Abstract
Non-Hodgkin’s lymphoma (NHL), which includes diffuse large B-cell lymphoma (DLBCL) and peripheral T-cell lymphoma, is a refractory malignant tumor originated from the lymphatic system. TNFAIP8L2 (TIPE2 or tumor necrosis-alpha-induced protein-8 like 2) is a negative regulator for inflammation and an inhibitor for carcinogenesis. However, whether TIPE2 plays a role in lymphomagenesis is unknown. In this study, we determined TIPE2 expression in NHL by immunohistochemistry and investigated its clinicopathological significance in DLBCL. We found that TIPE2 expression was upregulated in both DLBCL and peripheral T-cell lymphoma. But the expression of TIPE2 in T lymphomas was weaker than that in DLBCL. Interestingly, among DLBCL, TIPE2 expression was significantly stronger in the germinal center B-cell (GCB) type than in the non-GCB type. These results suggest that the expression of TIPE2 protein could be a predictor of better prognosis for DLBCL.
Keywords: diffuse large B-cell lymphoma, germinal center, non-Hodgkin’s lymphoma, TNFAIP8L2
Introduction
Non-Hodgkin’s lymphoma (NHL) including diffuse large B-cell lymphoma (DLBCL) and peripheral T-cell lymphoma is considered as a refractory malignant tumor originated in the lymphatic system.1 DLBCL, which traditionally has been defined as a diffuse proliferation of large B lymphoid cells, is the most common type of NHL comprising of greater than 30% of adult NHLs in the West and an even higher percent in developing countries,2,3 whereas peripheral T-cell lymphoma appears to be more common in China.4 However, the exact mechanism of NHL remains unclear. More and more evidence has showed that inflammatory response gene may play an important role in lymphomagenesis.5–7
The malignant B cells of DLBCL show extensive morphological and phenotypic similarities. Traditionally, DLBCL has been classified by the morphology and immunophenotype of the malignant B cells. However, more and more recent studies report molecular classification for DLBCL. Cell-of-origin classification based on gene expression profiling (GEP) has demonstrated that a germinal center (GC) phenotype is associated with a better survival than an activated B-cell-like phenotype.8,9 However, GEP is not readily applicable in routine clinical practice. According to Hans’ criteria, the combined immunostaining of CD10, BCL-6, and MUM-1/IRF4 (multiple myeloma oncogene 1/interferon regulatory factor 4)has been developed for use on paraffin-embedded tissues, with division into GC DLBCL and non-GC DLBCL subtypes.10,11 However, Hans’ criteria are unsatisfactory for subclassification into better and poorer prognosis groups in the rituximab era.12,13 Therefore, it is important to investigate novel clinicopathological factors associated with clinical prognosis of DLBCL. Ichikawa et al.14 reported that the expression level of BACH2 is a promising predictor of prognosis for DLBCL. Here, we report that TIPE2 may be a novel predictor associated with clinical prognosis of DLBCL.
TNFAIP8L2, tumor necrosis-alpha-induced protein-8 like 2 (also known as TIPE2), is a new member of TNFAIP8 (also known as TIPE) family. TIPE2 was originally identified as a negative regulator for immune homeostasis, which is mainly expressed in lymphoid tissues and inflammatory tissues.15 Subsequent work has shown that murine TIPE2 protein is also expressed in endocrine cells and germ cells of the reproductive organs and possesses a unique structure shared by all members of the TIPE family,16,17 whereas human TIPE2 was detected in a variety of non-immune cell types, including hepatocytes, neurons, and epithelial cells.18 Its germline deletion resulted in fatal inflammation and hypersensitivity to Toll-like receptor and T-cell receptor signaling.15,19 In human, TIPE2 is abnormal in chronic inflammatory disease such as hepatitis,20,21 atherosclerosis,22,23 and colitis.24 Furthermore, TIPE2 could promote cell death and significantly inhibited tumorigenesis in mice.25–27 Thus, TIPE2 is not only a negative regulator for inflammation but also an inhibitor for carcinogenesis.15,25,26,28 However, it remains unclear whether TIPE2 plays roles in lymphomagenesis. Therefore, in this article, we determine TIPE2 expression in NHL by immunohistochemistry and investigate its clinicopathological significance in DLBCL. To our knowledge, this is the first report about the association of TIPE2 and NHL. Therefore, findings of this study were performed to contribute to our understanding of the significance of TIPE2 in the genesis and progression of DLBCL.
