Abstract
Few studies have investigated the relationship between chronic rhinosinusitis (CRS) and erectile dysfunction (ED). This case-control study aimed to investigate the association between CRS and the risk of ED in a large national sample. Tapping Taiwan’s National Health Insurance Research Database, we identified people 30 years or older with a new primary diagnosis of CRS between 1996 and 2007. The cases were compared with sex- and age-matched controls. We identified 14 039 cases and recruited 140 387 matched controls. Both groups were followed up in the same database until the end of 2007 for instances of ED. Of those with CRS, 294 (2.1%) developed ED during a mean (SD) follow-up of 3.20 (2.33) years, while 1 661 (1.2%) of the matched controls developed ED, mean follow up 2.97 (2.39) years. Cox regression analyses were performed adjusting for sex, age, insurance premium, residence, hypertension, hyperlipidemia, diabetes, obesity, coronary heart disease, chronic kidney disease, chronic obstructive pulmonary disease, asthma, allergic rhinitis, arrhythmia, ischemic stroke, intracerebral hemorrhage, and medications. CRS was revealed to be an independent predictor of ED in the fully adjusted model (HR = 1.51; 95% CI = 1.33–1.73; P < 0.0001).
Chronic rhinosinusitis (CRS) is an inflammatory disease of the upper airway that can impact quality of life. It affects more than 30 million people in the United States, where the annual prevalence of CRS is reported to range from 13% to 16%1. The costs associated with treatment of CRS there are estimated to reach about US$4.3 billion annually1. Previous studies have suggested that upper airway diseases such as allergic rhinitis (AR) and lower airway diseases such as asthma and chronic obstructive pulmonary disease (COPD) may be associated with an increased risk for erectile dysfunction (ED)2,3,4,5. Low-grade subclinical inflammation affects endothelial function and is involved in all stages of the atherosclerotic process6. In fact, several studies have associated increased expressions of various markers of inflammation with the onset and severity of ED7.
Erectile dysfunction (ED), defined by the National Institutes of Health as “the inability to attain and maintain an erection of sufficient quality to permit satisfactory sexual intercourse,” affects ~5% of the male population in the United States8,9. More than ten million men there are affected by erectile dysfunction, and worldwide it is estimated to affect 100 million men8,10.
Some reports have suggested that there is a possible relationship between CRS and ED11,12. Patients with CRS have been found to be at increased risk of stroke and acute myocardial infarction (AMI)13,14,15. The risk factors for cardiovascular disease and ED are known to be similar7,10, but there has been no large investigation evaluating the risk of ED after a CRS diagnosis. This study tapped a nationwide population-based database in Taiwan to test the hypothesis that CRS is a risk factor for ED.
Materials and Methods
Sample
A retrospective cohort study was conducted using data collected from Taiwan’s National Health Insurance Research Database (NHIRD), an insurance claims database managed by Taiwan’s National Health Research Institute (NHRI) for research purposes. It contains demographic information, diagnoses, health services provided, and healthcare costs and prescription data for almost all outpatients and inpatients as well as patients receiving dental services in Taiwan. Implemented in 1995, the National Health Insurance (NHI) program provides compulsory universal health insurance, covering the delivery of virtually all health care (98%) to the entire population. In cooperation with the Bureau of the NHI, Taiwan’s NHRI extracted a randomly sampled set of representative data for one million people from the 2005 registry of all NHI enrollees to create a subset of the NHIRD for research purposes. The enrollees in this subset, known as the Longitudinal Health Insurance Database (LHID), are not significantly different from all NHI enrollees with regard to age, sex, or health care costs16.
CRS was identified in this study by the claims records showing a primary diagnosis of CRS designated with the use of the International Classification of Diseases, Ninth Revision (ICD-9) codes CRS15,17 (ICD-9 codes: 473, 473.0, 473.1, 473.2, 473.3, 473.8, and 473.9) from 1996 to 2007. Date of diagnosis was set as the index date. Nasal polyps (NP) were identified by a diagnosis of NP ICD-9 codes: 471, 471.0, 471.1, 471.8, and 471.9. CRS without NP (CRSsNP) was defined in subjects who had CRS but no NP. CRS with NP (CRSwNP) was defined in subjects who had CRS with comorbid NP.
The comparison cohort consisted of individuals who had no diagnosis of CRS in the LHID. These individuals were randomly frequency matched with the cases by age and sex at a ratio of 1:10. Their index dates were randomly assigned to months and days within the same year of the index date of the patients in the CRS cohort. We excluded subjects with pre-existing ED (ICD-9 code: 607.84) in both groups.
Both CRS cases and controls were followed up until they were diagnosed with ED, our outcome. ED cases were identified based on a recorded diagnosis of ED ICD-9 code 607.84. All medical claims containing this diagnostic code between 1996 and 2007 were collected from the NHIRD for further analysis. To improve the diagnostic validity of ED, only those patients who had been diagnosed with ED at least twice in an outpatient service or once in an inpatient service by urologists were included in the analysis.
