Abstract
Aims
Pulmonary congestion is a common and important finding in heart failure (HF). While clinical examination and chest radiography are insensitive, lung ultrasound (LUS) is a novel technique that may detect and quantify subclinical pulmonary congestion. We sought to independently relate LUS and clinical findings to 6-month HF hospitalizations and all-cause mortality (composite primary outcome).
Methods
We used LUS to examine 195 NYHA class II–IV HF patients (median age 66, 61% men, 74% white, ejection fraction 34%) during routine cardiology outpatient visits. Lung ultrasound was performed in eight chest zones with a pocket ultrasound device (median exam duration 2 min) and analysed offline.
Results
In 185 patients with adequate LUS images in all zones, the sum of B-lines (vertical lines on LUS) ranged from 0 to 13. B-lines, analysed by tertiles, were associated with clinical and laboratory markers of congestion. Thirty-two per cent of patients demonstrated ≥3 B-lines on LUS, yet 81% of these patients had no findings on auscultation. During the follow-up period, 50 patients (27%) were hospitalized for HF or died. Patients in the third tertile (≥3 B-lines) had a four-fold higher risk of the primary outcome (adjusted HR 4.08, 95% confidence interval, CI 1.95, 8.54; P < 0.001) compared with those in the first tertile and spent a significantly lower number of days alive and out of the hospital (125 days vs. 165 days; adjusted P < 0.001).
Conclusions
Pulmonary congestion assessed by ultrasound is prevalent in ambulatory patients with chronic HF, is associated with other features of clinical congestion, and identifies those who have worse prognosis.
Keywords: Heart failure, Pulmonary congestion, Lung ultrasound, Prognosis
See page 1252 for the editorial comment on this article (doi:10.1093/eurheartj/ehw094)
Introduction
Heart failure (HF) remains an important healthcare concern because of its high prevalence, associated morbidity, short- and long-term mortality, and costs.1 Most HF exacerbations are related to a progressive rise in cardiac filling pressures that precipitates pulmonary congestion and symptomatic decompensation.2 In the ambulatory setting, the physical examination is typically used to evaluate pulmonary congestion in HF patients; however, auscultation is qualitative, subjective, and abnormal findings are frequently absent in patients with chronic HF despite haemodynamic congestion.3 Since assessment of pulmonary congestion remains challenging without a gold standard, there is a critical need for quantitative markers of pulmonary congestion to increase the speed and accuracy of diagnosis, facilitate early treatment, inform treatment titration, and potentially improve risk stratification.4
Lung ultrasound (LUS) provides a semi-quantitative assessment of pulmonary congestion in HF that has been identified as a useful point-of-care tool in the evaluation of undifferentiated dyspnea.5,6 ‘B-lines’ are vertical lines on LUS which, when quantified, provide a graded measure of pulmonary congestion. Although LUS has been identified as more sensitive and specific than physical examination and chest X-ray in the acute care setting, the prognostic significance of LUS in ambulatory patients with chronic HF is unclear.5,6
Technological advances over the past two decades have made ultrasound equipment portable with functionality and image quality similar to high-end ultrasound systems.7 Recently, pocket size ultrasound devices have been developed by various vendors with sizes approaching those of smart phones.
To assess the effectiveness of LUS with a pocket device in ambulatory HF patients, we related LUS measures of pulmonary congestion to clinical characteristics and 6-month outcomes in patients with HF.
Methods
Patient population
This was a prospective, single centre, observational study in adults with NYHA II–IV, and HF hospitalization within the past 12 months irrespective of left ventricular ejection fraction (EF). Exclusion criteria are detailed in the Supplementary material online. Non-consecutive patients were recruited from ambulatory cardiology clinics of an academic hospital between December 2011 and October 2014. After obtaining informed consent, an investigator not involved in the patient's clinical care performed the LUS. The treating cardiology providers were blinded to the LUS findings. This study complies with the Declaration of Helsinki and the local institutional review committee approved the research protocol.
Lung ultrasound imaging protocol and analysis
Lung ultrasound examinations were performed by trained investigators employing a standardized imaging protocol with a pocket ultrasound device (VScan, General Electric) with a phased array transducer at an imaging depth of 18 cm in sitting/semirecumbent position. Two second ultrasound clips were recorded for each of the eight LUS zones (four on each hemithorax) as recommended by an international guideline.8 Offline image analysis was performed on de-identified videos by two investigators (E.P., J.P.) with experience in LUS analysis who were blinded to the clinical and outcome data. The highest number of B-lines (vertical lines arising from the pleural line) visualized in a single intercostal space was recorded for each zone. The sum of B-lines in all eight zones was used for the primary analysis. Inter- and intra-rater agreement is described in the Supplementary material online.