Materials and Methods
Human Subjects and Specimens
Tissue specimens from 82 DLBCL and 30 peripheral T-cell lymphomas of patients who were admitted to Shandong Qilu Hospital between 2009 and 2013 were investigated in this study. No patients had received prior treatment. The diagnosis of lymphoma was made in accordance with the “WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues.” The specimens were fixed in 10% buffered formalin and paraffin embedded, and serial sections about 4 mm were made for each case. Histological grade was graded according to hematoxylin and eosin staining. Histopathological diagnosis was carried out according to the criteria of the World Health Organization.29 Among DLBCL, 34 cases were assigned to the germinal center B-cell (GCB) group and 31 cases to the non-GCB group according to the phenotype determined by Hans’ algorithm.11 A total of 16 normal lymph nodes were used as controls. All procedures were preapproved by the Institutional Review Board of the Shandong University.
Immunohistochemistry and Pathological Examination
In most cases, immunohistochemistry was performed using the following primary antibodies: antibodies against the expression of pan-B-lineage markers such as CD19, CD20, and CD79a, and of other routine phenotype markers such as CD10, CD23, CD30, BCL-2, BCL-6, and MUM-1 (ZSGB-BIO, Beijing, China). Among DLBCL cases, GCB and non-GCB types were determined according to criteria presented by Hans et al.11 Utilizing the remaining paraffin-embedded sections described above, investigational immunohistochemical analyses for TIPE2 were performed.
Immunostaining was performed as described with minor modifications.16 Paraffin-embedded sections were deparaffinized by rehydration with decreasing concentrations of alcohol. After inhibition of endogenous peroxidase with 3% hydrogen peroxide (Sigma, St. Louis, MO) and blocking of nonspecific staining by incubation in goat serum (Invitrogen, Carlsbad, CA), the sections were incubated with primary antibodies (rabbit anti-TIPE2, 1:200, or rabbit lgG as isotype-matched control antibodies, 1:200; Invitrogen) for 3 hr at room temperature. Slides were washed in PBS solution, and antirabbit horseradish peroxidase–conjugated antibody (HRP-streptavidin system; Invitrogen) was applied for 30 min. After washing, immunoperoxidase staining was performed using a diaminobenzidine chromogen kit (ZSGB-BIO). Nuclei were stained with hematoxylin (ZSGB-BIO). The sections were examined under an optical microscopy (Olympus BX51, Tokyo, Japan).
The distribution of TIPE2 protein in tumor cells was determined and scored independently by two hematopathologists (Y.H.C. and Z.G.Y.). For each stained slide, the percentage of tumor cells showing positive staining for TIPE2 was recorded and the intensity of staining was scored as follows: (−) = no staining cell detected; (1+) and (2+) = weak staining and moderate staining, respectively; and (3+) = strong staining. A case was scored as positive if at least 50% of tumor cells stained positive for TIPE2 with an intensity of 1+, 2+, or 3+. Positive staining cells showed reactivity for TIPE2 in both the cytoplasm and nucleus.
Statistical Analysis
Data were presented as means ± standard deviations. The Wilcoxon two-sample test was used to determine the significance of the differences in TIPE2-positive cells. A value of p<0.05 was considered statistically significant. All analyses were performed using the SAS for Windows 9.13.
Results
TIPE2 Expression Was Upregulated in Non-Hodgkin’s Lymphoma
Immunohistochemical analysis for TIPE2 revealed moderate to strong staining of malignant B cells in DLBCL. TIPE2 expression in these cells localized to the cytoplasm and, to a variable extent, the nuclei. DLBCL is the most common large cell lymphoma and commonly identified by pan-B-cell antigen expressed by malignant B cells. We stained 82 cases of DLBCL for TIPE2 protein and detected positive expression in the tumor cells for 75 of 82 cases (Table 1). As shown in Fig. 1A and B and Fig. 2, TIPE2 protein was detected mainly in the cytoplasm of tumor cells. Strong and moderate staining of TIPE2 were detected in 21 and 35 cases, respectively. Low levels or weak staining of TIPE2 protein was detected in 19 cases and was not detectable in seven cases. The results suggest that TIPE2 is commonly expressed by malignant B cells of DLBCL.
