Abstract
Background: Historically, US women started smoking at a later age than men and had lower relative risks for smoking-related cancers. However, more recent birth cohorts of women and men have similar smoking histories and have now reached the high-risk age for cancer. The impact of these changes on cancer incidence has not been systematically examined.
Methods: Relative risks (RR), 95% confidence intervals (CI) and attributable fractions were calculated for cigarette smoking and incidence of 20 smoking-related cancers in 186 057 women and 266 074 men of the National Institutes of Health-AARP cohort, aged 50 to 71 years in 1995 and followed for 11 years.
Results: In the cohort, which included participants born between 1924 and 1945, most women and men started smoking as teenagers. RRs for current vs never smoking were similar in women and men for the following cancers: lung squamous-cell (RR women: 121.4, 95% CI: 57.3–257.4; RR men:114.6, 95% CI: 61.2–214.4), lung adenocarcinoma (RR women: 11.7, 95% CI: 9.8–14.0; RR men: 15.6, 95% CI: 12.5–19.6), laryngeal (RR women: 37.0, 95% CI: 14.9–92.3; RR men: 13.8, 95% CI: 9.3–20.2), oral cavity-pharyngeal (RR women:4.4, 95% CI: 3.3–6.0; RR men: 3.8, 95% CI: 3.0–4.7), oesophageal squamous cell (RR women: 7.3, 95% CI: 3.5–15.5; RR men: 6.2, 95% CI: 2.8–13.7), bladder (RR women: 4.7, 95% CI: 3.7–5.8; RR men: 4.0, 95% CI: 3.5–4.5), colon (RR women: 1.3, 95% CI: 1.2–1.5; RR men: 1.3, 95% CI: 1.1–1.4), and at other sites, with similar attributable fractions.
Conclusions: RRs for current smoking and incidence of many smoking-related cancers are now similar in US women and men, likely reflecting converging smoking patterns.
Keywords: Cigarette smoking, cohort, cancer, men and women
Key Messages
In the late 19th and early 20th centuries, US women typically started smoking cigarettes later in life than men and tended to have lower relative risks for cigarette smoking and different types of incident cancer.
By the 1960s, age at initiation in women had decreased to that of men. The NIH-AARP cohort is one of the first to include these birth cohorts of women, allowing direct comparisons of disease risks in men and women with largely similar lifetime exposures.
In the NIH-AARP cohort that included women and men with similar lifetime smoking histories, women and men had similar relative risks for cigarette smoking and many types of incident smoking-related cancer.
Introduction
The 1964, the US Surgeon General’s Report concluded that cigarette smoking causes lung cancer in men. 1 Later studies extended these findings to women and to numerous other cancer sites. 2,3 However, dramatic subsequent changes in smoking patterns 2 may have affected the magnitude of smoking-related cancer risks.
In the 1920s, most US cigarette smokers were men who had started smoking in their teenage years. 4,5 In contrast, cigarette smoking did not become common among women until World War II. Furthermore, age at initiation tended to be later in women than in men until the 1960s. 4–6 These birth cohorts have only recently reached the high-risk age for cancer. Previous epidemiological studies largely included women who started smoking later in life, and therefore may underestimate the contemporary risks of currently smoking women. 6 Indeed, recent data for overall mortality and the most common causes of death, including mortality from lung cancer, indicate that the RRs for current vs never smoking increased in US women over the course of the 20th centuryto reach that of men. 7 However, few comparable data for the incidence of different cancer types by sex are available.
Other historical changes may also have affected cancer risks, including widespread use of filters, blended tobacco and other changes to cigarettes. 2,8,9 Such changes are thought to have contributed to changes in the histological distribution of lung cancer and also may have affected disease risks. Contemporary smokers in the USA also tend to have lower socioeconomic status than smokers of the past. 10
We examined associations between cigarette smoking and incidence of 20 smoking-related cancers 3 in the women and men of the NIH-AARP study, 11 a large contemporary cohort in which most women and men began smoking in their teenage years. To examine possible changes in RRs over time, we systematically reviewed US studies of smoking and histological subtype of lung cancer (adenocarcinoma, small-cell, squamous-cell and undifferentiated) and anatomical subtype of head and neck cancer (larynx, oral cavity, and pharynx), sites with strong-associations and substantial previous literature.
Methods
Study population
The NIH-AARP (National Institutes of Health-AARP) Diet and Health Study 11 was initiated in 1995–96 when a questionnaire was mailed to 3.5 million AARP members between the ages of 50 and 71, who lived in: California; Florida; Louisiana; New Jersey; North Carolina; Pennsylvania; Atlanta, Georgia; or Detroit, Michigan. Questionnaires were returned by 617 119 participants, 566 398 of whom completed them in satisfactory detail.
The NIH-AARP was approved by the Special Studies Institutional Review Board of the NCI and participants gave informed consent by virtue of completing and returning the questionnaire.
We excluded proxy respondents ( n = 15 760), those with prevalent (except non-melanoma skin cancer) cancer at baseline ( n = 51 234), those lacking information on tobacco smoking ( n = 31 867), those who did not smoke cigarettes but smoked pipes or cigars ( n = 15 367) and those who died before their completed questionnaire was scanned, which was set as the first day of follow-up ( n = 40). Our analytical cohort included 186 057 women and 266 074 men.
Cohort follow-up
Cohort members were followed by annual updates to the US Postal Service National Change of Address database and participant change of address requests. Vital status was assessed via the Social Security Administration Death Master File and National Death Index.
‘Smoking-related cancers’, as defined by the International Agency for Research on Cancer (IARC), 3 were identified by the cancer registries of 11 states (eight baseline states plus Arizona, Nevada and Texas). 12 Site of first primary incident cancer was defined using the International Classification of Disease for Oncology site 13 and oesophagus, lung and ovary histology codes. We also combined cancers of the oral cavity and pharynx together to facilitate comparisons with previous studies.
Follow-up time ended at incident cancer diagnosis, death, movement out of the catchment area or 31 December 2006, whichever came first.
