Abstract
Honeybee colony survival strongly relies on the queen to overcome worker losses exposed to combined stressors like pesticides and parasites. Queen’s capacity to withstand these stressors is however very little known. The effects of the common neonicotinoid pesticide imidacloprid in a chronic and sublethal exposure together with the wide distributed parasite Nosema ceranae have therefore been investigated on queen’s physiology and survivorship in laboratory and field conditions. Early physiological changes were observed on queens, particularly the increase of enzyme activities (catalase [CAT] and glutathione-S-transferase [GST] in the heads) related to protective responses to xenobiotics and oxidative stress against pesticide and parasite alone or combined. Stressors also alter the activity of two other enzymes (carboxylesterase alpha [CaE α] and carboxylesterase para [CaE p] in the midguts) involved in metabolic and detoxification functions. Furthermore, single and combined effects of pesticide and parasite decrease survivorship of queens introduced into mating hives for three months. Because colony demographic regulation relies on queen’s fertility, the compromise of its physiology and life can seriously menace colony survival under pressure of combined stressors.
Survival of honeybee colonies strongly relies upon the queen, the one individual whose life is crucial for colony development1. In the nature, the queen and the whole colony are constantly under the pressure of multiple stressors like agro-chemicals, pathogens and poor food resources, suspected to underlie the decline of their populations around the world2. Compared with workers, queens usually live longer, which means they can be exposed to environmental stressors over long time periods with lethal consequences. The loss of the queen seems to be the Achilles heel of superorganism resilience, the ability to tolerate the loss of somatic cells (worker bees) as long as the germ line (reproduction) is maintained. It therefore represents a key factor to understanding colony fate challenged by combined environmental factors3. Resilience was recently demonstrated in field conditions where individual honeybees near neonicotinoid-treated fields disappeared at a faster rate. The impact of this was however buffered by the colonies’ demographic regulation response4.
Insecticides from the neonicotinoid class have been in the center of controversy for the last years due to their high toxicity to non-target organisms like domestic and wild pollinators2. Because of their systemic action, they can reach bee food resources like pollen and nectar of treated crops2,4,5. The negative effects of neonicotinoids have been demonstrated, including imidacloprid on insect pollinators5,6,7,8, even though honeybee colony field trials have been incapable of detecting laboratory predicted sublethal effects with conventionally accepted levels of certainty9. The interactive effects between neonicotinoids and pathogens are now also well known in honeybees and bumble bees2. However, in natural conditions interaction effects are not clearly demonstrated10. Particularly, in honeybees, the impact of the endoparasite N. ceranae and imidacloprid were proved to be enhanced when both stressors were present11. Interactions between imidacloprid and N. ceranae can be expected, as both have the potential to disturb similar metabolic functions related to immunity, energetic resources and antioxidant responses11,12,13.
In honeybee queens, a few recent studies show increased supersedure (the natural process by which an old or failing queen is replaced), probably driven by a reduced reproductive performance in neonicotinoid exposed honeybee queens6,14. In bumble bee colonies, similar exposure decreased queen’s production and survival by 85% and 37% respectively associated to reduced workers foraging efficiency15,16. In bumble bee queens, a significant interaction that increased mortality was also observed between the parasite Crithidia bombi and a neonicotinoid17. Given this, we hypothesized that honeybee queens chronically exposed to an environmental relevant sublethal dose of imidacloprid during their first days of life, in combination with the common parasite N. ceranae, experience physiological changes and shorter lives. To test this hypothesis, survivorship trials were carried out: four groups of queens were settled in the laboratory: N. ceranae infected queens (N), imidacloprid exposed queens (I), both infected and pesticide exposed queens (NI) and non-treated queens as controls (C). The same experiment was repeated in 2010 and 2012. Queens from groups N and NI only were inoculated orally at birth with 200,000 spores/queen of N. ceranae, as infection process starts in the midgut after spore ingestion13. Queens were then kept in cages with a group of attendance bees18. Same spore charge under similar conditions was proved to be sublethal to queens in cage experiments during comparable time lapse18. Queens from groups I and NI were then exposed for 8 days to sugar syrup contaminated with 0.7 μg/l of imidacloprid11. Similar levels of imidacloprid were found in royal jelly (0.6 μg/kg in average) produced by colonies fed with contaminated diet patties after a chronic sublethal field exposure mimicking imidacloprid treatments applied to crops during bloom5. Because in nature a queen rarely feeds herself, but is fed by the attendance bees (nurses) with the royal jelly they produced in their hypopharyngeal glands, we expected an indirect exposure of queens to the pesticide1. After exposure, queens were transferred into small hives used to rear queens (mating nuclei) placed in the field to naturally mate and lay eggs for 3 months (Fig. 1).
