Abstract
Growth hormone (GH) plays an essential role in controlling somatic growth and in regulating multiple physiological processes in humans and other species. Insulin-like growth factor I (IGF-I), a conserved, secreted 70-amino acid peptide, is a critical mediator of many of the biological effects of GH. Previous studies have demonstrated that GH rapidly and potently promotes IGF-I gene expression in rodents and in some other mammals through the transcription factor STAT5b, leading to accumulation of IGF-I mRNAs and production of IGF-I. Despite this progress, very little is known about how GH or other trophic factors control human IGF1 gene expression, in large part because of the absence of any cellular model systems that robustly express IGF-I. Here, we have addressed mechanisms of regulation of human IGF-I by GH after generating cells in which the IGF1 chromosomal locus has been incorporated into a mouse cell line. Using this model, we found that physiological levels of GH rapidly stimulate human IGF1 gene transcription and identify several potential transcriptional enhancers in chromatin that bind STAT5b in a GH-regulated way. Each of the putative enhancers also activates a human IGF1 gene promoter in reconstitution experiments in the presence of the GH receptor, STAT5b, and GH. Thus we have developed a novel experimental platform that now may be used to determine how human IGF1 gene expression is controlled under different physiological and pathological conditions.
Keywords: growth hormone, insulin-like growth factor I, signal transducer and activator of transcription 5b, gene transcription, chromatin immunoprecipitation, epigenetics
growth hormone (GH) and insulin-like growth factor I (IGF-I) are secreted proteins that play key roles in many physiological processes in humans and other species. Both molecules are critical for postnatal somatic growth, are essential for normal tissue repair, and are important secondary regulators of intermediary metabolism (40, 44, 54). GH and IGF-I also have been implicated as being involved in cancer and in human aging (5, 21, 31, 52, 63), indicating that their activities must be tightly controlled to minimize pathogenic effects, particularly in adults (28, 65). Thus it is important to understand the mechanisms of regulation and action of both GH and IGF-I to devise strategies that will enhance their positive effects while limiting their negative outcomes.
GH and IGF-I have been interconnected ever since 1957, when the somatomedin hypothesis first postulated the existence of a secreted factor under control of GH (58). Subsequent experiments broadened the biological roles of both proteins (reviewed in Refs. 16, 42, and 44) and also defined a mechanistic biochemical relationship between GH and IGF-I (reviewed in Refs. 10 and 56). It is now known that GH promotes IGF-I gene transcription, leading to production of IGF-I mRNAs and synthesis and secretion of IGF-I (10, 56).
The signaling pathways through which GH acts now have been established. Like related cytokines, GH binds to and activates a transmembrane receptor and engages the JAK-STAT signal transduction pathway (71). GH binding stimulates GH receptor-associated activity of Jak2 (8, 72), causing phosphorylation of tyrosine residues within the intracellular segment of the receptor, leading to recruitment and activation of STATs and other signaling intermediates (71, 72). Although several signaling cascades contribute to the biological actions of GH (9, 40, 72), recent observations have determined that STAT5b is the key agent responsible for many of the critical biological effects of GH, including production of IGF-I (reviewed in Refs. 10 and 56). For example, individuals with inactivating mutations in the STAT5B gene have growth impairment and reduced expression of IGF-I (20, 30, 39, 59, 66). In addition, molecular studies have shown that GH activates transcription of the rat Igf1 gene via a series of dispersed STAT5b-binding enhancer elements (2, 11, 12, 67), leading to production of Igf1 mRNAs and synthesis of IGF-I (56).
Despite progress in understanding regulation of Igf1 gene transcription in rodents, very little is known about how GH or other trophic factors control human IGF1 gene expression (33, 38, 46, 60, 64).
Human IGF1 is structurally similar to IGF-I genes from other mammalian species, being composed of six exons subdivided by five introns (55, 61) and possessing tandem promoters, each with its own unique leader exon (see Fig. 1A) (60). Moreover, the DNA sequences of all exons and both proximal promoter regions are highly conserved among mammals (55, 61). Yet to date there are minimal insights about regulatory mechanisms. A key problem preventing understanding of human IGF1 gene regulation is the absence of any cell systems that robustly express IGF1. Here, we have overcome this limitation by generating recombinant cell lines in which the human IGF1 chromosomal region has been incorporated into the mouse genome. Using these cells, we show that GH potently activates IGF1 gene transcription through STAT5b and identify the first putative transcriptional enhancers in the human IGF1 locus.
Fig. 1.
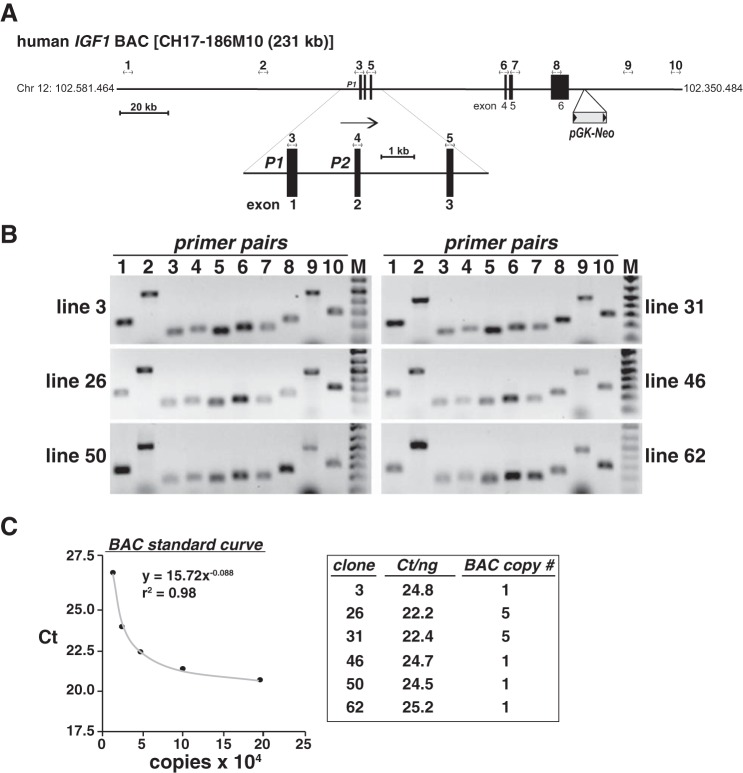
Developing recombinant cell lines incorporating the human IGF1 locus. A: schematic of human IGF-I-containing bacterial artificial chromosome (BAC) CH17-186M10. The location of the pGK-Neo selection cassette, which was introduced into the BAC by recombineering (43, 78), is indicated, as is a scale bar. Nos. 1–10 above the map indicate the locations of primer pairs used to test incorporation of the intact BAC into mouse C3H10T1/2 cells. The enlargement below the main map depicts the two IGF1 promoters, P1 and P2, and exons 1–3. Arrow indicates the direction of gene transcription. B: results of PCR experiments for the presence of the human IGF1 locus using DNA isolated from the listed recombinant cell lines (M = DNA size markers). Primer pairs are in Table 1. C: results of copy number determination for recombinant cell lines encoding the human IGF1 BAC. Left: standard curve of serial dilutions of IGF1 plasmid DNA (see materials and methods for details). Right: estimated copy numbers for cell lines 3, 26, 31, 46, 50, and 62. CT, cycle threshold.
MATERIALS AND METHODS
Materials.
TransIT LT1 transfection reagent was purchased from Mirus (Madison, WI), G418 was from Sigma-Aldrich (St. Louis, MO), okadaic acid was from Alexis Biochemicals (San Diego, CA), and micrococcal nuclease was from Active Motif (Carlsbad, CA). Immobilon-FL membranes were obtained from Millipore (Billerica, MA), protease inhibitor tablets were from Roche Applied Sciences (Indianapolis, IN), and AquaBlock EIA/WIB solution was from East Coast Biologicals (North Berwick, ME). Dulbecco's modified Eagle's medium (DMEM), trypsin-EDTA solution, SuperScript III reverse-transcriptase (RT) kit, protein A-Sepharose beads, SYBR Green Platinum quantitative PCR (qPCR) mix, and pGL3-basic were from Invitrogen-Life Technologies (Carlsbad, CA). Fetal calf serum and phosphate-buffered saline (PBS) were obtained from Mediatech-Cellgrow (Herndon, VA). The BCA protein assay kit was purchased from Pierce Biotechnologies (Rockford, IL), the Qia-Quick PCR purification kit was from Qiagen (Valencia, CA), and restriction enzymes, buffers, ligases, and polymerases were from New England Biolabs (Beverly, MA). Recombinant rat GH was purchased from the National Hormone and Pituitary Program, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, and was stored dry in aliquots at −80°C until use, when it was reconstituted at 2.5 mg/ml (∼100 μM). Primary antibodies were purchased from the following vendors: STAT5 (C17), Santa Cruz Biotechnology (Santa Cruz, CA); phospho-STAT5 (clone 8-5-2), Millipore (Billerica, MA); and Flag M2 monoclonal and α-tubulin, Sigma-Aldrich. Secondary antibodies were from Rockland Immunochemical (Gilbertsville, PA; goat-anti-rabbit IgG-IR800 and goat anti-mouse IgG-IR680) and Invitrogen (goat anti-mouse IgG1-Alexa 488). Custom oligonucleotides were obtained from Thermo Scientific (Waltham, MA). BAC clone no. CH17-186M10 was purchased from Children's Hospital Oakland Research Institute (Oakland, CA), and materials for recombineering were obtained from the National Cancer Institute (Frederick, MD). Human liver RNA was obtained from pooled surgical samples. All other materials were from commercial sources.