Physicians in Taiwan generally adhere to standardized CRS definitions and management guidelines, such as those provided by the Rhinosinusitis Task Force18 and Clinical Practice Guidelines: Adult Sinusitis19. The diagnosis of ED in Taiwan is based on criteria established by the American Urological Association20,21.
The covariates considered in this analysis were sex, age, insurance premium, area of residence (metropolitan, class I cities, class II cities, class I counties, class II counties, and unclassified), hypertension, hyperlipidemia, diabetes, obesity, coronary heart disease, chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), asthma, allergic rhinitis, arrhythmia, ischemic stroke, intracerebral hemorrhage, and medications [angiotensin-converting enzyme inhibitors (ACEIs), beta-adrenergic blockers, statins, and steroid]. The insurance premium served as an indicator of economic status and subjects were classified into 1 of 3 categories: fixed premium and dependent, NTD20,000 (income per month) or less, and more than NTD20,000 (US$1 = NTD32.8 in 2007).
This study was exempt from full review by the Institutional Review Board of Kaohsiung Medical University Hospital because the NHIRD only contains de-identified secondary data released to the public for research purposes (KMUHIRB-EXEMPT-20150003).
Statistical analysis
The baseline characteristics and comorbidities of the CRS cohort and non-CRS cohort were analyzed descriptively. All data were expressed as mean ± standard deviation (SD) or percentage. Chi-square and t-tests were used to test differences in categorical and continuous variables between the two cohorts. Univariate and multivariable Cox proportional hazards regression were used to assess the hazard ratio (HR) and 95% confidence interval (CI) of ED associated with CRS, compared with the non-CRS cohort. The multivariable analysis model was adjusted for sex, age, insurance premium, residence, hypertension, hyperlipidemia, diabetes, obesity, coronary heart disease, chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), asthma, allergic rhinitis, arrhythmia, ischemic stroke, intracerebral hemorrhage, and medications of ACEIs, beta-adrenergic blockers, statins, and steroid. This study also investigated the relationship between CRS w/s NP and ED as well as CRS and ED. To find out if CRS is an age-dependent risk factor for ED, we also analyzed the effect of CRS on ED stratified by age group.
The cumulative incidence of ED between the CRS cohort and the non-CRS cohort was analyzed using the Kaplan–Meier method, and the difference was examined by log-rank test. SAS software (version 9.3 for Windows; SAS Institute Inc., Cary, NC) was used for all statistical operations. A P value < 0.05 was considered significant.
Results
Characteristics of the subjects
The two cohorts were comprised of 14 039 people with newly diagnosed CRS and 140 387 (nearly ten for every patient in the CRS group) sex-, age-matched controls, both identified from the NHIRD 1996–2007. As can be seen in Table 1, a summary of cohort characteristics, most cases of CRS occurred in people 36–50 years old. Compared to the controls, more patients with CRS were diagnosed with hypertension, hyperlipidemia, diabetes, obesity, coronary heart disease, chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), asthma, allergic rhinitis, arrhythmia, ischemic stroke, and intracerebral hemorrhage (P < 0.0001) (Table 1). The patients in the CRS cohort were also more frequent users of ACEIs, beta-adrenergic blockers, statins, and steroid during the follow-up period than those without (P < 0.0001) (Table 1).
Table 1. Characteristics of CRS cases and matcheda controls in Taiwan, 1996–2007.
| Characteristic | Class | Total | CRS | Non-CRSa | P-value | ||
|---|---|---|---|---|---|---|---|
| N | % | N | % | ||||
| Total | 154426 | 14039 | 9.1 | 140387 | 90.9 | ||
| Age group | 30–35 | 27684 | 2518 | 17.9 | 25176 | 17.9 | 1.0000 |
| 36–50 | 68050 | 6185 | 44.1 | 61865 | 44.1 | ||
| 51–65 | 34418 | 3130 | 22.3 | 31288 | 22.3 | ||
| >65 | 24264 | 2206 | 15.7 | 22058 | 15.7 | ||
| Follow-up time (years) (Mean ± SD) | 3.20 ± 2.33 | 2.97 ± 2.39 | 0.1396 | ||||
