Abstract
The marker of neuronal activation, c-Fos, can be used to visualize spatial patterns of neural activity in response to taste stimulation. Because animals will not voluntarily consume aversive tastes, these stimuli are infused directly into the oral cavity via intraoral cannulae, whereas appetitive stimuli are given in drinking bottles. Differences in these 2 methods make comparison of taste-evoked brain activity between results that utilize these methods problematic. Surprisingly, the intraoral cannulae experimental conditions that produce a similar pattern of c-Fos activity in response to taste stimulation remain unexplored. Stimulation pattern (e.g., constant/intermittent) and hydration state (e.g., water-restricted/hydrated) are the 2 primary differences between delivering tastes via bottles versus intraoral cannulae. Thus, we quantified monosodium glutamate (MSG)-evoked brain activity, as measured by c-Fos, in the nucleus of the solitary tract (nTS; primary taste nucleus) across several conditions. The number and pattern of c-Fos neurons in the nTS of animals that were water-restricted and received a constant infusion of MSG via intraoral cannula most closely mimicked animals that consumed MSG from a bottle. Therefore, in order to compare c-Fos activity between cannulae-stimulated and bottle-stimulated animals, cannulated animals should be water restricted prior to stimulation, and receive taste stimuli at a constant flow.
Key words: drinking bottle, intraoral cannulae, taste
Introduction
The immediate early gene, c-Fos, is often used as an anatomical marker of neuronal activation, which enables measurement of differences in the spatial distribution of neuronal activity between different taste qualities (Harrer and Travers 1996; King et al. 1999; Travers 2002; Chan et al. 2004; Stratford and Finger 2011). In common experimental practice, water-restricted animals consume a taste stimulus from a drinking bottle in a short period (e.g., 30min; Wilkins and Bernstein 2006; Boughter et al. 2007; Chen et al. 2011; Stratford and Finger 2011; Stratford and Thompson 2014). However, because animals will not voluntarily consume aversive tastes, unpalatable stimuli must be directly infused into the oral cavity via intraoral cannulae. Thus, differences in methodology between these 2 approaches (i.e., bottle vs. intraoral cannulae stimulation), may complicate comparison between results that utilize these 2 methods.
In particular, there are 2 primary differences when taste stimuli are given in drinking bottles versus intraoral cannulae. First, animals consume fluids from drinking bottles intermittently in brief “bouts” (Stellar and Hill 1952; Gannon et al. 1992; Boughter et al. 2007; Johnson et al. 2010; Barkley-Levenson and Crabbe 2012); whereas, taste stimuli are usually infused at a constant flow rate through intraoral cannulas (Harrer and Travers 1996; Houpt et al. 1996; King et al. 1999, 2000, 2003, 2014, 2015; Travers 2002; Chan et al. 2004; Jarrett et al. 2007; Travers and Travers 2007; Biondolillo et al. 2009; Wilmouth and Spear 2009; Haino et al. 2010; Nagy et al. 2012; Zhao et al. 2012; Riley and King 2013; Tokita et al. 2014). Second, in order to encourage animals to consume fluids, animals are placed on water-restriction (e.g., 1h of water/24h for 2–3 days prior to experiment), but animals are rarely water deprived prior to receiving taste stimuli via intraoral cannulae. Thus, the pattern used to deliver taste stimuli (e.g., constant stimulation vs. intermittent, “bout” stimulation) or the hydration state of the animal (e.g., water-restricted vs. water-replete) may influence experimental measurements, independent of taste stimulation.
In this regard, auditory stimulation patterns are known to influence c-Fos activity in the cerebellum, with faster stimulation patterns (i.e., 40 Hz) producing more c-Fos positive cells than slower stimulation patterns (i.e., 10 Hz; Tian and Bishop 2002). Moreover, dehydration and rehydration are also known to influence c-Fos activity specifically within in the nucleus of the solitary tract (nTS), the primary taste/viscerosensory nucleus (Ji et al. 2007; Gottlieb et al. 2011). In particular, both prolonged (e.g., >45h) water deprivation as well as brief rehydration (e.g., <2h) significantly increase the number of c-Fos as compared with control animals. Together, these results suggest that both the stimulation pattern as well as the physiological state of the animal (i.e., dehydrated vs. rehydration) can produce increased c-Fos activity independent of stimulus.
In light of this information, it is logical to expect that cannulated animals should be water-restricted prior to taste stimulation for c-Fos immunohistochemistry, with taste stimuli infused into intraoral cannulas in an intermittent—“bout”—pattern in order to mimic the animal’s natural pattern of ingestion. Yet, to-date, no study has systematically compared taste-evoked c-Fos activity in the gustatory and viscerosensory processing areas of the brain across various physiological and stimulation parameters.
Therefore, the current study measured c-Fos activity in the nTS across several different parameters to determine which experimental conditions produce c-Fos activity that mirrors that observed when animals consume taste stimuli from drinking bottles. First, we compared c-Fos activity in groups that were water-restricted or water-replete to c-Fos activity in animals that consumed a taste stimulus from a drinking bottle. Second, we compared c-Fos activity in groups that received taste stimuli via intra oral cannulae at a constant flow rate or at an intermittent rate to c-Fos activity in animals that consumed a taste stimulus from a drinking bottle. Third, because the amount of fluid intermittently infused into intraoral cannulae is half the amount infused at a constant rate during the same infusion time, we also compared c-Fos activity between an intermittently stimulated group and a group that was intermittently stimulated for twice as long (i.e., “volume-matched” to our other experimental groups). Finally, we also measured c-Fos activity in water-restricted, cannulated mice that received no taste stimulation to serve as a control for both cannulation surgery as well as water restriction.
Materials and methods
Animals
Adult c57BL/6 female mice (n = 34; age 5–10 months) purchased from The Jackson Laboratory were used in this study. The animals were housed in a vivarium with a 12h light/dark cycle with lights on at 0500. Food (Teklad Global Rodent Diet # 2918) was available ad libitum throughout the course of the experiment. Water was also available ad libitum as well, except as noted.
All animal procedures were performed in accordance with NIH guidelines and were approved by the Institutional Animal Care and Use Committee at the University of Colorado Denver School of Medicine.
Cannula surgery
Bilateral intraoral cannulae were implanted into all experimental animals. Mice were anesthetized with an intramuscular (IM) injection of a combination of medetomidine hydrochloride (Domitor; 0.4mg/kg; Pfizer) and ketamine hydrocholoride (40mg/kg; Bioniche Pharma). Once sedated, the surgical site was shaved and prepped with iodine (7.5%; Professional Disposables International) and isopropyl alcohol (70%; Tyco Healthcare). Animals were then placed on a heating pad, and the topical analgesic, bupivacaine (Marcaine; 0.5%; Hospira), was applied to the surgical site.
