Th17 cells are stalled in inflamed lungs, delaying the onset of EAE
Keywords: autoimmunity, autophagy, CCL20, T-cell recruitment
Abstract
Recruiting pathogenic T cells to the central nervous system (CNS) is a critical step during the development of experimental autoimmune encephalomyelitis (EAE). Here, we report that the absence of autophagy and microtubule-associated protein 1A/1B-light chain 3-associated phagocytosis significantly delayed the onset of EAE in Atg7 conditional knockout (Atg7 CKO) mice in myeloid cells. T-helper cell-cell priming appeared to be normal in the Atg7 CKO mice, but the mice showed significant accumulation of Th17 cells in the lung. The data suggested that the stalling of Th17 cells in the lung en route to the CNS caused the delay. The lung of Atg7 CKO mice, in which we previously demonstrated spontaneous mild inflammation, showed high expression of CCL20, a chemokine that attracts Th17 cells. We have also shown that LPS intranasal instillation delayed EAE onset, suggesting that pulmonary inflammation has an impact on EAE development. Based on our data, therapeutic immunomodulation targeted to the lung, rather than systemically, might be a possible future option to treat multiple sclerosis.
Introduction
The lung possesses a unique immune environment that maintains homeostasis by inhibiting excessive immune responses against harmless environmental stimuli taken up by inhalation (1). However, it is still largely unknown how pulmonary immune activation impacts the development of autoimmune inflammation in the central nervous system (CNS). A previous report demonstrated that the lung is a ‘hub’ organ that licenses T cells migrating into the CNS during the development of experimental autoimmune encephalomyelitis (EAE) (2), an animal model of multiple sclerosis (MS). Indeed, epidemiological studies have implied a relationship between the lung and the pathology of MS, such as smoking increasing the risk of MS (3, 4), although the molecular mechanism is not fully elucidated. Therefore, it is highly possible that modulating the lung microenvironment alters the pathology of neuroinflammatory diseases.
ATG7 is required for autophagy and microtubule-associated protein 1A/1B-light chain 3 (LC3)-asscociated phagocytosis (LAP). The authors along with another group have recently found that the lack of ATG7 in myeloid cells can disturb the delicate balance of immune homeostasis and induce spontaneous and subclinical inflammation in the lung (1, 5). Our previous study demonstrated that pulmonary inflammation in Atg7 myeloid cell conditional knockout (Atg7 CKO; Atg7 fl/fl LysM-Cre) mice is caused by increased bacterial burdens in the lung during the suckling period (1). (Adult mice do not show detectable levels of bacterial burdens both in wild type (WT) and in Atg7 CKO mice (1).) In addition, Atg7 CKO mice showed increased sensitivity of alveolar macrophages (AMs) to TLR4 ligands, suggesting AM hypersensitivity as another cause of the lung inflammation (1). Since intranasally instilled antibiotics ameliorated the spontaneous lung inflammation, bacteria in the lung are a major trigger of inflammation (1). Interestingly, the spontaneous inflammation in Atg7 CKO mice was limited to the lung and was not observed in other organs including the mesenteric lymph node, colon, small intestine, liver, kidney, skin, spleen, brain and spinal cord (1). The lung-specific inflammatory phenotype of Atg7 CKO mice is considered to be a reflection of the lung’s constant exposure to the outer environment and the subsequent hyperresponsiveness to harmless stimuli.
In this study, we sought to elucidate the impact of lung inflammation on EAE development. CD4+ T-cell priming and T-helper cell polarization were normal in the draining lymph nodes DLNs of Atg7 CKO mice. However, T-helper cells, and Th17 cells in particular, were entrapped in the lungs of Atg7 CKO mice, resulting in delayed EAE onset. Intranasal instillation of LPS in WT mice also resulted in delayed EAE onset with Th17 accumulation in lungs. Therefore, lung inflammation, rather than autophagy and/or LAP, was sufficient to modulate the disease course of EAE. These results strongly suggest the involvement of the lung in the EAE pathophysiology, particularly in Th17-cell migration into the CNS. Thus, modulating local immune responses in the lung may be a novel therapeutic approach to treat MS.