| Insurance premium | Fixed premium and dependent | 20386 | 1722 | 12.3 | 18664 | 13.3 | <0.0001 |
| ≤NTDb 20,000 | 78974 | 6453 | 46.0 | 72521 | 51.7 | ||
| >NTD20,000 | 55066 | 5864 | 41.8 | 49202 | 35.0 | ||
| Residence | metropolitan | 39079 | 3633 | 25.9 | 35446 | 25.2 | <0.0001 |
| class I cities | 12480 | 1289 | 9.2 | 11191 | 8.0 | ||
| class II cities | 6519 | 725 | 5.2 | 5794 | 4.1 | ||
| class I counties | 67369 | 6324 | 45.0 | 61045 | 43.5 | ||
| class II counties | 27830 | 1982 | 14.1 | 25848 | 18.4 | ||
| Unclassified | 1149 | 86 | 0.6 | 1063 | 0.8 | ||
| Comorbidities | |||||||
| Hypertensionc | No | 132635 | 11383 | 81.1 | 121252 | 86.4 | <0.0001 |
| Yes | 21791 | 2656 | 18.9 | 19135 | 13.6 | ||
| Hyperlipidemiad | No | 144244 | 12687 | 90.4 | 131557 | 93.7 | <0.0001 |
| Yes | 10182 | 1352 | 9.6 | 8830 | 6.3 | ||
| Diabetes mellituse | No | 144497 | 12985 | 92.5 | 131512 | 93.7 | <0.0001 |
| Yes | 9929 | 1054 | 7.5 | 8875 | 6.3 | ||
| Obesityf | No | 154234 | 14002 | 99.7 | 140232 | 99.9 | <0.0001 |
| Yes | 192 | 37 | 0.3 | 155 | 0.1 | ||
| Coronary heart diseaseg | No | 146359 | 12920 | 92.0 | 133439 | 95.1 | <0.0001 |
| Yes | 8067 | 1119 | 8.0 | 6948 | 4.9 | ||
| Chronic kidney disease (CKD)h | No | 151382 | 13616 | 97.0 | 137766 | 98.1 | <0.0001 |
| Yes | 3044 | 423 | 3.0 | 2621 | 1.9 | ||
| Chronic obstructive pulmonary disease (COPD)i | No | 145941 | 12000 | 85.5 | 133941 | 95.4 | <0.0001 |
| Yes | 8485 | 2039 | 14.5 | 6446 | 4.6 | ||
| Asthmaj | No | 150035 | 12939 | 92.2 | 137096 | 97.7 | <0.0001 |
| Yes | 4391 | 1100 | 7.8 | 3291 | 2.3 | ||
| Allergic rhinitis11 | No | 145077 | 10223 | 72.8 | 134854 | 96.1 | <0.0001 |
| Yes | 9349 | 3816 | 27.2 | 5533 | 3.9 | ||
| Arrhythmial | No | 149443 | 13188 | 93.9 | 136255 | 97.1 | <0.0001 |
| Yes | 4983 | 851 | 6.1 | 4132 | 2.9 | ||
| Ischemic strokem | No | 151753 | 13729 | 97.8 | 138024 | 98.3 | <0.0001 |
| Yes | 2673 | 310 | 2.2 | 2363 | 1.7 | ||
| Intracerbral hemorrhagen | No | 150882 | 13594 | 96.8 | 137288 | 97.8 | <0.0001 |
| Yes | 3544 | 445 | 3.2 | 3099 | 2.2 | ||
| Medications | |||||||
| ACEIs | No | 148450 | 13348 | 95.1 | 135102 | 96.2 | <0.0001 |
| Yes | 5976 | 691 | 4.9 | 5285 | 3.8 | ||
| Beta-adrenergic blockers | No | 146248 | 12932 | 92.1 | 133316 | 95.0 | <0.0001 |
| Yes | 8178 | 1107 | 7.9 | 7071 | 5.0 | ||
| Statins | No | 151899 | 13727 | 97.8 | 138172 | 98.4 | <0.0001 |
| Yes | 2527 | 312 | 2.2 | 2215 | 1.6 | ||
| Steroid | No | 119028 | 8629 | 61.5 | 110399 | 78.6 | <0.0001 |
| Yes | 35398 | 5410 | 38.5 | 29988 | 21.4 | ||
| Development of EDo during follow-up | 1955 | 294 | 2.1 | 1661 | 1.2 | <0.0001 | |
aMatched by sex and age (±1 years old).
b1US$ = 32.8 NTD in 2007.
cICD-9: Hypertension (401-405).
dICD-9: Hyperlipidemia (272.2, 272.4).
eICD-9: Diabetes mellitus (250).
fICD-9: Obesity (278, 278.0, 278.00, 278.01).
gICD-9: Coronary heart disease (410-414, 429.2).
hICD-9: Chronic kidney disease (CKD) (580-587).
iICD-9: Chronic obstructive pulmonary disease (COPD)(491, 492, 494, 496).
jICD-9: Asthma (493).
kICD-9: Allergic rhinitis (477, 477.0, 477.1, 477.8, 477.9).
lICD-9: Arrhythmia (427, 785.0, 785.1).
mICD-9: Ischemic stroke (433-434, 436, 437.1).
nICD-9: Intracerbral hemorrhage (430-462.9).
oICD-9: Erectile dysfunction (ED) (607.84).
Association between CRS and risk of ED
Of the 154 426 subjects, 1955 (cases 294, 2.1%; controls 1661, 1.2%) were diagnosed with ED during the follow-up period. Twenty patients (2.6%) in the CRSwNP cohort and 274 (2.1%) in the CRSsNP cohort were later diagnosed with ED. The mean (SD) follow-up interval for the CRS cohort was 3.20 (2.33) years and for the non-CRS cohort was 2.97 (2.39) years. As shown in Table 2, CRS was positively associated with ED (HR = 1.51; 95% CI = 1.33–1.73; P < 0.0001) in the fully adjusted Cox regression model adjusted for sex, age, insurance premium, residence, hypertension, hyperlipidemia, diabetes, obesity, coronary heart disease, chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), asthma, allergic rhinitis, arrhythmia, ischemic stroke, intracerebral hemorrhage, and medications.