Intraoral cannulae were inserted using a procedure modified from Grill and Norgren (1978) (in rats) and Kiefer et al. (1998) (in mice). Briefly, a midline incision was made on the dorsal surface of the animal, immediately caudal to the pinnae. Then a sterile, 1.5 inch, stainless steel hypodermic needle (19 gauge; Hamilton) was inserted in the back of the neck and guided subcutaneously ventral to the auricle and eye, and then to the oral cavity. The needle was then inserted in the oral cavity lateral to the first maxillary molar.
Polyethylene tubing (22 gauge; Becton Dickinson and Company) was flared using a cautery iron, and then fed though a small washer and then through the tip of the needle. The washer, a #0 small nylon flat washer (Product Components Corporation), served to secure the tubing in the oral cavity. The needle was then withdrawn, the wound closed with Dexon II 6.0mm suture (Syneture), and a second nylon washer secured the loose end of the tubing firmly in place against the skin on the back of the neck. The remaining tubing was flared by heating the exposed tubing with a cautery iron (Fine Science Tools). Finally, a blunt 1″ hypodermic needle (25 gauge; McKesson) was secured to the loose end of the tubing and served as a metal fistula.
Following implantation of intraoral cannulae, animals were injected IM with atipamezole (Antisedan; 2mg/kg; Pfizer) to reverse the sedative effects of Domitor. To minimize postoperative pain, animals were injected subcutaneously with carprofen (Rimadyl; 5mg/kg; Pfizer) immediately before surgery and for 48h after surgery. Animals were allowed to recover for 5 days prior to training (see Taste stimuli through intraoral cannula).
Taste stimuli in drinking bottles
We first quantified c-Fos activation in the nTS of animals that were allowed to freely ingest taste stimuli from a drinking bottle. Three days prior to taste stimulation, mice (Bottle, n = 6) were placed on 23h/day water restriction. During this time, animals were given 1h access to water in a single drinking bottle at the same time each day to train animals to consume fluids in a relatively short period. On stimulation day, animals were given 150mM monosodium glutamate (MSG) in a single drinking bottle in their home cage. At the end of 30min, fluid intake was recorded and animals were left undisturbed for 45min prior to perfusion (see c-Fos immunohistochemistry).
All cannulated, water-restricted animals consumed ~3mL (in mL per day ± SD: 3.1±0.4) of MSG from their drinking bottles. Thus, to ensure that all experimental animals received an equal volume of MSG taste stimulation regardless of experimental condition (i.e., via bottle or infusion through intraoral cannula), 3mL of fluid was infused, via intraoral cannula, into the oral cavity of mice in all other experimental groups except where noted (see Taste stimuli through intraoral cannula for details).
Taste stimuli through intraoral cannula
Syringe pump and prestimulation training
Fluids were delivered into intraoral cannulae via a 5 cc syringe fitted with a blunted 25 gauge needle (Becton Dickinson and Company) connected to a syringe pump (Model R99-E, Razel Scientific Instruments). To acclimate mice to stimulation procedures, all animals were placed on 23h/day water restriction schedule 3 days prior to stimulation, except where noted (see Stimulation groups for details). Then, each day during training, all groups received 3mL of dH2O infused over the course of 30min into 1 of 2 intraoral cannulae at a flow rate of 0.101mL/min. Although this flow rate is slightly slower than the ingestion rate of fluids in mice (e.g., 0.3–0.5mL/min; Murakami 1977; Boughter et al. 2007), it is similar to that used by other researchers to deliver tastants for c-Fos stimulation (e.g., 0.1mL/min; Travers et al. 2007).
Stimulation groups
We measured c-Fos activity in the nTS of animals in 4 different experimental groups to thoroughly characterize how different stimulation conditions affect c-Fos activity in the nTS. First, in order to determine whether the hydration state of the animal affected c-Fos activity, the number of c-Fos positive cells in the nTS was compared between nonrestricted animals (Nondeprived Constant, n = 5) and animals that were deprived of water for 23 hrs (Deprived Constant, n = 6). To do this, Nondeprived Constant mice were given full access to water after 2 water training days (see Syringe pump and prestimulation training); whereas the Deprived group was maintained on 23h water restriction throughout training and stimulation. On stimulation day, 3mL of MSG was infused into a single intraoral cannula of mice in both groups at a constant flow rate (0.101mL/min) over the course of 30min. Given that Nondeprived Constant animals may not be motivated to consume the entire 3mL of MSG, unlike the Deprived Constant animals, MSG consumption was closely monitored in both groups throughout the entire 30min stimulation by an in-room observer. All animals robustly consumed fluids throughout the stimulus presentation, which may be attributed, especially in the Nondeprived Constant group, both to the palatability of MSG as well as the novelty of the stimulus.
Second, to determine whether the pattern of stimulation influences c-Fos activity, we also quantified c-Fos activity in the nTS of animals that were stimulated with MSG using an intermittent stimulation pattern that approximates the average “bout” length for fluid consumption by mice (e.g., ~1min; Gannon et al. 1992; Boughter et al. 2007; Deprived Intermit, n = 5). During training and stimulation, Deprived Intermit mice were maintained on 23h water restriction. On stimulation day, 1.5mL of MSG was infused into a single cannula of each mouse with a 1min on and 1min off stimulation protocol (0.101mL/min). However, to control for the amount of fluid infused into the oral cavity, an additional group of animals received the same intermittent taste stimulation with a duration that was twice as long (2min on, 1 off; 3mL total; Deprived Intermit 2X, n = 5). As observed with the Deprived Constant and Nondeprived Constant groups, all animals robustly drank MSG during the intermittent times it was available.
Finally, to determine whether bilateral cannula surgery alone affects c-Fos activity, 7 additional animals (Deprived Unstim) were implanted with bilateral cannulas and underwent water 23-h restriction, but were left undisturbed on stimulation day.
c-Fos immunohistochemistry
Forty-five minutes following taste stimulation, animals were deeply anesthetized with Fatal-Plus (50mg/kg intraperitoneally; MWI) and perfused transcardially first with 0.9% saline and then with 4% paraformaldehyde in 0.1M phosphate buffer. Following perfusion, the brain was removed and post-fixed in 4% paraformaldehyde for 3h. Then tissue was placed in 20% sucrose overnight at 4 °C. The olfactory bulbs (control) and brainstem were isolated, embedded in optimal cutting temperature (OCT) compound (Fisher Scientific) and frozen rapidly on dry ice. Coronal sections (40 µM) were cut on a cryostat and divided into a series of 3 adjacent sets. Sections were either reacted immediately or placed in cryoprotectant and stored at −20 °C for later processing (Watson et al. 1986). Sections stored in cryoprotectant were thoroughly rinsed in 0.1M phosphate-buffered saline (PBS) before staining.