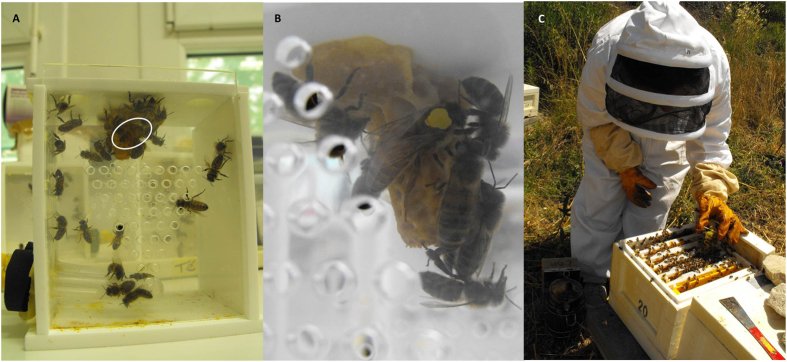
Figure 1. Queen rearing in the laboratory and subsequent transfer to mating nuclei.
Experimental cage with an open queen cell stuck on the inside top: queen, attending workers and feeders on the left side bottom are visible (A) Detail of a queen color marked over the thorax and a queen cell inside a cage (B) Introduction of the queen into the nucleus (C).
Results and Discussion
Survivorship curves (Fig. 2A,B) show the negative effect of combined stressors, while the impact of each stressor alone varied between the two years. On 2010, I, N and NI had statistically similar survivorship between them, but were all significantly lower than C. On 2012, C, I and N were statistically similar, and only NI was significantly different from C. That year, 66.7% of the control queens were accepted after introduction into the nuclei (Fig. 2B), which is considered as a common yield in beekeeping19. This control mortality was kept unchanged in the statistical analysis (see Methods). Survivorship curves (Fig. 2A,B) allow also inferring the queens’ median lifespan (T50). In 2010 and 2012, 50% of the queens from NI group were dead 45 and 15 days after introduction in the nuclei respectively. These were the lowest T50 from both years with exception of N on 2012 which also showed a T50 of 15 days. Queens from I group had a T50 of 75 days both years as well as N group from 2010. Survivorship differences between years might be related to the genetic background of sister queens used in the trials, that each year were reared from a different source colony accounting for genetic variability. In fact, the susceptibility to neonicotinoids can vary depending on the genetic basis of queens6. The variable susceptibility of honeybees of different genotypes to neonicotinoids has also been observed among worker bees20. Particularly imidacloprid sensitivity can change from one honeybee colony to another because of differences in the oxidative metabolism8. Regarding N. ceranae, our results show that early infections in queens can seriously compromise survival, which agrees with Chaimanee et al.21 who observed that young queens are more susceptible to the parasite than older queens because the immune responses to N. ceranae develop as queens age. Supersedure of queens is suspected to be due to changes induced by N. ceranae on the queen mandibular pheromones (QMP) that regulates the functioning of the colony stimulating queen attendance by workers, inhibiting worker ovary development and regulating worker behavioral maturation18. The observed differences between years might also be linked to environmental factors like climate that can impact the quality of honeybee food resources which can negatively affect bee’s immunity2.
Figure 2. Kaplan Meier survivorship curves of honeybee queens in the field.
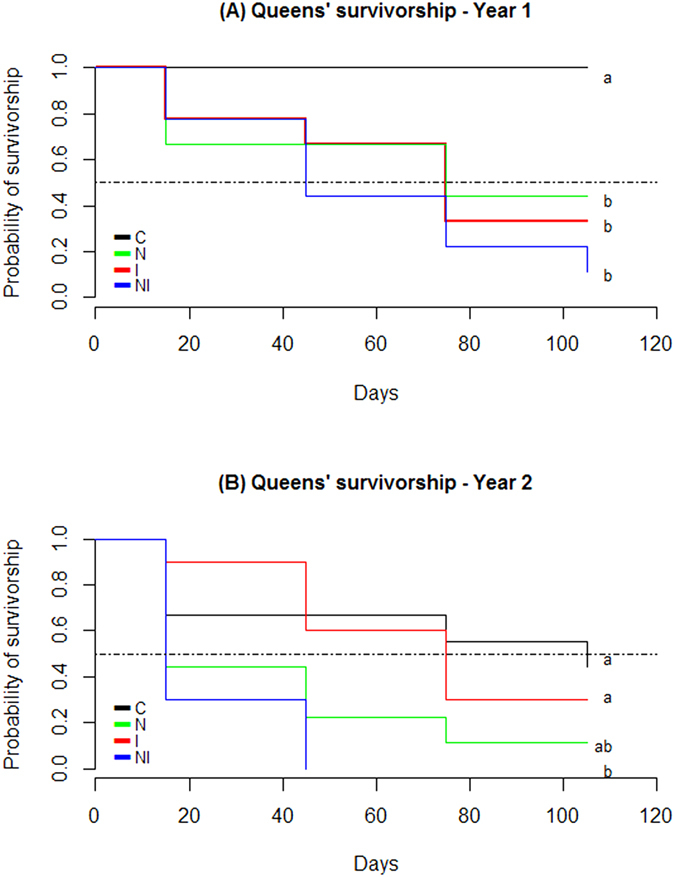
In laboratory conditions queens were divided in four groups to be parasite-inoculated and pesticide-exposed as follow: N. ceranae infected queens (N), imidacloprid exposed queens (I), both infected and exposed to imidacloprid queens (NI) and control queens (C). Queens were then introduced into mating nuclei placed in the field for 3 months. (A) Year 1 survivorship curves (n = 8, 9, 9, 9 for C, I, N and NI) and (B) Year 2 survivorship curves (n = 9, 10, 9, 10 for C, I, N and NI). Day 0 corresponds to the introduction of queens in the nuclei. Median lifespan (T50) is represented by a dot-dash line. Different letters denotes differences less than 5% of significance. Log-Rank test for 2010: C vs. N: p – value = 0.0151; C vs. I: p – value = 0.0058; C vs. NI: p – value = 0.0002; I vs. N: p – value = 0.774; N vs. NI: p – value = 0.212; I vs. NI: p – value = 0.312; and Log-Rank test for 2012: C vs. N: p – value = 0.0778; C vs. I: p – value = 0.643; C vs. NI: p – value = 0.0046; I vs. N: p – value = 0.0787; N vs. NI: p – value = 0.219; I vs. NI: p – value = 0.001.
