Abstract
We report on a 16-year-old boy with a maternally inherited ~ 18.3 Mb Xq13.2-q21.31 duplication delimited by aCGH. As previously described in patients with similar duplications, his clinical features included intellectual disability, developmental delay, speech delay, generalized hypotonia, infantile feeding difficulties, self-injurious behavior, short stature and endocrine problems. As additional findings, he presented recurrent seizures and pubertal gynecomastia. His mother was phenotypically normal and had completely skewed inactivation of the duplicated X chromosome, as most female carriers of such duplications. Five previously reported patients with partial Xq duplications presented duplication breakpoints similar to those of our patient. One of them, a fetus with multiple congenital abnormalities, had the same cytogenetic duplication breakpoint. Three of the reported patients shared many features with our proband but the other had some clinical features of the Prader-Willi syndrome. It was suggested that ATRX overexpression could be involved in the major clinical features of patients with partial Xq duplications. We propose that this gene could also be involved with the obesity of the patient with the Prader-Willi-like phenotype. Additionally, we suggest that the PCDH11X gene could be a candidate for our patient's recurrent seizures. In males, the Xq13-q21 duplication should be considered in the differential diagnosis of Prader-Willi syndrome, as previously suggested, and neuromuscular diseases, particularly mitochondriopathies.
Abbreviations: 5-BrdU, 5-bromodeoxyuridine; aCGH, array comparative genomic hybridization; ATRX, alpha thalassemia/mental retardation syndrome X-linked; CNV, copy number variation; CKT, creatinine kinase-phospho-total; CT, computed tomography; FISH, fluorescence in situ hybridization; HDAC8, histone deacetylase 8; JPX, JPX transcript; XIST, activator; NMR, nuclear magnetic resonance; OFC, occipitofrontal circumference; PCHD7, protocadherin 7; PCDH11X, protocadherin 11 X-linked; PCDH11Y, protocadherin 11 Y-linked; PCDH19, protocadherin 19; PWS, Prader–Willi syndrome; SLC16A2, solute carrier family 16, member 2
Keywords: Xq13-q21 duplication, Prader-Willi syndrome, Mitochondrial disease, ATRX protein, PCDH11X protein
Highlights
-
•
We report a boy with inherited Xq13.2-q21.31 duplication.
-
•
As additional findings, the patient presented seizures and pubertal gynecomastia.
-
•
We compared our patient phenotype with five similar previously reported patients.
-
•
We propose novel roles for ATRX gene related to Prader-Willi-like phenotype.
-
•
The PCDH11X gene may be associated with recurrent seizures in dup(X) patients.
1. Introduction
Males with Xq13-q21 duplications have short stature, intellectual disability, developmental delay, speech delay, generalized hypotonia, infantile feeding difficulties, endocrine problems, short palpebral fissures, epicanthic folds, ptosis, tented vermilion of the upper lip and downturned corners of the mouth, and genital anomalies such as hypoplastic genitalia with undescended testes. On the other hand, most of the female carriers are asymptomatic with an inactive duplication-bearing X chromosome.
Interestingly, patients with X-linked intellectual disability associated or not with alpha-thalassemia (MIM 301040, MIM 309580) have a phenotype similar to those with the Xq13-q21 duplication, including features such as short stature, intellectual disability, developmental delay, hypotonia, hypogonadism, cryptorchidism, epicanthic folds, ptosis and inverted V-shaped upper lip (Lugtenberg et al., 2009). This disorder comprises several syndromes reported separately, including Chudley-Lowry, Juberg-Marsidi, Carpenter-Waziri, Holmes-Gang and Smith-Fineman-Myers, found to be caused by mutations in the ATRX gene, located at Xq21.1 (alpha thalassemia/mental retardation syndrome X-linked, MIM 300032).
We report on a boy with an Xq13.2-q21.31 duplication inherited from his phenotypically normal mother. His chromosome rearrangement was identified by routine chromosome analysis and characterized by high resolution karyotyping and oligonucleotide aCGH analysis. We compare the patient's phenotype to five previously reported patients with molecularly delimited similar duplications.
2. Materials and methods
This study was approved by the Research Ethics Committee of the Universidade Federal de Minas Gerais (project number 0007.0.203.000-10). The written informed consent was undersigned by the patient's parents.
