Abstract
Introduction
Warfarin is the most commonly used antithrombotic drug. Single nucleotide polymorphisms (SNPs) of CYP2C9, CYP4F2, VKORC1 1173 and VKORC1-1639 influence warfarin maintenance dosage. We aimed to determine the impact of SNPs of these genes on mean daily warfarin dosage (MDWD) in Han-Chinese patients.
Methods
Strict literature inclusion criteria were established, and literature searching was performed on PubMed, Embase and Cochrane Library for English articles and CNKI, CBM and Wanfang database for Chinese articles before September 2, 2014. Revman 5.3 was used to analyze the relationship between gene SNPs and MDWD in Han-Chinese subjects.
Results
We included 33 studies researching the impact of gene SNPs on MDWD in Han-Chinese subjects. CYP2C9 *3/*3, *1/*3 and *3 carriers needed a 72% (95% confidence interval [CI]: 62.0%–81.0%), 28% (22.0%–33.0%) and 26% (21.0%–32.0%) lower MDWD, respectively, than CYP2C9 *1/*1 carriers. CYP4F2 TT, CT and T carriers required a 18% (7.0%–30.0%), 7% (7.0%–7.0%) and 11% (7.0%–14.0%) higher MDWD, respectively, than CYP4F2 CC carriers. VKORC1 1173 CC, CT and C carriers required a 98% (78.0%–118.0%), 49% (37.0%–62.0%) and 56% (44.0%–67.0%) higher MDWD, respectively, than VKORC1 1173 TT carriers. VKORC1-1639 GG, GA and G carriers needed a 101% (53.0%–149.0%), 40% (36.0%–45.0%) and 38% (35.0%–42.0%) higher MDWD, respectively, than VKORC1-1639 AA carriers.
Conclusions
This meta-analysis is the first to report the relationship between genotypes and MDWD among Han-Chinese patients. The results showed that SNPs of CYP2C9, CYP4F2, VKORC1 1173 and VKORC1-1639 significantly influenced the MDWD in Han-Chinese patients.
Abbreviations: INR, International Normalized Ratio; CYP2C9, Cytochrome P450 Complex Subunit 2C9; CYP4F2, Cytochrome P450 Complex Subunit 4F2; VKORC1, Vitamin K Epoxide Reductase Complex Subunit 1; SNPs, Single Nucleotide Polymorphisms; MDWD, Mean Daily Warfarin Dose; CI, Confidence Interval; MD, Mean Difference; SD, Standard Deviation; VTE, Venous Thromboembolism; AF, Atrial Fibrillation; DVT, Deep Vein Thrombosis; AVR, Atrial Valve Replacement; HVR, Heart Valve Replacement; MHVR, Mechanical Heart Valve Replacement; MVR, Mitral Valve Replacement; PE, Pulmonary Embolism; RHD, Rheumatic Heart Disease; NVAF, Non Valvular Atrial Fibrillation
Keywords: Warfarin, Han-Chinese, Meta-analysis, CYP2C9, VKORC1
Highlights
-
•
This meta-analysis examined the effects of genotype on mean daily warfarin dosage.
-
•
CYP2C9, CYP4F2 and VKORC1 genotypes were studied in Han-Chinese patients.
-
•
CYP2C9, CYP4F2, VKORC1-1173 and VKORC1-1639 polymorphisms affected warfarin dosage.
-
•
VKORC1-1173 C and VKORC1-1639 G mutations had similar frequencies and effects.
-
•
Either genotype can be tested for to guide drug usage and lower medical costs.
1. Introduction
Warfarin is the most commonly used oral anticoagulant. Its therapeutic window is rather narrow, and its dose must be adjusted according to the international normalized ratio (INR). A high target INR leads to a high risk of bleeding, while embolism events will occur if the target INR is too low. Many clinical and environmental factors, including age, sex, race, body size, co-morbidities and co-medications, as well as gene mutations affect warfarin dose requirements (Xie et al., 2001, Cavallari et al., 2010, Carlquist et al., 2006, Cheng et al., 2009, Klein et al., 2009, Yoshizawa et al., 2009). Cytochrome P450 2C9 (CYP2C9, rs1057910), cytochrome P450 4F2 (CYP4F2, also knew as V433M, rs2108622) and vitamin K epoxide reductase complex subunit 1 (VKORC1, include VKORC1 1173, rs9934438 and VKORC1-1639 also known as 3673, rs9923231) gene polymorphisms are widely considered to be associated with interindividual variations in warfarin dosage.
CYP2C9, VKORC1 and CYP4F2 gene polymorphisms can explain 40%–60% of the variation in interindividual warfarin doses (Klein et al., 2009, Rieder et al., 2005, Krishna Kumar et al., 2014, Lee et al., 2006, Veenstra et al., 2005, Wadelius et al., 2005), while non-genetic factors such as age and sex are considered to account for < 15% of this variation (Visscher et al., 2009). The US Food and Drug Administration recommends that genotyping be carried out before the prescription of warfarin in order to improve its therapeutic effect (FDA, 2007).
Several meta-analyses (Sanderson et al., 2005, Lindh et al., 2009a, Yang et al., 2010, Jorgensen et al., 2012, Liang et al., 2012a) have explored the impact of CYP2C9, CYP4F2, VKORC1 1173 and VKORC1-1639 gene polymorphisms on mean daily warfarin dosage (MDWD) in Caucasian, African and Asian subjects. As the first meta-analysis of the impact of gene polymorphism warfarin dosage requirement, Sanderson et al. (2005) included 19 studies in their research and found that patients with CYP2C9*2 and CYP2C9*3 alleles need lower MDWD than wild-type homozygotes CYP2C9*1*1. Subsequently, Lindh et al., (2009a) and Jorgensen et al., (2012) also the drew similar conclusions by conducting meta-analysis separately. Yang et al. (2010) found that the impacts of gene polymorphism on warfarin dosage requirement were significantly different between Caucasian and Asian population. Patients with VKORC1 1173 CTand 1173 CC required 44% (95% CI, 32%, 56%) and 97% (95% CI, 73%, 122%) higher MDWD than 1173 TT carriers. VKORC1-1639GA and -1639 GG carriers required 52% (95% CI, 41%, 64%) and 102% (95% CI, 85%, 118%) higher MDWD than -1639AA carriers. Liang et al. (2012a) reported that carriers of CYP4F2 CT, TT genotypes required 10.0% (95% CI, 4.0–15.0) and 21.0% (95% CI, 9.0–33.0) higher warfarin doses than homozygous CC respectively. Although genetic associations with warfarin response vary between ethnicities, but most of the previous meta-analyses were conducted by including Caucasian, African and Asian subjects.
However, no such meta-analysis has been conducted of studies involving only Han-Chinese subjects. Such an analysis is required because the size of the Han-Chinese population is up to 1.37 billion (M-S Wen.pdf). We therefore conducted a systematic review and meta-analysis to clarify the relationship between gene polymorphisms and MDWD in the Han-Chinese, and to determine which genotype must be tested for before prescribing warfarin. Our study includes 33 papers published in recent years about CYP2C9, CYP4F2, VKORC1 1173 and VKORC1-1639 gene polymorphisms in the Han-Chinese.
2. Medthods
2.1. Search strategy
We searched the PubMed, Embase, Cochrane Library, Chinese National Knowledge Infrastructure (CNKI), China Biology Medicine (CBM) and Wanfang databases for articles published before September 2, 2014. Print periodicals were also searched. The literature search was limited to studies published in English and Chinese. Studies written in English were searched for on the Cochrane Library, PubMed and Embase databases, and studies written in Chinese were searched for on the CNKI, CBM and Wanfang database or identified through the print periodical search. The keywords were warfarin (MeSH Terms) and Chinese (MeSH Terms) plus any of the following terms: genes (MeSH Terms, mutation (MeSH Terms), polymorphisms (MeSH Terms), Genetic Polymorphism (MeSH Terms), pharmacogenetics (MeSH Terms), CYP2C9 (MeSH Terms), CYP4F2 (MeSH Terms), VKORC1 (MeSH Terms), VKORC1 1173 (Free Terms) and VKORC1-1639 (Free Terms). Corresponding Chinese medical terms were used when we searched on CNKI databases for literatures written in Chinese.
2.2. Study selection
To be included, studies had to meet the following criteria: (1) patients received warfarin treatment, (2) patients were Han-Chinese, (3) at least one of the four target genes was tested and (4) warfarin maintenance dosage was mentioned along with the target gene(s). There were no special limits on INR range, patient characteristics (diseases, age, weight and height) and use of other drugs.
2.3. Data extraction
The research data were extracted and sorted by two reviewers (Chen CM and Chen ZJ) independently. Information such as year of publication, location (province or city), disease types, target INR, gene frequencies (wild type and variant type) and average warfarin maintenance dosage was extracted from the selected studies. Then, the two reviewers checked the integrity and accuracy of the extracted data, and resolved any differences or common points of confusion by discussion. In case of disagreements, other researchers read the literature and decided whether or not to include the study. If there was unclear or missing information in any of the studies, we were to contact the authors via phone or e-mail to obtain additional information.
2.4. Study quality assessment
We applied the checklist recommended by the Cochrane handbook (Julian and Green, 2011) as well as other methods recommended in related literature (Little et al., 2002, Jüni et al., 1999) to assess the quality of the selected papers: (1) study purpose, (2) validity of genetic analysis, including type of study sample, time of sample collection, definition of each genotype and genotyping methods used, (3) subject selection, including geographic area from which the subjects were recruited, subjects' age (mean age and standard deviation or age range), sex ratio, and (4) statistical issues, e.g., number of subjects included and analysis method.
2.5. Statistics
The data extracted from each eligible study were inputted into a computer. Revman 5.3 (Cochrane Collaboration) was used to analyze the relationship between MDWD and gene single nucleotide polymorphisms (SNPs) in the Han-Chinese. Four independent analyses were carried out for the four target genes (CYP2C9, CYP4F2, VKORC1 1173 and VKORC1-1639). Each analysis contained at least three studies. We referred to previously described methods (Lindh et al., 2009a, Liang et al., 2012a) to normalize the maintenance dose by using the homozygous wild-type group as a reference. In each study, the mean dose and standard deviation for each gene type was divided by the dose for the homozygous wild type. For clearer expression, we defined CYP2C9 *1/*3 or *3/*3 patients as “CYP2C9 *3 carriers,” CYP4F2 CT or TT patients as “CYP4F2 T carriers,” VKORC1 1173 CT or CC patients as “VKORC1 1173 C carriers” and VKORC1-1639 GA or GG patients as “VKORC1-1639 G carriers”.