Methods
Animals and reagents
All mice used in this study were on the C57BL/6 background. Atg7 fl/fl mice were described previously (1, 6, 7). Lysozyme M (LysM)cre/cre and 2D2 TCR transgenic mice specific to myelin oligodendrocyte glycoprotein (MOG) were purchased from Jackson Laboratories. All the experiments were performed as approved by the Institutional Animal Care and Use Committee. Antibodies against CD45, CD4, CD3, CCR6, IL-17, Foxp3 and IFN-γ were purchased from BioLegend. MOG35–55 peptide was synthesized by New England Peptides. Recombinant IL-6 (rIL-6), rIL-23 and rTGF-β were purchased from BD Biosciences. Neutralizing antibody for IFN-γ was purchased from BD Biosciences.
EAE induction and LPS instillation
Active EAE was induced by immunizing the mice with MOG35–55 peptide (100 μg) emulsified in complete Freund’s adjuvant (Sigma) containing heat-killed Mycobacterium tuberculosis H37Ra (200 μg per mouse, DIFCO Laboratories) on day 0, and intraperitoneal injection of 200ng pertussis toxin (PTx; List Biological Laboratories) on days 0 and 2. To induce Th17-mediated passive EAE, CD4+ T cells, obtained from 2D2 mice, were polarized with rIL-6 (20ng ml–1), rIL-23 (10ng ml–1), rTGF-β (3ng ml–1) and anti-IFN-γ antibody (4 μg ml–1) on a plate coated with anti-CD3 and anti-CD28 antibodies for 5 days and adoptively transferred to sublethally irradiated (450 rads) WT and Atg7 CKO mice. PTx (200ng) was intraperitoneally administrated on days 0 and 2. Intranasal instillation of LPS (2.5 μg per instillation) was performed on days 6 and 9 after EAE induction. EAE severity was scored as previously described (8, 9).
Flow cytometry analyses
Lung tissues were cut into small pieces and incubated in a 1-mg ml–1 collagenase D solution at 37°C for 30min. Cells were then enriched by Percoll gradient (GE Healthcare). After staining with specific antibodies, cells were analyzed with FACS Canto™ II (BD Biosciences) and the FlowJo software (Treestar Inc.). For intracellular cytokine staining, cells were stimulated with PMA (50ng ml–1) and ionomycin (500ng ml–1) for 5h and treated with GolgiPlug (BD Biosciences) for the last 3h. Cell surface markers were stained first, then intracellular cytokines were stained as previously described (8, 9).
Real-time PCR analysis
Total mRNA were reverse transcribed to cDNA, and gene expression levels were determined by using the −ΔΔC t method of real-time PCR as previously described (1, 7–9) using primers for Ccl20 (forward: 5ʹ-AAGACAGATGGCCGATGAAG-3ʹ, reverse: 5ʹ-TCTTGACTCTTAGGCTGAGGA-3ʹ), Tnfa (forward: 5ʹ-CCCTCACACTCAGATCATCTTCT-3ʹ, reverse: 5ʹ-GCTACG ACGTGGGCTACAG-3ʹ), Il6 (forward: 5ʹ-GAGGATACCACT CCCAACAGACC-3ʹ, reverse: 5ʹ-AAGTGCATCATCGTTGTTCATACA-3ʹ), Il10 (forward: 5ʹ-GGTTGCCAAGCCTTATCGGA-3ʹ, reverse: 5ʹ-ACCTGCTCCACTGCCTTGCT-3ʹ), Cxcl1 (forward: 5ʹ-TGGGATTCACCTCAAGAACA-3ʹ, reverse: 5ʹ-TTTCTGAAC CAAGGGAGCTT-3ʹ), Ccl3 (forward: 5ʹ-TGCTTCTCCTACAGC CGGAAGATT-3ʹ, reverse: 5ʹ-TCAGGCATTCAGTTCCAGGT CAGT-3ʹ) and Actb (forward: 5ʹ-GTTACCAACTGGGAC GACA-3ʹ, reverse: 5ʹ-CTGGGTCATCTTTTCACGGT-3ʹ). Actb expression was used as the internal control. Results shown are representatives from multiple independent experiments with similar results.
