Abstract
The continued degradation of primate habitat worldwide is forcing many primate populations into small protected forest islands surrounded by high-density human populations. One well-studied example is the critically endangered mountain gorilla (Gorilla beringei beringei). Decades of monitoring and research on Rwanda's mountain gorillas offer a unique opportunity to use non-invasive endocrine analysis to address pressing questions about the conservation of this endangered population. The aims of our study were as follows: (i) to validate field and laboratory methods for assessing stress through faecal glucocorticoid metabolite (FGM) analysis using inter-social unit interactions as a natural stressor; (ii) to determine the excretion lag times between interactions and detectable stress response in faeces; and (iii) to determine whether there are circadian patterns of FGM excretion. We collected ~6000 faecal samples from 127 known gorillas in 10 habituated groups, monitored by the Dian Fossey Gorilla Fund's Karisoke Research Center over 21 months in 2011 and 2012. Extracted FGMs were measured using a cortisol enzyme immunoassay (R4866; C. J. Munro). Results revealed cause–effect relationships between inter-unit interactions and increased FGMs (relative to individual pre-event samples) between 20 and 140 h after interactions, with the peak most often occurring on day 3. There was no evidence of circadian patterns in FGM concentrations, as previously shown in many species with long gut passage times. However, baseline FGM concentrations were lower in adult males than in adult females, and variation was associated with the collection month, indicating possible seasonal variation. This study provides a biologically validated, field-friendly faecal hormone metabolite extraction and laboratory enzyme immunoassay analysis method for non-invasive monitoring of adrenocortical activity in Virunga mountain gorillas. The methods are useful for future evaluation of a variety of environmental and human-induced potential stressors in this critically endangered population.
Keywords: Ape, circadian pattern, faecal sample, interaction, lag time
Introduction
‘Stress’ is a widely discussed topic because of its potential negative health effects, especially when it is chronic. Physiological stress occurs when stimuli lead to ‘allostatic overload’ (McEwen and Wingfield, 2003); that is, energetic demands to maintain or re-establish homeostasis exceed available energy supplies. However, allostatic overload can also occur when an organism experiences social disruptions or conflicts even when energy balance is neutral or positive. In mammals, allostatic overload causes a host of physiological responses, including the release of a cascade of hormones by glands constituting the hypothalamic–pituitary–adrenal axis. The adrenal glands secrete glucocorticoids (GCs), which then signal back to the hypothalamic–pituitary–adrenal axis, creating a negative feedback loop (Romero, 2004). Glucocorticoid secretion leads to a rapid increase in gluconeogenesis, which mobilizes energy reserves to respond adaptively to stressors through a ‘fight-or-flight’ response (reviewed by Sapolsky et al., 2000). Prolonged allostatic overload disrupts the negative feedback mechanism and can lead to deleterious health outcomes for the individual (Sapolsky et al., 2000; McEwen and Wingfield, 2003; Romero, 2004; Tarlow and Blumstein, 2007; Tort and Teles, 2007). Negative effects are variable and include reproductive inhibition, immune system suppression, neuron loss and impaired cognitive function, and impaired growth and development (Sapolsky et al., 2000; McEwen and Wingfield, 2003; Charmandari et al., 2005; Tort and Teles, 2007). Such detrimental effects on individual organisms and, potentially, populations can threaten the survival of endangered species.
The integration of hormone analysis into wildlife conservation research is a prevailing and widely accepted approach (Ganswindt et al., 2012). Hormones such as GCs provide precise measures of adrenocortical activity in captive and wild animal populations and, thus, crucial information for addressing important conservation questions and developing effective interventions (Wielebnowski and Watters, 2008; Ayres et al., 2012; Ganswindt et al., 2012). For example, Ayres and colleagues (2012) assessed a combination of triiodothyronine (an indicator of nutritional stress) and GC in endangered killer whales (Orcinus orca). Their analyses revealed that a marked reduction in availability of the whales’ key prey, Chinook salmon (Oncorhynchus tshawtscha), was related to the decline of the whale population, as opposed to psychological stress associated with noise disturbance from growing vessel traffic.
Techniques for measuring GC metabolites in faeces have been used to monitor physiological stress responses to environmental and social changes in wild animal populations (Ganswindt et al., 2012). This non-invasive method allows for long-term physiological monitoring in natural settings with minimal animal disturbance. It is typically used concurrently with other biological measures, such as behaviour, biotic (e.g. predator pressure, food availability) and abiotic (e.g. temperature, rainfall) environmental factors (Ayres et al., 2012), anthropogenic disturbances (Tarlow and Blumstein, 2007), and demography and health (e.g. parasite load; Muehlenbein, 2006). Faeces are particularly suitable for long-term monitoring and capturing seasonal and chronic stressors. They contain pooled hormone metabolite concentrations rather than a discrete amount of hormone secreted at a specific time, as occurs in blood samples (Millspaugh and Washburn, 2004).
Faecal hormone metabolite extraction at field sites offers multiple advantages over shipping preserved or dried faecal samples to laboratories for analysis (Ziegler and Wittwer, 2005; Santymire and Armstrong, 2010; Palme et al., 2013). For example, using preservatives or drying samples increases the risk of degrading steroid metabolites (see Santymire and Armstrong 2010; Palme et al., 2013). Shipping small vials containing extracted hormones rather than preserved faecal samples also reduces transportation costs, eliminates the complications of shipping flammable preservatives and, typically, does not require import permission for countries where analysis laboratories are based. Developing extraction laboratories at field sites also contributes to capacity building of local scientists.
Although it has many advantages, faecal hormone analysis measures hormone metabolites, not the native hormone, so it requires extensive biological and biochemical validation before application. Each species and hormone must be validated individually owing to inter-specific differences in steroid hormone metabolism and route before secretion into faecal matter (Reimers et al., 1981; Heistermann et al., 2006). Once validated, faecal hormone metabolite analysis can be used to evaluate environmental and social variables that may be limiting the maintenance or growth of endangered populations. Such information facilitates effective conservation policy.
The Virunga mountain gorilla (Gorilla beringei beringei) is a critically endangered species; only 880 individuals remain in two isolated populations (Gray et al., 2013; Roy et al., 2014). In the protected area they inhabit, multiple conservation management strategies are used; these include regular patrols to target persistent illegal activities, gorilla habituation for eco-tourism, and long-term research and health monitoring combined with an on-site veterinary intervention programme. These collective long-term conservation efforts have resulted in a steady population increase (Gray et al., 2010, 2013). Although mountain gorillas are a unique success story in ape conservation, this success is accompanied by challenges. There is an ever-growing human presence in the forest—specifically near the gorillas—and the animals are experiencing ongoing changes in population dynamics as their numbers increase in a space-restricted forest island. Changes in the population dynamics include an up to 6-fold increase of inter-social unit interactions in some areas (Caillaud et al., 2014). Inter-unit interactions can be very violent and cause severe injuries or death of involved males or dependent offspring (Fossey, 1984; Watts, 1989; Sicotte, 1993). To ensure a sustainable, healthy population, non-invasive assessments of gorilla health are an essential aspect of long-term monitoring programmes. Ongoing monitoring of the gorillas and their habitat allows for the study of hormone patterns and their relationship to myriad social and environmental factors. Given that samples can be obtained from individually identified gorillas, analyses can control for factors such as sex (Goymann, 2012), age (Boscaro et al., 1998), social status (Goymann and Wingfield, 2004; Gesquiere et al., 2011; Markham et al., 2013) and reproductive status (Hoffman et al., 2010).