Table 2. Adjusted Cox regression analyses on erectile dysfunction (ED) in Taiwan, 1996–2007.
| Crude HRa | 95% CIb | P-value | Adjusted HR | 95% CIb | P-value | |
|---|---|---|---|---|---|---|
| Non-CRS | 1 | — | — | 1 | — | — |
| CRS | 1.78 | 1.57–2.02 | <0.0001 | 1.51 | 1.33–1.73 | <0.0001 |
| CRSsNP | 1.77 | 1.56–2.01 | <0.0001 | 1.50 | 1.31–1.72 | <0.0001 |
| CRSwNP | 1.95 | 1.26–3.04 | 0.0029 | 1.78 | 1.15–2.78 | 0.0106 |
*Contrast of CRSsNP versus CRSwNP, Estimate = 0.84 (0.54–1.32), P-value = 0.4552.
aHR: hazard ratio.
bCI: Confidence interval.
CRS w/s NP and risk of ED
Both CRSwNP and CRSsNP were associated with ED (HR = 1.78; 95% CI = 1.15–2.78; P = 0.0106 and HR = 1.50; 95% CI = 1.31–1.72, respectively; P < 0.0001) in the same fully adjusted Cox regression models. In the stratified analysis, however, there was no significant difference between the associations of CRSsNP and CRSwNP with ED [contrast of CRSsNP versus CRSwNP, estimate = 0.84 (0.54–1.32), P-value = 0.4552] (Table 2).
Stratified by age
To evaluate whether CRS is an age-dependent risk factor for ED, we divided the CRS patients into four age groups: 30–35 years, 36–50 years, 51–65 years, and >65 years. After adjusting Cox regression models for potential confounding factors, the highest adjusted HR for ED in the patients with CRS compared with the controls was 1.71 (95% CI = 1.36–2.14, P < 0.0001) in the patients aged 36–50 years (Table 3).
Table 3. Effecta of CRS on ED in Taiwan by age group, 1996–2007.
| Development of ED | Age group |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30–35 |
36–50 |
51–65 |
>65 |
|||||||||
| Study group | Comparison | P-Value | Study group | Comparison | P-Value | Study group | Comparison | P-Value | Study group | Comparison | P-Value | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |||||
| Yes | 19 (0.76) | 121 (0.48) | 105 (1.70) | 512 (0.83) | 94 (3.01) | 584 (1.87) | 76 (3.45) | 444 (2.02) | ||||
| Crude HRb (95% CIc) | 1.57 (0.97–2.55) | 1 | 0.0672 | 2.07 (1.68–2.55) | 1 | <0.0001 | 1.62 (1.31–2.02) | 1 | <0.0001 | 1.73 (1.35–2.20) | 1 | <0.0001 |
| Adjusted HR (95% CI) | 1.39 (0.83–2.34) | 1 | 0.2134 | 1.71 (1.36–2.14) | 1 | <0.0001 | 1.27 (1.03–1.60) | 1 | 0.0485 | 1.47 (1.14–1.91) | 1 | 0.0036 |
aAdjusted Cox regression analyses controlling by sex, age, insurance premium, residence, hypertension, hyperlipidemia, diabetes, obesity, coronary heart disease, chronic kidney disease, chronic obstructive pulmonary disease, asthma, allergic rhinitis, arrhythmia, ischemic stroke, intracerebral hemorrhage, and medications.
bHR: hazard ratio.
cCI: Confidence interval.
Kaplan–Meir analysis of cumulative incidence from ED revealed that patients with CRS had a significantly higher incidence of ED than the non-CRS group (P < 0.0001, based on a modified log-rank test) (Fig. 1). The cumulative incidence of ED in the CRS w/s NP cohort was also higher than that in the non-CRS cohort (P < 0.0001) (Fig. 2).
Figure 1. Cumulative incidence on erectile dysfunction by study groups [CRS (dashed line) VS Non-CRS (solid line)].
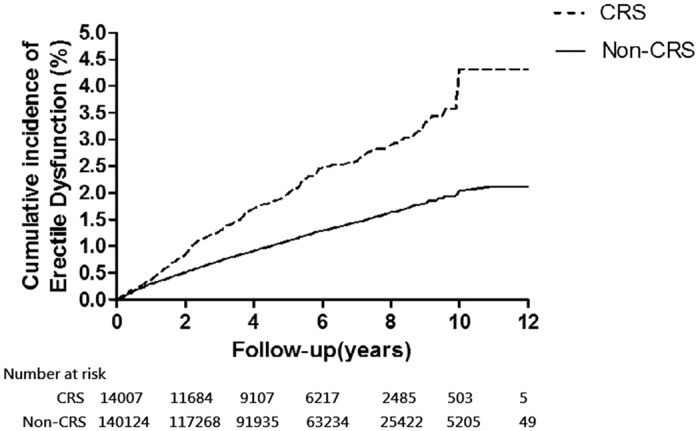
Modified log-rank test p value < 0.0001.