All steps were conducted at room temperature unless otherwise indicated. Sections were first washed 3 times in 0.1M PBS. Sections were then blocked in 2% normal donkey serum (NDS, Jackson ImmunoResearch) for 1h and then incubated in rabbit anti-Fos (1:3000), diluted in antibody media (AB media; 0.3% triton, 0.15M sodium chloride, and 1% bovine serum albumin; for details about anti-sera see c-Fos antisera) at 4 °C for 48h.
Following primary antibody incubation and 3 washes in 0.1M PBS, the anti-Fos antibody was detected with a Rhodamine Red-X FAB fragment donkey anti-rabbit antibody (Jackson ImmunoResearch Laboratories catalog # 711-297-003; Lot # 103577; diluted 1:800), incubated with tissue for 2h. Sections were washed with PBS between each reaction. Following an additional 3 washes in PBS, sections were mounted onto Fisher Superfrost Plus slides and cover slipped with Fluormount G (Southern Biotechnology).
c-Fos antisera
Sections were processed for c-Fos-like immunoreactivity (Fos-LI) using a rabbit polyclonal anti-c-Fos antibody from Milipore (formerly sold by Calbiochem and Oncogene). The c-Fos antibody (Ab-5, catalog # PC38; lot #s D00134698, D00148958; diluted 1: 3000) was prepared against a peptide mapping at residues 4–17 of the human c-Fos protein. This antiserum stains a single band at ~50–55 kDa as observed by western blot analysis of fibroblast-like BHK 21 C13 cells (Archer et al. 1999). Moreover, omission of rabbit anti-c-Fos antibody resulted in no labeled cells (data not shown).
Microscopic analysis
Whole slide images were photographed using the imaging software, Surveyor by Objective Imaging, which controls both the microscope stage as well as enables image acquisition, with a black and white Leica DFC 365FX camera on a Leica DM6000B microscope. For each slide, using the multiscan option in the imaging software, a series of 10× images, aligned in a grid, were obtained for each fluorophore. Within each grid, each channel (Texas Red, Cy-5) was obtained sequentially and merged together to prevent side-band excitation of the different fluorophores. Images were then stitched together in real time using the Best Focus algorithm in the Surveyor software, which yielded a mosaic image of the whole microscope slide. Images of individual fluorescent, RBG brain sections were then obtained using the Region of Interest tool in the Surveyor Viewer Software.
c-Fos cell counting and representative levels of the nTS
To characterize thoroughly c-Fos activity across all experimental conditions, the number of c-Fos positive cells was quantified in 9 rostrocaudal coronal levels with 6 subfields in each coronal plane (dorsal medial, dorsal intermediate, dorsal lateral, ventral medial, ventral intermediate, and ventral lateral). The rostrocaudal levels were designated as rostral (r1–r5) and intermediate (i1–i4); situated respectively at −6.36, −6.48, −6.72, −6.96, −7.08, −7.20, −7.32, −7.48, and −7.56 from bregma (Paxinos and Franklin 2001; Figure 1). The boundaries of the nTS were outlined based on cell size and cell density using a Nissl counterstain (NeuroTrace 640/680 1:100; lot # 927003, Invitrogen), which was included in the antibody media during secondary antibody incubation.
Figure 1.
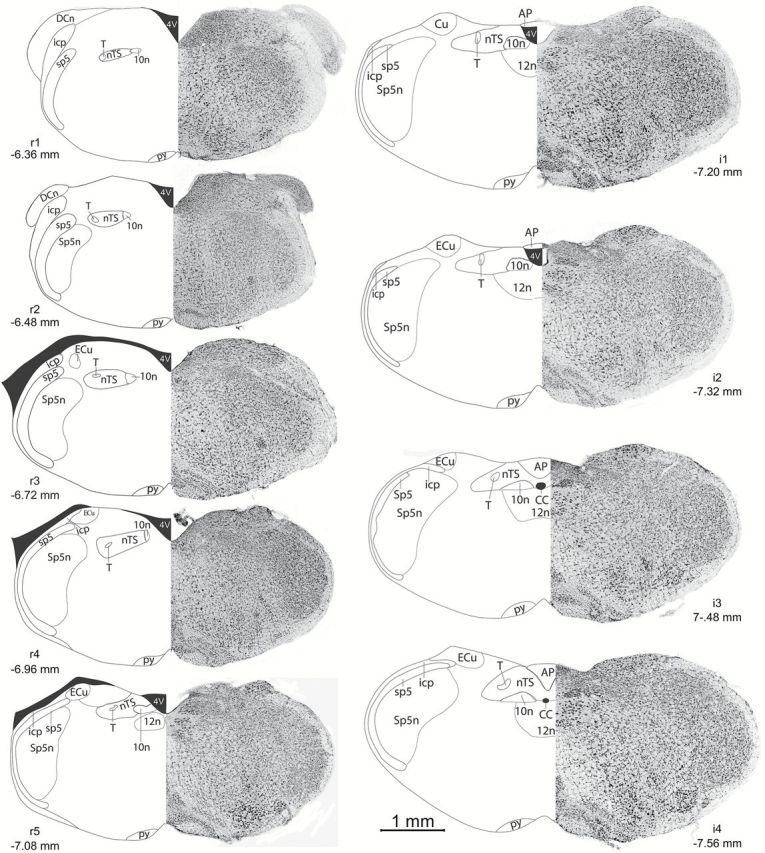
Standard counting planes of the nTS. For each column, Left: rostrocaudal nTS levels designated as rostral (r1–r5), and intermediate (i1–i4). Coordinates from Bregma are listed for each nTS level. AP, area postrema; CC, central canal; ECu, external cuneate nucleus; icp, inferior peduncle; nTS, nucleus of the solitary tract; py, pyramidal tract; sp5, spinal trigeminal tract; Sp5n, spinal trigeminal nucleus; T, solitary tract; 4V, 4th ventricle; 10n, dorsal motor nucleus of the vagus; 12n, hypoglossal nucleus. Images modified from Paxinos The Mouse Brain in Stereotaxic Coordinates, 2nd edition. Right: Photomicrographs of fluorescent Nissl staining in the brainstem of an experimental animal. Images converted to greyscale colors for clarity.
To quantify the number of c-Fos positive cells, the red (c-Fos) color channel in each image was first thresholded based on a stringent threshold (mean + 2 standard deviations [SDs] of background pixel intensity level), and the red color channel was then converted to a binary BW image using ImageBWconvertGUI—a custom-made program running in the 2013a Matlab Computing Environment with the Image Processing Toolbox (The MathWorks; program available on Github: https://github.com/neuropil/ImageBWconvert/, last accessed January 6, 2016.). This resulted in individual, thresholded binary black and white images of c-Fos staining > 2 SDs of background staining for each animal. Then, the number of c-Fos positive cells was quantified using the cell counter plugin in ImageJ (version 1.47). Substantial Fos-LI was observed in the olfactory bulb of all experimental, as c-Fos expression is robust in the olfactory bulb in all animals, and served as a positive control for our primary antibody (Guthrie et al. 1993).