2.1. Clinical report
The 16-year-old boy (Fig. 1) was the fourth child of a non-consanguineous healthy couple. He was born at term by elective Cesarean section, with weight of 3020 g (25–50th centile), length of 45 cm (10–25th centile) and head circumference of 35 cm (75–90th centile). A previous male sibling died of an unknown cause, 17 h after birth, and two sisters were phenotypically normal. In the newborn period he presented feeding difficulties, with poor sucking, and at age 15 days he weighed 1900 g (< 3rd centile). Neonatal screening for hypothyroidism and phenylketonuria were negative.
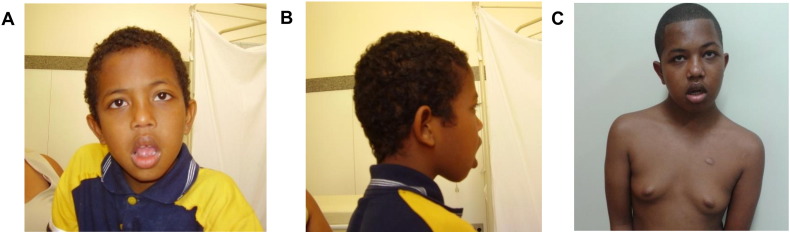
Fig. 1.
(A, B) Frontal and lateral views of patient at 12-years of age, showing facial hypotonia, bilateral ptosis, low-set protruding ears and tented mouth. (C) Patient at 16-years of age, showing gynecomastia.
His growth and neuropsychomotor development were delayed; he walked without support and spoke his first words at age 3 years. Although calm, he had a self-injurious behavior (for instance, he bit himself and hit his head on the wall), first noted when he was 4 years old. At age 2 years, the diagnosis of hypothyroidism was established, and he made use of levothyroxine until the age of 5 years and 6 months, when the thyroid function normalized. He underwent surgical correction of cryptorchidism and bilateral inguinal hernia at 6 years. From age 7 to 13 years he presented recurrent seizures, treated by carbamazepine since the first episode.
Examined at 10 years of age, his height was 118.2 cm (< 3rd centile), and his weight was 20.2 kg (< 3rd centile). He presented intellectual disability, impaired social interaction, language impairment (spoke a few words), facial and generalized hypotonia, normal tendon reflexes, bilateral ptosis, tented mouth, high-arched palate, low-set protruding ears, pectus excavatum, genu varum and joint hyperextensibility. Ophthalmologic examination and brain computed tomography (CT) scan did not show abnormalities; nuclear magnetic resonance (NMR) of the brain documented mild dilatation of lateral ventricles, spectroscopy showed an increased lactate peak and the electroencephalogram showed the presence of nonspecific changes. Venous blood gases, ammonia, lactate, ions, complete blood count with platelets, blood glucose, uric acid, creatine phosphokinase-total (CKT), urine qualitative reactions and chromatography of oligosaccharides in urine and blood amino acid were normal. TSH, T3, T4 and T3-reverse were performed at 12-years of age and the results were within the normal range. He entered puberty at age 12 and since then presented bilateral generalized gynecomastia (Fig. 1). Currently at age 16, his height is 152 cm (< 3rd centile), his weight is 53 kg (10–25th centile) and he has adult genitalia, axillary hair, and thin mustache (beardless).
Chromosome analysis revealed a 46,XY,dup(X)(q13q23) karyotype. His mother presented the same tandem duplication. His maternal grandmother karyotype was normal, and his grandfather refused examination. Recently, his sister had a hypotonic male child that needed gastrostomy diet due to poor sucking; his karyotype showed the same duplication.
2.2. Cytogenetic analysis
Chromosome preparations from the patient and his mother were obtained from cultured peripheral blood lymphocytes. In order to obtain high-resolution chromosomes we combined thymidine cell synchronization with ethidium bromide addition. Chromosome analysis was performed after GTG-banding and fluorescence in situ hybridization (FISH) was carried out with a flow-sorted whole-human X chromosome probe labeled and detected according to standard procedures.
