Abstract
Imeglimin is a promising new oral antihyperglycemic agent that has been studied in clinical trials as a possible monotherapy or add-on therapy to lower fasting plasma glucose and improve hemoglobin A1c (1–3, 9). Imeglimin was shown to improve both fasting and postprandial glycemia and to increase insulin secretion in response to glucose during a hyperglycemic clamp after 1-wk of treatment in type 2 diabetic patients. However, whether the β-cell stimulatory effect of imeglimin is solely or partially responsible for its effects on glycemia remains to be fully confirmed. Here, we show that imeglimin directly activates β-cell insulin secretion in awake rodents without affecting hepatic insulin sensitivity, body composition, or energy expenditure. These data identify a primary amplification rather than trigger the β-cell mechanism that explains the acute, antidiabetic activity of imeglimin.
Keywords: imeglimin, β-cell, glucose-stimulated insulin secretion
imeglimin is a new oral antihyperglycemic agent that has been studied in clinical trials as a possible monotherapy or add-on therapy to lower fasting plasma glucose and improve hemoglobin A1c (2, 3, 9). Imeglimin was shown to improve both fasting and postprandial glycemia (1) and increase insulin secretion in response to glucose during a hyperglycemic clamp after 1 wk of treatment in type 2 diabetic patients (8). Improvements in glucose tolerance have been reported in both humans and rodents treated with imeglimin and have been ascribed to multiple mechanisms: lower hepatic lipids (12), improved liver and muscle insulin signaling (12), and improved β-cell function (3, 12). However, prior studies have not utilized the gold-standard technique to evaluate hepatic insulin sensitivity: the hyperinsulinemic euglycemic clamp. Of note is that, although the mechanism of action of imeglimin has been ascribed at least in part to improved insulin sensitivity (12), improved insulin sensitivity would be expected to result in reductions in plasma insulin concentrations, not the increases that have been noted in multiple human (8, 9) and rodent (12) studies. Importantly, previous papers have not included in vitro studies to determine whether imeglimin increases glucose-stimulated insulin secretion directly or indirectly. In addition, most of the prior studies performed with imeglimin were long-term (months-long) treatment studies, which do not allow researchers to identify the first beneficial effects of the drug. To this end, we performed intraperitoneal glucose tolerance tests (GTT) and hyperinsulinemic euglycemic clamps in chow- and high-fat fed rats treated with imeglimin for 2 wk, thereby conclusively demonstrating the mechanism by which imeglimin improves glycemia in the high-fat fed rat using a comprehensive toolkit of gold-standard in vitro and in vivo techniques. Furthermore, we assessed glucose-stimulated insulin secretion in isolated rat islets and identified a direct, glucose-dependent, amplifying mechanism of action that would explain the absence of hypoglycemia when used at recommended pharmacological concentrations in humans.
METHODS
Animals.
All animal protocols were approved by the Yale University Institutional Animal Care and Use Committee, and all guidelines in the Guide for the Care and Use of Laboratory Animals (8th Ed.) were followed. Because metabolic cages could be used only for mice, male C57BL/6 mice were purchased from The Jackson Laboratory at 8 wk of age and were placed on chow or a lard-based high-fat diet (HFD). They were treated twice daily with imeglimin (150 mg/kg) mixed into a small amount of peanut butter or an equal amount of peanut butter vehicle. Mice underwent metabolic cage analysis (Columbus Instruments, Columbus, OH) after 1 and 3 wk of treatment in the chow-fed group or after 1 and 5 wk of treatment in the HFD-fed group.
All other animal studies involved Male Sprague-Dawley rats that were purchased from Charles River Laboratories at 350 g and were placed on chow or a safflower oil-based HFD. After 2 wk on the diet, they were treated twice daily for 2 wk with imeglimin (150 mg/kg) in peanut butter or peanut butter vehicle while they were maintained on the diet. After the first week of treatment, they underwent surgery under general isoflurane anesthesia to place polyethylene catheters in the carotid artery and jugular vein, and after 1 wk of recovery and an overnight fast they underwent intraperitoneal GTT (1 g/kg glucose). The final dose of imeglimin was given 15 min prior to the start of the GTT. Blood (200 μl at each draw) was taken from the venous catheter 0, 5, 10, 15, 30, 45, 60, and 90 min after the glucose bolus and immediately placed in heparin-coated tubes, and plasma was isolated after centrifugation for analysis of plasma glucose and insulin concentrations.
In the clamp studies, rats were again studied after a total of 4 wk of diet feeding, during the last 2 wk of which they were treated with imeglimin (150 mg/kg twice daily in peanut butter or peanut butter vehicle). Following an overnight fast, a 120-min basal infusion of [6,6-2H2]glucose (1 mg·kg−1·min−1 prime for 5 min, followed by a 0.25 mg·kg−1·min−1 continuous infusion) was administered, with 200 μl of whole blood taken after 100, 110, and 120 min of infusion to measure glucose turnover, as described below. The final dose of imeglimin was given 15 min prior to the start of the basal infusion. Immediately following the basal infusion, 120-min hyperinsulinemic euglycemic clamps were performed. Rats received arterial infusions of insulin (prime: 40 mU/kg over 5 min; followed by a continuous dose: 4 mU·kg−1·min−1) for the duration of the clamp. Euglycemia (100–110 mg/dl) was maintained with a variable infusion of D20 [6,6-2H2]glucose (99 atom% excess). Blood samples (50 μl) were taken every 15 min for measurement of glucose using a YSI glucose analyzer (Yellow Springs Instruments, Yellow Springs, OH). After 120 min, 20 mCi of 2-[14C]deoxyglucose was injected through the venous line, and glucose uptake was measured in skeletal muscle (quadriceps), as described previously (4). Twenty minutes after administration of the 2-[14C]deoxyglucose, rats were euthanized with intravenous pentobarbital, and liver and quadriceps were rapidly freeze-clamped in aluminum tongs precooled in liquid N2.