Developing recombinant cell lines containing the human IGF1 chromosomal locus.
Bacterial artificial chromosome (BAC) clone no. CH17-186M10 encodes 230,981 base pairs of human chromosome 12 and spans coordinates 102.350.484 to 102.581.464, including the ∼80-kb IGF1 gene on the reverse DNA strand from 102.482.382 (5′ end, exon 1) to 102.402.508 (3′ end, exon 6) and both 5′ and 3′ flanking regions (Fig. 1A). A DNA cassette encoding the neomycin resistance gene driven by a combined prokaryotic and eukaryotic promoter (derived from plasmid pL452) was inserted into the BAC at position 102.390.054 (Fig. 1A) by homologous recombination (recombineering) using E. coli strain SW102 and selection on kanamycin-containing agar plates (43, 78). Modified BAC DNA was purified, and the presence of the neomycin resistance cassette and the IGF-I gene and flanking DNA were validated by PCR. The human DNA part of the BAC was incorporated into the genome of mouse C3H10T1/2 cells (ATCC no. CCL226) following transfection using Transit LT1 and selection in medium containing G418 at 400 μg/ml. Individual colonies were isolated using cloning cylinders, expanded, checked for an intact IGF-I gene and locus by PCR (Fig. 1B; primers are listed in Table 1), and stored in liquid N2 until use. Transgene copy number was assessed by quantitative (q)PCR, as described (15, 35), with standard curves being generated with serial dilutions of plasmid DNA containing segments of the human IGF1 gene (Fig. 1C).
Table 1.
DNA sequences of oligonucleotide primers for mapping BAC cell lines
| Genomic Location (5′ End) | Primer Pair No. | Top Strand (5′ to 3′) | Bottom Strand (5′ to 3′) | Amplicon (bp) |
|---|---|---|---|---|
| 102.577.557 | 1 | TAGTATTTCGTTAAAGAGGGGCAG | TAGTTCCTTAGCATTCATCTGAGC | 246 |
| 102.520.484 | 2 | CATTCCTATCCCCTTCTTTACTGCCT | TCCATCACCTCCACTTTGATGCCTT | 501 |
| 102.480.320 | 3 | CTTCAAGAAATCACAAAAGCAGCA | ATGTGACATTGCTCTCAACATCTC | 162 |
| 102.478.439 | 4 | AATACTCACTGTAGGTGTAATCAT | GCATACCTGCCTGGGTGTCCAAAT | 174 |
| 102.475.664 | 5 | TGAAATAAAAGCCCCTGTCTC | GTGAAGATGCACACCATGTCCTCC | 157 |
| 102.419.512 | 6 | TCTGGGTCTTGGGCATGTCGGTGT | ACAAGCCCACAGGGTATGGCTCCA | 180 |
| 102.417.832 | 7 | TTGCCTCTGCATTCAGCATTTCTA | GTTTACAGTATCAGCCCCCATCT | 181 |
| 102.402.327 | 8 | CAAATCACTCCTAAAGACAATGTT | GAAGTACATTTGAAGAACGCAAGT | 241 |
| 102.370.484 | 9 | ACCTTTGCTGTAGTTCCCAAGAAT | TTTGCTGGGAACTTCTCCTTGCCAC | 501 |
| 102.350.620 | 10 | CAGTCATCTTCCACCTGCCCAAGG | AGACAGCCTATTGTGGGACTTCAC | 303 |
BAC, bacterial artificial chromosome; bp, base pairs.
Analysis of recombinant cell lines.
Cells were incubated in antibiotic-free DMEM with 10% fetal bovine serum and 200 μg/ml G418 at 37°C in humidified air with 5% CO2, and at ∼50% of confluent density they were coinfected with recombinant adenoviruses encoding the mouse GH receptor [Ad-GHR, multiplicity of infection (MOI) of 200; rat STAT5b (Ad-STAT5b), MOI of 200; and a tetracycline-inhibited transcriptional activator (Ad-tTA), MOI of 100], as described (75). The latter is required for expression of Stat5b (75). At 1 day after infection, cells were washed with PBS and incubated in serum-free medium (SFM) for 2 h, followed by the addition of SFM and 1% bovine serum albumin plus vehicle or recombinant rat GH (40 nM) for varying intervals (15 min to 24 h).
Analysis of gene expression.
Whole cell and nuclear RNA were isolated as described (3, 12). RNA integrity was assessed by agarose gel electrophoresis, and concentrations were determined spectrophotometrically at 260 nm. Total RNA (2 μg) was reverse-transcribed with oligo-dT primers in a final volume of 20 μl using the SuperScript III RT kit (3, 12). The cDNA (0.5 μl) was used as a template for conventional PCR (3, 12) after pilot studies were performed to establish a cycle number for each primer set that achieved amplification in the linear range (∼20–27 cycles, depending on the primers). Primer pairs are listed in Table 2. Products were visualized after agarose gel electrophoresis and staining with ethidium bromide. Results are representative of at least three independent RT-PCR experiments. Nuclear RNA (2.5 μg) was reverse-transcribed with random hexamers in a final volume of 20 μl using the SuperScript III RT kit, and 1 μl of cDNA was used as template for PCR (3, 12). Primer pairs are in Table 3. For conventional PCR, pilot studies showed that 20–26 cycles achieved amplification in the linear range for each primer pair. Products were visualized after agarose gel electrophoresis by ethidium bromide staining. The results are representative of three or more independent experiments. For quantitative RT-PCR, first-strand cDNA was diluted 100-fold (25 μl into final volume of 2.5 ml), and 1 μl was used as template for PCR (the equivalent of ∼1.0 ng of nuclear RNA input). Quantitative PCR was performed as described (11, 12) using primers listed in Table 3 and a Bio-Rad Chromo4 Real-Time PCR detection system. A standard curve was constructed for each primer pair by plotting cycle threshold vs. amount of input DNA (log10 scale), and the slope and correlation coefficient for each curve were determined (11, 12). Standards included serial dilutions of human or mouse genomic DNA (7 concentrations ranging from 0.06 to 40 ng). Primer sets were judged to be acceptable if the slope of the linear part of the curve approximated −3.3 (theoretical maximum efficiency of amplification) and the r2 coefficient was >0.98. The cycle threshold for each sample was extrapolated to the standard curve to determine the amount of nascent transcript (in ng). Results for mouse Mrps17 (S17) were used to normalize samples at each time point (Fig. 3).
Table 2.
Primers used for RT-PCR of total RNA
| Gene (Amplicon) | DNA Sequence |
|---|---|
| Human IGF-I (173 bp) | |
| Exon 1 | 5′-TTGCTAAATCTCACTGTCACTGC |
| Exon 4 | 5′-GCTCCGGAAGCAGCACT |
| Human IGF-I (122 bp) | |
| Exon 2 | 5′-TGGATGCTCTTCAGTTCGTG |
| Exon 4 | 5′-GCTCCGGAAGCAGCACT |
| Human IGF-I (119 bp) | |
| Exon 3 | 5′-GATGCTCTTCAGTTCGTGTGT |
| Exon 4 | 5′-GCTCCGGAAGCAGCACT |
| Mouse Igf1 (213 bp) | |
| Exon 3 | 5′-CTCTTCAGTTCGTGTGTGGACC |
| Exon 4 | 5′-GTCTTGGGCATGTCAGTGTGG |
| Mouse Igfals (246 bp) | |
| Exon 2 | 5′-GATAGCATCCCAGTCAGCA |
| Exon 3 | 5′-TGAGTGAAGCCAGACTTGGT |
| Mouse Socs2 (212 bp) | |
| Exon 3 | 5′-GAATGGAGCGGACAGGAC |
| Exon 4 | 5′-ATCCTGTTTGACTGAG |
| Mouse Mrps17 (328 bp) | |
| Exon 2 | 5′-ATCCCCAGCAAGAAGCTTCGGAACA |
| Exon 3 | 5′-TATGGCATAACAGATTAAACAGCTC |
Socs2, suppressor of cytokine signaling 2; Mrps17, mitochondrial ribosomal protein S17; Igfals, insulin-like growth factor-binding protein acid labile subunit.
Table 3.