2.3. X chromosome inactivation
The X chromosome inactivation pattern was analyzed in the patient's mother after 5-bromodeoxyuridine (5-BrdU) incorporation and acridine orange staining, according to Latt (1973) with modifications. Briefly, leukocytes were cultured for 40 h in medium with 0.2 mg/ml 5-BrdU (Sigma-Aldrich, Saint Louis, MO, EUA) and then for 6 h in 5-BrdU free medium containing 0.2 mg/ml thymidine (Sigma-Aldrich). The X inactivation pattern was analyzed in 50 cells. The methylation status of the androgen-receptor gene was determined in a DNA sample extracted from peripheral blood, as previously described (Allen et al., 1992). The resulting PCR products were analyzed on an ABI-310 Genetic Analyzer, and product length and peak areas were obtained using the Gene Mapper Software v4.0 (Applied Biosystems, Foster City, CA).
2.4. aCGH analysis
Genomic DNA was isolated from the patient's and his mother's blood cells using the Qiagen DNA extraction kit (Santa Clara, CA). Microarray-based comparative genomic hybridization (aCGH) was performed using an X-chromosome dedicated 44K microarray (custom design 2008) and the Whole Human Genome CGH Microarray 60K (Agilent Technologies Inc., Santa Clara, CA, USA), following the manufacturer's protocol. Scanned images of the arrays were processed with the Feature Extraction software (Agilent Technologies). We applied the Genomic Workbench software (Agilent Technologies) for calling CNVs using the Aberration Detection Method 2 statistical algorithm (sensitivity threshold of 6.7). Duplications or deletions were considered when the log2 ratio of the Cy3/Cy5 intensities of a region encompassing at least three probes was > 0.3 or <− 0.3, respectively. Mapping data were analyzed using the UCSC genome browser - NCBI Build 37, hg19.
3. Results
High resolution chromosome analysis of the patient showed a partial duplication of the X chromosome long arm [46,XY,dup(X)(q13.3q22.1)]. The same duplication was detected in his mother. FISH with an X-chromosome probe showed labeling exclusively over the entire X chromosomes, pointing to an intrachromosomal rearrangement (Fig. 2). The duplication was delimited in the patient by aCGH using an oligonucleotide platform devoted to the X chromosome that revealed a duplication at Xq13.2q21.31 with minimum size of 18.28 Mb (spanning from probe A_16_P21495742 located at chrX:73,207,744-73,207,804 to probe A_16_P21518961 located at chrX:91,485,284–91,485,344) and maximum size of 18.32 Mb (spanning from probe A_16_P41702986 located at chrX:73,167,151–73,167,211 to probe A_16_P21518972 located at chrX:91,488,728-91,488,788). No other copy number changes were detected in the patient or his mother by aCGH analysis (Fig. 3).
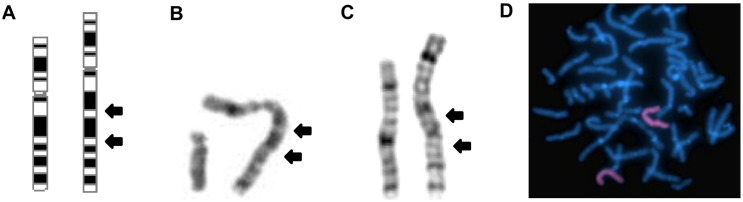
Fig. 2.
(A) Ideogram of the duplication. GTG-banded (B), X and Y chromosomes of the patient, and (C) X chromosomes of the patient's mother; the arrows indicate the duplication of bands q13.3 to q22.1. (D) FISH with a human X chromosome painting probe showing labeling exclusively over the entire length of the der(X) and its normal homologue in a metaphase from the proband's mother.
