Abstract
Hepatitis C virus (HCV) infection is a growing problem, disproportionately affecting those born between 1945 and 1965. Here, we demonstrate the wide geographic reach and surveillance potential of emergency department–based screening and identify areas of elevated HCV infection in central Alabama that were socioeconomically disadvantaged compared with surrounding communities.
Keywords: hepatitis C, screening, emergency department, geography, epidemiology
Chronic hepatitis C virus (HCV) infection afflicts more than 2.5 million persons in the United States and is associated with substantial morbidity and mortality, including cirrhosis and hepatocellular carcinoma, representing a leading cause of liver transplantation [1, 2]. Baby boomers, those born between 1945 and 1965, are affected disproportionately, accounting for 75% of all infections [3]. The advent of new antiviral treatments has transformed clinical management of HCV and renewed public health urgency to find individuals unknowingly infected [4]. Pilot efforts have demonstrated the high yield of targeted screening in the emergency department (ED) and the need for improved linkage to care for new cases [5]. However, the utility of ED-based screening for identification of previously unrecognized HCV infection within the larger community surrounding such a program as well as geospatial patterns of HCV prevalence are unclear.
In this analysis, we examine the geographic reach of ED-based HCV screening and the potential for screening initiatives to inform public health surveillance using data from an urban, academic ED in central Alabama.
METHODS
We conducted a geospatial analysis of newly diagnosed HCV-infected patients presenting to the University of Alabama at Birmingham (UAB) Hospital ED. The institutional review board of UAB approved the study.
Previously described in detail, the UAB ED HCV screening program offered opt-out screening to all “baby boomer” patients as well as select high-risk individuals (eg, intravenous drug users [IDUs]) [5]. Patients were excluded if they were medically unstable, unable to complete a prescreening questionnaire, or reported known HCV status. Antibody testing used the Abbott ARCHITECT i1000 platform and all test results were recorded in the electronic health record (EHR). We included tests from September 2013 to February 2015. We identified the zone improvement plan (ZIP) code of each patient's residence from the EHR and used a crosswalk file to link each ZIP code to a tabulation area (a representative geographic unit). We calculated HCV prevalence for each ZIP code as the number of antibody-positive tests divided by the total. We linked data with areal characteristics at the ZIP code level from the 2009–2013 American Community Survey [6].
To characterize areal HCV prevalence, we identified geospatial clusters using reliability-adjusted empirical Bayes (EB) rates [7–9]. This approach allows for adjustment of prevalence estimates based on local averages, with the amount of adjustment relative to the total number screened in a given ZIP code. Specifically, by using EB rates, we were able to achieve more valid comparisons across ZIP codes with a small number of HCV tests performed. We also performed local index of spatial autocorrelation (LISA) analysis, classifying areas as part of a high–high cluster, low–low cluster, high outlier surrounded by low prevalence, and low outlier surrounded by high prevalence [7, 8, 10]. We classified a ZIP code as high risk if it was in the highest EB rate quartile and part of a high–high LISA cluster. Thus, a high-risk ZIP code was one that had an elevated HCV prevalence and had neighboring communities with elevated prevalence. These methods are described in greater detail in Supplementary Table 1. In order to obtain valid prevalence estimates, analysis was limited to ZIP codes with ≥10 tests and areas mapped were limited to those with at least 5 positives.
ZIP code–level demographic characteristics were compared by high-risk status using Wilcoxon rank sum tests of equal distribution and Fisher exact tests. Specifically, we compared the percentage aged ≥65 years, African American, unemployed, having less than a high school education, living below the federal poverty line, receiving supplemental social security income, and without insurance (aged <65 years). We also compared the percentage of single female-headed households, median household income, and population setting (defined as metropolitan or non-metropolitan using rural urban commuting area codes). In order to fully characterize high-risk ZIP codes, we also listed the characteristics individually. We used GeoDa 1.6.7 (GeoDa Center, Tempe, Arizona), ArcGIS 10.2.2 (ESRI, Redlands, California), and Stata 13.1 (College Station, Texas) for all analyses.