Statistical analysis
The two-tailed Student’s t-test was used for statistical analyses.
Results and discussion
Atg7 CKO mice delayed EAE onset by accumulating Th17 cells in the lung
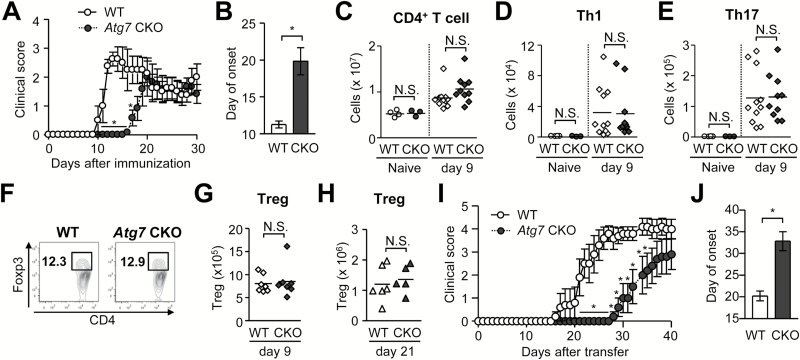
ATG7 is essential for autophagy and LAP (10, 11). To evaluate the impact of ATG7 in myeloid cells during EAE, we induced EAE in Atg7 fl/fl LysM cre/+ mice (hereafter denoted as ‘Atg7 CKO mice’) with LysM cre/+ mice as control (hereafter denoted as ‘WT mice’). Atg7 CKO mice develop spontaneous, but subclinical, pulmonary inflammation (1). We found that Atg7 CKO mice showed significantly delayed onset of EAE (Fig. 1A and B). However, on day 9 when T cells have been primed but not infiltrated in CNS, the mice did not show altered numbers of total CD4+ T cells, Th1, Th17 (Fig. 1C, D and E) and Treg cells (Fig. 1F and G) in DLNs (8). Even at a later stage (day 21), the number of Tregs in DLNs in Atg7 CKO mice was comparable with that in WT mice (Fig. 1H). The result suggests that CD4+ T-cell priming and T-helper cell polarization were normal in the DLNs of Atg7 CKO mice. We had initially expected reduced Th17 cell development from the lack of autophagy, as autophagy inhibits NLRP3 inflammasome activity and reduces the expression of IL-1β, which induces Th17 responses (12). However, Th17 cell development in DLNs appeared to be normal even without ATG7 in myeloid cells. To evaluate the possibility that Atg7 deficiency in myeloid cells changes T-cell pathogenicity and whether it causes the delayed EAE onset, we induced EAE in WT and Atg7 CKO mice by adoptively transferring Th17-polarized MOG-specific 2D2 CD4+ T cells (Fig. 1I and J). Atg7 CKO recipients exhibited significantly delayed onset, suggesting that the delay of EAE onset in Atg7 CKO mice is rather due to the lack of ATG7 in recipients.
Fig. 1.
Delayed onset and mild EAE in Atg7 CKO mice. (A, B) Time-course of EAE score (A) and average day of EAE onset (B). WT (n = 6). Atg7 CKO (n = 4). Error bars denote mean ± SD. *p < 0.05. (C–E) Numbers of CD4+ T cell (C), Th1 (D), Th17 (E) cells in DLNs on days 0 and 9 after EAE induction. (F) Representative flow cytometry charts of CD3-positive pre-gated cells in DLNs on day 9. (G and H) Numbers of Tregs (Foxp3+CD4+) in DLNs on day 9 (G) or day 21 (H). (I and J) Clinical score (I) and onset (J) of passive EAE. CD4+ T cells obtained from 2D2 mice were cultured with plate-coated anti-CD3 and anti-CD28 antibodies (5 μg ml−1 each) in Th17-polarizing condition for 5 days and adoptively transferred to sub-lethally irradiated WT and Atg7 CKO recipients. n = 5 per group. One data point reflects a result from one mouse. Horizontal lines denote average values. Data are representative of two independent experiments. N.S.: not significant.