Table 1.
Analysis of TIPE2 Expression in Biopsies by Immunostaining.
| Patients | TIPE2 Expression |
|||
|---|---|---|---|---|
| Intense (+++) | Moderate (++) | Weak (+) | Negative (−) | |
| T lymphoma (n=30) | 0 | 10 | 15 | 5 |
| DLBCL (n=82) | 21 | 35 | 19 | 7 |
| GCB types (n=38)* | 17 | 15 | 6 | 0 |
| Non-GCB types (n=44)* | 4 | 20 | 13 | 7 |
Abbreviations: TIPE2, tumor necrosis-alpha-induced protein-8 like 2; DLBCL, diffuse large B-cell lymphoma; GCB, germinal center B cell; Non-GCB, non-germinal center B cell.
p<0.0001.
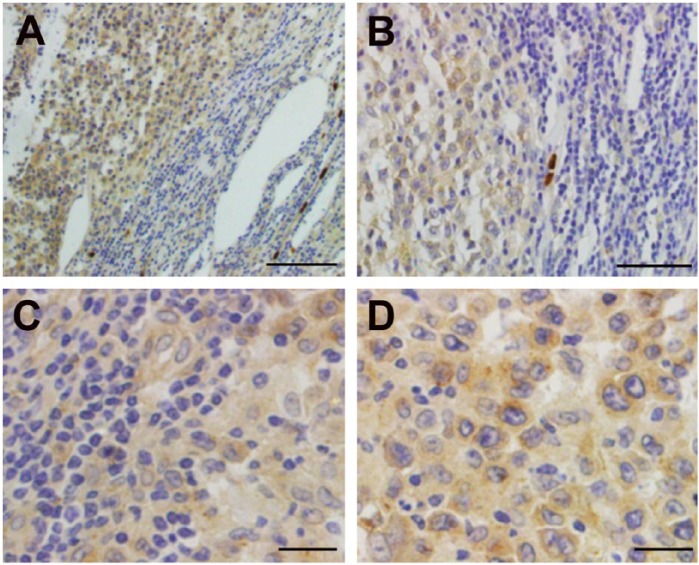
Figure 1.
Tumor necrosis-alpha-induced protein-8 like 2 (TIPE2) expression in non-Hodgkin’s lymphoma. A and B show the detection of TIPE2 protein in diffuse large B-cell lymphoma (DLBCL) by immunohistochemistry (A, ×400; B, ×200). C and D show that moderate levels of TIPE2 protein were detected in T lymphomas (C, ×400; D, ×200). E and F show that the staining of TIPE2 protein was detected in the human normal lymph node (×400). G, H, and I: No staining was detectable with the rabbit isotype antibody for DLBCL, T lymphomas, and normal lymph nodes, respectively (×400).
Figure 2.
Tumor necrosis-alpha-induced protein-8 like 2 (TIPE2) expression in diffuse large B-cell lymphoma by immunohistochemistry. A and B show that the staining of TIPE2 was detected in malignant cells (A, ×100; B, ×200). C and D show that TIPE2 protein was expressed mainly in the cytoplasm of tumor cells (×400).
The expression of TIPE2 protein in T lymphomas was weaker than that in DLBCL cells (Fig. 1C and D, Fig. 3). Only moderate or low-level staining of TIPE2 protein was detected in 25 cases of total of 30 cases (Table 1).
Figure 3.
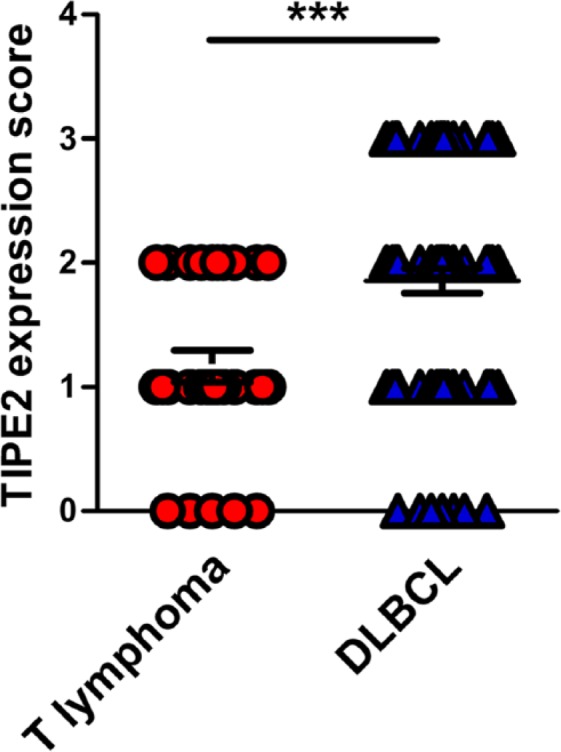
Statistical analysis on the levels of tumor necrosis-alpha-induced protein-8 like 2 (TIPE2) expression in malignant cells between diffuse large B-cell lymphoma (DLBCL) and T lymphoma patients. ***p<0.001.