Biochemical analysis.
Plasma glucose concentrations were measured biochemically using a YSI Glucose Analyzer (Yellow Springs Instruments), and plasma insulin was measured by radioimmunoassay by the Yale Diabetes Research Core.
Western blots.
Pan-acetyl-CoA carboxylase (ACC) phosphorylation and AMP-activated protein kinase (AMPK) activation were measured by Western blot (14) in overnight-fasted rat liver 2 h following the last imeglimin dose, with antibodies obtained from Cell Signaling Technology. Equal protein loading was confirmed by measuring GAPDH protein content (antibody from Santa Cruz Biotechnology) in each blot.
Insulin secretion in isolated islets.
Intact islets isolated from ∼300-g Sprague-Dawley rats were hand-picked and layered between acrylamide gel column beads [Bio-Gel P4G (156-4124)] in perifusion/static incubation media [DMEM (Sigma D5030) supplemented with NaHCO3 as per the manufacturer's instructions, 10 mM HEPES, 4 mM glutamine, 0.2% fatty acid free BSA, and 2.5 mM glucose]. Eighty islets for each condition were perifused at a rate of 100 μl/min on an eight- or 12-channel BioRep Technologies (Miami, FL) perifusion device. Islets were equilibrated on the instrument in basal (2.5 mM) glucose perifusion media with or without imeglimin (100 μM) for 45 min prior to sample collection in a 5% CO2-95% air, 37°C constant environment. For static secretion assays, intact islets were dispersed using accutase (Gibco) and reaggregated to form single pseudoislet aggregates. Following dispersion into single cells, the cell suspension was seeded at 5,000 cells/well of a 96-well V-bottom plate and cultured under the same conditions as the intact islets. Pseudoislet aggregates formed 12–24 h after incubation at 37°C and 5% CO2-95% air. Rat insulin was measured in the