Primers used for qRT-PCR of nuclear RNA
| Gene (Amplicon) | DNA Sequence |
|---|---|
| Human IGF-I (263 bp) | |
| Exon 1 | 5′-TTGCTAAATCTCACTGTCACTGC |
| Intron 1 | 5′-ACTTCAAAGAGTAAGAAATATTTAC |
| Human IGF-I (69 bp) | |
| Exon 2 | 5′-TGGATGCTCTTCAGTTCGTG |
| Intron 2 | 5′-ACATTGAGAGGGAGGGCTACTTAC |
| Human IGF-I (192 bp) | |
| Exon 3 | 5′-GATGCTCTTCAGTTCGTGTGT |
| Intron 3 | 5′-GCTTACTTTAAGGTGAGGAAT |
| Mouse Igfals (189 bp) | |
| Exon 2 | 5′-GATAGCATCCCAGTCAGCA |
| Intron 2 | 5′-GTTCCGTTCCAGGTGCAGAT |
| Mouse Socs2 (122 bp) | |
| Exon 2 | 5′-GAATGGAGCGGACAGGAC |
| Intron 2 | 5′-CCAGCTCCCTACCTGTTTGA |
| Mouse Mrps17 (114 bp) | |
| Exon 2 | 5′-AGGAGCGAGACCCCACTAAC |
| Intron 3 | 5′-CAGCTTTCCGGCCACTTAC |
qRT-PCR, quantitative real-time PCR.
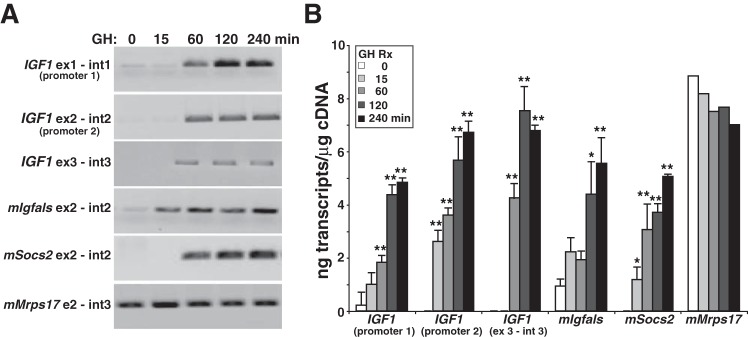
Fig. 3.
Rapid induction of IGF1 gene transcription by GH and STAT5b in a human IGF-I BAC-containing cell line. A: results on an individual experiment showing time course of appearance and accumulation of nascent transcripts for the human IGF1 gene and for several mouse genes, as measured by RT-PCR using nuclear RNA isolated at different times after the addition of GH to clone 3 cells. B: quantitative analysis of accumulation of nascent transcripts over time from human IGF1 promoters 1 and 2, from exon 3 and intron 3, and from mouse (m) Igfals, Socs2, and Mrps17, which were measured after addition of GH to clone 3 cells by quantitative RT-PCR (means ± SD of n = 4 independent experiments). *P < 0.01, **P < 0.001 vs. time 0. Primer sets are listed in Table 3.
Immunobloting and immunocytochemistry.
Whole cell protein lysates were prepared as described (47). Protein samples (15 μg/lane) were separated by SDS-PAGE, transferred to Immobilon-FL membranes, blocked with 50% AquaBlock solution for 1 h, and incubated with primary and secondary antibodies (47). Primary antibodies were used at 1:2,000 (anti-Flag M2, anti-phospho-STAT5) or 1:15,000 dilutions (anti-α-tubulin), and secondary antibodies were used at 1:5,000. Results were visualized and images captured using the LiCoR Odyssey and version 3.0 analytical software (Lincoln, NE). For immunocytochemistry, cells were fixed, made permeable, blocked, and incubated in buffer with primary antibodies (anti-Flag M2, 1:2,000 dilution) for 18 h and then with secondary antibodies (goat anti-mouse IgG1-Alexa 488, 1:2,000) for 1 h (47). Nuclei were stained with Hoechst 33258 dye. Images were captured using a Nikon DS-Qi1Mc camera attached to a Nikon Eclipse Ti-U inverted microscope using NIS element 3.1 software. Image analysis was performed with the same software.
Quantitative chromatin immunoprecipitation assays.
For each time point, 1 × 107 nuclei were incubated on a rotating platform in 500 μl of DMEM with 1% formaldehyde for 2 min, followed by the addition of 250 μl of 1 M glycine and incubation for an additional 1 min. After centrifugation at 200 g for 10 min at 20°C, the pellet was washed in PBS and suspended in 350 μl of digestion buffer (plus protease inhibitors) for 5 min at 37°C. Micrococcal nuclease was added to a final concentration of 200 U/ml and incubated for 10 min at 37°C, and sheared chromatin was extracted as described (2, 12, 13). DNA was isolated from an aliquot, and the sheared chromatin was adjusted to 1 mg/ml DNA in immunoprecipitation (IP) buffer (50 mM Tris·HCl, 5 mM EDTA, 150 mM NaCl, 0.1% SDS, and 1% Triton X-100, pH 8.1, plus protease inhibitors). Chromatin was stored in 100-μl aliquots at −80°C until use. IPs were performed with sheared chromatin containing ∼100 μg of DNA and 2 μg of the anti-STAT5 antibody as described (2, 12, 13). DNA was extracted using the QiaQuick PCR purification kit (DNA fragments of average size of 500 ± 100 bp were generated) and analyzed for purity and concentration using a 2100 Bio-analyzer (Agilent Technologies, Santa Clara, CA). DNA was used as template in qPCR reactions with the primer pairs listed in Table 4. PCR assays were performed with a Bio-Rad Chromo4 Real-Time PCR detection system. Reactions (20 μl volume) contained 1× SYBR Green mix, 200 nM primers, and chromatin immunoprecipitation (ChIP)-enriched DNA and were performed in eight-well strips in a 96-well format. Standard curves (with 0.01–1.25 ng of genomic DNA) were included in each experiment for each primer pair. Values are expressed as percent input necessary to achieve the identical cycle threshold. Results are the mean ± SD of three or more independent qPCR experiments from independent IPs.
Table 4.
DNA sequences of oligonucleotide primers for ChIP assays
| Element | Top Strand (5′ to 3′) | Bottom Strand (5′ to 3′) | Amplicon, bp |
|---|---|---|---|
| H8 9 | GCCAAACACAGATGAGTAGTTGG | GACAGAAATAGCTTTTCTGC | 100 |
| H17 | TTTACAGATCCAGTGGTAGCTC | TGGAAAGAAGGATAGATTCAATG | 120 |
| H60 | CATGCAGGTGGCCATCAGA | CATCTTTCTGCTATTCCTAG | 89 |
| H75–76 | GTAACCATGGATAGGTCCTGTGA | GGCCTTTTGGCCTTCTGGCCAA | 114 |
| H77–78 | ATCGATGCCTAAATTTGATAGG | CTTTGGCATCTTAAAAGTGA | 98 |
| Mouse Socs2 | GCTGGCGGGGGCTGCGGTCA | AGCCGAGTGTGCGCGCGACT | 145 |
| Mouse Igfals | AGCTATGATGACTACACAGAT | CCACAGAGCCCTGGTGCTGAC | 99 |
| Mouse Mrps17 | GAATACAGCTGTGATAGGGCATG | GCTACATAGAGTGACCCT | 115 |
ChIP, chromatin immunoprecipitation.
Promoter-reporter assays.
Expression plasmids in pcDNA3 for mouse GH receptor and flag epitope-tagged wild-type rat STAT5b have been described previously (67, 75, 76). A reporter gene plasmid was constructed in pGL3-basic containing a 121-base pair segment of human IGF1 promoter 2 cloned into 5′ BglII and 3′ HindIII sites of the vector [IGF1 promoter 2-luciferase (P2-Luc)]. To generate this promoter DNA, the following oligonucleotide primers were used to amplify human genomic DNA: 5′-ATagatctCCAAATGTAACTAGATGCTTT-3′ (BglII site underlined) and 5′-ATaagcttCCAGGTCTTTTACAGCAGGTC-3′ (HindIII site underlined). Additional reporter gene plasmids were generated by cloning 5′ to IGF1 P2-Luc individual DNA segments amplified by PCR from human genomic DNA that spanned STAT5b-binding domains from the IGF1 locus (see Table 5 for coordinates). Cos-7 cells (ATCC NO. CRL-1651) in 12-well dishes were incubated in antibiotic-free DMEM with 10% fetal bovine serum at 37°C in humidified air with 5% CO2 and cotransfected with mouse GH receptor (25 ng), STAT5b (100 ng), and individual promoter-reporter plasmids (187.5 ng) as described (67). Twenty-four hours later, cells were incubated for 16–18 h in SFM and 1% bovine serum albumin ± rat GH (40 nM). Cells were then harvested and lysates used for luciferase assays (67). Results were normalized to total cellular protein concentrations.
Table 5.