Notably, a previous report demonstrated that Atg7 deficiency in DCs (Atg7 fl/flCD11c-Cre) did not alter the time of EAE onset, but the disease severity was significantly milder than in WT mice (13). The difference between the phenotype of their results and ours is intriguing, but ultimately, they should not be compared due to differences in doses of reagents used in EAE induction. (We have previously demonstrated that the method of EAE induction has significant impact on disease development (9).) Nevertheless, the different outcome in our result from the DC-specific Atg7-deficient mice suggests that macrophages, AMs (1, 5) in particular, are involved in delaying EAE onset.
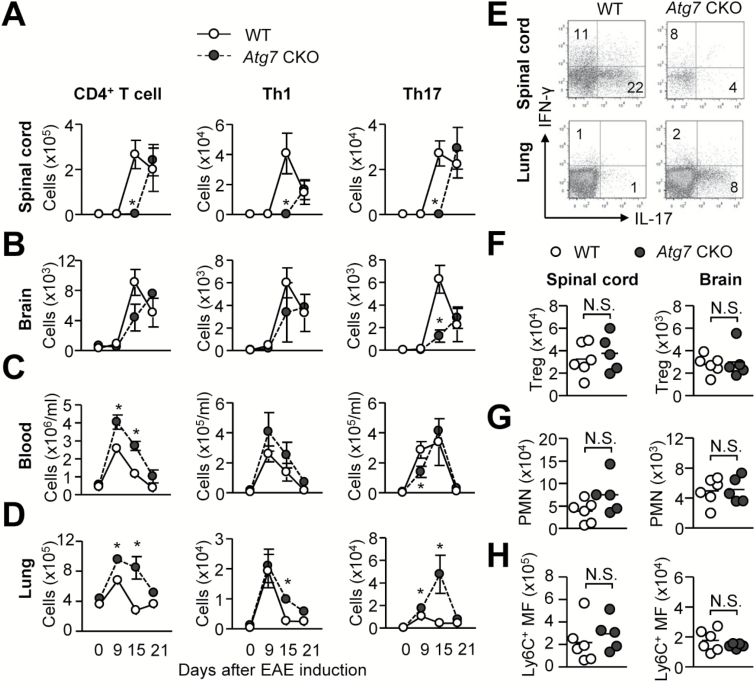
To understand why the onset is delayed in Atg7 CKO mice, we examined the numbers of total CD4+ T cells, Th1, and Th17 cells during EAE development in spinal cords, brains, circulating blood and lungs. We evaluated lungs because a previous publication demonstrated that T cells transiently reside within the lung during migration to the CNS (2). First, we noticed the absence of total CD4+ T cells, Th1 and Th17 cells in spinal cords of Atg7 CKO mice on day 15, although these cells were found on day 21 (Fig. 2A), suggesting a significant delay in T-helper cell migration. Atg7 CKO brains also showed significant delay in T-cell accumulation, although only with Th17 cells (Fig. 2B). In contrast, lungs and circulating blood of Atg7 CKO mice generally showed more CD4+ T cells than WT mice on days 9 and 15 (Fig. 2C and D). A closer look at the time-course data indicated a notable stall of Th17 cells in lungs of Atg7 CKO mice (Fig. 2D). Thus, Atg7 CKO mice accumulated Th17 cells in the lung, but were yet to fully recruit Th17 cells in the CNS as WT mice did on day 15 (Fig. 2A, B, D and E). The retention of CD4+ T cells is considered to be mediated mainly by chemokine receptors and adhesion molecules; therefore, T-cell encephalitogenicity itself is most probably not a major factor of their retention in the lung. At a later stage of EAE on day 21, CD4+ T cells, including Tregs, eventually achieved their migration to the CNS (Fig. 2A, B, F). On day 21, neutrophils and monocytes/macrophages also successfully migrated to the CNS in Atg7 CKO mice to the comparable level to those in WT mice (Fig. 2G–H). In sum, the results strongly suggested that the delay in EAE onset in Atg7 CKO mice was due to the accumulation of Th17 cells in the lung.
Fig. 2.
