Abstract
Pulmonary tumor thrombotic microangiopathy (PTTM) is a fatal malignancy-related condition that involves rapidly progressing hypoxia and pulmonary hypertension. We report a case of PTTM caused by prostate carcinoma, which was diagnosed before autopsy in an 81-year-old man. Computed tomography showed diffuse ground-glass opacities, consolidation, and small nodules in the peripheral regions of the lung. Autopsy showed adenocarcinoma cells embolizing small pulmonary arteries with fibrocellular intimal proliferation, which was consistent with PTTM caused by prostate carcinoma.
Keywords: Prostate, adenocarcinoma, autopsy, lung – pathology, hypertension, pulmonary – etiology, thrombotic microangiopathies – complications
Introduction
Pulmonary tumor thrombotic microangiopathy (PTTM), which was first reported by von Herbay et al. in 1990 (1), is a rare but fatal complication of malignancy that presents as rapidly progressing hypoxia and pulmonary hypertension. It is pathologically characterized by intimal fibrocellular proliferation of the pulmonary arteries and consequent vascular stenosis caused by small tumor emboli, which may not always occlude the pulmonary arteries (1,2). Computed tomography (CT) findings of PTTM are non-specific and a definitive diagnosis requires lung biopsy; therefore, antemortem diagnosis is difficult in critical ill patients. The most common cancer associated with PTTM is gastric cancer, followed by lung cancer (1,3). Here, we report a case of PTTM caused by prostate adenocarcinoma, which is a rare origin (4).
Case report
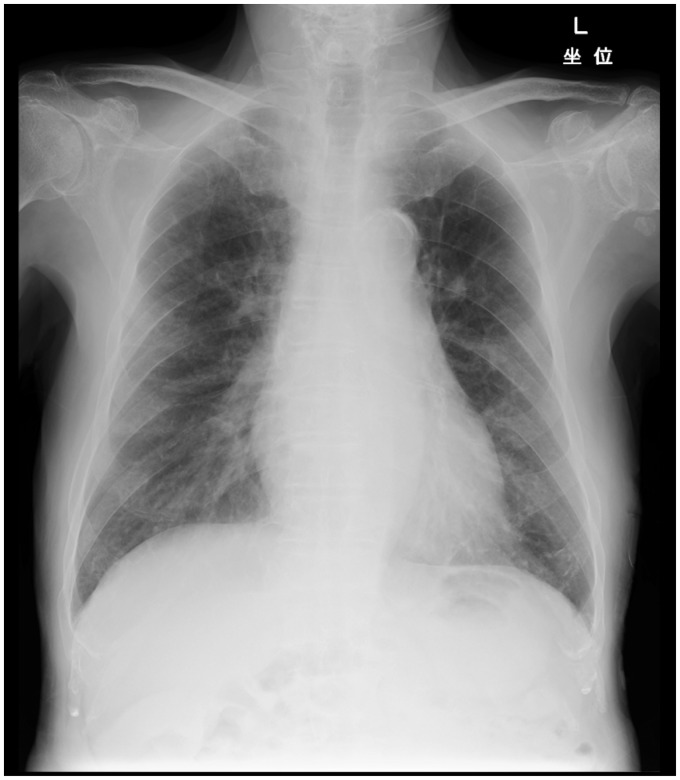
An 81-year-old man (non-smoker) presented with a 3-day history of progressive dyspnea on exertion and palpitation before admission. He had advanced prostate carcinoma and had received androgen-deprivation therapy. Further, he had undergone percutaneous coronary intervention 6 years previously. Prostatic biopsy examination indicated an adenocarcinoma with a Gleason score of 9 (5 + 4). Magnetic resonance imaging (MRI) of the pelvis, abdominal CT, and bone scans showed tumor invasion of the bladder and multiple bone metastases. The clinical stage was c-T4N0M1. On examination, the patient was afebrile but had tachycardia (98 bpm) and tachypnea (28 breaths/min). Oxygen saturation had decreased to 88% in room air. Electrocardiography showed normal findings. Laboratory tests showed elevated levels of prostate-specific antigen (25.24 ng/mL; normal, <4.0 ng/mL), compared to the level noted 1 month previously (3.62 ng/mL). Chest radiograph showed bilateral oligemia in the peripheral lungs without cardiomegaly or pleural effusion (Fig. 1).