Clinical and demographic data
Clinical and demographic data were abstracted from medical records by a single investigator (see the Supplementary material online for definitions). A binary congestion score was computed and considered positive if any of the following was present: Crackles, jugular venous distension (JVD), lower extremity oedema. Laboratory test results were only reported if they were obtained within 7 days of the cardiology clinic visit with the exception of NT-proBNP which was also reported within 30 days of the visit. Estimated GFR (eGFR) was calculated using the modification of diet in renal disease (MDRD) formula. Left ventricular EF was reported if the patient had a recent examination documenting EF up to 12 months prior to the clinic visit.
Outcomes
Patients were followed for 6 months and time to first event was used for all outcome measures. The primary endpoint was a composite of HF hospitalization or all-cause mortality. The secondary endpoint was a composite of urgent HF visit, HF hospitalization, or all-cause mortality. Heart failure hospitalizations were confirmed through patient follow-up phone calls, contacting primary care physicians/cardiologists, and review of patients’ electronic medical records. All-cause mortality was confirmed through the institution's electronic medical records and the social security death index. All prespecified primary and secondary endpoints were based on standardized criteria for endpoint events in cardiovascular trials and were adjudicated by three cardiologists (E.F.L., P.S.J., S.C.) with extensive experience in endpoint adjudication who were blinded to the LUS data (see Supplementary material online for definitions).9
During the study period (May 2013), an ambulatory clinic for short-term intravenous therapy for HF patients opened at the study site.10 Since this new treatment venue could have prevented HF hospitalizations in patients who previously would have been hospitalized, we included these urgent HF visits into a secondary composite outcome and adjusted for the availability of this venue in all secondary outcome analyses. A sensitivity analysis evaluating the impact of the availability of this clinic on the primary outcome is described in the Supplementary material online.
Statistical analyses
For the main data analysis, we divided patients into three groups on the basis of the sum of B-lines (in tertiles) in all eight zones: Tertile 1: 0 B-lines, Tertile 2: 1–2 B-lines, Tertile 3: ≥3 B-lines. This approach was chosen to assess potential trends in baseline characteristics across tertiles with potentially lower B-line numbers than reported with high-end systems.11 We assessed trends across B-line tertiles with modified Wilcoxon rank-sum tests. Continuous variables are presented as medians (interquartile range, IQR) unless otherwise noted, and categorical variables as counts and percentages.
Cumulative incidence functions were calculated for each of the three groups to describe the event rate for each outcome. Cox proportional hazard models (unadjusted and adjusted) were used to assess the effects of B-line number (by tertile) and comorbidities on event-free survival.
Models were adjusted for potential confounding variables, including age, sex, NYHA class, and congestion score. These covariates were chosen based on their clinical importance in relation to the outcome, using a limited number of variables to prevent statistical overfitting.12 The direction and significance of the results remained stable when adjusting for: days since last HF hospitalization, body mass index (BMI), and creatinine. Harrell's C statistic was calculated for each of the models. All models were checked for interaction by B-line tertile. The assumption of proportionality of hazards was tested by allowing a time-varying coefficient for the primary exposure variable (B-line tertile). In none of the models presented was this assumption violated.
Similar unadjusted and adjusted analyses were performed using restricted mean survival time (RMST) as the response variable, where 180 days were used as the truncation time for calculating days alive and out of the hospital for the three groups.13,14
Patients in whom LUS images could not be interpreted in one or several zones (n = 10) were excluded from the main analysis, but baseline characteristics were reported. Sensitivity analyses were performed for these patients and are described in the Supplementary material online.
The incremental diagnostic utility of B-lines in identifying subjects with increased pulmonary or haemodynamic congestion at Day 180 was assessed comparing the physical examination findings (crackles on auscultation; congestion score) vs. LUS findings (presence vs. absence of ≥3 B-lines in eight zones) using the incremental discrimination improvement (IDI) with 10-fold cross validation and the area under the ROC curve (AUC) with bias corrected 95% confidence intervals (CIs).15,16 Two-sided significance levels of 0.05 were used for all analyses. Data were analysed using Stata SE, version 12.0 (StataCorp, Texas 2011).
Results
Baseline characteristics
All 200 enrolled patients underwent LUS (median duration: 2 min per patient) and 5 patients were excluded after enrolment based on exclusion criteria: Two patients had been previously enrolled in our study, two were later found not to have HF as determined by the treating cardiologist and one had pneumonia (Figure 1). Of the remaining 195 patients, 185 (95%) had adequate LUS data in all 8 zones and were included in the main analysis. Baseline characteristics for this cohort, stratified by tertiles of B-line number, are presented in Table 1.
Figure 1.
Study flow chart. Detailing heart failure patients included in the analysis. HF, heart failure; LUS, lung ultrasound.
Table 1.
