.01, or three-way interaction, F < 1 partial eta2 = 0.02. When the difference scores from the experimental group were tested against zero, the decrease in control exerted by the previously over-selected stimuli was statistically significant, t(29) = 7.35, p < 0.001, but the emergence of the previously under-selected stimulus was not significantly different from zero, t < 1. When the mean scores from the control group were tested against zero, neither the decrease in control exerted by the previously over-selected stimuli, nor the emergence of the previously under-selected stimuli, were statistically significant, both t < 1.
Re-test phase: predictors
A multiple regression was conducted, with the difference between most-selected stimulus at test and re-test as the target (re-test score minus test score), and the participants’ age, IQ, and WCST scores, as predictors. This was not statistically significant, F(3,56) = 1.24, p > 0.30, R2 = 0.062, and none of the predictors was independently significant: age (β = 0.043, t = 1.32,p > 0.10): IQ (β = 0.156, t 1, p > 0.50); WCST (β = −0.518, t = 1.09, p > 0.20). A multiple regression was conducted with the difference between least-selected stimulus at test and re-test as the target (re-test score minus test score), and the participants’ age, IQ, and WCST scores as predictors. This was not statistically significant, F(3,56) = 1.03, p > 0.30, R2 = 0.052, and none of the predictors was independently significant: age (β = 0.034, t = 1.64 ,p > 0.10); IQ (β = 0.100, t < 1, p > 0.50); WCST (β = −0.537, t < 1, p > 0.60).
Discussion
The current experiment demonstrated the presence of stimulus over-selectivity in a simple visual discrimination task with an added distractor in each of the three older age groups. This finding replicated the results reported by McHugh and Reed (2007), as well as research exploring the effects of increased cognitive load on levels of over-selectivity in a non-clinical population (Broomfield et al. 2010; Reed and Gibson 2005; Reed et al. 2012; Reynolds and Reed 2011a, b; Reynolds et al. 2012). In addition, an age trend in over-selective responding emerged, where over-selectivity increased as a function of chronological age. Specifically, the oldest age group (80–89 years) displayed higher levels of over-selectivity than those in the 70–79-year-old group, and the 70–79-year-old group displayed greater over-selective responding than the 60–69-year-old group.
This effect was the product of decreasing scores in terms of the least-selected stimulus, with performance for the most-selected stimulus remaining unaltered. This finding suggests that as participants become older, then they can still perfectly capable of acquiring the necessary information to perform the task. However, they do so increasingly by focusing on narrower aspects of the stimulus that they are required to learn about – such that, when tested on all of the cues, they respond as well to one element as younger individuals, but perform worse to other aspects of the stimulus that may be regarded as redundant at the time, but which may be needed at a subsequent time.
A number of possibilities exist in terms of which cognitive abilities are implicated in producing this effect. Although the level of over-selectivity was related to the participants’ ages, it was neither related to the IQ (WAIS-IV) nor the level of cognitive flexibility (WCST) demonstrated by the participant. This was despite the fact that age and both intellectual ability and cognitive flexibility were noted to negatively co-vary (see also Finucane et al. 2000; Park 2000; Tales and Porter 2008; Tales et al. 2011; Traykov et al. 2007).
The results from the extinction manipulation do suggest that the current over-selectivity effects are the result of a deficit in initial attentional processing, rather than post-processing mechanisms. The comparator theory of over-selectivity predicts that post-learning manipulations of the over-selected cue should enhance the performance of the under-selected cue (Reed 2007, 2011). However, as in the current study, McHugh and Reed (2007) found no recovery effects in a 70–80-year-old group. Thus, it is possible that the older participants displayed an initial pre-conditioning attentional deficit and, thus, there was no suppressed learning about the under-selected stimulus to be revealed by extinguishing the over-selected stimulus. This suggests corresponds to a number of papers that have posited an attentional problem with increasing age (Chao and Knight 1997).