Fig. 1.
Chest radiograph showing bilateral oligemia in the peripheral lung field without cardiomegaly or pleural effusion.
Transthoracic echocardiography showed that the ejection fraction was sufficient, but the tricuspid regurgitation pressure gradient (TR-PG) had increased to 53 mmHg. The diameter of the inferior vena cava was slightly larger than normal, with reduced respiratory variation. Pulmonary hypertension caused by left ventricular failure was suspected. After admission, the patient started treatment with diuretic agents for acute left heart failure. Although the urine volume was adequate, echocardiography showed an increased TR-PG, right ventricular enlargement and flattening of the ventricular septum. This aggravation of pulmonary hypertension despite diuresis indicated pulmonary thromboembolism rather than left heart failure.
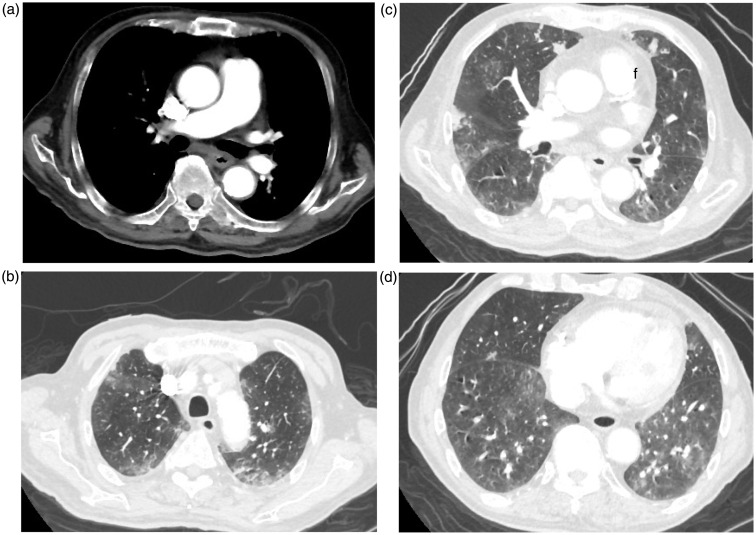
A CT scan was performed to identify filling defects in the pulmonary arteries. Contrast-enhanced CT showed that the diameter of the main pulmonary artery had increased to 33 mm, but no filling defects were found in the central pulmonary arteries (Fig. 2a). Diffuse ground glass opacities (GGO) and small nodular opacities were seen predominantly in the periphery in the lung window setting (Fig 2b–d). Since in cancer patients rapidly progressing hypoxia and pulmonary hypertension without pulmonary thromboembolism are indicative of PTTM, this condition was clinically diagnosed. On the 6th day after admission, the patient died from hypoxia. Autopsy was performed 23 h after death.
Fig. 2.
(a) Contrast-enhanced CT showing an increase in the diameter of the main pulmonary artery to 33 mm without filling defects. (b–d) Thin-section CT in the lung window setting showing diffuse GGO predominantly in the peripheral lung regions. Focal consolidation and nodular opacities are also seen in the periphery.
