Summary
Chronic inflammation and proinflammartory mediators contribute tumor-associated immunosuppression. An immunosuppressive microenvironment allows advantages for tumor formation, growth and progression. In this review, we highlight recent advances in our understanding of how proinflammatory mediators modulate an immunosuppressive microenvironment via attraction of immunosuppressive cells and activation of immune checkpoint pathways.
Abstract
Chronic inflammation contributes to cancer development via multiple mechanisms. One potential mechanism is that chronic inflammation can generate an immunosuppressive microenvironment that allows advantages for tumor formation and progression. The immunosuppressive environment in certain chronic inflammatory diseases and solid cancers is characterized by accumulation of proinflammatory mediators, infiltration of immune suppressor cells and activation of immune checkpoint pathways in effector T cells. In this review, we highlight recent advances in our understanding of how immunosuppression contributes to cancer and how proinflammatory mediators induce the immunosuppressive microenvironment via induction of immunosuppressive cells and activation of immune checkpoint pathways.
Introduction
Inflammation is typically referred to as either acute or chronic. Acute inflammation caused by physical or chemical injury or by an infectious agent is meant to provide an early beneficial response that helps eliminate pathogens and necrotic cells as well as initiates the healing process at the site of tissue injury. This inflammatory process is self-limiting and resolves after tissue repair or elimination of pathogens. During the resolution of inflammation, the levels of proinflammatory mediators and infiltrated immune cells decline and resolvins are produced. Resolvins are generated from eicosapentaenoic acid and docosahexaenoic acid via cyclooxygenase (COX) pathway and exhibit both anti-inflammatory and proresolving actions. By contrast, chronic inflammation caused by infectious or autoimmune diseases is a prolonged abnormal immune response that is not terminated by the normal feedback mechanisms. Clinical and epidemiologic evidence indicates that chronic inflammation is a risk factor for several gastrointestinal malignancies, including esophageal, gastric, hepatic, pancreatic and colorectal cancer (CRC). For example, it has been long known that patients with persistent hepatitis B infection, Helicobacter pylori infection or autoimmune disorders such as inflammatory bowel diseases (IBD) face an increased lifetime risk of developing liver, gastric and CRC. For example, more than 20% of patients with ulcerative colitis were reported to develop colitis-associated CRC within 30 years of diagnosis (1). It has been estimated that chronic inflammation contributes to the development of ~15–20% of malignancies worldwide (2). The observation that non-steroidal anti-inflammatory drugs have beneficial effects on reducing the incidence, metastasis and mortality of various solid tumors (3–6) supports the concept that chronic inflammation promotes tumor initiation, growth and progression.
It is generally thought that chronic inflammation promotes tumor initiation, progression and metastasis by providing a tumor-supporting microenvironment. In addition, tumors are referred to as ‘wounds that do not heal’ and chronic inflammation is clearly found in the tumor microenvironment that is probably initiated by the presence of malignant cells. The common pathological features of chronic inflammatory diseases and solid cancers include elevation of proinflammatory mediators such as cytokines, chemokines and prostaglandins; massive infiltration of deregulated immune cells and recruitment of endothelial cells and fibroblasts (7–9). The proinflammatory mediators orchestrate crosstalk between various cells to create a tumor-supporting microenvironment, including immunosuppression and angiogenesis, which allows tumor formation, growth and progression. In this review, we mainly focus on recent insights of how chronic inflammation contributes to tumor initiation and how immunosuppression induced by chronic inflammation and malignant cells promotes tumor growth and progression. Understanding these mechanisms may provide a rationale for developing more effective therapeutic strategies to eliminate cancer stem-like cells and to subvert tumor-induced immunosuppression for patients with cancer.
Inflammatory microenvironment
In the normal gut, the immune system maintains a balance between tolerance to gut flora and protection from harmful pathogens by providing multiple safeguards for immune homeostasis. In IBD, chronic inflammation is thought to result from disruption of immune homeostasis in response to the gut flora, which contains foreign luminal antigens from food and commensal bacteria. The common pathological changes associated with chronic inflammation in IBD, H.pylori-associated gastritis and autoimmune gastritis include elevation of proinflammatory mediators, massive infiltration of dysregulated immune cells and diminished epithelial barrier integrity. This inflammatory microenvironment is thought to initiate epithelial cell transformation and to promote tumor growth and progression (10).