In the analysis of the impact of the CYP2C9 gene, the normalized dose for the genotypes CYP2C9 *1/*3, *3/*3 and *3 were compared to those for the genotype CYP2C9 *1/*1. Furthermore, the data for the CYP2C9 *1/*3 group were compared to that for the CYP2C9 *3/*3 group. Thus, there were four independent analyses for the gene CYP2C9. Similarly, we carried out four analyses for the other genes as well: CYP4F2, CT, TT and T carriers vs. CC carriers and CT vs. TT carriers; VKORC1 1173, CT, CC and C carriers vs. TT carriers and CT vs. CC carriers; and VKORC1-1639, GA, GG and G carriers vs. AA carriers and GA vs. GG carriers. Studies were weighted using the inverse variance method, and the effect of each genotype on MDWD was presented as the mean difference.
Since the data were normalized, the calculated mean difference represented the relative difference rather than the actual difference in maintenance dose. For example, a mean difference of 0.5 indicates a 50% increase in warfarin dose. In each analysis, the sum of the mean differences in every study was equal to the total weighted mean difference. We used the Z test to examine the impact of SNPs on MDWD, and the level of statistical significance was set to P < 0.05. Heterogeneity among studies was tested using Cochran's Q test (Mantel–Haenszel chi-square test), and measured using the variation across studies attributable to heterogeneity rather than due to chance (I2). A P-value ≥ 0.1 or an I2 value ≤ 25% indicated that no or low heterogeneity existed among the studies. In this case, the fixed-effects model was used; otherwise, the random-effects model was selected.
We performed sensitivity analyses by deselecting the studies one by one in chronological order. We also conducted the funnel plots by using Revman 5.3 to check for publication bias. Software STATA 12.0 was used to explore the source of heterogeneity in the analysis results via meta-regression analysis. Software Comprehensive Meta Analysis V2 was used to conduct a cumulative meta analysis of gene CYP 4F2.
3. Results
3.1. Study selection
The literature screening process is illustrated in Fig. 1. Initially, 541 studies were retrieved, of which only 33 eligible studies were included in this systematic review and meta-analysis. Of the eligible studies, 16 were in English (Veenstra et al., 2005, Cen et al., 2010, Chen et al., 2014, Gu et al., 2010, Huang et al., 2009a, Li et al., 2012, Liang et al., 2012b, Liang et al., 2013, Lu et al., 2013, Ma et al., 2012, Miao et al., 2007, Tan et al., 2013, Wei et al., 2012, Yang et al., 2011, Yuan et al., 2005, Zhang et al., 2012a), the another 17 were in Chinese (Cheng et al., 2009, Du et al., 2010, Gao et al., 2010, Huo et al., 2008, Jiang et al., 2007, Li and Sheng, 2010, Liu and Zhang, 2010, Lou et al., 2012, Meng et al., 2011, Tang et al., 2009, Wang and Zhong, 2013, Wang et al., 2011, Zhang et al., 2012b, Zhang et al., 2007a, Zhang et al., 2007b, Zheng et al., 2008, Zhuang et al., 2014).
Fig. 1.
Flow diagram showing the number of citations identified, retrieved, extracted and included in the final analysis.
3.2. Study characteristics
All patients included in this meta-analysis were Han-Chinese from mainland China, Hong Kong and Taiwan. A total of 6495 patients were included in the current meta-analysis. The indications for warfarin in these patients were venous thromboembolism, deep vein thrombosis, atrial fibrillation, rheumatic heart disease, atrial valve replacement, mitral valve replacement, mechanical heart valve replacement and pulmonary embolism. Some eligible studies analyzed two or more genotypes. Of the 33 studies, 24 (Cheng et al., 2009, Veenstra et al., 2005, Cen et al., 2010, Chen et al., 2014, Gu et al., 2010, Huang et al., 2009a, Liang et al., 2012b, Liang et al., 2013, Lu et al., 2013, Ma et al., 2012, Miao et al., 2007, Tan et al., 2013, Wei et al., 2012, Zhang et al., 2012a, Du et al., 2010, Gao et al., 2010, Huo et al., 2008, Li and Sheng, 2010, Liu and Zhang, 2010, Zhang et al., 2007a, Zhang et al., 2007b, Zheng et al., 2008, Zhuang et al., 2014, Yang and Han, 2012) investigated CYP2C9 gene polymorphisms, 11 (Cen et al., 2010, Chen et al., 2014, Li et al., 2012, Liang et al., 2012b, Liang et al., 2013, Ma et al., 2012, Tan et al., 2013, Wei et al., 2012, Wang et al., 2011, Zhang et al., 2012b, Zhuang et al., 2014) assessed CYP4F2 gene polymorphisms, 8 (Huang et al., 2009a, Wei et al., 2012, Yang et al., 2011, Zhang et al., 2012a, Jiang et al., 2007, Meng et al., 2011, Tang et al., 2009, Zhuang et al., 2014) examined VKORC1 1173 gene polymorphisms and 16 (Cheng et al., 2009, Veenstra et al., 2005, Gu et al., 2010, Liang et al., 2012b, Lu et al., 2013, Ma et al., 2012, Miao et al., 2007, Tan et al., 2013, Yuan et al., 2005, Du et al., 2010, Gao et al., 2010, Li and Sheng, 2010, Lou et al., 2012, Meng et al., 2011, Wang and Zhong, 2013, Zhang et al., 2007b) analyzed VKORC1-1639 gene polymorphisms. The characteristics of the studies are shown in Table 1.
Table 1.
Characteristics of included studies.
| Studies | Study location | Indication of warfarin | Number of sample(ALL/M) | Age | INR target range | Genotype frequencies |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CYP2C9 |
CYP4F2 |
VKORC1 1173 |
VKORC1-1639 |
|||||||||||||||
| *1/*1 | *1/*3 | *3/*3 | CC | CT | TT | TT | TC | CC | AA | GA | GG | |||||||
| 1 | Cen et al. (2010) | Guangdong | MHVR | 222/104 | 45 ± 12 | 1.8–2.4 | 91.0 | 8.0 | 1.0 | 52.0 | 41.0 | 7.0 | – | – | – | – | – | – |
| 2 | Chen et al. (2014) | Beijing | HVR,AF | 551/308 | 51 (43–60) | 1.6–2.5 | 91.2 | 8 | 0.2 | 52.3 | 38.8 | 8.9 | – | – | – | – | – | – |
| 3 | Cheng et al. (2009) | Fujian | AF, HVR | 248/132 | 68.86 ± 6.09 | 1.5–3.0 | 86.7 | 12.1 | 1.2 | – | – | – | – | – | – | 65.3 | 26.6 | 8.1 |
| 4 | Du et al. (2010) | Beijing | AF,DVT,HVR,PE | 190/109 | 60 (18–89) | 1.5–3.0 | 91.58 | 8.42 | 0 | – | – | – | – | – | – | 85.79 | 13.16 | 1.05 |
| 5 | Gao et al. (2010) | Fujian | MHVR | 119/50 | 44.58 | 1.5–2.5 | 92.44 | 7.56 | 0 | – | – | – | – | – | – | 84.03 | 15.97 | 0 |
| 6 | Gu et al. (2010) | Chongqing | MHVR | 127/59 | 44.3 ± 17.6 | 1.5–2.0 | 85 | 12.6 | 2.4 | – | – | – | – | – | – | – | – | – |