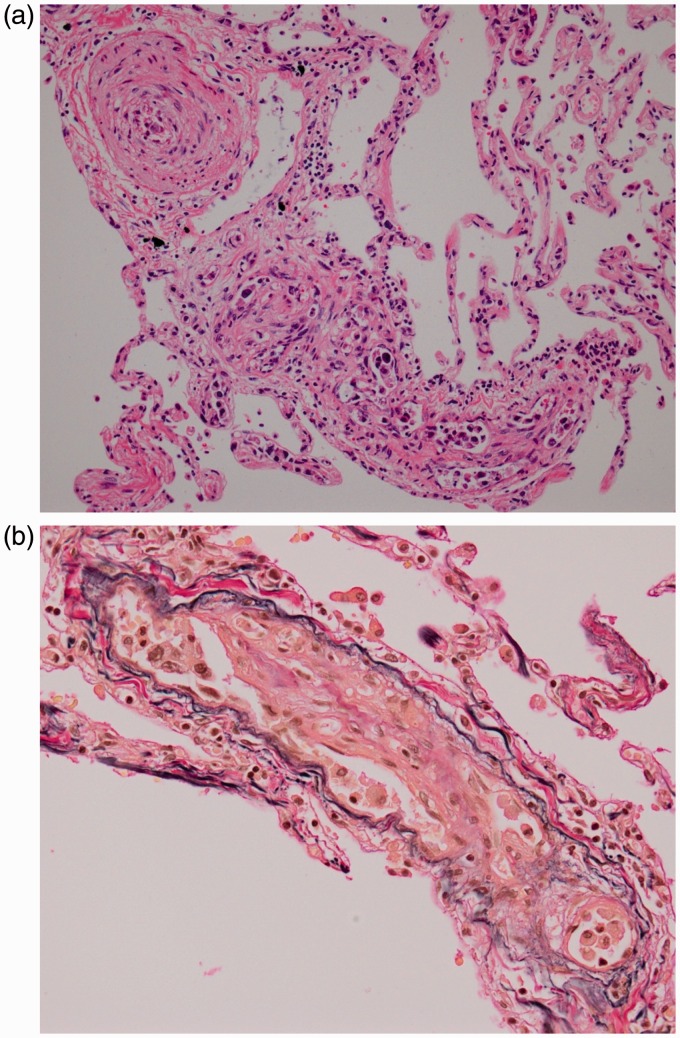
Hematoxylin & eosin (H&E) staining of the autopsied lung showed adenocarcinoma cells embolizing small pulmonary arteries with fibrocellular intimal proliferation. No hemorrhage or infiltration of inflammatory cells was noted in the alveoli (Fig. 3a). Elastica van Gieson staining showed apparent fibrous thickening of endothelial cells on the elastic membrane (Fig. 3b). These findings are pathognomonic for PTTM.
Fig. 3.
(a) H&E staining of the lung showing adenocarcinoma cells embolizing small pulmonary arteries with fibrocellular intimal proliferation. No hemorrhage or infiltration of inflammatory cells can be seen. (b) Elastica van Gieson staining showing fibrous thickening and fibrocellular intimal proliferation of endothelial cells on the internal elastic membrane.
Discussion
PTTM was first defined by von Herbay et al. in 1990 (1). Patients with PTTM present progressive cough, dyspnea, and hypoxia, and clinical examination shows progressive pulmonary hypertension. These patients usually die from respiratory failure within a few days to weeks of disease manifestation.
In PTTM, metastatic tumor cells occlude small pulmonary arteries and induce microthrombosis and fibrocellular intimal proliferation by activating a coagulation cascade and inflammatory mediators such as vascular endothelial growth factor or tissue factors. Diffuse narrowing and occlusion of the small pulmonary arteries lead to elevation of vascular resistance, resulting in secondary pulmonary hypertension (1,2,5).
The most frequent causative malignancy for PTTM is gastric cancer, and the histological tumor type in cases of PTTM is usually adenocarcinoma (1,3). Other less common causes include lung, breast, esophageal, gallbladder, colon, pancreatic, liver, ureteral, urinary bladder, and ovarian cancer (1,3,6–8). To our knowledge, only two cases of PTTM caused by prostate cancer have been reported thus far (1,4).
CT findings of PTTM are non-specific, including consolidation, diffuse GGO, small nodules, and a tree-in-bud appearance in the lung window settings (3). The tree-in-bud appearance usually suggests infectious bronchiolitis and is also seen in patients with PTTM caused by tumor embolism or fibrocellular intimal proliferation in the small arteries or arterioles (9). Interlobular septal thickening, centrilobular ground glass opacities, reticular nodular opacities, and cavitation are also seen in PTTM (10–12). Dilatation of the main pulmonary artery suggests secondary pulmonary hypertension. In the present case, we observed diffuse GGO, patchy consolidation, and nodular opacities. Autopsy showed no hemorrhage or infiltration of inflammatory cells in the alveoli. We believe that the GGO in the present case might represent diffuse alveolar damage without hemorrhage caused by inflammatory mediators.