Furthermore, we found that TIPE2 protein was detectable in both T-cell zone and GC of the normal lymph node by immunohistochemistry (Fig. 1E and F). The staining was specific for TIPE2 because isotype-matched antibody controls showed no staining (Fig 1G, H, and I).
Clinicopathological Characteristics of DLBCL Patients
The clinical features of the DLBCL patients are summarized in Table 2. There were 39 females and 43 males with a median age of 59 years (range, 23–91 years). Thirty-seven patients had nodal DLBCL, and 45 samples were obtained from extranodal sites, such as the stomach, small intestine, colon, skin, ovary, lung, brain, adrenal gland, liver, and breast. All patients received no treatment. Among DLBCL, according to immunohistochemistry algorithm described by Hans et al.,11 38 patients with MUM-1 (−), CD10 (−), and BCL-6 (+), or MUM-1 (−), CD10 (+), and BCL-6 (+/−), were diagnosed with the GCB subtype, whereas 44 patients with MUM-1 (+), CD10 (−), and BCL-6 (+/−) were non-GCB phenotype (data not shown).
Table 2.
Clinicopathological Characteristics of Diffuse Large B-Cell Lymphoma.
| Characteristics | Patients (n) |
|---|---|
| Gender | |
| Female | 39 |
| Male | 43 |
| Age in years, median (range) | 59 (23–91) |
| Phenotype | |
| Germinal center | 38 |
| Non-germinal center | 44 |
| Ki67 ≥ 80% | 39 |
| Presentation | |
| Nodal | 37 |
| Extranodal | 45 |
The Staining of TIPE2 Protein Was Stronger in GCB Phenotype Than That in Non-GCB Type
Among DLBCL, according to the phenotype determined by Hans’ algorithm, cases with immunostaining of CD10 positive, BCL-6 positive, and MUM-1 negative were considered as the GCB phenotype, whereas CD10 negative, BCL-6 negative, and MUM-1 positive cases were considered as the non-GCB type (data not shown). As a result, 38 cases were assigned to the GCB phenotype and 44 cases to the non-GCB phenotype. We found that TIPE2 staining was much stronger in GCB groups than that in non-GCB groups (Figs. 4 and 5). In patients with the GCB phenotype, strong and moderate staining of TIPE2 protein were detected in 17 cases (45%) and 15 cases (39%), respectively. Low-level or weak staining of TIPE2 protein was detected in six cases (16%), and no negative case was found, whereas in patients with the non-GCB phenotype, only four cases (9%) with strong staining were detected. Moderate and low levels of TIPE2 protein were detected in 20 cases (45%) and 13 cases (30%), respectively. There was a significant difference about TIPE2 levels between GCB and non-GCB phenotypes (p<0.0001; Table 1).
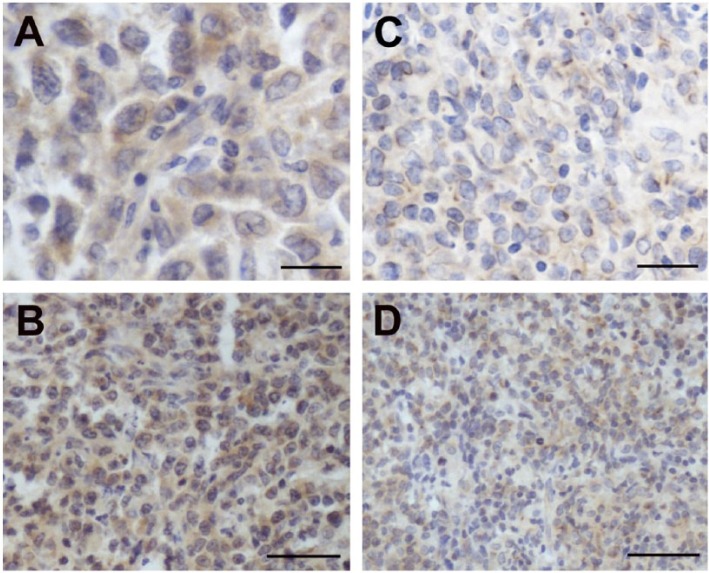
Figure 4.