Faecal hormone metabolite analysis has been validated for both captive and wild western lowland gorillas (Gorilla gorilla gorilla; captive: Heistermann et al., 2006; Nizeyi et al., 2011,a,b; Shutt et al., 2012; Jacobs et al., 2014; wild: Shutt et al., 2012). Adrenocorticotrophic hormone challenge tests validated GC analysis techniques in zoo-housed western lowland gorillas (Heistermann et al., 2006; Nizeyi et al., 2011a; Shutt et al., 2012; Jacobs et al., 2014). Performing an adrenocorticotrophic hormone challenge in mountain gorillas is impractical, because there is not an established captive population. However, comparing GC concentrations of samples collected before and after natural biological stressors in wild populations provides an alternative approach for validating a GC assay. Faecal glucocorticoid metabolite (FGM) analysis from wild mountain gorillas living in Bwindi Impenetrable Forest National Park in Uganda was biologically validated using samples from before and after gorillas raided crops and were chased back into the forest by local people (Nizeyi et al., 2011a). Although characterization of stress physiology of the Bwindi mountain gorilla population provides useful insights for both populations, this population differs from the Virunga population in several key aspects, including diet and altitudinal range (Watts, 1984; Ganas et al., 2004; Harcourt and Stewart, 2007). These differences may affect hormone metabolism (Beehner and McCann, 2008; Goymann, 2012), so additional Virunga population-specific validation is necessary.
The specific objectives of our study were as follows: (i) to validate standardized field and laboratory methods to characterize the stress physiology of Virunga mountain gorillas using FGMs for analysis and inter-social unit interactions; (ii) to determine the excretion lag times between interaction and detectable stress response in faeces; and (iii) to determine circadian patterns of FGM excretion. By developing non-invasive methods to characterize stress physiology in the largest population of this endangered species, we can learn more about how environmental pressures, including anthropogenic, social and ecological change, are impacting the health and long-term population success of this critically endangered species.
Materials and methods
Study site and animals
Between April 2011 and December 2012, behavioural data and faecal samples were collected on 127 known individual Virunga mountain gorillas monitored by the Dian Fossey Gorilla Fund's Karisoke Research Center (KRC). The animals lived in 10 social units in Volcanoes National Park, Rwanda. Volcanoes National Park comprises the Rwandan portion of the Virunga massif, a montane cloud forest ranging from 2300 to 4500 m altitude, which is shared with Democratic Republic of Congo and Uganda. Temperatures are mild year round. Rainfall is bi-modally distributed, with a long and short wet season lasting from September to December and March to May, respectively (Mehta and Katee, 2005). The principal dry season lasts 2–4 months between May and September, whereas the short dry season from January to March is characterized by reduced rain (Mehta and Katee, 2005).
Terminology
Throughout, we refer to animals by the established names for their appropriate age/sex class. ‘Silverbacks’ are adult males age ≥12 years; ‘blackbacks’ are post-adolescent males age 8–11 years; adult females are those age ≥8 years; subadults are animals of either sex between 6 and 8 years old; and infants are animals ages 0–3.5 years (Williamson and Gerald-Steklis, 2001; Weber and Vedder, 1983; Fletcher, 1994; Watts and Pusey, 1993). Males living in groups with multiple silverbacks have strong dominance hierarchies (e.g. Stoinski et al., 2009). We refer to the male at the top of the dominance hierarchy as the dominant male, and any male second ranked or lower as subordinate. Adult males on their own without a social unit are referred to as solitary silverbacks.
Inter-social unit interactions typically involve ritualized behaviours that can either be performed individually or simultaneously/in quick succession (e.g. vocalizing while engaging in a physical movement). These are usually, but not always, an excited performance by subadult or adult males used to intimidate rivals. When behaviours are performed simultaneously or in quick succession, they are referred to as ‘displays’. Behaviours that may be used individually or in displays include strut-stances (a stiff-appendage posture), chest-beating (alternately hitting the pectoral muscles with both fists), smashing/dragging plants, pounding the ground, wagging the head and/or symbolic feeding (nipping off a leaf and holding it between lips before spitting it out; Fossey, 1983; Fletcher, 1994). Vocalizations performed during displays specifically and interactions more generally include screaming, which is an aggressive vocalization, or hooting, which is often done simultaneously with chest-beating (Fossey, 1972).
Faecal collection and storage
Faecal samples were collected by KRC field staff between 07.15 and 16.00 h. We aimed to collect one baseline hormone sample per gorilla per week, and ad libitum samples from all gorillas affected by inter-unit social interactions for 5 days consecutively post-event (hereafter referred to as event samples). Baseline samples refer to samples that were collected before any event, and ≥6 days after any observed event. Immediately after defecation, subsamples were taken from any section of the faeces, because the distribution of glucocorticoids in mountain gorilla faeces is relatively even (Nizeyi et al., 2011b). Small plastic bags were used as gloves to collect and transport the samples. Samples contaminated by urine or exposed to water were not collected, because both factors may affect FGM concentrations (Wasser et al., 1998; Washburn and Millspaugh, 2002; Ganswindt et al., 2010). Each sample was labelled with the collection date and time and the gorilla's identification code. Samples were kept on ice packs once they reached a car for transport to the KRC laboratory (~1–6 h after collection).
Upon arrival at the laboratory, samples were stored at −20°C and sample label details were noted in a logbook, including the time of storage in the freezer. Samples were typically frozen at ~15.00 h (~5–15 h after collection). A delay in faecal sample freezing can affect the FGM concentration, which may be influenced by environmental conditions and bacterial enzymes (Möstl et al., 2005, 1999; Palme et al., 2005). Previous reports from the Bwindi mountain gorilla population have shown that FGMs are stable in their natural environment up to 60 h post-defecation (Nizeyi et al., 2011b). Note that no information is available about faecal exposure to rain, but given Bwindi's rainy environment (Nizeyi et al., 2011b) and that samples for the study by Nizeyi and colleagues (2011b) were deliberately left in the forest environment for resampling for several days, some or most samples were almost certainly exposed.
Three factors suggest that FGMs in the present study should have remained stable for the 5–15 h lag between collection and freezing. First, the annual mean temperatures in the Virungas are lower than those of the section of Bwindi where Nizeyi and colleagues (2011b) collected their samples (Ganas et al., 2004), meaning that sample degradation should be slower. Second, none of the faecal material we used was exposed to water (which again may degrade samples), whereas samples in the previous study probably were. Finally, the time from defecation to freezing was randomly distributed amongst the samples, so although this may have added noise to the data, it should not create systematic bias. After approximately 2–3 months of frozen storage, FGMs were extracted from wet faeces in KRC's laboratory by M.R.U. using the faecal hormone extraction protocol described previously by Santymire and Armstrong (2010). This field protocol has been used for other wild apes (Murray et al., 2013).
Field hormone extraction method and extract storage
Each faecal sample was thawed to room temperature and mixed in the plastic bag. We then weighed out 0.5 ± 0.02 g of wet faecal material free from plant parts and other debris, and placed it in the bottom of a labelled 16 mm × 125 mm polypropylene tube. The weight of each sample, label details and any unusual observations were noted on a recording sheet. We then added 5 ml of 90% ethanol:distilled water, and homogenized the sample (OMNI International homogenizer, Kennesaw, GA, USA) for 1 min. The resulting mixture was poured through a funnel-shaped filter paper (VWR, 11 cm diameter, cut in half and rolled) into a clean identical tube. This filtrate resulted in a 1:5 sample dilution in ethanol. Two 1 ml aliquots were pipetted into two new 12 mm × 75 mm polypropylene tubes that were labelled with a sample code, gorilla name, collection date and collection time. Both aliquots were then placed in a rack in a sunny location (when possible) until completely dry (approximately 1–2 weeks). During extreme rainy periods, we used a hair dryer to apply low heat to the hormone extracts (e.g. Loeding et al., 2011; Jacobs et al., 2014) for ~15 min each day to avoid moulding. Dry samples were capped and stored at −20°C until transport to the Davee Center for Epidemiology and Endocrinology at Lincoln Park Zoo (Chicago, IL, USA) for hormone analysis.