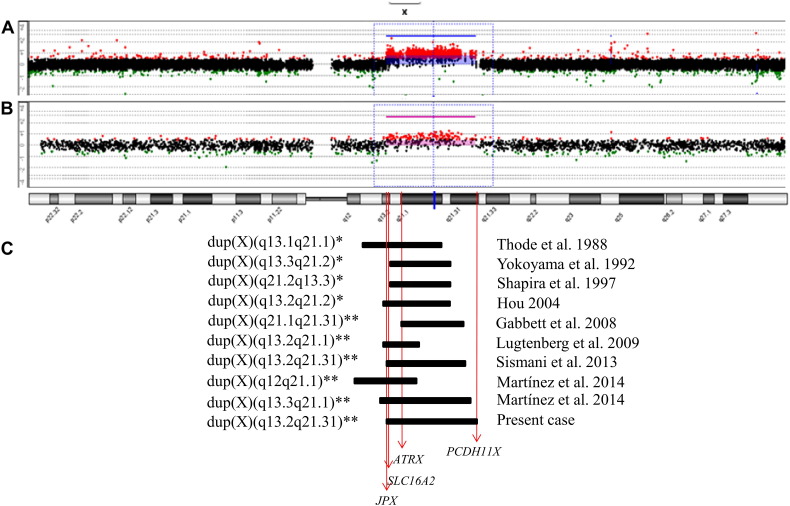
Fig. 3.
(A) Mapping of the patient's ~ 18.3 Mb Xq13.2-q21.31 duplication on a 44K oligoarray dedicated to the X chromosome. (B) The same duplication in the mother is shown on a 60K Whole Human Genome platform. The duplication is indicated by the blue-dashed square. Images obtained from Workbench Software (Agilent). (C) Schematic representation of the duplicated segments in the patients previously reported with rearrangements similar to that of our patient. The gene positions are indicated by the red arrows, according to UCSC Genome Browser (GRCh37). *Based on the GTG-banded karyotype; **Delimited by aCGH.
The duplicated X chromosome was shown to be late replicating (inactive) after 5-BrdU incorporation in each of the 50 examined cells from the mother. Analysis of the androgen receptor gene methylation status also revealed a completely skewed X-inactivation (Fig. 4).
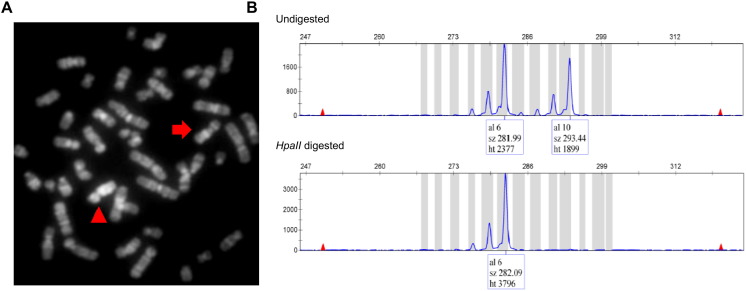
Fig. 4.
(A) Metaphase from the patient's mother, after 5-BrdU treatment during the initial S-phase and acridine-orange staining, showing the late replicating duplicated X chromosome (arrowhead). The arrow points to the normal X chromosome. (B) X-inactivation assay based on the methylation status of the androgen-receptor alleles. After HpaII digestion only one methylated allele was amplified, indicating completely skewed X-inactivation.
4. Discussion
We report on a 16-year old boy with an ~ 18.3 Mb maternally inherited Xq13.2q21.31 duplication extending from approximate positions 73.2 Mb to 91.5 Mb from Xpter (hg19). The presence of growth and developmental delays, hypotonia, ptosis, intellectual disability and transitory hypothyroidism in our patient led to the initial diagnostic hypothesis of mitochondrial disease. Notably, Apacik et al. (1996) also raised the possibility of neuromuscular disease in their patients that had a maternally inherited inverted insertion of the segment (X)(q12q13.3) into Xq21.2.
Our patient's duplication contains around 131 genes (including pseudogenes, hypothetical genes and microRNA – NCBI Map Viewer Build GRCh37.p13), comprising 38 OMIM genes. Mutations in some of the duplicated genes (ATRX, KIAA2022, ZDHHC15, MAGT1, ATP7A, BRWD3, ZNF711, RPS6KA6) have been associated with X-linked intellectual disability. The SLC16A2 gene (solute carrier family 16, member 2, MIM 300095) is located at chrX:73,641,327–73,753,763 (GRCh37; Xq13.2) and has been related to thyroid hormone cell transporter deficiency (Schwartz et al., 2005). Interestingly, our patient was diagnosed with medically responsive hypothyroidism. TSH, T3, T4 and T3-reverse were performed but the results were normal at age 12 years. In addition, Shapira et al. (1997) described a patient with a duplication of Xq21.2 to Xq13.3 and compensated primary hypothyroidism. This duplication was characterized only by GTG-banding and may involve the SLC16A2 gene.