Detection of tumor necrosis-alpha-induced protein-8 like 2 (TIPE2) protein in germinal center B-cell (GCB) phenotype and non-GCB type of diffuse large B-cell lymphoma (DLBCL) patients. A and B show the expression of TIPE2 protein in non-GCB phenotype of DLBCL by immunohistochemistry (A, ×400; B, ×200). C and D show TIPE2 expression in GCB phenotype (C, ×400; D, ×200).
Figure 5.
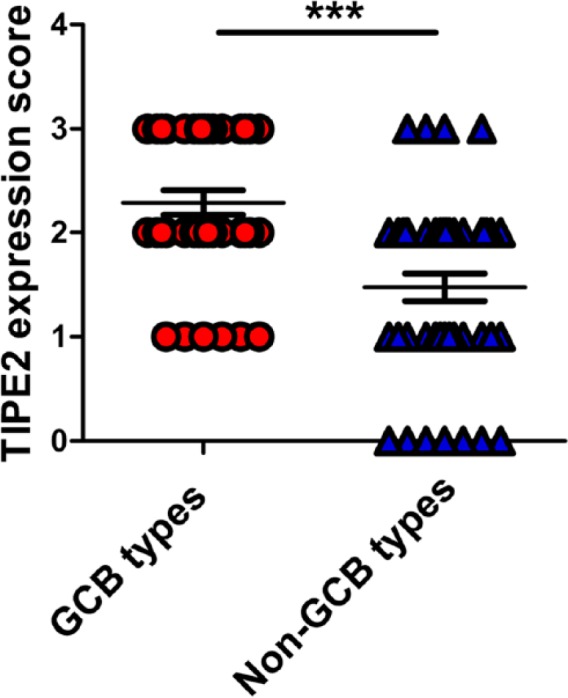
Statistical analysis on the levels of tumor necrosis-alpha-induced protein-8 like 2 (TIPE2) expression between germinal center B-cell (GCB) type and non-GCB type. ***p<0.001.
Discussion
NHLs, solid tumors of lymphocyte origin, are the most common hematopoietic cancers in both men and women in the developed world.29,30 More and more evidence suggests that the occurrence of NHL is associated with abnormal homeostasis, such as severe immunodeficiency including both hereditary immunodeficiency disorders and acquired conditions, and autoimmune conditions, which is a strong risk factor for NHL.31,32 In this report, we used a specific antibody and a standard immunohistochemical staining technique to determine the expression of TIPE2 protein in malignant cells of NHL, including DLBCL and peripheral T-cell lymphoma. We show that TIPE2 protein is mainly expressed in the malignant B cells and T cells of NHL.
First, we found that TIPE2 expression was upregulated in malignant B and T cells. TIPE2 was originally identified as a negative regulator for immune homeostasis, which is mainly expressed in lymphoid tissues and inflammatory tissues.15 Within the lymphoid compartment, T cells appear to express high level of TIPE2 protein, whereas B cells and B-cell zones of lymphoid organs were devoid of TIPE2.16 This expression pattern can explain why TIPE2 deficiency preferentially affected cellular but not humoral immunity in mice.15 In this article, we reported that the levels of TIPE2 protein were markedly upregulated in malignant B cells and T cells, especially malignant B cells. Immunohistochemical analysis revealed that moderate to strong staining of TIPE2 protein was detected in malignant B cells of most DLBCL patients, whereas in T lymphoma cells, TIPE2 staining was weaker than that in DLBCL cells. These data suggest that TIPE2 is commonly expressed by malignant B cells and T cells. But much work needs to be done about how TIPE2 protein was upregulated during lymphomagenesis in the future.