Cortisol enzyme immunoassay
The dried hormone extracts were reconstituted with 1 ml phosphate-buffered saline following established protocols (Murray et al., 2013). The FGMs were quantified using a cortisol enzyme immunoassay (EIA; provided by C. J. Munro, University of California Davis, Davis, CA, USA) employing horseradish peroxidase (1:8500 dilution) ligands and polyclonal antiserum (R4866; 1:20 000 dilution) with previously described methods (Loeding et al., 2011). The cortisol EIA cross-reactivities were previously published (Young et al., 2004): cortisol, 100%; prednisone, 6.3%; corticosterone, 0.7%; 21-deoxycorticosterone, 0.5%; progesterone, 0.2%; pregnenolone, 0.1%; androstenedione, 0.1%; dehydroisoandrosterone-3-sulfate, 0.1%; estradiol-17β, 0.1%; estriol, 0.1%; cholesterol, 0.1%; prednisolone, 9.9%; cortisone, 5.0%; deoxycorticosterone, 0.3%; 11-desoxycortisol, 0.2%; 17α-hydroxyprogesterone, 0.2%; 17α-hydroxypregnenolone, 0.1%; testosterone, 0.1%; dehydroepiandrosterone, 0.1%; aldosterone, 0.1%; estrone, 0.1%; and spironolactone, 0.1%.
Validation of adrenocortical activity
High-performance liquid chromatography previously established the presence of FGM in mountain gorilla faeces (Nizeyi et al., 2011a). For the present study, the cortisol EIA was validated in the Virunga mountain gorillas by demonstrating the following: (i) parallelism between binding inhibition curves of faecal extract dilutions (r = 0.976); and (ii) significant recovery (>90%) of exogenous cortisol added to faecal extracts (y = 1.15x + 3.59; R² = 0.998). Assay sensitivity was 3.9 pg/well, and intra- and inter-assay coefficients of variation were <10%.
Biological validation
To validate our methods in wild Virunga mountain gorillas, we used faecal samples from 11 gorillas before and after four independent inter-unit interactions. These are usually aggressive and pose a high risk of infanticide and injury (Fossey, 1984; Watts, 1989; Sicotte, 1993). These individuals and interactions were selected for validation analyses because they had the best faecal collection coverage pre- and post-event. Detailed behavioural observations during all observed interactions were recorded by trained KRC field staff who could identify known individuals.
Inter-unit interaction 1
The first interaction took place on 21 December 2011 from 09.00 to 12.00 h and involved two groups, Bwenge (BG) and Inshuti (IG). The two groups contained nine and six gorillas, respectively, each with one silverback and associated females and offspring. The interaction started when both silverbacks exchanged chest-beats while ~300 m apart. Inshuti group moved toward BG until they had visual contact with one another at 09.20 h. The silverback in IG strut-stanced and screamed at BG group. When a nulliparous adult female in IG tried to move toward BG, IG's silverback hit and dragged her. During the 3 h interaction, IG's silverback displayed at BG 28 times. The silverback in BG performed chest-beats three times and moved away from IG continuously, while IG followed slowly. Both groups travelled ~1.5 km during the interaction. At 12.00 h, when the groups were separated by ~400 m, IG's silverback stopped displaying and changed directions, followed by his group. For the biological validation, pre- and post-event faecal samples were used from the six gorillas in IG.
Inter-unit interaction 2
The second interaction took place on 17 September 2011 at 10.15 h between Ugenda group (UG) and a solitary silverback, and lasted ~5 min. Ugenda group contained 11 individuals, including two silverbacks. The group was apparently surprised by the sudden appearance of the solitary silverback. The solitary silverback bit an infant female who, along with her mother, lagged behind the main group. The infant's finger was severed in the attack. When the infant screamed, group members ran to the interaction site, led by the subordinate silverback in UG. The subordinate silverback bit the solitary silverback, which ran away shortly thereafter. The dominant and subordinate males continued displaying for another 2 min. The subordinate male sustained a small wound on his neck. Faecal samples before and after the event were retrieved from the injured infant, her mother and the group's subordinate male.
Inter-unit interaction 3
The third interaction, on 12 January 2012, involved a solitary silverback and Kuryama group (KG). Kuryama group contained 14 individuals, including two silverbacks. The subordinate male in KG gave a series of 23 hooting vocalizations combined with chest-beats from 08.50 to 09.40 h, while the rest of the group continued normal foraging behaviour. During this period, the dominant male in KG displayed four times. At 10.30 h, the solitary silverback approached to within 10 m of KG and displayed, including strut-stancing and pounding the ground with his fists. The three males faced each other in strut-stance posture. After 15 min, the dominant male moved toward the solitary silverback. He was followed by the subordinate male and a blackback in KG, while the rest of the group remained in place and continued feeding. The solitary silverback retreated quickly, followed closely by the KG males. The KG males displayed at the solitary male, including chest-beating, symbolic feeding, smashing plants and strut-stancing. At 11.00 h, the dominant male and blackback returned to the rest of the group and left the subordinate male alone with the solitary silverback. The two males continued to display at each other. The subordinate male rejoined the group 15 min later, leaving the solitary silverback 250 m behind. The dominant male continued hooting and chest-beating periodically until 12.15 h. In total, the dominant and subordinate males displayed 12 and 32 times, respectively. There was no physical contact observed between any of the participants. Faecal samples were obtained from the dominant male before the interaction and during 5 days consecutively after the interaction.
Inter-unit interaction 4
In the fourth interaction, groups KG and IG encountered one another on 11 August 2011 at 11.30 h. Inshuti group's silverback moved downhill towards KG, leaving the IG females and infants ~800 m behind. He moved to ~20 m distance from KG and stood in strut-stance posture, at which point KG's subordinate silverback, a blackback and a subadult male noticed his presence. A series of approaches and retreats between KG's dominant silverback and IG's silverback followed. The blackback and two female infants approached the IG silverback closely, while both of KG's silverbacks stood in front. At 11.55 h, KG's dominant silverback returned to the rest of the group, which by this point was slightly behind, and started feeding. IG's silverback, and the subadult male and blackback from KG continued displaying at one another. At 12.17 h the IG silverback retreated towards his group, which remained uphill ~800 m away. KG moved further downhill away from IG, except for the blackback, who followed IG for another 50 min before rejoining KG. Faecal samples before and after the interaction were obtained from the mother of one of the infants that closely approached IG's silverback.
Data analysis
Calculation of hormonal baseline values of FGM concentrations
As the gorillas are not monitored 24 h per day, it is impossible to record all potentially stressful events that occur. Unsurprisingly, some samples classified as baseline samples revealed high FGM concentrations, which might indicate the occurrence of unobserved stressful events. To control for these values, we used an iterative process that excluded baseline samples with values greater than the mean + 1.5 standard deviations (SD) for each individual gorilla. This process was repeated until all baseline samples were either equal to or less than the mean ± 1.5 SD (Moriera et al., 2001).
Circadian patterns
In order to determine whether FGM concentrations followed a circadian pattern, we divided baseline samples by collection time into morning (before noon) and afternoon samples. Adult gorillas (≥8 years old) with fewer than three baseline samples in either of the two categories were excluded from this analysis. Collection time was entered as a fixed effect in a linear mixed-effects model, with baseline hormone values as the response variable and gorilla identification nested within social groups as random effects. We also included two potentially confounding variables as fixed effects, namely sex of the adult gorillas (44 females and 28 males) and the collection month, because FGM concentrations in mammals can vary according to sex (Touma and Palme, 2005) and season (Touma and Palme, 2005; Gesquiere et al., 2008). These and all other statistical tests were run using R (version 3.1.2; R Core Team, 2015).
Time gap between event and elevated FGM concentration
In order to determine excretion lag times between exposure to a stressor and elevated FGM concentrations, we used a larger data set involving 25 inter-unit interactions and 233 samples collected from a total of 34 gorillas 1.8–31.9 years of age (Table 1) 1 week before (pre-event) and up to 10 days after inter-unit interactions. For analysis of the time lag between observed events and elevated FGM concentration, we excluded gorillas without evidence of elevated FGM concentrations following an interaction (284 out of 406 cases). Unaltered post-event FGM concentrations might indicate a lack of interaction-induced stress in some gorillas or insufficient post-event sampling. Seventy-two other cases were omitted because of missing pre-event samples 1 week before the interaction. We ran a linear mixed-effects model, with the lag time in relationship to the interaction event as a fixed effect. These values were divided into pre-event samples, collected before the interaction, and 20 h post-event interval categories starting with >0–20 h and extending up to >200–220 h post-event. The response variable was log-transformed FGM concentrations of the pre-event and post-event samples. Gorilla identity nested within social group identity were entered as random effects.