However, it is worth noting that, the current pattern of findings in terms of the predictors of initial over-selectivity is similar to that obtained from individuals with ASD (Frankel et al. 1984; Kelly et al. 2015; Reed et al. 2009). In these studies of this particular clinical sample, no significant association was revealed between IQ and over-selective responding. In fact, in these studies, the participants’ verbal abilities were the key predictor of over-selective performance, and this possibility could be investigated with the current population.
A potential limitation of this study was that it did not include a young-persons control group and focused on individuals between 60–89 years of age. However McHuch and Reed (2007) demonstrated the impact of ageing on stimulus over-selectivity and investigated individuals within the age ranges of 18–22, 47–55, and 70–80. Therefore the focus of this study was individuals in the older age range.
Taken together, the results showed that as chronological age increased, three effects were revealed: levels of stimulus over-selectivity increased, IQ scores decreased, and cognitive flexibility decreased. Ageing is, thus, related to significant declines in effective stimulus control, intellectual functioning, and cognitive functioning. Furthermore these findings may have implication more broadly in relation to cognition and ageing. It has been noted in the literature that older adults may seek less information, and focus quickly on well-learned “rules of thumb” (Finucane et al. 2005). This may have implications in relation to decision making or making decision given multiple alternatives. This study and other have demonstrated that over-selectivity is a deficit which increases with age. This may account for older people’s hampered adaption to task demands (McHugh et al. 2010). These effects can have a serious impact on the physical and psychological health of older adults, as well as their quality of life, and, therefore, this area of research certainly warrants further exploration.
References
- Bailey SL. Stimulus over selectivity in learning disabled children. J Appl Behav Anal. 1981;14:239–248. doi: 10.1901/jaba.1981.14-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayne C, Gill C, Huppert FA, Barkley C, Gehlhaar E, Girling DM, O’Connor DW, Paykel ES. Incidence of clinically diagnosed subtypes of dementia in an older population. Cambridge Project for Later Life. Br J Psychiatry. 1995;167:255–262. doi: 10.1192/bjp.167.2.255. [DOI] [PubMed] [Google Scholar]
- Broomfield L, McHugh L, Reed P. Factors impacting emergence of behavioral control by underselected stimuli in humans after reduction of control by overselected stimuli. J Exp Anal Behav. 2010;94:125–133. doi: 10.1901/jeab.2010.94-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Knight RT. Prefrontal deficits in attention and inhibitory control with aging. Cereb Cortex. 1997;7:63–69. doi: 10.1093/cercor/7.1.63. [DOI] [PubMed] [Google Scholar]
- Dube WV. Stimulus overselectivity in discrimination learning. In: Reed P, editor. Behavioral theories and interventions for autism. New York: Nova Science Publishers; 2009. pp. 23–46. [Google Scholar]
- Dube WV, McIlvane WJ. Reduction of stimulus over selectivity with nonverbal differential observing responses. J Appl Behav Anal. 1999;32:25–33. doi: 10.1901/jaba.1999.32-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube WV, Lombard KM, Farren KM, Flusser D, Balsamo LM, Fowler TR. Eye tracking assessment of stimulus over selectivity in individuals with mental retardation. Exp Anal Hum Behav Bull. 1999;13:267–271. [Google Scholar]
- Fabio RA, Giannatiempo S, Antonietti A, Budden S. The role of stereotypies in overselectivity process in Rett syndrome. Res Dev Disabil. 2009;30:136–145. doi: 10.1016/j.ridd.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Feeney S. Breadth of cue utilization and ability to attend selectively in schizophrenics and normals. (Doctoral dissertation) Los Angeles: University of California; 1972. [Google Scholar]