Exposure assessment
We assessed tobacco smoking, demographics and other exposures via baseline questionnaire. Ever smokers were participants who reported smoking 100 cigarettes during their lifetime. We assessed the typical number of cigarettes smoked per day, current or former smoking status and years since cessation for former smokers. Participants reporting quitting in the previous year were considered to be current smokers. To calculate duration of smoking, we used data on age at initiation that were available for a majority of the cohort ( n = 290 242; n = 152 644 ever smokers) who answered a later questionnaire in 2004–06. For those lacking age at initiation, we imputed 17 years as this was the mid point of both female and male participants’ most common response category (15 to 19 years) and corresponds with national surveys. Pack-years of smoking were determined by multiplying cigarettes per day by smoking duration.
Statistical methods
Analyses were conducted using SAS version 9.1. Statistical tests were two-sided. Age-standardized incidence rates per 100 000 person-years and 95% confidence intervals (CI) were calculated for men and women separately, using 5-year age bands standardized to the entire NIH-AARP study population.
Sex-stratified RRs and 95% CIs were obtained from Cox proportional hazards regression models, with adjustment for age, education, alcohol, self-reported ethnicity and smoking pipes or cigars. We provide relative risks for categories of smoking with three or more cases. Inclusion of additional lifestyle and dietary variables, including: consumption of fruits, vegetables and red meat; body mass index; physical activity; and menstrual and reproductive factors (women), had only minor effects on relative risks and were excluded to facilitate comparisons with earlier studies.
Population attributable fractions (AFs) for ever smoking were calculated from multivariate-adjusted beta-coefficients with 95% CIs.
Systematic review of previous studies
Due to strong associations between smoking and each major histological subtype of lung cancer, as well as anatomical sites of head and neck cancer, we systematically reviewed previous US studies of these associations to identify temporal changes in relative risk. Details of our search and selection criteria are provided in the Supplement (available as Supplementary data at IJE online). We identified 21 lung cancer studies providing relative risks by histological subtype and 24 studies of head and neck cancer that provided relative risks by anatomical sub-site. These studies were conducted between 1949 and 2009.
Results
At baseline, 17% of women and 13% of men were current smokers, and 44% of women and 26% of men were never smokers ( Supplementary Table 1 , available as Supplementary data at IJE online). Current smokers tended to have less education than never smokers. Among participants with data on age of initiation, the most common category of initiation was age 15–19 (43% of female and 41% of male current smokers), although more men (33%) started smoking before age 15 than women (16%). Few men (2%) or women (6%) started smoking after age 25.
Incidence rates among current smokers were higher than among never smokers for all 20 examined cancer sites ( Supplementary Tables 2–3 , available as Supplementary data at IJE online) in both women and men. Among female current smokers, the highest incidence rates were observed for lung cancer, followed by colon, bladder, pancreas, and oral cavity and pharynx. Among male current smokers, the most common cancers, in order, were lung, bladder, colon, oral cavity and pharynx, and kidney. In general, incidence rates tended to be higher in men than women, among both current smokers and among never smokers.
RRs for current vs never smoking nearly always reached statistical significance, even for hypopharynx, renal pelvis and other sites with relatively low incidence ( Figure 1 ; Supplementary Tables 2–3 ). The strongest associations were observed for cancers of the respiratory tract, including larynx and lung; RRs for oesophageal squamous-cell, oral cavity and urinary tract cancers also reached 4 or higher.
Figure 1.
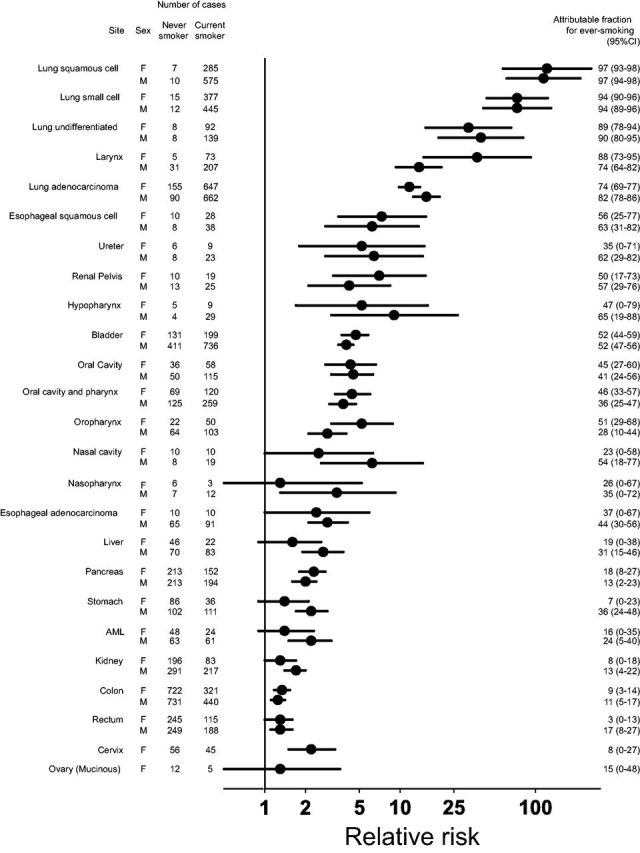
Associations of current vs never cigarette smoking with 20 smoking-related cancer sites in women and men. Relative risks on the log scale are on the x-axis, with each cancer site listed on the y-axis. Plotted circles represent the point estimate and whiskers reflect the 95% confidence intervals. Horizontal line represents a relative risk of 1. Relative risks are adjusted for age, education, alcohol intake, self-reported ethnicity and smoking pipes or cigars. Never smokers are participants who did not smoke cigarettes, pipes or cigars. Attributable fractions and associated 95% confidence intervals for ever smoking are also included.