Table 1:
Age and sex distribution of the gorillas used in analysis of the time lag between inter-unit interaction events and elevated faecal glucocorticoid metabolite concentrations
| Age/sex category | Age range (years) | Females (n) | Males (n) | Total (n) |
|---|---|---|---|---|
| Full-grown silverback | >15 | – | 5 | 5 |
| Young silverback | >12–15 | – | 2 | 2 |
| Blackback | >8–12 | – | 0 | 0 |
| Adult female | >8.0 | 16 | – | 16 |
| Sub-adult | >6.0–8.0 | 0 | 0 | 0 |
| Juvenile | >3.5–6.0 | 4 | 3 | 7 |
| Infant | 0–3.5 | 2 | 2 | 4 |
Age/sex classifications are from Table II of Breuer et al. (2009).
Results
Baseline sample variation
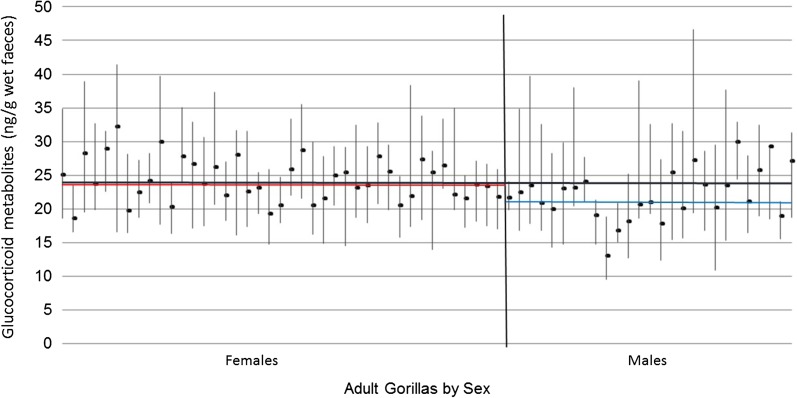
The mean baseline FGM concentration in adult mountain gorillas ranged from 13.0 to 32.2 ng/g wet faeces (range for females, 18.5–32.2 ng/g wet faeces and for males, 13.0–29.9 ng/g wet faeces; Fig. 1).
Figure 1:
Adult male mountain gorillas (n = 28) had lower mean baseline faecal glucocorticoid metabolite concentrations than adult females (n = 44; F = 13.20, P < 0.001, n = 1936 faecal samples). Individual points represent the mean ± 1.5 SD for each animal; the vertical black lines are the upper and lower 95% confidence intervals. The blue line represents the mean of the mean ± 1.5 SD for all adult males; the red line represents the mean of the mean ± 1.5 SD for all adult females.
Circadian, monthly and sex-specific patterns of FGM concentrations
For baseline samples from adult gorillas, there was no difference between FGM concentrations of samples collected in the morning vs. the afternoon, after controlling for sex and collection month (morning samples, 18.18 ± 4.31 ng/g wet faeces, n = 1299; afternoon samples, 18.23 ± 4.15 ng/g wet faeces, n = 637; β = 2.16, SE = 1.63, F = 1.74, d.f. = 1, P = 0.186).
Adult males (n = 28) had lower baseline FGM concentrations than adult females (n = 44; F = 13.20, d.f. = 1; β = 1.69, SE = 0.46, n = 1936 samples, P < 0.001; Fig. 1). Baseline FGM concentrations varied with the month in which the faecal sample was collected (F = 9.19, d.f. = 11; n = 1936 samples, P < 0.001).
Biological validation
Inter-unit interaction 1
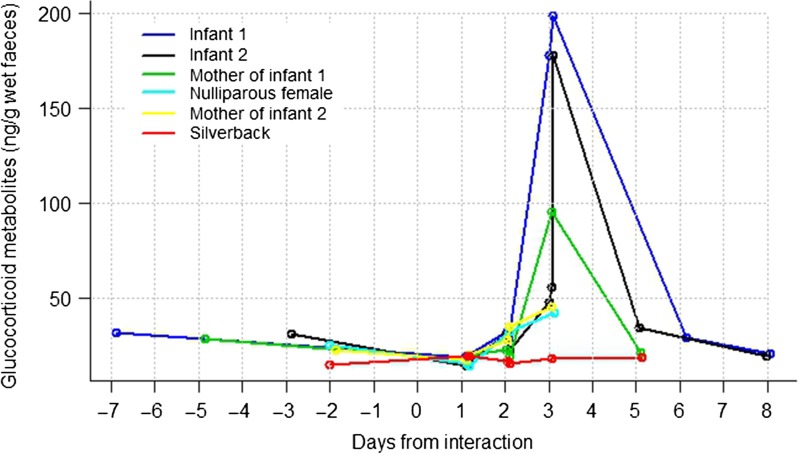
In Inshuti group, five of the six gorillas had elevated FGM concentrations post-event (absolute mean 57 ± 54.5 ng/g wet faeces) relative to their pre-event FGM level (that is, the last FGM sample collected before the interaction event; Fig. 2). Across animals, FGM peaked ~3 days after the interaction and returned to pre-event concentrations by day 5 or 6. However, we were unable to evaluate faeces from post-event day 4. The two infants in the group showed the largest FGM elevation, with an 8- and 7.5-fold increase, respectively, followed by the mothers of the infants and the nulliparous female (Fig. 2). The silverback's FGM concentration remained at this pre-event level throughout. However, we were unable to obtain faecal samples from the silverback during post-event days 3–5, so we may have missed samples with elevated FGM concentrations.
Figure 2:
Faecal glucocorticoid metabolite concentration profiles of individual gorillas before and after an inter-unit interaction in six animals from Inshuti group.
Inter-unit interaction 2
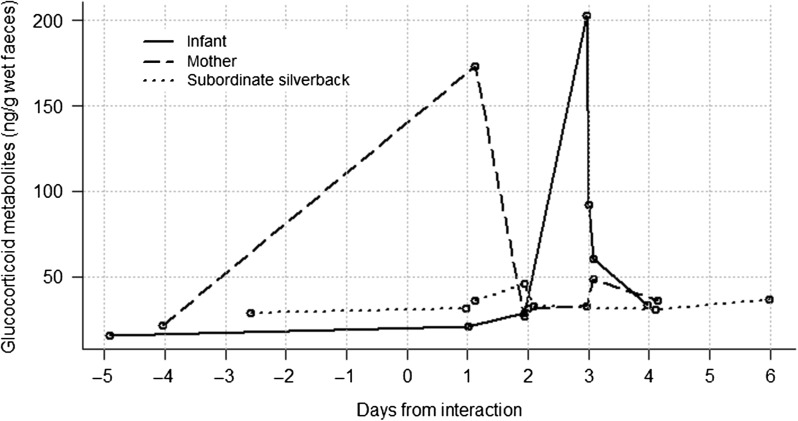
Faecal samples were collected from the injured infant, her mother and the subordinate silverback; that is, the three animals that were primarily involved in the interaction. The injured infant and mother had clearly elevated FGM concentrations after the interaction (69.7 ± 61.9 ng/g wet faeces), up to 8.8- and 7.3-fold above pre-event levels, respectively (Fig. 3). The mother's FGM concentration peaked shortly after the interaction day (27 h post-event), and fell to pre-event concentration on day 2. The infant's FGM peaked on day 3 and then declined steeply, nearly back to her pre-event value within a few hours after the peak. The subordinate silverback's FGM peaked at 2-fold above the pre-event level (absolute elevation 46.2 ng/g wet faeces) around day 2, then returned nearly to the pre-event level (absolute value 32.6 ng/g wet faeces).
Figure 3:
Faecal glucocorticoid metabolite concentration profiles of three individual gorillas from Ugenda group before and after an interaction with a solitary silverback. The infant lost a finger in the interaction.