Figure 2. Cumulative incidence of erectile dysfunction in CRSwNP (fine dashed line), CRSsNP (thick dashed line), and Non-CRS (solid line).
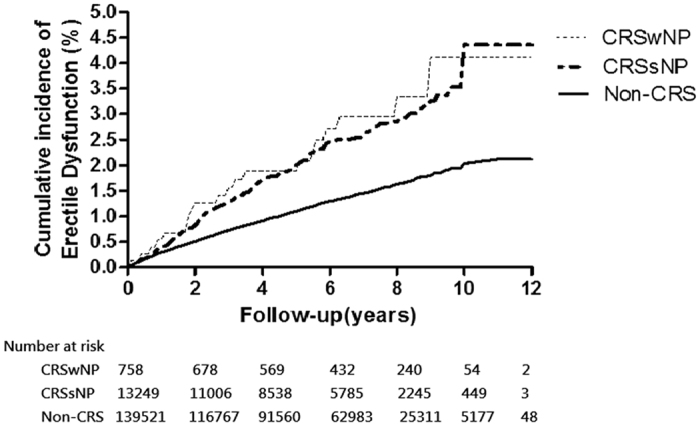
Modifed log-rank test p value < 0.0001.
Discussion
In this large-scale nationwide study using claims data from Taiwan, we observed that although comorbid diseases were more likely to be prevalent in the CRS cohort than in the control cohort, CRS remained an independent risk factor for the development of ED after adjustment for covariates. Patients with CRS were found to be at significantly increased risk of ED (HR = 1.51; 95% CI = 1.33–1.73; P < 0.0001), after adjustment for possible confounding factors.
Treatment of CRS has been found to improve ED. A hospital-based study by Gunhan et al. showed that ED was more common in patients with CRSwNP than in control patients, subjectively measured using the International Index of Erectile Function (IIEF-EF) (P = 0.009) and objectively measured by nocturnal penile tumescence (NPT) (P = 0.0118)12. Their results indicated the presence of ED in 34% (IIEF-EF) and 24% (NPT) of their patients with CRSwNP. After comparing the pre- and postoperative results of the CRSwNP patients, the authors further found erectile function to be significantly improved after functional endoscopic sinus surgery12. A hospital-based study by Benninger et al., who used the Rhinosinusitis Disability Index, a validated instrument that measures the physical, functional, and emotional impact of CRS on a person’s quality of life (QoL), found that patients with CRS had significantly improved sexual function scores after surgery for their CRS (P < 0.001)11.
The exact mechanism underlying the development of ED in CRS patients remains unknown. The relationship between CRS and ED could conceivably be influenced by a number of pathophysiologic factors, including QoL, hypoxia, and inflammation. The inflammatory biomarker and cytokines that link CRS and ED may include C-reactive protein (CRP), tumor necrosis factor alpha (TNF-α), and transforming growth factor beta (TGF-β). Some consequences of CRS, including low QoL, hypoxia, and cardiovascular disorders, might increase the risk of ED in a select subpopulation of patients with CRS.
Videler et al., administering the Short-Form health survey (SF-36), found that patients with CRS had significantly worse QoL scores than patients with other chronic conditions, including head and neck cancer, hypertension, angina pectoris, and migraine22. Interestingly, Ottaviano et al. observed a significant association between olfactory sensitivity and sexual desire in young adults (P = 0.02)23. Thus, the CRS symptoms of nasal obstruction, rhinorrhea, smell dysfunction, facial pressure, headache, and postnasal drainage might reduce libido leading to ED.
Ozdemir et al. found that patients with CRSwNP had hypoxia and nasal surgery led to increased blood oxygen saturation24. Hypoxia increases afferent sympathetic activation, resulting in increased vasoconstriction and significantly reduced nitric oxide (NO) synthase activity, which is a rate-limiting factor for NO production in the penile corpus cavernosum25. Studies have suggested that NO is a bronchodilator and vasodilator involved in mucocililary-regulating activities26. Lindberg et al. reported that their patients with CRS had lower concentrations of nasal NO than their healthy control patients27. Studies have also shown an inverse correlation between nasal NO and nasal polyp size28. Ragab et al. observed that nasal NO levels increased after the medical and surgical treatment of CRS26. NO plays a crucial role in the molecular mechanism of penile smooth muscle relaxation9, and decreased levels of NO can contribute to ED. In addition, Verratti et al. revealed that men who are sexually potent at sea level had pathological rigidometric records at a high altitude in a hypoxic environment25. Therefore, a selected subpopulation of CRS patients with hypoxia may develop ED.
