Abstract
Testosterone deficiency is associated with a higher incidence of cardiovascular diseases in men. However, its effect on cell senescence, which plays a causal role in vascular aging, remains unclear. Here, we tested the hypothesis that testosterone alleviated vascular smooth muscle cell (VSMC) senescence and collagen synthesis via growth arrest-specific protein 6 (Gas6)/Axl- and Akt/FoxO1a-dependent pathways. Testosterone significantly ameliorated angiotensin II-induced VSMC senescence and collagen overexpression. In addition, testosterone inhibited angiotensin II-induced matrix metalloproteinase-2 (MMP-2) activity, which played a pivotal role in facilitating age-related collagen deposition. Testosterone increased the expression of tissue inhibitor of metalloproteinase-2 but decreased the expression of MMP-2 and membrane type-1 metalloproteinase which contributed to increase MMP-2 activity. The effects on VSMCs senescence and collagen synthesis were mediated by restoration of angiotensin II-induced downregulation of Gas6 and Axl expression and a subsequent reduction of Akt and FoxO1a phosphorylation. The effects of testosterone were reversed by a Gas6 blocker, Axl-Fc, and a specific inhibitor of Axl, R428. Treatment of VSMCs with PI3K inhibitor LY294002 abrogated the downregulating effect of testosterone on MMP-2 activity. Furthermore, when FoxO1a expression was silenced by using a specific siRNA, the inhibitory effect of testosterone on MMP-2 activity was revered as well, that indicated this process was Akt/FoxO1a dependence. Taken together, Gas6/Axl and Akt/FoxO1a were involved in protective effects of testosterone on VSMCs senescence and collagen synthesis. Our results provide a novel mechanism underlying the protective effect of testosterone on vascular aging and may serve as a theoretical basis for testosterone replacement therapy.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-016-9910-5) contains supplementary material, which is available to authorized users.
Keywords: Testosterone, Growth arrest-specific protein 6 (Gas6)/Axl, Vascular smooth muscle cell, Cellular senescence, Collagen
Introduction
Vascular aging is a major independent risk factor for cardiovascular diseases (Lakatta and Levy 2003; Yildiz 2007a). Different pathophysiological stimuli are involved in its development and mediate extracellular matrix (ECM) remodeling leading to vascular stiffness (Lakatta 2003; Wang and Bennett 2012). Cellular senescence is considered to play a causal role in the pathogenesis and development of vascular aging (Kovacic et al. 2011a, b; Minamino and Komuro 2007). Cellular senescence causes disorders of ECM metabolism, initiates vascular remodeling, and finally results in a reduction in compliance and an increase in stiffness (Kovacic et al. 2011a; Minamino and Komuro 2007). The circulating level of testosterone decreases with age gradually. Testosterone deficiency is associated with a higher incidence of cardiovascular disease in men (Jones and Saad 2009; Lopes et al. 2012; Vlachopoulos et al. 2014). Furthermore, it is associated with the markers of vascular aging such as increased carotid intima-media thickness and aortic calcification (Vlachopoulos et al. 2014). The newest large observational cohort with extended follow-up shows that normalization of total testosterone level after testosterone replacement therapy (TRT) is associated with a significant reduction in all-cause mortality, myocardial infarction, and stroke (Sharma et al. 2015). The cardiovascular protective effects of testosterone partly attributes to its vasodilation effect and beneficial effect on blood lipid profile and against atheroma formation (Seyrek et al. 2007; Yildiz and Seyrek 2007b). However, the underlying relevance of testosterone delaying vascular aging as well as cellular senescence remains poorly understood.
Gas6 is a member of the vitamin K-dependent protein family and interacts with receptor tyrosine kinases of the TAM (Tyro3, Axl, Mer) family (Claudio Schneider et al. 1988; Lemke 2013). Gas6 has the highest affinity for Axl among TAM receptors and is often called Gas6/Axl pathway. Numerous studies have identified that Gas6/Axl pathway plays a pivotal role in vascular biology and diseases such as vascular calcification, vascular remodeling, and atherosclerosis (Hurtado et al. 2011; Korshunov et al. 2006; Lutgens et al. 2008; Son et al. 2010). Moreover, the polymorphism of gas6 gene has been associated with stroke and acute coronary syndrome in humans (Hurtado et al. 2010; Munoz et al. 2007). Clinical research shows that the plasma concentration of Gas6 decreases with age and is associated with the testosterone level (Hung et al. 2010). Moreover, Son et al. (2010) finds that there are functional androgen-response elements in the Gas6 promoter, so androgen receptor pathway could regulate Gas6 transcription directly. More importantly, we demonstrate that Gas6/Axl plays an important role in regulating cell cycle arrest and delaying VSMC senescence recently (Jin et al. 2015).
Thus, we hypothesized that testosterone might delay VSMC senescence and decrease collagen expression via Gas6/Axl pathway. Firstly, we established the induced senescence model by angiotensin II and determined the relationships among testosterone, Gas6 and Axl expression, VSMC senescence, and collagen expression. To further elucidate the role of Gas6 and Axl in testosterone delaying VSMC senescence, we used specific inhibitors Axl-Fc and R428 to explore the function of Gas6 and Axl in testosterone delaying VSMC senescence. Finally, we detected the effects of downstream signal molecules, Akt and FoxO1a, and used a specific inhibitor of Akt and FoxO1a gene silencing to confirm the role of Akt/FoxO1a in testosterone delaying VSMC senescence.
Materials and methods
Reagents and antibodies
DMEM/F12, fetal bovine serum (FBS), and trypsin were purchased from Gibco (Grand Island, NY, USA). Recombinant mouse Gas6 (Gas6), recombinant mouse Axl Fc chimera (Axl-Fc), and ELISA kit for Gas6 were purchased from R&D Systems (St. Paul, MN, USA). R428 (BGB324) was purchased from Selleck (Houston, TX, USA). LY294002 was purchased from Merck (Darmstadt, Hessen, Germany). Testosterone and angiotensin II were purchased from Sigma-Aldrich (St, Louis, MO, USA). The small interfering RNA (siRNA) specific for FoxO1a and the negative control siRNA sequence were designed and synthesized by GenePharma (Shanghai, China). Lipofectamine™ 2000 was purchased from Invitrogen (Carlsbad, CA, USA). The anti-Axl, anti-Akt, anti-p-Akt, anti-FoxO1a, anti-p-FoxO1a antibodies, and SA-β-Gal Staining Kit were purchased from Cell Signaling Technology (Beverly, MA, USA). The anti-type I collagen, anti-type III collagen, and anti-MMP-2 antibodies were obtained from Abcam (Cambidg, MA, USA). β-Actin antibody was obtained from Zhongshan Biotech (Guangzhou, China). Other antibodies referred to in this paper were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Other chemicals were of the highest purity grade.