RRs for current smoking in women were largely comparable to that for men, with overlapping CIs ( Figure 1 ; Supplementary Tables 2–3 ). AFs for ever smoking were also similar ( Figure 1 ). We observed this pattern for major lung cancer subtypes: squamous-cell (women, RR: 121.4, 95% CI: 57.3–257.4, AF for ever smoking: 97%; men, RR: 114.6, 95% CI: 61.2–214.4, AF: 97%), small-cell (women, RR: 73.1, 95% CI: 43.6–122.8, AF: 94%; men, RR: 73.1, 95% CI: 41.1–130.2, AF: 94%), undifferentiated (women, RR: 32.0, 95% CI: 15.4–66.2, AF: 89%; men, RR: 39.5, 95% CI: 19.2–81.2, AF: 90%), adenocarcinoma (women, RR: 11.7, 95% CI: 9.8–14.0, AF: 74%; men, RR: 15.6, 95% CI: 12.5–19.6, AF: 82%) and other sites as well, although confidence intervals for less common sites were sometimes wide.
For example, similar RRs and AFs were observed in women and men for oesophageal squamous-cell (women, RR: 7.3, 95% CI: 3.5–15.5, AF: 56%; men, RR: 6.2, 95% CI: 2.8–13.7, AF: 63%), oesophageal adenocarcinoma (women, RR: 2.4, 95% CI: 1.0–5.9, AF: 37%; men, RR: 2.9, 95% CI: 2.1–4.1, AF: 44%), cancers of the bladder (women, RR: 4.7, 95% CI: 3.7–5.8, AF: 52%; men, RR: 4.0, 95% CI: 3.5–4.5, AF: 52%), renal pelvis (women, RR: 7.0, 95% CI: 3.2–15.4, AF: 50%; men, RR: 4.2, 95% CI: 2.1–8.4, AF: 57%), oral cavity and pharynx (women, RR: 4.4, 95% CI: 3.3–6.0, AF: 46%; men, RR: 3.8, 95% CI: 3.0–4.7, AF: 36%), pancreas (women, RR: 2.3, 95% CI: 1.8–2.8, AF: 18%; men, RR: 2.0, 95% CI: 1.6–2.4, AF: 13%) and colon (women: RR: 1.3, 95% CI: 1.2–1.5, AF: 9%; men, RR: 1.3, 95% CI: 1.1–1.4, AF: 11%). Although RRs were higher in men for some sites, such as liver (men, RR: 2.7, 95% CI: 1.9–3.8, AF: 31%; women, RR: 1.6, 95% CI: 0.9–2.6, AF: 19%), RRs for others, such as larynx, were higher in women (women, RR: 37.0, 95% CI: 14.9–92.3, AF: 88%; men, RR: 13.8, 95% CI: 9.3–20.2, AF: 74%).
We observed a dose-response for nearly all examined sites, with higher incidence rates and RRs observed with smoking more cigarettes per day. RRs for cigarettes per day ( Tables 1 and 2 ) and pack-years ( Supplementary Tables 4–5 , available as Supplementary data at IJE online) were generally similar between women and men for each cancer site.
Table 1.
Cigarettes per day among current smokers and cancer in women
| Smoking use | Never a |
1–10 cigarettes per day
|
11–20 cigarettes per day
|
20–40 cigarettes per day
|
> 40 cigarettes per day
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Number in cohort | 82 032 |
9784
|
13 513
|
8017
|
630
|
||||
| Cancer type | Case ( n ) | Case ( n ) | Relative risk b(95% CI) | Case ( n ) | Relative risk b(95% CI) | Case ( n ) | Relative risk b(95% CI) | Case ( n ) | Relative risk b(95% CI) |
| Oral cavity and pharynx | 69 | 23 | 2.9 (1.8–4.8) | 56 | 5.2 (3.6–7.4) | 37 | 5.8 (3.8–8.8) | 4 | 8.0 (2.9–22.2) |
| Oral cavity | 36 | 8 | 2.2 (1–4.7) | 30 | 5.6 (3.4–9.2) | 20 | 6.5 (3.7–11.4) | 0 | nd |
| Oropharynx | 22 | 11 | 3.8 (1.8–7.9) | 21 | 5.3 (2.8–9.7) | 15 | 6.1 (3.1–12.2) | 3 | 15.2 (4.4–52) |
| Hypopharynx | 5 | 1 | nd | 5 | 7.6 (2.1–27.1) | 2 | nd | 1 | nd |
| Nasopharynx | 6 | 3 | 4.3 (1–17.9) | 0 | nd | 0 | nd | 0 | nd |
| Respiratory tract | |||||||||
| Nasal cavity | 10 | 1 | nd | 3 | 1.6 (0.4–5.9) | 6 | 5.5 (1.9–16) | 0 | nd |
| Larynx | 5 | 11 | 18.4 (6.3–53.3) | 29 | 37.1 (14.3–96.6) | 29 | 65.2 (24.9–170.9) | 4 | 118.8 (31.4–449.4) |
| Lung | 281 | 375 | 12.2 (10.4–14.2) | 800 | 19.8 (17.3–22.7) | 643 | 28.4 (24.6–32.8) | 75 | 44.2 (34.2–57.2) |
| Lung adenocarcinoma | 155 | 131 | 7.4 (5.9–9.4) | 287 | 12.4 (10.2–15.2) | 204 | 15.7 (12.7–19.5) | 25 | 25.7 (16.8–39.4) |