Inter-unit interaction 3
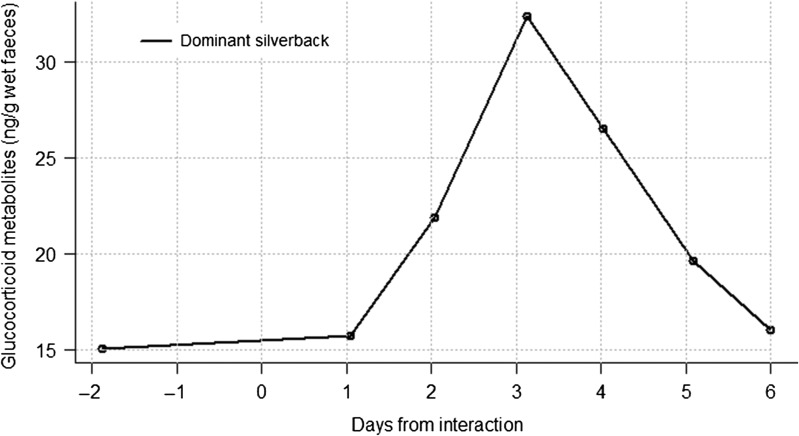
Kuryama group's dominant silverback had an elevated FGM concentration that peaked at 1.6-fold above the pre-event level between days 3 and 4, with a mean (absolute value) elevated FGM of 26.9 ± 5.3 ng/g wet faeces (Fig. 4). The elevation lasted until day 6, when the pre-event value was reached again.
Figure 4:
Faecal glucocorticoid metabolite concentration profile of the dominant silverback from Kuryama group before and after an interaction with a solitary silverback.
Inter-unit interaction 4
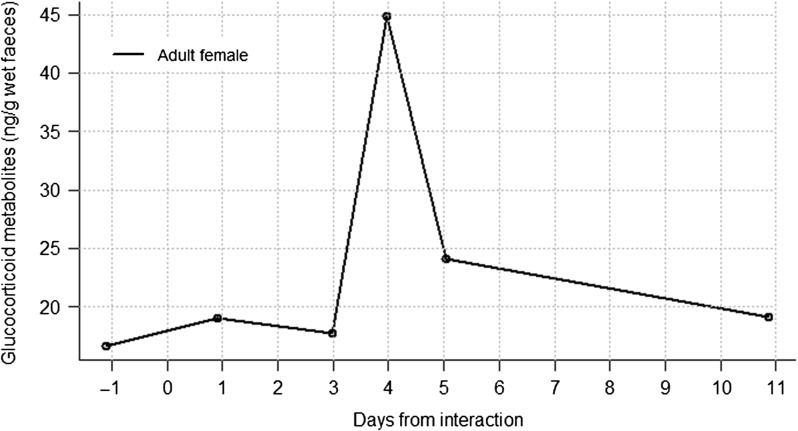
The adult female's post-event FGM level peaked with a 2.2-fold increase at day 3 after the interaction, followed by a steep decline at day 5 (Fig. 5). Her absolute mean elevated FGM concentration was 34.5 ± 14.7 ng/g wet faeces after the interaction.
Figure 5:
Faecal glucocorticoid metabolite concentration profile of an adult female in Kuryama group before and after an interaction with Inshuti group.
Time gap of elevated FGM concentrations post-events
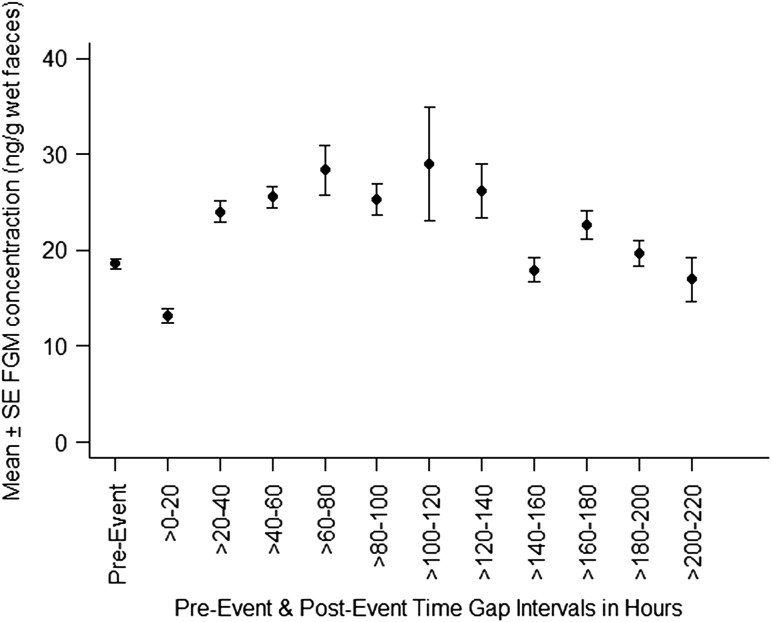
Individuals exhibiting elevated FGM concentrations after inter-unit interactions were from all age–sex classes (Table 1). The distribution of sample points suggested it was most appropriate to divide data into 20 h intervals rather than 24 h intervals for analysis. There was a significant elevation of FGM concentrations from pre-event samples between 20 and 140 h after events (Table 2 and Fig. 6). The peak most often occurred on day 3, between 60 and 80 h after an interaction.
Table 2:
Mixed-effects model parameter estimates showing the relationship between faecal glucocorticoid metabolite concentrations and excretion lag times (presented in 20 h intervals) after inter-unit interactions
| Time gap intervals (h) | b ± SE | t | P-value |
|---|---|---|---|
| >0–20 | −0.002 ± 0.059 | −0.531 | 0.596 |
| >20–40 | 0.260 ± 0.069 | 3.788 | 0.002 |
| >40–60 | 0.288 ± 0.068 | 4.202 | <0.001 |
| >60–80 | 0.371 ± 0.073 | 5.076 | <0.001 |
| >80–100 | 0.332 ± 0.089 | 3.725 | <0.001 |
| >100–120 | 0.381 ± 0.146 | 2.607 | 0.010 |
| >120–140 | 0.353 ± 0.108 | 3.268 | 0.001 |
| >140–160 | −0.068 ± 0.121 | −0.561 | 0.575 |
| >160–180 | 0.226 ± 0.130 | 1.739 | 0.084 |
| >180–200 | 0.032 ± 0.250 | 0.130 | 0.897 |
| >200–220 | −0.162 ± 0.178 | −0.910 | 0.364 |
Reference level is the last sample from each animal in the week leading up to the event. n = 233 samples from 25 inter-unit interactions involving 34 gorillas; F = 5.69, d.f. = 11, P < 0.001. Significant effects at P = .05 are bold.
Figure 6:
Mean (±SEM) of faecal glucocorticoid metabolite (FGM) concentration (removal of the two highest values above 150 ng/g wet faeces to adjust scale) of pre-event samples and post-event samples, collected >0–220 h after inter-unit interactions.
Discussion
Biological validation
This study used a field-friendly faecal hormone metabolite extraction method (Santymire and Armstrong, 2010; Murray et al., 2013) successfully to measure and validate FGM as a measure of stress in endangered Virunga mountain gorillas. We detected cause–effect relationships between inter-unit interactions and increased FGMs with elevations up to 8.8-fold above pre-interaction concentrations, indicating that such social events can be extremely stressful. In primates, inter-unit interactions can result in female transfers, male takeovers and severe injuries or death, but so far elevated FGM concentrations following such events have been reported only in adult male white-faced capuchins (Cebus capucinus; Schoof and Jack, 2013) and adult male and female chacma baboons (Papio hamadryas ursinus; Beehner et al., 2005; Bergman et al., 2005; Engh et al., 2006). To our knowledge, this is the first study demonstrating that this natural event can also cause a physiological stress response in juveniles and infants, who are vulnerable to the threat of infanticide (Fossey, 1984; Watts, 1989; Sicotte, 1993; Robbins et al., 2013).