Cell culture
VSMCs were extracted from the aortas of 8–10-week-old C57BL/6J wild-type mice purchased from Vital River, Inc. All experimental procedures were performed in accordance with the Animal Management Rules of the Chinese Ministry of Health and approved by the Institutional Animal Care and Use Committee of Shandong University. Briefly, mice were sacrificed, and aortas were isolated under sterile conditions. The aortas were minced, and tissue blocks were spread on the bottom of culture flasks without medium for about 2 h until the tissue blocks were attached. Then, culture medium was added, and a large number of cells grew from blocks 3–5 days after. Then, cells at passages 3–5 were used for Western blot analysis and SA-β-Gal staining, and the supernatant was used for gelatin zymography and ELISA. The mouse vascular smooth muscle cell lines (MOVAS) were obtained from the American Type Culture Collection. All cells were maintained in DMEM/F12 supplemented with 10 % FBS in 5 % CO2 and 95 % humidified air at 37 °C.
Establishment and identification of cell senescence model
It was served as an induced senescence model that VSMCs were serially passaged four times and treated with angiotensin II (10−6 mol/L) for 48 h. We evaluated the degree of cell senescence by SA-β-Gal staining and Western blot analysis of p16INK4a and p21Cip1.
Western blotting analysis
Proteins from VSMCs and MOVASs were extracted by using cell lysis buffer (Beyotime Institute of Biotechnology, China) and 1 mM phenyl-methanesulfonyl fluoride. The samples were separated on 10 % SDS-polyacrylamide gels and then transferred to polyvinylidene difluoride membranes. After being incubated in blocking solution (5 % nonfat milk) for 1 h, the membranes were incubated separately with a 1:1000 dilution of antibodies of Axl, collagen I, collagen III, MMP-2, p-Akt, Akt, p-FoxO1a, and FoxO1a; with a 1:500 dilution of p16INK4a, p21Cip1, tissue inhibitor of metalloproteinase-2 (TIMP-2), and membrane type-1 metalloproteinase (MT1-MMP); and with a 1:200 dilution of Gas6 overnight at 4 °C. The membranes were washed with 1× TBST solution and treated with horseradish peroxidase-conjugated secondary antibodies for 1 h. Then, the membranes were treated with ECL solution (Millipore, Darmstadt, Germany) according to the manufacturer’s instructions and detected by using an ImageQuant™ LAS 4000 imager (GE, CT, USA). The relative intensities of protein bands were analyzed by the ImageJ software (National Institutes of Health, USA).
Senescence-associated β-galactosidase staining
The culture medium was removed, and the cells were washed one time with precooled PBS. Then, cells were fixed with fixative solution for 15 min at room temperature. After the β-galactosidase staining solution was added, the cells were incubated at 37 °C without CO2 overnight. Cells which had blue-green granules in their cytoplasm were regarded as positive staining. For each group, three fields of cells were selected randomly to calculate the positive staining rate using Image-Pro Plus 6.0 software (Bethesda, MD, USA).
ELISA
The cell culture supernatants were collected and frozen at −80 °C. All reagents and samples were brought to room temperature before use. Add 50 μL of Assay Diluent RD1-43 to each well and add 50 μL of standard, control, or sample per well. Mix by gently tapping the plate frame for 1 min, and incubate for 3 h at 2–8 °C. After five washes, 100 μL of Mouse Gas6 Conjugate was added to each well and incubated for 1 h at 2–8 °C. After another five washes, 100 μL of substrate solution was added to each well and incubated for 30 min at room temperature without light. After 100 μL of stop solution was added, determine the optical density of each well within 30 min by using a microplate reader.
Gelatin zymography
The enzymatic activity of MMPs in VSMCs was assayed by gelatin zymography. SDS-polyacrylamide gel (10 %) with 1 mg/ml gelatin was used for electrophoresis. Gels were renatured at room temperature by renaturing buffer (50 mM Tris–HCl, 100 mM NaCl, 2.5 % Triton X-100), then followed by a developing buffer (50 mM Tris–HCl, 200 mM NaCl, 5 mM CaCl2, 0.02 % Brij-35, pH 7.6) incubation in a 37 °C for 20 h. Gels were stained with Coomassie brilliant blue R-250 and then destained with an appropriate Coomassie R-250 destaining solution (methanol/acetic acid/water = 50:10:40).
Transfection of siRNA
A siRNA (5′-GAGGAUUGAACCAGUAUAATT-3′) specific for FoxO1a was used to inhibit FoxO1a synthesis, and a randomly mixed sequences siRNA (5′-UUCUCCGAACGUGUCACGUTT-3′) was used as negative control. The oligoribonucleotides were transfected into MOVAS grown in a six-well plate using Lipofectamine™ 2000 transfection according to the manufacturer’s protocol.
Statistical analyses
All data were presented as mean ± SD. SPSS 20.0 (SPSS, Chicago, IL) was used for statistical analysis. Differences between two groups were analyzed by independent samples t test, and one-way ANOVA was used to compare results among groups. Statistical analyses were performed using GraphPad 5.01 software program (San Diego, CA, USA). A P < 0.05 was considered significant.
Results
Testosterone delays angiotensin II-induced VSMC senescence
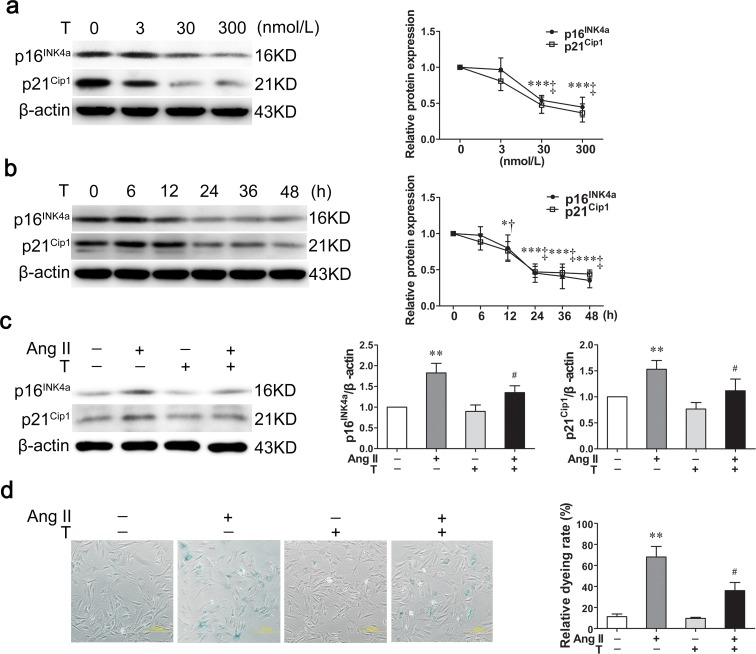
To explore the suitable testosterone concentration which could inhibit the expression of p16INK4a and p21Cip1 in VSMCs, cells were maintained in DMEM with 0, 3, 30, and 300 nM testosterone for 24 h. The protein expression of p16INK4a and p21Cip1 significantly decreased after VSMCs were maintained in 30 nM testosterone (P < 0.001, P < 0.001) and 300 nM (P < 0.001, P < 0.001) (Fig. 1a), so 30 nM testosterone was used as a suitable concentration in subsequent experiments. Then, VSMCs were maintained in DMEM with 30 nM testosterone for 0, 6, 12, 24, 36, and 48 h. The protein expression of p16INK4a and p21Cip1 markedly reduced after VSMCs were exposed to testosterone for 12 h (P < 0.05, P < 0.05), 24 h (P < 0.001, P < 0.001), 36 h (P < 0.001, P < 0.001), and 48 h (P < 0.001, P < 0.001) (Fig. 1b); therefore, 24 h was used in the following experiments.