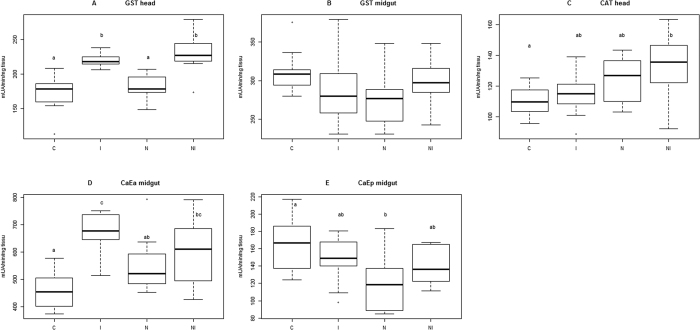
To address physiological responses to stressor agents, infected or non-infected 8-days-old queens not yet mated, were analyzed just after pesticide exposure to a set of biochemical markers already tested on worker bees: CAT with a strong antioxidant action, GST involved in multiple metabolic functions including antioxidant activity and CaE α and CaE p implicated in detoxification22,23. GST in head tissue is clearly induced by imidacloprid exposure and not by N. ceranae infection (Fig. 3A) despite GSTs have antioxidant functions and that parasite infection induces oxidative stress13, (ANOVA, N = 43, F = 18.83, p – value = 1.04e-07; Tukey post-hoc test at 95% confidence level, C vs. I: p – value = 0.0000255; C vs. N: p – value = 0.7537960; C vs. NI: p – value = 0.0000026; N vs. I: p – value = 0.0003927; N vs. NI: p – value = 0.0000352; I vs. NI: p – value = 0.7522841). The observed activity in imidacloprid exposed queens is probably related to phase-II GST enzyme involved in detoxification process, which transforms xenobiotics in more polar compounds to be excreted or further metabolized24,25. In fact, GSTs don’t participate directly in the detoxification of imidacloprid which is mainly metabolized by phase-I enzymes25. Nevertheless other metabolic functions might be also affected because the superfamily of GST enzymes is composed of six different types, some of them found to be well represented in honeybee brain tissue24. Unfortunately, its specific function there remains unknown24. In contrast GST activity in the midgut of treated queens is very variable and no significant differences were observed between groups, though there is a general downward trend that seems to be induced by each stressor especially N. ceranae (Fig. 3B) (ANOVA, N = 43, F = 2.244, p – value = 0.0984). Concerning the pesticide, the lack of significant differences of GST activity in the midgut of treated queens compare to C was not expected considering that in honeybee species GST is mainly localized in that organ which is the main place of metabolisation of imidacloprid25. One explanation can be that two of the more toxic imidacloprid metabolites responsible for lethal effects, olefin derivative and 4/5- hydroxyimidacloprid, are detected in the head rather than the midgut, in addition to 3 other metabolites, all of them with a long persistence25. In the midgut, only 3 less toxic compounds are present25. However, to our knowledge, the metabolism of imidacloprid has been studied only in worker bees25 and differences in the physiology between workers and queens can underlie different responses. In fact, Dahlgren et al.26 observed that for some acaricides queens are more tolerant than workers. Apart from imidacloprid, workers also respond differently than queens to N. ceranae infection by increasing GST activity in the midgut to fight oxidative stress13, contrary to the decreasing tendency we observed in queens (Fig. 3B). This result agrees with observations that queens and workers might have different responses against infection. For example, workers bees infected by N. ceranae, show lower levels of gene expression of vitellogenin (Vg), that probably render bees more susceptible to infection, as Vg is a yolk protein capable to reduce oxidative stress12. In turn, in infected queens, Vg titers and antioxidant capacity increase, which might help to fight infection18. Although our results represent the physiological response at a specific point in time, early in the life of queens, one could expect that the observed tendency to decrease GST activity in the midgut will not recover given the reduction of survivorship of Nosema infected queens (Fig. 2A).