Although the molecular mechanisms underlying contribution of chronic inflammation to carcinogenesis are not fully understood, nuclear factor-kappaB (NF-κB) signaling and certain cytokines, such as interleukin (IL)-6, IL-17, IL-22 and IL-23, have been shown to be essential to link inflammation to tumorigenesis in mouse models of colitis-associated CRC (11–13). H.pylori infection that is associated with gastric chronic inflammation and cancer activates NF-κB, which in turn induces proinflammatory genes such as IL-1, IL-6, IL-8 [C-X-C motif chemokine ligand 8 (CXCL8)], tumor necrosis factor-α and COX-2 as well as inducible nitric oxide synthase and vascular endothelial growth factor (14). These proinflammatory mediators can induce expression of chemokines that are responsible for recruitment of leukocytes from the circulation system to local tissue sites. For example, a recent study showed that COX-2-derived prostaglandin E2 (PGE2) secreted from mouse colonic epithelial calls and macrophages stimulated macrophages to produce CXCL1, C–C motif chemokine ligand 2 (CCL2), CCL3, CCL4, IL-6, and IL-1β in a mouse model of IBD (15). CXCL1, CCL2, CCL3 and CCL4 were shown to correlate with the severity of disease in IBD patients (16). Genetic and pharmacologic studies have demonstrated that CXCL1, CCL2, CCL3 or CCL4 signaling promotes inflammation in models of injury-induced experimental colitis (17–20).
Chronic inflammation and tumor initiation
Chronic inflammation initiates tumor formation through induction of reactive oxygen and nitrogen species and/or DNA methylation. Inflammation-induced oxidative stress may increase the risk of developing colorectal, gastric and liver cancer (21–24). Reactive oxygen and nitrogen species produced by inflammatory cells are associated with mutation of key genes such as tumor suppressor and DNA repair genes (25). In addition, proinflammatory mediators such as IL-6 and PGE2 stimulate tumor initiation by silencing tumor suppressor and/or DNA repair genes via induction of DNA methylation (26,27). Activation of NF-κB can enhance Wnt-signaling leading to the dedifferentiation of non-stem tumor epithelial cells into tumor-initiating cells in mouse intestine (28).
Chronic inflammation and immunosuppressive cells
Emerging evidence indicates that chronic inflammation also induces immunosuppression via induction of proinflammatory mediators and accumulation/activation of immune suppressor cells (29). Myeloid-derived suppressor cells (MDSCs), one type of immune suppressor cell, are greatly expanded in autoimmune diseases such as IBD (30). Since MDSCs are a heterogenous population of immature myeloid cells including progenitors of macrophages, dendritic cells (DCs) and granulocytes, expansion of MDSCs disrupts the normal homeostasis by interrupting maturation of macrophages, DCs and granulocytes.
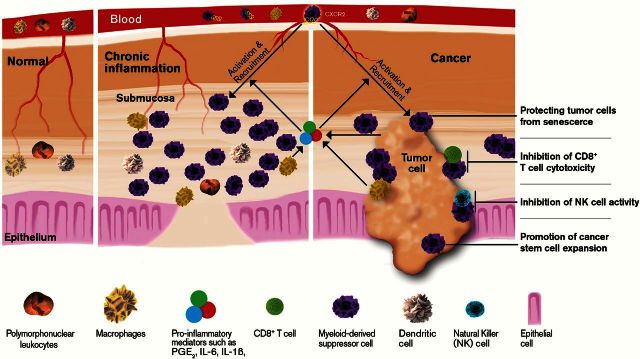
Several animal studies demonstrate that MDSCs are a targetable link between chronic inflammation and cancer (Figure 1). Depletion of MDSCs during colonic inflammation attenuated colitis-associated tumorigenesis in a mouse model of IBD-associated carcinogenesis (31). By contrast, transfer of MDSCs promoted chronic inflammation in the colon and colitis-associated tumor formation, growth and progression via suppression of colonic CD8+ T-cell cytotoxicity against tumor cells (18). These findings suggest that chronic inflammation might promote tumor initiation and progression by induction of immunosuppression via MDSCs. Moreover, proinflammatory mediators induce MDSC expansion and recruitment (Figure 1). For example, IL-1β, IL-6 and PGE2 have been shown to induce MDSC accumulation and activation (32,33). Moreover, a chemokine receptor, CXCR2, is required for infiltration of MDSCs from the circulatory system to inflamed colonic mucosa and colitis-associated tumors in a mouse model of colitis-associated tumorigenesis (18). Similarly, CXCL8-overexpressing transgenic mice exacerbated inflammation and promoted inflammation-associated tumorigenesis with more infiltration of MDSCs into colonic and gastric mucosa in mouse models of colitis-associated carcinogenesis and H. felis-induced gastritis (34). CXCL8 is one of the CXCR2 ligands. Stomach-specific overexpression of IL-1β in mice resulted in spontaneous gastric inflammation and cancer with infiltration of MDSCs into the stomach (35). Collectively, these results suggest that proinflammatory mediators promote chronic inflammation and inflammation-associated tumorigenesis via MDSCs. However, it is unclear why MDSCs in a chronic inflammatory environment do not function as immunosuppressive cells.