Baseline characteristics by B-line tertilesa (n = 195)
| Incomplete B-line data (n = 10) | 0 B-lines (n = 72) | 1–2 B-lines (n = 54) | ≥3 B-lines (n = 59) | P (trend)† | |
|---|---|---|---|---|---|
| Sum of B-line in eight zones (median, IQR) | N/A | 0 | 1.5 (1–2) | 5 (4–8) | N/A |
| Demographics | |||||
| Age | 76 (69–80) | 61 (51–73) | 64 (55–72) | 73 (65–82) | <0.001 |
| Male | 6 (60) | 37 (51) | 33 (61) | 42 (71) | 0.021 |
| Race | 0.992 | ||||
| White | 9 (90) | 51 (71) | 42 (78) | 42 (71) | |
| Black | 1 (10) | 15 (21) | 9 (17) | 10 (17) | |
| Hispanic | 0 | 4 (6) | 3 (6) | 7 (12) | |
| Asian | 0 | 2 (3) | 0 | 0 | |
| NYHA class III or IV (n, %) | 5 (50) | 19 (27) | 12 (22) | 31 (53) | 0.003 |
| Days since last HF admission (n = 180) | 40 (9–123) | 94 (35–172) | 89 (26–159) | 40 (14–161) | 0.171 |
| HF admission within past 6 months (n, %) | 7 (70) | 50 (70) | 43 (80) | 44 (75) | 0.555 |
| Medical history | |||||
| Hypertension | 7 (70) | 49 (68) | 40 (74) | 43 (73) | 0.526 |
| Diabetes mellitus | 4 (40) | 35 (49) | 26 (48) | 31 (53) | 0.666 |
| Atrial fibrillation | 6 (60) | 30 (42) | 27 (50) | 38 (64) | 0.010 |
| Current smoker | 0 | 7 (10) | 6 (11) | 3 (5) | 0.371 |
| COPD | 2 (20) | 17 (24) | 16 (30) | 14 (24) | 0.952 |
| Interstitial lung disease | 0 | 0 | 1 (2) | 1 (2) | 0.335 |
| Obstructive sleep apnoea | 3 (30) | 23 (32) | 12 (22) | 11 (19) | 0.076 |
| PCI | 4 (40) | 14 (19) | 14 (26) | 15 (25) | 0.405 |
| CABG | 7 (70) | 14 (19) | 14 (26) | 18 (31) | 0.143 |
| Myocardial infarction | 6 (60) | 16 (22) | 14 (26) | 18 (31) | 0.284 |
| Pacemaker | 0 | 8 (11) | 8 (15) | 12 (20) | 0.145 |
| CRT | 3 (30) | 13 (18) | 9 (17) | 14 (24) | 0.436 |
| ICD | 5 (50) | 28 (39) | 27 (50) | 28 (48) | 0.305 |
| Stroke | 1 (10) | 9 (13) | 4 (7) | 11 (19) | 0.337 |
| Cancer | 6 (60) | 11 (15) | 13 (24) | 18 (31) | 0.038 |
| Medications | |||||
| β-Blocker | 10 (100) | 65 (90) | 48 (89) | 51 (86) | 0.495 |
| ACE-I/ARB | 6 (60) | 54 (75) | 38 (70) | 33 (56) | 0.022 |
| Digoxin | 2 (20) | 12 (17) | 12 (22) | 15 (25) | 0.218 |
| Diuretic | 9 (90) | 65 (90) | 48 (89) | 58 (98) | 0.097 |
| Spironolactone | 1 (10) | 24 (33) | 12 (22) | 20 (34) | 0.990 |
| Calcium channel blocker | 1 (10) | 13 (18) | 5 (9) | 8 (14) | 0.425 |
| Amiodarone | 3 (30) | 12 (17) | 12 (22) | 11 (19) | 0.745 |
| Lipid-lowering drug | 10 (100) | 49 (68) | 33 (61) | 38 (64) | 0.638 |
| Aspirin/antiplatelet agent | 9 (90) | 48 (67) | 37 (69) | 33 (56) | 0.222 |
| Oral anticoagulation | 8 (80) | 35 (49) | 30 (56) | 38 (64) | 0.072 |
| Laboratory results | |||||
| Sodium (mmol/L) (n = 141) | 138 (136–141) | 139 (137–140) | 138 (137–139) | 137 (135–139) | 0.017 |
| Potassium (mmol/L) (n = 140) | 4.0 (3.5–4.4) | 4.1 (3.8–4.5) | 3.9 (3.6–4.3) | 4.1 (3.7–4.4) | 0.501 |
| Haemoglobin (g/dL) (n = 76) | – | 13 (11–13) | 12 (11–13) | 11 (10–13) | 0.052 |
| Haematocrit (%) (n = 76) | – | 38 (34–40) | 37 (33–40) | 34 (31–39) | 0.041 |
| BUN (mg/dL) (n = 140) | 33 (31–44) | 23 (17–37) | 29 (21–45) | 38 (27–64) | <0.001 |
| Creatinine (mg/dL) (n = 141) | 1.4 (0.9–1.8) | 1.2 (0.9–1.5) | 1.4 (1.1–1.9) | 1.5 (1.2–2.2) | <0.001 |
| eGFR (mL/min/1.73 m2) (n = 141) | 38 (35–72) | 61 (42–76) | 47 (33–63) | 44 (29–56) | 0.001 |
| NT-proBNP (pg/mL) (+/− 7 days) (n = 56) | – | 1070 (239–3017) | 1986 (369–3018) | 5395 (3262–8570) | <0.001 |
| NT-proBNP (pg/mL) (+/−30 days) (n = 92) | – | 1095 (294–2562) | 1651 (467–4049) | 5086 (3025–9428) | <0.001 |
| LVEF | |||||
| EF (%) (n = 181) | 46 (28–60) | 36 (25–55) | 33 (22–49) | 32 (23–43) | 0.052 |
| EF ≥ 45% (n, %) | 5 (50) | 38 (54) | 27 (58) | 23 (43) | 0.223 |
PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; CRT, cardiac resynchronization therapy; ICD; implantable cardioverter de brillator; BUN, blood urea nitrogen; LVEF, left ventricular ejection fraction.