Recent epidemiological studies have identified an increased risk of subsequent stroke and acute myocardial infarction in patients with CRS13,14,15. In previous studies on CRS patients, the HRs of stroke, ischemic stroke, and acute myocardial infarction were 1.34 (95% CI = 1.04–1.74)13, 1.34 (95% CI = 1.18–1.53), and 1.48 (95% CI = 1.32–1.67)15, respectively, after adjustment for potential confounding factors. CRS is defined as a group of disorders associated with inflammation of the nasal mucosa and sinuses lasting at least 12 weeks29. Inflammation is thought to play a central role in the pathogenesis of atherosclerotic initiation, plaque rupture, thrombosis, and stroke30. In the first stages of atherosclerosis, the penis is typically first affected, manifesting as ED, making ED a possible warning sign that a heart attack or stroke is imminent, often within 3 to 5 years31. Thompson et al. proposed that ED is a sentinel symptom in patients with occult cardiovascular disease10, suggesting that CRS could be a risk factor for ED.
Elevation of the systemic inflammatory marker, CRP, has been reported to correlate significantly with increasing severity of penile vascular disease in men with ED as measured by penile Doppler32. In a study by Giugliano et al., circulating CRP levels were significantly higher in obese men with ED than in obese men without ED (P < 0.05)33. In other studies, patients with sinusitis have had higher CRP levels compared with control patients34,35. Hirshoren et al. observed a significant association between CRP levels and the severity of sinusitis36, suggesting that CRP might play a direct role in the pathogenesis of atherosclerosis. These findings have led to the hypothesis that CRS might induce local inflammation in the upper airway and induce systemic inflammatory effects that contribute to ED. CRP might thus play a role in both CRS and ED.
TNF-α might also play a key role in inducing ED or contribute to the pathophysiology of ED. Oyer et al. reported that the mucus of patients with CRSwNP had higher levels of the proinflammatory cytokine TNF-α than their control patients37. Carneiro et al. found increased serum TNF-α levels in patients with moderate to severe ED38.
The fibrogenic cytokine TGF-β plays key roles in CRS and ED. Van Crombruggen et al. observed upregulated TGF-β expression in patients with CRSsNP39. In a study by Ryu et al., plasma TGF-β expression was significantly higher in ED patients than in control patients40. Previous studies have also revealed that TGF-β is involved in cavernous fibrosis, promoting the development of vasculogenic ED in men41.
Our results indicate a stronger association between CRS and ED men aged ≥35 years than in other age groups, particularly in men aged 36–50 years (Table 3). However, the mechanism underlying these age-associated effects remains unclear.
Strengths and limitations
The key strengths of this study are its nationally representative sample, physician-based diagnosis to identify CRS cases, longitudinal analysis, and range of covariates considered, including sociodemographic factors and general health status. However, this study has some limitations, particularly those caused by the use of routine claims data, which do not include information on other potential confounders such as severity of CRS status, stressful life events, smoking, air pollution, body mass index, and family history. This study was conducted in Taiwan. Whether our findings can be extrapolated to other countries is not known.
Conclusion
In summary, this study found an association between having CRS and a significantly higher risk of developing ED than the general population, regardless of age, presence of comorbidity, and medications. Because of the high (and potentially increasing) prevalence of CRS, clinicians should be aware of the risk of ED in CRS patients. Further research is required to determine the underlying mechanism linking these two adverse health outcomes.
Additional Information
How to cite this article: Tai, S.-Y. et al. Chronic Rhinosinusitis Associated with Erectile Dysfunction: A Population-Based Study. Sci. Rep. 6, 32195; doi: 10.1038/srep32195 (2016).
Acknowledgments
The authors thank the Statistical Analysis Laboratory, Department of Medical Research, Kaohsiung Medical University Hospital, Kaohsiung Medical University, for their assistance. This work was supported by grants from the Kaohsiung Municipal Hsiao-Kang Hospital (Kmhk-103–032), Kaohsiung Medical University Hospital (KMUH103-3R35), the Ministry of Science and Technology (MOST 104-2314-B-037-033), and Research Center for Environmental Medicine, Kaohsiung Medical University (KMU-TP104A34). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
Footnotes
Author Contributions Conceived and designed the experiments: S.-Y.T., L.-F.W., C.-F.T., Y.-T.H. and C.-Y.C. Performed the experiments: S.-Y.T. and C.-Y.C. Analyzed the data: S.-Y.T. and Y.-T.H. Prepare Tables and Figure: S.-Y.T., L.-F.W. and C.-Y.C. Wrote the paper: S.-Y.T., L.-F.W., C.-F.T., Y.-T.H. and C.-Y.C. All authors reviewed the manuscript.