| 7 | Huang et al. (2009a) | Guangdong | HVR,AF,DVT | 266/123 | 51.5 ± 15.0 | 1.8–3.0 | 90.2 | 9.4 | 0.4 | – | – | – | 77.4 | 21.1 | 1.5 | – | – | – |
| 8 | Huo et al. (2008) | Guangdong | HVR | 93/45 | 21–62 | 1.5–2.0 | 84.95 | 15.05 | 0 | – | – | – | – | – | – | – | – | – |
| 9 | Jiang et al. (2007) | Jiangsu | / | 102/51 | 53.6 ± 16 | 1.5–2.5 | – | – | – | – | – | – | 81.4 | 15.7 | 2.9 | – | – | – |
| 10 | Li et al. (2012) | Jiangxi | MVR,AVR,DVR | 352/123 | 61.8 ± 6.6 | 1.8–2.5 | – | – | – | 58 | 34.9 | 7.1 | – | – | – | – | – | – |
| 11 | Li and Sheng (2010) | Jiangsu | HVR,AF | 73/41 | 54.98 ± 14.10 | 1.5–3.0 | 80.82 | 16.44 | 2.74 | – | – | – | – | – | – | 76.71 | 19.18 | 4.11 |
| 12 | Liang et al. (2012b) | Beijing | AF,DVT,PE,HVR | 115/71 | 64.9 ± 13.0 | 2.0–3.0 | 92.2 | 7.8 | 0 | 41.7 | 47.8 | 10.4 | – | – | – | 85.2 | 14.8 | 0 |
| 13 | Liang et al. (2013) | Yunnan | – | 300/138 | 47.9 ± 12.5 | 1.5–3.0 | 92 | 7.3 | 0.7 | 57.3 | 40 | 2.7 | – | – | – | – | – | – |
| 14 | Liu and Zhang (2010) | Beijing | PE | 108/46 | 59.02 | 2.0–3.0 | – | – | – | – | – | – | – | – | – | 77.78 | 21.3 | 0.92 |
| 15 | Lou et al. (2012) | Beijing | HVR,AF | 161/89 | 64.53 | 1.5–3.0 | 87.58 | 12.42 | 0 | – | – | – | – | – | – | – | – | – |
| 16 | Lu et al. (2013) | Jiangsu | MHVR | 197/82 | 47.0 (18–76) | 1.5–2.8 | 94.4 | 5.6 | 0 | – | – | – | – | – | – | 80.7 | 18.8 | 0.5 |
| 17 | Ma et al. (2012) | Beijing | AF,DVT,PE,HVR | 312/119 | 56.6 ± 16.0 | 1.6–3.0 | 87.8 | 12.2 | 56.1 | 43.9 | – | – | – | 84.9 | 15.1 | |||
| 18 | Meng et al. (2011) | Jiangsu | RHD, AF, DVT | 125/48 | 51.16 | 1.8–3.0 | – | – | – | – | – | – | 87.2 | 12 | 0.8 | 87.2 | 12 | 0.8 |
| 19 | Miao et al. (2007) | Jiangsu | AF,DVT,PE,HVR | 178/74 | 54.7 | 1.5–3.0 | 91 | 9 | 0 | – | – | – | – | – | – | 83.7 | 15.7 | 0.6 |
| 20 | Tan et al. (2013) | Hunan | MHVR. | 317/95 | 45.2 ± 10.5 | 2.1–2.8 | 91.5 | 7.9 | 0.6 | 63.4 | 33.75 | 2.84 | – | – | – | 80.7 | 18 | 1.3 |
| 21 | Tang et al. (2009) | Beijing | VTE | 205/108 | 60.1 ± 13.8 | 2.0–3.0 | – | – | – | – | – | – | 86.8 | 12.2 | 1 | – | – | – |
| 22 | Veenstra et al. (2005) | Hongkong | AF, DVT, RHD | 69/32 | 58.0 ± 10.0 | 1.8–3.2 | 94.2 | 5.8 | 0 | – | – | – | – | – | – | 76.8 | 20.3 | 2.9 |
| 23 | Wang and Zhong (2013) | Sichuan | Orthopedic Surgery | 214/114 | 51.6 ± 7.5 | 2.0–3.0 | 90.7 | 8.4 | 0.9 | – | – | – | – | – | – | 79.9 | 19.2 | 0.9 |
| 24 | Wang et al. (2011) | Liaoning | / | 196/80 | 61.89 | 1.8–3.0 | – | – | – | 50.51 | 42.86 | 6.63 | – | – | – | – | – | – |
| 25 | Wei et al. (2012) | Jiangsu | NVAF,DVT,MHVR | 325/153 | 66.5 ± 12.9 | 1.5–3.0 | 90.8 | 9.2 | 0 | 56 | 33.3 | 10.8 | 86.8 | 12.9 | 0.3 | |||
| 26 | Yang et al. (2011) | Jiangsu | / | 178/74 | 55.3 | 1.5–3.0 | – | – | – | – | – | – | 86.5 | 12.9 | 0.56 | – | – | – |
| 27 | Yuan et al. (2005) | Taiwan | / | 104/56 | 58.6 ± 14.4 | 1.58–2.55 | – | – | – | – | – | – | – | – | – | 79.8 | 18.3 | 1.9 |
| 28 | Zhang et al. (2012b) | Jiangsu | MHVR | 197/82 | 52.92 ± 11.76 | 1.5–2.8 | – | – | – | 58.38 | 37.06 | 4.57 | – | – | – | – | – | – |
| 29 | Zhang et al. (2007a) | Xinjiang | HVR | 88/41 | 45.1 | 1.5–2.0 | 77.27 | 14.77 | 0 | – | – | – | – | – | – | – | – | – |
| 30 | Zhang et al. (2012a) | Beijing | VTE,PE | 297/148 | 64 | 2.0–3.0 | 91.2 | 8.8 | 0 | – | – | – | 85.5 | 13.8 | 0.7 | – | – | – |
| 31 | Zhang et al. (2007b) | Fujian | RHD,AF | 129/52 | 45.79 ± 12.06 | 1.5–3.0 | 90.7 | 8.15 | 0.8 | – | – | – | – | – | – | 74.42 | 23.26 | 2.3 |
| 32 | Zheng et al. (2008) | Beijing | / | 123 | / | / | 92.68 | 6.5 | 0 | – | – | – | – | – | – | – | – | – |
| 33 | Zhuang et al. (2014) | Shanghai | AF,DVT,HVR | 214/110 | 65.72 ± 10.59 | 1.5–3.0 | 92.52 | 7.48 | 0 | 57.94 | 42.06 | 82.71 | 17.29 | 0 | – | – | – | |
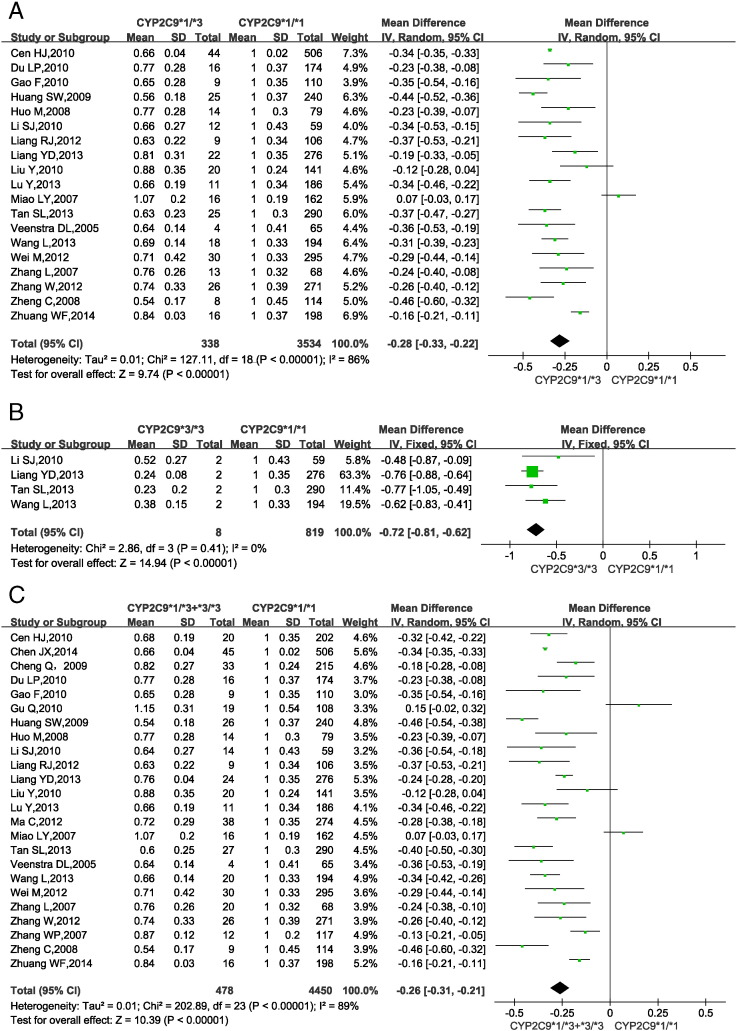
3.3. CYP2C9 gene polymorphisms and warfarin dosage requirement
Fig. 2 shows the impact of CYP2C9 gene SNPs on warfarin dosage requirements in Han-Chinese patients. 5 studies (Cheng et al., 2009, Cen et al., 2010, Gu et al., 2010, Ma et al., 2012, Zhang et al., 2007b) only provided pooled data for genotypes CYP2C9 *1/*3 and *3/*3 rather than separate data for each genotype. So, the data from these studies were included in only one analysis (CYP2C9 *1/*3 + *3/*3 vs. *1/*1). Genotype CYP2C9 *3/*3 could not be found in 12 studies (Veenstra et al., 2005, Liang et al., 2012b, Lu et al., 2013, Miao et al., 2007, Wei et al., 2012, Zhang et al., 2012a, Du et al., 2010, Gao et al., 2010, Huo et al., 2008, Liu and Zhang, 2010, Zhuang et al., 2014) or only 1 patient was found in 3 studies (Chen et al., 2014, Huang et al., 2009a, Zheng et al., 2008), So the data from these studies were excluded from the analysis of CYP2C9 *3/*3 vs. *1/*1.
Fig. 2.
Forest plots of impact of CYP2C9 SNPs on warfarin dosage requirements.
(A) Relative warfarin dosage requirements of CYP2C9 *1/*3 carriers compared to those of wild-type CYP2C9 *1/*1 carriers. (B) CYP2C9 *3/*3 vs. *1/*1 carriers. (C) CYP2C9 *3 carriers (*1/*3 or *3/*3) vs. *1/*1 carriers. SD: standard deviation of normalized warfarin doses associated with each genotype. CI: confidence interval.
Statistical homogeneity was found among studies comparing CYP2C9 *3/*3 vs. *1/*1 (P = 0.41, I2 = 0%; Fig. 2B), so the fixed-effects model was applied. In contrast, significant heterogeneity was found among studies comparing CYP2C9 *1/*3 vs. *1/*1 (P < 0.00001, I2 = 86%, Fig. 2A) and among those comparing CYP2C9 *1/*3 + *3/*3 vs. *1/*1 (P < 0.00001, I2 = 89%; Fig. 2C), so the random-effects model was applied. The results showed that compared with CYP2C9 *1/*1 carriers, CYP2C9 *1/*3 and *3/*3 carriers required a 28% (95% confidence interval: 22.0%–33.0%) and 72% (62.0%–81.0%) lower MDWD. CYP2C9 *3 carriers required a 26% (21.0%–31.0%) lower MDWD than CYP2C9 *1/*1 carriers. All P-values from the test for overall effect were < 0.05.
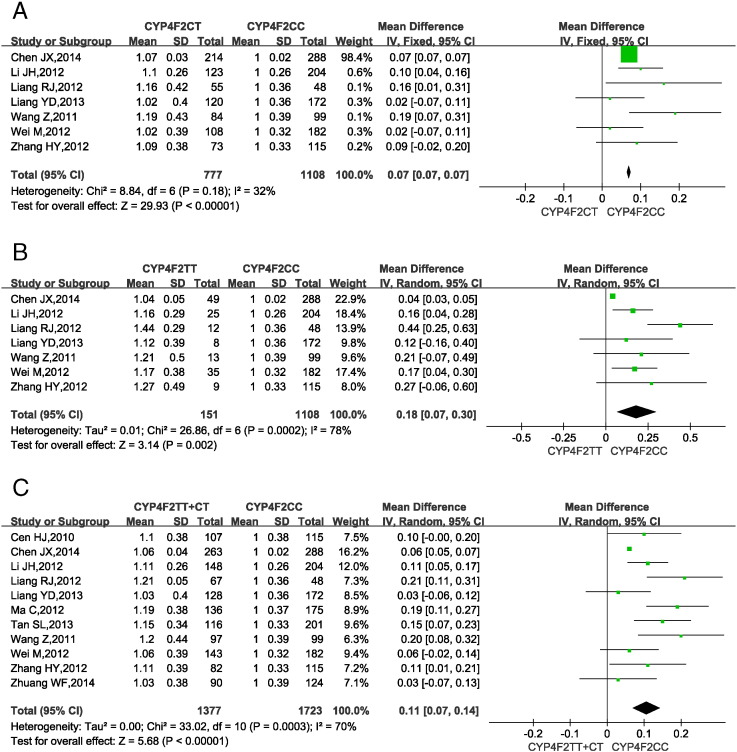
3.4. CYP4F2 gene polymorphisms and warfarin dosage requirement
Fig. 3 shows the impact of CYP4F2 gene SNPs on warfarin dosage requirements in Han-Chinese patients. 4 studies (Cen et al., 2010, Ma et al., 2012, Tan et al., 2013, Zhuang et al., 2014) only provided pooled data for the genotypes CYP4F2 CT and TT, so the data from these studies were used in a single analysis, i.e., CYP4F2 CT + TT vs. CC.