DNA sequences of oligonucleotide primers for promoter-reporter studies
| Element | Size, bp | Genomic Location (5′ End) | Top Strand (5′ to 3′)* | Bottom Strand (5′ to 3′)* |
|---|---|---|---|---|
| H17 | 297 | 102.548.544 | ATBTCTAGGCCACCCCTTGCTGAT | ATSAAGTTCATTTTGACAGAGTTTGAA |
| H75–76 | 258 | 102.429.729 | ATBGACATTAGGTCTCACATTGCGT | ATSGAGAAATCATACCCTTTCTTATTT |
| H77–78 | 327 | 102.425.157 | ATBATGGGGATTTAGTAGCTGAGA | ATSCCTTATACTTTGCTAGGAGAC |
B = GAGCTC (SacI restriction enzyme site); S = CCCGGG (SmaI site).
Statistical analysis.
Data are presented as means ± SD. Paired and unpaired Student's t-tests were performed using GraphPad Prism 5.0, with Bonferonni corrections used for multiple comparisons.
RESULTS
Developing cells with a chromosomally integrated transgene containing the human IGF-I locus.
IGF-I plays a central role in controlling somatic growth in children (54) and is important in tissue regeneration, aging, and disease pathogenesis in adults (9, 21, 52, 73), yet there are no robust experimental systems available to study regulation of the human IGF-I gene or protein expression. Of the few cell lines or primary cells that produce measureable amounts of IGF-I, very few respond even minimally to GH or to other factors essential for the production of IGF-I in vivo (42, 44). As a consequence, despite long-standing study, progress has been minimal in defining the mechanisms responsible for controlling human IGF-I biosynthesis. Since we recently developed recombinant cell lines in which the rat Igf1 locus was integrated in chromatin in cultured cells and responded rapidly and potently to GH (2), we now have attempted to develop analogous cell lines for analysis of human IGF-I gene regulation. To generate a transgene containing the human IGF1 locus, we first modified the bacterial artificial chromosome BAC CH17-186M10 by incorporating a selectable marker for neomycin resistance, encoded by pGK-Neo, into a position ∼12.5 kb downstream from the 3′ end of IGF1 (Fig. 1A). CH17-186M10 contains the 80-kb six-exon human IGF-I gene and ∼99 kb of the 5′ and ∼52 kb of the 3′ flanking DNA (Fig. 1A). The transgene was incorporated into mouse C3H10T1/2 cell chromatin after selection with G418, followed by isolation of individual clones, expansion of cells, and testing for an intact human IGF1 locus by PCR (Fig. 1B). Six of 10 cell lines screened had complete BAC inserts (Fig. 1B), of which four had a single copy number per haploid genome (Fig. 1C). All six lines showed an increase in IGF-I mRNA after cells were treated with GH (data not shown). We selected clones 3 and 50 for further analysis.
Control of IGF-I gene expression by GH.
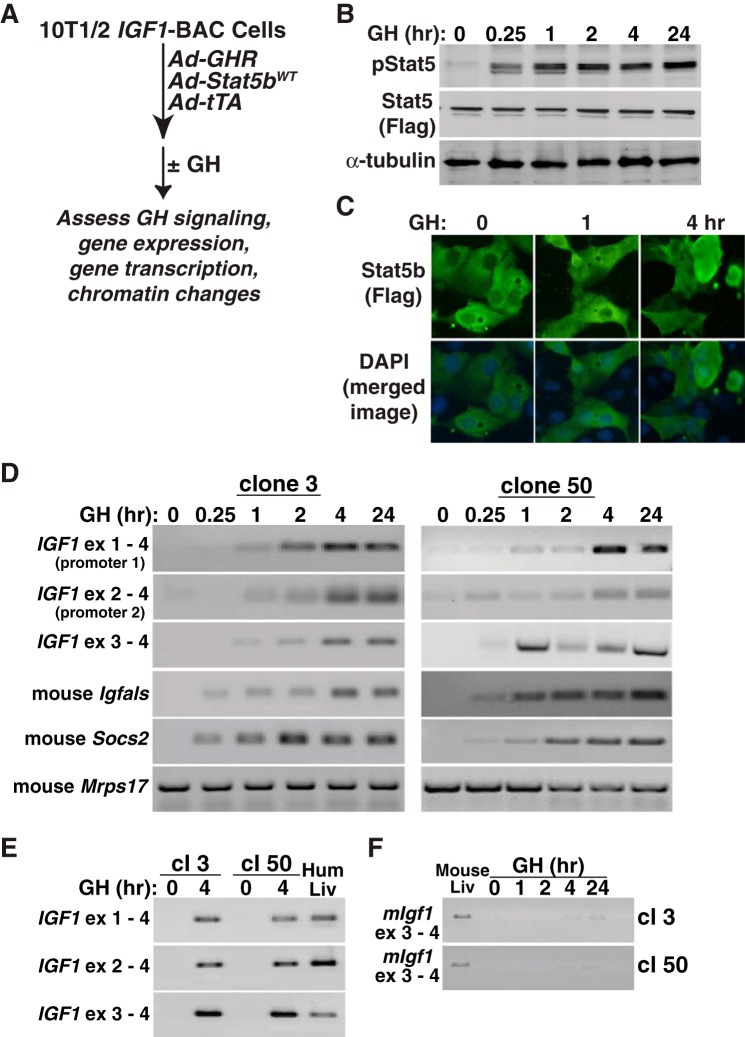
To test hormonal regulation of IGF1 gene expression, we first delivered the mouse GH receptor and rat STAT5b to cells by acute adenoviral transduction and showed that incubation with hormone (40 nM) created a GH-responsive system in which STAT5b was rapidly tyrosine phosphorylated and translocated to the nucleus (Fig. 2, A–C). Under these conditions, GH also rapidly induced expression of mRNAs from endogenous mouse insulin-like growth factor-binding protein acid labile subunit (Igfals) and suppressor of cytokine signaling 2 (Socs2) genes but had no effect on Mrps17 (Fig. 2D). In the same cells, GH treatment stimulated accumulation of human IGF-I mRNAs with kinetics of production similar to mouse Igfals and Socs2 transcripts (Fig. 2D). IGF-I mRNA was first detected within 60 min of exposure to GH and was sustained for ≤24 h (Fig. 2D). Incubation with GH for 4 h led to accumulation of IGF1 transcripts that were comparable with levels detected in human liver RNA (Fig. 2E). In contrast to results observed with mouse Igfals and Socs2, mouse Igf1 gene expression was minimal, even in cells incubated with GH for ≤24 h (Fig. 2F). Thus, under the conditions of our experiments, the integrated human IGF1 gene is functional in mouse C3H10T1/2 cells and is activated by GH, whereas the endogenous mouse Igf1 gene is nearly transcriptionally inert. These results indicate that the human IGF-I locus resides in an open chromatin environment in the recombinant cell lines, whereas the endogenous mouse Igf1 locus appears to be in closed chromatin.
Fig. 2.
Rapid induction of human IGF1 gene expression by GH in a BAC-containing cell line. A: experimental plan outlining steps to test IGF1 gene regulation in response to GH and STAT5b in C3H10T1/2 IGF1 BAC clones. B: induction of tyrosine-phosphorylated STAT5b (p-STAT5), total STAT5b (using antibody to Flag), and α-tubulin, as assessed by immunoblotting after addition of recombinant rat GH (40 nM) to clone 3 cells (similar results were seen with clone 50). C: addition of GH causes accumulation of STAT5b in the nucleus of IGF1 BAC clone 3, as detected by immunocytochemistry. Hoescht dye was used to stain nuclei (similar results were seen with clone 50). D: time course of accumulation of human IGF-I mRNAs and mouse Igfals, Socs2, and mitochondrial ribosomal protein S17 (Mrps17) transcripts, as assessed by RT-PCR using whole cell RNA isolated at different times after GH addition to clones 3 and 50. E: appearance of IGF-I mRNAs in clones (cl) 3 and 50, as assessed by RT-PCR using RNA isolated prior to or 4 h after GH addition to cells. Human liver RNA serves as a positive control. F: minimal expression of mouse Igf1 mRNA after GH treatment in recombinant cell lines 3 or 50. Mouse liver RNA serves as a positive control. Primer pairs in D–F are listed in Table 2. DAPI, 4,6-diamidino-2-phenylindole.
Rapid stimulation of human IGF-I gene transcription by GH.
Having identified recombinant cell lines with regulated expression of human IGF-I mRNA, we next focused on quantifying the response to GH at the level of transcriptional activation, since GH has been shown to stimulate IGF-I gene transcription in vivo, at least in rodents (6, 41, 74, 75) and other mammalian species (19, 70). We performed a series of time course studies, harvesting nuclear RNA from 15 to 240 min after exposure of cells to GH (Fig. 3). Under these conditions, nascent nuclear IGF-I transcripts could be detected in nuclear RNA by quantitative RT-PCR within 15 min of hormone treatment (Fig. 3B). In addition, levels increased for ≤4 h (Fig. 3B), indicating that the transcriptional response to GH was both rapid and sustained.