perifusate/incubation media using a high-range rat insulin ELISA (Alpco, Salem, NH) and normalized to total islet DNA concentration using Quant-it PicoGreen dsDNA Assay Kit (Life Technologies, Carlsbad, CA). Secretagogues included the glucokinase activator (MK-0941), tolbutamide, exendin-4 (Tocris), l-leucine, forskolin, and monomethyl ester of succinic acid (SAME) at the indicated concentrations.
RESULTS
Imeglimin treatment does not alter body composition or energetics.
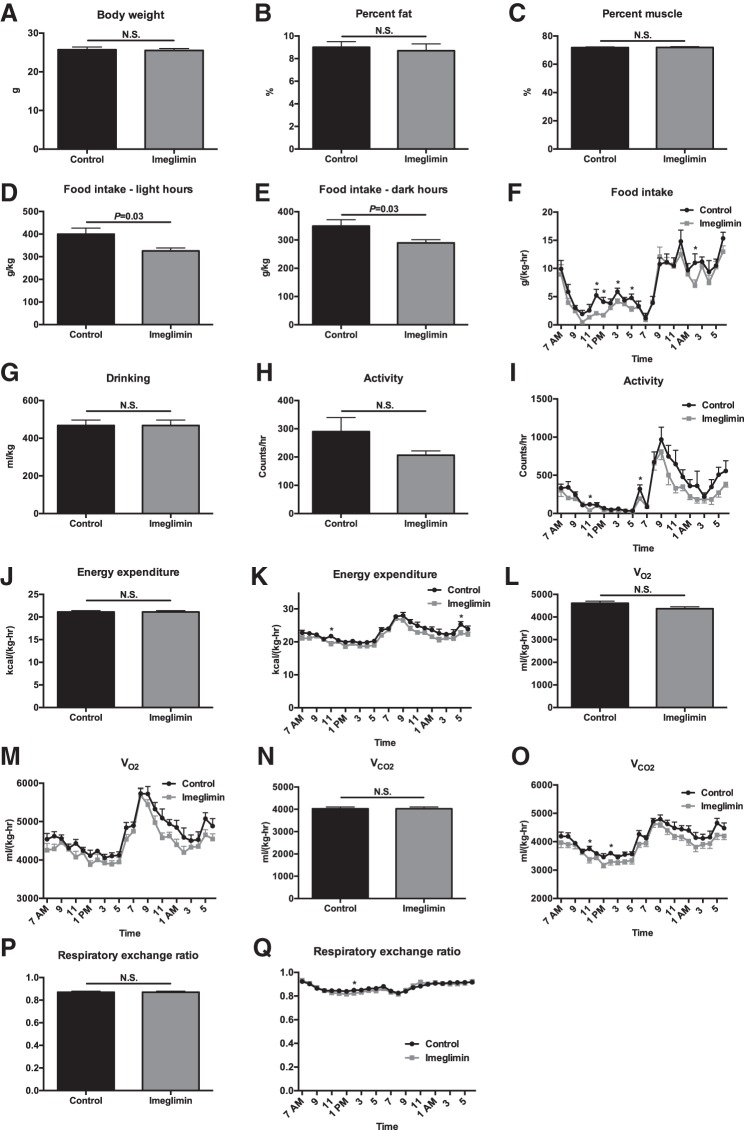
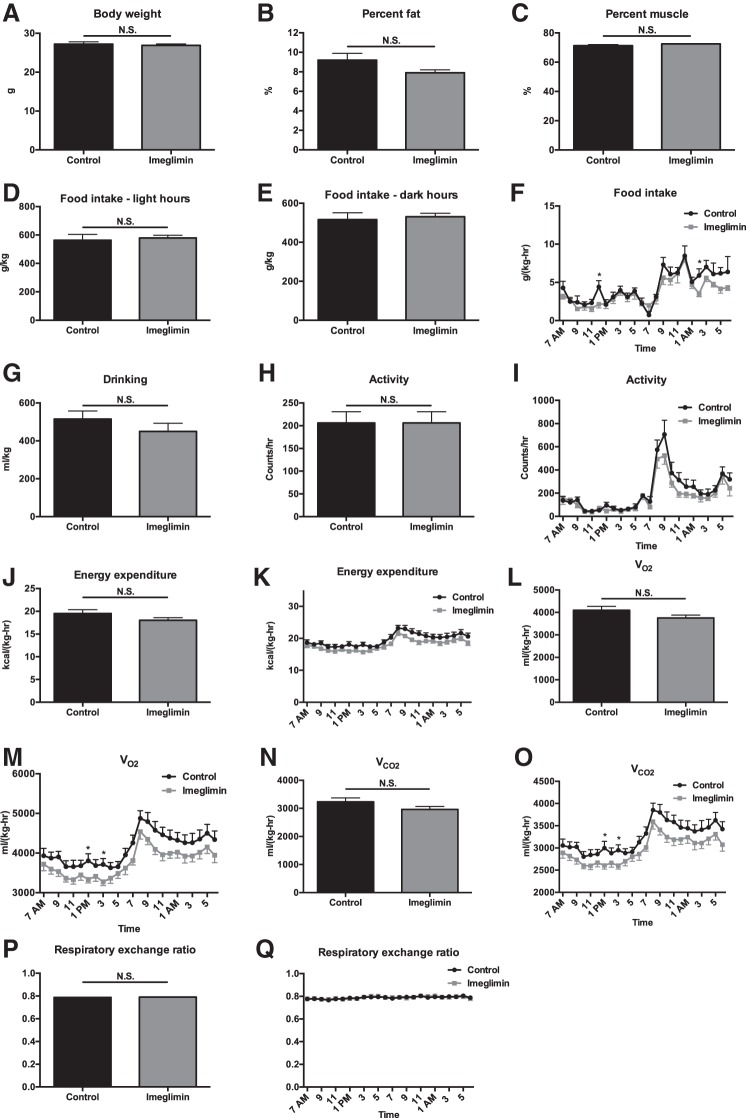
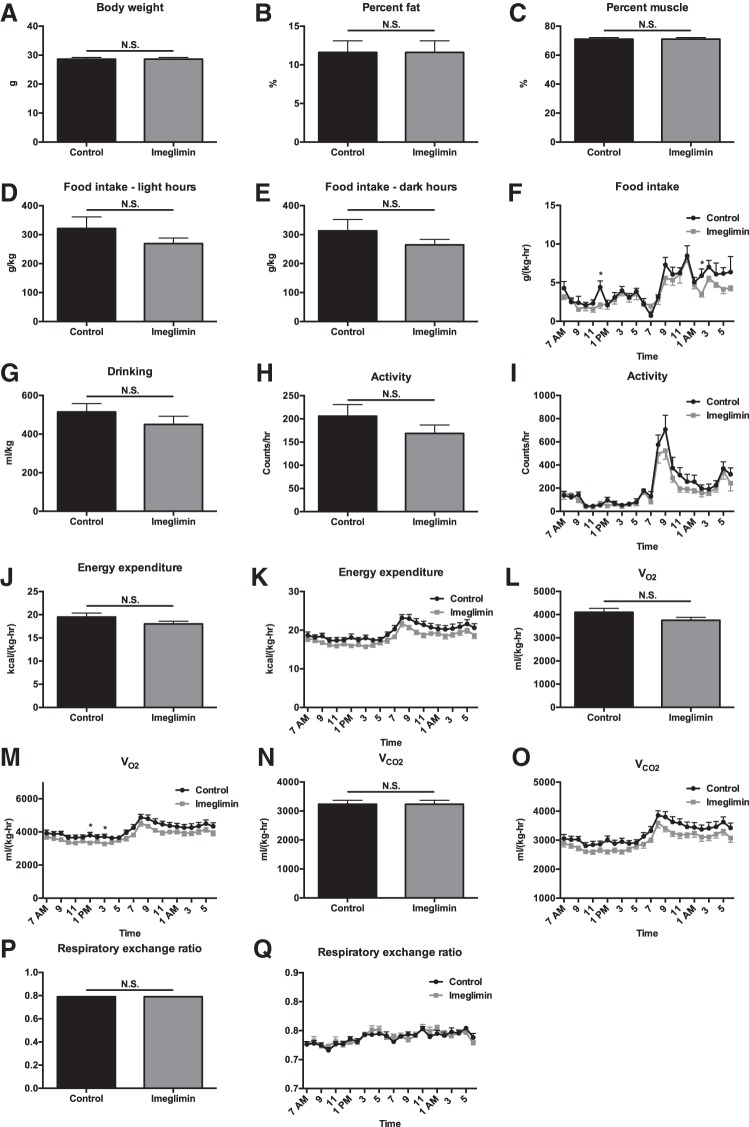
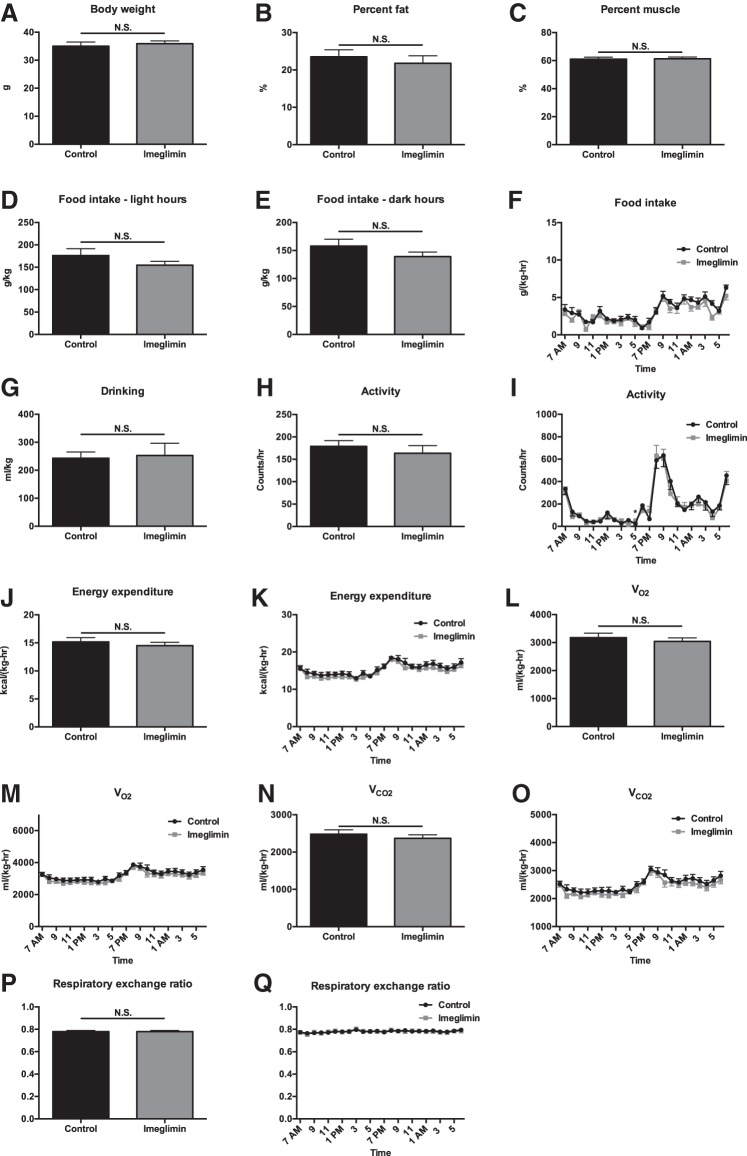
Metabolic cage analysis revealed no difference in any parameter studied in chow- or high-fat-fed mice (HFD) treated for 1 wk with imeglimin, with the exception of food intake, which was modestly lower in imeglimin-treated mice. Food intake was different only after 1 wk of treatment (Figs. 1 and 2). Similarly, in chow- and HFD-fed mice treated chronically (3–5 wk) with imeglimin, there were no differences seen in body composition, food and water intake, or energetics compared with vehicle-treated controls (Figs. 3 and 4).
Fig. 1.
One week of imeglimin treatment does not alter body weight, basal energetics, body composition, or food intake in chow-fed mice. A–C: body weight, %body fat, and %body muscle. D and E: food intake during light and dark hours. F: food intake time course. G: water drinking. H: 24-h activity. I: activity time course. J: 24-h energy expenditure. K: energy expenditure time course. L and M: average V̇o2 and V̇o2 time course. N and O: average V̇co2 and V̇co2 time course. P and Q: average respiratory exchange ratio (RER) and RER time course. Data are means ± SE of n = 8/group. *P < 0.05. NS, not significant.
Fig. 2.