Fig. 3.
Forest plots of impact of CYP4F2 T > C SNPs on warfarin dosage requirements.
(A) Relative warfarin dosage requirements of CYP4F2 CT carriers compared to those of homozygous wild-type CYP4F2 CC carriers. (B) CYP4F2 TT vs. CC carriers. (C) CYP4F2 T carriers (CT or TT) vs. CC carriers. SD: standard deviation of normalized warfarin doses associated with each genotype. CI: confidence interval.
Statistical homogeneity was found among studies comparing CYP4F2 CT vs. CC (P = 0.18, I2 = 32%; Fig. 3A), so the fixed-effects model was applied. In contrast, significant heterogeneity was found among studies comparing CYP4F2 TT vs. CC (P < 0.0002, I2 = 78%, Fig. 3B) and among those comparing CYP4F2 TT + CT vs. CC (P < 0.0003, I2 = 70%; Fig. 3C), so the random-effects model was applied. The results showed that the MDWD was 7% (7.0%–7.0%) and 18% (7.0%–30.0%) higher in CYP4F2 CT and TT carriers, respectively, than in CYP4F2 CC carriers. CYP4F2 T carriers (CT or TT) needed a 11% (7.0%–14.0%) higher MDWD than CC carriers. All P-values from the test for overall effect were < 0.05.
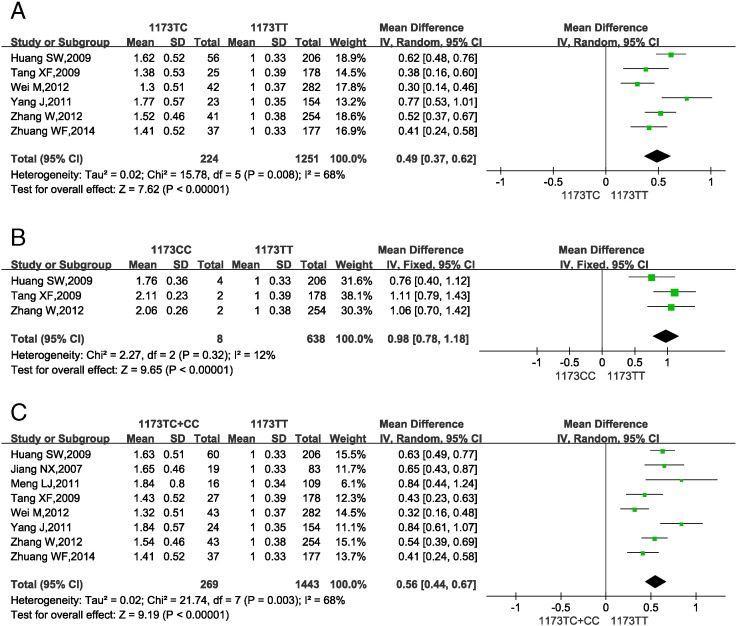
3.5. VKORC1 1173 gene polymorphisms and warfarin dosage requirement
Fig. 4 shows the impact of VKORC1 1173 gene SNPs on warfarin dosage requirements in Han-Chinese subjects. 2 studies (Jiang et al., 2007, Meng et al., 2011) reported the pooled MDWD for VKORC1 1173 CT and CC carriers, so the data from these studies were used in a single analysis (VKORC1 1173 CT + CC vs. TT). Genotype VKORC1 1173 CC was not found in 1 study and only 1 patient was found in 1 studies (Wei et al., 2012, Yang et al., 2011). So the data from these studies were excluded from the analysis of VKORC1 1173 CC vs. TT.
Fig. 4.
Forest plots of impact of VKORC1 1173 C > T SNPs on warfarin dosage requirements.
(A) Relative warfarin dosage requirements of CT carriers compared to those of wild-type VKORC1 1173 TT carriers. (B) VKORC1 1173 CC vs. TT carriers. (C) VKORC1 1173 C carriers (TC or CC) vs. TT carriers.SD: standard deviation of normalized warfarin doses associated with each genotype. CI: confidence interval.
Statistical homogeneity was found among studies comparing VKORC1 1173 CC vs. TT (P = 0.32, I2 = 12%; Fig. 4B), so the fixed-effects model was applied. Significant heterogeneity was seen among studies assessing VKORC1 1173 TC vs. TT (P = 0.008, I2 = 68%; Fig. 4A) and VKORC1 1173 TC + CC vs. TT (P = 0.003, I2 = 68%; Fig. 4C), so the random-effects model was used. The results showed that VKORC1 1173 TC and CC carriers required a 49% (37.0%–62.0%) and 98% (78.0%–118.0%) higher MDWD, respectively, than TT carriers. VKORC1 1173 C carriers (CT or CC) needed a 56% (44.0%–67.0%) higher MDWD than TT carriers. All P-values from the test for overall effect were < 0.05.
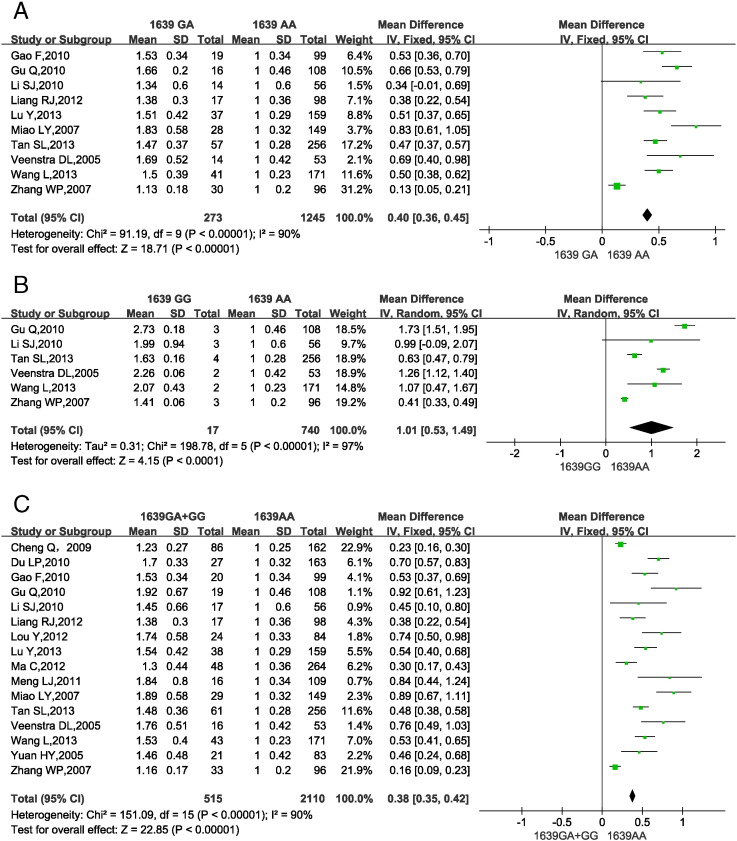
3.6. VKORC1-1639 gene polymorphisms and warfarin dosage requirement
Fig. 5 shows the impact of VKORC1-1639 gene SNPs on warfarin dosage requirements in Han-Chinese subjects. 2 (Liang et al., 2012b, Gao et al., 2010) studies did not report the genotype VKORC1-1639 GG only were excluded from the analysis of VKORC1-1639 GG vs. AA. 6 studies (Cheng et al., 2009, Ma et al., 2012, Yuan et al., 2005, Du et al., 2010, Lou et al., 2012, Meng et al., 2011) provided pooled data for VKORC1-1639 GA and GG carriers, so the data from these studies were included in a single analysis of VKORC1-1639 GA + GG vs. AA.
Fig. 5.
Forest plots of impact of VKORC1-1639 G > A SNPs on warfarin dosage requirements.
(A) Relative warfarin dosage requirements of VKORC1-1639 GA carriers compared to VKORC1-1639 AA carriers. (B) VKORC1-1639 GG vs. AA carriers. (C) VKORC1-1639 G (GA or GG) vs. AA carriers.SD: standard deviation of normalized warfarin doses associated with each genotype. CI: confidence interval.
Significant heterogeneity was found among studies assessing VKORC1-1639 gene polymorphisms (Fig. 5A–5C; P < 0.00001, I2 = 93%; P < 0.00001, I2 = 97%; P < 0.00001, I2 = 90%), so the random-effects model was selected. The results showed that compared to VKORC1-1639 AA carriers, GA and GG carriers required a 40% (36.0%–45.0%) and 101% (53.0%–149.0%) higher MDWD, respectively. VKORC1-1639 G carriers (GA or GG) needed a 38% (35.0%–42.0%) higher MDWD than AA carriers. All P-values from the test for overall effect were < 0.05.
3.7. Heterogeneity and sensitivity analysis
Sensitivity analysis was performed by deselecting the studies one by one in chronological order. The results were not changed greatly when any study was deselected, and no study was found to be significantly associated with statistical heterogeneity, which indicated that the results of the analysis were stable and reliable.
