The p53-VEGF pathway is critical for the growth and metastases of soft tissue sarcomas. Inhibition of VEGFR is one way to disrupt this pathway and mutations in TP53 may predict response to VEGFR inhibition in metastatic STSs.
Keywords: sarcoma, TP53, VEGFR, pazopanib, next-generation sequencing
Abstract
Background
To investigate whether TP53 DNA mutational status impacts progression-free survival (PFS) in patients with advanced sarcomas (soft tissue sarcoma) treated with vascular endothelial growth factor receptors (VEGFR) inhibition.
Patients and methods
We retrospectively reviewed 19 cases of patients treated at the Ohio State James Comprehensive Cancer Center with advanced sarcoma treated with VEGFR inhibition who also had next-generation sequencing of their tumors (via FoundationOne Heme panel). We evaluated TP53 as well as mutations that were observed in at least 20% of patients and evaluated its contribution to PFS using the Kaplan–Meier survival analysis of available radiology end points.
Results
Mutations that were observed in at least 20% of patients included TP53 and Rb1. Only TP53 was predictive of PFS in the context of VEGFR inhibition. The PFS of patients with TP53 mutations was significantly greater than TP53 wild-type tumors with the median PFS of 208 versus 136 days, respectively [P = 0.036, hazards ratio 0.38 (95% confidence interval 0.09–0.83)].
Conclusions
Mutations in TP53 may serve as a predictive biomarker of response to VEGFR inhibition in patients with advanced sarcoma. Larger, prospective studies are necessary to confirm these findings.
introduction
Bone and soft tissue sarcomas (STS) comprise a diverse group of rare tumors originating from the embryonic mesoderm. They account for 1% of all new adult malignancies diagnosed annually [1, 2]. The mainstay of curative therapy is surgical resection of localized disease with or without adjuvant chemoradiation. Overall survival has changed little for patients with advanced sarcomas in the past few decades, albeit with few exceptions such as in gastrointestinal stromal tumors. The median overall survival remains 12 months in the metastatic setting, and cytotoxic chemotherapeutic options have not significantly altered this landscape.
Pazopanib, an orally available multitargeted tyrosine kinase inhibitor, has recently been shown to improve progression-free survival (PFS) for patients with non-adipocytic STS in the metastatic setting. It has activity against vascular endothelial growth factor receptors (VEGFR) 1, 2, 3 and platelet-derived growth factor receptors. EORTC 62043 was a single-arm, phase II study that characterized the activity of pazopanib in the metastatic setting in four different strata: leiomyosarcomas, adipocytic STS, synovial sarcoma and other STS. In 142 patients, the predefined primary end point of progression-free rate of 40% of patients at 12 weeks was met in the leiomyosarcoma, synovial and other STS cohorts. The predefined PFR12 weeks was not met in the adipocytic cohort [3]. The PALETTE study was a randomized, phase III, multi-institutional trial designed to confirm the efficacy of pazopanib observed in EORTC 62043. In this trial, pazopanib 800 mg once daily was directly compared with placebo in non-adipocytic STSs. The patients in the pazopanib arm experienced an increase of 3 months in PFS versus patients on placebo, thus establishing pazopanib as a new treatment option for patients with advanced STS [4].
Given the modest, albeit significant, increase in PFS observed in the PALETTE trial, it would be ideal to identify patients who are likely to benefit from pazopanib before initiating therapy. Kasper et al. used the pooled data from EORTC 62043 (n = 118) and the PALETTE trial (n = 226) to compare patient characteristics among long-term survivors on pazopanib, defined as a PFS of ≥6 months. Based on this definition, 36% of patients were considered long-term survivors. They found that performance status, low–intermediate histology and normal baseline hemoglobin were advantageous for long-term outcome. Interestingly, 12 patients (3.5%) had a response to pazopanib for more than 2 years. These patients tended to be young, female and have low–intermediate histologies [5].