aData are presented as median (IQR) for continuous and as count (%) for categorical data.
†P(trend) for B-line tertile 1–3.
The median age of all 195 study subjects was 66 years (range 24–93), 61% were men, 74% Caucasian, median EF within the past 12 months was 34% (IQR 23–51). The sum of B-lines in eight zones ranged from 0 to 13 (median 1, IQR 0–4).
Patients with a higher number of B-lines were more likely to be in a higher NYHA class, have prior atrial fibrillation, cancer, less likely to be prescribed ACE inhibitors or angiotensin receptor blockers, have lower sodium and haematocrit, worse renal function, and higher NT-proBNP. There was no significant difference in history of chronic obstructive pulmonary disease (COPD) or EF across B-line tertiles.
Baseline physical examination
Of all 195 study subjects, 17 (9%) had crackles on auscultation (Table 2). Patients with a higher B-line number were more likely to have a lower BMI, lower diastolic blood pressure, elevated JVD, and more likely to have crackles on auscultation. A stratified analysis of markers of advanced HF by BMI tertiles is presented in the Supplementary material online.
Table 2.
Physical exam findings by B-line tertilesa
| Incomplete B-line data (n = 10) | 0 B-lines (n = 72) | 1–2 B-lines (n = 54) | ≥3 B-lines (n = 59) | P (trend)† | |
|---|---|---|---|---|---|
| BMI (kg/m2) | 33 (24–37) | 31 (27–39) | 29 (26–36) | 26 (22–31) | <0.001 |
| Heart rate (bpm) | 71 (59–75) | 75 (64–86) | 78 (65–85) | 75 (67–83) | 0.845 |
| Systolic blood pressure (mmHg) | 125 (109–136) | 124 (113–136) | 124 (110–133) | 117 (108–130) | 0.084 |
| Diastolic blood pressure (mmHg) | 69 (62–74) | 74 (68–82) | 72 (65–80) | 67 (61–75) | 0.001 |
| Pulse pressure (mmHg) | 50 (42–61) | 46 (40–57) | 48 (40–62) | 48 (39–62) | 0.384 |
| Respiratory rate (breaths per min) | – | 16 (14–18) | 16 (15–18) | 16 (15–18) | 0.211 |
| JVD (≥10 cm) | 6 (60) | 15 (21) | 16 (30) | 22 (37) | 0.048 |
| S3/4 | 2 (20) | 2 (3) | 4 (7) | 5 (9) | 0.175 |
| Crackles (any) | 0 | 1 (1) | 5 (9) | 11 (19) | 0.001 |
| Leg oedema (any) | 6 (60) | 12 (33) | 18 (33) | 29 (49) | 0.074 |
aData are presented as median (IQR) for continuous and as count (%) for categorical data.
†P(trend) for B-line tertile 1–3.
Primary outcome
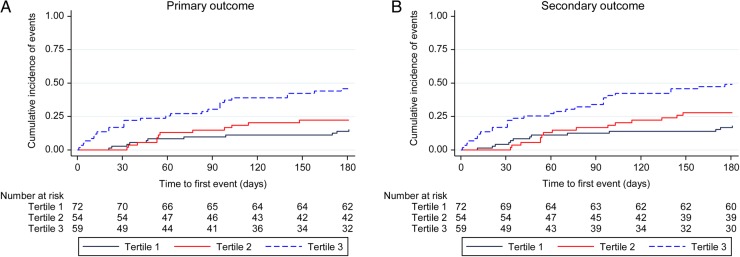
There were 50 primary outcome events during the 6-month follow-up time period. The unadjusted and adjusted risk of the composite primary outcome of HF hospitalization and all-cause mortality is shown in Table 3. The risk of the primary outcome increased with increasing B-line number. Patients in the third tertile (≥3 B-lines) had a four-fold higher risk (adjusted HR 4.08; 95% CI 1.95, 8.54; P < 0.001) of the primary outcome when compared with the first tertile. This difference was mostly driven by the increased number of HF hospitalizations in the two higher tertiles (Table 3). After adjusting for BMI in addition to age, sex, NYHA class III/IV, and congestion score, the main results remained stable (B-line Tertile 3: HR 4.64, 95% CI: 2.09, 10.31, P < 0.001). There was no significant interaction between BMI and B-line number (P = 0.166). The number of days alive and out of the hospital (up to 180 days), as assessed by the RMST, was significantly lower in the third tertile (125 days) when compared with the first (165 days; adjusted P < 0.001).