Since high statistical heterogeneity was found among some analyses of genes CYP2C9, CYP4F2, VKORC1 1173 C > T and VKORC1-1639 G > A, the software STATA 12.0 was used to explore the source of heterogeneity via meta-regression analysis (Liang et al., 2012a). The year of publication, language of publication, location of patients, mean age of patients, number of patients, proportion of men and median INR were used as covariates of the mean difference in MDWD in each meta-regression analysis. In order to improve this process, we set certain values for the variables. For language, English was set as 0 and Chinese as 1. For location, we divided the whole country into five regions: the region of Taiwan and Hong Kong, southeast China, southwest China, northeast China and northwest China, which were set as 0, 1, 2, 3 and 4, respectively. The meta-regression results for each genotype are shown in Table 2, Table 3, Table 4, Table 5.
Table 2.
Results of meta-regression analysis of various covariates from studies on CYP2C9.
| Covarirate | Coef. | Sta. Err | t | p > | t | | I-squared_res (%) | Adj R-squared (%) |
|---|---|---|---|---|---|---|
| Published year | − 0.015594 | 0.0117369 | − 1.33 | 0.198 | 85.82 | 5.70 |
| Language | 0.0412391 | 0.0603107 | − 0.68 | 0.502 | 85.60 | − 1.74 |
| Location | 0.0352089 | 0.0292356 | 1.20 | 0.242 | 86.68 | − 0.42 |
| Age | 0.0005659 | 0.0036818 | 0.15 | 0.879 | 88.31 | − 5.53 |
| Number of patients | − 0.0003186 | 0.000255 | − 1.25 | 0.225 | 82.73 | 4.85 |
| Male ratio | − 0.2032788 | 0.4329343 | − 0.47 | 0.644 | 86.50 | − 4.94 |
| Median INR | − 0.205372 | 0.127046 | − 1.62 | 0.121 | 88.83 | 7.13 |
None of the covariates was significantly correlated with heterogeneity.
Table 3.
Results of meta-regression analysis of various covariates from studies on CYP4F2.
| Covarirate | Coef. | Sta. err | t | p > | t | | I-squared_res (%) | Adj R-squared (%) |
|---|---|---|---|---|---|---|
| Published year | − 0. 0264162 | 0.0144864 | − 1.82 | 0.102 | 42.13 | 46.43 |
| Language | 0. 0020607 | 0.0493276 | 0.04 | 0.968 | 70.96 | − 16.98 |
| Location | − 0. 0381552 | 0.0229246 | − 1.66 | 0.130 | 71.90 | − 5.00 |
| Age | 0. 0009953 | 0.0025719 | 0.39 | 0.708 | 67.34 | − 16.15 |
| Number of patients | − 0.0002609 | 0.0002848 | − 0.92 | 0.387 | 49.99 | − 10.66 |
| Male ratio | − 0.1809945 | 0.2084318 | − 0.87 | 0.408 | 53.38 | 7.61 |
| Median INR | 0.0554843 | 0.1109978 | 0.50 | 0.629 | 60.80 | − 3.88 |
None of the covariates was significantly correlated with heterogeneity.
Table 4.
Results of meta-regression analysis of various covariates from studies on VKORC1 1173.
| Covarirate | Coef. | Sta. err | t | p > | t | | I-squared_res (%) | Adj R-squared (%) |
|---|---|---|---|---|---|---|
| Published year | − 0.0300199 | 0. 0305746 | − 0.98 | 0.364 | 64.70 | 6.64 |
| Language | − 0.0247233 | 0.1403689 | − 0.18 | 0.866 | 71.93 | − 26.42 |
| Location | 0.0950507 | 0.15246227 | 0.62 | 0.556 | 71.89 | − 24.05 |
| Age | − 0.0162225 | 0.0071395 | − 2.27 | 0.063 | 51.09 | 49.93 |
| Number of patients | − 0.0013617 | 0.0007849 | − 1.73 | 0.133 | 62.14 | 26.79 |
| Male ratio | − 2.884117 | 1.343607 | − 2.15 | 0.075 | 57.16 | 42.96 |
| Median INR | − 0.1214239 | 0.4457683 | − 0.27 | 0.794 | 72.39 | − 26.28 |
None of the covariates was significantly correlated with heterogeneity.
Table 5.
Results of meta-regression analysis of various covariates from studies on VKORC1–1639.
| Covarirate | Coef. | Sta. err | t | p > | t | | I-squared_res (%) | Adj R-squared (%) |
|---|---|---|---|---|---|---|
| Published year | − 0.1861517 | 0.1581693 | − 1.18 | 0.259 | 95.83 | 3.99 |
| Language | − 0.0325182 | 0.0307319 | − 1.06 | 0.308 | 96.67 | 1.30 |
| Location | − 0.0849311 | 0.0726921 | − 1.17 | 0.262 | 92.10 | 6.16 |
| Age | − 0.0011833 | 0.0108254 | − 0.11 | 0.915 | 96.93 | − 7.83 |
| Number of patients | − 0.0012891 | 0.0010766 | − 1.20 | 0.251 | 96.93 | 2.46 |
| Male ratio | − 0.4980054 | 0.9576941 | − 0.52 | 0.611 | 96.67 | − 5.93 |
| Median INR | − 0.6058801 | 0.4032263 | − 1.50 | 0.155 | 95.51 | 9.90 |
None of the covariates was significantly correlated with heterogeneity.
3.8. Publication bias
Initially, publication bias between the studies was checked via Revman 5.3, and the results did not indicate significant publication bias (data not shown).
4. Discussion
Polymorphisms of genes CYP2C9, VKORC1 1173 T > C and VKORC1-1639 G > A account for 40%–60% of the interindividual variation in warfarin dosage (Klein et al., 2009, Rieder et al., 2005, Krishna Kumar et al., 2014, Lee et al., 2006, Veenstra et al., 2005, Wadelius et al., 2005). Recently, another gene, CYP4F2, was found to be moderately associated with the interindividual variation in warfarin dosage requirements (Liang et al., 2012a, Cen et al., 2010). The frequencies of these four genes differ among people of different ethnicities (Table 6).
Table 6.
Frequencies of the target genotypes in different regions.
| Population | CYP2C9 genotype frequencies(%) |
No. of subjects | Reference | |||||
|---|---|---|---|---|---|---|---|---|
| *1/*1 | *1/*3 | *3/*3 | *1/*2 | *2/*2 | *2/*3 | |||
| Han-Chinese | 90.41 | 8.94 | 0.58 | 0.06 | – | – | 4928 | Current review |
| Japanese | 95.26 | 4.41 | 0.09 | – | – | – | 2277 | Gaikwad et al. (2014) |
| Korean | 90.82 | 9.10 | 0.08 | – | – | – | 1151 | Gaikwad et al. (2014) |
| Indian | 76.84 | 13.48 | 1.65 | 6.80 | 0.39 | 0.84 | 3510 | Gaikwad et al. (2014) |
| Caucasian | 66 | 12 | 0.5 | 19 | 1.4 | 1.3 | 1490 | Wadelius et al. (2009); Daneshjou et al. (2013) |
| African | 93.71 | 2.32 | – | 3.97 | – | – | 302 | Daneshjou et al. (2013); Mushiroda et al. (2006) |
| Population | VKORC1 1173 genotype frequencies (%) | No. of subjects | Reference | ||
| TT | TC | CC | |||
| Han-Chinese | 84.29 | 14.89 | 0.82 | 1712 | Current review |
| Japanese | 83.33 | 15.94 | 0.72 | 828 | Mushiroda et al. (2006); Choi et al. (2011) |
| Korean | 87.41 | 11.7 | 0.89 | 564 | Choi et al. (2011); Kumar et al. (2013) |
| Indian | 77.47 | 21.26 | 1.26 | 470 | Kumar et al. (2013); Krajciova et al. (2014) |
| Caucasian | 17.38 | 46.67 | 35.95 | 420 | Krajciova et al. (2014); Limdi et al. (2008) |
| African | 0.9 | 18.7 | 80.4 | 225 | Limdi et al. (2008); Chin et al. (2013) |
| Population | VKORC1-1639 genotype frequencies (%) | No. of subjects | Reference | ||
| AA | GA | GG | |||
| Han-Chinese | 79.87 | 17.77 | 2.36 | 2524 | Current review |
| Japanese | 83.21 | 16.06 | 0.72 | 800 | Mushiroda et al. (2006); Choi et al. (2011) |
| Korean | 86.91 | 13.09 | – | 298 | Chin et al. (2013); Scott et al. (2008) |
| Indian | 77.45 | 37.3 | 1.35 | 470 | Kumar et al. (2013); Krajciova et al. (2014) |
| Caucasian | 15 | 49 | 36 | 1461 | Wadelius et al. (2009); Daneshjou et al. (2013) |
| African | 2.0 | 17.7 | 80.3 | 300 | Scott et al. (2008); Cha et al. (2010) |
| Puopulation | CYP4F2 genotype frequencies (%) | No. of subjects | Reference | ||
| CC | CT | TT | |||
| Han-Chinese | 55.30 | 37.86 | 6.83 | 3100 | Current review |
| Japanese | 52.95 | 39.77 | 7.27 | 440 | Cha et al. (2010); Wypasek et al. (2014) |
| Korean | 41.69 | 46.65 | 11.66 | 403 | Choi et al. (2011); Kumar et al. (2013) |
| Indian | 33.5 | 49.4 | 17.1 | 445 | Kumar et al. (2013); Krajciova et al. (2014) |
| Caucasian | 7.31 | 41.75 | 50.94 | 479 | Wypasek et al. (2014); Bress et al. (2012) |
| African | 88.76 | 10.85 | 0.39 | 258 | Bress et al. (2012); Ye et al. (2014) |
Some meta-analyses (Sanderson et al., 2005, Lindh et al., 2009a, Yang et al., 2010, Jorgensen et al., 2012, Liang et al., 2012a) have researched the impact of these four genes on the MDWD in Caucasian, African and Asian patients. Thus far, however, no meta-analysis has specifically analyzed the impact of these genes in the Han-Chinese. Our study is the first to explore the impact of these four genes on MDWD in Han-Chinese patients.