To our knowledge, about 95 patients from 42 families have been reported with different isolated partial Xq duplications, none of which had the same breakpoints as ours, but seven of them had similar rearrangements, exclusively involving the Xq13-q21 region (Fig. 3C) (Gabbett et al., 2008, Hou, 2004, Lugtenberg et al., 2009, Martinez et al., 2014, Shapira et al., 1997, Sismani et al., 2013, Thode et al., 1988, Yokoyama et al., 1992). Nevertheless, the extent of the duplication had been determined by aCGH only by Gabbett et al. (2008); Lugtenberg et al. (2009); Sismani et al. (2013) and Martinez et al. (2014). Comparison of the clinical findings associated to these five duplications and the one described herein is shown in Supplementary Table 1.
The patient described by Lugtenberg et al. (2009) had an Xq13.2q21.1 duplication, extending from approximately 72.4 to 79.6 Mb (within BAC clone CTD-2003H23 to RP11-608A14). This ~ 7 Mb duplication could not be detected on the routine chromosome analysis not even retrospectively at a 500-banded karyotype. The patient presented many features also observed in the boy described herein, including feeding difficulties in infancy, generalized hypotonia, psychomotor retardation, intellectual disability, language impairment, short stature, bilateral ptosis, high-arched palate, low-set ears, pectus excavatum, genu valgus and cryptorchidism. This patient's duplication narrows down the critical region responsible for the shared clinical features with our patient.
Lugtenberg et al. (2009) performed a thorough review of the clinical findings of previously reported patients with overlapping Xq duplications and hypothesized that their shared phenotype was caused by the overexpression of the ATRX gene. Their hypothesis relied on the evidence that all previously reported patients had duplications encompassing the ATRX gene, and on findings in animal models with overexpression of the ATRX gene. In the mouse, neurodevelopmental defects and growth retardation were observed, as well as seizures and mild craniofacial anomalies (Berube et al., 2002). This gene is located at chrX:76,760,355–77,041,754 (GRCh37; Xq21.1) and has been shown to be mutated in patients with X-linked intellectual disability associated or not with alpha-thalassemia. Affected males present severe intellectual disability, developmental delay, genital abnormalities, limited language, a tented upper lip and facial hypotonia; which are features shared with those carrying duplications encompassing the ATRX gene (Lugtenberg et al., 2009).
Gabbett et al. (2008) reported a boy with an interstitial tandem duplication of Xq21.1q21.31, extending from approximately 76.7 to 87.9 Mb (BAC probes RP5-875J14 to RP4-542O23). The boy had clinical features of the Prader–Willi syndrome (PWS, MIM 176270), such as developmental delay, hypotonia, obesity and food-seeking behavior. This case raised the possibility that duplication of gene(s) on cytoband Xq21 might be responsible for a PWS-like phenotype. One of the main features of PWS is obesity, which is not present in our patient. Mild obesity is a rare clinical finding in patients with X-linked intellectual disability associated or not with alpha-thalassemia, leading us to hypothesize that the ATRX gene could be involved with the PWS-like phenotype of the patient described by Gabbett et al. (2008). The authors of studies describing patients with X-linked intellectual disability and obesity (the Chudley-Lowry syndrome) concluded that these patients had phenotypes reminiscent of, but distinct from the Prader-Willi syndrome (Chudley et al., 1988, Vasquez et al., 1979). Remarkably, the first proximal duplicated probe of the patient described by Gabbett et al. (2008) (probe RP5-875J14) is located in the region of the ATRX gene. A possible explanation for the patient's PWS-like exceptional phenotype is that the phenotypic effect of the duplicated segment including the ATRX gene may be modulated by some specific regions of the rest of the genome.
Sismani et al. (2013) described a male fetus with a maternally inherited 14.8 Mb duplication extending from Xq13.2 to Xq21.31 (73,187,033–88,124,189; GRCh37/hg19). After elected termination of the pregnancy at 28 weeks, a detailed autopsy of the fetus revealed multiple congenital abnormalities, including facial dysmorphic features, ventriculomegaly, hepatosplenomegaly and protruding thorax. A comparison of his phenotypic findings with our case is difficult because he was prenatally ascertained.