There was strong inter- and intra-individual variation in elevated FGM levels after inter-unit interactions, demonstrating that individual mountain gorillas are affected differently by the same event. A comprehensive investigation is needed to determine what factors explain such variation (e.g. length of interactions, group composition, relatedness between interacting groups, presence of cycling females, severity of wounding, vulnerability of individuals during interactions). Since 2007, the annual interaction rate in the study population has increased 6-fold as a result of continuing population growth in an isolated forest island, resulting in more groups and a higher group density (Caillaud et al., 2014). The long-term impact of increasing exposure to these stressful events may have lasting effects on the reproduction, health and, thus, survival of the Virunga mountain gorillas, and needs to be monitored closely. The biological validation described here paves the way for future studies examining the relationship between adrenocortical activity and a variety of social, environmental and human-induced potential stressors for this gorilla population.
Time gap between event and stress response
Our results revealed significant elevation of FGM from pre-event levels until 20–140 h after inter-unit interactions, with the peak most often occurring 3 days post-event. This elevation is comparable to FGM analysis from captive western lowland gorillas following adrenocorticotrophic hormone challenge tests (Heistermann et al., 2006; Nizeyi et al., 2011a; Shutt et al., 2012) and transportation between zoos for breeding purposes (e.g. an adult female showed a 7.6-fold increase in FGM; Jacobs et al., 2014). Individual FGM profiles before and after inter-unit interactions showed that elevated FGM concentrations can quickly drop towards pre-event levels, implying that intensive faecal collection, especially around day 3 post-event, is crucial to assess the magnitude of any stressor.
Individual differences and biotic and environmental effects
Basal FGM concentrations varied between adult male and female gorillas. Males excreted lower levels than females, replicating results from a study in captive animals involving one adult male and one female western lowland gorilla (Jacobs et al., 2014). There are three potential explanations for this difference. Adult females may indeed be more vulnerable to stress, but alternatively, the differences might reflect higher energetic demands and physiological differences related to female reproductive states. For female mammals, reproductive state can be another source of inter- and intra-individual differences in basal FGM concentrations (chacma baboons, P. hamadryas ursinus, Weingrill et al., 2004; baboons, Gesquiere et al., 2008; chimpanzees, Thompson et al., 2010; rhesus macaques, Macaca mulatta, Hoffman et al., 2010). For example, higher FGM concentrations were associated with pregnancy in chacma baboons (Weingrill et al., 2004) and ring-tailed lemurs (Lemur catta; Cavigelli, 1999) and with lactation in spotted hyenas (Goymann et al., 2001).
Finally, there may also simply be species-specific sex differences in hormone metabolism and excretion (see Touma et al., 2003; Touma and Palme, 2005; Goymann, 2012). Other authors have proposed that biological and physiological validation of hormone metabolite measures should be conducted for both sexes separately (Touma and Palme, 2005; Goymann, 2012). Based on the present results, we can conclude that the field and laboratory methods applied in this study allow detection of cause–effect relationships in both sexes, but direct comparisons between male and female FGM concentrations in mountain gorillas may not be meaningful except when comparing relative changes in FGM concentrations.
Individual variation in basal FGM concentrations also occurred within adult males and females, which might be driven by social rank. An association between social rank and GC concentrations has been demonstrated in many primate species (olive baboons, Papio anubis, Sapolsky, 1982; Japanese macaques, Macaca fuscata, Barrett et al., 2002; review by Goymann and Wingfield, 2004; baboons, Papio cynocephalus, Gesquiere et al., 2011; review by Creel et al., 2013; chimpanzees, Pan troglodytes schweinfurthii, Markham et al., 2013) and other social mammals, birds and fish (reviews by Goymann and Wingfield, 2004; Creel et al., 2013). Future studies need to explore factors that are associated with individual variation in both sexes of wild mountain gorillas.
Consistent with results from the Bwindi mountain gorilla population (Nizeyi et al., 2011b) and other species with long gut passage times and slow FGM excretion, our data showed no circadian patterns. This may be because of complete dilution of pooled faeces in the digestive tract before excretion (Millspaugh and Washburn, 2004). However, our results did indicate variation in FGM concentrations between collection months, which may point to temporal changes in basal FGM concentrations and needs to be investigated further. In primates, seasonal cortisol variation is associated with multiple factors, which are often interrelated and difficult to disentangle. Temporal GC production in the Virunga gorilla population might be associated with variation in rainfall and temperature, which determines the four main seasons (long and short wet season and long and short dry season; for similar examples in other primates, see Beehner and McCann, 2008; Gesquiere et al., 2008; Foerster et al., 2012). The Virunga mountain gorillas’ habitat covers a wide altitudinal range, from approximately 2200 to 3800 m (McNeilage, 2001). Different altitudes have different rainfall and temperatures and contain different types of vegetation, which leads to changes in diet composition and thus may also contribute to temporal GC changes (Beehner and McCann, 2008; Goymann, 2012). Annual fluctuations in the number of tourist visits and the occurrence of illegal activities (e.g. snares in the home ranges of the study groups) might also be associated with temporal variation in stress levels. To increase our knowledge of the Virunga mountain gorilla stress physiology and inter-individual differences more generally, future research also needs to examine age-related patterns, plus social factors such as dominance rank, group size and group composition.
This study provides the foundation—a biologically validated, field-friendly faecal hormone metabolite extraction and laboratory EIA analysis method (Santymire and Armstrong, 2010; Murray et al., 2013)—for non-invasive monitoring of stress in the endangered Virunga mountain gorilla population. In future studies, this method can be used to examine the relationship between animal population growth, increasing anthropogenic pressure, and stress physiology in this critically endangered ape. The integration of a long-term stress-monitoring programme into existing research practices will provide crucial information for decision-makers in the conservation community, which will assist in answering important conservation questions and developing effective interventions.
Acknowledgements
We thank the Rwandan government and the Rwandan Development Board for their long-term support of the research, monitoring and protection activities of the Dian Fossey Gorilla Fund International's Karisoke Research Center. We are indebted to all Karisoke field staff for their tireless support in collecting faecal samples and long-term behavioural data and to Diane Armstrong, Erin Loeding and Michael Landeche for their support in the laboratory at Lincoln Park Zoo's Davee Center for Epidemiology & Endocrinology. We would like to extend special thanks to the anonymous reviewers for their valuable contributions to improve this article.
Funding
This work was supported by Dian Fossey Gorilla Fund International; Lincoln Park Zoo's Davee Center for Epidemiology & Endocrinology; United States Fish and Wildlife Service (grant number F12AP01120); the Wenner-Gren Foundation (grant number 20110146); the National Science Foundation Doctoral Dissertation Improvement Grant (grant number 1122321); the Leakey Foundation and Sharon and Herb Lurie.