Finally, adopting methodology used previously to visualize patterns of cell activation in the nTS (Stratford and Finger 2011), we generated “heat-maps,” similar to fMRI activity maps showing the relative level of activation (number of c-Fos positive cells) for each subfield of the nTS.
Statistics
Data are presented as group means ± SD. Data were analyzed using appropriate 1- or 2-way ANOVAs (Statistica; StatSoft). Tukey’s honest significant difference tests were used to assess statistically significant (P < 0.05) main effects or interactions.
Results
Total number of c-Fos positive cells
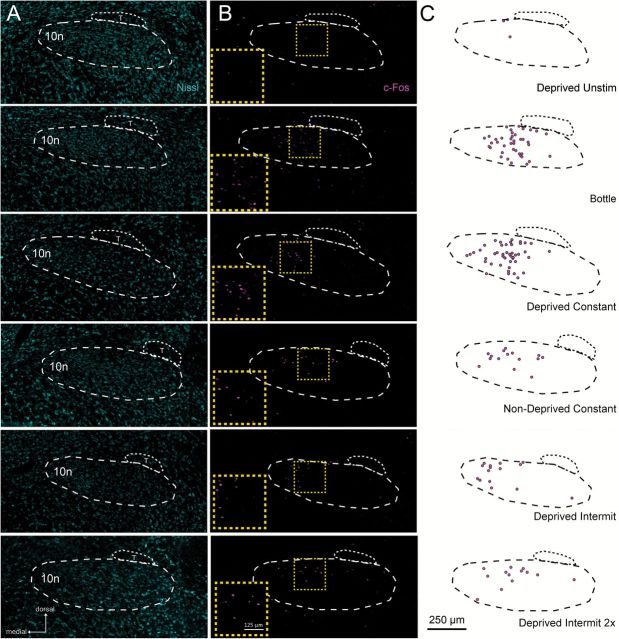
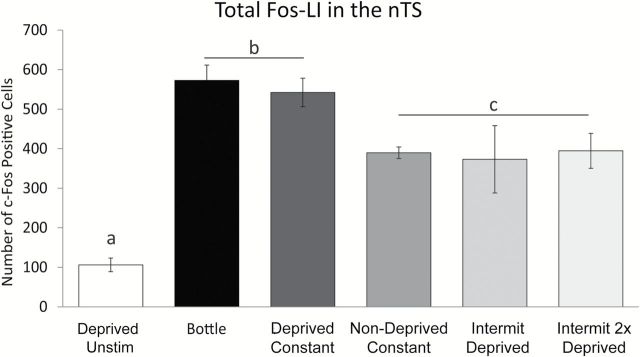
As shown in Figures 2 and 3, different experimental conditions produced a diverse number and pattern of c-Fos positive cells in the nTS. Overall, the total amount of Fos-LI in the nTS (e.g., sum of c-Fos positive cells across all 9 nTS levels) varied in a graded fashion between experimental groups [Figure 3; 1-way (group) ANOVA, F(1, 28) = 18.2, P < 0.001]. As expected, Fos-LI in the nTS of Deprived Unstim animals was significantly less than compared with all other groups (all post hoc comparison Ps < 0.05). Moreover, total Fos-LI was not different between the Nondeprived Constant, Deprived Intermit, and Deprived Intermit 2X groups (Ps = 0.99, 0.86, 0.72, respectively), although the number of c-Fos positive cells was more variable in both the Deprived Intermit and Deprived Intermit 2X groups than the Nondeprived Constant group (Deprived Intermit SD: 85.2; Deprived Intermit 2X SD: 44.14; Nondeprived Constant SD: 14.6). Moreover, Fos-LI in the Nondeprived Constant, Deprived Intermit, and Deprived Intermit 2X groups was significantly less than that observed in either the Bottle and Deprived groups (all post hoc comparison Ps < 0.05). Finally, Fos-LI was similar between the Bottle and Deprived groups (P = 0.99).
Figure 2.
Fos-LI varies significantly across experimental conditions. Photomicrographs (A, B) and chartings (C) of Fos-LI cells in the nTS in response to no stimulation (Deprived Unstim; top), MSG stimulation with a drinking bottle (Bottle; 2nd row), constant MSG infusion via intraoral cannula to a water-restricted animal (Deprived Constant; 3rd row), constant MSG infusion via intraoral cannula to an animal that received no water restriction (Non-Deprived Constant; 4th row), intermittent MSG infusion via intraoral cannula to a water-restricted animal MSG (Deprived Intermit; 5th row), or intermittent MSG infusion via intraoral cannula to a water-restricted animal MSG that was twice as long (e.g., “fluid matched”; Deprived Intermit 2X; bottom). (A) Photomicrographs counterstained with a fluorescent Nissl (cyan) in the r3 nTS level. (B) Photomicrographs of Fos-LI in the nTS. Yellow boxes denote the location of the enlarged Fos-LI inset for each row. (C) Outlines of the nTS and chartings showing c-Fos positive cells from panel B. T, solitary tract; 10n, dorsal motor nucleus of the vagus.
Figure 3.
Fos-LI in the nTS of bottle-stimulated animals is most similar to Fos-LI in the nTS of water-restricted, constantly stimulated animals. Unstimulated animals (Deprived Unstim) had few c-Fos positive cells in the nTS as compared with all other groups. Fos-LI is not different between Nondeprived Constant, constantly stimulated (Nondeprived Constant), deprived intermittently stimulated (Deprived Intermit) or intermittently stimulated twice as long (Deprived Intermit 2X) animals, but Fos-LI in all 3 groups is less than deprived, constantly stimulated (Deprived Constant) and bottle stimulated (Bottle) animals. Deprived Unstim < (Nondeprived Constant = Deprived Intermit = Deprived Intermit 2X) < (Deprived Constant = Bottle). Fos-LI is comparable between Deprived Constant and Bottle groups. a, Deprived Unstim significantly less than all other groups; b, Bottle and Deprived Constant groups significantly greater than all other groups; c, Nondeprived Constant, Deprived Intermit, and Deprived Intermit 2X groups significantly greater than Deprived Unstim, but significantly less than Bottle and Deprived Constant groups. All Ps < 0.05.
Pattern of Fos-LI across nTS levels
Overall, total Fos-LI in the nTS of the Deprived Constant group was similar to that found in bottle-stimulated animals (Bottle), and Fos-LI in both groups was greater than the total Fos-LI in the Nondeprived Constant group (Figure 3). However, this measurement does not provide detailed analysis of Fos-LI at each of the 9 nTS levels (i.e., r1, r2, … etc.), which is particularly important for comparison of Fos-LI in our constantly stimulated groups (i.e., Deprived Constant and Nondeprived Constant). Therefore, we also quantified Fos-LI within each nTS level (i.e., r1, r2, … etc.) in the constantly stimulated groups and the bottle stimulated groups. Moreover, the total number of Fos-LI does not provide the resolution to determine whether constant MSG intraoral cannula stimulation (e.g., Deprived Constant group) produced a similar pattern of c-Fos expression to that observed when animals consume MSG via a drinking bottle (Bottle group). Thus, we measured Fos-LI within each nTS subfield (dorsal-medial, ventral-medial, etc.) in the Deprived Constant and Bottle experimental groups.