Table 3.
Primary and secondary outcomes and mean event free times up to 180 days
| Incomplete B-line data (n = 10) | 0 B-lines (n = 72) | 1–2 B-lines (n = 54) | ≥3 B-lines (n = 59) | |
|---|---|---|---|---|
| Primary outcome | ||||
| 6-month HF hospitalization | 4 (40) | 10 (14) | 10 (19) | 24 (41) |
| Death | 2 (20) | 1 (1) | 5 (9) | 7 (12) |
| Primary composite outcome (first event) | 4 (40) | 11 (15) | 12 (22) | 27 (46) |
| Unadjusted HR (95% CI) | – | 1 | 1.50 (0.66, 3.41) P = 0.328 | 3.78 (1.88, 7.63) P<0.001 |
| Adjusted HR* (95% CI) | – | 1 | 1.58 (0.70, 3.59) P = 0.275 | 4.08 (1.95, 8.54) P < 0.001 |
| RMST* (95% CI) | – | 165 days (155, 175) | 156 days (143, 169) P = 0.285 (adj. P = 0.173) | 125 days (107, 144) P < 0.001 (adj. P < 0.001) |
| Secondary outcome | ||||
| 6-month urgent HF visits | 1 (25) | 2 (5) | 3 (7) | 3 (9) |
| Secondary composite outcome (first event) | 4 (40) | 13 (18) | 15 (28) | 29 (49) |
| Unadjusted HR (95% CI) | – | 1 | 1.56 (0.74, 3.31) P = 0.242 | 3.44 (1.79, 6.64) P < 0.001 |
| Adjusted HR* (95% CI) | – | 1 | 1.61 (0.75, 3.42) P = 0.219 | 3.45 (1.72, 6.93) P < 0.001 |
| RMST* (95% CI) | – | 161 days (149, 172) | 152 days (139, 166) P = 0.346 (adj. P = 0.287) | 121 days (102, 139) P < 0.001 (adj. P < 0.001) |
| Censored events | ||||
| VAD placement | 0 | 2 (3) | 0 | 0 |
| Loss to follow-up (for HF hospitalization) | 0 | 3 (4) | 0 | 1 (2) |
VAD, ventricular assist device.
Primary outcome: *Unadjusted model: c-statistic 0.655. Adjusted model: covariates: age, gender, NYHA class III or IV, and congestion score (c-statistic: 0.723).
Secondary outcome: All analyses were adjusted for availability of ambulatory unit for urgent HF visits. *Unadjusted model: c-statistic 0.643. Adjusted model: covariates: age, gender, NYHA class III or IV, and congestion score (c-statistic: 0.700).
Secondary outcome
There were 57 secondary outcome events (urgent HF visit, HF hospitalization, or all-cause mortality) during the follow-up period. The unadjusted and adjusted risk of the composite secondary outcome is shown in Table 3. Similar to the primary outcome, the risk of the primary outcome increased with increasing B-line number. Patients in the third tertile (≥3 B-lines) had a 3.5-fold higher risk (adjusted HR 3.45; 95% CI 1.72, 6.93; P < 0.001) of the primary outcome when compared with the first tertile. The composite secondary outcome was also mainly driven by the number of HF hospitalizations (Table 3). The number of days alive and out of the hospital (up to 180 days by RMST) was significantly lower in the third tertile (121 days) when compared with the first (161 days; adjusted P < 0.001).
Sensitivity analyses for both the primary and the secondary outcome were performed including the 10 patients with incomplete B-line data (Supplementary material online). The direction and significance of the main results remained stable.