References
- Zara M. & Patel P. H. H. In Bailey’s head and neck surgery-otolaryngology Vol. 1 (ed Clark A. Rosen., Jonas T. Johnson) Ch. 35, 535–549 (Lippincott Williams & Wilkins, 2014). [Google Scholar]
- Su V. Y. et al. Allergic rhinitis and risk of erectile dysfunction–a nationwide population-based study. Allergy 68, 440–445, doi: 10.1111/all.12100 (2013). [DOI] [PubMed] [Google Scholar]
- Chou K. T. et al. Asthma and risk of erectile dysfunction–a nationwide population-based study. J Sex Med 8, 1754–1760, doi: 10.1111/j.1743-6109.2011.02242.x (2011). [DOI] [PubMed] [Google Scholar]
- Shen T. C. et al. The risk of erectile dysfunction in chronic obstructive pulmonary disease: a population-based cohort study in Taiwan. Medicine (Baltimore) 94, e448, doi: 10.1097/MD.0000000000000448 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadag F., Ozcan H., Karul A. B., Ceylan E. & Cildag O. Correlates of erectile dysfunction in moderate-to-severe chronic obstructive pulmonary disease patients. Respirology 12, 248–253, doi: 10.1111/j.1440-1843.2006.01042.x (2007). [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C., Rokkas K., Ioakeimidis N. & Stefanadis C. Inflammation, metabolic syndrome, erectile dysfunction, and coronary artery disease: common links. European urology 52, 1590–1600, doi: 10.1016/j.eururo.2007.08.004 (2007). [DOI] [PubMed] [Google Scholar]
- Gandaglia G. et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. European urology 65, 968–978, doi: 10.1016/j.eururo.2013.08.023 (2014). [DOI] [PubMed] [Google Scholar]
- Droller MJ A. J., Beck JC et al. NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence. JAMA 270, 83–90 (1993). [PubMed] [Google Scholar]
- McVary K. T. Clinical practice. Erectile dysfunction. N Engl J Med 357, 2472–2481, doi: 10.1056/NEJMcp067261 (2007). [DOI] [PubMed] [Google Scholar]
- Thompson I. M. et al. Erectile dysfunction and subsequent cardiovascular disease. JAMA 294, 2996–3002, doi: 10.1001/jama.294.23.2996 (2005). [DOI] [PubMed] [Google Scholar]
- Benninger M. S., Khalid A. N., Benninger R. M. & Smith T. L. Surgery for chronic rhinosinusitis may improve sleep and sexual function. Laryngoscope 120, 1696–1700, doi: 10.1002/lary.21010 (2010). [DOI] [PubMed] [Google Scholar]
- Gunhan K., Zeren F., Uz U., Gumus B. & Unlu H. Impact of nasal polyposis on erectile dysfunction. Am J Rhinol Allergy 25, 112–115, doi: 10.2500/ajra.2011.25.3585 (2011). [DOI] [PubMed] [Google Scholar]
- Wu C. W., Chao P. Z., Hao W. R., Liou T. H. & Lin H. W. Risk of stroke among patients with rhinosinusitis: a population-based study in Taiwan. Am J Rhinol Allergy 26, 278–282, doi: 10.2500/ajra.2012.26.3783 (2012). [DOI] [PubMed] [Google Scholar]
- Kang J. H., Wu C. S., Keller J. J. & Lin H. C. Chronic rhinosinusitis increased the risk of stroke: a 5-year follow-up study. Laryngoscope 123, 835–840, doi: 10.1002/lary.23829 (2013). [DOI] [PubMed] [Google Scholar]
- Wang P. C., Lin H. C. & Kang J. H. Chronic rhinosinusitis confers an increased risk of acute myocardial infarction. Am J Rhinol Allergy 27, e178–182, doi: 10.2500/ajra.2013.27.3952 (2013). [DOI] [PubMed] [Google Scholar]
- Institutes N. H. R. Introduction to the National Health Insurance Research Database (NHIRD), Taiwan http://nhird.nhri.org.tw/en/index.htm. Last accessed: December 2013 (2013). [Google Scholar]
- Chien C. Y., Tai S. Y., Wang L. F. & Lee C. T. Chronic obstructive pulmonary disease predicts chronic rhinosinusitis without nasal polyps: A population-based study. Am J Rhinol Allergy 29, e75–80, doi: 10.2500/ajra.2015.29.4172 (2015). [DOI] [PubMed] [Google Scholar]
- Lanza D. C. & Kennedy D. W. Adult rhinosinusitis defined. Otolaryngol Head Neck Surg 117, S1–7 (1997). [DOI] [PubMed] [Google Scholar]
- Rosenfeld R. M. et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg 137, S1–31, doi: 10.1016/j.otohns.2007.06.726 (2007). [DOI] [PubMed] [Google Scholar]
- Montague D. K. et al. Chapter 1: The management of erectile dysfunction: an AUA update. J Urol 174, 230–239, doi: 10.1097/01.ju.0000164463.19239.19 (2005). [DOI] [PubMed] [Google Scholar]
- Lue T. F. et al. Summary of the recommendations on sexual dysfunctions in men. J Sex Med 1, 6–23, doi: 10.1111/j.1743-6109.2004.10104.x (2004). [DOI] [PubMed] [Google Scholar]