Furthermore, we demonstrated that TIPE2 expression may be a predictor of better prognosis in cases of DLBCL. Studies showed that a GC phenotype might be associated with a better survival.8,9 According to the phenotype determined by Hans’ algorithm, cases with immunostaining of CD10 positive, BCL-6 positive, and MUM-1 negative were considered as the GCB phenotype. Bellas et al.33 also suggested that BCL-6 protein expression conferred a trend toward better outcomes. In this study, we found that the staining of TIPE2 protein was stronger in the GCB phenotype compared with the non-GCB type (p<0.0001) of DLBCL patients. Furthermore, TIPE2 staining in T lymphoma cells was weaker than that in DLBCL cells. This expression pattern suggests that TIPE2 expression may be a predictor of better prognosis in lymphomas. Much work is required to confirm this notion.
TIPE2 plays important roles in controlling inflam-mation and tumorigenesis by suppressing RAS signaling.15,26 Studies showed that TIPE2 plays atheroprotective roles by regulating the functions of macrophages and vascular smooth muscle cells (VSMCs).22,23 In the physical circumstances, TIPE2 is highly expressed in macrophages but very low in VSMCs.16 Interestingly, TIPE2 was downregulated in macrophages, whereas it was upregulated in VSMCs during atherogenesis.22,23 TIPE2 is also an inhibitor for carcinogenesis by promoting cell death and was found to be downregulated in hepatoma cells.25,26 Similar events have been observed in this article. TIPE2 is highly expressed in T cells, but downregulated in T lymphoma cells, the same as the downregulation in Hepatitis B virus–specific T cells,21 while upregulated in DLBCL, especially was stronger in GC-subtype than that in non-GC type. These data suggest that TIPE2 may be a new inhibitor for carcinogenesis with a different expression pattern from other tumor inhibitory genes. It is very interesting to study the mechanism of TIPE2 expression, especially under physiological and pathological conditions.
Collectively, our data demonstrate that TIPE2 expression was upregulated in malignant cells of DLBCL and peripheral T-cell lymphoma. Among DLBCL patients, TIPE2 expression was significantly stronger in the GCB phenotype compared with that in the non-GCB type. These results suggest that TIPE2 protein may be a predictor of better prognosis in cases of DLBCL.
Acknowledgments
We thank Prof. Gengyin Zhou (Department of Pathology, Shandong University School of Medicine) for good suggestions for lymphoma determination.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the grants from the National Natural Science Foundation of China (No. 81171578, No. 81100205) and supported by the Fundamental Research Funds of Shandong University (2014JC010).
References
- 1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics. CA Cancer J Clin. 2008;58:71–96. [DOI] [PubMed] [Google Scholar]
- 2. Lenz G, Wright GW, Emre NC, Kohlhammer H, Dave SS, Davis RE, Carty S, Lam LT, Shaffer AL, Xiao W, Powell J, Rosenwald A, Ott G, Muller-Hermelink HK, Gascoyne RD, Connors JM, Campo E, Jaffe ES, Delabie J, Smeland EB, Rimsza LM, Fisher RI, Weisenburger DD, Chan WC, Staudt LM. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci U S A. 2008;105: 13520–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC Press; 2008. p. 233–7. [Google Scholar]
- 4. Li X, Li G, Gao Z, Zhou X, Zhu X. The relative frequencies of lymphoma subtypes in China: a nationwide study of 10002 cases by the Chinese Lymphoma Study Group. Ann Oncol. 2011;22:141. [Google Scholar]