Figure 3. Effect of imidacloprid exposure and N. ceranae infection on queens’ tissue enzyme activity.
Unmated queens of 8-days-old were analyzed by spectrophotometry to measure GST in the head (A) and midgut (B) CAT in the head (C) CaE α (D) and CaE p (E) in the midgut. Four experimental groups were tested: N. ceranae infected queens (N), imidacloprid exposed queens (I), both infected and exposed to imidacloprid queens (NI) and control queens (C). Boxplots show 1st and 3rd interquartile range with line denoting median, whiskers encompass 90% of the individuals beyond which outliers are represented by circles. Different letters denote statistically significant differences <5% of significance after ANOVA and Tukey post-hoc test. For C, N, I and NI, n = 11, 12, 11, 9 respectively.
Queens under the pressure of both stressors (NI) present a significantly higher CAT activity in the heads, while CAT activity of I and specially N was higher than C but not statistically different (Fig. 3C) (ANOVA, N = 43, F = 3.461, p – value = 0.0253; Tukey post-hoc test at 95% confidence level, C vs. I: p – value = 0.8856685; C vs. N: p – value = 0.2361979; C vs. NI: p – value = 0.0234735; N vs. I: p – value = 0.6440953; N vs. NI: p – value = 0.6081494; I vs. NI: p – value = 0.1139478). Even if infected queens enhance CAT activity (our results) and develop a higher total antioxidant enzyme activity and an elevated Vg titer18, it seems these protective responses are not enough to cope with the physiological stress of N. ceranae over the long-term. This is especially true when combined with neonicotinoid exposure (Fig. 3C), and is consistent with previous studies27. CAT is the primary defense against the overproduction of reactive oxygen species (ROS) that can be originated following N. ceranae infection as a defense response. ROS are however not specific and when not sufficiently reduced, they damage the organism by reacting with macromolecules of biological importance such as lipids, proteins, nucleic acids, and carbohydrates, eventually leading to cell death13.
CaE α activity in the midgut also reflects the stress level of queens, which is significantly more elevated in I and NI compared to C (Fig. 3D), (ANOVA, N = 43, F = 10.52, p – value = 3.33e-05; Tukey post-hoc test at 95% confidence level, C vs. I: p – value = 0.0000167; C vs. N: p – value = 0.1138786; C vs. NI: p – value = 0.0077867; N vs. I: p – value = 0.0114687; N vs. NI: p – value = 0.5681849; I vs. NI: p – value = 0.3053523). CaE α is a phase-I enzyme that would therefore be directly involved in the detoxification of imidacloprid or might also responded to the production of other xenobiotics during intoxication and infection processes22,23,25. Additionally, the expression of CaEs during development might also be regulated by other factors such as hormones28. However, even in mammalians, the physiological mechanisms which control them are relatively little known28. Finally, CaE p in the midgut (Fig. 3E) presents a decreasing trend, similar to GST response in the midgut, especially with N. ceranae infection (ANOVA, N = 43, F = 4.238, p – value = 0.011; Tukey post-hoc test at 95% confidence level, C vs. I: p – value = 0.620222; C vs. N: p – value = 0.0062021; C vs. NI: p – value = 0.3849647; N vs. I: p – value = 0.1255768; N vs. NI: p – value = 0.3510227; I vs. NI: p – value = 0.9680898). The reduction of CaE p activity deserves additional attention since it might render queens more vulnerable to xenobiotics or affect other metabolic functions.