Tandem promoters govern human IGF1 gene expression, and each promoter controls a unique 5′ exon (Fig. 1A and Refs. 55 and 61). As seen in Fig. 2D, IGF-I mRNAs containing each promoter-specific 5′ exon increased in abundance after exposure of cells to GH. A more direct assessment of dual promoter usage is shown in Fig. 3B, which illustrates that the kinetics of IGF1 gene transcription in response to GH from each promoter is equivalent. Transcription was minimal in the absence of GH but rose from both promoters five- to 15-fold within 15 min after hormone addition to cell culture medium and 10- to 25-fold by 60 min, with promoter 2 showing higher relative stimulation than promoter 1 (Fig. 3B). Transcriptional activation of the human IGF-I transgene was comparable with induction of endogenous mouse Igfals and Socs2 genes, and at 4 h after GH administration, the level of nascent human IGF1 gene transcripts directed by each promoter was equivalent to mouse Mrps17, a highly expressed gene that is not regulated by GH (Fig. 3B). The results in Fig. 3 thus show that human IGF1 gene transcription is rapidly and robustly induced by GH in transgenic cells and support the idea that we have developed a useful model system to dissect mechanisms of IGF1 gene regulation by GH.
Identifying potential GH-activated transcriptional enhancers in the human IGF-I locus.
In previous studies, using a combined approach involving bioinformatics and biochemical and molecular analyses, we identified seven regions in the rat Igf1 locus that possessed epigenetic properties associated with transcriptional enhancers (12). Each of these DNA elements was shown to bind STAT5b in rat liver chromatin in response to GH-activated signals and was found to bind several transcriptional cofactors (12). We also demonstrated that each DNA segment functioned as a GH-activated transcriptional regulator in promoter-reporter assays in cultured cells and that this activity required intact STAT5b-binding sequences (12, 67).
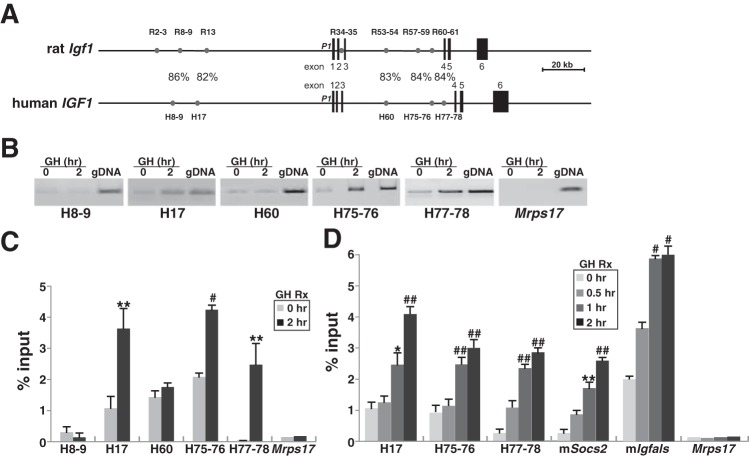
Comparison of these seven putative enhancer elements in the rat Igf1 gene with the human genome revealed that five were conserved (82–86% DNA sequence identity, including putative STAT5-binding sites; Fig. 4A), and were present in analogous positions within the human IGF1 locus (Fig. 4A). Based on these results, we next tested each human DNA element for its ability to bind STAT5b in a GH-regulated way in chromatin. By quantitative ChIP assays, we found that STAT5b was present at low levels at each segment in the absence of GH but was rapidly increased in abundance by GH at three regions, H17, H75-76, and H77-78 (Fig. 4, B–D). In contrast, little inducible binding of STAT5b was observed at H8-9 and H60 despite a similar degree of DNA sequence conservation, as was observed for H17, H75-76, and H77-78 (Fig. 4A). These results indicate that DNA sequence similarity is not the only parameter that determines conservation of function. Also, no binding was seen at a segment of mouse Mrps17, a negative control region for ChIP. The rate and extent of binding of STAT5b to chromatin at H17, H75-76, and H77-78 was comparable with what was detected at the STAT5b-binding elements of mouse Igfals and Socs2 (Fig. 4D), which reside in the proximal promoter regions of their respective genes (11). Taken together, these results support the idea that these three STAT5b-binding elements might be transcriptional enhancers for the human IGF1 gene.
Fig. 4.
GH induces recruitment of STAT5b to potential binding elements in chromatin within the human IGF-I locus. A: comparison of rat Igf1 and human IGF1 loci. Depicted in addition to the genes are 7 previously identified GH-regulated STAT5b-binding elements in the rat Igf1 locus and 5 analogous regions in the human IGF1 locus (gray circles) and a scale bar. %Identity for each element between species is shown. B–D: results of chromatin immunoprecipitation experiments using chromatin harvested from C310T1/2 human IGF1 BAC clone 3 after addition to cells of recombinant rat GH (40 nM) for 0, 0.5, 1, or 2 h are depicted. Primer pairs are listed in Table 4. B: results from 1 experiment of binding of STAT5b to DNA in chromatin at the 5 conserved putative STAT5-binding elements depicted in A plus a control primer pair from mouse Mrps17 in cells prior to and 2 h after GH addition. C: results of binding of STAT5b to DNA in chromatin at the 5 conserved putative STAT5-binding elements seen in A plus a control primer pair from mouse Mrps17 in cells before and 2 h after GH addition (means ± SD of 4 independent experiments: **P < 0.02 and #P < 0.01 vs. time 0). D: time course of binding after GH treatment of STAT5b to DNA in chromatin at the 5 conserved putative STAT5-binding elements shown in A plus the control primer pair from mouse Mrps17 (means ± SD of 4 independent experiments: **P < 0.02, #P < 0.01, and ##P < 0.005 vs. time 0).
In an effort to broaden identification of potential GH-regulated enhancers in the human IGF1 locus, we performed a series of STAT5b ChIP-DNA sequencing experiments using chromatin-isolated from cells before and 1 and 2 h after GH exposure. Results did not lead to additional elements being found (data not shown). Thus, under the conditions of our studies, there are at least three putative GH-regulated STAT5b-binding DNA elements in human IGF1 chromatin.
Functional properties of STAT5b-binding sequences in the human IGF-I locus.
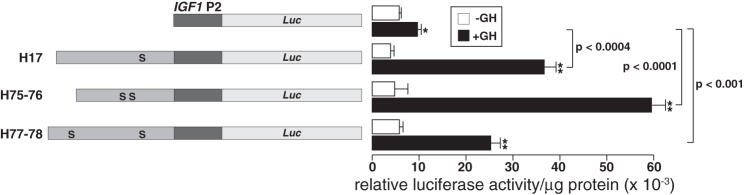
We next tested the three identified inducible STAT5b binding segments by examining their ability to stimulate human IGF1 promoter 2 (P2) in response to GH in reconstitution experiments in transiently transfected cells (Fig. 5). Each DNA sequence was able to enhance the activity of IGF1 P2 five- to 12-fold in cells coexpressing the GH receptor and STAT5b and incubated with GH. In contrast, human IGF1 P2 showed a <50% response to GH. Thus, these results show that each element has the potential to function as a GH-activated transcriptional enhancer.
Fig. 5.
Transcriptional activation by GH and STAT5b of human IGF1 STAT5-binding elements. Left: schematic of human IGF1 promoter 2 (P2)-exon 2 fused to a luciferase (Luc) gene (top) and diagrams of human STAT5-binding elements H17, H75–76, or H77–78 cloned 5′ to the IGF1 P2-exon 2 luciferase fusion gene (bottom). Each S represents the location of a STAT5 binding sequence. Right: results of luciferase assays in Cos-7 cells transiently transfected with individual human IGF1 P2-reporter plasmids and with expression plasmids encoding the mouse GH receptor and rat STAT5b and incubated with vehicle or GH (40 nM) for 16–18 h. Results are expressed as relative luciferase enzymatic activity/μg protein in cells treated with GH and represent the mean ± SD of 4 independent experiments, each performed in duplicate. Raw luciferase values for the IGF1 P2-reporter plasmid ranged from 3,200 to 5,800 light units/10 s in the absence of GH and from 7,800 to 61,000 light units/10 s in cells incubated with GH (*P < 0.05 and **P < 0.005 vs. no GH).
DISCUSSION
Inactivating mutations in several genes within the GH-IGF-I axis have unambiguously defined central roles for GH and IGF-I in postnatal somatic growth in children (reviewed in Refs. 20 and 54) and in sustaining bone strength, maintaining muscle mass, and minimizing fat mass in adults (40, 73). Molecular genetic studies in experimental animals and biochemical analyses in cultured cells have further established that GH is a key regulator of IGF-I expression (10) and that it potently activates IGF-I gene transcription, leading to production of IGF-I mRNAs and protein (10, 56). Despite these advances and other observations showing that the transcription factor STAT5b is a critical mediator of GH-stimulated IGF-I gene activity in several species (6, 19, 41, 74, 75) and is essential for GH- and IGF-I-mediated somatic growth in humans (20, 30, 39, 59, 66), the mechanisms by which GH controls human IGF1 gene expression have remained largely unexplored, primarily because no experimental models have been available to evaluate this problem in any detail. Here, we have developed a robust system to study human IGF1 gene regulation by incorporating the chromosomal IGF1 locus into cultured mouse C3H10T1/2 cells (Fig. 1). In the presence of the GH receptor and STAT5b, we saw rapid induction of endogenous GH- and STAT5b-dependent genes, including mouse Socs2 and Igfals, and equivalent activation of human IGF1 (Figs. 2 and 3), with the magnitude of IGF1 gene expression being comparable with what is detected in human liver (Fig. 2). Using this experimental system, we have identified a cohort of GH-regulated STAT5b-binding chromatin elements within the IGF1 locus (Fig. 4) and have shown that these DNA segments can each mediate GH- and STAT5b-stimulated gene activity when fused to a human IGF1 promoter (Fig. 5). Collectively, our results show that GH activates human IGF1 gene transcription and present a novel experimental platform that now may be used to determine how human IGF1 gene expression is controlled under different physiological and pathological conditions.