Recently, there has been a convergence of evidence to suggest that STSs with mutations in the TP53 gene may respond better to VEGFR inhibition than TP53 wild-type (WT). In the preclinical setting, Pollock et al. demonstrated that wild-type p53 suppresses angiogenesis by transcriptional suppression of vascular endothelial growth factor (VEGF) expression in human leiomyosarcoma and synovial sarcoma cell lines. As such, loss of function p53 mutant cells produced significantly more VEGF, which contribute directly to angiogenesis, metastasis and growth [6]. Farhang Ghahremani et al. subsequently found that the p53-VEGF pathway is dependent on a functional Rb1. They showed that p53 WT promotes VEGF expression and angiogenesis in the absence of an intact p21-Rb pathway in mouse embryonic fibroblasts exposed to hypoxic conditions [7].
Additionally, there is mounting clinical evidence that TP53 mutations may predict for response to VEGFR inhibition. A recent phase I study from MD Anderson demonstrated that VEGFR inhibition in combination with histone deacetylase (HDAC) inhibition appeared to be more effective in TP53 mutant versus TP53 WT tumors [8]. In their cohort of 78 patients with mostly metastatic colorectal or STS, they showed an increase in stable disease beyond 6 months for patients with mutant TP53 versus wild-type (45% versus 16%, P = 0.096), as well as trends toward increased PFS and OS (3.5 versus 2 months, P = 0.042; 12.7 versus 7.4 months, P = 0.1).
Based on these compelling preliminary data, we hypothesized that TP53 mutations resulting in loss of function in p53 would be a positive predictor of response to pazopanib as measured by PFS in advanced sarcoma patients.
materials and methods
We carried out a retrospective chart review of patients with advanced STS treated with pazopanib at the Ohio State Comprehensive Cancer Center (IRB 2014E0450). We stratified patients by TP53 and Rb1 mutational status based on next-generation sequencing (FoundationOne Heme/Sarcoma panel) and used PFS as our primary end point.
patients
From 1 January 2012 to 9 January 2014, we treated over 70 patients with advanced sarcomas with pazopanib. Of these, 19 patients had next-generation sequencing carried out through Foundation Medicine (sarcoma/heme panel) and were included in this retrospective review.
treatment
Eighteen patients were treated with oral pazopanib at the maximal tolerated doses. One patient was treated with sunitinib. Some patients had dose reductions of 400 mg daily based on tolerability. Eleven of 19 had been treated with prior doxorubicin-based therapy.
clinical assessment
Patients were evaluated for PFS based on imaging modality chosen by the treating physician (PET CT, CT or MRI). Responses were assessed using RECIST or PERCIST criteria, depending on the imaging modality. Time to progression was measured in days. One cycle of pazopanib was defined as 28 days. Patients were gauged for response after two cycles of drug and subsequently every two cycles thereafter. Patients who had to discontinue therapy due to drug toxicities were excluded from this analysis.
statistical analysis
Descriptive statistics were used to summarize the overall patient mutational statuses. The Kaplan–Meier survival estimates were generated using Graphpad Prism 6.0 by log-rank (Mantel Cox) test.
results
Patient characteristics are listed in Table 1 (male/female 8/11; mean age 49). Histologies included leiomyosarcoma (5), liposarcoma (3), mesenchymal chondrosarcoma (2), desmoblastic small round cell (1), myxofibrosarcoma (2), epithelioid (1), myxoid chondrosarcoma (2), synovial (1), angiosarcoma (1), undifferentiated pleomorphic sarcoma (1). Mutations seen in 10% or more of patients are summarized in Table 2. A total of 233 mutations were observed in this cohort of patients. TP53 mutations were the most common mutations present with 53% (10/19) of patients, predicting loss of function in p53. Thirty-two percent (6/19) patients had mutations in Rb1. All other mutations were present in <20% of cases.
Table 1.