In summary, we show here that both chronic exposure to very low doses of neonicotinoid and infection by a common honeybee parasite, affects queens’ physiology with a trade-off on queens’ survival. Specifically, we found a protective response against imidacloprid through the increased activity of GST in the head and CaE α in the midgut. A protective response to oxidative stress was also observed from CAT activity in the head, especially in the case of N. ceranae infection combined with imidacloprid. However, in the midgut, which is the first barrier to parasite development and oral pesticide exposure25,27, there was a decreasing trend in the activity of GST and CaE p particularly during infection. This decrease might negatively affect other metabolic and detoxification functions which are probably linked to increased mortality. The reduced lifespan of stressed queens, suggests that the physiological protective responses to oxidative stress and xenobiotics detoxification, observed before mating, are not sufficient in the long term to cope the negative effects of pesticide exposure and parasite infection. This could explain previous reports of queen supersedure6, of decreased worker production by the queen15, of queen and workers death27, as well as the loss of colony resilience3, essential to colony survival under adverse conditions4.
Methods
Experimental design
Four groups of 10 emerging queens from local breeds of A. mellifera, were treated as follow: (i) inoculated with spores of N. ceranae at emergence (N), (ii) exposed to a sublethal dose of pesticide from emergence until they were 8-days-old (I), (iii) both inoculated with N. ceranae spores and exposed to the pesticide (NI), and (iv) neither inoculated with spores nor exposed to the pesticide corresponding to the control group (C). This part of the experiment was performed in laboratory conditions. When the queens were 8-days-old, they were transferred to small hives specially designed to rear queens (mating nuclei) and settled in a non-cultivated field surrounded by an urban forest located at the south-east border of the city of Avignon, in the South of France. The experiment was repeated in 2010 and 2012, between spring and autumn and queens’ survival in the nuclei was monitored for 3 months. Just before transfer to the nuclei, some of the queens were sacrificed for analysis of a set of five physiological biomarkers (see further). Three additional measurements were made: (i) the success of N. ceranae experimental infection (n = 12 for each experimental group) (Supplementary Fig. S1); (ii) sugar consumption during queen rearing in the laboratory (Supplementary Table S1); (iii) fat body content (n = 10, 12, 10, 9 for C, I, N, NI respectively) as an indicator of the nutritional status of queens. The later was used to test if the pesticide induced behavioral changes in workers bees affecting their queens’ attending capacities29,30,31 (Supplementary Fig. S2).
Queen rearing
Sister queens were reared according to standard beekeeping methods32 by grafting young larvae less than 48-hours-old from a single source colony and reared as queens in a queenless colony32. The source colony being different between year 1 and year 2, natural genetic variation has been considered. One to two days before emergence capped queen cells were stuck individually on the inside top of Plexiglas cages (10.5 × 7.5 × 11.5 cm) and kept in incubators at 33 °C. Humidity was maintained between 50–60% through natural evaporation of water in a container. Each queen cell was provided with 30 one-day-old attending workers bees obtained from combs of last stage pupae, which were incubated until emergence and then transferred into the cages (Fig. 1).
Pesticide exposure
A sublethal dose of the neonicotinoid imidacloprid [1−(6-chloro-3-pyridylmethyl)- N-nitro-imidazolidin-2-ylidene amine] was given to the attending bees and the queen, through contaminated sugar syrup for 10 hours per day. Contaminated sugar syrup was made from a pure standard of imidacloprid (Cluzeau, France) diluted with dimethyl sulfoxide (DMSO) and water to obtain a stock solution of 7 μg/l. The stock solution was aliquoted for daily needs and frozen at −20 °C until use. Syrup was prepared at a concentration of 50% (w/v) sucrose and used to dilute the stock solution to get a final solution of 0.7 μg/l imidacloprid with 0.1% (w/v) DMSO11. This concentration was known to cause less than 10% worker bees’ mortality under similar conditions, and no evident effects were observed on physiological markers related to individual and social immunity11. The theoretical effective dose of imidacloprid was 0.0083 ng/bee/day for I and 0.0095 ng/bee/day for NI estimated upon the daily consumption of syrup (Supplementary Table S1). Solutions containing sucrose and 0.1% DMSO, were used as controls. The concentration of an aliquot of stock solution was verified from a froze sample by UHPLC-MS/MS. Feeders with sugar syrup were replaced each day at the same time of the day and weighted before and after feeding to estimate syrup consumption. Pesticide exposure began when attending worker bees were introduced in the cages along with the queen cell and continued until queens were 8-days-old, when they were ready to start orientation and mating flights32. After daily pesticide exposure, bees were provided ad libitum with water, pollen free of Nosema sp. spores (checked under microscope), and a sucrose candy (Apifonda® mixed with powder sugar). Food and water were changed every two days.