Fig. 1.
Testosterone delays the senescence process induced by angiotensin II in VSMCs. a, b Western blot demonstrated that testosterone decreased the protein expression of p16INK4a and p21Cip1 in a concentration- and time-dependent manner. c The cells passaged four times and treated with angiotensin II (10 mol/L) for 48 h were served as an induced senescence model. Testosterone (30 nmol/L, 24 h) could decrease p16INK4a and p21Cip1 expression induced by angiotensin II. d Representative SA-β-Gal staining of VSMCs (scale bar 200 m). Ang II angiotensin II, T testosterone. Values are mean ± SD of three measurements. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control group; †P < 0.05 and ‡P < 0.001 compared with control group; #P < 0.05 compared with angiotensin II-treated group
Angiotensin II greatly increased the expression of p16INK4a (P < 0.01) and p21Cip1 (P < 0.01) and SA-β-Gal staining rate (P < 0.01) compared with control group (Fig. 1c, d). With testosterone treatment, the increased expression of p16INK4a (P < 0.05) and p21Cip1 (P < 0.05) were significantly decreased (Fig. 1c), as the same of SA-β-Gal staining rate (P < 0.05) (Fig. 1d).
Gas6/Axl is involved in testosterone-mediated anti-senescence effects
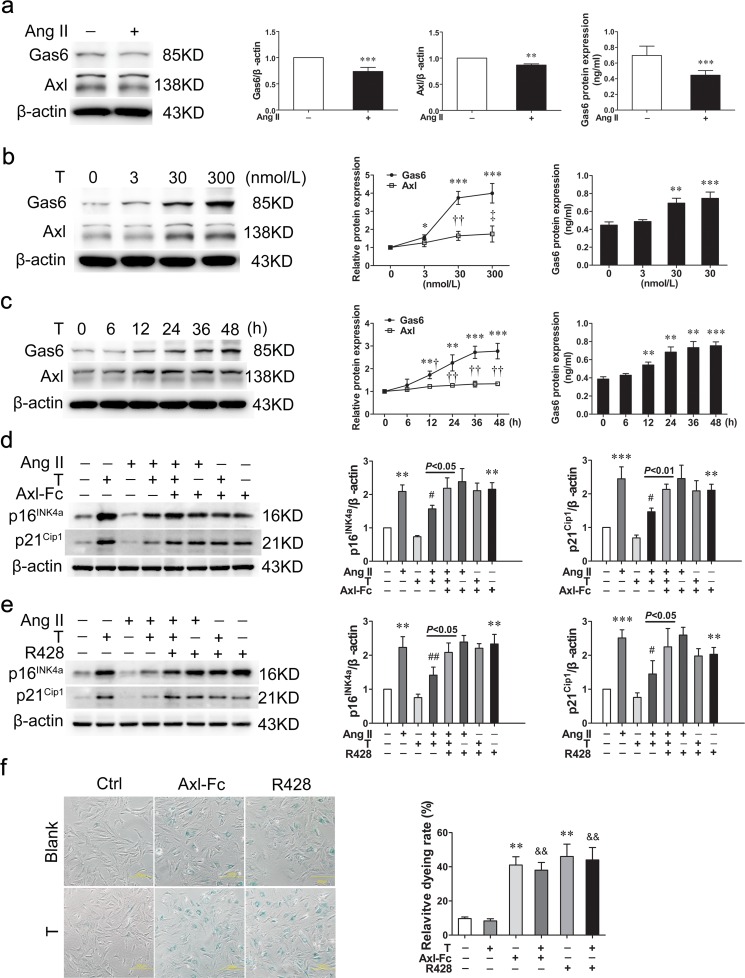
In accord with previous study, angiotensin II (10−6 mol/L, 48 h) decreased the protein expression of Gas6 (P < 0.001) and Axl (P < 0.01) in VSMCs and reduced the Gas6 content in supernatant (P < 0.001) compared with control group (Fig. 2a). On the contrary, testosterone elevated the Gas6 and Axl protein expression in VSMCs and supernatant in a concentration- and time-dependent manner (P < 0.05∼P < 0.001) (Fig. 2b, c).
Fig. 2.
Gas6/Axl is the primary pathway in the testosterone-mediated anti-senescence effect. a Western blot and ELISA analysis demonstrated that angiotensin II decreased the protein expression of Gas6 and Axl. b, c Testosterone promoted the Gas6 and Axl protein expression in a concentration- and time-dependent manner. d The Axl-Fc protein, a soluble form of Axl, could neutralize Gas6 within the supernatant, reversed the downregulating effect of testosterone on p16INK4a and p21Cip1 expression induced by angiotensin II. e R428, a specific inhibitor of Axl, reversed the downregulating effect of testosterone on p16INK4a and p21Cip1 expression induced by angiotensin II as well. f Representative SA-β-Gal staining of VSMCs (scale bar 200 μm). Ang II angiotensin II, T testosterone. Values are mean ± SD of three measurements. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control group; †P < 0.05, ††P < 0.01, and ‡P < 0.001 compared with control group; #P < 0.05 and ##P < 0.01 compared with angiotensin II-treated group; &&P < 0.01 compared with testosterone-treated group
To investigate whether the anti-senescence effects of testosterone mediated by Gas6, we used Axl-Fc protein, a soluble form of Axl, to neutralize Gas6 within the supernatant at a dilution of 10 μg/ml for 24 h. After Axl-Fc treatment, the expression of p16INK4a (P < 0.01) and p21Cip1 (P < 0.01) as well as the staining rate of SA-β-Gal (P < 0.01) increased significantly compared with control group, regardless of whether testosterone was added (Fig. 2d, f). Furthermore, Axl-Fc could reverse the downregulating effects of testosterone on p16INK4a (P < 0.05) and p21Cip1 (P < 0.01) expression induced by angiotensin II (Fig. 2d). R428, a specific inhibitor of Axl, was used to evaluate whether Axl was involved in the anti-senescence process. With R428 (1 μM, 24 h) treatment, the p16INK4a (P < 0.01) and p21Cip1 (P < 0.01) expression as well as the SA-β-Gal staining rate (P < 0.01) elevated markedly, in spite of whether testosterone was added (Fig. 2e, f). Likewise, R428 reversed inhibitory effects of testosterone on p16INK4a (P < 0.05) and p21Cip1 (P < 0.05) expression induced by angiotensin II (Fig. 2e).