Patient characteristics
| Patient | Histology | Sites of metastases | TP53 | RB1 | Time to progression (days) |
|---|---|---|---|---|---|
| 1 | LMS | Liver, abdominal lymph nodes | MUT | WT | 334 |
| 2 | Angiosarcoma | Scalp lesion | MUT | WT | 447 |
| 3 | Mesenchymal chondro | Intraabdominal mesenteric tumors | WT | WT | 245 |
| 4 | LMS | Lungs, mediastinum | MUT | WT | 249 |
| 5 | LPS | Lungs | WT | MUT | 153 |
| 6 | Synovial | Lungs | WT | WT | 112 |
| 7 | Desmoplastic small round cell | Intraabdominal lymph nodes, lungs | MUT | MUT | 153 |
| 8 | LMS | Peritoneum, retroperitoneum | MUT | WT | 133 |
| 9 | LMS | Left pelvic soft tissue, hilar lymph nodes | MUT | MUT | 169 |
| 10 | Myxofibrosarcoma | Lungs | MUT | MUT | 98 |
| 11 | Epithelioid | Lungs, supraclavicular LNs, acetabulum | WT | WT | 111 |
| 12 | Osteosarcoma | Pelvic mass, lungs | WT | WT | 85 |
| 13 | Small blue round cell | Sacrum, lungs | WT | WT | 62 |
| 14 | Myxo | Vertebral lesions | MUT | MUT | 92 |
| 15 | LMS | Lungs, mediastinum, liver | MUT | MUT | 132 |
| 16 | Mesenchymal chondro | Cervical and thoracic spine metastases | WT | WT | 162 |
| 17 | LPS | Liver, retroperitoneum | WT | WT | 136 |
| 18 | LPS-DD | Retroperitoneum | WT | WT | 163 |
| 19 | UPS | Right gluteal mass, lungs | MUT | WT | 168 |
Table 2.
Frequency of mutations (n = patients)
| Mutations detected in >20% of patients | Mutations detected in >15% of patients | Mutations detected in >10% of patients |
|---|---|---|
| TP53 (10), RB1 (6) | BCL6 (3), BLM (3), BRCA1 (3), CIC (3), MAP3K1 (3), NF1 (3), POT1 (3), WDR90 (3), WHSC1 (3) | APC (2), ARID2 (2), ASXL1 (2), ATRX (2), BRCA2 (2), CCT6B (2), CDK12 (2), CDKN2A (2), CHD2 (2), CHEK2 (2), CIITA (2), CPS1 (2), EP300 (2), EXOSC6 (2), FANCE (2), FRS2 (2), GRIN2A (2), HDAC7 (2), IRS2 (2), JAK1 (2), LRP1B (2), LRRK2 (2), MDM2 (2), MED12 (2), NOTCH1 (2), NTRK1 (2), PIK3R2 (2), RAD50 (2), SDHC (2), TET2 (2) |
All of the mutations in TP53 were predicted to be loss of function mutations by Foundation Medicine. The most common mutations were those within the DNA binding domain (DBD) (3/10 patients) or that affected the tetramerization domain (4/10 patients). One patient had a mutation predicted to affect both the DBD and tetramerization domain. Two patients had homozygous deletion resulting in complete loss of p53.
Foundation Medicine next-generation sequencing does not differentiate between germline and somatic mutations. No patients in this cohort had been diagnosed with LiFraumeni syndrome or concurrent malignancies, however, and all TP53 mutations observed were assumed to be somatic in origin.
There were no documented CRs to pazopanib in our 19 patients included in this analysis. The best response was a partial remission, which 1 of 11 responders to pazopanib patient achieved. Ten of the 11 responders otherwise had stable disease.
discussion
To our knowledge, this retrospective review is the first study to show that response to VEGFR inhibition with single-agent pazopanib can be predicted by TP53 mutational status in advanced non-adipocytic STS.
Preclinical studies have established the importance of the p53-VEGF pathway for angiogenesis in tumor biology. It has previously been shown that wild-type TP53 suppresses angiogenesis by transcriptional suppression of VEGF expression in human leiomyosarcoma and synovial sarcoma cell lines [6]. As such, cells with loss-of-function TP53 produce significantly more VEGF, thus directly contributing to the angiogenesis, metastases and growth of tumor cells. Moreover, mutations in TP53 have been shown to directly increase the level of hypoxia-inducible factor 1α (HIF-1α) and augment HIF-1α-dependent transcriptional activation of the VEGF gene in response to hypoxia [9, 10].
Additionally, the p53-VEGF pathway appears to be dependent on a functional Rb1, as increased levels of VEGF have been observed in Rb1-null mouse fibroblasts with a functional p53 [7]. The loss of function mutations in either TP53 or RB1 would therefore hypothetically directly contribute to the angiogenesis of the malignant cells, thus making tumors with mutations in either gene potentially sensitive to anti-angiogenic therapy.