We found that among Han-Chinese subjects, CYP2C9 *3*3, *1/*3 and *3 carriers require 72% (62.0%–81.0%), 28% (22.0%–33.0%) and 26% (21.0%–31.0%) less warfarin maintenance dosage, respectively, than CYP2C9 *1/*1 carriers. Although the homozygous variant CYP2C9 *3*3 was very rare among the Han-Chinese (0.39%), the warfarin maintenance dosage was much lower in these subjects than in homozygous wild-type carriers. Thus, if given the same loading dose, patients with the CYP2C9 *3*3 genotype will have a higher risk of bleeding than CYP2C9 *1/*1 carriers (Ma et al., 2012). Lindh et al. (2009b) reported that among Caucasians, the warfarin maintenance dosage was 78.1% (72.0%–84.3%) and 35.1% (29.4%–38.1%) lower in CYP2C9 *3/*3 and *1/*3 patients, respectively, than in *1/*1 patients. It appears that the influence of CYP2C9 gene polymorphisms on warfarin maintenance dosage is greater in Caucasian patients than in Han-Chinese patients. However, the distribution of the CYP2C9 *3 genotype in the Han-Chinese significantly differs from that in Caucasians, Africans (Table 5) and South and West Asians (Gaikwad et al., 2014). In this meta-analysis, among 4928 patients, the allele frequencies of CYP2C9 *1/*1, *1/*3 and *3/*3 were 91.0%, 8.44% and 0.55% respectively. The frequency of mutant *3 is lower in the Han-Chinese than in Caucasians and Indians. Furthermore, mutant *2 is common in Caucasians and South and West Asians, but it is very rare in East Asians, including the Han-Chinese, Koreans and Japanese. Genome-wide association studies concerning the Han-Chinese seldom provide data on CYP2C9 *2 patients. We therefore had no occasion to conduct an analysis to determine the influence of CYP2C9 *2 on MDWD in Han-Chinese subjects.
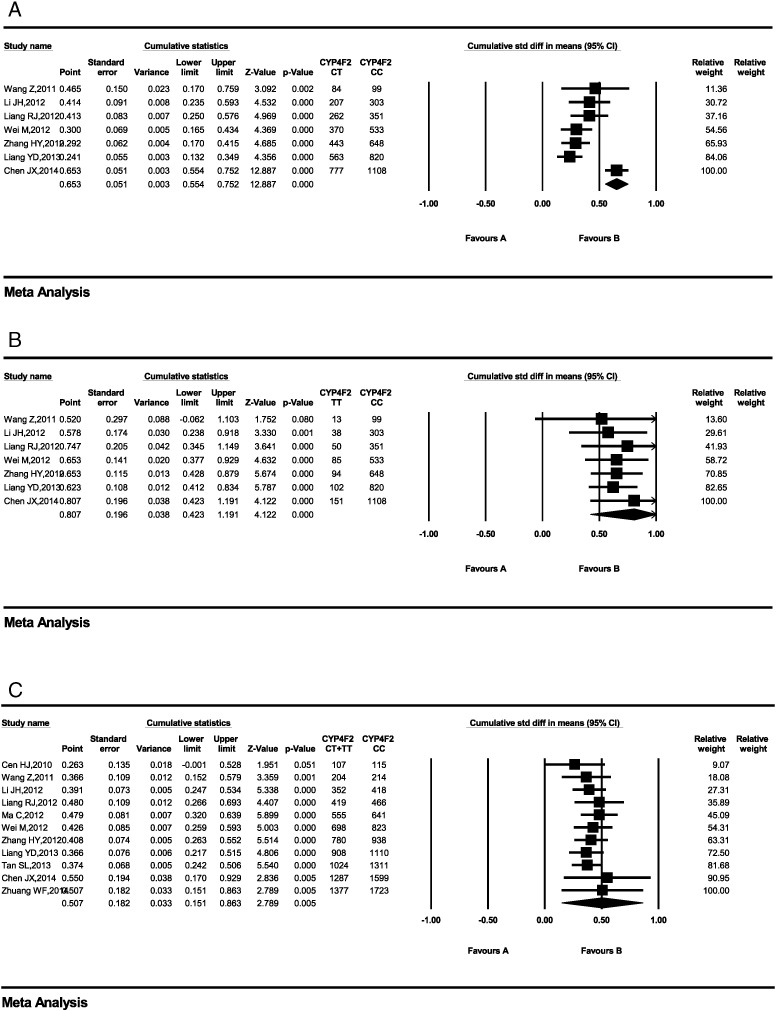
Although Kringen et al. (2011) and Lee et al. (2009) have found that the CYP4F2 gene has little effect on warfarin maintenance dosage in Caucasians and the Han-Chinese, many other researchers consider that this impact is not negligible and that CYP4F2 SNPs should be taken into account before prescribing warfarin (Kringen et al., 2011). In the included studies, there were significant differences in MDWD among subjects with different CYP4F2 genotypes. We found that in Han-Chinese subjects, the MDWD was 18% (7.0%–30.0%), 7% (7.0%–7.0%) and 11% (7.0%–14.0%) higher in CYP4F2 TT, CT and T carriers, respectively, than in CYP4F2 CC patients. The corresponding values in Caucasians were 23.0% (7.0%–39.0%), 10.0% (4.0%–15.0%) and 11.0% (7.0%–15.0%) (Liang et al., 2012a). The Forest plots for CYP 4F2 showed some studies that were not statistically significant, yet the aggregate meta analysis data showed statistical difference. So we performed a cumulative meta analysis of CYP 4F2 on software Comprehensive Meta Analysis V2. But the results showed that the studies were not statistically significant (Fig. 6A–C).
Fig. 6.
Cumulative meta-analysis of CYP 4F2 on warfarin dosage requirements in chrononologic order. (A) CYP4F2 CT vs. CC carriers. (B) CYP4F2 TT vs. CC carriers. (C) CYP4F2 T carriers (CT or TT) vs. CC carriers. CI: confidence interval.
We also found that in Han-Chinese subjects, the warfarin maintenance dosage was 98% (78.0%–118.0%), 49% (37.0%–62.0%) and 56% (44.0%–67.0%) higher in VKORC1 1173 CC, TC and C carriers (TC or CC), respectively, than in TT carriers. The warfarin maintenance dosage was 101% (53.0%–149.0%), 45% (41.0%–49.0%) and 38% (35.0%–42.0%) higher in Han-Chinese VKORC1-1639 GG, GA and G carriers (GA or GG), respectively, than in AA carriers. In 1712 subjects, the frequencies of VKORC1 1173 TT, CT and CC were 84.29%, 14.89% and 0.82% respectively, while in 2625 subjects, the frequencies of VKORC1-1639 AA, GA and GG were 79.87%, 17.77% and 2.36%, respectively. Not only VKORC1 1173 C mutations and VKORC1-1639 G mutations have similar frequencies in the Han-Chinese, but also both mutations had a similar impact on MDWD in Han-Chinese patients. Physicians can test for any one of these two genotypes to ensure rational drug usage and save hospitalization costs.
It is well-known that Caucasians and Africans require a higher MDWD than the Han-Chinese, who require a nearly 40% lower MDWD than Caucasian patients (Xie et al., 2001). One black male patient was reported to require a 60-mg daily dose of warfarin to elicit a therapeutic anticoagulant response (Hallak et al., 1993). Caucasian or African patients who need > 15 mg/day warfarin are considered warfarin resistant (Osinbowale et al., 2009), whereas for the Han-Chinese, the resistance level is 6 mg (Yuan et al., 2005). The frequencies of the VKORC1 1173 C and VKORC1-1639 G alleles, which increase the expression of VKORC1 mRNA and render patients insensitive to warfarin (Rieder et al., 2005), in the Han-Chinese are quite different from those in Caucasians and Africans. Approximately 16% Han-Chinese patients are VKORC1 1173 C or VKORC1-1639 G carriers, while most Caucasian and African patients are VKORC1 1173 C or VKORC1-1639 G carriers (Table 6). This may explain why Caucasians and Africans require a higher MDWD than the Han-Chinese to achieve the same target INR range, despite all other factors being equal. Huang SW's study have shown that the pharmacogenetics-based dosing algorithm improve the time to reach the stable dosing of warfarin in Han-Chinese patients (Huang et al., 2009b). And pharmacogenetics-based dosing algorithm may be useful in helping the clinicians to prescribe warfarin with greater safety and efficiency (Huang et al., 2009b). But it still need to constuct a pharmacogenetics-based dosing algorithm for Han-Chinese patients by muti-center.
It is a stern reality that gene sequencing is very expensive and is not covered by medical insurance providers in many countries, and the average patient cannot bear the expenses of testing all four genes. After the warfarin loading dose and the subsequent INR test, physicians can estimate whether or not a patient is sensitive to warfarin. From the results of this meta-analysis, we deduced that testing for the CYP2C9 gene can reveal warfarin-sensitive patients, while testing for the VKORC1 gene can reveal warfarin-insensitive patients. Therefore, conversely, doctors can select warfarin-sensitive patients for CYP2C9 testing, and warfarin-insensitive patients for VKORC1 testing. Thus, long-term warfarin users will save on medical costs while still receiving appropriate treatment.
4.1. Limitations and perspectives
We found that during warfarin treatment to prevent embolization, the target INR set by doctors varies greatly among different regions. For example, during warfarin therapy to prevent thrombosis in heart valve replacement patients, Gu et al. (2010), Huo et al. (2008) and Zhang et al. set the target INR to 1.5–2.0, but Tan et al. (2013) set this to 2.1–2.8. Most of the included studies were from southeast China and northeast China, and more samples from other regions should be analyzed to confirm the results of our meta-analysis.
5. Conclusions
Polymorphisms of CYP2C9, VKORC1 1173 and VKORC1-1639 significantly affect warfarin maintenance dosage in Han-Chinese patients. CYP4F2 gene polymorphisms explain part of the dosage difference, though the effect of this gene is lower than that of the other three. The distribution of these four genes in the Han-Chinese is different from that in other major populations, which could explain the differences in the required warfarin dosage between Han-Chinese and other populations.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgment
The Project Sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (20141685) and Fujian medical innovation project (2014-CX-18).