Testosterone reduces angiotensin II-induced collagen expression in VSMCs via Gas6/Axl
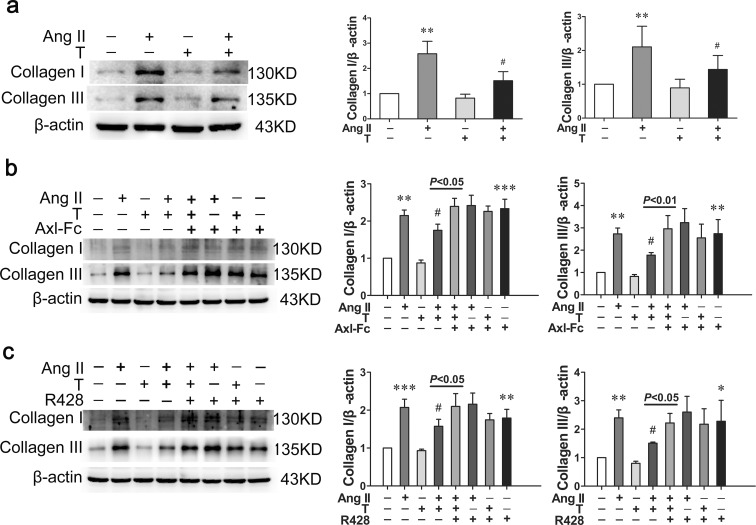
When stimulated by angiotensin II, the protein expression of collagen I (P < 0.01) and III (P < 0.01) increased significantly compared with control group (Fig. 3a). With testosterone treatment, the elevated collagen I (P < 0.05) and III (P < 0.05) expression decreased significantly compared with angiotensin II-treated group (Fig. 3a). When treated with Axl-Fc, the expression of collagen I (P < 0.001) and collagen III (P < 0.01) increased significantly compared with control group, regardless of whether testosterone was added (Fig. 3b). Moreover, Axl-Fc could reverse the downregulating effects of testosterone on collagen I (P < 0.05) and collagen III (P < 0.01) expression induced by angiotensin II (Fig. 3b). With R428 treatment, the collagen I (P < 0.01) and collagen III (P < 0.05) expression elevated markedly, in spite of whether testosterone was added (Fig. 3c). Likewise, R428 reversed inhibitory effects of testosterone on collagen I (P < 0.05) and collagen III (P < 0.05) expression induced by angiotensin II (Fig. 3c).
Fig. 3.
Testosterone decreases the expression of collagen I and collagen III via Gas6/Axl. a Western blot showed that testosterone decreased the protein expression of collagen I and III induced by angiotensin II. b Axl-Fc reversed the downregulating effect of testosterone on collagen I and III expression induced by angiotensin II. c R428 reversed the downregulating effect of testosterone on collagen I and III expression induced by angiotensin II. Ang II angiotensin II, T testosterone. Values are mean ± SD of three measurements. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control group; #P < 0.05 compared with angiotensin II-treated group
Testosterone regulates the expression of MMP-2, TIMP-2, and MT1-MMP through Gas6/Axl pathway
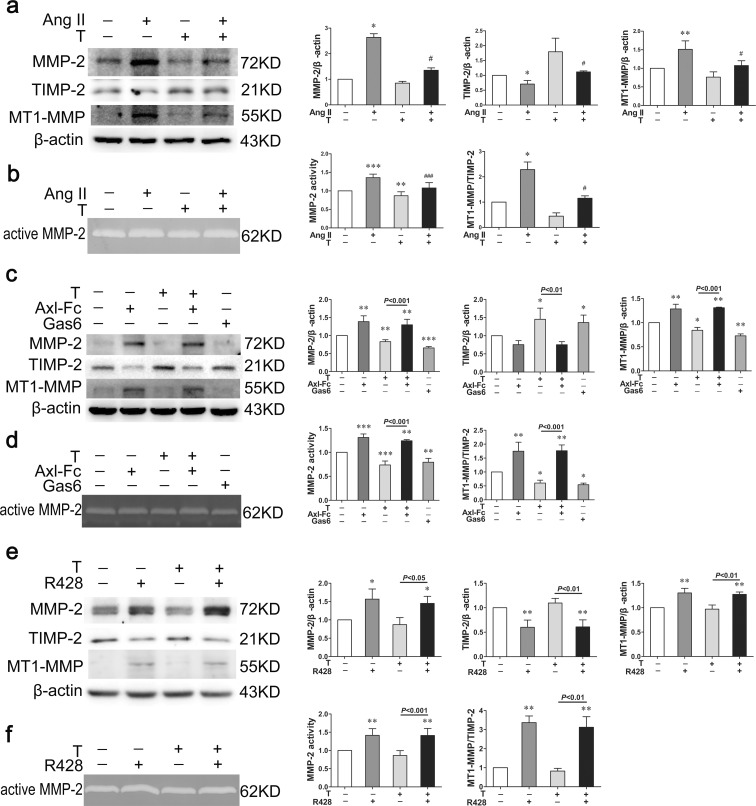
Angiotensin II reduced the protein expression of TIMP-2 (P < 0.05) while elevated the protein expression of MMP-2 (P < 0.05) and MT1-MMP (P < 0.01), as well as the MT1-MMP to TIMP-2 ratio (P < 0.05) compared with control group (Fig. 4a). In keeping with the elevated MMP-2 expression, the MMP-2 activity was increased in angiotensin II-treated group as well (P < 0.001) (Fig. 4b). With testosterone treatment, the MMP-2 (P < 0.05) and MT1-MMP (P < 0.05) expression, the MT1-MMP to TIMP-2 ratio (P < 0.05) and the MMP-2 activity (P < 0.001) were markedly decreased while the expression of TIMP-2 (P < 0.05) was increased compared with angiotensin II-treated group (Fig. 4a, b).
Fig. 4.
Gas6/Axl is involved in the regulating effect of testosterone on MMP-2, TIMP-2, and MT1-MMP expression. a, b Western blot demonstrated that testosterone reduced the MMP-2 and MT1-MMP protein expression as well as MT1-MMP to TIMP-2 ratio increased by angiotensin II, while elevated the TIMP-2 expression decreased by angiotensin II. b Representative gelatin zymography of MMP-2 activity. c Axl-Fc reversed the regulating effect of testosterone on MMP-2, TIMP-2, and MT1-MMP expression, as well as MT1-MMP to TIMP-2 ratio. The expression of MMP-2, TIMP-2, and MT1-MMP, as well as the MT1-MMP to TIMP-2 ratio had no significant difference between the two Axl-Fc-treated groups. d Representative gelatin zymography of MMP-2 activity. e R428 reversed the regulating effect of testosterone on MMP-2, TIMP-2, and MT1-MMP expression and MT1-MMP to TIMP-2 ratio. The expression of MMP-2, TIMP-2, and MT1-MMP and MT1-MMP to TIMP-2 ratio had no significant difference between the two R428-treated groups. f Representative gelatin zymography of MMP-2 activity. Ang II angiotensin II, T testosterone. Values are mean ± SD of three measurements. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control group; #P < 0.05 and ###P < 0.001 compared with angiotensin II-treated group
Then, we used Axl-Fc to block the function of Gas6. The regulating effects of testosterone on MMP-2 (P < 0.001), TIMP-2 (P < 0.05), and MT1-MMP (P < 0.05) expression, as well as the MT1-MMP to TIMP-2 ratio (P < 0.01) were reversed by Axl-Fc (Fig. 4c). Simultaneously, the inhibitory effect of testosterone on the MMP-2 activity was reversed by Axl-Fc (P < 0.001) (Fig. 4d). Moreover, there was no difference in the expression of MMP-2, TIMP-2, and MT1-MMP, the MT1-MMP to TIMP-2 ratio, and MMP-2 activity between the two Axl-Fc-treated groups (Fig. 4c, d).