Three weeks of imeglimin treatment does not alter body weight, basal energetics, body composition, or food intake in chow-fed mice. A–C: body weight, %body fat, and %body muscle. D and E: food intake during light and dark hours. F: food intake time course. G: water drinking. H: 24-h activity. I: activity time course. J: 24-h energy expenditure. K: energy expenditure time course. L and M: average V̇o2 and V̇o2 time course. N and O: average V̇co2 and V̇co2 time course. P and Q: average RER and RER time course. Data are means ± SE of n = 8/group. *P < 0.05.
Fig. 3.
One week of imeglimin treatment does not alter basal energetics, body composition, or food intake in high-fat-fed mice. A–C: body weight, %body fat, and %body muscle. D and E: food intake during light and dark hours. F: food intake time course. G: water drinking. H: 24-h activity. I: activity time course. J: 24-h energy expenditure. K: energy expenditure time course. L and M: average V̇o2 and V̇o2 time course. N and O: average V̇co2 and V̇co2 time course. P and Q: average RER and RER time course. Data are means ± SE of n = 8/group, with comparisons by t-test. *P < 0.05.
Fig. 4.
Five weeks of imeglimin treatment does not alter basal energetics, body composition, or food intake in high-fat-fed mice. A–C: body weight, %body fat, and %body muscle. D and E: food intake during light and dark hours. F: food intake time course. G: water drinking. H: 24-h activity. I: activity time course. J: 24-h energy expenditure. K: energy expenditure time course. L and M: average V̇o2 and V̇o2 time course. N and O: average V̇co2 and V̇co2 time course. P and Q: average RER and RER time course. Data are means ± SE of n = 8/group. *P < 0.05.
Imeglimin-treated rats exhibit improved glucose tolerance due to increased insulin secretion in vivo.