Nosema ceranae experimental infection of queens
Just after queens emerged in the cages (see queen rearing), they were individually inoculated with spores of N. ceranae. For spore inoculation, a solution was prepared containing 50% (w/v) of sucrose in water and a concentration of 100,000 spores/μl in suspension, and each queen bee was fed 2 μl of this solution using a micropipette18. From a naturally infected colony, spores of N. ceranae were isolated by crushing the abdomens of infected bees in distilled water, filtering the suspension and centrifuging it to collect the spores33. Molecular identification of N. ceranae in the bees from the infected colony was confirmed by standard PCR11. The concentration of spores was estimated with a haemocytometer in the feeding solution and in the midgut of 8-days-old queens to verify the success of infection32. Verification was done from 9 up to 12 queens per treatment group from both years (Supplementary Fig. S1).
Queen introduction in mating nuclei
After N. ceranae spore inoculation at birth and exposure to the pesticide in the laboratory for 8 days, each queen was color marked in the thorax according to the experimental group they belong (N, I, NI and C) and introduced into a mating nucleus (Fig. 1). Each year, 40 nuclei (Mini Plus “Stehr”®), ten for each experimental group, were established one day before queen’s introduction. To build the nuclei, young workers were collected from the upper hive bodies of large colonies. Upper hives were separated from the brood hive with a wire mesh queen excluder. Each mating nucleus contained approximately 400 g of bees, 4 small frames with foundations, and one feeding frame filled with sugar candy. After introduction in the nuclei queens were left undisturbed for at least 15 days, so they could mate and start lying eggs, which can take place when queens are between 10 to 19-days-old depending on the time of the season32. Queens’ acceptance by the bee population from the nucleus was verified fifteen days after introduction. For C groups queens’ acceptance over 65% was considered as satisfactory19. Every month, for 3 months, nuclei were carefully observed frame by frame to determine the presence of the queen either by direct observation or by searching for freshly-laid eggs and the presence of brood. We also recorded the unusual presence of drones cells, dead larvae, abnormal brood pattern or workers behavior and signs of diseases or pests. Queen’s survival was the only variable taken into account, since mating nuclei have a reduced space compared to regular hives, therefore less suitable to follow colony development.
The mating hives were set in pairs with their entrances facing opposite directions and painted different colors to facilitate queen’s orientation to return to the correct nucleus.
Physiological markers
We examined the physiological conditions of 8-days-old queens from the second year, after treatment and just before introduction into the nuclei. For each experimental group, we analyzed the enzymatic activity of CAT in head tissue, GST in midgut and head tissue and CaE α and CaE p in midgut tissue. Enzyme extraction method was modified from Badiou-Bénéteau et al.23.
Enzymes were obtained from heads and midguts from queens that were numbed at 4 °C. All tissues were stored at −80 °C until analysis. The analyses were done on each queen individually (for C, N, I and NI, n = 11, 12, 11, 9 respectively). Samples were homogenized at 4 °C with a TissuLyser (Qiagen) (5610 s at 30 Hz) in phosphate buffer pH 7.4 (40 mM sodium phosphate, 10 mM NaCl, 1% (w/v) Triton X-100, containing a mixture of 2 mg/ml of antipain, leupeptin and pepstatin A, 25 units/ml of aprotinin and 0.1 mg/ml of trypsin inhibitor), to make a 10% (w/v) extract. The homogenates were then centrifuged at 15,000 g for 20 min at 4 °C and resulting supernatants were used immediately for analysis of enzyme activities.
GST activity was monitored by following the conjugation of reduced glutathione to 1-chloro-2,4-dinitrobenzene using a method adapted from Habig, Pabst and Jakoby34. GST activity was measured by adding enzymatic extract to the reaction mixture containing 1 mM EDTA, 2.5 mM reduced glutathione, 1 mM 1-chloro-2,4-dinitrobenzene and 100 mM Na/K-phosphate pH 7.4. GST activity was followed spectrophotometrically at 340 nm for 5 min at 25 °C.
CAT was measured according to a procedure modified from Beers and Sizer35 in a medium containing 30 mM H2O2 and 100 mM NA-phosphate at pH 7.0. The reaction was monitored by the decrease in absorbance at 240 nm due to the consumption of H2O2 for 5 min at 25 °C.
CaE α (type one) and CaE p (type three) are classified according to their substrate specificity corresponding to the hydrolysis of α - naphtyl acetate (α - NA) and p - nitrophenyl acetate (p - NA), respectively. For CaE α the enzyme reaction was performed for 1 min then stopped with 10% SDS and 4 mg/mL Fast Garnet and kept 10 min in the dark. The reaction product was then measured at 568 nm in a medium containing 0.1 mM α-naphtyl acetate as the substrate, 0.01 mM BW284C51 as an AChE inhibitor and 100 mM Na-phosphate pH 7.0. For CaE p, the reaction was monitored continuously at 410 nm in a medium containing 0.1 mM p-nitrophenyl acetate as the substrate, 0.01 mM BW284C51 as an AChE inhibitor and 100 mM Na-phosphate pH 7.0. Modified by Carvalho et al.22.