After that, R428 was used to inhibit Axl. With R428 treatment, the regulating effects of testosterone on MMP-2 (P < 0.05), TIMP-2 (P < 0.01), and MT1-MMP (P < 0.01) expression, as well as the MT1-MMP to TIMP-2 ratio (P < 0.01) were reversed (Fig. 4e). Similarly, the inhibiting effect of testosterone on the MMP-2 activity was reversed by R428 as well (P < 0.001) (Fig. 4f).
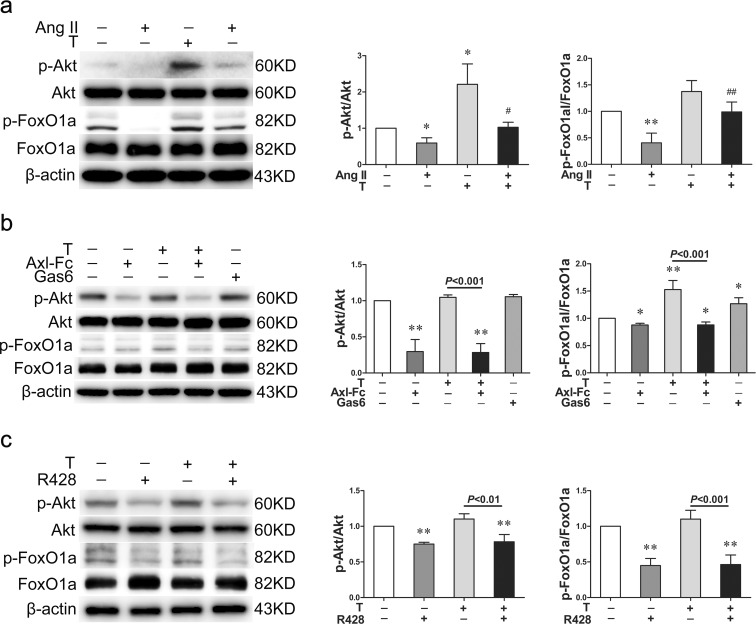
Akt/FoxO1a is involved in the protective effects of testosterone
To explore the downstream signaling pathways of Gas6/Axl, cells were serum-starved for 12 h first. Then, angiotensin II, Axl-Fc, or R428 was added. An hour later, testosterone was added and maintained for an hour. The phosphorylation of Akt (P < 0.05) and FoxO1a (P < 0.01) were decreased in angiotensin II-treated group compared with the control group (Fig. 5a). Testosterone markedly restored the suppressed phosphorylation of Akt (P < 0.05) and FoxO1a (P < 0.01) (Fig. 5a). Axl-Fc reversed the enhancing effects of testosterone on the phosphorylation of Akt (P < 0.001) and FoxO1a (P < 0.001) (Fig. 5b). Moreover, there was no difference in the phosphorylation of Akt and FoxO1a between the two Axl-Fc-treated groups (Fig. 5b). Furthermore, the enhancing effects of testosterone on the phosphorylation of Akt (P < 0.01) and FoxO1a (P < 0.001) were reversed by R428 (Fig. 5c), and no difference was found in the phosphorylation of Akt and FoxO1a between the two R428-treated groups (Fig. 5c).
Fig. 5.
The Akt/FoxO1a signaling pathway is implicated in the protective effect of testosterone. a Western blot showed that testosterone restored the phosphorylation levels of p-Akt and p-FoxO1a decreased by angiotensin II. b Axl-Fc reversed the upregulating effect of testosterone on the phosphorylation levels of p-Akt and p-FoxO1a. The phosphorylation levels of p-Akt and p-FoxO1a had no significant difference between the two Axl-Fc-treated groups. c R428 reversed the upregulating effect of testosterone on the phosphorylation levels of p-Akt and p-FoxO1a as well. The phosphorylation levels of p-Akt and p-FoxO1a had no significant difference between the two R428-treated groups. Ang II angiotensin II, T testosterone, p-Akt phosphorylated Akt, p-FoxO1a phosphorylated FoxO1a. Values are mean ± SD of three measurements. *P < 0.05 and **P < 0.01 compared with control group; #P < 0.05 and ##P < 0.01 compared with angiotensin II-treated group
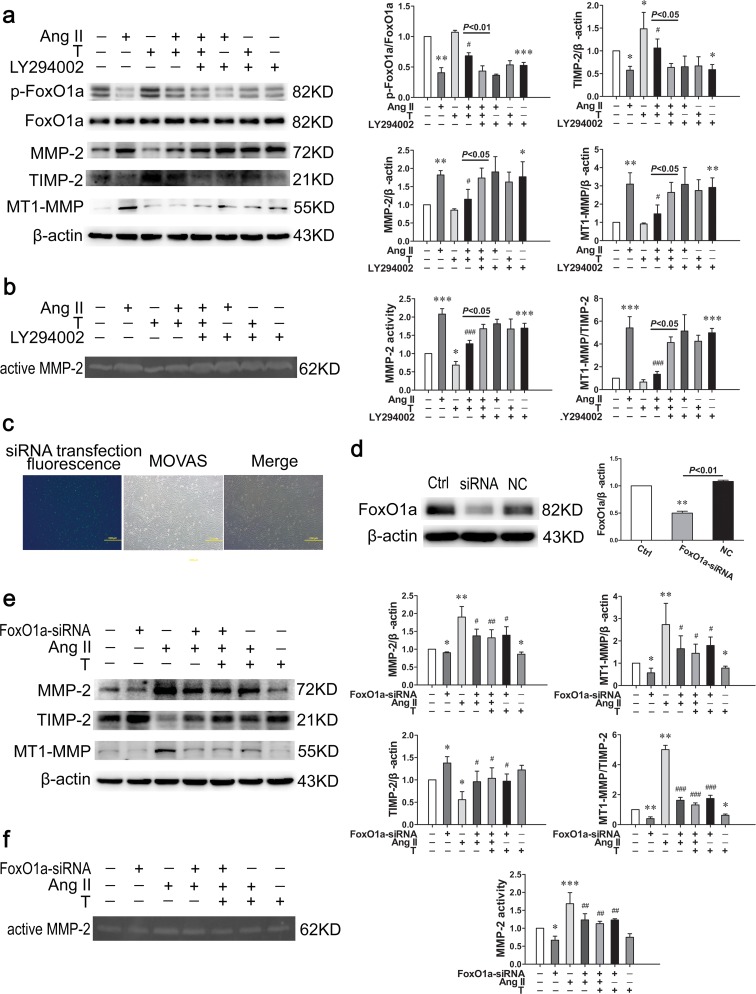
Akt/FoxO1a is implicated in the regulating effects of testosterone on MMP-2, TIMP-2, and MT1-MMP expression
To demonstrate the role of Akt in the regulating effects of testosterone on MMP-2, TIMP-2, and MT1-MMP expression, we inhibited PI3K activity by LY294002 (10 μM). LY294002 was added one hour before testosterone. The upregulating effect of testosterone on the phosphorylation of FoxO1a was reversed by LY294002 (P < 0.01) (Fig. 6a), which suggested that FoxO1a was a downstream transcription factor of Akt in testosterone-mediated anti-senescence process. Similarly, the regulating effects of testosterone on MMP-2 (P < 0.05), TIMP-2 (P < 0.05), and MT1-MMP expression, the MT1-MMP to TIMP-2 ratio (P < 0.05), and MMP-2 activity (P < 0.05) were reversed by LY294002 compared with angiotensin II + testosterone group (Fig. 6a, b).