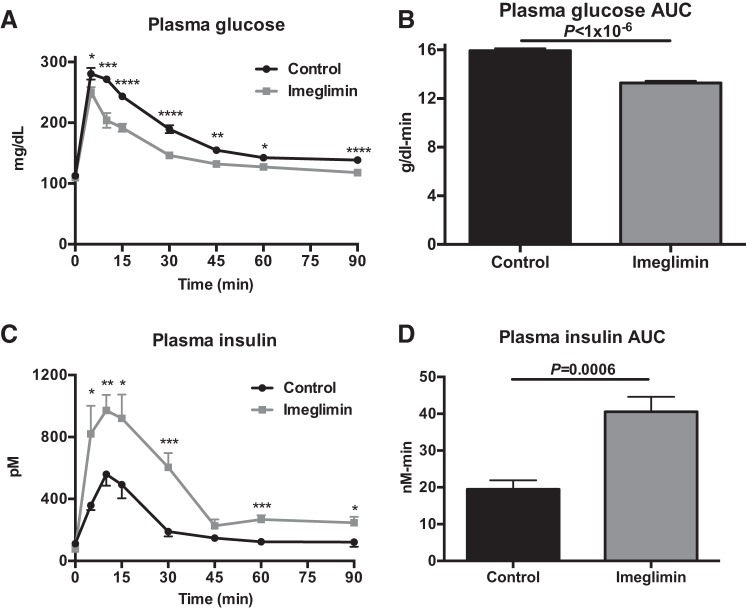
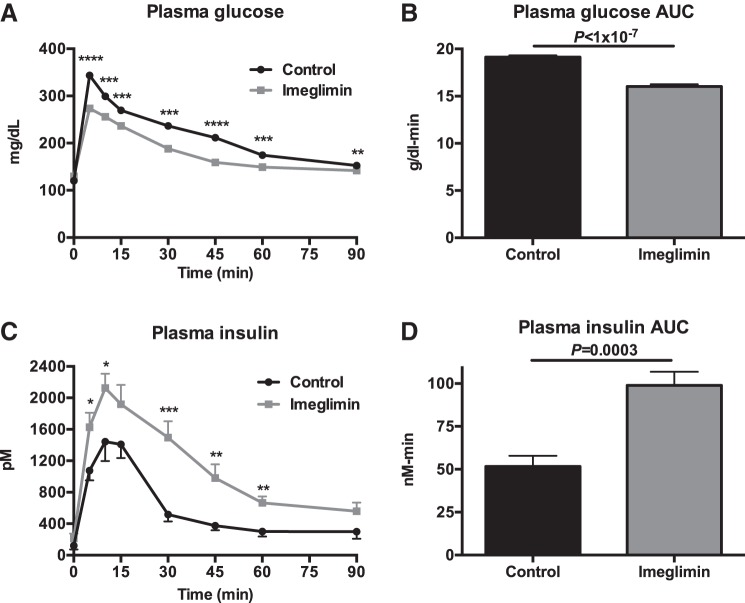
In chow-fed rats, 2 wk of imeglimin treatment resulted in a striking improvement in glucose tolerance, with lower plasma glucose concentrations throughout the GTT (Fig. 5, A and B). Imeglimin-treated rats exhibited 30–100% increases in insulin secretion at each time point during the GTT, with more than a doubling in the insulin area under the curve during the GTT (Fig. 5, C and D). These results were replicated in high-fat-fed (HFD) rats; imeglimin improved glucose tolerance by increasing insulin secretion throughout a GTT after a 2-wk treatment period (Fig. 6, A–D).
Fig. 5.
Imeglimin increases glucose-stimulated insulin secretion in vivo and in vitro in chow-fed rats. A and B: plasma glucose and glucose area under the curve (AUC) during a glucose tolerance test. C and D: plasma insulin and insulin AUC during a glucose tolerance test. Data are means ± SE of n = 6/group. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 by t-test.
Fig. 6.
Imeglimin increases glucose-stimulated insulin secretion in vivo in high-fat-fed rats. A and B: plasma glucose and glucose AUC during a glucose tolerance test. C and D: plasma insulin and insulin AUC during a glucose tolerance test. Data are means ± SE of n = 6/group. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 by t-test.
Imeglimin does not alter insulin sensitivity in chow- or high-fat-fed rats.
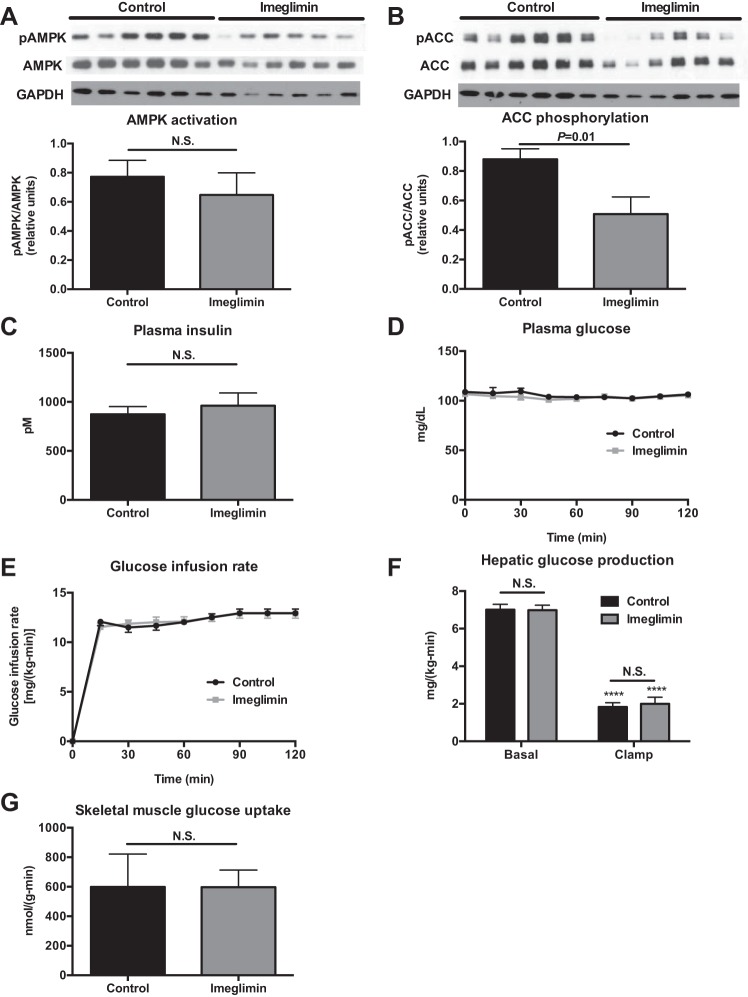
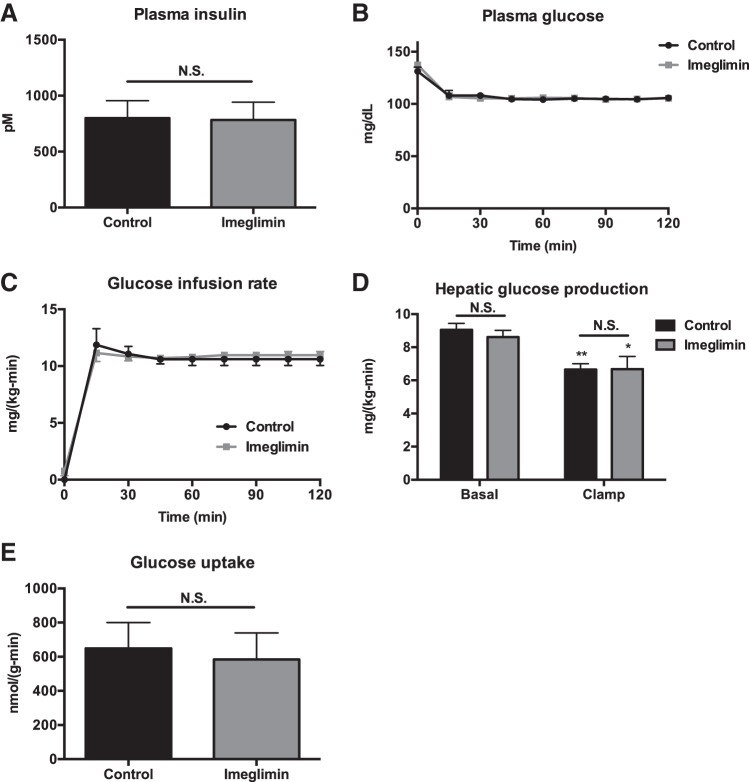
The improvements in glucose tolerance observed in imeglimin-treated rats were not associated with hepatic AMPK activation in chow-fed rats; however, we observed a 50% reduction in ACC phosphorylation as predicted by imeglimin-treated rats' higher plasma insulin concentrations (Fig. 7, A and B) (13). To determine whether imeglimin treatment was associated with improved hepatic or peripheral muscle insulin sensitivity, we performed hyperinsulinemic euglycemic clamps and observed no difference in the glucose infusion rate required to maintain euglycemia, in basal or clamped hepatic glucose production, or in peripheral muscle glucose disposal during the clamp (Fig. 7, C–G). Similarly, in HFD rats, there were no differences in hepatic or muscle insulin sensitivity, as indicated by the absence of any differences in glucose infusion rate, hepatic glucose production, or muscle glucose uptake after 2 wk of imeglimin treatment (Fig. 8, A–E).
Fig. 7.
Imeglimin does not alter insulin sensitivity in chow-fed rats. A and B: AMP-activated protein kinase (AMPK) activation and acetyl-CoA carboxylase (ACC) phosphorylation. C: plasma insulin at the end of the clamp. D: plasma glucose. E: glucose infusion rate to maintain euglycemia. F: hepatic glucose production. ****P < 0.0001 relative to basal. G: skeletal muscle glucose uptake during the clamp. Data are means ± SE of n = 6/group, with comparisons by t-test.
Fig. 8.
Imeglimin does not alter insulin sensitivity in high-fat-fed rats. A: plasma insulin at the end of the clamp. B: plasma glucose. C: glucose infusion rate to maintain euglycemia. D: hepatic glucose production. *P < 0.05 and **P < 0.01 relative to basal. E: skeletal muscle glucose uptake during the clamp. Data are means ± SE of n = 6/group, with comparisons by t-test.
Imeglimin amplifies rather than triggers insulin secretion.
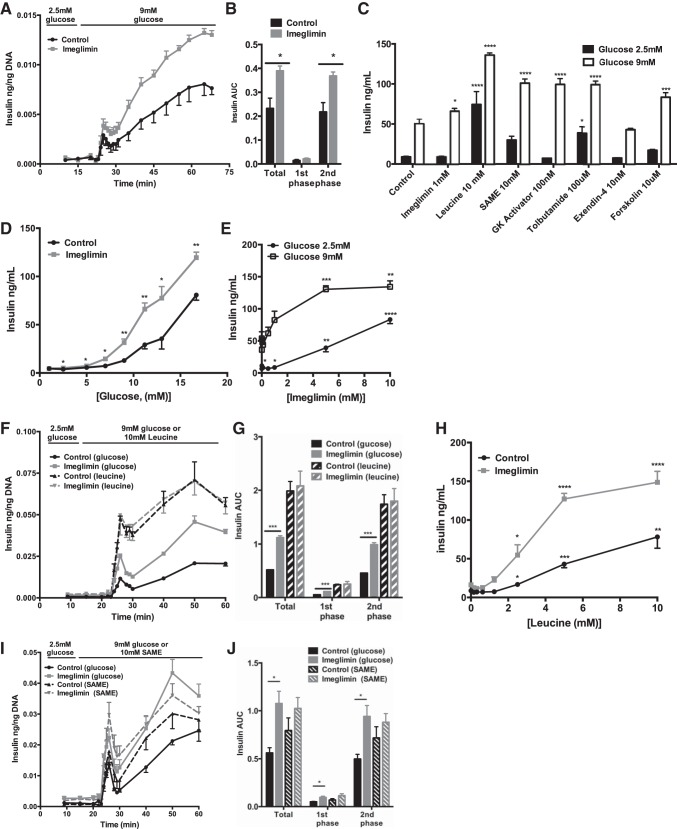