For CaE α and p, and CAT one unit of enzyme activity was defined as the quantity of enzyme that, under the assay conditions, hydrolysed 1 μmol of substrate per min. For GST, one unit of activity corresponded to the quantity of enzyme conjugating 1 μmol of GSH per min. Results were expressed in terms of tissue activities, as defined above, per quantity of tissue (mUA/min/mg).
Statistical analysis
Statistical analysis and figures were generated in R version 3.0.2. Survival data was transformed in a Kaplan-Meier table. Log-Rank test was used to compare survival curves using R function {survdiff} and the package {survival}36. Survival data from 2012 was not transformed considering the small number of individuals (n = 9 queens in the control group), which brings uncertainty to the normalization of data, keeping the unchanged mortality of controls in the statistical analysis. Enzyme activities were compared between groups with ANOVA and Tukey post-hoc test after assumptions of normality and homogeneity of variance were checked with Shapiro-Wilk test and Bartlett’s test respectively.
Additional Information
How to cite this article: Dussaubat, C. et al. Combined neonicotinoid pesticide and parasite stress alter honeybee queens’ physiology and survival. Sci. Rep. 6, 31430; doi: 10.1038/srep31430 (2016).
Supplementary Material
Acknowledgments
Special thanks to Louise Clochey and Ilyas Boudia for technical assistance during their professional training at INRA of Avignon Laboratory, Camille Vourch for English revision and referee for comments that improved the manuscript. AM and YLC were supported by Human Frontier Science Program (RGP0042/2007-C101). CD was supported by a CONICYT/French Embassy of Chile grant.
Footnotes
Author Contributions C.D., A.M., J.-L.B. and Y.L.C. conceived the study and designed the experiment; C.D., A.M. and D.C. carried out the field experiment; C.D. and S.T. carried out the laboratory experiment; C.D., M.B. and M.C. carried out the laboratory analysis; C.D. analyzed the data and prepared the manuscript; A.M., J.-L.B., A.K. and Y.L.C. discussed the results and commented on the manuscript.
References
- Winston M. L. The Biology of the Honey Bee (Harvard University Press, Cambridge, Mass, 1987). [Google Scholar]
- Sánchez-Bayo F. et al. Are bee diseases linked to pesticides? — A brief review. Environ. Int. 89–90, 7–11 (2016). [DOI] [PubMed] [Google Scholar]
- Straub L., Williams G. R., Pettis J., Fries I. & Neumann P. Superorganism resilience: Eusociality and susceptibility of ecosystem service providing insects to stressors. Curr. Opin. Insect Sci. 12, 109–112 (2015). [Google Scholar]
- Henry M. et al. Reconciling laboratory and field assessments of neonicotinoid toxicity to honeybees. Proc. R. Soc. B 282, 20152110 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dively G. P., Embrey M. S., Kamel A., Hawthorne D. J. & Pettis J. S. Assessment of chronic sublethal effects of imidacloprid on honey bee colony health. PLoS One 10, e0118748 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrock C. et al. Impact of chronic neonicotinoid exposure on honeybee colony performance and queen supersedure. PLoS One 9, e103592 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M. et al. A common pesticide decreases foraging success and survival in honey bees. Science 336, 348–350 (2012). [DOI] [PubMed] [Google Scholar]
- Suchail S., Guez D. & Belzunces L. P. Discrepancy between acute and chronic toxicity induced by imidacloprid and its metabolites in Apis mellifera. Environ. Toxicol. Chem. 20, 2482–2486 (2001). [DOI] [PubMed] [Google Scholar]
- Cresswell J. E. A meta-analysis of experiments testing the effects of a neonicotinoid insecticide (imidacloprid) on honey bees. Ecotoxicology 20, 149–157 (2011). [DOI] [PubMed] [Google Scholar]
- Retschnig G. et al. Effects, but no interactions, of ubiquitous pesticide and parasite stressors on honey bee (Apis mellifera) lifespan and behaviour in a colony environment. Environ. Microbiol. 17, 4322–4331 (2015). [DOI] [PubMed] [Google Scholar]
- Alaux C. et al. Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera). Environ. Microbiol. 12, 774–782 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antúnez K. et al. Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environ. Microbiol. 11, 2284–2290 (2009). [DOI] [PubMed] [Google Scholar]