Fig. 6.
Testosterone regulates MMP-2, TIMP-2, and MT1-MMP expression through Akt/FoxO1a signaling pathway. a Western blot showed that the phosphorylation level of p-FoxO1a and the expression of TIMP-2 reduced while MMP-2, MT1-MMP expression, and MT1-MMP to TIMP-2 ratio increased after PI3K was inhibited by LY94002. b Representative gelatin zymography of MMP-2 activity. c The transfection efficacy of FoxO1a siRNA (scale bar 200 μm). d The silence efficacy of selected FoxO1a-siRNA sequence measured by western blot. e The inhibiting of FoxO1a reversed the increased of MMP-2, MT1-MMP expression, and MT1-MMP to TIMP-2 ratio, as well as the decreased TIMP-2 expression induced by angiotensin II. In the presence of FoxO1a-siRNA, testosterone cannot play an additional role in regulating MMP-2, TIMP-2, and MT1-MMP expression induced by angiotensin II. f Representative gelatin zymography of MMP-2 activity. Ang II angiotensin II, T testosterone, NC negative control siRNA. Values are mean ± SD of three measurements. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control group; #P < 0.05, ##P < 0.01, and ###P < 0.001compared with angiotensin II group
To explore the role of FoxO1a in the regulating effects of testosterone on MMP-2, TIMP-2, and MT1-MMP expression, we inhibited the expression of FoxO1a by a specific FoxO1a-siRNA. The transfection efficacy of FoxO1a-siRNA into MOVASs reached 85 % (Fig. 6c). To test the efficacy of the selected siRNA sequence, we measured the protein expression of FoxO1a after transfection with FoxO1a-siRNA for 48 h. The expression of FoxO1a in the cells transfected with siRNA was significantly lower than in the cells transfected with the negative control-siRNA (P < 0.01) (Fig. 6d). After the expression of FoxO1a was inhibited, the regulating effects of testosterone on MMP-2 (P < 0.05), TIMP-2 (P < 0.05), and MT1-MMP (P < 0.01) expression, as well as the MT1-MMP to TIMP-2 ratio (P < 0.001) were reversed (Fig. 6e). Simultaneously, the increased MMP-2 activity induced by angiotensin II was also decreased significantly after FoxO1a was inhibited (P < 0.01) (Fig. 6f). Moreover, no difference was found in the MMP-2, TIMP-2, and MT1-MMP expression, MT1-MMP to TIMP-2 ratio, and MMP-2 activity between angiotensin II + FoxO1a-siRNA group and angiotensin II + FoxO1a-siRNA + testosterone group (Fig. 6e, f). In the presence of FoxO1a-siRNA, testosterone cannot play an additional role in regulating MMP-2, TIMP-2, and MT1-MMP expression induced by angiotensin II.
Discussion
In this study, we have identified a novel molecular mechanism for testosterone in protecting against VSMC senescence. Firstly, our findings revealed that testosterone alleviated VSMC senescence and collagen synthesis mainly by modulating Gas6/Axl pathway. We then showed that the dramatic reduction in collagen expression was attributed to the decreased MMP-2 activity. Furthermore, we found that Akt/FoxO1a signaling pathway was involved in the protective effects of testosterone on VSMC senescence and collagen synthesis. Our results suggest that testosterone plays a pivotal role in cell senescence, which could be a critical mechanism for testosterone delay vascular aging.
Testosterone deficiency is known to be associated with age-related vascular changes in men (Jones et al. 2005; Vlachopoulos et al. 2014). Although there are controversies in the TRT, the newest large observational cohort shows that TRT reduced the all-cause mortality, myocardial infarction, and stroke (Sharma et al. 2015). However, the underlying mechanisms remain unclear. Proteins changing with age and testosterone level are considered as potential mediators in the anti-aging effects of testosterone. An example of such a protein is the vitamin K-dependent protein Gas6. The serum Gas6 level decreases with age and are positively correlated with testosterone level (Hung et al. 2010). In this study, we established the model of VSMC senescence induced by angiotensin II, for chronic inflammation mediated by angiotensin II was considered to play a pivotal role in age-related vascular remodeling and cellular senescence (Herbert et al. 2008; Kunieda et al. 2006; Nguyen Dinh Cat et al. 2013; Wang et al. 2014). The p16INK4a and p21Cip1 protein expression levels and SA-β-Gal staining rate were used to evaluate the degree of cellular senescence (Campisi 2005; Goberdhan et al. 1995). We found that testosterone markedly alleviated VSMC senescence induced by angiotensin II. When challenged with angiotensin II, Gas6 and Axl protein levels were significantly downregulated in VSMC and culture supernatant. In accordance with previous study, testosterone could increase Gas6 and Axl protein expression in a concentration- and time-dependent manner (Gao et al. 2008). When we used the Axl-Fc protein, a soluble form of the Axl, to neutralize Gas6 within the supernatant, the anti-senescence effects of testosterone were reversed, as the same of R428, a specific inhibitor of Axl. These results were in accord with our previous findings (Jin et al. 2015). Together, these data suggest a pivotal role for testosterone in delaying VSMC senescence by modulating Gas6/Axl pathway.
Vascular stiffness is the most important manifestation of vascular aging, which is attributed to ECM remodeling. Previous studies showed that Gas6 was implicated in the inhibitory effect of testosterone on calcification on VSMC, an important reason of ECM remodeling during vascular aging (Son et al. 2010). Son et al. also have found that testosterone could directly and transcriptionally regulate Gas6 expression by binding to the androgen-response elements in the Gas6 promoter. However, the relationship among testosterone, Gas6/Axl, and collagen synthesis was not well elucidated. In the present study, we indicated that testosterone significantly decreased the protein expression of collagen I and collagen III induced by angiotensin II. With Axl-Fc or R428 treatment, the modulating effects of testosterone on collagen expression were markedly reversed. Therefore, activation of Gas6/Axl pathway may contribute to the downregulating effects of testosterone on collagen synthesis.
To explore how testosterone treatment affects collagen synthesis. We evaluated the regulating effects of testosterone on MMP-2 activity, a critical downstream molecule of angiotensin II system (Wang et al. 2003). Both the expression level and activity of MMP-2 were dramatically elevated in the central arterial wall with advancing age and played pivotal roles in facilitating age-associated ECM remodeling (Jiang et al. 2012; Wang et al. 2012). The activated MMP-2 not only digested elastin and collagen fibers, but more importantly, it also cleaved extracellular bioactive factors such as TGF-β1 and pro-ET-1, increased their activity and reinforces their downstream molecule Smad-2/3 phosphorylation, and then upregulated collagen synthesis in VSMC (Wang et al. 2012). In this study, we found that testosterone attenuated the MMP-2 protein expression and activity induced by angiotensin II. Meanwhile, testosterone also had the regulating effects on TIMP-2 and MT1-MMP expression, as well as the MT1-MMP to TIMP-2 ratio. The increased MMP-2 activity was due not only to enhanced transcription and translation but also to a shift in the ratio of its activator MT1-MMP and inhibitor TIMP-2 (Jiang et al. 2012). Similarly, when treated with Axl-Fc and R428, the modulating effects of testosterone were significantly reversed. Furthermore, testosterone also had the regulating effect on MMP-9 and TIMP-1 expression levels, but the MMP-9 activity was much weaker in VSMC (data not shown). So, MMP-2 might play a more important role in this process. Taken together, testosterone attenuated MMP-2 activity as well as MT1-MMP to TIMP-2 ratio by activating Gas6/Axl pathway. Combined with previous findings, the regulating effects of testosterone on collagen I and collagen III expression are partly attributed to the modulation in MMP-2 activity.