To determine whether imeglimin directly stimulates β-cell insulin secretion, we measured glucose-stimulated insulin secretion by isolated islet perifusion and static incubations. Adding imeglimin to previously untreated rat islets at 100 μM, a dose previously demonstrated to stimulate glucose-stimulated insulin secretion (12), caused an immediate increase in glucose-stimulated insulin secretion (Fig. 9A). An area under the curve analysis identified the mitochondrial metabolism-dependent second phase of insulin secretion accounting for the bulk of the increase (Fig. 9B). Compared with other islet secretagogues, insulin secretion appeared to be dependent upon glucose metabolism up to a concentration of 1 mM imeglimin (Fig. 9C). The glucose dependency displayed a graded amplifying behavior, except at concentrations >5 mM (well above its therapeutic target range), where it triggered insulin release even at low glucose (Fig. 9, D and E). To determine whether imeglimin was dependent on glycolytic and/or mitochondrial metabolism, islet perifusions were performed using obligate mitochondrial fuels such as leucine and succinate (Fig. 9, F, G, I, and J). In the presence of glutamine, 10 mM leucine stimulates insulin secretion by activating glutamate dehydrogenase and supplies both anaplerotic and oxidative pathways of mitochondrial metabolism. Leucine-stimulated secretion was higher than glucose-stimulated secretion and was not amplified further by 1 mM imeglimin (Fig. 9, F and G). However, in contrast to the perifusions at increasing concentrations of leucine in static incubations, imeglimin actually amplified secretion, suggesting that the lack of an observed difference in the perifusion could be attributed to having reached maximal insulin release (Fig. 9H). Like leucine, the methyl ester of succinate (SAME) has both anaplerotic and oxidative metabolism but showed only a trend toward being amplified by imeglimin (Fig. 9, I and J). Taken together, these data imply that the primary mechanism of action of imeglimin is via an amplification of mitochondrial metabolism-dependent signals that stimulate insulin release.
Fig. 9.
Imeglimin amplifies insulin secretion in rat islets. A and B: insulin release and AUC in perifusions from intact islets at 100 μmol/l imeglimin (n = 4 replicates/group). C: insulin secretion from static rat islet aggregates following 2 h of stimulation at indicated concentrations of glucose and/or stimulus (n = 4 replicates/group). D: acute static insulin secretion following 2-h incubations at increasing concentrations of glucose as indicated for control and imeglimin (1 mM; n = 3 replicates/group). E: acute static insulin secretion following 2-h incubations at increasing concentrations of imeglimin at indicated glucose concentrations (n = 4 replicates/group). F, G, I, and J: insulin secretion from islet perifusions at the indicated times (F and I) or AUC (G and J) in the presence of 1 mM imeglimin and the indicated substrate and concentration [monomethyl ester of succinic acid (SAME)]. Glutamine was present at 4 mM in all perifusions and n = 3 replicates/group. H: acute static insulin secretion following 2-h incubations with 1 mM imeglimin at increasing concentrations of leucine at 2.5 mM glucose (n = 4 replicates/group). Data are means ± SE of n = 6/group, with comparisons by t-test or 1-way ANOVA.
DISCUSSION