References
- Bress A., Patel S.R., Perera M.A., Campbell R.T., Kittles R.A., Cavallari L.H. Effect of NQO1 and CYP4F2 genotypes on warfarin dose requirements in Hispanic-Americans and African-Americans. Pharmacogenomics. 2012;13(16):1925–1935. doi: 10.2217/pgs.12.164. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlquist J.F., Horne B.D., Muhlestein J.B. Genotypes of the cytochrome p450 isoform, CYP2C9, and the vitamin K epoxide reductase complex subunit 1 conjointly determine stable warfarin dose: a prospective study. J. Thromb. Thrombolysis. 2006;22(3):191–197. doi: 10.1007/s11239-006-9030-7. [DOI] [PubMed] [Google Scholar]
- Cavallari L.H., Langaee T.Y., Momary K.M. Genetic and clinical predictors of warfarin dose requirements in African Americans. Clin. Pharmacol. Ther. 2010;87(4):459–464. doi: 10.1038/clpt.2009.223. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Cen H., Zeng W., Leng X. CYP4F2 rs2108622: a minor significant genetic factor of warfarin dose in Han Chinese patients with mechanical heart valve replacement. British Journal Of Clinical Pharmacology. 2010;70(2):234–240. doi: 10.1111/j.1365-2125.2010.03698.x. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha P.C., Mushiroda T., Takahashi A. Genome-wide association study identifies genetic determinants of warfarin responsiveness for Japanese. Hum. Mol. Genet. 2010;19(23):4735–4744. doi: 10.1093/hmg/ddq389. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Chen J., Shao L., Gong L. A pharmacogenetics-based warfarin maintenance dosing algorithm from northern chinese patients. PLoS One. 2014;9(8):e105250. doi: 10.1371/journal.pone.0105250. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q., Chen H., Luo J.W., Zhang W.P., Wu X.Y. Effects of VKORC1 and CYP2C9 gene polymorphisms on metabolism and pharmacodynamics of warfarin in elderly with artrial fibrillation. Chin. J. Clin. Neurosci. 2009;17:573–579. (Article in Chinese) [Google Scholar]
- Chin D.-K., Han I.-B., Ropper A.E. Association of VKORC1-1639G > A polymorphism with susceptibility to ossification of the posterior longitudinal ligament of the spine: a Korean study. Acta Neurochir. 2013;155(10):1937–1942. doi: 10.1007/s00701-013-1747-4. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Choi J.R., Kim J.-O., Kang D.R. Proposal of pharmacogenetics-based warfarin dosing algorithm in Korean patients. Eur. J. Hum. Genet. 2011;56(4):290–295. doi: 10.1038/jhg.2011.4. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Daneshjou R., Tatonetti N.P., Karczewski K.J. Pathway analysis of genome-wide data improves warfarin dose prediction. BMC Genomics. 2013;14(Suppl. 3):S11. doi: 10.1186/1471-2164-14-S3-S11. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L.P., Mei D., Liu C.W., Liu B., Su W. Influence of CYP2C9 and VKORC1 pol ymorphisms on warfarin dose and anti-coagulative effect in chinese population. Chin. Pharm. J. 2010;45:1628–1633. (Article in Chinese) [Google Scholar]
- FDA FDA Approves Updated Warfarin (Coumadin) Prescribing Information. 2007. http://www.fda.gov/bbs/topics/NEWS/2007/NEW01684.html
- Gaikwad T., Ghosh K., Shetty S. VKORC1 and CYP2C9 genotype distribution in Asian countries. Thromb. Res. 2014 doi: 10.1016/j.thromres.2014.05.028. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Gao F., Song H.T., Feng Y.L. . Effects of CYP2C9 and VKORC1 genetic polymorphisms on maintenance dosage and anticoagulation effect of warfarin in patients after undergoing mechanical heart valve prostheses replacement. China Pharmacy. 2010;21:2053–2057. (Article in Chinese) [Google Scholar]
- Gu Q., Kong Y., Schneede J. VKORC1-1639G/A, CYP2C9, EPHX1691A/G genotype, body weight, and age are important predictors for warfarin maintenance doses in patients with mechanical heart valve prostheses in southwest China. Eur. J. Clin. Pharmacol. 2010;66(12):1217–1227. doi: 10.1007/s00228-010-0863-9. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Hallak H.O., Wedlund P.J., Modi M.W. High clearance of (S)-warfarin in a warfarin-resistant subject. Br. J. Clin. Pharmacol. 1993;35(3):327–330. doi: 10.1111/j.1365-2125.1993.tb05703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.W., Chen H.S., Wang X.Q. Validation of VKORC1 and CYP2C9 genotypes on interindividual warfarin maintenance dose: a prospective study in Chinese patients. Pharmacogenet. Genomics. 2009;19(3):226–234. doi: 10.1097/FPC.0b013e328326e0c7. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Huang S.W., Chen H.S., Wang X.Q. Validation of VKORC1 and CYP2C9 genotypes on interindividual warfarin maintenance dose: aprospective study in Chinese patients. Pharmacogenet. Genomics. 2009;19(3):226–234. doi: 10.1097/FPC.0b013e328326e0c7. [DOI] [PubMed] [Google Scholar]
- Huo M., Liu C., Yang C. Study on the association between the maintenance dose of warfarin anticoagulation therapy and the genetic mutation of cytochrome P450 (CYP2C9) Chin. J. Prev. Contr. Chron. Non-commun. Dis. 2008;16:35–37. (Article in Chinese) [Google Scholar]
- Jiang N.X., Song J., Xu B. In: Vitamin K Epoxide Reductase Complex 1 Gene Polymorphism and Warfarin Dose Requirement in Chinese Patients. Xin Z., Guan X., Zhi B.Z., editors. Vol. 35. 2007. pp. 652–654. (published Online First: Epub Date Article in Chinese) [PubMed] [Google Scholar]
- Jorgensen A.L., FitzGerald R.J., Oyee J., Pirmohamed M., Williamson P.R. Influence of CYP2C9 and VKORC1 on patient response to warfarin: a systematic review and meta-analysis. Plos One. 2012;7(8):e44064. doi: 10.1371/journal.pone.0044064. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian P.T.H., Green S. 2011. The Cochrane Handbook: v5.1.0; 2011. Part 2:8.5. [Google Scholar]
- Jüni P., Witschi A., Bloch R., Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. J. Am. Med. Assoc. 1999;282(11):1054–1060. doi: 10.1001/jama.282.11.1054. [DOI] [PubMed] [Google Scholar]
- Klein T.E., Altman R.B., Eriksson N. Estimation of the warfarin dose with clinical and pharmacogenetic data. The New England Journal Of Medicine. 2009;360(8):753–764. doi: 10.1056/NEJMoa0809329. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajciova L., Deziova L., Petrovic R., Luha J., Turcani P., Chandoga J. Frequencies of polymorphisms in CYP2C9 and VKORC1 genes influencing warfarin metabolism in Slovak population: implication for clinical practice. Bratisl. Lek. Listy. 2014;115(9):563–568. doi: 10.4149/bll_2014_109. [DOI] [PubMed] [Google Scholar]
- Kringen M.K., Haug K.B., Grimholt R.M. Genetic variation of VKORC1 and CYP4F2 genes related to warfarin maintenance dose in patients with myocardial infarction. J. Biomed. Biotechnol. 2011:739751. doi: 10.1155/2011/739751. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna Kumar D., Shewade D.G., Loriot M.-A. Effect of CYP2C9, VKORC1, CYP4F2 and GGCX genetic variants on warfarin maintenance dose and explicating a new pharmacogenetic algorithm in South Indian population. Eur. J. Clin. Pharmacol. 2014;70(1):47–56. doi: 10.1007/s00228-013-1581-x. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Kumar D.K., Shewade D.G., Manjunath S., Ushakiran P., Reneega G., Adithan C. Inter and intra ethnic variation of vitamin K epoxide reductase complex and cytochrome P450 4F2 genetic polymorphisms and their prevalence in South Indian population. Indian Journal Of Human Genetics. 2013;19(3):301–310. doi: 10.4103/0971-6866.120817. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.C., Ng S.S., Oldenburg J. Interethnic variability of warfarin maintenance requirement is explained by VKORC1 genotype in an Asian population. Clin. Pharmacol. Ther. 2006;79(3):197–205. doi: 10.1016/j.clpt.2005.11.006. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Lee M.T.M., Chen C., Chou C. Genetic determinants of warfarin dosing in the Han-Chinese population. Pharmacogenomics. 2009;10(12):1905–1913. doi: 10.2217/pgs.09.106. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Li S.J., Sheng H.Z. CYP2C9 and VKORC1 polymorphisms are associated with Warfarin Anticoagulation-Dose Requirements. Tianjin Med. J. 2010;38:670–673. (Article in Chinese) [Google Scholar]
- Li J.H., Ma G.G., Zhu S.Q. Correlation between single nucleotide polymorphisms in CYP4F2 and warfarin dosing in Chinese valve replacement patients. J. Cardiothorac. Surg. 2012:1749–8090. doi: 10.1186/1749-8090-7-97. ((Electronic) doi: D - NLM: PMC3487995 EDAT- 2012/09/28 06:00 MHDA- 2013/01/17 06:00 CRDT- 2012/09/28 06:00 PHST- 2012/06/15 [received] PHST- 2012/09/23 [accepted] PHST- 2012/09/27 [aheadofprint] AID - 1749-8090-7-97 [pii] AID - 10.1186/1749-8090-7-97 [doi] PST - epublish[published Online First: Epub Date]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang R., Wang C., Zhao H., Huang J., Hu D., Sun Y. Influence of CYP4F2 genotype on warfarin dose requirement-a systematic review and meta-analysis. Thromb. Res. 2012;130(1):38–44. doi: 10.1016/j.thromres.2011.11.043. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Liang R., Li L., Li C. Impact of CYP2C9*3, VKORC1-1639, CYP4F2rs2108622 genetic polymorphism and clinical factors on warfarin maintenance dose in Han-Chinese patients. Journal Of Thrombosis And Thrombolysis. 2012;34(1):120–125. doi: 10.1007/s11239-012-0725-7. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Liang Y., Chen Z., Guo G. Association of genetic polymorphisms with warfarin dose requirements in Chinese patients. Genet. Test. Mol. Biomarkers. 2013;17(12):932–936. doi: 10.1089/gtmb.2013.0303. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limdi N.A., McGwin G., Goldstein J.A. Influence of CYP2C9 and VKORC1 1173C/T genotype on the risk of hemorrhagic complications in African-American and European-American patients on warfarin. Clin. Pharmacol. Ther. 2008;83(2):312–321. doi: 10.1038/sj.clpt.6100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindh J.D., Holm L., Andersson M.L., Rane A. Influence of CYP2C9 genotype on warfarin dose requirements-a systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 2009;65(4):365–375. doi: 10.1007/s00228-008-0584-5. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Lindh J.D., Holm L., Andersson M.L., Rane A. Influence of CYP2C9 genotype on warfarin dose requirements—a systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 2009;65(4):365–375. doi: 10.1007/s00228-008-0584-5. [DOI] [PubMed] [Google Scholar]
- Little J., Bradley L., Bray M.S. Reporting, appraising, and integrating data on genotype prevalence and gene-disease associations. Am. J. Epidemiol. 2002;156(4):300–310. doi: 10.1093/oxfordjournals.aje.a000179. [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhang J. Effects of CYP2C9 gene polymorphisms and age in Chinese Han patients on warfarin dose. Adverse Drug Reactions Journal. 2010;12:240–245. (Article in Chinese) [Google Scholar]
- Lou Y., Liu H., Han L.L., Xie S., Gao X.J., Duan B. Influence of CYP2C9 and VKORC1 genetic polymorphisms on maintenance warfarin dose in Chinese pulmonary embolism ptients. Chinese Journal of Pharmacovigilance. 2012;9:202–204. (Article in Chinese) [Google Scholar]
- Lu Y., Yang J., Zhang H., Yang J. Prediction of warfarin maintenance dose in Han Chinese patients using a mechanistic model based on genetic and non-genetic factors. Clin. Pharmacokinet. 2013;52(7):567–581. doi: 10.1007/s40262-013-0054-9. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Ma C., Zhang Y., Xu Q. Influence of warfarin dose-associated genotypes on the risk of hemorrhagic complications in Chinese patients on warfarin. Int. J. Hematol. 2012;96(6):719–728. doi: 10.1007/s12185-012-1205-8. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Meng L.J., Song J., Wang D.J. Effect of multiplesigle-nucleotide variants in VKORC1 gene on the maintenance doses of warfarin. Jiangsu Med. J. 2011;37:185–187. (Article in Chinese) [Google Scholar]
- Miao L., Yang J., Huang C., Shen Z. Contribution of age, body weight, and CYP2C9 and VKORC1 genotype to the anticoagulant response to warfarin: proposal for a new dosing regimen in Chinese patients. Eur. J. Clin. Pharmacol. 2007;63(12):1135–1141. doi: 10.1007/s00228-007-0381-6. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- M-S Wen.pdf. doi: 10.1038/sj.clpt.6100453[published Online First: Epub Date].