Previous researches demonstrated that Akt/FoxO was implicated in the regulating effects of Gas6 on cell survival and senescence (Ganopolsky et al. 2008; Jin et al. 2015). In VSMCs, Gas6 delayed cell senescence by promoting G1/S phase transition and affecting cell cycle arrest, which was attributed to the activation of Akt/FoxO3a (Jin et al. 2015). In this study, we found that testosterone could restore the phosphorylation of Akt/FoxO1a inhibited by angiotensin II, and this effect was Gas6/Axl dependent. So, we thought that Akt/FxoO1a was involved in the anti-senescence effect of testosterone as well.
In addition to regulating cell cycle arrest, Akt/FoxO1a was implicated in the regulation of MMP and TIMP expression. Akt could stimulate TIMP-1 expression in erythroid cells (Kadri et al. 2005). PI3Kγ, the molecule upstream of Akt, was proved to inhibit the expression and activity of MMP-2 and MT1-MMP induced by biomechanical stress (Guo et al. 2010). Akt2 inhibited MMP-9 expression while stimulated TIMP-1 expression by enhancing phosphorylation of FoxO1 in cultured VSMCs (Shen et al. 2013). Furthermore, they found that there were FoxO1a binding sites in MMP-9 and TIMP-1 promoters. In addition, they also showed that Akt2-deficient mice revealed increased expression of MMP-2, but they did not carry out further investigation. To explore whether Akt/FoxO1a was involved in the regulation of testosterone on MMP and TIMP expression, we used PI3K inhibitor LY294002 to inhibit Akt activity firstly. With LY294002 treatment, the regulating effects of testosterone on MMP-2, TIMP-2, and MT1-MMP expression, MT1-MMP to TIMP-2 ratio, as well as MMP-2 activity were reversed, which suggested that Akt was implicated in the protective effects of testosterone. Then, we silenced the expression of FoxO1a by using a specific siRNA. MOVAS cells were used for siRNA transfection, because the transfection efficacy in primary VSMCs was unsatisfactory. With FoxO1a silencing, the regulating effects of testosterone on MMP-2, TIMP-2, and MT1-MMP expression, the MT1-MMP to TIMP-2 ratio, as well as MMP-2 activity were reversed. Moreover, in the presence of FoxO1a-siRNA, testosterone could not play an additional role in regulating MMP-2, TIMP-2, and MT1-MMP expression induced by angiotensin II. Together, the improvement of VSMCs senescence and MMP-2 activity mediated by testosterone partly attributes to the activation of Akt/FoxO1a.
In conclusion, Gas6/Axl played a pivotal role in alleviating VSMC senescence and collagen synthesis by testosterone through modulating Akt/FoxO1a pathway. The present study revealed a molecular mechanism underlying the protective effects of testosterone on cell senescence and provided a theoretical basis for TRT. The anti-senescence effects of testosterone might contribute to its improving effect on vascular aging and aged-related cardiovascular diseases. Future studies are required to confirm whether testosterone/Gas6/Axl signaling pathway is involved in VSMC senescence and vascular remolding in animal models.
Electronic supplementary material
The primary data of Fig. 1 (GIF 260 kb)
The primary data of Fig. 2 (GIF 303 kb)
The primary data of Fig. 3 (GIF 205 kb)
The primary data of Fig. 4 (GIF 200 kb)
The primary data of Fig. 5 (GIF 254 kb)
The primary data of Fig. 6 (GIF 227 kb)
The primary data of ESM 8 (GIF 88 kb)
The Akt signaling pathway is involved in the anti-senescence effect of testosterone (A) The PI3K inhibitor, LY294002 could reverse the downregulating effects of testosterone on the expression of p16INK4a and p21Cip1 (P<0.05) induced by angiotensin II. But the regulating effect of LY294002 on p16INK4a expression was much weaker. Angiotensin II (Ang II), testosterone (T). Values are mean±SD of three measurements. *P<0.05 and **P<0.01 compared with control group; #P<0.05 and ##P<0.01compared with angiotensin II-treated group; †P<0.05 compared with testosterone-treated group (GIF 54 kb)
Acknowledgments
This work was supported by the research grants from the National Basic Research Program of China (973 Program, Grant No. 2013CB530700), the National Natural Science Foundation of China (81100605, 81270352, 81270287, 81300168, 81471036, 81470560, and 81570400), the Natural Science Foundation of Shandong Province (BS2013YY017, ZR2014HQ037), the Key Research and Development Program of Shandong Province (2015GSF118062), cardiovascular exploration research foundation of Chinese Medical Doctor Association (DFCMDA201320), and the Specialized Research Fund for the Doctoral Program of Higher Education (SRFDP 20130131120065).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Contributor Information
Li Li, Email: lili_8599@163.com.
Ming Zhong, Email: zhongmingzm@gmail.com.