The attractive safety and efficacy profile of imeglimin in poorly controlled diabetic patients raises the question of how and why it is so safe and effective. Improved glucose tolerance in rodents suggests the possibility of a phenotype in the β-cell (8); however, definitive studies had not yet been performed to assess hepatic and peripheral muscle insulin sensitivity or whole body energetics. We determined that short-term imeglimin treatment modulates glucose-stimulated insulin secretion in isolated islets and in vivo in rats during a GTT without altering hepatic or peripheral insulin sensitivity, AMPK activity, or energetics. The lack of any difference in hepatic insulin sensitivity in high-fat-fed rats using the gold-standard technique, the hyperinsulinemic euglycemic clamp, shows that after 2 wk of treatment, imeglimin's benefit on glucose tolerance in this HFD rat model is due to its ability to increase glucose-stimulated insulin secretion. This result contrasts with the improvement in insulin sensitivity shown by Vial et al. (12) in the high-fat, high-sucrose diet mouse model (HFHSD) and by Fouqueray et al. (1) in streptozotocin-treated diabetic rats. These differences could be explained by the difference in treatment duration (2 wk in the present study vs. 6–7 wk in the previous studies) and/or by differences in imeglimin effects in the models. Imeglimin decreased liver lipid content in HFHSD mice by 30% (12) but had no effect on liver triglycerides in the present study (data not shown).
Treatment of isolated rodent islets with imeglimin acutely potentiated insulin secretion, indicating that there is a primary and direct effect of the compound on intact islets. Similarly, following the treatment of healthy rodents in vivo for 2 wk there was noticeable improvement in insulin secretion consistent with a primary islet effect. It is not surprising and perhaps anticipated that there are also potential secondary benefits following the improved glucose homeostasis from augmented insulin secretion. These might include improvements in insulin sensitivity after a longer treatment (6 wk), as observed in a high-fat rat model of insulin resistance. It is also possible that chronic imeglimin treatment may secondarily improve liver and muscle insulin sensitivity in diabetic rodents and patients with type 2 diabetes through reversal of glucose toxicity (10).
In isolated islet perifusions from healthy rats, imeglimin displayed a potent metabolism-dependent stimulation that resembled more the amplifying effect observed with cell surface receptors (e.g., GLP-1R or GPR40) rather than triggering by increasing metabolism or directly closing KATP channels (e.g., glucokinase activators or sulfonylureas) (5, 6). This pharmacological behavior links the enhanced insulin secretion to metabolic coupling factors. As such, the amplifying effect is minimal when on the low end of the physiological range. This may help explain imeglimin's observed efficacy without causing hypoglycemia in humans. As glucose levels climb, there is a progressive increase in secretion, as would be needed to reestablish homeostasis following a meal. By acting only when glucose levels are high, the intermittent responsivity prevents basal hyperinsulinism and potentially allows the β-cell more of a chance to rest between challenges. These may afford a long-term advantage of this novel class of oral agents to protect and/or preserve islet health.