Martinez et al. (2014) reported two unrelated male patients with maternally inherited duplication at Xq13.3–q21.1. Their duplication size was about 12.6 Mb and 18.7 Mb and their phenotype was very similar to our patient's, including severe intellectual disability, language impairment, early hypotonia, postnatal growth deficiency, cryptorchidism, low-set ears, and downslanting palpebral fissures. Notably, they presented behavior problems (hyperactivity, repetitive self-stimulatory behavior and aggressiveness) and one of them also had a self-injurious behavior (biting fingers), as does our patient. The authors showed that these clinical findings are also usually present among patients with loss-of-function mutations of the ATRX gene and they agreed with Lugtenberg et al. (2009) that duplication of the ATRX gene is the main pathogenic mechanism for the patients' phenotype. Martinez et al. (2014) highlighted that ATRX belongs to a list of dose-sensitive genes. Thus, besides deletions and point mutations, duplications also cause a similar phenotype.
Differently from the five individuals with similar rearrangements previously described (Gabbett et al., 2008, Lugtenberg et al., 2009, Sismani et al., 2013, Martinez et al., 2014), our patient has recurrent seizures and a partial duplication of the PCDH11X gene (protocadherin 11 X-linked, MIM 300246), located at chrX:91,034,259–91,878,228 (GRCh37; Xq21.31q21.32). This duplication could compromise its function, since in the der(X) chromosome there is a normal copy and an additional segment of this gene that may be fused with part of the JPX gene [JPX transcript, XIST activator (non-protein coding); MIM 300832], which is also partially duplicated. PCDH11X, which is part of a human-specific X/Y gene pair together with PCDH11Y (protocadherin 11 Y-linked, MIM 400022), is predominantly expressed in the brain and is a member of the protocadherin subfamily of calcium-dependent cell adhesion and recognition proteins (Blanco et al., 2000). It has been suggested that impaired Ca2 + homeostasis in nerve cells may be correlated with seizures (Wheal et al., 1998). Indeed, mutations in PCDH19 (also located on the X chromosome, protocadherin 19, MIM 300460) and a microdeletion in PCHD7 (located at 4p15.1, protocadherin 7, MIM 602988) have been associated with epilepsy in females (Dibbens et al., 2008, Lal et al., 2015). PCDH11X thus appears as a candidate gene for seizures in our patient. Further investigation is necessary in order to test this assumption.
As an additional finding, our patient presents a seemingly pathologic bilateral generalized pubertal gynecomastia, probably unrelated to the rare side effect of carbamazepine (Novartis®, 2013) as he has been taking this medicine for seizures since he was 7 year-old and only developed gynecomastia at 12. This phenotype is possibly related to the syndrome, though it has not been reported in patients with similar duplications. Some families with X-linked mental retardation syndrome and gynecomastia have been described (Wilson-Turner Syndrome, MIM 309585). However, in one family X-exome sequencing analysis identified a variant in the HDAC8 gene (histone deacetylase 8, MIM 300269, chrX:71,549,366–71,792,953). Since this gene is located at chrXq13.1, outside of our patient's duplication, we believe that there is another gynecomastia-related gene within the duplicated region.
In conclusion, the Xq13-q21 duplication is a rare condition that can be easily misdiagnosed as a neuromuscular disorder. We report on a patient initially suspected of having a mitochondrial disease, but whose chromosome analysis has allowed establishing the causal diagnosis. All things considered, the dup(X)(q13q21) in men should be included in the differential diagnosis of Prader-Willi syndrome, as previously suggested, but also in neuromuscular diseases, especially mitochondriopathies.
The following is the supplementary data related to this article.
Clinical features of our patient and of the five previously described probands with Xq duplication breakpoints similar to those of our patient.
Conflict of interests
The authors declare that there is no conflict of interests regarding the publication of this article.
Acknowledgements
This work was supported by FAPEMIG grants to MS, a FAPESP grant to AMV and CR (CEPID 98/14254-2 and 2009/00898-1) and the Laboratório de Erros Inatos do Metabolismo of HC-UFMG supported ERV. The authors are grateful to the patient and his family for their precious cooperation in this study.