Time-course of CD4+ T-cell numbers during EAE development. Numbers of total CD4+ T cells, Th1 and Th17 cells were assessed in spinal cords (A), brains (B), circulating blood (C) and lungs (D) on day 0 (n = 3), 9 (n = 8), 15 (n = 5) and day 21 (n = 3). (E) Representative flow plots of CD4+ T cells (gated on CD45+CD3+CD4+) in the lung and spinal cord of WT and Atg7 CKO mice on day 15. (F–H) Numbers of Tregs (F), neutrophils (PMN) (G) and Ly6C+ monocytes (H) in the spinal cord and brain on day 21. n = 6 per group. Data are representative of two independent experiments. Error bars denote mean ± SD. *p < 0.05.
Ccl20 mRNA is highly expressed in the lung of Atg7 CKO mice
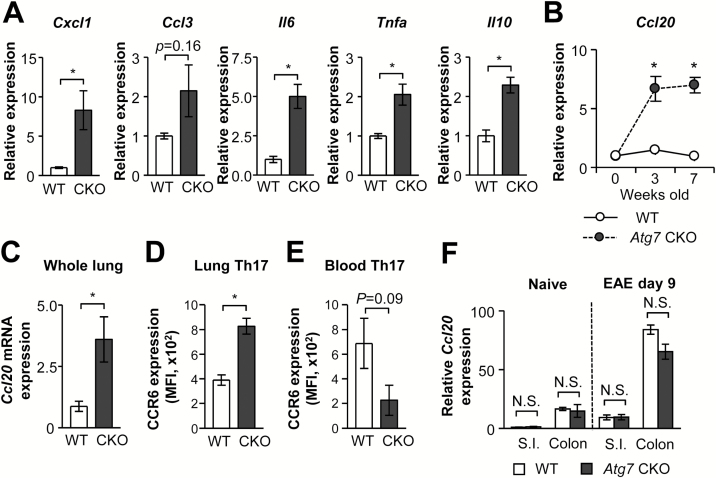
To investigate this ‘stalling’ of Th17 cells in the lung of Atg7 CKO mice, we examined cytokine gene expression in total lung tissues. The genes examined were Cxcl1, Ccl3, Il6, Tnfa and Il10, and most of them were up-regulated in Atg7 CKO lungs (Fig. 3A). As we have previously reported, the activation of these genes was due to the spontaneous pulmonary inflammation in Atg7 CKO mice (1). Because of the accumulation of Th17 cells in the lung during EAE development (Fig. 2D and E), we then examined the gene expression of CCL20, a Th17-cell chemoattractant, in Atg7 CKO lungs. CCL20 is a ligand of CCR6, highly expressed in Th17 cells, and particularly plays a critical role in EAE development by attracting Th17 cells to the CNS (13, 14). We found elevated Ccl20 mRNA expression in lungs of naive (Fig. 3B) and EAE-induced (Fig. 3C) Atg7 CKO lungs. We also found that Th17 cells in Atg7 CKO lungs expressed higher levels of CCR6, compared with Th17 cells in WT lungs on day 9 (Fig. 3D). CCR6 expression is also critical to progress EAE, as CCR6 deficiency in pathogenic CD4+ T cells abates their recruitment into CNS and EAE development (13, 15). In contrast, circulating Th17 cells in Atg7 CKO mice did not show elevated CCR6 expression (Fig. 3E). We also examined other major mucosal tissues that have direct contacts to the outer environment—the small intestine and colon. In contrast to the lung, no difference was found in Ccl20 mRNA expression in the gut between WT and Atg7 CKO mice regardless of EAE induction (Fig. 3F), suggesting that enhanced Ccl20 mRNA expression in Atg7 CKO mice is specific to the lung. These results suggest involvement of the CCL20/CCR6 axis in the lung to cause the delay of EAE onset.
Fig. 3.