Future studies will be needed to identify the receptor and/or pathway(s) through which this compound acts. In particular, given that both anaplerotic and oxidative mitochondrial metabolism are coupled to insulin secretion, it will be important to determine whether imeglimin's action is dependent on one and/or the other (7, 11). Regardless, here we demonstrate that the primary mechanism of imeglimin appears to be via islet amplification rather than improving insulin sensitivity in the high-fat-fed rat.
GRANTS
This study was funded by grants from the National Institutes of Health (R01-DK-40936, R01-AG-23686, P30-DK-45735, U24-DK-59635, T32-DK-101019, and R01-DK-92606), The Novo Nordisk Foundation Center for Basic Metabolic Research, and investigator-initiated support from Poxel SA (K. F. Petersen and R. G. Kibbey).
DISCLOSURES
This work was supported in part by an investigator-sponsored grant from Poxel SA. Pascale Fouqueray, Sophie Hallakou-Bozec, and Sébastien Bolze are Poxel employees and may own stock in the company. No other potential conflicts of interest relevant to this article are reported.
AUTHOR CONTRIBUTIONS
R.J.P., P.F., S.H.-B., S.B., G.I.S., K.F.P., and R.G.K. conception and design of research; R.J.P., R.L.C., M.C.P., and D.Z. performed experiments; R.J.P., R.L.C., M.C.P., D.Z., P.F., S.H.-B., S.B., G.I.S., K.F.P., and R.G.K. analyzed data; R.J.P., R.L.C., M.C.P., D.Z., P.F., S.H.-B., S.B., G.I.S., K.F.P., and R.G.K. interpreted results of experiments; R.J.P. and R.L.C. prepared figures; R.J.P. drafted manuscript; R.J.P., R.L.C., M.C.P., D.Z., P.F., S.H.-B., S.B., G.I.S., K.F.P., and R.G.K. edited and revised manuscript; R.J.P., R.L.C., M.C.P., D.Z., P.F., S.H.-B., S.B., G.I.S., K.F.P., and R.G.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jianying Dong, Xiaojian Zhao, Wanling Zhu, Ali Nasiri, Gina Butrico, and Maria Batsu for their expert technical support.
REFERENCES
- 1.Fouqueray P, Leverve XIM, Fontaine E, Baquié M, Wollheim C, Lebovitz H, Bozec S. Imeglimin - A New Oral Antidiabetic that Targets the Three Key Defects of Type 2 Diabetes. J Diabetes Metab 2: 1–8, 2011. [Google Scholar]
- 2.Fouqueray P, Pirags V, Diamant M, Schernthaner G, Lebovitz HE, Inzucchi SE, Bailey CJ. The efficacy and safety of imeglimin as add-on therapy in patients with type 2 diabetes inadequately controlled with sitagliptin monotherapy. Diabetes Care 37: 1924–1930, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Fouqueray P, Pirags V, Inzucchi SE, Bailey CJ, Schernthaner G, Diamant M, Lebovitz HE. The efficacy and safety of imeglimin as add-on therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy. Diabetes Care 36: 565–568, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, Goodyear LJ, Kraegen EW, White MF, Shulman GI. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes 48: 1270–1274, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Henquin JC. The dual control of insulin secretion by glucose involves triggering and amplifying pathways in beta-cells. Diabetes Res Clin Pract 93, Suppl 1: S27–S31, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 49: 1751–1760, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Jitrapakdee S, Wutthisathapornchai A, Wallace JC, MacDonald MJ. Regulation of insulin secretion: role of mitochondrial signalling. Diabetologia 53: 1019–1032, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pacini G, Mari A, Fouqueray P, Bolze S, Roden M. Imeglimin increases glucose-dependent insulin secretion and improves beta-cell function in patients with type 2 diabetes. Diabetes Obes Metab 17: 541–545, 2015. [DOI] [PubMed] [Google Scholar]
- 9.Pirags V, Lebovitz H, Fouqueray P. Imeglimin, a novel glimin oral antidiabetic, exhibits a good efficacy and safety profile in type 2 diabetic patients. Diabetes Obes Metab 14: 852–858, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest 79: 1510–1515, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stark R, Pasquel F, Turcu A, Pongratz RL, Roden M, Cline GW, Shulman GI, Kibbey RG. Phosphoenolpyruvate cycling via mitochondrial phosphoenolpyruvate carboxykinase links anaplerosis and mitochondrial GTP with insulin secretion. J Biol Chem 284: 26578–26590, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vial G, Chauvin MA, Bendridi N, Durand A, Meugnier E, Madec AM, Bernoud-Hubac N, Pais de Barros JP, Fontaine E, Acquaviva C, Hallakou-Bozec S, Bolze S, Vidal H, Rieusset J. Imeglimin normalizes glucose tolerance and insulin sensitivity and improves mitochondrial function in liver of a high-fat, high-sucrose diet mice model. Diabetes 64: 2254–2264, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Witters LA, Kemp BE. Insulin activation of acetyl-CoA carboxylase accompanied by inhibition of the 5′-AMP-activated protein kinase. J Biol Chem 267: 2864–2867, 1992. [PubMed] [Google Scholar]
- 14.Zhang D, Christianson J, Liu ZX, Tian L, Choi CS, Neschen S, Dong J, Wood PA, Shulman GI. Resistance to high-fat diet-induced obesity and insulin resistance in mice with very long-chain acyl-CoA dehydrogenase deficiency. Cell Metab 11: 402–411, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]