References
- Allen R.C., Zoghbi H.Y., Moseley A.B., Rosenblatt H.M., Belmont J.W. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am. J. Hum. Genet. 1992;51:1229–1239. [PMC free article] [PubMed] [Google Scholar]
- Apacik C., Cohen M., Jakobeit M., Schmucker B., Schuffenhauer S., Thurn und Taxis E., Genzel-Boroviczeny O., Stengel-Rutkowski S. Two brothers with multiple congenital anomalies and mental retardation due to disomy (X) (q12–>q13.3) inherited from the mother. Clin. Genet. 1996;50:63–73. doi: 10.1111/j.1399-0004.1996.tb02350.x. [DOI] [PubMed] [Google Scholar]
- Berube N.G., Jagla M., Smeenk C., De Repentigny Y., Kothary R., Picketts D.J. Neurodevelopmental defects resulting from ATRX overexpression in transgenic mice. Hum. Mol. Genet. 2002;11:253–261. doi: 10.1093/hmg/11.3.253. [DOI] [PubMed] [Google Scholar]
- Blanco P., Sargent C.A., Boucher C.A., Mitchell M., Affara N.A. Conservation of PCDHX in mammals; expression of human X/Y genes predominantly in brain. Mamm. Genome. 2000;11:906–914. doi: 10.1007/s003350010177. [DOI] [PubMed] [Google Scholar]
- Chudley A.E., Lowry R.B., Hoar D.I. Mental retardation, distinct facial changes, short stature, obesity, and hypogonadism: a new X-linked mental retardation syndrome. Am. J. Med. Genet. 1988;31:741–751. doi: 10.1002/ajmg.1320310404. [DOI] [PubMed] [Google Scholar]
- Dibbens L.M., Tarpey P.S., Hynes K., Bayly M.A., Scheffer I.E., Smith R., Bomar J., Sutton E., Vandeleur L., Shoubridge C., Edkins S., Turner S.J., Stevens C., O'Meara S., Tofts C., Barthorpe S., Buck G., Cole J., Halliday K., Jones D., Lee R., Madison M., Mironenko T., Varian J., West S., Widaa S., Wray P., Teague J., Dicks E., Butler A., Menzies A., Jenkinson A., Shepherd R., Gusella J.F., Afawi Z., Mazarib A., Neufeld M.Y., Kivity S., Lev D., Lerman-Sagie T., Korczyn A.D., Derry C.P., Sutherland G.R., Friend K., Shaw M., Corbett M., Kim H.G., Geschwind D.H., Thomas P., Haan E., Ryan S., McKee S., Berkovic S.F., Futreal P.A., Stratton M.R., Mulley J.C., Gecz J. X-linked protocadherin 19 mutations cause female-limited epilepsy and cognitive impairment. Nat. Genet. 2008;40:776–781. doi: 10.1038/ng.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbett M.T., Peters G.B., Carmichael J.M., Darmanian A.P., Collins F.A. Prader-Willi syndrome phenocopy due to duplication of Xq21.1-q21.31, with array CGH of the critical region. Clin. Genet. 2008;73:353–359. doi: 10.1111/j.1399-0004.2007.00960.x. [DOI] [PubMed] [Google Scholar]
- Hou J.W. Inherited tandem duplication of the X chromosome: dup(X)(q13.2-q21.2) in a family. Chang Gung Med. J. 2004;27:685–690. [PubMed] [Google Scholar]
- Lal D., Ruppert A.K., Trucks H., Schulz H., de Kovel C.G., Kasteleijn-Nolst T.D., Sonsma A.C., Koeleman B.P., Lindhout D., Weber Y.G., Lerche H., Kapser C., Schankin C.J., Kunz W.S., Surges R., Elger C.E., Gaus V., Schmitz B., Helbig I., Muhle H., Stephani U., Klein K.M., Rosenow F., Neubauer B.A., Reinthaler E.M., Zimprich F., Feucht M., Moller R.S., Hjalgrim H., De Jonghe P., Suls A., Lieb W., Franke A., Strauch K., Gieger C., Schurmann C., Schminke U., Nurnberg P., Sander T. Burden analysis of rare microdeletions suggests a strong impact of neurodevelopmental genes in genetic generalised epilepsies. PLoS Genet. 2015;11 doi: 10.1371/journal.pgen.1005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latt S.A. Microfluorometric detection of deoxyribonucleic acid replication in human metaphase chromosomes. Proc. Natl. Acad. Sci. U. S. A. 1973;70:3395–3399. doi: 10.1073/pnas.70.12.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg D., de Brouwer A.P., Oudakker A.R., Pfundt R., Hamel B.C., van Bokhoven H., Bongers E.M. Xq13.2q21.1 duplication encompassing the ATRX gene in a man with mental retardation, minor facial and genital anomalies, short stature and broad thorax. Am. J. Med. Genet. A. 2009;149A:760–766. doi: 10.1002/ajmg.a.32742. [DOI] [PubMed] [Google Scholar]