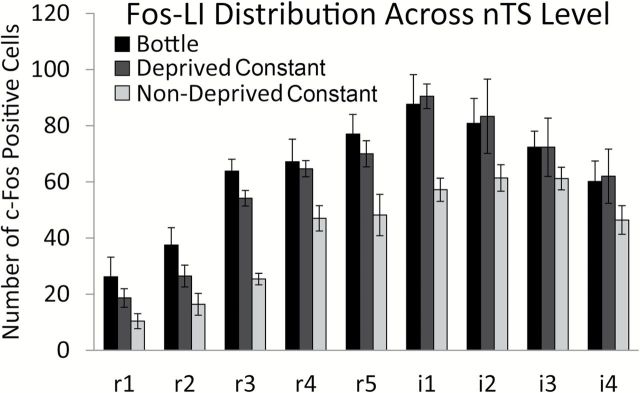
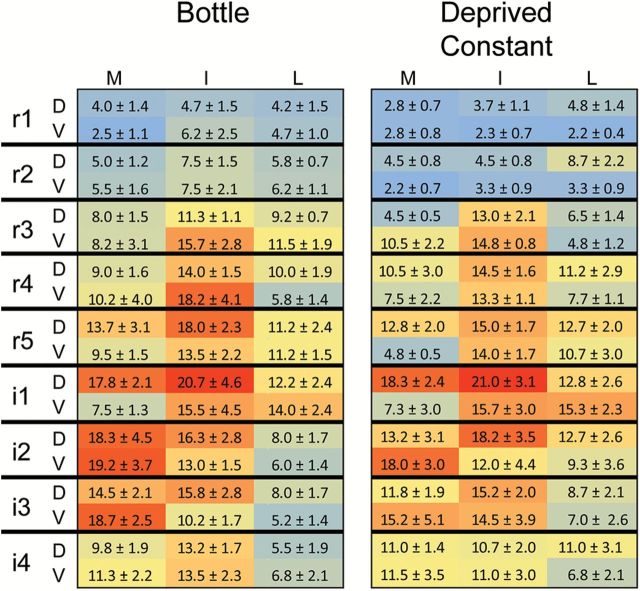
Fos-LI in the Deprived Constant group was greater than the Fos-LI in the Nondeprived Constant group [F(1, 9) = 28.15, P < 0.001], suggesting that water restriction increases MSG-evoked Fos-LI throughout the nTS. Moreover, across the 9 nTS levels, the number of c-Fos positive cells was comparable between Deprived Constant and Bottle groups [Figure 4; F(8, 80) = 0.38, P = 0.93]. Furthermore, the topography of Fos-LI within each nTS subfield (dorsal-ventral, medial-lateral) of Deprived Constant mice resembled that found in the nTS of Bottle animals [Figure 5; F(8, 40) = 1.34, P = 0.40]. Together, these results suggest that constant infusion of MSG into intraoral cannulae of water-restricted mice results in not only a similar total number of c-Fos positive cells as does bottle stimulation (Figure 3), but also evokes a comparable pattern of Fos-LI across the entire nTS (Figures 4 and 5).
Figure 4.
Water restriction increases Fos-LI in the nTS and Fos-LI is similar between bottle and water-restricted, constantly stimulated animals across all nTS levels. Nonwater restricted, constantly stimulated animals (light grey) had significantly less Fos-LI than water restricted, constantly stimulated animals (dark grey; P < 0.001). Moreover, Fos-LI was similar between Bottle (black) and Deprived Constant (dark grey) groups across all nTS levels (P = 0.93).
Figure 5.
Density plots of the nTS show that MSG-evoked Fos-LI is similar between Bottle (left) and Deprived Constant (right) groups. Within each chart, each 3×2 box represents 1 level of the nTS subdivided into the component subfields: medial-mid-lateral in dorsal and ventral tiers. Numbers shown are the mean number of c-Fos positive cells for each subfield ± SD. Each density map is color coded (blue = minimal; red = maximal) with red denoting the maximum number of c-Fos positive cells across both groups. D, dorsal; V, ventral; M, mid, I, intermediate; L, lateral. There was no difference in Fos-LI between the groups (P = 0.40).
Discussion
c-Fos is commonly used to visualize spatial patterns of neuronal activity in response to taste stimulation. However, because animals naturally avoid consuming unpalatable tastes, intraoral cannulae are used to deliver aversive taste stimuli to experimental animals; whereas, appetitive stimuli are commonly given to animals in drinking bottles. Beyond the obvious volitional differences between the 2 paradigms (i.e., voluntary vs. involuntary), there are 2 critical disparities between taste exposure from a drinking bottle versus taste consumption from intraoral cannulae that relate to the physiology and ethology of rodent drinking behavior. First, mice and rats naturally drink fluids from bottles in brief, intermittent “bouts,” but taste stimuli are often infused into intraoral cannulae at a constant, sustained rate. Second, in order to encourage experimental animals to drink from bottles, water access is restricted (e.g., 23h/day). Therefore, methodological differences between these 2 methods may prevent direct comparison between studies.
In this regard, the only research to ever compare c-Fos activity between bottle stimulation and intraoral cannula stimulation did so in the context of conditioned taste aversions. These 2 previous studies compared the strength of a conditioned taste aversion, and associated c-Fos activation, when a conditioning stimulus was given in a drinking bottle versus via intraoral cannula (Spray et al. 2000; Wilkins and Bernstein 2006). Both studies reported that the strength of a conditioned taste aversion was not affected by stimulus delivery method, but the 2 studies found opposing results regarding conditioned stimulus-induced c-Fos activity. Spray et al. (2000) reported an increase, whereas Wilkins and Bernstein (2006) showed a decrease, in c-Fos activity in the nTS when a stimulus was infused via intraoral cannula versus consumed from a bottle. Although the cause of this disparity remains unknown, both results suggest that the method in which tastants are given to an animal may influence c-Fos activity independent from the taste stimulation itself. However, whether taste-induced c-Fos activity is affected by fluid delivery method remains unexplored.
To address this issue, the current study compared (Fos-LI) in the nTS across various intraoral cannulae fluid delivery methods (constant vs. intermittent, “bout”) and physiological states (water-restricted vs. water-replete) to Fos-LI observed when animals drink stimuli from a bottle.