Although the experimental model described here may be considered artificial, as it is composed of components from two different species, its utility may be related in part to some distinctive features of mouse C3H10T1/2 cells (53). In contrast, transduction of other cell lines did not lead to clones containing intact BACs and thus did not produce any lines with human IGF1 gene expression (results not shown). Another component of our initial success may be secondary to the transient delivery of the GH receptor and STAT5b, although little IGF1 gene expression was observed until after cells were incubated with physiological concentrations of GH (Figs. 2 and 3). Despite these caveats, our results present an opportunity to gain new insights into the biology of GH-regulated enhancer function and provide a way to study an important human gene that cannot be addressed in any detail in vivo.
IGF-I gene regulation and speciation.
The observations made in this article highlight some potentially important initial differences between rodent Igf1 and human IGF1 loci. Although the overall topology of both genes is very similar, as each is composed of six exons subdivided by five introns and is controlled by dual promoters regulating distinct leader exons (55, 61), GH controls rat and mouse Igf1 gene transcription through the actions of up to seven dispersed STAT5b-binding elements (2, 12, 41). In the human, only five of these seven regions could be detected by DNA sequence conservation (Fig. 1), and in our studies only three were able to bind STAT5b in chromatin in a GH-dependent way (Fig. 4). Although these results may be considered preliminary and, unlike in the rat or mouse (12, 41), have not been confirmed in vivo, the potential decline in the number of GH response elements may reflect important physiological differences between species. As a parallel example, loss of an enhancer sequence during speciation may account for changes in developmental expression of the human GDF6 gene, which encodes a member of the BMP family (32). Its new pattern of gene expression may be responsible for the alterations in bone size and shape in human feet that are necessary for bipedalism (32). From the perspective of IGF-I, statural growth ends in humans with the completion of puberty, as the epiphyses of long bone close (4, 54). In contrast, in rats somatic growth persists throughout life, although it slows in the adult compared with the juvenile (37). Thus, reduction in the number of GH-stimulated transcriptional enhancers in the human IGF1 locus may reflect a necessary adaptation in the complexity of GH-mediated gene activation during human evolution, a hypothesis that clearly will require active testing and analysis of other mammalian species to confirm or refute.
Testing other pathways potentially involved in IGF-I gene regulation.
IGF-I is produced in a variety of tissues (14, 24, 29, 45) and is under control of other signaling molecules in addition to GH (36, 57). For example, previous studies had shown that IGF-I gene expression could be increased by some steroid hormones, including androgens (36, 57) and estrogens (26), but reduced by glucocortoids (18), although the supporting evidence in human studies has been limited to either induction of IGF-I mRNAs by estradiol (23, 79) or stimulation of promoter-reporter activity by testosterone (77). Now it is possible to test directly how these transcriptional activators control IGF-I gene expression and to determine the locations and functions of putative estrogen, androgen, and glucocorticoid response elements in chromatin in the human IGF1 locus. Moreover, because several hormones using signaling pathways involving G protein-coupled cyclic AMP production also stimulate IGF-I gene expression (7, 25, 27), their mechanisms of action may be elucidated as well.
IGF-I is produced in different lineages derived from mesenchymal progenitors, including skeletal muscle, bone, cartilage, and fat (reviewed in Ref. 10). Since C3H10T1/2 cells can be converted readily to each of these cell types (17, 62, 69), our model also offers the opportunity to identify mechanisms of cell type-specific transcriptional control of IGF-I biosynthesis in a chromosomal context.
The liver is the organ with the highest levels of IGF-I gene and protein expression (14, 24, 29, 45). In previous studies, it was found that several liver-enriched transcription factors, including C/EBPα, C/EBPβ, HNF-1α, and HNF-3β, could enhance the activity of cotransfected human IGF1 promoter 1 (48–50). It will now be possible to determine whether these proteins can promote IGF1 gene expression in chromatin and also to learn whether these regulatory molecules might control transcription from IGF1 promoter 2.
DNA sequence polymorphisms and IGF-I gene regulation.
Each individual human genome contains multiple DNA sequence polymorphisms (51), many of which have the potential to alter gene expression, as they reside within promoters, enhancers, or other regulatory elements (1). A single nucleotide polymorphism (SNP) in human IGF1 promoter 1 has been shown to modify both basal activity and responses to cyclic AMP, although this has been tested only in promoter-reporter assays in transiently transfected cells (64). The three functional STAT5b-binding elements identified in this report each reside within chromosomal regions that encode multiple SNPs (19a), with one being located within a STAT5b-binding site in domain H77-78. It will now be possible to determine what influence this latter single nucleotide change has on binding of STAT5b to the region and on GH-activated IGF1 gene transcription. Functional consequences of SNPs in other elements may be tested similarly, as may additional DNA sequence alterations when found in individuals with growth deficiency syndromes.
Human evolution and the genetic control of height.
Statural growth in children reflects a complex combinatorial interplay of genetic modifiers and environmental influences, including effects of nutritional intake, acute and chronic disease, and other factors (4). Multiple classes of genes, in addition to components of the GH-IGF-I system and other hormones and growth factors, influence growth rates and final adult height (4), with all ultimately acting on the cartilage of the epiphyseal growth plate at the ends of long bones (4). During recent human evolutionary history, there were many interactions among different populations, such that non-Africans contain ∼2% of Neanderthal DNA and variable amounts of genetic admixture from Denisovans (22, 34, 68). Some of this introgressed DNA clearly influences traits in modern humans, such as hair color, skin pigmentation, and disease risk (68). It is thus conceivable that Neanderthal- or Denisovan-based alterations within the human IGF1 locus at GH response elements or at other locations could modify the response to GH or to other trophic factors and that this influences production of IGF-I and ultimately growth rates. We are now in the position to test such hypotheses.
Final comments.
The pivotal roles of GH and IGF-I in human physiology and disease make it understandable that GH-regulated IGF-I gene expression is complicated and multifaceted and provide a plausible explanation for the existence of several STAT5b-binding elements within the human IGF1 locus. The precise roles of these elements as potential transcriptional enhancers may be broad and dynamic, as they could each respond independently to varying GH doses or exposures, and yet they could function collectively as a GH dose-dependent transcriptional rheostat. Alternatively, individual STAT5b-binding modules could act as “negative enhancers” to limit the access of GH-stimulated STAT5b to more positive elements and thus provide a feedback mechanism to reduce the overall stimulatory effects of GH on IGF1 gene transcription. We can now test these and other hypotheses in our model and begin to determine how GH and other factors regulate human IGF-I gene expression.
GRANTS
These studies were supported by National Institute of Diabetes and Digestive and Kidney Diseases Research Grant R01-DK-069703-08 (to P. Rotwein).
DISCLOSURES
The authors declare that they have no conflicts of interest pertinent to the contents of this article.
AUTHOR CONTRIBUTIONS
A.M., D.A., and P.R. conception and design of research; A.M. and D.A. performed experiments; A.M., D.A., and P.R. analyzed data; A.M., D.A., and P.R. interpreted results of experiments; A.M., D.A., and P.R. edited and revised manuscript; A.M., D.A., and P.R. approved final version of manuscript; P.R. prepared figures; P.R. drafted manuscript.