- 5. Dong LM, Potter JD, White E, Ulrich CM, Cardon LR, Peters U. Genetic susceptibility to cancer: the role of polymorphisms in candidate genes. JAMA. 2008;299:2423–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lan Q, Zheng T, Rothman N, Zhang Y, Wang SS, Shen M, Berndt SI, Zahm SH, Holford TR, Leaderer B, Yeager M, Welch R, Boyle P, Zhang B, Zou K, Zhu Y, Chanock S. Cytokine polymorphisms in the Th1/Th2 pathway and susceptibility to non-Hodgkin lymphoma. Blood. 2006;107:4101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Purdue MP, Lan Q, Kricker A, Grulich AE, Vajdic CM, Turner J, Whitby D, Chanock S, Rothman N, Armstrong BK. Polymorphisms in immune function genes and risk of non-Hodgkin lymphoma: findings from the New South Wales non-Hodgkin Lymphoma Study. Carcinogenesis. 2006;28:704–12. [DOI] [PubMed] [Google Scholar]
- 8. Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, Powell JI, Yang L, Marti GE, Moore T, Hudson J, Jr, Lu L, Lewis DB, Tibshirani R, Sherlock G, Chan WC, Greiner TC, Weisenburger DD, Armitage JO, Warnke R, Levy R, Wilson W, Grever MR, Byrd JC, Botstein D, Brown PO, Staudt LM. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–11. [DOI] [PubMed] [Google Scholar]
- 9. Shipp MA, Ross KN, Tamayo P, Weng AP, Kutok JL, Aguiar RC, Gaasenbeek M, Angelo M, Reich M, Pinkus GS, Ray TS, Koval MA, Last KW, Norton A, Lister TA, Mesirov J, Neuberg DS, Lander ES, Aster JC, Golub TR. Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat Med. 2002;8:68–74. [DOI] [PubMed] [Google Scholar]
- 10. Choi WW, Weisenburger DD, Greiner TC, Piris MA, Banham AH, Delabie J, Braziel RM, Geng H, Iqbal J, Lenz G, Vose JM, Hans CP, Fu K, Smith LM, Li M, Liu Z, Gascoyne RD, Rosenwald A, Ott G, Rimsza LM, Campo E, Jaffe ES, Jaye DL, Staudt LM, Chan WC. A new immunostain algorithm classifies diffuse large B-cell lymphoma into molecular subtypes with high accuracy. Clin Cancer Res. 2009;15:5494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E, Braziel RM, Jaffe ES, Pan Z, Farinha P, Smith LM, Falini B, Banham AH, Rosenwald A, Staudt LM, Connors JM, Armitage JO, Chan WC. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–82. [DOI] [PubMed] [Google Scholar]
- 12. Benesova K, Forsterova K, Votavova H, Campr V, Stritesky J, Velenska Z, Prochazka B, Pytlik R, Trneny M. The Hans algorithm failed to predict outcome in patients with diffuse large B-cell lymphoma treated with rituximab. Neoplasma. 2013;60:68–73. [DOI] [PubMed] [Google Scholar]
- 13. Castillo JJ, Beltran BE, Song MK, Ilic I, Leppa S, Nurmi H, Seki R, Uccella S, Li JM, Treaba DO, Stachurski D, Butera JN. The Hans algorithm is not prognostic in patients with diffuse large B-cell lymphoma treated with R-CHOP. Leuk Res. 2012;36:413–7. [DOI] [PubMed] [Google Scholar]
- 14. Ichikawa S, Fukuhara N, Katsushima H, Takahashi T, Yamamoto J, Yokoyama H, Sasaki O, Fukuhara O, Nomura J, Ishizawa K, Ichinohasama R, Muto A, Igarashi K, Harigae H. Association between BACH2 expression and clinical prognosis in diffuse large B-cell lymphoma. Cancer Sci. 2014;105:437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sun H, Gong S, Carmody RJ, Hilliard A, Li L, Sun J, Kong L, Xu L, Hilliard B, Hu S, Shen H, Yang X, Chen YH. TIPE2, a novel negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell. 2008;133:415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang G, Hao C, Lou Y, Xi W, Wang X, Wang Y, Qu Z, Guo C, Chen YH, Zhang Y, Liu S. Tissue-specific expression of TIPE2 provides insights into its function. Mol Immunol. 2010;47:2435–42. [DOI] [PubMed] [Google Scholar]
- 17. Zhang X, Wang J, Fan C, Li H, Sun H, Gong S, Chen YH, Shi Y. Crystal structure of TIPE2 provides insights into immune homeostasis. Nat Struct Mol Biol. 2009;16:89–90. [DOI] [PubMed] [Google Scholar]