Fos-LI and stimulation pattern
Fos-LI varied significantly between stimulation patterns. In water-restricted groups (i.e., “Deprived Constant,” “Deprived Intermit,” “Deprived Intermit 2X”, and “Bottle”), the total number of c-Fos positive cells was significantly less in the nTS of mice that were stimulated intermittently (Deprived Intermit and Deprived Intermit 2X) as compared with those that received constant taste stimulation (Deprived Constant) or consumed MSG from a drinking bottle (Bottle; Figure 3). In fact, the total number of c-Fos positive cells in the nTS of the Deprived Constant group most closely matched that measured in bottle-stimulated animals (Bottle; Figure 3). Even more importantly, the Deprived Constant group also produced a similar amount (Figure 4) and spatial pattern (Figure 5) of Fos-LI to that of the Bottle group throughout the entire rostral-intermediate nTS, suggesting that this effect is not limited to one level or subfield of the nTS, and further strengthens our conclusions.
Our results are somewhat unexpected as an intermittent, “bout”-like pattern of stimulation may simulate the temporal conditions experienced when an animal drinks fluids from a bottle. However, intermittent taste stimulation may not evoke the same Fos-LI as bottle stimulation does for several reasons. One concern with an intermittent stimulation protocol is that animals consume half the amount of fluid as compared with constant taste stimulation. To address this idea, we infused MSG for twice as long (i.e., volume matched to other experimental groups) in a second group of intermittently stimulated animals (Deprived Intermit 2X). We found that the total volume of fluid instilled into the oral cavity does not affect Fos-LI as the total number of c-Fos positive cells was similar between animals that received the same intermittent taste stimulation with a duration that was twice as long (2min on, 1 off; 3mL total) and our Deprived Intermit (1min on, 1 off; 1.5mL total) group (see Materials and methods). This suggests that the amount of fluid consumed does not significantly impact Fos-LI (although we did observe a reduction in variability between the Deprived Intermit 2X and Deprived Intermit groups; Figures 2 and 3).
Second, electromyography recordings of the digastric muscle in the jaw and behavioral observations find that animals lick and swallow naturally during intraoral infusions—even when fluids are infused at a constant rate (Kaplan and Grill 1989; Kaplan et al. 1995). Thus, the intermittent stimulation pattern we used may interfere with an animal’s ability to naturally lick and swallow fluids (although intermittently stimulated animals were able to consume all infused fluids during the stimulation session; see Materials and methods). Moreover, although animals naturally consume fluids in “bouts,” these short bursts of drinking are sporadic; whereas taste stimuli were infused at regular, tonic intervals (i.e., 1min on; 1min off) in this study.
Finally, c-Fos activity is dependent upon the novelty of a stimulus, as Fos-LI decreases with repeated exposure to the same stimulus (Montag-Sallaz et al. 1999; Amin et al. 2006; Lin et al. 2012). Yet, it is unlikely that decreased novelty can account for the decreased Fos-LI observed in both intermittent groups (Deprived Intermit and Deprived Intermit 2X) as the kinetics of c-Fos activity are much longer than our stimulation protocol (i.e., hours vs. minutes). Furthermore, the effects of novelty on c-Fos activity occur after hours of prolonged stimulation (i.e., 4.5h to days); whereas our total stimulation protocol lasts between 30 and 45min. In this regard, one concern is that, in order to infuse the same amount of fluid as other experimental groups, animals in our Deprived Intermit 2X group are stimulated 15min longer than other groups (i.e., 45min vs. 30min; 1h 45min vs. 1h 30min stimulation/perfusion). Yet, this longer stimulation/perfusion timeframe is still well within the 1–2h post-stimulation window for peak c-Fos protein activity (Zangenehpour and Chaudhuri 2002), providing further evidence to suggest that an intermittent stimulation procedure may not evoke a similar pattern and number of c-Fos positive cells as does bottle stimulation.
Fos-LI and hydration state
Animals are often water-restricted in order to measure behavioral taste responses. Although numerous studies report that food restriction enhances the acquisition of various conditioned behaviors, including conditioned taste aversions and conditioned flavor preferences, few studies have explored whether dehydration (i.e., water-restriction) affects taste preferences (Cohen and Hachey 1980; Drucker et al. 1994; Eckel and Ossenkopp 1995; Grill et al. 1996; Grigson et al. 1999; Berridge 2000). In addition, whether gustatory-induced brain activity changes during water restriction is unknown.
We found that overnight (23h) water restriction increased taste-evoked Fos-LI in the rostral and intermediate nTS (Figures 3 and 4). Although our results are in line with previous research that report increased Fos-LI in the nTS following prolonged water restriction alone (Ji et al. 2007; Gottlieb et al. 2011), our study has 3 primary advantages over these other studies. First, our study is the first to compare brain activation in response to taste stimulation, as measured by Fos-LI, between water-restricted and water-replete animals. Second, Ji et al. (2007) only quantified Fos-LI in extremely caudal, enteric nTS, which does not receive primary gustatory information. Third, the length of water restriction animals underwent in our study is much shorter than the duration used in these previous studies (i.e., 23h vs. 48h). Thus, water restriction may increase overall brainstem activity, in addition to increasing taste-specific brain responses.
Applications and conclusions
Despite good concordance between our Deprived Constant and Bottle groups, several additional parameters were beyond the scope of the present study. First, we focused on Fos-LI evoked by only one appetitive taste stimulus, which is important for our past and current studies (e.g., MSG; Stratford and Finger 2011). Although it would be informative to compare Fos-LI evoked by an aversive stimulus (e.g., Denatonium), it is challenging to prompt animals to consume aversive tastes in a short period from a drinking bottle without prolonged water restriction (St John and Spector 1998). Thus, whether our stimulation parameters apply to other taste stimuli is unknown, though no evidence suggests that individual taste qualities require different stimulation parameters.
Second, because our goal was to determine which intraoral experimental conditions produced Fos-LI similar to that observed when animals consumed fluids from a drinking bottle, we quantified all Fos-LI in awake, freely behaving animals. Yet, some previous studies measured Fos-LI in urethane-anesthetized animals, and report a much larger overall number of c-Fos positive cells in response to taste stimulation (i.e., >4000 vs. ~800; Kwak et al. 2011, 2015). Therefore, our optimal experimental parameters may not be appropriate for anesthetized preparations.
Third, we utilized fluorescent immunocytochemistry to visualize and quantify Fos-LI, but c-Fos activity is also commonly visualized using an avidin-biotin DAB reaction (Harrer and Travers 1996; Houpt et al. 1996; King et al. 1999, 2000, 2003, 2014, 2015; Spray et al. 2000; Travers 2002; Chan et al. 2004; Amin et al. 2006; Wilkins and Bernstein 2006; Ji et al. 2007; Travers and Travers 2007; Travers et al. 2007; Haino et al. 2010; Chen et al. 2011; Kwak et al. 2011, 2015). To address this idea, we compared MSG- evoked Fos-LI in the nTS visualized with either fluorescent immunocytochemistry or by an avidin-biotin DAB reaction between animals stimulated via bottle and undeprived animals stimulated via intraoral cannula (with the same 3mL volume of MSG infused as in the current study). Our preliminary results found that, although the total number of c-Fos positive cells was less when quantified using fluorescent immunocytochemistry versus DAB (fluorescent ~600–400 vs. DAB ~1400–800), the overall pattern of Fos-LI was similar between the 2 techniques (i.e., bottle stimulation > nondeprived cannula stimulation; unpublished observation). It is interesting to note that the lower number of fluorescent c-Fos positive cells may reflect an overall decreased sensitivity of this technique, which may have a smaller “working range” for quantification as compared with the avidin DAB procedure.