- Videler W. J., van Drunen C. M., van der Meulen F. W. & Fokkens W. J. Radical surgery: effect on quality of life and pain in chronic rhinosinusitis. Otolaryngol Head Neck Surg 136, 261–267, doi: 10.1016/j.otohns.2006.08.010 (2007). [DOI] [PubMed] [Google Scholar]
- Ottaviano G. et al. Olfactory sensitivity and sexual desire in young adult and elderly men: an introductory investigation. Am J Rhinol Allergy 27, 157–161, doi: 10.2500/ajra.2013.27.3879 (2013). [DOI] [PubMed] [Google Scholar]
- Ozdemir R. et al. Loss of nocturnal decline of blood pressure in patients with nasal polyposis. Blood pressure 8, 165–171 (1999). [DOI] [PubMed] [Google Scholar]
- Verratti V. et al. The role of hypoxia in erectile dysfunction mechanisms. Int J Impot Res 19, 496–500, doi: 10.1038/sj.ijir.3901560 (2007). [DOI] [PubMed] [Google Scholar]
- Ragab S. M., Lund V. J., Saleh H. A. & Scadding G. Nasal nitric oxide in objective evaluation of chronic rhinosinusitis therapy. Allergy 61, 717–724, doi: 10.1111/j.1398-9995.2006.01044.x (2006). [DOI] [PubMed] [Google Scholar]
- Lindberg S., Cervin A. & Runer T. Nitric oxide (NO) production in the upper airways is decreased in chronic sinusitis. Acta Otolaryngol 117, 113–117 (1997). [DOI] [PubMed] [Google Scholar]
- Colantonio D., Brouillette L., Parikh A. & Scadding G. K. Paradoxical low nasal nitric oxide in nasal polyposis. Clin Exp Allergy 32, 698–701 (2002). [DOI] [PubMed] [Google Scholar]
- Benninger M. S. et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg 129, S1–32 (2003). [DOI] [PubMed] [Google Scholar]
- Elkind M. S. Inflammatory mechanisms of stroke. Stroke 41, S3–8, doi: 10.1161/STROKEAHA.110.594945 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz B. G. & Kloner R. A. Cardiology patient page: cardiovascular implications of erectile dysfunction. Circulation 123, e609–611, doi: 10.1161/CIRCULATIONAHA.110.017681 (2011). [DOI] [PubMed] [Google Scholar]
- Billups K. L. et al. Relation of C-reactive protein and other cardiovascular risk factors to penile vascular disease in men with erectile dysfunction. Int J Impot Res 15, 231–236, doi: 10.1038/sj.ijir.3901012 (2003). [DOI] [PubMed] [Google Scholar]
- Giugliano F. et al. Erectile dysfunction associates with endothelial dysfunction and raised proinflammatory cytokine levels in obese men. J Endocrinol Invest 27, 665–669, doi: 10.1007/BF03347500 (2004). [DOI] [PubMed] [Google Scholar]
- Hansen J. G., Hojbjerg T. & Rosborg J. Symptoms and signs in culture-proven acute maxillary sinusitis in a general practice population. APMIS 117, 724–729, doi: 10.1111/j.1600-0463.2009.02526.x (2009). [DOI] [PubMed] [Google Scholar]
- Cals J. W., Schot M. J., de Jong S. A., Dinant G. J. & Hopstaken R. M. Point-of-care C-reactive protein testing and antibiotic prescribing for respiratory tract infections: a randomized controlled trial. Annals of family medicine 8, 124–133, doi: 10.1370/afm.1090 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshoren N., Hirschenbein A. & Eliashar R. Risk stratification of severe acute rhinosinusitis unresponsive to oral antibiotics. Acta Otolaryngol 130, 1065–1069, doi: 10.3109/00016481003645727 (2010). [DOI] [PubMed] [Google Scholar]
- Oyer S. L., Mulligan J. K., Psaltis A. J., Henriquez O. A. & Schlosser R. J. Cytokine correlation between sinus tissue and nasal secretions among chronic rhinosinusitis and controls. Laryngoscope 123, E72–78, doi: 10.1002/lary.24305 (2013). [DOI] [PubMed] [Google Scholar]
- Carneiro F. S., Webb R. C. & Tostes R. C. Emerging role for TNF-alpha in erectile dysfunction. J Sex Med 7, 3823–3834, doi: 10.1111/j.1743-6109.2010.01762.x (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Crombruggen K., Zhang N., Gevaert P., Tomassen P. & Bachert C. Pathogenesis of chronic rhinosinusitis: inflammation. J Allergy Clin Immunol 128, 728–732, doi: 10.1016/j.jaci.2011.07.049 (2011). [DOI] [PubMed] [Google Scholar]
- Ryu J. K. et al. Plasma transforming growth factor-beta1 levels in patients with erectile dysfunction. Asian J Androl 6, 349–353 (2004). [PubMed] [Google Scholar]
- Ryu J. K. et al. Expression of cavernous transforming growth factor-beta1 and its type II receptor in patients with erectile dysfunction. Int J Androl 27, 42–49 (2004). [DOI] [PubMed] [Google Scholar]