- Finucane ML, Alhakami A, Slovic P, Johnson SM. The affect heuristic in judgments of risks and benefits. J Behav Decis Mak. 2000;13:1–17. doi: 10.1002/(SICI)1099-0771(200001/03)13:1<1::AID-BDM333>3.0.CO;2-S. [DOI] [Google Scholar]
- Finucane ML, Mertz CK, Schmidt ES (2005) Task complexity and older adults’ decision-making competence. Psychol Aging 20:71–84 [DOI] [PubMed]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J P Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frankel F, Simmons JQ, Fichter M, Freeman BJ. Stimulus overselectivity in autistic and mentally retarded children: a research note. J Child Psychol Psychiatry. 1984;25:147–155. doi: 10.1111/j.1469-7610.1984.tb01727.x. [DOI] [PubMed] [Google Scholar]
- Gard T, Hölzel BK, Lazar SW. The potential effects of meditation on age-related cognitive decline: a systematic review. Ann N Y Acad Sci. 2014;1307:89–103. doi: 10.1111/nyas.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant DA, Berg E. A behavioral analysis of degree of reinforcement and ease of shifting to new responses in Weigl-type card-sorting problem. J Exp Psychol. 1948;38:404–411. doi: 10.1037/h0059831. [DOI] [PubMed] [Google Scholar]
- Hill EL. Evaluating the theory of executive dysfunction in autism. Dev Rev. 2004;24:189–233. doi: 10.1016/j.dr.2004.01.001. [DOI] [Google Scholar]
- Hsieh PC, Yeh TL, Lee IH, Huang HC, Chen PS, Yang YK, Liao M. Correlation between errors on the Wisconsin Card Sorting Test and the availability of striatal dopamine transporters in healthy volunteers. J Psychiatry Neurosci. 2010;35:90–94. doi: 10.1503/jpn.090007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MP, Leader G, Reed P. Stimulus over-selectivity and extinction-induced recovery of performance as a product of intellectual impairment and autism severity. J Autism Dev Disord. 2015;45:3098–3106. doi: 10.1007/s10803-015-2466-x. [DOI] [PubMed] [Google Scholar]
- Koegel RL, Wilhelm H. Selective responding to multiple cues by autistic children. J Exp Child Psychol. 1973;15:442–453. doi: 10.1016/0022-0965(73)90094-5. [DOI] [PubMed] [Google Scholar]
- Leader G, Loughnane A, Mc Moreland C, Reed P. The effect of stimulus salience on over-selectivity. J Autism Dev Disord. 2009;39:330–338. doi: 10.1007/s10803-008-0626-y. [DOI] [PubMed] [Google Scholar]
- Lovaas OI, Schreibman L, Koegel R, Rehm R. Selective responding by autistic children to multiple sensory inputs. J Abnorm Psychol. 1971;7(77):211–222. doi: 10.1037/h0031015. [DOI] [PubMed] [Google Scholar]
- Matzel LD, Schachtman TR, Miller RR. Recovery of an overshadowed association achieved by extinction of the overshadowing stimulus. Learn Motiv. 1985;16:398–412. doi: 10.1016/0023-9690(85)90023-2. [DOI] [Google Scholar]
- McHugh L, Reed P. Age trends in stimulus overselectivity. J Exp Anal Behav. 2007;88:369–380. doi: 10.1901/jeab.2007.88-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh L, Simpson A, Reed P. Mindfulness as a potential intervention for stimulus over-selectivity in older adults. Res Dev Disabil. 2010;31:178–184. doi: 10.1016/j.ridd.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Park DC. Medication adherence: is and why is older wiser? J Am Geriatr Soc. 2000;48:458–459. doi: 10.1111/j.1532-5415.2000.tb04711.x. [DOI] [PubMed] [Google Scholar]
- Ploog BO. Stimulus overselectivity four decades later: a review of the literature and its implications for current research in autism spectrum disorder. J Autism Dev Disord. 2010;40:1332–1349. doi: 10.1007/s10803-010-0990-2. [DOI] [PubMed] [Google Scholar]
- Reed P. Comparator deficits in autism during discrimination learning: theory to treatment. In: Carlisle PC, editor. Progress in autism research. New York: Nova Science Publishers, Inc; 2007. [Google Scholar]
- Reed, P. (2011). Comparator mechanisms and autistic spectrum conditions. In. T.R. S Schachtman& S.R. Reilly (Eds.), Associative Learning and Conditioning: Human and Animal Applications. Oxford University Press.