REFERENCES
- 1.Albert FW, Kruglyak L. The role of regulatory variation in complex traits and disease. Nat Rev Genet 16: 197–212, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Alzhanov D, Mukherjee A, Rotwein P. Identifying growth hormone-regulated enhancers in the Igf1 locus. Physiol Genomics 47: 559–568, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alzhanov DT, McInerney SF, Rotwein P. Long-range interactions regulate Igf2 gene transcription during skeletal muscle differentiation. J Biol Chem 285: 38969–38977, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron J, Savendahl L, De Luca F, Dauber A, Phillip M, Wit JM, Nilsson O. Short and tall stature: a new paradigm emerges. Nat Rev Endocrinol 11: 735–746, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berryman DE, Christiansen JS, Johannsson G, Thorner MO, Kopchick JJ. Role of the GH/IGF-1 axis in lifespan and healthspan: lessons from animal models. Growth Horm IGF Res 18: 455–471, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bichell DP, Kikuchi K, Rotwein P. Growth hormone rapidly activates insulin-like growth factor I gene transcription in vivo. Mol Endocrinol 6: 1899–1908, 1992. [DOI] [PubMed] [Google Scholar]
- 7.Bichell DP, Rotwein P, McCarthy TL. Prostaglandin E2 rapidly stimulates insulin-like growth factor-I gene expression in primary rat osteoblast cultures: evidence for transcriptional control. Endocrinology 133: 1020–1028, 1993. [DOI] [PubMed] [Google Scholar]
- 8.Brooks AJ, Dai W, O'Mara ML, Abankwa D, Chhabra Y, Pelekanos RA, Gardon O, Tunny KA, Blucher KM, Morton CJ, Parker MW, Sierecki E, Gambin Y, Gomez GA, Alexandrov K, Wilson IA, Doxastakis M, Mark AE, Waters MJ. Mechanism of activation of protein kinase JAK2 by the growth hormone receptor. Science 344: 1249783, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Brooks AJ, Waters MJ. The growth hormone receptor: mechanism of activation and clinical implications. Nat Rev Endocrinol 6: 515–525, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Chia DJ. Minireview: mechanisms of growth hormone-mediated gene regulation. Mol Endocrinol 28: 1012–1025, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chia DJ, Rotwein P. Defining the epigenetic actions of growth hormone: acute chromatin changes accompany GH-activated gene transcription. Mol Endocrinol 24: 2038–2049, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chia DJ, Varco-Merth B, Rotwein P. Dispersed Chromosomal Stat5b-binding elements mediate growth hormone-activated insulin-like growth factor-I gene transcription. J Biol Chem 285: 17636–17647, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chia DJ, Young JJ, Mertens AR, Rotwein P. Distinct alterations in chromatin organization of the two IGF-I promoters precede growth hormone-induced activation of IGF-I gene transcription. Mol Endocrinol 24: 779–789, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Ercole AJ, Stiles AD, Underwood LE. Tissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proc Natl Acad Sci USA 81: 935–939, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'haene B, Vandesompele J, Hellemans J. Accurate and objective copy number profiling using real-time quantitative PCR. Methods 50: 262–270, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Daughaday WH, Rotwein P. Insulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr Rev 10: 68–91, 1989. [DOI] [PubMed] [Google Scholar]
- 17.Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell 51: 987–1000, 1987. [DOI] [PubMed] [Google Scholar]
- 18.Delany AM, Durant D, Canalis E. Glucocorticoid suppression of IGF I transcription in osteoblasts. Mol Endocrinol 15: 1781–1789, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Eleswarapu S, Gu Z, Jiang H. Growth hormone regulation of insulin-like growth factor-I gene expression may be mediated by multiple distal signal transducer and activator of transcription 5 binding sites. Endocrinology 149: 2230–2240, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a.Ensembl. Ensemble Release 83 (Online). http://useast.ensembl.org/index.html [Dec 2015]. [Google Scholar]
- 20.Feigerlova E, Hwa V, Derr MA, Rosenfeld RG. Current issues on molecular diagnosis of GH signaling defects. Endocr Dev 24: 118–127, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Gallagher EJ, LeRoith D. Minireview: IGF, Insulin, and Cancer. Endocrinology 152: 2546–2551, 2011. [DOI] [PubMed] [Google Scholar]
- 22.Gallego Llorente M, Jones ER, Eriksson A, Siska V, Arthur KW, Arthur JW, Curtis MC, Stock JT, Coltorti M, Pieruccini P, Stretton S, Brock F, Higham T, Park Y, Hofreiter M, Bradley DG, Bhak J, Pinhasi R, Manica A. Ancient Ethiopian genome reveals extensive Eurasian admixture throughout the African continent. Science 350: 820–822, 2015. [DOI] [PubMed] [Google Scholar]
- 23.Giudice LC, Dsupin BA, Jin IH, Vu TH, Hoffman AR. Differential expression of messenger ribonucleic acids encoding insulin-like growth factors and their receptors in human uterine endometrium and decidua. J Clin Endocrinol Metab 76: 1115–1122, 1993. [DOI] [PubMed] [Google Scholar]
- 24.Hall LJ, Kajimoto Y, Bichell D, Kim SW, James PL, Counts D, Nixon LJ, Tobin G, Rotwein P. Functional analysis of the rat insulin-like growth factor I gene and identification of an IGF-I gene promoter. DNA Cell Biol 11: 301–313, 1992. [DOI] [PubMed] [Google Scholar]
- 25.Hatey F, Langlois I, Mulsant P, Bonnet A, Benne F, Gasser F. Gonadotropins induce accumulation of insulin-like growth factor I mRNA in pig granulosa cells in vitro. Mol Cell Endocrinol 86: 205–211, 1992. [DOI] [PubMed] [Google Scholar]
- 26.Hewitt SC, Li Y, Li L, Korach KS. Estrogen-mediated regulation of Igf1 transcription and uterine growth involves direct binding of estrogen receptor alpha to estrogen-responsive elements. J Biol Chem 285: 2676–2685, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofbauer LC, Rafferzeder M, Janssen OE, Gartner R. Insulin-like growth factor I messenger ribonucleic acid expression in porcine thyroid follicles is regulated by thyrotropin and iodine. Eur J Endocrinol 132: 605–610, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Horne HN, Sherman ME, Pfeiffer RM, Figueroa JD, Khodr ZG, Falk RT, Pollak M, Patel DA, Palakal MM, Linville L, Papathomas D, Geller B, Vacek PM, Weaver DL, Chicoine R, Shepherd J, Mahmoudzadeh AP, Wang J, Fan B, Malkov S, Herschorn S, Hewitt SM, Brinton LA, Gierach GL. Circulating insulin-like growth factor-I, insulin-like growth factor binding protein-3 and terminal duct lobular unit involution of the breast: a cross-sectional study of women with benign breast disease. Breast Cancer Res 18: 24, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoyt EC, Van Wyk JJ, Lund PK. Tissue and development specific regulation of a complex family of rat insulin-like growth factor I messenger ribonucleic acids. Mol Endocrinol 2: 1077–1086, 1988. [DOI] [PubMed] [Google Scholar]
- 30.Hwa V. STAT5B deficiency: Impacts on human growth and immunity. Growth Horm IGF Res 28: 16–20, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ibrahim YH, Yee D. Insulin-like growth factor-I and cancer risk. Growth Horm IGF Res 14: 261–269, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Indjeian VB, Kingman GA, Jones FC, Guenther CA, Grimwood J, Schmutz J, Myers RM, Kingsley DM. Evolving new skeletal traits by cis-regulatory changes in bone morphogenetic proteins. Cell 164: 45–56, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jansen E, Steenbergh PH, van Schaik FM, Sussenbach JS. The human IGF-I gene contains two cell type-specifically regulated promoters. Biochem Biophys Res Commun 187: 1219–1226, 1992. [DOI] [PubMed] [Google Scholar]
- 34.Jones ER, Gonzalez-Fortes G, Connell S, Siska V, Eriksson A, Martiniano R, McLaughlin RL, Gallego Llorente M, Cassidy LM, Gamba C, Meshveliani T, Bar-Yosef O, Muller W, Belfer-Cohen A, Matskevich Z, Jakeli N, Higham TF, Currat M, Lordkipanidze D, Hofreiter M, Manica A, Pinhasi R, Bradley DG. Upper Palaeolithic genomes reveal deep roots of modern Eurasians. Nat Commun 6: 8912, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joshi M, Keith Pittman H, Haisch C, Verbanac K. Real-time PCR to determine transgene copy number and to quantitate the biolocalization of adoptively transferred cells from EGFP-transgenic mice. Biotechniques 45: 247–258, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Kamanga-Sollo E, Pampusch MS, Xi G, White ME, Hathaway MR, Dayton WR. IGF-I mRNA levels in bovine satellite cell cultures: effects of fusion and anabolic steroid treatment. J Cell Physiol 201: 181–189, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Kilborn SH, Trudel G, Uhthoff H. Review of growth plate closure compared with age at sexual maturity and lifespan in laboratory animals. Contemp Top Lab Anim Sci 41: 21–26, 2002. [PubMed] [Google Scholar]