Together, our results suggest that both physiological state and fluid delivery pattern produce changes in taste-evoked brain activity. Moreover, in order to produce a similar number and spatial pattern of Fos-LI, in the nTS, as that produced when animals drink taste stimuli from bottles, cannulated animals should be water restricted overnight (23h) prior to stimulation, and receive taste stimuli at a constant flow rate.
Funding
This work was supported by an NIH grant [5F32DC012025-03 to J.M.S.] with additional support from Dr Thomas E. Finger [5R01DC012931-03], and the Rocky Mountain Taste and Smell Center [P30 DC04657].
Acknowledgments
We thank Dr Thomas E. Finger for his generous advice and comments. In addition, special thanks to Jason Parnes and Nicole Shultz for immunohistochemistry technical support.
References
- Amin E, Pearce JM, Brown MW, Aggleton JP. 2006. Novel temporal configurations of stimuli produce discrete changes in immediate-early gene expression in the rat hippocampus. Eur J Neurosci. 24(9):2611–2621. [DOI] [PubMed] [Google Scholar]
- Archer S, Li TT, Evans AT, Britland ST, Morgan H. 1999. Cell reactions to dielectrophoretic manipulation. Biochem Biophys Res Commun. 257(3):687–698. [DOI] [PubMed] [Google Scholar]
- Barkley-Levenson AM, Crabbe JC. 2012. Ethanol drinking microstructure of a high drinking in the dark selected mouse line. Alcohol Clin Exp Res. 36(8):1330–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. 2000. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 24(2):173–198. [DOI] [PubMed] [Google Scholar]
- Biondolillo JW, Williams LA, King MS. 2009. Blocking glutamate receptors in the waist area of the parabrachial nucleus decreases taste reactivity behaviors in conscious rats. Chem Senses. 34(3):221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughter JD, Jr, Baird JP, Bryant J, St John SJ, Heck D. 2007. C57BL/6J and DBA/2J mice vary in lick rate and ingestive microstructure. Genes Brain Behav. 6(7):619–627. [DOI] [PubMed] [Google Scholar]
- Chan CY, Yoo JE, Travers SP. 2004. Diverse bitter stimuli elicit highly similar patterns of Fos-like immunoreactivity in the nucleus of the solitary tract. Chem Senses. 29(7):573–581. [DOI] [PubMed] [Google Scholar]
- Chen K, Yan J, Li J, Lv B, Zhao X. 2011. c-Fos expression in rat brainstem following intake of sucrose or saccharin. Front Med. 5(3):294–301. [DOI] [PubMed] [Google Scholar]
- Cohen JS, Hachey GJ. 1980. Development of sucrose preferences under two levels of water deprivation. Psychol Rep. 46(3 Pt 1):820–822. [DOI] [PubMed] [Google Scholar]
- Drucker DB, Ackroff K, Sclafani A. 1994. Nutrient-conditioned flavor preference and acceptance in rats: effects of deprivation state and nonreinforcement. Physiol Behav. 56(4):701–707. [DOI] [PubMed] [Google Scholar]
- Eckel LA, Ossenkopp KP. 1995. Cholecystokinin reduces ingestive taste reactivity responses to water in fluid-replete but not fluid-deprived rats. Physiol Behav. 57(3):599–603. [DOI] [PubMed] [Google Scholar]
- Gannon KS, Smith JC, Henderson R, Hendrick P. 1992. A system for studying the microstructure of ingestive behavior in mice. Physiol Behav. 51(3):515–521. [DOI] [PubMed] [Google Scholar]
- Gottlieb HB, Ji LL, Cunningham JT. 2011. Role of superior laryngeal nerve and Fos staining following dehydration and rehydration in the rat. Physiol Behav. 104(5):1053–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigson PS, Lyuboslavsky PN, Tanase D, Wheeler RA. 1999. Water-deprivation prevents morphine-, but not LiCl-induced, suppression of sucrose intake. Physiol Behav. 67(2):277–286. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. 1978. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 143(2):263–279. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Roitman MF, Kaplan JM. 1996. A new taste reactivity analysis of the integration of taste and physiological state information. Am J Physiol. 271(3 Pt 2):R677–R687. [DOI] [PubMed] [Google Scholar]
- Guthrie KM, Anderson AJ, Leon M, Gall C. 1993. Odor-induced increases in c-Fos mRNA expression reveal an anatomical “unit” for odor processing in olfactory bulb. Proc Natl Acad Sci U S A. 90(8):3329–3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haino T, Hironaka S, Ooka T, Tokita K, Kubota Y, Boughter JD, Jr, Inoue T, Mukai Y. 2010. Orosensory deprivation alters taste-elicited c-Fos expression in the parabrachial nucleus of neonatal rats. Neurosci Res. 67(3):228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrer MI, Travers SP. 1996. Topographic organization of Fos-like immunoreactivity in the rostral nucleus of the solitary tract evoked by gustatory stimulation with sucrose and quinine. Brain Res. 711(1–2):125–137. [DOI] [PubMed] [Google Scholar]
- Houpt TA, Philopena JM, Joh TH, Smith GP. 1996. c-Fos induction in the rat nucleus of the solitary tract by intraoral quinine infusion depends on prior contingent pairing of quinine and lithium chloride. Physiol Behav. 60(6):1535–1541. [DOI] [PubMed] [Google Scholar]
- Jarrett MM, Scantlebury J, Parker LA. 2007. Effect of delta9-tetrahydrocannabinol on quinine palatability and AM251 on sucrose and quinine palatability using the taste reactivity test. Physiol Behav. 90(2–3):425–430. [DOI] [PubMed] [Google Scholar]
- Ji LL, Gottlieb HB, Penny ML, Fleming T, Toney GM, Cunningham JT. 2007. Differential effects of water deprivation and rehydration on Fos and FosB/DeltaFosB staining in the rat brainstem. Exp Neurol. 203(2):445–456. [DOI] [PubMed] [Google Scholar]
- Johnson AW, Sherwood A, Smith DR, Wosiski-Kuhn M, Gallagher M, Holland PC. 2010. An analysis of licking microstructure in three strains of mice. Appetite. 54(2):320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JM, Grill HJ. 1989. Swallowing during ongoing fluid ingestion in the rat. Brain Res 499(1): 63–80. [DOI] [PubMed] [Google Scholar]
- Kaplan JM, Roitman MF, Grill HJ. 1995. Ingestive taste reactivity as licking behavior. Neurosci Biobehav Rev 19(1): 89–98. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Hill KG, Kaczmarek HJ. 1998. Taste reactivity to alcohol and basic tastes in outbred mice. Alcohol Clin Exp Res. 22(5):1146–1151. [PubMed] [Google Scholar]