| Lung small-cell | 15 | 62 | 38.4 (21.8–67.6) | 148 | 70.2 (41.2–119.7) | 151 | 129.3 (75.7–220.7) | 16 | 185.8 (91.4–377.7) |
| Lung squamous-cell | 7 | 57 | 74 (33.7–162.6) | 126 | 127.6 (59.5–273.9) | 93 | 173.8 (80.3–376.3) | 9 | 233.7 (86.6–630.6) |
| Lung undifferentiated | 8 | 24 | 26.2 (11.7–58.6) | 44 | 37 (17.3–79.2) | 23 | 34.1 (15–77.4) | 1 | nd |
| Alimentary tract | |||||||||
| Oesophageal squamous-cell | 10 | 3 | 2.1 (0.6–8) | 20 | 12.6 (5.7–28.1) | 5 | 5.4 (1.8–16.7) | 0 | nd |
| Oesophageal adenocarcinoma | 10 | 1 | nd | 3 | 1.6 (0.4–6.2) | 6 | 5.4 (1.8–16.2) | 0 | nd |
| Stomach | 86 | 10 | 1.1 (0.6–2.2) | 17 | 1.6 (0.9–2.6) | 7 | 1.2 (0.5–2.6) | 2 | nd |
| Colon | 722 | 101 | 1.3 (1.1–1.6) | 146 | 1.5 (1.2–1.8) | 73 | 1.4 (1.1–1.7) | 1 | nd |
| Rectum | 245 | 30 | 1.1 (0.7–1.6) | 55 | 1.5 (1.1–2) | 24 | 1.2 (0.8–1.8) | 6 | 4.0 (1.7–9) |
| Liver | 46 | 6 | 1.3 (0.5–3) | 11 | 1.7 (0.9–3.4) | 4 | 1.1 (0.4–3.2) | 1 | nd |
| Pancreas | 213 | 58 | 2.6 (2.0–3.5) | 60 | 2.1 (1.6–2.8) | 32 | 2.0 (1.4–2.9) | 2 | nd |
| Urinary tract | |||||||||
| Bladder | 131 | 50 | 3.5 (2.5–4.9) | 82 | 4.5 (3.4–5.9) | 63 | 6.2 (4.5–8.4) | 4 | 5.2 (1.9–14.1) |
| Renal pelvis | 10 | 3 | 3.4 (0.9–12.4) | 9 | 7.6 (3–19.3) | 7 | 10.7 (3.9–29.4) | 0 | nd |
| Ureter | 6 | 2 | nd | 5 | 6.3 (1.8–21.7) | 2 | 4.3 (0.8–22.7) | 0 | nd |
| Kidney | 196 | 25 | 1.1 (0.8–1.8) | 36 | 1.3 (0.9–1.8) | 18 | 1.2 (0.7–1.9) | 4 | 3.5 (1.3–9.4) |
| Other | |||||||||
| AML | 48 | 12 | 2.4 (1.2–4.5) | 8 | 1.1 (0.5–2.4) | 4 | 1.0 (0.3–2.7) | 0 | nd |
| Cervical | 56 | 14 | 2.1 (1.2–3.8) | 16 | 1.9 (1.1–3.3) | 15 | 3.2 (1.8–5.7) | 0 | nd |
| Ovary (mucinous) | 12 | 2 | nd | 3 | 1.6 (0.4–5.8) | 0 | nd | 0 | nd |
nd, not determined; AML, acute myeloid leukemia.
a Referent group: never smokers are participants who did not smoke cigarettes, pipes or cigars.
b Adjusted for age, education, alcohol intake, self-reported ethnicity and smoking pipes or cigars.
Table 2.
Cigarettes per day among current smokers and cancer in men
| Smoking use | Never a |
1–10 cigarettes per day
|
11–20 cigarettes per day
|
21–40 cigarettes per day
|
> 40 cigarettes per day
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Number in cohort | 68 748 |
7184
|
13 314
|
13 421
|
1982
|
||||
| Cancer type | Case ( n ) | Case ( n ) | Relative risk b (95% CI) | Case ( n ) | Relative risk b (95% CI) | Case ( n ) | Relative risk b (95% CI) | Case ( n ) | Relative risk b (95% CI) |
| Oral cavity and pharynx | 125 | 32 | 2.4 (1.6–3.6) | 89 | 3.6 (2.7–4.8) | 117 | 4.5 (3.4–6.0) | 21 | 5.4 (3.3–8.7) |
| Oral cavity | 50 | 12 | 2.5 (1.3–4.8) | 45 | 5.0 (3.2–7.7) | 51 | 5.5 (3.6–8.4) | 7 | 5.0 (2.2–11.2) |
| Oropharynx | 64 | 18 | 2.7 (1.5–4.6) | 31 | 2.4 (1.5–3.8) | 44 | 3.2 (2.1–4.9) | 10 | 4.9 (2.4–9.8) |
| Hypopharynx | 4 | 1 | nd | 8 | 7.8 (2.2–27.3) | 17 | 15.2 (4.7–48.4) | 3 | 17.4 (3.6–83.2) |
| Nasopharynx | 7 | 1 | nd | 5 | 2.8 (0.8–10.1) | 5 | 3.2 (0.9–11.6) | 1 | nd |
| Respiratory tract | |||||||||
| Nasal cavity | 8 | 2 | nd | 9 | 7.6 (2.7–21.3) | 7 | 5.9 (2.0–17.7) | 1 | nd |
| Larynx | 31 | 28 | 9.4 (5.5–15.8) | 72 | 13.1 (8.5–20.3) | 87 | 16 (10.4–24.6) | 20 | 26.2 (14.6–46.8) |
| Lung | 175 | 299 | 17.3 (14.3–20.9) | 851 | 27.9 (23.7–33) | 1076 | 37.7 (32–44.4) | 209 | 53.0 (43.2–65.2) |
| Lung adenocarcinoma | 90 | 99 | 11.5 (8.5–15.4) | 248 | 16.2 (12.6–20.8) | 274 | 19.1 (14.9–24.5) | 41 | 20.9 (14.3–30.6) |
| Lung small-cell | 12 | 51 | 40.8 (21.6–77.1) | 153 | 67.9 (37.5–123) | 203 | 93.6 (51.9–168.9) | 38 | 125.5 (65.0–242.3) |