Expression of Ccl20 mRNA and CCR6 protein. (A) mRNA expression of Cxcl1, Ccl3, Il6, Tnfa and Il10 in the lungs of WT and Atg7 CKO mice 9 days after EAE induction. n = 3 per group. (B) Ccl2 mRNA expression in the lungs of ‘naive’ WT and Atg7 CKO mice. n = 3 per group. (C) Ccl20 mRNA expression in lungs on day 9 after EAE induction. Three mice per group. (D, E) CCR6 expression on Th17 cells in lungs (D) and circulating blood (E) on day 9. Three mice per group. (F) Ccl20 expression in small intestines (S.I.) and colons obtained from days 0 and 9 after EAE induction. n = 3 per group. Data are representative of two independent experiments. Error bars denote mean ± SD. *p < 0.05. N.S.: not significant.
Lung inflammation was sufficient to delay EAE onset by stalling Th17 cells in the lung
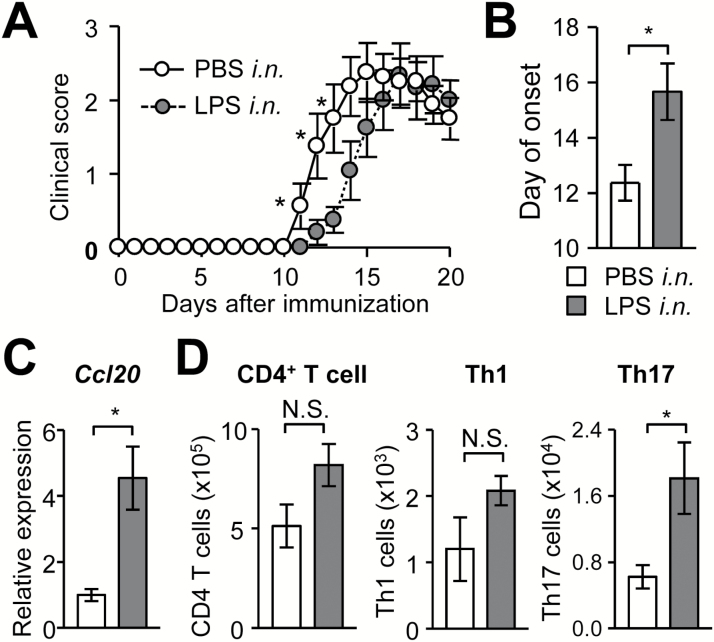
We have shown that Th17-cell stalling in the lung is a mechanism that delays EAE onset in Atg7 CKO mice. However, it is not clear which is important to stall Th17 cells in the lung, the absence of autophagy (or LAP) in myeloid cells or just the mild lung inflammation (1). To test whether mild lung inflammation delays the EAE onset, we performed intranasal instillation of a low-dose LPS to WT mice on days 6 and 9 after EAE induction. LPS instillation indeed delayed the onset of EAE in WT mice (Fig. 4A and B), albeit to a lesser extent to that in Atg7 CKO mice (Fig. 1A). The level of LPS instillation induced transient inflammation and may explain why delayed EAE onset in LPS-treated WT mice was less significant than that seen in Atg7 CKO mice that have chronic lung inflammation. A previous LPS-induced lung inflammation study showed no change in TNFα levels in plasma after LPS instillation, in which a dose of LPS per mouse weight was similar to our condition (16). The study also showed that TNFα levels in bronchoalveolar lavage fluid (BALF) were transiently up-regulated 4 and 24h after LPS instillation. This suggests that lung inflammation was ongoing but transient, because BALF TNFα levels came back to normal in 72h (16). In our study, elevated Ccl20 mRNA expression and increased Th17-cell accumulation in lungs were observed in LPS-treated mice on day 9 (Fig. 4C). CCL20 expression in bronchial epithelial cells is known to be induced by a wide variety of stimulations including pathogens, allergens and environmental pollutions (17, 18). Therefore, it is highly possible that bronchial epithelial cells produced CCL20 after LPS instillation. LPS-mediated mild lung inflammation caused lungs to accumulate Th17 cells, but not total CD4+ T and Th1 cells (Fig. 4D). These results suggest that mild lung inflammation is sufficient to stall Th17 cells in the lung to delay the onset of EAE.
Fig. 4.