- 38.Kim SW, Lajara R, Rotwein P. Structure and function of a human insulin-like growth factor-I gene promoter. Mol Endocrinol 5: 1964–1972, 1991. [DOI] [PubMed] [Google Scholar]
- 39.Kofoed EM, Hwa V, Little B, Woods KA, Buckway CK, Tsubaki J, Pratt KL, Bezrodnik L, Jasper H, Tepper A, Heinrich JJ, Rosenfeld RG. Growth hormone insensitivity associated with a STAT5b mutation. N Engl J Med 349: 1139–1147, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Lanning NJ, Carter-Su C. Recent advances in growth hormone signaling. Rev Endocr Metab Disord 7: 225–235, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Laz EV, Sugathan A, Waxman DJ. Dynamic in vivo binding of STAT5 to growth hormone-regulated genes in intact rat liver. Sex-specific binding at low- but not high-affinity STAT5 sites. Mol Endocrinol 23: 1242–1254, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le Roith D, Bondy C, Yakar S, Liu JL, Butler A. The somatomedin hypothesis: 2001. Endocr Rev 22: 53–74, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Lee EC, Yu D, Martinez de Velasco J, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics 73: 56–65, 2001. [DOI] [PubMed] [Google Scholar]
- 44.LeRoith D. Clinical relevance of systemic and local IGF-I: lessons from animal models. Pediatr Endocrinol Rev 5, Suppl 2: 739–743, 2008. [PubMed] [Google Scholar]
- 45.Lowe WL Jr, Roberts CT Jr, Lasky SR, LeRoith D. Differential expression of alternative 5′ untranslated regions in mRNAs encoding rat insulin-like growth factor I. Proc Natl Acad Sci USA 84: 8946–8950, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mittanck DW, Kim SW, Rotwein P. Essential promoter elements are located within the 5′ untranslated region of human insulin-like growth factor-I exon I. Mol Cell Endocrinol 126: 153–163, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Mukherjee A, Wilson EM, Rotwein P. Selective signaling by Akt2 promotes bone morphogenetic protein 2-mediated osteoblast differentiation. Mol Cell Biol 30: 1018–1027, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nolten LA, Steenbergh PH, Sussenbach JS. Hepatocyte nuclear factor 1 alpha activates promoter 1 of the human insulin-like growth factor I gene via two distinct binding sites. Mol Endocrinol 9: 1488–1499, 1995. [DOI] [PubMed] [Google Scholar]
- 49.Nolten LA, Steenbergh PH, Sussenbach JS. The hepatocyte nuclear factor 3beta stimulates the transcription of the human insulin-like growth factor I gene in a direct and indirect manner. J Biol Chem 271: 31846–31854, 1996. [DOI] [PubMed] [Google Scholar]
- 50.Nolten LA, van Schaik FM, Steenbergh PH, Sussenbach JS. Expression of the insulin-like growth factor I gene is stimulated by the liver-enriched transcription factors C/EBP alpha and LAP. Mol Endocrinol 8: 1636–1645, 1994 [DOI] [PubMed] [Google Scholar]
- 51.Ott J, Wang J, Leal SM. Genetic linkage analysis in the age of whole-genome sequencing. Nat Rev Genet 16: 275–284, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer 12: 159–169, 2012. [DOI] [PubMed] [Google Scholar]
- 53.Reznikoff CA, Brankow DW, Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res 33: 3231–3238, 1973. [PubMed] [Google Scholar]
- 54.Rosenfeld RG, Hwa V. The growth hormone cascade and its role in mammalian growth. Horm Res 71, Suppl 2: 36–40, 2009. [DOI] [PubMed] [Google Scholar]
- 55.Rotwein P. Molecular biology of IGF-I and IGF-II. In: The IGF System, edited by Rosenfeld R, Roberts CJ. Totowa, NJ: Humana, 1999, p. 19–35. [Google Scholar]
- 56.Rotwein P. Mapping the growth hormone—Stat5b—IGF-I transcriptional circuit. Trends Endocrinol Metab 23: 186–193, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sahlin L, Norstedt G, Eriksson H. Androgen regulation of the insulin-like growth factor-I and the estrogen receptor in rat uterus and liver. J Steroid Biochem Mol Biol 51: 57–66, 1994. [DOI] [PubMed] [Google Scholar]
- 58.Salmon WD, Daughaday WH. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J Lab Clin Med 49: 825–836, 1957. [PubMed] [Google Scholar]
- 59.Scalco RC, Hwa V, Domene HM, Jasper HG, Belgorosky A, Marino R, Pereira AM, Tonelli CA, Wit JM, Rosenfeld RG, Jorge AA. STAT5B mutations in heterozygous state have negative impact on height: another clue in human stature heritability. Eur J Endocrinol 173: 291–296, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Steenbergh PH, Jansen E, van Schaik FM, Sussenbach JS. Functional analysis of the human IGF-I gene promoters. Mol Reprod Dev 35: 365–367, 1993. [DOI] [PubMed] [Google Scholar]
- 61.Sussenbach JS, Steenbergh PH, Jansen E, Holthuizen P, Meinsma D, van Dijk MA, Gloudemans T. Structural and regulatory aspects of the human genes encoding IGF-I and -II. Adv Exp Med Biol 293: 1–14, 1991. [DOI] [PubMed] [Google Scholar]
- 62.Tang QQ, Otto TC, Lane MD. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci USA 101: 9607–9611, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science 299: 1346–1351, 2003. [DOI] [PubMed] [Google Scholar]
- 64.Telgmann R, Dordelmann C, Brand E, Nicaud V, Hagedorn C, Pavenstadt H, Cambien F, Tiret L, Paul M, Brand-Herrmann SM. Molecular genetic analysis of a human insulin-like growth factor 1 promoter P1 variation. FASEB J 23: 1303–1313, 2009. [DOI] [PubMed] [Google Scholar]
- 65.Travis RC, Appleby PN, Martin RM, Holly JM, Albanes D, Black A, Bueno-de-Mesquita HB, Chan JM, Chen C, Chirlaque MD, Cook MB, Deschasaux M, Donovan JL, Ferrucci L, Galan P, Giles GG, Giovannucci EL, Gunter MJ, Habel LA, Hamdy FC, Helzlsouer KJ, Hercberg S, Hoover RN, Janssen JA, Kaaks R, Kubo T, Le Marchand L, Metter EJ, Mikami K, Morris JK, Neal DE, Neuhouser ML, Ozasa K, Palli D, Platz EA, Pollak MN, Price AJ, Roobol M, Schaefer C, Schenk JM, Severi G, Stampfer MJ, Stattin P, Tamakoshi A, Tangen CM, Touvier M, Wald NJ, Weiss NS, Zeigler RG, Key TJ, Allen NE; Endogenous Hormones, Nutritional Biomarkers and Prostate Cancer Collaborative Group. A Meta-analysis of Individual Participant Data Reveals an Association Between Circulating Levels of IGF-I and Prostate Cancer Risk. Cancer Res 76: 2288–2300, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Varco-Merth B, Feigerlova E, Shinde U, Rosenfeld RG, Hwa V, Rotwein P. Severe growth deficiency is associated with STAT5b mutations that disrupt protein folding and activity. Mol Endocrinol 27: 150–161, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Varco-Merth B, Mirza K, Alzhanov DT, Chia DJ, Rotwein P. Biochemical characterization of diverse Stat5b-binding enhancers that mediate growth hormone-activated insulin-like growth factor-I gene transcription. PLoS One 7: e50278, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vattathil S, Akey JM. Small Amounts of Archaic Admixture Provide Big Insights into Human History. Cell 163: 281–284, 2015. [DOI] [PubMed] [Google Scholar]
- 69.Wang EA, Israel DI, Kelly S, Luxenberg DP. Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Factors 9: 57–71, 1993. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, Jiang H. Identification of a distal STAT5-binding DNA region that may mediate growth hormone regulation of insulin-like growth factor-I gene expression. J Biol Chem 280: 10955–10963, 2005. [DOI] [PubMed] [Google Scholar]
- 71.Waters MJ. The growth hormone receptor. Growth Horm IGF Res 28: 6–10, 2016. [DOI] [PubMed] [Google Scholar]
- 72.Waters MJ, Brooks AJ. JAK2 activation by growth hormone and other cytokines. Biochem J 466: 1–11, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Waters MJ, Hoang HN, Fairlie DP, Pelekanos RA, Brown RJ. New insights into growth hormone action. J Mol Endocrinol 36: 1–7, 2006. [DOI] [PubMed] [Google Scholar]
- 74.Waxman DJ, O'Connor C. Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol 20: 2613–2629, 2006. [DOI] [PubMed] [Google Scholar]
- 75.Woelfle J, Billiard J, Rotwein P. Acute control of insulin-like growth factor-1 gene transcription by growth hormone through STAT5B. J Biol Chem 278: 22696–22702, 2003. [DOI] [PubMed] [Google Scholar]
- 76.Woelfle J, Chia DJ, Rotwein P. Mechanisms of growth hormone (GH) action. Identification of conserved Stat5 binding sites that mediate GH-induced insulin-like growth factor-I gene activation. J Biol Chem 278: 51261–51266, 2003. [DOI] [PubMed] [Google Scholar]
- 77.Wu Y, Zhao W, Zhao J, Pan J, Wu Q, Zhang Y, Bauman WA, Cardozo CP. Identification of androgen response elements in the insulin-like growth factor I upstream promoter. Endocrinology 148: 2984–2993, 2007. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y, Muyrers JP, Testa G, Stewart AF. DNA cloning by homologous recombination in Escherichia coli. Nat Biotechnol 18: 1314–1317, 2000. [DOI] [PubMed] [Google Scholar]
- 79.Zhou J, Dsupin BA, Giudice LC, Bondy CA. Insulin-like growth factor system gene expression in human endometrium during the menstrual cycle. J Clin Endocrinol Metab 79: 1723–1734, 1994. [DOI] [PubMed] [Google Scholar]