Incremental value of lung ultrasound
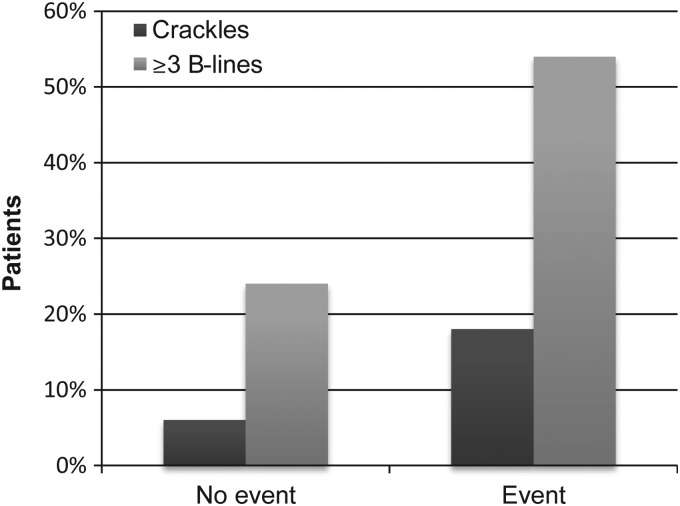
The incremental prognostic value of LUS when compared with auscultation as assessed by the IDI was 6.4% (95% CI 1.0, 14.4) for the primary outcome and 4.9% (95% CI 0.6, 12.5) for the secondary outcome (see also Figure 2). Similarly, the AUC improved significantly both for the primary (AUC delta: 0.194, 95% CI 0.147, 0.315; P < 0.001) and the secondary outcome (AUC delta: 0.132, 95% CI 0.078, 0.213; P = 0.001). The incremental prognostic value of LUS when compared with a congestion score by the IDI was 6.6% (95% CI: 1.9, 15.1) for the primary outcome and 5.5% (95% CI: 0.6, 15.5) for the secondary outcome. The AUC improved both for the primary (AUC delta: 0.136, 95% CI 0.082, 0.228; P = 0.002) and the secondary outcome (AUC delta: 0.078, 95% CI 0.024, 0.167; P = 0.043).
Figure 2.
Percentage of patients with and without primary outcome event and findings on auscultation (crackles) vs. lung ultrasound (B-lines).
Discussion
In this study of stable patients with NYHA class II–IV HF during an outpatient visit, LUS measures of pulmonary congestion were associated with traditional clinical markers of congestion, regardless of EF, but were more prevalent than auscultation findings. A higher number of B-lines on LUS identified patients with a four-fold risk of HF hospitalizations or death from any cause and a more than three-fold risk of urgent HF visits, HF hospitalizations, or death from any cause over 6 months independent of age, sex, NYHA class, and clinical congestion score. Over 180 days, patients with the highest B-line number spent on average 40 days less alive and out of hospital compared to those with the fewest B-lines. Finally, we found that the presence of B-lines may provide incremental prognostic information over traditional methods of pulmonary congestion assessment in patients with chronic HF.
Prior research suggests that ambulatory patients with chronic HF who demonstrate crackles on auscultation are at higher risk for HF hospitalizations and death.12,17 However, auscultation findings are qualitative, subjective, and frequently absent in ambulatory patients with chronic HF, with as few as 4% demonstrating these findings.17 Pulmonary congestion is an important target of HF therapy and, in the outpatient setting, treatment is commonly adjusted based on clinical signs and symptoms. In the absence of a sensitive, specific, and quantitative gold standard for the assessment of pulmonary congestion both the clinical treatment and validation of new methods for its assessment remain challenging. These facts make it necessary to relate novel techniques of pulmonary congestion assessment to outcomes relevant in the population of interest.
Association with clinical characteristics
In this study, only 19% of patients in the highest B-line tertile had crackles on auscultation. Therefore, LUS has the potential to detect subclinical pulmonary congestion even in a stable outpatient population with known HF.18 B-lines were also associated with other clinical and laboratory markers of congestion, including physical exam findings and, in a subset, NT-proBNP. Interestingly, patients with a higher number of B-lines also had a significantly lower BMI, which is similar but more pronounced than in a prior LUS study from our group in which high-end ultrasound systems were used.18 This difference could be due to more advanced HF in the highest B-line group with associated cachexia, a known marker of morbidity and mortality risk in this population.12 Both prior atrial fibrillation and prior cancer were more common in patients with a higher number of B-lines in accordance with their older age. Since patients with known primary or secondary lung or pleural cancer were excluded, other cancer history was unlikely to impact the number of B-lines in our study. Importantly, the prevalence of COPD did not differ between the three groups. Lung ultrasound could present an especially useful diagnostic tool in patients with concomitant HF and COPD (Figure 3).
Figure 3.
Cumulative incidence of primary and secondary outcome by B-line tertiles.
Prognostic value of lung ultrasound
There are limited data on the prognostic value of LUS in HF. Three studies of hospitalized patients, in which study populations were either heterogeneous and not limited to those with HF, or focused on patients with acute coronary syndrome, showed that a higher B-line number was associated with an increased morbidity and mortality risk in multivariable analyses.19–21 These studies employed high-end ultrasound systems for the assessment of LUS findings. Only one prior study, involving 104 HF patients, related B-lines in addition to pleural effusions to HF hospitalization or death during a median follow-up time of ∼1.5 years.22 The investigators found that, after multivariable adjustment, patients with >3 B-lines in five zones or pleural effusion(s) had an almost five-fold risk of experiencing the primary outcome.