RESULTS
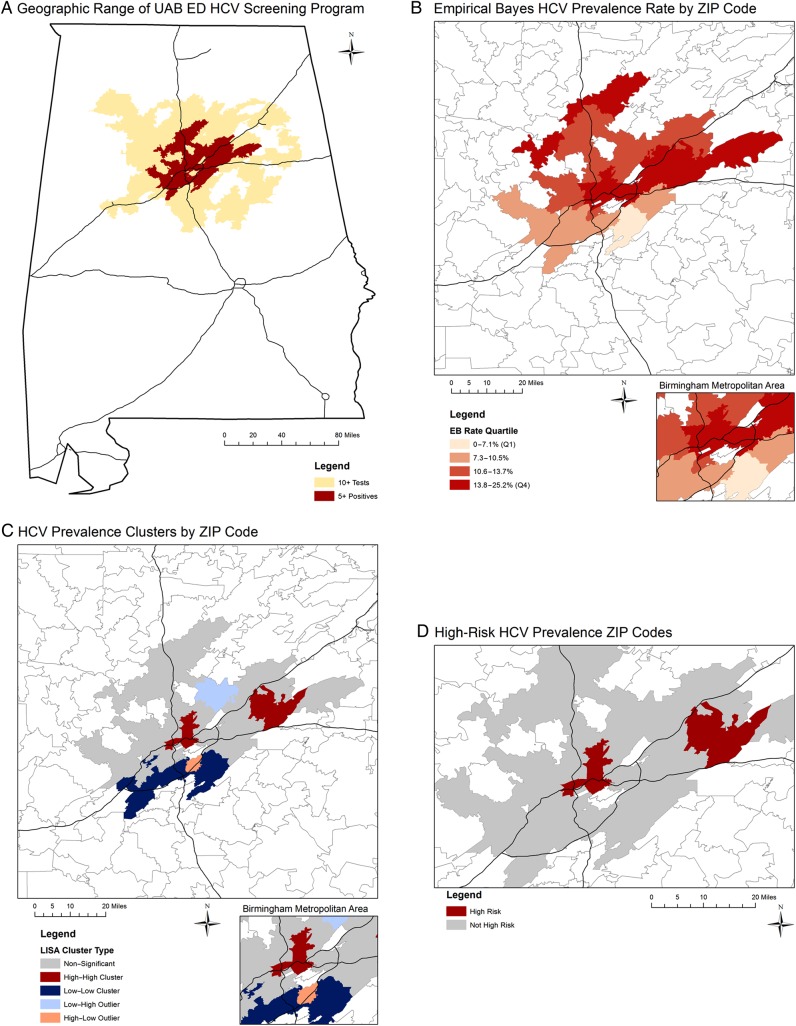
A total of 8742 HCV tests were performed (representing 391 of 604 ZIP codes [64.7%] across Alabama), with 6888 among those born between 1945 and 1965 (79%). The overall prevalence was 11.6%. We analyzed 120 ZIP codes with ≥10 tests, and there were 41 with ≥5 positives (Figure 1A). Median HCV prevalence ranged from 3.5% in the lowest EB quartile to 15.9% in the highest EB quartile (Figure 1B). LISA analysis identified 8 high–high, 11 low–low, 5 low–high, and 3 high–low outliers (Figure 1C). All 8 high–high ZIP codes were in the highest EB rate quartile and classified as high risk (Figure 1D).
Figure 1.
Geographic distribution of hepatitis C virus prevalence in central Alabama by ZIP code. Geospatial analysis includes tests performed from 2013 to 2015 at the University of Alabama at Birmingham Emergency Department. Analysis was limited to ZIP codes with at least 10 tests; data are not shown in maps for ZIP codes with fewer than 5 positives. Black lines represent major interstate highways. Abbreviations: EB, empirical Bayes; ED, emergency department; HCV, hepatitis C virus; LISA, local index of spatial autocorrelation; UAB, University of Alabama at Birmingham.
Compared with non–high-risk areas, high-risk ZIP codes had higher median percentage unemployed (16.1% vs 10.6%; P = .031), higher median percentage living below the federal poverty level (32.4% vs 17.7%; P = .003), lower median household income ($33 078 vs $42 392; P = .049), higher median percentage of single female-headed households (38.7% vs 18.5%; P = .007), higher median percentage receiving supplementary social security income (9.1% vs 6.0%; P = .012), and higher median percentage of population aged <65 years uninsured (20.6% vs 16.1%; P = .016). However, we did not observe significant differences for the percentage aged ≥65 years (13.1% vs 14.7%; P = .535), African American (51.6% vs 11.4%; P = .115), or percent with less than a high school education (21.3% vs 18.0%; P = .081). There was no difference in population setting between high-risk and non–high-risk ZIP codes (12.5% vs 19.6% non-metropolitan; P = .524). More detailed results for all demographic characteristics examined are provided in Supplementary Tables 2 and 3.