- Reed P, Gibson E. The effects of concurrent task load on stimulus overselectivity. J Autism Dev Disord. 2005;35:601–614. doi: 10.1007/s10803-005-0004-y. [DOI] [PubMed] [Google Scholar]
- Reed P, McCarthy J. Cross-modal attention-switching is impaired in autism spectrum disorders. J Autism Dev Disord. 2012;42:947–953. doi: 10.1007/s10803-011-1324-8. [DOI] [PubMed] [Google Scholar]
- Reed P, Broomfield L, McHugh L, McCausland A, Leader G. Extinction of overselected stimuli causes emergence of underselected cues in higher-functioning children with autistic spectrum disorders. J Autism Dev Disabil. 2009;39:290–298. doi: 10.1007/s10803-008-0629-8. [DOI] [PubMed] [Google Scholar]
- Reed P, Savile A, Truzoli R. Event related potential analysis of stimulus over-selectivity. Res Dev Disabil. 2012;33:655–662. doi: 10.1016/j.ridd.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Reynolds G, Reed P. Effects of schedule of reinforcement on over-selectivity. Res Dev Disabil. 2011;32:2489–2501. doi: 10.1016/j.ridd.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Reynolds G, Reed P. The strength and generality of stimulus over-selectivity in simultaneous discrimination procedures. Learn Motiv. 2011;42:113–122. doi: 10.1016/j.lmot.2010.12.001. [DOI] [Google Scholar]
- Reynolds G, Watts J, Reed P. Lack of evidence for inhibitory processes in over-selectivity. Behav Process. 2012;89:14–22. doi: 10.1016/j.beproc.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Smeets PM, Hoogeveen FR, Striefel S, Lancioni GE. Stimulus o overselectivity in TMR children: establishing functional control of simultaneous multiple stimuli. Anal Interv Dev Disabil. 1985;5:247–267. doi: 10.1016/0270-4684(85)90014-X. [DOI] [Google Scholar]
- Solomon M, Smith AC, Frank MJ, Ly S, Carter C. Probabilistic reinforcement learning in adults with autism spectrum disorders. Autism Res. 2011;4:1–12. doi: 10.1002/aur.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tales A, Porter G. Visual attention-related processing in Alzheimer’s disease. Rev Clin Gerontol. 2008;18:229–243. doi: 10.1017/S0959259809002792. [DOI] [Google Scholar]
- Tales A, Bayer AJ, Haworth J, Snowden RJ, Philips M, Wilcock G. Visual search in mild cognitive impairment: a longitudinal study. J Alzheimers Dis. 2011;24:151–160. doi: 10.3233/JAD-2010-101818. [DOI] [PubMed] [Google Scholar]
- Traykov L, Raoux N, Latour F, Gallo L, Hanon O, Baudic S, Rigaud AS. Executive functions deficit in mild cognitive impairment. Cogn Behav Neurol. 2007;20:219–224. doi: 10.1097/WNN.0b013e31815e6254. [DOI] [PubMed] [Google Scholar]
- Wayland S, Taplin JE. Nonverbal categorisation in fluent and non-fluent anomic aphasics. Brain Lang. 1982;16:87–108. doi: 10.1016/0093-934X(82)90074-8. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale—fourth edition. Texas: Pearson: San Antonio; 2008. [Google Scholar]
- White KG, Ruske AC. Memory deficits in Alzheimer’s disease: the encoding hypothesis and cholinergic function. Psychon Bull Rev. 2002;9:426–437. doi: 10.3758/BF03196301. [DOI] [PubMed] [Google Scholar]
- Wilhelm H, Lovaas OI. Stimulus over selectivity: a common feature in autism and mental retardation. Am J Ment Defic. 1976;81:26–31. [PubMed] [Google Scholar]
- Wilkie DM, Masson ME. Attention in the pigeon: a re-evaluation. J Exp Anal Behav. 1976;26:207–212. doi: 10.1901/jeab.1976.26-207. [DOI] [PMC free article] [PubMed] [Google Scholar]