| Lung squamous-cell | 10 | 68 | 64.5 (33.0–126.0) | 185 | 102.0 (53.7–193.7) | 270 | 160.6 (84.9–303.7) | 52 | 222.5 (112.3–440.9) |
| Lung undifferentiated | 8 | 18 | 24.7 (10.6–57.9) | 37 | 28.7 (13.1–62.6) | 73 | 61.7 (29.1–130.6) | 11 | 67.5 (26.6–171.0) |
| Alimentary tract | |||||||||
| Oesophageal squamous-cell | 8 | 4 | 3.2 (0.9–11) | 12 | 6.0 (2.3–15.4) | 19 | 9.7 (3.9–23.7) | 3 | 9.8 (2.5–39.3) |
| Oesophageal adenocarcinoma | 65 | 10 | 1.6 (0.8–3.3) | 37 | 3.2 (2.1–5) | 39 | 3.5 (2.2–5.4) | 5 | 3.2 (1.3–8.1) |
| Stomach | 102 | 27 | 2.5 (1.6–3.9) | 38 | 2.1 (1.4–3.1) | 43 | 2.5 (1.7–3.7) | 3 | 1.3 (0.4–4.0) |
| Colon | 731 | 93 | 1.3 (1.1–1.7) | 161 | 1.3 (1.1–1.5) | 161 | 1.3 (1.1–1.6) | 25 | 1.4 (0.9–2.1) |
| Rectum | 249 | 35 | 1.1 (0.8–1.7) | 75 | 1.4 (1.0–1.8) | 64 | 1.2 (0.9–1.6) | 14 | 1.7 (1.0–3.0) |
| Liver | 70 | 20 | 2.8 (1.7–4.8) | 34 | 2.9 (1.8–4.5) | 26 | 2.3 (1.4–3.8) | 3 | 1.9 (0.6–6.1) |
| Pancreas | 213 | 39 | 1.7 (1.2–2.5) | 63 | 1.6 (1.2–2.2) | 78 | 2.1 (1.6–2.8) | 14 | 2.6 (1.5–4.6) |
| Urinary tract | |||||||||
| Bladder | 411 | 122 | 3.2 (2.6–4.0) | 284 | 4.2 (3.5–4.9) | 288 | 4.4 (3.7–5.2) | 42 | 4.6 (3.3–6.4) |
| Renal pelvis | 13 | 4 | 4.2 (1.3–13.5) | 12 | 6.9 (2.9–16.3) | 8 | 4.9 (1.9–12.9) | 1 | nd |
| Ureter | 8 | 3 | 4.7 (1.2–18.4) | 13 | 10.5 (4.1–26.6) | 7 | 5.6 (1.9–16.4) | 0 | nd |
| Kidney | 291 | 53 | 1.9 (1.4–2.6) | 67 | 1.4 (1.0–1.8) | 88 | 1.9 (1.4–2.4) | 9 | 1.4 (0.7–2.7) |
| Other | |||||||||
| AML | 63 | 13 | 2.1 (1.1–3.9) | 23 | 2.1 (1.3–3.6) | 23 | 2.3 (1.4–3.9) | 2 | nd |
nd, not determined; AML, acute myeloid leukemia.
a Referent group: never smokers are participants who did not smoke cigarettes, pipes or cigars.
b Adjusted for age, education, alcohol intake, self-reported ethnicity and smoking pipes or cigars.
Similar patterns for current vs never smoking and each incident cancer were also observed among each stratum of educational status ( Supplementary Tables 6–7 , available as Supplementary data at IJE online), although our confidence intervals were often wide.
To evaluate possible historical changes, we compared our results for lung, laryngeal, oral cavity and pharyngeal cancers with previously published US studies by systematic review (plotted chronologically in Figures 2 and 3 and listed in Supplementary Tables 8–12 , available as Supplementary data at IJE online). As most previous studies combined cancers of the oral cavity and pharynx together, we did the same. In studies conducted before 1970, that included participants born in the late 19th and early 20th centuries, RRs for current vs never smoking were lower in women than in men (RRs for lung adenocarcinoma: 0.6–1.5 in women vs 1.5–4.6 in men; RRs for lung squamous-cell cancer: 2.6 and 3.0 in women vs 8.3 and 22.9 in men; RRs for laryngeal cancer: 3.8 in women vs 5.8–23.4 in men; RRs for oral cavity and pharyngeal cancer: 2.0 and 2.6 in women vs 3.1–6.3 in men).
Figure 2.
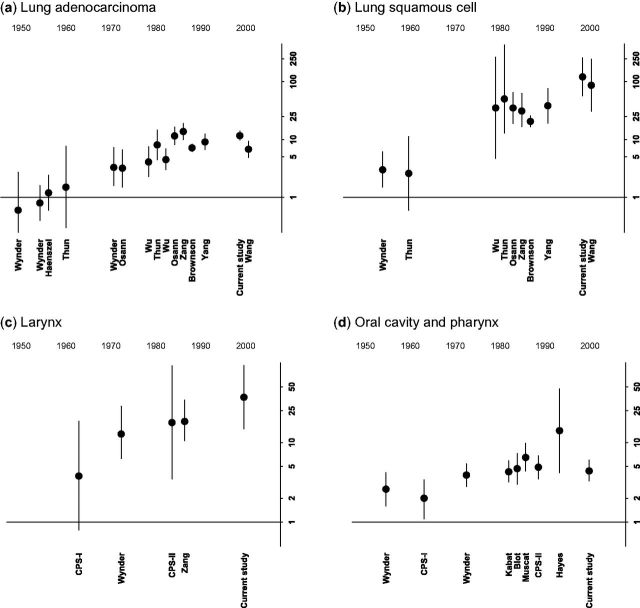
Current cigarette use and lung and head and neck cancer by study year, in women. Relative risks for current vs never smoking are plotted on the y-axis, using a log scale. Year is on the x-axis. Plotted circles represent the point estimate and whiskers reflect the 95% confidence intervals. Each study is plotted by the mid point of when it was conducted, and labelled with the first author of the associated publication. Horizontal line represents a relative risk of 1. (a) Lung adenocarcinoma; (b) lung squamous cell carcinoma; (c) larynx; (d) oral cavity and pharynx.
Figure 3.