- Dussaubat C. et al. Gut pathology and responses to the microsporidium Nosema ceranae in the honey bee Apis mellifera. PLoS One 7, e37017 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. R. et al. Neonicotinoid pesticides severely affect honey bee queens. Sci. Rep. 5, 14621 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehorn P. R., O’Connor S., Wackers F. L. & Goulson D. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336, 351–352 (2012). [DOI] [PubMed] [Google Scholar]
- Scholer J. & Krischik V. Chronic exposure of imidacloprid and clothianidin reduce queen survival, foraging, and nectar storing in colonies of Bombus impatiens. PLoS One 9, e91573 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauser-Misslin A., Sadd B. M., Neumann P. & Sandrock C. Influence of combined pesticide and parasite exposure on bumblebee colony traits in the laboratory. J. Appl. Ecol. 51, 450–459 (2014). [Google Scholar]
- Alaux C. et al. Pathological effects of the microsporidium Nosema ceranae on honey bee queen physiology (Apis mellifera). J. Invertebr. Pathol. 106, 380–385 (2011). [DOI] [PubMed] [Google Scholar]
- Pérez-Sato J. A. & Ratnieks F. L. W. Comparing alternative methods of introducing virgin queens (Apis mellifera) into mating nucleus hives. Apidologie. 37, 571–576 (2006). [Google Scholar]
- Laurino D. L., Anino A. M., Atetta A. P. & Orporato M. P. Toxicity of neonicotinoid insecticides on different honey bee genotypes. Bull. Insectology 66, 119–126 (2013). [Google Scholar]
- Chaimanee V., Chantawannakul P., Chen Y., Evans J. D. & Pettis J. S. Effects of host age on susceptibility to infection and immune gene expression in honey bee queens (Apis mellifera) inoculated with Nosema ceranae. Apidologie 45, 451–463 (2014). [Google Scholar]
- Carvalho S. M., Belzunces L. P., Carvalho G. a., Brunet J. L. & Badiou-Beneteau A. Enzymatic biomarkers as tools to assess environmental quality: A case study of exposure of the honeybee Apis mellifera to insecticides. Environ. Toxicol. Chem. 32, 2117–2124 (2013). [DOI] [PubMed] [Google Scholar]
- Badiou-Bénéteau A. et al. Development of biomarkers of exposure to xenobiotics in the honey bee Apis mellifera: Application to the systemic insecticide thiamethoxam. Ecotoxicol. Environ. Saf. 82, 22–31 (2012). [DOI] [PubMed] [Google Scholar]
- Claudianos C. et al. A deficit of detoxification enzymes: Pesticide sensitivity and environmental response in the honeybee. Insect Mol. Biol. 15, 615–636 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchail S., De Sousa G., Rahmani R. & Belzunces L. P. In vivo distribution and metabolisation of 14C-imidacloprid in different compartments of Apis mellifera L. Pest Manag. Sci. 60, 1056–1062 (2004). [DOI] [PubMed] [Google Scholar]
- Dahlgren L., Johnson R. M., Siegfried B. D. & Ellis D. Comparative toxicity of acaricides to honey bee (Hymenoptera: Apidae) workers and queens. Apic. Soc. Insects 105, 1895–1902 (2012). [DOI] [PubMed] [Google Scholar]
- Higes M., Martín-Hernández R., García-Palencia P., Marín P. & Meana A. Horizontal transmission of Nosema ceranae (Microsporidia) from worker honeybees to queens (Apis mellifera). Environ. Microbiol. Rep. 1, 495–498 (2009). [DOI] [PubMed] [Google Scholar]
- Satoh T. & Hosokawa M. Structure, function and regulation of carboxylesterases. Chem. Biol. Interact. 162, 195–211 (2006). [DOI] [PubMed] [Google Scholar]
- Naug D. & Gibbs A. Behavioral changes mediated by hunger in honeybees infected with Nosema ceranae. Apidologie 40, 595–599 (2009). [Google Scholar]
- Wright G. A., Softley S. & Earnshaw H. Low doses of neonicotinoid pesticides in food rewards impair short-term olfactory memory in foraging-age honeybees. Sci. Rep. 5, 15322 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrese E. L. & Soulages J. L. Insect fat body: energy, metabolism, and regulation. Annu. Rev. Entomol. 55, 207–225 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laidlaw H. & Page R. Queen rearing and bee breeding (Wicwas Press, 1997). [Google Scholar]
- Fries I. et al. Standard methods for nosema research. J. Apic. Res. 52, 1–28 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J. & Jakoby W. B. Glutathione S-transferase: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249, 7130–7139 (1974). [PubMed] [Google Scholar]
- Beers R. F. & Sizer I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 195, 133–140 (1952). [PubMed] [Google Scholar]
- Corrillon P. A. et al. Statistiques avec R (Presses Universitaires de Rennes, Rennes, 2008). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.