There is mounting clinical evidence that demonstrates the importance of TP53 mutations in predicting response to anti-angiogenic therapy. Recently, Fu et al. published the results of a phase I study of pazopanib in combination with the HDAC inhibitor, vorinostat, in advanced solid malignancies. Analysis of 78 unselected patients (23 sarcoma) with combination pazopanib and vorinostat did not lead to antitumor activity (5% PR and 14% SD ≥ 6 months). However, when stratified by TP53 mutational status, patients with detected mutations tended to respond more favorably with greater SD ≥ 6 months (45% versus 16%, P = 0.096) [8]. A significantly longer median PFS (3.5 versus 2.0 months, P = 0.042) was also observed in the group with TP53 mutations, which supports our observation that patients with TP53 mutations are more likely to respond to VEGFR inhibition (Figure 1).
Figure 1.
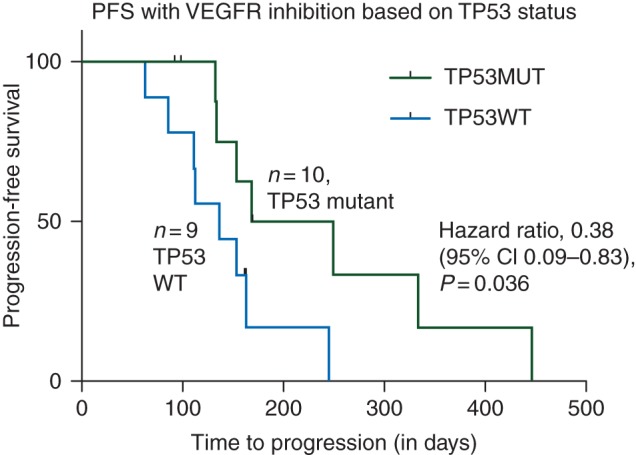
Progression-free survival of VEGFR inhibition stratified by TP53 mutation status. Ten patients with mutations in TP53 with longer progression-free survival than nine patients with no mutation in TP53.
Of additional interest is whether the type of TP53 mutation relates to the sensitivity to VEGFR inhibition. All of the mutations in our small population were predicted to be loss of function. The two most common mutations in TP53 that result in loss of function are those involving the DBD or tetramerization domain. We did not observe a significant difference in PFS when stratifying between p53 mutations in the DBD versus tetramerization domain; however, our study is limited by small sample size and this should be an area of future clinical study.
The PALETTE study showed an improvement of 3 months in PFS in patients with advanced non-adipocytic STS versus placebo. Interestingly, in a subset analysis, patients with leiomyosarcoma were noted to have greater PFS on pazopanib versus other histologies (hazards ratio 0.88) [4]. We suspect that this may relate to the prevalence of TP53 mutations in this tumor group. According to the COSMIC database, 24% of LMS have mutations in TP53, making it the most common mutation in this histology (cancer.sanger.ac.uk, query date: 6 October 2015) [11]. Although liposarcomas were not included in the PALETTE trial as the adipocytic strata in EORTC 62043 did not demonstrate a clinical benefit in this group of patients, in the post-marketing survey, the PFS rate at 12 weeks was 26% (5 of 19 LPS patients) and higher than the threshold for study continuation [12]. In fact, pazopanib is approved in Japan and ongoing clinical trials are exploring these PALETTE ineligible cohorts [13]. Given these data, we included three adipocytic histologies in this retrospective review as our assumption was that response to treatment would be predicted by genetic alterations and not histology.
In this study, we were also curious as to whether the TP53 mutational status may be relevant to predicting response to doxorubicin, a common cytotoxic chemotherapy used in the treatment of advanced STSs. The mechanism of action of doxorubicin is likely highly dependent on a functional p53 since, as a topoisomerase II inhibitor, it prevents G1 to S phase. There are additionally data to suggest that tumors with TP53 mutations are less sensitive to doxorubicin. Aas et al. [14] have previously reported specific TP53 mutations associated with de novo resistance to doxorubicin in breast cancer patients. Therefore, we predicted that p53 WT tumors would respond better to doxorubicin. Of our 19 patients included in our analysis, 11 received doxorubicin previously. In this small patient sample, there was no observed significance difference in response to doxorubicin. Larger studies will be necessary to confirm these results.