Similarly, in our study with a larger number of patients and events we found that ambulatory HF patients with ≥3 B-lines in eight chest zones are at higher risk of experiencing a HF hospitalization or dying by 180 days. Given that only a small proportion of these patients had findings on auscultation, the detection of subclinical pulmonary congestion has the potential to allow for earlier treatment modification and possibly reduction of HF hospitalizations. Beyond the clinical utility, this method could be used in trials to identify and quantify the degree of pulmonary congestion at baseline and monitor the effect of treatment on pulmonary congestion in an objective manner.4
Incremental value of lung ultrasound
Since this technique can be either performed with a point-of-care device or with standard high-end ultrasound equipment routinely used in clinical practice the added cost would be low as long as ultrasound equipment is available. The average time to perform this examination was 2 min in our study which makes integration in standard clinical examinations even in time pressed settings feasible. High reproducibility is another important feature of this method for both the clinical and research arena, with a mean difference of 0.3 B-lines between readers in our study.6,23
Our findings suggest that LUS provides incremental prognostic value when compared with both auscultation and a clinical congestion score assessed by IDI and AUC analyses. While LUS has many advantages over auscultation, our findings should be considered hypothesis generating and will need to be investigated further in larger trials in chronic HF populations.
Limitations
This was a single centre study of a well-characterized sample of ambulatory HF patients. Two HF patients met neither the NYHA class nor the HF hospitalization criteria. However, given the substantial event rate in our cohort, we do believe that all included patients were at high risk of experiencing the primary and secondary outcomes. The ultrasound device employed in this study only allowed for recording of 2 s clips. Recent literature suggests that clip length may impact the quantification of B-lines during offline image analysis.11 We thus may have underestimated the number of B-lines in this cohort. As pocket devices with longer clip duration have since become available, these could be utilized in future studies. Moreover, when LUS images are interpreted in real-time, clip length would be irrelevant. In both situations, different (likely higher) cut-off values may need to be used. Although the linear trend towards a lower BMI with increasing B-line number may be due to more advanced HF with associated lower BMI, we cannot exclude that ultrasound device characteristics may have contributed to the identification of fewer B-lines in obese patients. In this study, we did not assess for the presence of pleural effusions in addition to B-lines.22 Finally, since only a small subset of patients had natriuretic peptides measured in proximity to their clinic visit we could not assess the incremental value of LUS over these biomarkers. However, prior literature suggests that B-lines may provide incremental prognostic information over NT-proBNP in HF patients.22
Conclusions
Pulmonary congestion assessed by ultrasound is prevalent in ambulatory patients with chronic HF, is associated with other features of clinical congestion, and identifies those who have a worse prognosis. Whether LUS findings of pulmonary congestion can be used to guide therapy and as a therapeutic target in HF deserves consideration.
Authors’ contributions
E.P., H.U.; performed statistical analysis; E.P., S.D.S. handled funding and supervision; J.P., Em.Pi., A.A.M., D.H., C.W., S.E.F. acquired the data; E.P.: conceived and designed the research; E.P. drafted the manuscript; E.F.L., H.U., Em.Pi., P.S.J., S.C., S.D.S. made critical revision of the manuscript for key intellectual content.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by grants from the Eleanor and Miles Shore Fellowship (E.P.) and the American Heart Association (grant number 13CRP14330000) (E.P.). The writing of this manuscript was supported by a grant from the National Heart, Lung and Blood Institute (grant number 1K23HL123533-01A1) (E.P.).
Conflict of interest: none declared.