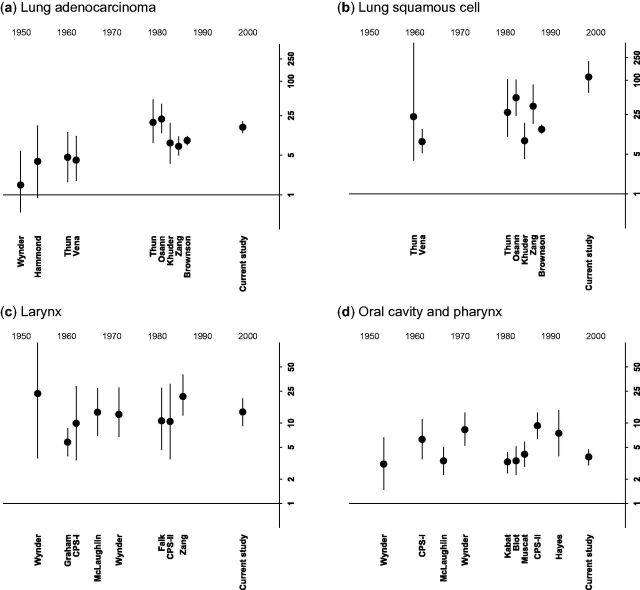
Current cigarette use and lung and head and neck cancer by study year, in men. Relative risks for current vs never smoking are plotted on the y-axis, using a log scale. Year is on the x-axis. Plotted circles represent the point estimate and whiskers reflect the 95% confidence intervals. Each study is plotted by the mid point of when it wasconducted, and labelled with the first author of the associated publication. Horizontal line represents a relative risk of 1. (a) lung adenocarcinoma; (b) lung squamous cell carcinoma; (c) larynx; (d) oral cavity and pharynx.
For women ( Figure 2 ), RRs increased over time for each of these cancer types (comparing RRs in studies conducted prior to 1970 with the current study: lung adenocarcinoma: 0.6–1.5 vs 11.7; lung squamous-cell cancer: 2.6 and 3.0 vs 121.4; laryngeal cancer: 3.8 vs 37.0; and oral cavity and pharyngeal cancer: 2.0 and 2.6 vs 4.4) and became similar to RRs in men. Similar patterns were also seen for small-cell lung cancer, although we identified fewer previous studies ( Supplementary Table 8 ).
In contrast to trends among women, RRs for current vs never smoking with laryngeal and oral cavity and pharyngeal cancer in men did not appear to change over time ( Figure 3 ). However, RRs for lung adenocarcinoma (for example, 15.6 in the current study) and lung squamous-cell cancer (114.6 in the current study) were higher in the more recent studies than in earlier studies, as we had observed for women. Similar patterns were again observed for small-cell lung cancer.
In both women and men, increasing RRs for cigarette smoking and lung cancer persisted among categories of cigarettes per day and pack-years of smoking ( Supplementary Tables 9 and Supplementary Data ).
Discussion
Relative risks for current smoking and each smoking-related cancer were broadly similar in the women and men of the NIH-AARP cohort, with largely overlapping confidence intervals. In contrast, data from our systematic review indicate substantially lower RRs in women relative to men in US studies conducted before 1970, which included participants born in the late 19th and early 20th centuries, for each major histological subtype of lung cancer, as well as for cancers of the larynx and oral cavity and pharynx. Before 1970, women at the ages of highest cancer risk tended to start smoking later in life than men, and thus had less cumulative exposure to cigarettes. 4,5 Typical age at initiation only reached that of men in the 1960s. 4–6,14 The NIH-AARP cohort is one of the first to include these more recent birth cohorts of women, allowing direct comparisons of disease risks in men and women with largely similar lifetime exposures. Our results indicate that the relative risks and population-attributable fractions for cigarette smoking and individual smoking-related cancers are now also similar for men and women. Although systematic investigations of cigarette smoking and incident cancers among these birth cohorts are not yet available from other US studies, we expect our results to be confirmed as they parallel recent findings for cigarette smoking with total and cause-specific mortality, which were consistent in our cohort and four others. 7,15 Together, these results indicate that with converging patterns of cigarette use, US female and male smokers now have similar cigarette-related RRs for disease.
Unlike women, men have been initiating smoking as teenagers for a century. 4,5 Perhaps reflecting this pattern, the RRs among men for laryngeal, oral cavity and pharyngeal cancer were stable over the years in our systematic review, whereas RRs among women strengthened over time. In contrast, RRs for cigarette smoking and each major histological subtype of lung cancer appeared to increase in men over the course of the 20th century, as we also observed for women. Such findings likely do not reflect changes in cigarette use per day. Higher RRs for lung cancer in the more recent studies persisted within specific levels of cigarettes per day or pack-years. Also, smokers in our cohort tended to smoke fewer cigarettes per day than in earlier US studies, reflecting similar declines for cigarettes per day in the US population. 5,16 Concordant with our results, mortality rates for each of these histological subtypes were higher in male current smokers in the Cancer Prevention Study (CPS)-II, with follow-up from 1982 to 1984, than in CPS-I with follow-up from 1959 to 1961. 8 Supporting these findings, modelling studies have also shown that the relative risks from CPS-I underestimated subsequent US lung cancer mortality rates, and in particular the rise of lung adenocarcinoma. 2,17,18
Analogous findings in the USA have been reported for incident bladder cancer, in which associations with smoking were stronger in the current cohort 19 and the New England Bladder Cancer Study 20 than in previous studies. Associations between smoking and total mortality also strengthened in the USA and the UK over the 20th century in both women and men. 6,7,21,22
Strengthening associations between cigarette smoking and some cancers may reflect widespread changes in the design and construction of cigarettes. 2,8 Such changes could have affected both smoking behaviour and the distribution of carcinogens present in tobacco smoke. 9 These changes might have differential effects across the spectrum of tobacco-related cancers, suggesting an explanation for higher smoking-related risks of lung and bladder cancer in men yet constant risks of laryngeal, oral cavity and pharyngeal cancer. Recent data suggesting specific associations between certain tobacco-specific nitrosamines and particular forms of cancer 23 support this hypothesis.