- 18. Zhang L, Shi Y, Wang Y, Zhu F, Wang Q, Ma C, Chen YH, Zhang L. The unique expression profile of human TIPE2 suggests new functions beyond its role in immune regulation. Mol Immunol. 2011;48:1209–15. [DOI] [PubMed] [Google Scholar]
- 19. Lou Y, Zhang G, Geng M, Zhang W, Cui J, Liu S. TIPE2 negatively regulates inflammation by switching arginine metabolism from nitric oxide synthase to arginase. PLoS ONE. 2014;9(5):e96508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xi W, Hu Y, Liu Y, Zhang J, Wang L, Lou Y, Qu Z, Cui J, Zhang G, Liang X, Ma C, Gao C, Chen Y, Liu S. Roles of TIPE2 in hepatitis B virus-induced hepatic inflammation in humans and mice. Mol Immunol. 2011;48:1203–8. [DOI] [PubMed] [Google Scholar]
- 21. Zhang W, Zhang J, Zhao L, Shao J, Cui J, Guo C, Zhu F, Chen YH, Liu S. TIPE2 protein negatively regulates HBV-specific CD8+ T lymphocyte functions in humans. Mol Immunol. 2015;64:204–9. [DOI] [PubMed] [Google Scholar]
- 22. Lou Y, Liu S, Zhang C, Zhang G, Li J, Ni M, An G, Dong M, Liu X, Zhu F, Zhang W, Gao F, Chen YH, Zhang Y. Enhanced atherosclerosis in TIPE2-deficient mice is associated with increased macrophage responses to oxidized low-density lipoprotein. J Immunol. 2013;191:4849–57. [DOI] [PubMed] [Google Scholar]
- 23. Zhang G, Zhang W, Lou Y, Xi W, Cui J, Geng M, Zhu F, Chen YH, Liu S. TIPE2 deficiency accelerates neointima formation by downregulating smooth muscle cell differentiation. Cell Cycle. 2013;12:501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lou Y, Sun H, Morrissey S, Porturas T, Liu S, Hua X, Chen YH. Critical roles of TIPE2 protein in murine experimental colitis. J Immunol. 2014;193:1064–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cao X, Zhang L, Shi Y, Sun Y, Dai S, Guo C, Zhu F, Wang Q, Wang J, Wang X, Chen YH, Zhang L. Human tumor necrosis factor (TNF)-alpha-induced protein 8-like 2 suppresses hepatocellular carcinoma metastasis through inhibiting Rac1. Mol Cancer. 2013;12:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gus-Brautbar Y, Johnson D, Zhang L, Sun H, Wang P, Zhang S, Zhang L, Chen YH. The anti-inflammatory TIPE2 is an inhibitor of the oncogenic Ras. Mol Cell. 2012;45:610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ruan Q, Wang P, Wang T, Qi J, Wei M, Wang S, Fan T, Johnson D, Wan X, Shi W, Sun H, Chen YH. MicroRNA-21 regulates T-cell apoptosis by directly targeting the tumor suppressor gene Tipe2. Cell Death Dis. 2014;5:e1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li XM, Su JR, Yan SP, Cheng ZL, Yang TT, Zhu Q. A novel inflammatory regulator TIPE2 inhibits TLR4-mediated development of colon cancer via caspase-8. Cancer Biomark. 2014;14:233–40. [DOI] [PubMed] [Google Scholar]
- 29. Jaffe ES. Anaplastic large cell lymphoma: the shifting sands of diagnostic hematopathology. Mod Pathol. 2001;14:219–28. [DOI] [PubMed] [Google Scholar]
- 30. Müller AM, Ihorst G, Mertelsmann R, Engelhardt M. Epidemiology of non-Hodgkin’s lymphoma (NHL): trends, geographic distribution, and etiology. Ann Hematol. 2005;84:1–12. [DOI] [PubMed] [Google Scholar]
- 31. Grulich AE, Vajdic CM, Cozen W. Altered immunity as a risk factor for non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16:405–8. [DOI] [PubMed] [Google Scholar]
- 32. Tio M, Cox MR, Eslick GD. Meta-analysis: coeliac disease and the risk of all-cause mortality, any malignancy and lymphoid malignancy. Aliment Pharmacol Ther. 2012;35:540–51. [DOI] [PubMed] [Google Scholar]
- 33. Bellas C, García D, Vicente Y, Kilany L, Abraira V, Navarro B, Provencio M, Martín P. Immunohistochemical and molecular characteristics with prognostic significance in diffuse large B-cell lymphoma. PLoS ONE. 2014;9(6):e98169. [DOI] [PMC free article] [PubMed] [Google Scholar]