References
- 1.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013;6:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JE, Cleland JG, Dickstein K, Drazner MH, Fonarow GC, Jaarsma T, Jondeau G, Sendon JL, Mebazaa A, Metra M, Nieminen M, Pang PS, Seferovic P, Stevenson LW, van Veldhuisen DJ, Zannad F, Anker SD, Rhodes A, McMurray JJ, Filippatos G. Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail 2010;12:423–433. [DOI] [PubMed] [Google Scholar]
- 3.Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. J Am Med Assoc 1989;261:884–888. [PubMed] [Google Scholar]
- 4.Platz E, Jhund PS, Campbell RT, McMurray JJ. Assessment and prevalence of pulmonary oedema in contemporary acute heart failure trials: a systematic review. Eur J Heart Fail 2015;17:906–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al Deeb M, Barbic S, Featherstone R, Dankoff J, Barbic D. Point-of-care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea: a systematic review and meta-analysis. Acad Emerg Med 2014;21:843–852. [DOI] [PubMed] [Google Scholar]
- 6.Pivetta E, Goffi A, Lupia E, Tizzani M, Porrino G, Ferreri E, Volpicelli G, Balzaretti P, Banderali A, Iacobucci A, Locatelli S, Casoli G, Stone MB, Maule MM, Baldi I, Merletti F, Cibinel GA. Lung ultrasound-implemented diagnosis of acute decompensated heart failure in the ED: a SIMEU multicenter study. Chest 2015;148:202–210. [DOI] [PubMed] [Google Scholar]
- 7.Prinz C, Voigt JU. Diagnostic accuracy of a hand-held ultrasound scanner in routine patients referred for echocardiography. J Am Soc Echocardiogr 2011;24:111–116. [DOI] [PubMed] [Google Scholar]
- 8.Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, Melniker L, Gargani L, Noble VE, Via G, Dean A, Tsung JW, Soldati G, Copetti R, Bouhemad B, Reissig A, Agricola E, Rouby JJ, Arbelot C, Liteplo A, Sargsyan A, Silva F, Hoppmann R, Breitkreutz R, Seibel A, Neri L, Storti E, Petrovic T. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 2012;38:577–591. [DOI] [PubMed] [Google Scholar]
- 9.Hicks KA, Hung HMJ, Mahaffey KW, Mehran R, Nissen SE, Stockbridge NL, Targum SL, Temple R, On Behalf of the Standardized Data Collection for Cardiovascular Trials Initiative. Draft definitions for testing. Standardized definitions for cardiovascular and stroke endpoint in clinical trials. Food Drug Admin 2012, Chapter 7, p16–17. [Google Scholar]
- 10.Desai AS, Stevenson LW. Rehospitalization for heart failure: predict or prevent? Circulation 2012;126:501–506. [DOI] [PubMed] [Google Scholar]
- 11.Platz E, Pivetta E, Merz AA, Peck J, Rivero J, Cheng S. Impact of device selection and clip duration on lung ultrasound assessment in patients with heart failure. Am J Emerg Med 2015;33:1552–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pocock SJ, Wang D, Pfeffer MA, Yusuf S, McMurray JJ, Swedberg KB, Ostergren J, Michelson EL, Pieper KS, Granger CB. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J 2006;27:65–75. [DOI] [PubMed] [Google Scholar]
- 13.Zhao L, Tian L, Uno H, Solomon SD, Pfeffer MA, Schindler JS, Wei LJ. Utilizing the integrated difference of two survival functions to quantify the treatment contrast for designing, monitoring, and analyzing a comparative clinical study. Clin Trials 2012;9:570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uno H, Wittes J, Fu H, Solomon SD, Claggett B, Tian L, Cai T, Pfeffer MA, Evans SR, Wei LJ. Alternatives to hazard ratios for comparing the efficacy or safety of therapies in noninferiority studies. Ann Intern Med 2015;163:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerr KF, McClelland RL, Brown ER, Lumley T. Evaluating the incremental value of new biomarkers with integrated discrimination improvement. Am J Epidemiol 2011;174:364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–172; discussion 207–112. [DOI] [PubMed] [Google Scholar]
- 17.Damy T, Kallvikbacka-Bennett A, Zhang J, Goode K, Buga L, Hobkirk J, Yassin A, Dubois-Rande JL, Hittinger L, Cleland JG, Clark AL. Does the physical examination still have a role in patients with suspected heart failure? Eur J Heart Fail 2011;13:1340–1348. [DOI] [PubMed] [Google Scholar]
- 18.Platz E, Hempel D, Pivetta E, Rivero J, Solomon SD. Echocardiographic and lung ultrasound characteristics in ambulatory patients with dyspnea or prior heart failure. Echocardiography 2014;31:133–139. [DOI] [PubMed] [Google Scholar]
- 19.Frassi F, Gargani L, Tesorio P, Raciti M, Mottola G, Picano E. Prognostic value of extravascular lung water assessed with ultrasound lung comets by chest sonography in patients with dyspnea and/or chest pain. J Card Fail 2007;13:830–835. [DOI] [PubMed] [Google Scholar]
- 20.Kimura BJ, Yogo N, O'Connell CW, Phan JN, Showalter BK, Wolfson T. Cardiopulmonary limited ultrasound examination for "quick-look" bedside application. Am J Cardiol 2011;108:586–590. [DOI] [PubMed] [Google Scholar]
- 21.Bedetti G, Gargani L, Sicari R, Gianfaldoni ML, Molinaro S, Picano E. Comparison of prognostic value of echographic [corrected] risk score with the thrombolysis in myocardial infarction (TIMI) and global registry in acute coronary events (GRACE) risk scores in acute coronary syndrome. Am J Cardiol 2010;106:1709–1716. [DOI] [PubMed] [Google Scholar]
- 22.Gustafsson M, Alehagen U, Johansson P. Imaging congestion with a pocket ultrasound device - prognostic implications in patients with chronic heart failure. J Card Fail 2015;21:548–554. [DOI] [PubMed] [Google Scholar]
- 23.Platz E, Lattanzi A, Agbo C, Takeuchi M, Resnic FS, Solomon SD, Desai AS. Utility of lung ultrasound in predicting pulmonary and cardiac pressures. Eur J Heart Fail 2012;14:1276–1284. [DOI] [PubMed] [Google Scholar]