- Martinez F., Rosello M., Mayo S., Monfort S., Oltra S., Orellana C. Duplication at Xq13.3-q21.1 with syndromic intellectual disability, a probable role for the ATRX gene. Am. J. Med. Genet. A. 2014;164A:918–923. doi: 10.1002/ajmg.a.36371. [DOI] [PubMed] [Google Scholar]
- Novartis® . 2013. Carbamazepine (Tegretol®), Product Monograph. (Internet link: www.novartis.com.au/PI_PDF/tgr.pdf) [Google Scholar]
- Schwartz C.E., May M.M., Carpenter N.J., Rogers R.C., Martin J., Bialer M.G., Ward J., Sanabria J., Marsa S., Lewis J.A., Echeverri R., Lubs H.A., Voeller K., Simensen R.J., Stevenson R.E. Allan-Herndon-Dudley syndrome and the monocarboxylate transporter 8 (MCT8) gene. Am. J. Hum. Genet. 2005;77:41–53. doi: 10.1086/431313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira M., Dar H., Bar-El H., Bar-Nitzan N., Even L., Borochowitz Z. Inherited inverted duplication of X chromosome in a male: report of a patient and review of the literature. Am. J. Med. Genet. 1997;72:409–414. doi: 10.1002/(sici)1096-8628(19971112)72:4<409::aid-ajmg7>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Sismani C., Donoghue J., Alexandrou A., Karkaletsi M., Christopoulou S., Konstantinidou A.E., Livanos P., Patsalis P.C., Velissariou V. A prenatally ascertained, maternally inherited 14.8 Mb duplication of chromosomal bands Xq13.2-q21.31 associated with multiple congenital abnormalities in a male fetus. Gene. 2013;530:138–142. doi: 10.1016/j.gene.2013.08.032. [DOI] [PubMed] [Google Scholar]
- Thode A., Partington M.W., Yip M.Y., Chapman C., Richardson V.F., Turner G. A new syndrome with mental retardation, short stature and an Xq duplication. Am. J. Med. Genet. 1988;30:239–250. doi: 10.1002/ajmg.1320300125. [DOI] [PubMed] [Google Scholar]
- Vasquez S.B., Hurst D.L., Sotos J.F. X-linked hypogonadism, gynecomastia, mental retardation, short stature, and obesity–a new syndrome. J. Pediatr. 1979;94:56–60. doi: 10.1016/s0022-3476(79)80350-9. [DOI] [PubMed] [Google Scholar]
- Wheal H.V., Bernard C., Chad J.E., Cannon R.C. Pro-epileptic changes in synaptic function can be accompanied by pro-epileptic changes in neuronal excitability. Trends Neurosci. 1998;21:167–174. doi: 10.1016/s0166-2236(97)01182-x. [DOI] [PubMed] [Google Scholar]
- Yokoyama Y., Narahara K., Tsuji K., Moriwake T., Kanzaki S., Murakami M., Namba H., Ninomiya S., Higuchi J., Seino Y. Growth hormone deficiency and empty sella syndrome in a boy with dup(X) (q13.3----q21.2) Am. J. Med. Genet. 1992;42:660–664. doi: 10.1002/ajmg.1320420506. [DOI] [PubMed] [Google Scholar]
Web Resources
- Ensembl database http://www.ensembl.org
- UCSC genome browser http://genome.ucsc.edu
- Online Mendelian Inheritance In Man (OMIM) http://www.ncbi.nlm.nih.gov/omim
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical features of our patient and of the five previously described probands with Xq duplication breakpoints similar to those of our patient.