As cancers of the head and neck are additionally caused by alcohol 24 and human papillomavirus (HPV), 25 it is also possible that historical changes in these or possibly other risk factors may have obscured the effects of changing cigarettes on these sites. As our study lacked biological samples, we were unable to test for HPV or other cancer risk factors requiring biological samples such as Helicobacter pylori , hepatitis B and hepatitis C. Future studies that include biological samples are needed to examine associations of cigarette smoking and cancer in the context of these risk factors in contemporary birth cohorts of women and men.
Higher RRs of certain smoking-related cancers could also reflect decreasing incidence rates in never smokers, which would cause the RRs for smoking to increase even if absolute risks were unchanged. However, data from three consortial projects 7,26,27 indicate that the rates of lung cancer in never smokers remained constant, or possibly increased, over the ast 50 years. Other factors, such as increases in fine particulate air pollution (PM2.5), may also have influenced lung cancer trends in never smokers, particularly trends in adenocarcinoma. 28
Current smokers in the US population tend to have less education than never smokers and this was reflected in our cohort. Concerns have been raised that such differences could confound associations between smoking and cancer. 29 However, we observed strong associations between smoking and cancer across strata of educational status, suggesting socioeconomic differences between smokers and non-smokers had little effect on our relative risks.
Strengths of our study include prospective assessment of cigarette use and very large size, allowing examination of nasopharynx, ureter and other relatively uncommon cancers that are rarely investigated in prospective cohort studies. We examined incident cancers, important as cigarette smoking can affect both cancer development and survival, 30 and comparisons of incident cancer are less affected by historical changes in treatment than are studies of cancer mortality. Unlike most mortality studies, we were able to investigate associations between cigarette smoking and specific histological subtypes of oesophageal and lung cancer, observing associations of substantially different magnitude for each subtype. Inclusion of both women and men allowed us to directly compare risks between them.
Limitations include a lack of information on smoking initiation for the full cohort and assessment of smoking at only a single time-point. During follow-up, some current smokers probably quit, which would attenuate observed associations for current smoking. However, as our RRs were only moderately higher in analyses restricted to the first 6 years of follow-up (data not shown), we present results for the entire follow-up period to maximize statistical power. Also, our results may not apply to other populations with different smoking prevalence and different cigarette composition. For example, recent data from the European Prospective Investigation into Cancer cohort revealed lower RRs in women than men for current smoking and different cancer types, 31 likely reflecting less lifetime smoking history in the women relative to the men of this cohort. However, our results do likely apply to the UK and other countries where women and men have similar smoking patterns.
In conclusion, our data indicate that falling age of smoking initiation in women and other historical changes in US cigarette smoking patterns have altered associations with incident cancer. With now similar lifelong smoking patterns, relative risks and population-attributable fractions for cigarette smoking and cancer in women are now broadly similar to those found in men. Cigarette smoking remains a critical determinant of cancer in the USA and elsewhere. Further reductions in cigarette smoking are urgently needed.
Funding
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics.
Conflict of interest: None declared.
Supplementary Material
Acknowledgements
The authors thank the participants in the NIH-AARP Diet and Health Study for their cooperation. The authors thank Sigurd Hermansen and Kerry Grace Morrissey from Westat Inc. (Rockville, MD) for study outcomes ascertainment and management, and Leslie Carroll at Information Management Services (Silver Spring, MD) for data support and analysis. These authors have confirmed their agreement.
Cancer incidence data from the Atlanta metropolitan area were collected by the Georgia Center for Cancer Statistics, Department of Epidemiology, Rollins School of Public Health, Emory University. Cancer incidence data from California were collected by the California Department of Health Services, Cancer Surveillance Section. Cancer incidence data from the Detroit metropolitan area were collected by the Michigan Cancer Surveillance Program, Community Health Administration, State of Michigan. The Florida cancer incidence data used in this report were collected by the Florida Cancer Data System (FCDS) under contract with the Florida Department of Health (FDOH). Cancer incidence data from Louisiana were collected by the Louisiana Tumor Registry, Louisiana State University Medical Center in New Orleans. Cancer incidence data from New Jersey were collected by the New Jersey State Cancer Registry, Cancer Epidemiology Services, New Jersey State Department of Health and Senior Services. Cancer incidence data from North Carolina were collected by the North Carolina Central Cancer Registry. Cancer incidence data from Pennsylvania were supplied by the Division of Health Statistics and Research, Pennsylvania Department of Health, Harrisburg, Pennsylvania. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions. Cancer incidence data from Arizona were collected by the Arizona Cancer Registry, Division of Public Health Services, Arizona Department of Health Services. Cancer incidence data from Texas were collected by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services. Cancer incidence data from Nevada were collected by the Nevada Central Cancer Registry, Center for Health Data and Research, Bureau of Health Planning and Statistics, State Health Division, State of Nevada Department of Health and Human Services.