References
- Ayres KL, Booth RK, Hempelmann JA, Koski KL, Emmons CK, Baird RW, Balcomb-Bartok K, Hanson MB, Ford MJ, Wasser SK (2012) Distinguishing the impacts of inadequate prey and vessel traffic on an endangered killer whale (Orcinus orca) population. PLoS One 7: e36842; doi:10.1371/journal.pone.0036842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett GM, Shimizu K, Bardi M, Asaba S, Mori A (2002) Endocrine correlates of rank, reproduction, and female-directed aggression in male Japanese macaques (Macaca fuscata). Horm Behav 42: 85–96. [DOI] [PubMed] [Google Scholar]
- Beehner JC, McCann C (2008) Seasonal and altitudinal effects on glucocorticoid metabolites in a wild primate (Theropithecus gelada). Physiol Behav 95: 508–514. [DOI] [PubMed] [Google Scholar]
- Beehner JC, Bergman TJ, Cheney DL, Seyfarth RM, Whitten PL (2005) The effect of new alpha males on female stress in free-ranging baboons. Anim Behav 69: 1211–1221. [Google Scholar]
- Bergman TJ, Beehner JC, Cheney DL, Seyfarth RM, Whitten PL (2005) Correlates of stress in free-ranging male chacma baboons, Papio hamadryas ursinus. Anim Behav 70: 703–713. [Google Scholar]
- Boscaro M, Paoletta A, Scarpa E, Barzon L, Fusaro P, Fallo F, Sonino N (1998) Age-related changes in glucocorticoid fast feedback inhibition of adrenocorticotropin in man. J Clin Endocrinol Metab 83: 1380–1383. [DOI] [PubMed] [Google Scholar]
- Breuer T, Hockemba MBN, Olejniczak C, Parnell RJ, Stokes EJ (2009) Physical maturation, life-history classes and age estimates of free-ranging western gorillas - Insights from Mbeli Bai, Republic of Congo. Am J Primatol 71: 106–119. [DOI] [PubMed] [Google Scholar]
- Caillaud D, Ndagijimana F, Giarrusso AJ, Vecellio V, Stoinski TS (2014) Mountain gorilla ranging patterns: influence of group size and group dynamics. Am J Primatol 76: 730–746. [DOI] [PubMed] [Google Scholar]
- Cavigelli SA. (1999) Behavioural patterns associated with faecal cortisol levels in free-ranging female ring-tailed lemurs, Lemur catta. Anim Behav 57: 935–944. [DOI] [PubMed] [Google Scholar]
- Charmandari E, Tsigos C, Chrousos G (2005) Endocrinology of the stress response. Annu Rev Physiol 67: 259–284. [DOI] [PubMed] [Google Scholar]
- Creel S, Dantzer B, Goymann W, Rubenstein DR (2013) The ecology of stress: effects of the social environment. Funct Ecol 27: 66–80. [Google Scholar]
- Engh A, Beehner J, Bergman T, Whitten P, Hoffmeier R, Seyfarth R, Cheney D (2006) Female hierarchy instability, male immigration and infanticide increase glucocorticoid levels in female chacma baboons. Anim Behav 71: 1227–1237. [Google Scholar]
- Fletcher AW. (1994) The social development of immature mountain gorillas (Gorilla gorilla beringei). PhD thesis, University of Bristol. [Google Scholar]
- Foerster S, Cords M, Monfort SL (2012) Seasonal energetic stress in a tropical forest primate: proximate causes and evolutionary implications. PLoS One 7: e50108; doi:10.1371/journal.pone.0050108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossey D. (1972) Vocalizations of the mountain Gorilla (Gorilla gorilla beringei). Anim Behav 20: 36–53. [Google Scholar]
- Fossey D. (1983) Gorillas in the Mist. Houghton Mifflin Company, New York. [Google Scholar]
- Fossey D. (1984) Infanticide in mountain gorillas (Gorilla gorilla beringei) with comparative notes on chimpanzees In Hausfater G, Hrdy SB, eds, Infanticide: Comparative and Evolutionary Perspectives. Aldine, New York, pp 217–235. [Google Scholar]
- Ganas J, Robbins MM, Nkurunungi JB, Kaplin BA, McNeilage A (2004) Dietary variability of mountain gorillas in Bwindi Impenetrable National Park, Uganda. Int J Primatol 25: 1043–1072. [Google Scholar]
- Ganswindt A, Münscher S, Henley M, Palme R, Thompson P, Bertschinger H (2010) Concentrations of faecal glucocorticoid metabolites in physically injured free-ranging African elephants Loxodonta africana. Wildlife Biol 16: 323–332. [Google Scholar]
- Ganswindt A, Brown JL, Freeman EW, Kouba AJ, Penfold LM, Santymire RM, Vick MM, Wielebnowski N, Willis EL, Milnes MR (2012) International Society for Wildlife Endocrinology: the future of endocrine measures for reproductive science, animal welfare and conservation biology. Biol Lett 8: 695–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesquiere LR, Khan M, Shek L, Wango TL, Wango EO, Alberts SC, Altmann J (2008) Coping with a challenging environment: effects of seasonal variability and reproductive status on glucocorticoid concentrations of female baboons (Papio cynocephalus). Horm Behav 54: 410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesquiere LR, Learn NH, Simao MCM, Onyango PO, Alberts SC, Altmann J (2011) Life at the top: rank and stress in wild male baboons. Science 333: 357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goymann W. (2012) On the use of non-invasive hormone research in uncontrolled, natural environments: the problem with sex, diet, metabolic rate and the individual. Methods Ecol Evol 3: 757–765. [Google Scholar]
- Goymann W, Wingfield JC (2004) Allostatic load, social status and stress hormones: the costs of social status matter. Anim Behav 67: 591–602. [Google Scholar]
- Goymann W, East ML, Wachter B, Höner OP, Möstl E, Van't Hof T, Hofer H (2001) Social, state-dependent and environmental modulation of faecal corticosteroid levels in free-ranging female spotted hyenas. Proc Biol Sci 268: 2453–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M, McNeilage A, Fawcett K, Robbins MM, Ssebide B, Mbula D, Uwingeli P (2010) Censusing the mountain gorillas in the Virunga Volcanoes: complete sweep method versus monitoring. Afr J Ecol 48: 588–599. [Google Scholar]
- Gray M, Roy J, Vigilant L, Fawcett K, Basabose A, Cranfield M, Uwingeli P, Mburanumwe I, Kagoda E, Robbins MM (2013) Genetic census reveals increased but uneven growth of a critically endangered mountain gorilla population. Biol Conserv 158: 230–238. [Google Scholar]
- Harcourt AH, Stewart KJ (2007) Gorilla Society – Conflict, Compromise and Cooperation between Sexes. University of Chicago Press, Chicago, IL. [Google Scholar]
- Heistermann M, Palme R, Ganswindt A (2006) Comparison of different enzyme-immunoassays for assessment of adrenocortical activity in primates based on fecal analysis. Am J Primatol 273: 257–273. [DOI] [PubMed] [Google Scholar]
- Hoffman CL, Ayala JE, Mas-Rivera A, Maestripieri D (2010) Effects of reproductive condition and dominance rank on cortisol responsiveness to stress in free-ranging female rhesus macaques. Am J Primatol 72: 559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs RM, Ross SR, Wagner KE, Leahy M, Meiers ST, Santymire RM (2014) Evaluating the physiological and behavioral response of a male and female gorilla (Gorilla gorilla gorilla) during an introduction. Zoo Biol 33: 394–402. [DOI] [PubMed] [Google Scholar]
- Loeding E, Thomas J, Bernier D, Santymire R (2011) Using fecal hormonal and behavioral analyses to evaluate the introduction of two sable antelope at Lincoln Park Zoo. J Appl Anim Welf Sci 14: 220–246. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC (2003) The concept of allostasis in biology and biomedicine. Horm Behav 43: 2–15. [DOI] [PubMed] [Google Scholar]
- McNeilage A. (2001) Diet and habitat use of two mountain gorilla groups in contrasting habitats in the Virungas In Robbins MM, Sicotte P, Stewart KJ, eds, Mountain Gorillas: Three Decades of Research at Karisoke. Cambridge University Press, Cambridge, pp 265–292. [Google Scholar]
- Markham AC, Santymire RM, Lonsdorf EV, Heintz MR, Lipende I, Murray CM (2013) Rank effects on social stress in lactating chimpanzees. Anim Behav 87: 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta H, Katee C (2005) Virunga Massif Sustainable Tourism Development Plan: D.R. Congo, Rwanda and Uganda. International Gorilla Conservation Programme (IGCP), Nairobi, Kenya. [Google Scholar]