LPS intranasal instillation delays EAE onset. (A, B) Time-course of EAE score (A) and average day of EAE onset (B). WT mice were intranasally instilled with LPS (2.5 μg per instillation) on days 6 and 9 after EAE induction. LPS-instilled (n = 12) and PBS-instilled control (n = 8) groups are shown. (C) Ccl20 mRNA expression in lungs on day 9. n = 5 per group. (D) Numbers of CD4+ T cells, Th1 and Th17 cells in lungs on day 10. LPS-instilled (n = 6) and PBS-instilled control (n = 7) groups are shown. Data are representative of two independent experiments. White and gray bars denote values obtained from PBS- or LPS-treated mice, respectively (B–D). Error bars denote mean ± SD. *p < 0.05. N.S.: not significant.
Our previous study showed that Atg7 deficiency in myeloid cells spontaneously increased bacterial loads in the lung and enhanced TLR4 sensitivity of AMs that results in mild spontaneous pulmonary inflammation (1). In this study, we demonstrated that Atg7 CKO mice show delayed onset of EAE specifically due to stalling of Th17 cells in the lung during their migration to the CNS. A very recent article reported that respiratory infection by bacteria reduces CNS inflammation, attenuates clinical symptoms of EAE and delays EAE onset (19). Their study also demonstrated that the pulmonary bacterial infection prevents T-cell infiltration into the CNS (19). Although the study did not particularly show accumulation of T cells in the lung, our results suggest that stalling of Th17 cells in the lung is possible during the respiratory infection. Indeed, intranasal instillation of a low-dose LPS delayed the EAE onset by the stalling of Th17 cells in the lung (Fig. 4). During delayed EAE development, Atg7 CKO mice and LPS-instilled WT mice both increase the expression of Ccl20 mRNA. Interestingly, cigarette smoking, a risk factor of MS, is known to reduce the expression of Ccl20 mRNA in human lungs (20), a quality that may allow Th17 cells to quickly migrate to the CNS. Results from the human study (20) and our data both suggest the involvement of lung CCL20 in controlling autoimmune responses in the CNS. We therefore intended to examine whether local CCL20 expression in the lung is sufficient to delay EAE and intra-tracheally instilled recombinant CCL20 (rCCL20) to WT mice. Unfortunately, the result of the experiment was inclusive, because we were unable to observe a delay in EAE onset after treatment (data not shown). It is still not clear whether the failure was technical (e.g. poor efficiency in rCCL20 delivery, short half-life, or insufficient amount of rCCL20), or whether rCCL20 alone is not sufficient to alter the in vivo biology of multiple cell behaviors (e.g. requirement in enhanced expression of adhesion molecules in vascular endothelial cells). Altering multiple factors in the lung may be essential to achieve the Th17-cell retention.
The majority of treatments for autoimmune diseases involve the administration of immunomodulating drugs that disseminate systemically. To minimize the side-effects during treatment, it is desirable to modulate the immune responses only in a certain organ or a limited area of a body. Our results suggest that the lung might be targeted to modulate T-cell migration to the sites of autoimmunity. In addition, potential therapeutics can be delivered to the lung relatively noninvasively through inhalation, as compared with the current drugs. This study demonstrated that lung inflammation specifically stalls Th17-cell migration and delays the onset of EAE that may be exploited to create therapeutic opportunities in lung-specific immune modifications to treat CNS inflammatory diseases such as MS.
Funding
This work was supported by the National Institutes of Health (grant number R01-AI08100 to M.L.S) and the National Multiple Sclerosis Society (grant number RG 4536B2/1 to M.L.S).
Acknowledgements
We thank Drs W. Jia, M. He and I. McLeod for their help in setting up the Atg7 fl/fl LysM cre/+ mouse line and experimental assistance on autophagy and Ms K. Moore and Mr J. Ashe for their help to maintain LysM cre/+ and Atg7 fl/fl LysM cre/+ mouse lines. We are also grateful to Mr W. Barclay for commenting and editing this manuscript.
Conflict of interest statement: The authors have no financial conflict of interest.