In addition to the TP53 mutational status, we were also interested in the Rb1 status. Rb1 is relevant to the p53-VEGF pathway and was the second most common observed mutation in our cohort. Farhang Ghahremani et al. [7] have previously shown that p53 promotes VEGF expression and angiogenesis in the absence of an intact p21-Rb pathway. We therefore hypothesized that tumors with Rb1 mutations would also be sensitive to VEGFR inhibition. However, our clinical observation was contrary to this prediction. Rb1 mutants tended to do worse in the context of a p53 mutation and when patients were stratified into four groups: TP53 MUT/Rb1 WT (n = 6), TP53 MUT/Rb1 MUT (n = 4), TP53 WT/Rb1 MUT (n = 1) and TP53 WT/Rb1 WT (n = 8). However, there was no significant difference in PFS observed in our patient sample.
In considering the clinical relevance of our findings, several limitations should be borne in mind. First, the retrospective nature of the analysis limits the validity of our observations. Additionally, the small patient sample of 19 patients and the wide-range histologies we included make conclusions difficult to draw. Thirdly, this is a single-institutional study and all the patients were treated at the Ohio State University. Despite this, we observed a significant increase in the PFS of patients with advanced STS and TP53 mutations that is hypothesis-generating and supported by the emerging relevance of targeting the p53-VEGF axis as a therapeutic modality in cancer patients. Larger correlative studies should be carried out to confirm these results in the future.
funding
SARC CDA grant was awarded to Chen by SARC (no grant number).
disclosure
JLC has carried out advisory board work for Novartis. All remaining authors have declared no conflicts of interest.
references
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65(1): 5–29. [DOI] [PubMed] [Google Scholar]
- 2.Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med 2005; 353(7): 701–711. [DOI] [PubMed] [Google Scholar]
- 3.Sleijfer S, Ray-Coquard I, Papai Z et al. . Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043). J Clin Oncol 2009; 27(19): 3126–3132. [DOI] [PubMed] [Google Scholar]
- 4.van der Graaf WT, Blay Jy, Chawla SP et al. . Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012; 379(9829): 1879–1886. [DOI] [PubMed] [Google Scholar]
- 5.Kasper B, Sleijfer S, Litiere S et al. . Long-term responders and survivors on pazopanib for advanced soft tissue sarcomas: subanalysis of two European Organisation for Research and Treatment of Cancer (EORTC) clinical trials 62043 and 62072. Ann Oncol 2014; 25(3): 719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L, Yu D, Hu M et al. . Wild-type p53 suppresses angiogenesis in human leiomyosarcoma and synovial sarcoma by transcriptional suppression of vascular endothelial growth factor expression. Cancer Res 2000; 60(13): 3655–3661. [PubMed] [Google Scholar]
- 7.Farhang Ghahremani M, Goossens S, Nittner D et al. . p53 promotes VEGF expression and angiogenesis in the absence of an intact p21-Rb pathway. Cell Death Differ 2013; 20(7): 888–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu S, Hou MM, Naing A et al. . Phase I study of pazopanib and vorinostat: a therapeutic approach for inhibiting mutant p53-mediated angiogenesis and facilitating mutant p53 degradation. Ann Oncol 2015; 26(5): 1012–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loges S, Mazzone M, Hohensinner P, Carmeliet P. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer Cell 2009; 15(3): 167–170. [DOI] [PubMed] [Google Scholar]
- 10.Ravi R, Mookerjee B, Bhujwalla ZM et al. . Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev 2000; 14(1): 34–44. [PMC free article] [PubMed] [Google Scholar]
- 11.Forbes SA, Beare D, Gunasekaran P et al. . COSMIC: exploring the world's knowledge of somatic mutations in human cancer. Nucleic Acids Res 2015; 43(Database issue): D805–D811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilky BA, Meyer CF, Trent JC. Pazopanib in sarcomas: expanding the PALETTE. Curr Opin Oncol 2013; 25(4): 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawai A. Post marketing surveillance in Japan for soft tissue sarcoma patients treated with pazopanib. Abstract 3438 In European Cancer Congress 2015 2015. Vienna, Austria. [Google Scholar]
- 14.Aas T, Borresen AL, Geisler S et al. . Specific P53 mutations are associated with de novo resistance to doxorubicin in breast cancer patients. Nat Med 1996; 2(7): 811–814. [DOI] [PubMed] [Google Scholar]