References
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Ganopolsky JG, Abid MR, Aird WC, Blostein MD. GAS6-induced signaling in human endothelial cells is mediated by FOXO1a. J Thromb Haemost. 2008;6:1804–1811. doi: 10.1111/j.1538-7836.2008.03114.x. [DOI] [PubMed] [Google Scholar]
- Gao BB, Stuart L, Feener EP. Label-free quantitative analysis of one-dimensional PAGE LC/MS/MS proteome: application on angiotensin II-stimulated smooth muscle cells secretome. Mol Cell Proteomics. 2008;7:2399–2409. doi: 10.1074/mcp.M800104-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goberdhan P, Dimri, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92(20):9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, et al. Loss of PI3Kgamma enhances cAMP-dependent MMP remodeling of the myocardial N-cadherin adhesion complexes and extracellular matrix in response to early biomechanical stress. Circ Res. 2010;107:1275–1289. doi: 10.1161/CIRCRESAHA.110.229054. [DOI] [PubMed] [Google Scholar]
- Herbert KE, Mistry Y, Hastings R, Poolman T, Niklason L, Williams B. Angiotensin II-mediated oxidative DNA damage accelerates cellular senescence in cultured human vascular smooth muscle cells via telomere-dependent and independent pathways. Circ Res. 2008;102:201–208. doi: 10.1161/CIRCRESAHA.107.158626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung YJ, Lee CH, Chu NF, Shieh YS. Plasma protein growth arrest-specific 6 levels are associated with altered glucose tolerance, inflammation, and endothelial dysfunction. Diabete Care. 2010;33:1840–1844. doi: 10.2337/dc09-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado B, et al. Association study between polymorphims in GAS6-TAM genes and carotid atherosclerosis. Thromb Haemost. 2010;104:592–598. doi: 10.1160/TH09-11-0787. [DOI] [PubMed] [Google Scholar]
- Hurtado B, et al. Expression of the vitamin K-dependent proteins GAS6 and protein S and the TAM receptor tyrosine kinases in human atherosclerotic carotid plaques. Thromb Haemost. 2011;105:873–882. doi: 10.1160/TH10-10-0630. [DOI] [PubMed] [Google Scholar]
- Jiang L, Zhang J, Monticone RE, Telljohann R, Wu J, Wang M, Lakatta EG. Calpain-1 regulation of matrix metalloproteinase 2 activity in vascular smooth muscle cells facilitates age-associated aortic wall calcification and fibrosis. Hypertension. 2012;60:1192–1199. doi: 10.1161/HYPERTENSIONAHA.112.196840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin CW, et al. Gas6 delays senescence in vascular smooth muscle cells through the PI3K/Akt/FoxO signaling pathway. Cell Physiol Biochem. 2015;35:1151–1166. doi: 10.1159/000373940. [DOI] [PubMed] [Google Scholar]
- Jones TH, Saad F. The effects of testosterone on risk factors for, and the mediators of, the atherosclerotic process. Atherosclerosis. 2009;207:318–327. doi: 10.1016/j.atherosclerosis.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Jones RD, Nettleship JE, Kapoor D, Jones HT, Channer KS. Testosterone and atherosclerosis in aging men: purported association and clinical implications. Am J Cardiovasc Drugs. 2005;5:141–154. doi: 10.2165/00129784-200505030-00001. [DOI] [PubMed] [Google Scholar]
- Kadri Z, et al. Phosphatidylinositol 3-kinase/Akt induced by erythropoietin renders the erythroid differentiation factor GATA-1 competent for TIMP-1 gene transactivation. Mol Cell Biol. 2005;25:7412–7422. doi: 10.1128/MCB.25.17.7412-7422.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korshunov VA, Mohan AM, Georger MA, Berk BC. Axl, a receptor tyrosine kinase, mediates flow-induced vascular remodeling. Circ Res. 2006;98:1446–1452. doi: 10.1161/01.RES.0000223322.16149.9a. [DOI] [PubMed] [Google Scholar]
- Kovacic JC, Moreno P, Hachinski V, Nabel EG, Fuster V. Cellular senescence, vascular disease, and aging: part 1 of a 2-part review. Circulation. 2011;123:1650–1660. doi: 10.1161/CIRCULATIONAHA.110.007021. [DOI] [PubMed] [Google Scholar]
- Kovacic JC, Moreno P, Nabel EG, Hachinski V, Fuster V. Cellular senescence, vascular disease, and aging: part 2 of a 2-part review: clinical vascular disease in the elderly. Circulation. 2011;123:1900–1910. doi: 10.1161/CIRCULATIONAHA.110.009118. [DOI] [PubMed] [Google Scholar]
- Kunieda T, et al. Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation. 2006;114:953–960. doi: 10.1161/CIRCULATIONAHA.106.626606. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.CIR.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “Set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.CIR.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Lemke G. Biology of the TAM receptors. Cold Spring Harb Perspect Biol. 2013;5:a009076. doi: 10.1101/cshperspect.a009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes RA, Neves KB, Carneiro FS, Tostes RC. Testosterone and vascular function in aging. Front Physiol. 2012;3:89. doi: 10.3389/fphys.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgens E, et al. Genetic loss of Gas6 induces plaque stability in experimental atherosclerosis. J Pathol. 2008;216:55–63. doi: 10.1002/path.2381. [DOI] [PubMed] [Google Scholar]
- Minamino T, Komuro I. Vascular cell senescence: contribution to atherosclerosis. Circ Res. 2007;100:15–26. doi: 10.1161/01.RES.0000256837.40544.4a. [DOI] [PubMed] [Google Scholar]
- Munoz X, Obach V, Hurtado B, de Frutos PG, Chamorro A, Sala N. Association of specific haplotypes of GAS6 gene with stroke. Thromb Haemost. 2007;98:406–412. [PubMed] [Google Scholar]
- Nguyen Dinh Cat A, Montezano AC, Burger D, Touyz RM. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid Redox Signal. 2013;19:1110–1120. doi: 10.1089/ars.2012.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C, King RM, Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988;54:787–793. doi: 10.1016/S0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- Seyrek M, Yildiz O, Ulusoy HB, Yildirim V. Testosterone relaxes isolated human radial artery by potassium channel opening action. J Pharmacol Sci. 2007;103:309–316. doi: 10.1254/jphs.FP0060883. [DOI] [PubMed] [Google Scholar]
- Sharma R, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015 doi: 10.1093/eurheartj/ehv346. [DOI] [PubMed] [Google Scholar]
- Shen YH, et al. AKT2 confers protection against aortic aneurysms and dissections. Circ Res. 2013;112:618–632. doi: 10.1161/CIRCRESAHA.112.300735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son BK, et al. Androgen receptor-dependent transactivation of growth arrest-specific gene 6 mediates inhibitory effects of testosterone on vascular calcification. J Biol Chem. 2010;285:7537–7544. doi: 10.1074/jbc.M109.055087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachopoulos C, et al. Testosterone deficiency: a determinant of aortic stiffness in men. Atherosclerosis. 2014;233:278–283. doi: 10.1016/j.atherosclerosis.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res. 2012;111:245–259. doi: 10.1161/CIRCRESAHA.111.261388. [DOI] [PubMed] [Google Scholar]
- Wang M, et al. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension. 2003;41:1308–1316. doi: 10.1161/01.HYP.0000073843.56046.45. [DOI] [PubMed] [Google Scholar]
- Wang M, et al. Chronic matrix metalloproteinase inhibition retards age-associated arterial proinflammation and increase in blood pressure. Hypertension. 2012;60:459–466. doi: 10.1161/HYPERTENSIONAHA.112.191270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Jiang L, Monticone RE, Lakatta EG. Proinflammation: the key to arterial aging. Trends Endocrinol Metab. 2014;25:72–79. doi: 10.1016/j.tem.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz O. Vascular smooth muscle and endothelial functions in aging. Ann N Y Acad Sci. 2007;1100:353–360. doi: 10.1196/annals.1395.038. [DOI] [PubMed] [Google Scholar]
- Yildiz O, Seyrek M. Vasodilating mechanisms of testosterone. Exp Clin Endocrinol Diabetes. 2007;115:1–6. doi: 10.1055/s-2007-949657. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The primary data of Fig. 1 (GIF 260 kb)
The primary data of Fig. 2 (GIF 303 kb)
The primary data of Fig. 3 (GIF 205 kb)
The primary data of Fig. 4 (GIF 200 kb)
The primary data of Fig. 5 (GIF 254 kb)
The primary data of Fig. 6 (GIF 227 kb)
The primary data of ESM 8 (GIF 88 kb)
The Akt signaling pathway is involved in the anti-senescence effect of testosterone (A) The PI3K inhibitor, LY294002 could reverse the downregulating effects of testosterone on the expression of p16INK4a and p21Cip1 (P<0.05) induced by angiotensin II. But the regulating effect of LY294002 on p16INK4a expression was much weaker. Angiotensin II (Ang II), testosterone (T). Values are mean±SD of three measurements. *P<0.05 and **P<0.01 compared with control group; #P<0.05 and ##P<0.01compared with angiotensin II-treated group; †P<0.05 compared with testosterone-treated group (GIF 54 kb)