- Millspaugh JJ, Washburn BE (2004) Use of fecal glucocorticoid metabolite measures in conservation biology research: considerations for application and interpretation. Gen Comp Endocrinol 138: 189–199. [DOI] [PubMed] [Google Scholar]
- Moreira N, Monteiro-Filho ELA, Moraes W, Swanson WF, Graham LH, Pasquali OL, Gombes MLF, Morais RN, Wildt DE, Brown JL (2001) Reproductive steroid hormones and ovarian activity in felids of the Leopardus genus. Zoo Biol 20: 103–116. [DOI] [PubMed] [Google Scholar]
- Möstl E, Messmann S, Bagu E, Robia C, Palme R (1999) Measurement of glucocorticoid metabolite concentrations in faeces of domestic livestock. J Vet Med A 46: 621–631. [DOI] [PubMed] [Google Scholar]
- Möstl E, Rettenbacher S, Palme R (2005) Measurement of corticosterone metabolites in birds’ droppings: an analytical approach. Ann NY Acad Sci 34: 17–34. [DOI] [PubMed] [Google Scholar]
- Muehlenbein MP. (2006) Intestinal parasite infections and fecal steroid levels in wild chimpanzees. Am J Phys Anthropol 130: 546–550. [DOI] [PubMed] [Google Scholar]
- Murray CM, Heintz MR, Lonsdorf EV, Parr LA, Santymire RM (2013) Validation of a field technique and characterization of fecal glucocorticoid metabolite analysis in wild chimpanzees (Pan troglodytes). Am J Primatol 75: 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizeyi JB, Czekala N, Monfort SL, Taha N, Cranfield M, Linda P, Gilardi K (2011. a) Detecting adreno-cortical activity in gorillas : a comparison of faecal glucocorticoid measures using RIA versus EIA. Int J Anim Vet Adv 3: 104–116. [Google Scholar]
- Nizeyi JB, Monfort SL, Taha NM, Cranfield M, Gilardi K, Ave S (2011. b) Non-invasive sampling strategy for monitoring free-ranging Mountain Gorilla (Gorilla beringei beringei) fecal corticoid excretion in Bwindi Impenetrable National Park, South-Western Uganda. Int J Anim Vet Adv 3: 93–102. [Google Scholar]
- Palme R, Rettenbacher S, Touma C, El-Bahr SM, Moestl E (2005) Stress hormones in mammals and birds: comparative aspects regarding metabolism, excretion and noninvasive measurement in fecal samples. Ann NY Acad Sci 1040: 162–171. [DOI] [PubMed] [Google Scholar]
- Palme R, Touma C, Arias N, Dominchin MF, Lepschy M (2013) Steroid extraction: get the best out of faecal samples. Wien Tierarztl Monatsschr 100: 238–246. [Google Scholar]
- R Core Team (2015) R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria: http://www.R-project.org. [Google Scholar]
- Reimers TJ, Cowan RG, Davidson HP, Colby ED (1981) Validation of radioimmunoassay for triiodothyronine, thyroxine, and hydrocortisone (cortisol) in canine, feline, and equine sera. Am J Vet Res 42: 2016–2021. [PubMed] [Google Scholar]
- Robbins AM, Gray M, Basabose A, Uwingeli P, Mburanumwe I, Kagoda E, Robbins MM (2013) Impact of male infanticide on the social structure of mountain gorillas. PLoS One 8. doi:10.1371/journal.pone.0078256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero LM. (2004) Physiological stress in ecology: lessons from biomedical research. Trends Ecol Evol 19: 249–255. [DOI] [PubMed] [Google Scholar]
- Roy J, Vigilant L, Gray M, Wright E, Kato R, Kabano P, Basabose A, Tibenda E, Kühl HS, Robbins MM (2014) Challenges in the use of genetic mark-recapture to estimate the population size of Bwindi mountain gorillas (Gorilla beringei beringei). Biol Conserv 180: 249–261. [Google Scholar]
- Santymire RM, Armstrong DM (2010) Development of a field-friendly technique for fecal steroid extraction and storage using the African wild dog (Lycaon pictus). Zoo Biol 29: 289–302. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. (1982) The endocrine stress-response and social status in the wild baboon. Horm Behav 16: 279–292. [DOI] [PubMed] [Google Scholar]
- Sapolsky R, Romero L, Munck A (2000) How do glucocorticoids influence stress responses? Preparative actions. Endocr Rev 21: 55–89. [DOI] [PubMed] [Google Scholar]
- Schoof VAM, Jack KM (2013) The association of intergroup encounters, dominance status, and fecal androgen and glucocorticoid profiles in wild male white-faced capuchins (Cebus capucinus). Am J Primatol 75: 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutt K, Setchell JM, Heistermann M (2012) Non-invasive monitoring of physiological stress in the Western lowland gorilla (Gorilla gorilla gorilla): validation of a fecal glucocorticoid assay and methods for practical application in the field. Gen Comp Endocrinol 179: 167–177. [DOI] [PubMed] [Google Scholar]
- Sicotte P. (1993) Inter-group encounters and female transfer in mountain gorillas – influence of group composition on male behavior. Am J Primatol 30: 21–36. [DOI] [PubMed] [Google Scholar]
- Stoinski TS, Rosenbaum S, Ngaboyamahina T, Vecellio V, Ndagijimana F, Fawcett K (2009) Patterns of male reproductive behaviour in multi-male groups of mountain gorillas: examining theories of reproductive skew. Behaviour 146: 1193–1215. [Google Scholar]
- Tarlow EM, Blumstein DT (2007) Evaluating methods to quantify anthropogenic stressors on wild animals. Appl Anim Behav Sci 102: 429–451. [Google Scholar]
- Thompson EM, Muller MN, Kahlenberg SM, Wrangham RW (2010) Dynamics of social and energetic stress in wild female chimpanzees. Horm Behav 58: 440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort L, Teles M (2007) The endocrine response to stress – a comparative view In Akin F, ed, Basic and Clinical Endocrinology Up-to-Date. InTech, pp 263–286. [Google Scholar]
- Touma C, Sachser N, Moestl E, Palme R (2003) Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen Comp Endocrinol 130: 267–278. [DOI] [PubMed] [Google Scholar]
- Touma C, Palme R (2005) Measuring fecal glucocorticoid metabolites in mammals and birds: the importance of validation. Ann NY Acad Sci 1046: 54–74. [DOI] [PubMed] [Google Scholar]
- Washburn BE, Millspaugh JJ (2002) Effects of simulated environmental conditions on glucocorticoid metabolite measurements in white-tailed deer feces. Gen Comp Endocrinol 127: 217–222. [DOI] [PubMed] [Google Scholar]
- Wasser SK, Risler L, Steiner RA (1998) Excreted steroids in primate feces over the menstrual cycle and pregnancy. Biol Reprod 39: 862–872. [DOI] [PubMed] [Google Scholar]
- Watts DP. (1984) Composition and variability of mountain gorilla diets in the Central Virungas. Am J Primatol 7: 323–356. [DOI] [PubMed] [Google Scholar]
- Watts D. (1989) Infanticide in mountain gorillas: new cases and a reconsideration of the evidence. Ethology 81: 1–18. [Google Scholar]
- Watts DP, Pusey AE (1993) Behavior of juvenile and adolescent great apes In: Pereira ME, Fairbanks LA, eds. Juvenile Primates: Life History, Development and Behavior. Oxford University Press, New York, pp 148–167. [Google Scholar]
- Weber A, Vedder A (1983) Population dynamics of the Virunga Gorillas: 1959–1978. Biol Conserv 26: 341–366. [Google Scholar]
- Weingrill T, Gray DA, Barrett L, Henzi SP (2004) Fecal cortisol levels in free-ranging female chacma baboons: relationship to dominance, reproductive state and environmental factors. Horm Behav 45: 259–269. [DOI] [PubMed] [Google Scholar]
- Wielebnowski N, Watters J (2008) Applying fecal endocrine monitoring to conservation and behavior studies of wild mammals: important considerations and preliminary tests. Isr J Ecol Evol 53: 439–460. [Google Scholar]
- Williamson E, Gerald-Steklis N (2001) Composition of Gorilla gorilla beringei groups monitored by Karisoke Research Centre, 2001. African Primates 5: 48–51. [Google Scholar]
- Young KM, Walker SL, Lanthier C, Waddell WT, Monfort SL, Brown JL (2004) Noninvasive monitoring of adrenocortical activity in carnivores by fecal glucocorticoid analyses. Gen Comp Endocrinol 137: 148–165. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Wittwer DJ (2005) Fecal steroid research in the field and laboratory: improved methods for storage, transport, processing, and analysis. Am J Primatol 67: 159–174. [DOI] [PubMed] [Google Scholar]