- Mushiroda T., Ohnishi Y., Saito S. Association of VKORC1 and CYP2C9 polymorphisms with warfarin dose requirements in Japanese patients. Eur. J. Hum. Genet. 2006;51(3):249–253. doi: 10.1007/s10038-005-0354-5. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Osinbowale O., Al Malki M., Schade A., Bartholomew J.R. An algorithm for managing warfarin resistance. Cleve. Clin. J. Med. 2009;76(12):724–730. doi: 10.3949/ccjm.76a.09062. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Rieder M.J., Reiner A.P., Gage B.F. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N. Engl. J. Med. 2005:1533–4406. doi: 10.1056/NEJMoa044503. (Electronic) [DOI] [PubMed] [Google Scholar]
- Sanderson S., Emery J., Higgins J. CYP2C9 gene variants, drug dose, and bleeding risk in warfarin-treated patients: a systematic review and meta-analysis. Genetics In Medicine. 2005;7(2):97–104. doi: 10.1097/01.gim.0000153664.65759.cf. [DOI] [PubMed] [Google Scholar]
- Scott S.A., Edelmann L., Kornreich R., Desnick R.J. Warfarin pharmacogenetics: CYP2C9 and VKORC1 genotypes predict different sensitivity and resistance frequencies in the Ashkenazi and Sephardi Jewish populations. American Journal Of Human Genetics. 2008;82(2):495–500. doi: 10.1016/j.ajhg.2007.10.002. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S.L., Li Z., Zhang W. Cytochrome P450 oxidoreductase genetic polymorphisms A503V and rs2868177 do not significantly affect warfarin stable dosage in Han-Chinese patients with mechanical heart valve replacement. Eur. J. Clin. Pharmacol. 2013;69(10):1769–1775. doi: 10.1007/s00228-013-1544-2. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Tang X.F., Zhang W., Zhang F.C. Effects of VKORC1 gene polymorphisms on warfarin doses in Han Chinese patients with venous thromboembolism. Clin. Med. J. 2009;7(3):44–50. (Article in Chinese) [Google Scholar]
- Veenstra D.L., You J.H., Rieder M.J. Association of Vitamin K epoxide reductase complex 1 (VKORC1) variants with warfarin dose in a Hong Kong Chinese patient population. Pharmacogenet. Genomics. 2005;15:687–691. doi: 10.1097/01.fpc.0000174789.77614.68. [DOI] [PubMed] [Google Scholar]
- Visscher P.M., Takeuchi F., McGinnis R. A genome-wide association study confirms VKORC1, CYP2C9, and CYP4F2 as principal genetic determinants of warfarin dose. PLoS Genet. 2009;5(3) doi: 10.1371/journal.pgen.1000433. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadelius M., Chen L.Y., Downes K. Common VKORC1 and GGCX polymorphisms associated with warfarin dose. Pharm. J. 2005;5(4):262–270. doi: 10.1038/sj.tpj.6500313. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Wadelius M., Chen L.Y., Lindh J.D. The largest prospective warfarin-treated cohort supports genetic forecasting. Blood. 2009;113(4):784–792. doi: 10.1182/blood-2008-04-149070. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Zhong W. Effects of CYP2C9*3 and VKORC1 gene polymorphisms on warfarinin prevention of deep venous thrombosis of lower limbs after orthopedic surgery. Journal of Shanghai Jiaotong University (Medical science) 2013;33:1360–1368. (Article in Chinese) [Google Scholar]
- Wang Z., Yang D., Li Y.L., Yuan B.X., Yuan H.Y.L. Impact of CYP4F2 genetic polymorphisms on warfarin maintenance dose. Guangdong Medical Journal. 2011;32:3091–3094. (Article in Chinese) [Google Scholar]
- Wei M., Ye F., Xie D. A new algorithm to predict warfarin dose from polymorphisms of CYP4F2, CYP2C9 and VKORC1 and clinical variables: derivation in Han Chinese patients with non valvular atrial fibrillation. Thromb. Haemost. 2012;107(6):1083–1091. doi: 10.1160/TH11-12-0848. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Wypasek E., Branicka A., Awsiuk M., Sadowski J., Undas A. Genetic determinants of acenocoumarol and warfarin maintenance dose requirements in Slavic population: a potential role of CYP4F2 and GGCX polymorphisms. Thromb. Res. 2014;134(3):604–609. doi: 10.1016/j.thromres.2014.06.022. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Xie H., Kim R.B., Wood A.J.J., Stein C.M. Molecular basis of ethnic differences in drug disposition and response. Annu. Rev. Pharmacol. Toxicol. 2001;41(1):815. doi: 10.1146/annurev.pharmtox.41.1.815. [DOI] [PubMed] [Google Scholar]
- Yang L., Han M.H. Master's Thesis of Kunming Medical University. 2012. CYP2C9 gene polymorphism influence patients with atrial fibrillations warfarin doses. (Article in Chinese) [Google Scholar]
- Yang L., Ge W., Yu F., Zhu H. Impact of VKORC1 gene polymorphism on interindividual and interethnic warfarin dosage requirement-a systematic review and meta analysis. Thromb. Res. 2010;125(4):e159–e166. doi: 10.1016/j.thromres.2009.10.017. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Yang J., Huang C., Shen Z., Miao L. Contribution of 1173C > T polymorphism in the VKORC1 gene to warfarin dose requirements in Han Chinese patients receiving anticoagulation. Int. J. Clin. Pharmacol. Ther. 2011;49(01):23–29. doi: 10.5414/cpp49023. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Ye C., Jin H., Zhang R. Variability of warfarin dose response associated with CYP2C9 and VKORC1 gene polymorphisms in Chinese patients. J. Int. Med. Res. 2014;42(1):67–76. doi: 10.1177/0300060513499094. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Yoshizawa M., Hayashi H., Tashiro Y. Effect of VKORC1-1639 G > A polymorphism, body weight, age, and serum albumin alterations on warfarin response in Japanese patients. Thromb. Res. 2009;124(2):161–166. doi: 10.1016/j.thromres.2008.11.011. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Yuan H.Y., Chen J.J., Lee M.T. A novel functional VKORC1 promoter polymorphism is associated with inter-individual and inter-ethnic differences in warfarin sensitivity. Hum. Mol. Genet. 2005;14(13):1745–1751. doi: 10.1093/hmg/ddi180. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Zhang L., He L., Du Y.K., Li M., Tang H.N. Relationship between warfarin maintainance dose after mechanical heart valve prostheses implantation and polymorphisms of CYP2C9 on low anticoagulant level. Chin. J. Health Lab. Technol. 2007;17:1193–1195. (Article in Chinese) [Google Scholar]
- Zhang W.P., Chen H., Luo J.W., Wu X.Y. Influence of VKORC1–1639G/A and CYP2C9 1061A/C polymorphisms on warfarin dose. J. Med. Mol. Biol. 2007;4:396–400. (published Online First: Epub Date (Article in Chinese)) [Google Scholar]
- Zhang W., Zhang W.J., Zhu J. Genetic polymorphisms are associated with variations in warfarin maintenance dose in Han Chinese patients with venous thromboembolism. Pharmacogenomics. 2012;13(3):309–321. doi: 10.2217/pgs.11.147. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Zhang H.Y., Luo W.W., Ma S.M., Xu L.H. Effect of CYP4F2 genetic polymorphisms on warfarin maintenance dose. Pharm. Clin. Res. 2012;18:338–340. (Article in Chinese) [Google Scholar]
- Zheng C., Mei D., Guan Y. Effect of CYP2C9 genetic polymorphisms on warfarin dosage and anticoagulation. Chin. J. Pharm. 2008;43:862–865. (Article in Chinese) [Google Scholar]
- Zhuang W., Wu D., Wang Z. Influence of warfarin related genes and non- genetic factors on administrative dose in Shanghai area. Chin. J. Hemotal. 2014;35:13–17. doi: 10.3760/cma.j.issn.0253-2727.2014.01.004. (Article in Chinese) [DOI] [PubMed] [Google Scholar]