Several nuclear medicine reports have indicated that in PTTM, perfusion lung scanning shows multiple small perfusion defects in both lung fields (13), and that in 18F-fluorodeoxyglucose positron emission tomography examination of PTTM shows multiple abnormal concentrations of the dye (14). Lung biopsy is essential for definitive diagnosis, but it is difficult to perform in critically ill patients.
In conclusion, various types of tumors, including prostate cancer, can cause PTTM; therefore, PTTM should be included in the differential diagnosis of rapidly progressing hypoxia and pulmonary hypertension in cancer patients.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.von Herbay A, Illes A, Waldherr R, et al. Pulmonary tumor thrombotic microangiopathy with pulmonary hypertension. Cancer 1990; 66: 587–592. [DOI] [PubMed] [Google Scholar]
- 2.Chinen K, Tokuda Y, Fujiwara M, et al. Pulmonary tumor thrombotic microangiopathy in patients with gastric carcinoma: an analysis of 6 autopsy cases and review of the literature. Pathol Res Pract 2010; 206: 682–689. [DOI] [PubMed] [Google Scholar]
- 3.Uruga H, Fujii T, Kurosaki A, et al. Pulmonary tumor thrombotic microangiopathy: a clinical analysis of 30 autopsy cases. Intern Med 2013; 52: 1317–1323. [DOI] [PubMed] [Google Scholar]
- 4.Nayyar D, Muthiah K, Hayward CS, et al. Pulmonary tumor thrombotic microangiopathy from metastatic prostate carcinoma. Case Rep Pulmonol 2015; 2015: 286962–286962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinen K, Kazumoto T, Ohkura Y, et al. Pulmonary tumor thrombotic microangiopathy caused by a gastric carcinoma expressing vascular endothelial growth factor and tissue factor. Pathol Int 2005; 55: 27–31. [DOI] [PubMed] [Google Scholar]
- 6.Ohno Y, Yamanishi H, Matsuura B, et al. Signet-ring cell carcinoma of the gallbladder complicated by pulmonary tumor thrombotic microangiopathy. Intern Med 2014; 53: 1125–1129. [DOI] [PubMed] [Google Scholar]
- 7.Marumo S, Sakaguchi M, Teranishi T, et al. Pulmonary tumor thrombotic microangiopathy induced by ureteral carcinoma: a necropsy case report. Case Rep Oncol 2014; 7: 605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chinen K, Fujino T, Horita A, et al. Pulmonary tumor thrombotic microangiopathy caused by an ovarian cancer expressing tissue factor and vascular endothelial growth factor. Pathol Res Pract 2009; 205: 63–68. [DOI] [PubMed] [Google Scholar]
- 9.Franquet T, Gimenez A, Prats R, et al. Thrombotic microangiopathy of tumors: a vascular cause of tree-in-bud pattern on CT. Am J Roentgenol 2002; 179: 897–899. [DOI] [PubMed] [Google Scholar]
- 10.Godbole R, Ghatol A, Betancourt J, et al. Pulmonary tumor thrombotic microangiopathy: clinical, radiologic, and histologic correlation. Clin Imaging Sci 2015; 5: 44–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAnearney S, Drain M. A case of pulmonary tumour thrombotic microangiopathy. Respir Med Case Rep 2015; 16: 7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bae K, Kwon WJ, Choi SH, et al. A case report: various caused by pulmonary tumor thrombotic microangiopathy in a patient with pancreatic intraductal papillary mucinous neoplasm. Korean J Radiol 2015; 16: 936–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen WL, Cherng SC, Hwang WS, et al. Perfusion scan in pulmonary tumor microembolism: report of a case. J Formos Med Assoc 1991; 90: 863–866. [PubMed] [Google Scholar]
- 14.Tashima Y, Abe K, Matsuo Y, et al. Pulmonary tumor thrombotic microangiopathy FDG-PET/CT findings. Clin Nucl Med 2009; 34: 175–177. [DOI] [PubMed] [Google Scholar]