DISCUSSION
The UAB ED screening program identified unrecognized HCV in 11.6% of ED patients tested and provided expansive coverage, testing individuals from 65% of ZIP codes in the state of Alabama. Although UAB Hospital is located in Birmingham, 2 distinct high-risk areas were identified. These findings suggest that screening at a single hospital ED may have wide geographic reach and provide vital surveillance data to guide organized HCV prevention or treatment efforts that target high-risk communities.
High-risk clusters identified in this analysis were socioeconomically disadvantaged, which is not surprising given the known epidemiology of HCV infection. Recent outbreaks of human immunodeficiency virus and HCV affecting young whites living in rural areas have been highly publicized [11–13]. Conversely, we highlight the heavy burden of HCV in a metropolitan area of the Deep South, where cases of African American baby boomers predominate. This is a population at elevated risk of developing symptoms related to chronic HCV infection that would benefit from treatment with new antiviral therapies. Local strategies to improve awareness and access to care will necessitate organized HCV interventions that target these high-risk communities. Given the broad geographic reach of this ED screening program and the challenges of identifying injection drug use risks among young populations, a universal (nontargeted) HCV screening approach in the ED setting could allow for similar analysis to identify high-prevalence IDU clusters where prevention strategies could be used.
These results should be interpreted in light of several limitations. Due to small numbers per ZIP code, we were unable to split the population screened into relevant subgroups. It is possible that the ZIP code recorded in the EHR may not necessarily represent where patients spend the majority of their time. We also note that UAB is 1 of 4 hospitals within Birmingham and handles most tertiary care cases; if screening were conducted at another hospital, these geographic patterns may shift and these results may not be generalizable to other regions.
In conclusion, our results reveal the potential of urban ED-based HCV screening to impact the US HCV epidemic through the expansive geographic radius covered and the potential impact on public health surveillance.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Authorship. All authors participated in the study design, analysis, and writing of the manuscript. No other persons participated in analyzing, drafting, or revising the manuscript.
Disclaimer. Members of the funding organizations had no role in the design, analysis, or presentation of the results.
Financial support. This project was supported by contract from the Centers for Disease Control and Prevention (CDC-PS10-10138). J. P. D. received support from the Agency for Healthcare Research and Quality (award number T32-HS013852). H. E. W. received support from the National Institute for Nursing Research (grant number R01-NR012726).
Potential conflicts of interest. R. A. F. received research support for clinical research and consulting fees from Gilead. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kim WR. The burden of hepatitis C in the United States. Hepatology 2002; 36:S30–4. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong GL, Wasley A, Simard EP et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med 2006; 144:705–14. [DOI] [PubMed] [Google Scholar]
- 3.Smith BD, Morgan RL, Beckett GA et al. Hepatitis C virus testing of persons born during 1945–1965: recommendations from the Centers for Disease Control and Prevention. Ann Intern Med 2012; 157:817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afdhal NH, Zeuzem S, Schooley RT et al. The new paradigm of hepatitis C therapy: integration of oral therapies into best practices. J Viral Hepat 2013; 20:745–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galbraith JW, Franco RA, Donnelly JP et al. Unrecognized chronic hepatitis C virus infection among baby boomers in the emergency department. Hepatology 2015; 61:776–82. [DOI] [PubMed] [Google Scholar]
- 6.National Historical Geographic Information System. American Community Survey, 2009–2013 American Community Survey 5 Year Estimates. Available at: www.nhgis.org Accessed August 2015.
- 7.Anselin L. GeoDa 0.9 User's Guide. Available at: https://geodacenter.org/downloads/pdfs/geoda093.pdf Accessed August 2015.
- 8.Anselin L, Syabri I, Kho Y. GeoDa: an introduction to spatial data analysis. Geogr Anal 2006; 38:5–22. [Google Scholar]
- 9.Marshall RJ. Mapping disease and mortality rates using empirical Bayes estimators. J R Stat Soc Ser C Appl Stat 1991; 40:283–94. [PubMed] [Google Scholar]
- 10.Anselin L. Local indicators of spatial association—LISA. Geogr Anal 1995; 27:93–115. [Google Scholar]
- 11.Conrad C, Bradley HM, Broz D et al. Community outbreak of HIV infection linked to injection drug use of oxymorphone—Indiana, 2015. MMWR Morb Mortal Wkly Rep 2015; 64:443–4. [PMC free article] [PubMed] [Google Scholar]
- 12.Zibbell JE, Iqbal K, Patel RC et al. Increases in hepatitis C virus infection related to injection drug use among persons aged </=30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morb Mortal Wkly Rep 2015; 64:453–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Suryaprasad AG, White JZ, Xu F et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006–2012. Clin Infect Dis 2014; 59:1411–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.