References
- 1. Kanayama M. He Y. W. and Shinohara M. L. 2015. The lung is protected from spontaneous inflammation by autophagy in myeloid cells. J. Immunol. 194:5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Odoardi F., Sie C., Streyl K., et al. 2012. T cells become licensed in the lung to enter the central nervous system. Nature 488:675. [DOI] [PubMed] [Google Scholar]
- 3. Handel A. E. Williamson A. J. Disanto G. Dobson R. Giovannoni G. and Ramagopalan S. V. 2011. Smoking and multiple sclerosis: an updated meta-analysis. PLoS One 6:e16149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pittas F., Ponsonby A. L., van der Mei I. A., et al. 2009. Smoking is associated with progressive disease course and increased progression in clinical disability in a prospective cohort of people with multiple sclerosis. J. Neurol. 256:577. [DOI] [PubMed] [Google Scholar]
- 5. Abdel Fattah E. Bhattacharya A. Herron A. Safdar Z. and Eissa N. T. 2015. Critical role for IL-18 in spontaneous lung inflammation caused by autophagy deficiency. J. Immunol. 194:5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jia W. Pua H. H. Li Q. J. and He Y. W. 2011. Autophagy regulates endoplasmic reticulum homeostasis and calcium mobilization in T lymphocytes. J. Immunol. 186:1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kanayama M. Inoue M. Danzaki K. Hammer G. He Y. W. and Shinohara M. L. 2015. Autophagy enhances NFκB activity in specific tissue macrophages by sequestering A20 to boost antifungal immunity. Nat. Commun. 6:5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Inoue M. Williams K. L. Gunn M. D. and Shinohara M. L. 2012. NLRP3 inflammasome induces chemotactic immune cell migration to the CNS in experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. U. S. A. 109:10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Inoue M. Williams K. L. Oliver T. Vandenabeele P. Rajan J. V. Miao E. A. and Shinohara M. L. 2012. Interferon-beta therapy against EAE is effective only when development of the disease depends on the NLRP3 inflammasome. Sci. Signal 5:ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Komatsu M., Waguri S., Ueno T., et al. 2005. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J. Cell Biol. 169:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martinez J., Almendinger J., Oberst A., et al. 2011. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc. Natl Acad. Sci. U. S. A. 108:17396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chung Y., Chang S. H., Martinez G. J., et al. 2009. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity 30:576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arima Y., Harada M., Kamimura D., et al. 2012. Regional neural activation defines a gateway for autoreactive T cells to cross the blood-brain barrier. Cell 148:447. [DOI] [PubMed] [Google Scholar]
- 14. Reboldi A., Coisne C., Baumjohann D., et al. 2009. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat. Immunol. 10:514. [DOI] [PubMed] [Google Scholar]
- 15. Yamazaki T., Yang X. O., Chung Y., et al. 2008. CCR6 regulates the migration of inflammatory and regulatory T cells. J. Immunol. 181:8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vernooy J. H. Dentener M. A. van Suylen R. J. Buurman W. A. and Wouters E. F. 2001. Intratracheal instillation of lipopolysaccharide in mice induces apoptosis in bronchial epithelial cells: no role for tumor necrosis factor-alpha and infiltrating neutrophils. Am. J. Respir. Cell Mol. Biol. 24:569. [DOI] [PubMed] [Google Scholar]
- 17. Reibman J. Hsu Y. Chen L. C. Bleck B. and Gordon T. 2003. Airway epithelial cells release MIP-3alpha/CCL20 in response to cytokines and ambient particulate matter. Am. J. Respir. Cell Mol. Biol. 28:648. [DOI] [PubMed] [Google Scholar]
- 18. Ito T. Carson W. F. IV Cavassani K. A. Connett J. M. and Kunkel S. L. 2011. CCR6 as a mediator of immunity in the lung and gut. Exp. Cell Res. 317:613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Edwards S. C. Higgins S. C. and Mills K. H. 2015. Respiratory infection with a bacterial pathogen attenuates CNS autoimmunity through IL-10 induction. Brain Behav. Immun. 50:41. [DOI] [PubMed] [Google Scholar]
- 20. Meuronen A. Majuri M. L. Alenius H. Mantyla T. Wolff H. Piirila P. and Laitinen A. 2008. Decreased cytokine and chemokine mRNA expression in bronchoalveolar lavage in asymptomatic smoking subjects. Respiration 75:450. [DOI] [PubMed] [Google Scholar]