References
- 1. United States Surgeon General's Advisory Committee on Smoking and Health . Smoking and Health; Report of the Advisory Committee to the Surgeon General of the Public Health Service . Washington, DC: : U.S. Dept. of Health, Education, and Welfare; , 1964. . [Google Scholar]
- 2. U.S. Department of Health and Human Services . The Health Consequences of Smoking: 50 Years of Progress: a Report of the Surgeon General . Atlanta, GA: : U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014 . [Google Scholar]
- 3. Secretan B, Straif K, Baan R, et al. . A review of human carcinogens. Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish . Lancet Oncol 2009. ; 10 : 1033 – 34 . [DOI] [PubMed] [Google Scholar]
- 4. Burns David M, et al. . “Cigarette smoking behavior in the United States,” in Changes in Cigarette-Related Disease Risks and Their Implications for Prevention and Control . Smoking and Tobacco Control Monograph no. 8, NIH publication no. 97–4213. Bethesda, MD.: Cancer Control and Population Sciences, National Cancer Institute; 1997. pp. 13–112 . [Google Scholar]
- 5. Burns D, Major J, Shanks T . “Changes in number of cigarettes smoked per day: cross-sectional and birth cohort analyses using NHIS,” in Those who continue to smoke: is achieving abstinence harder and do we need to change our interventions? Smoking and Tobacco Control Monograph no. 15, NIH publication no. 03–5370. Bethesda, MD: National Cancer Institute; 2003. pp. 83–99 . [Google Scholar]
- 6. Pirie K, Peto R, Reeves GK, Green J, Beral V . The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK . Lancet 2013. ; 381 : 133 – 41 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thun MJ, Carter BD, Feskanich D, et al. . 50-year trends in smoking-related mortality in the United States . N Engl J Med 2013. ; 368 : 351 – 64 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thun MJ, Lally CA, Flannery JT, Calle EE, Flanders WD, Heath CW, Jr . Cigarette smoking and changes in the histopathology of lung cancer . J Natl Cancer Inst 1997. ; 89 : 1580 – 86 . [DOI] [PubMed] [Google Scholar]
- 9. Hoffmann D, Djordjevic MV, Hoffmann I . The changing cigarette . Prev Med 1997. ; 26 : 427 – 34 . [DOI] [PubMed] [Google Scholar]
- 10. Kanjilal S, Gregg EW, Cheng YJ, et al. . Socioeconomic status and trends in disparities in 4 major risk factors for cardiovascular disease among US adults, 1971–2002 . Arch Intern Med 2006. ; 166 : 2348 – 55 . [DOI] [PubMed] [Google Scholar]
- 11. Schatzkin A, Subar AF, Thompson FE, et al. . Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health-American Association of Retired Persons Diet and Health Study . Am J Epidemiol 2001. ; 154 : 1119 – 25 . [DOI] [PubMed] [Google Scholar]
- 12. Michaud DS, Midthune D, Hermansen S, et al. . Comparison of cancer registry case ascertainment with SEER estimates and self-reporting in a subset of the NIH-AARP Diet and Health Study . J Registry Manag 2005. ; 32 : 70 – 75 . [Google Scholar]
- 13. Fritz AG . International Classification of Diseases for Oncology, ICD-O . 3rd edn . Geneva: : World Health Organization; , 2000. . [Google Scholar]
- 14. Agaku IT, King BA, Dube SR . Current cigarette smoking among adults – United States, 2005-2012 . MMWR Morb Mortal Wkly Rep 2014. ; 63 : 29 – 34 . [PMC free article] [PubMed] [Google Scholar]
- 15. Carter BD, Abnet CC, Feskanich D, et al. . Smoking and mortality—beyond established causes . N Engl J Med 2015. ; 372 : 631 – 40 . [DOI] [PubMed] [Google Scholar]
- 16. Shiffman S . Light and intermittent smokers: background and perspective . Nicotine Tob Res 2009. ; 11 : 122 – 25 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burns DM, Anderson CM, Gray N . Has the lung cancer risk from smoking increased over the last fifty years? Cancer Causes Control 2011. ; 22 : 389 – 97 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burns DM, Anderson CM, Gray N . Do changes in cigarette design influence the rise in adenocarcinoma of the lung? Cancer Causes Control 2011. ; 22 : 13 – 22 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC . Association between smoking and risk of bladder cancer among men and women . JAMA 2011. ; 306 : 737 – 45 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baris D, Karagas MR, Verrill C, et al. . A case-control study of smoking and bladder cancer risk: emergent patterns over time . J Natl Cancer Inst 2009. ; 101 : 1553 – 61 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Doll R, Peto R, Wheatley K, Gray R, Sutherland I . Mortality in relation to smoking: 40 years' observations on male British doctors . BMJ 1994. ; 309 : 901 – 11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jha P, Ramasundarahettige C, Landsman V, et al. . 21st-century hazards of smoking and benefits of cessation in the United States . N Engl J Med 2013. ; 368 : 341 – 50 . [DOI] [PubMed] [Google Scholar]
- 23. Stepanov I, Sebero E, Wang R, Gao YT, Hecht SS, Yuan JM . Tobacco-specific N-nitrosamine exposures and cancer risk in the Shanghai cohort study: Remarkable coherence with rat tumor sites . Int J Cancer 2014. ; 134:2278 – 83 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Freedman ND, Schatzkin A, Leitzmann MF, Hollenbeck AR, Abnet CC . Alcohol and head and neck cancer risk in a prospective study . Br J Cancer 2007. ; 96 : 1469 – 74 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. D'Souza G, Kreimer AR, Viscidi R, et al. . Case-control study of human papillomavirus and oropharyngeal cancer . N Engl J Med 2007. ; 356 : 1944 – 56 . [DOI] [PubMed] [Google Scholar]
- 26. Thun MJ, Hannan LM, Adams-Campbell LL, et al. . Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies . PLoS Med 2008. ; 185 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wakelee HA, Chang ET, Gomez SL, et al. . Lung cancer incidence in never smokers . J Clin Oncol 2007. ; 25 : 472 – 78 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Raaschou-Nielsen O, Andersen ZJ, Beelen R, et al. . Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE) . Lancet Oncol 2013. ; 14 : 813 – –22 . [DOI] [PubMed] [Google Scholar]
- 29. Thun MJ, Apicella LF, Henley SJ . Smoking vs other risk factors as the cause of smoking-attributable deaths: confounding in the courtroom . JAMA 2000. ; 284 : 706 – 12 . [DOI] [PubMed] [Google Scholar]
- 30. Gritz ER, Demark-Wahnefried W . Health behaviors influence cancer survival . J Clin Oncol 2009. ; 27 : 1930 – 32 . [DOI] [PubMed] [Google Scholar]
- 31. Agudo A, Bonet C, Travier N, et al. . Impact of cigarette smoking on cancer risk in the European prospective investigation into cancer and nutrition study . J Clin Oncol 2012. ; 30 : 4550 – 57 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.