- King CT, Deyrup LD, Dodson SE, Galvin KE, Garcea M, Spector AC. 2003. Effects of gustatory nerve transection and regeneration on quinine-stimulated Fos-like immunoreactivity in the parabrachial nucleus of the rat. J Comp Neurol. 465(2):296–308. [DOI] [PubMed] [Google Scholar]
- King CT, Garcea M, Spector AC. 2000. Glossopharyngeal nerve regeneration is essential for the complete recovery of quinine-stimulated oromotor rejection behaviors and central patterns of neuronal activity in the nucleus of the solitary tract in the rat. J Neurosci. 20(22):8426–8434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CT, Garcea M, Spector AC. 2014. Restoration of quinine-stimulated Fos-immunoreactive neurons in the central nucleus of the amygdala and gustatory cortex following reinnervation or cross-reinnervation of the lingual taste nerves in rats. J Comp Neurol. 522(11):2498–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CT, Hashimoto K, Blonde GD, Spector AC. 2015. Unconditioned oromotor taste reactivity elicited by sucrose and quinine is unaffected by extensive bilateral damage to the gustatory zone of the insular cortex in rats. Brain Res. 1599:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CT, Travers SP, Rowland NE, Garcea M, Spector AC. 1999. Glossopharyngeal nerve transection eliminates quinine-stimulated fos-like immunoreactivity in the nucleus of the solitary tract: implications for a functional topography of gustatory nerve input in rats. J Neurosci. 19(8):3107–3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak Y, Han J, Rhyu MR, Nam TS, Leem JW, Lee BH. 2015. Different spatial expressions of c-Fos in the nucleus of the solitary tract following taste stimulation with sodium, potassium, and ammonium ions in rats. J Neurosci Res. 93(2):340–349. [DOI] [PubMed] [Google Scholar]
- Kwak Y, Rhyu MR, Bai SJ, Sa YH, Kwon MJ, Lee BH. 2011. c-Fos expression in the nucleus of the solitary Tract in response to salt stimulation in rats. Korean J Physiol Pharmacol. 15(6):437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Roman C, Arthurs J, Reilly S. 2012. Taste neophobia and c-Fos expression in the rat brain. Brain Res. 1448:82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag-Sallaz M, Welzl H, Kuhl D, Montag D, Schachner M. 1999. Novelty-induced increased expression of immediate-early genes c-Fos and arg 3.1 in the mouse brain. J Neurobiol. 38(2):234–246. [PubMed] [Google Scholar]
- Murakami H. 1977. Rhythmometry on licking rate of the mouse. Physiol Behav. 19(6):735–738. [DOI] [PubMed] [Google Scholar]
- Nagy B, Takacs G, Szabo I, Lenard L, Karadi Z. 2012. Taste reactivity alterations after streptozotocin microinjection into the mediodorsal prefrontal cortex. Behav Brain Res. 234(2):228–232. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KB. 2001. The mouse brain in stereotaxic coordinates. 2nd ed San Diego (CA): Academic Press. [Google Scholar]
- Riley CA, King MS. 2013. Differential effects of electrical stimulation of the central amygdala and lateral hypothalamus on fos-immunoreactive neurons in the gustatory brainstem and taste reactivity behaviors in conscious rats. Chem Senses. 38(8):705–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray KJ, Halsell CB, Bernstein IL. 2000. c-Fos induction in response to saccharin after taste aversion learning depends on conditioning method. Brain Res. 852(1):225–227. [DOI] [PubMed] [Google Scholar]
- St John SJ, Spector AC. 1998. Behavioral discrimination between quinine and KCl is dependent on input from the seventh cranial nerve: implications for the functional roles of the gustatory nerves in rats. J Neurosci. 18(11):4353–4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellar E, Hill JH. 1952. The rats rate of drinking as a function of water deprivation. J Comp Physiol Psychol. 45(1):96–102. [DOI] [PubMed] [Google Scholar]
- Stratford JM, Finger TE. 2011. Central representation of postingestive chemosensory cues in mice that lack the ability to taste. J Neurosci. 31(25):9101–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford JM, Thompson JA. 2014. Beta-galactosidase staining in the nucleus of the solitary tract of Fos-Tau-LacZ mice is unaffected by monosodium glutamate taste stimulation. PLoS One. 9(9):e107238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian JB, Bishop GA. 2002. Stimulus-dependent activation of c-Fos in neurons and glia in the rat cerebellum. J Chem Neuroanat. 23(3):157–170. [DOI] [PubMed] [Google Scholar]
- Tokita K, Armstrong WE, St John SJ, Boughter JD., Jr 2014. Activation of lateral hypothalamus-projecting parabrachial neurons by intraorally delivered gustatory stimuli. Front Neural Circuits. 8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers JB, Herman K, Yoo J, Travers SP. 2007. Taste reactivity and Fos expression in GAD1-EGFP transgenic mice. Chem Senses. 32(2):129–137. [DOI] [PubMed] [Google Scholar]
- Travers SP. 2002. Quinine and citric acid elicit distinctive Fos-like immunoreactivity in the rat nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol. 282(6):R1798–R1810. [DOI] [PubMed] [Google Scholar]
- Travers SP, Travers JB. 2007. Taste-evoked Fos expression in nitrergic neurons in the nucleus of the solitary tract and reticular formation of the rat. J Comp Neurol. 500(4):746–760. [DOI] [PubMed] [Google Scholar]
- Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. 1986. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 7(1):155–159. [DOI] [PubMed] [Google Scholar]
- Wilkins EE, Bernstein IL. 2006. Conditioning method determines patterns of c-Fos expression following novel taste-illness pairing. Behav Brain Res. 169(1):93–97. [DOI] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. 2009. Hedonic sensitivity in adolescent and adult rats: taste reactivity and voluntary sucrose consumption. Pharmacol Biochem Behav. 92(4):566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangenehpour S, Chaudhuri A. 2002. Differential induction and decay curves of c-Fos and zif268 revealed through dual activity maps. Brain Res Mol Brain Res. 109(1–2):221–225. [DOI] [PubMed] [Google Scholar]
- Zhao XL, Yan JQ, Yang XJ, Chen K, Li JR, Zhang Y. 2012. Fos positive neurons in the brain stem and amygdala mostly express vesicular glutamate transporter 3 after bitter taste stimulation. Brain Res. 1445:20–29. [DOI] [PubMed] [Google Scholar]