Figure 1.
The role of MDSCs in chronic inflammation and cancer.
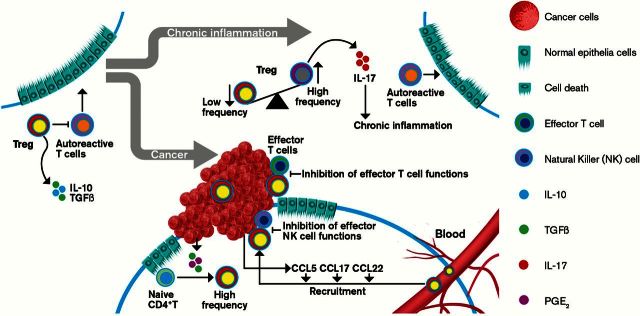
Regulatory T cells (Tregs) can also serve as immune suppressor cells and are mainly a subset of CD4+ T cells that express high levels of CD25 and Foxp3. Tregs are essential for maintaining self-tolerance and suppressing immune responses by regulating the activity of other immune cells in prevention and control of autoimmune diseases (Figure 2). The functions of Tregs are dependent on both cell–cell contact and secretion of immunosuppressive cytokines IL-10 and transforming growth factor (TGF)-β (36). In contrast to MDSCs, Tregs play a key role in prevention and control of IBD and gastritis (Figure 2). In IBD patients, reduction of Tregs and elevation of Th17 cells were observed in the peripheral blood and proinflammatory cytokines such as IL-17a, IL-1β and IL-6 were elevated in intestinal mucosa (37). It is unclear whether IL-1β and/or IL-6 regulate the ratio between Tregs and Th17 cells in IBD. In H.pylori-infected patients, the numbers of Tregs in gastric mucosa or peripheral blood are negatively correlated with the level of inflammation (38). Moreover, in vivo studies have demonstrated that Tregs function as immunosuppressive cells in IBD, H.pylori-associated gastritis and autoimmune gastritis. Transfer of Tregs completely prevented and ameliorated inflammation in a murine T-cell transfer model of colitis (39). Transfer of iTregs that were induced by treatment of naive T cells with TGF-β1 and IL-2 in vitro into mice with the late stages of autoimmune gastritis suppressed disease progression (40). By contrast, depletion of Tregs exacerbated gastric inflammation and elevated proinflammatory cytokine expression in H.pylori-infected mice (41). These studies suggest that Tregs play a key role in the prevention of autoimmune responses and H.pylori-associated gastritis. However, the function of Tregs in connecting chronic inflammation to cancer remains unknown. Emerging evidence revealed that Tregs which expanded in intestinal adenomas no longer produced IL-10 and instead switched to production of IL-17 in vivo (42). The number of IL-17-producing Tregs was found to be significantly increased in inflamed mucosa of IBD patients compared with healthy individuals and associated with colitis-associated tumorigenesis (43,44), suggesting that proinflammatory mediators produced by chronic inflammation may convert Tregs to IL-17-producing Tregs (Figure 2). Indeed, IL-1β–IL-1R1 signaling induced the conversion of IL-17-producing Tregs from Tregs (45,46). Since IL-17-producing Tregs lose their anti-inflammatory function and promote CRC development (47), it is conceivable that IL-17-producing Tregs induced by proinflammatory cytokines are a potential cellular link between chronic inflammation and carcinogenesis (Figure 2). A recent observation that transient depletion of Tregs during inflammation inhibited colitis-associated tumorigenesis in vivo (48) supports this hypothesis.
Figure 2.
The role of Tregs in chronic inflammation and cancer.
Inflammation and immune checkpoint molecules
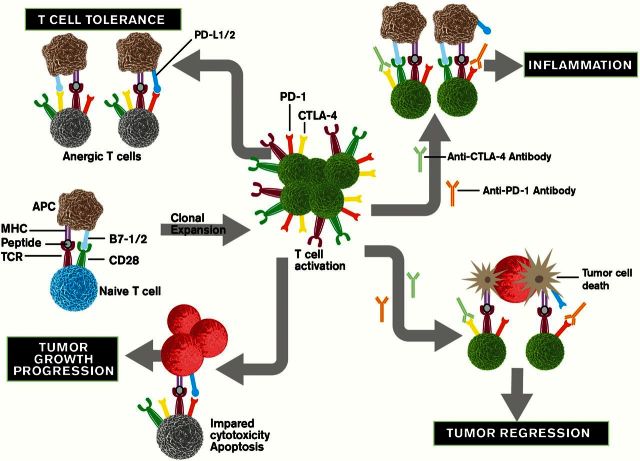
T-cell activation requires antigen-specific TCR stimulation and activation of an antigen-independent costimulatory receptor (CD28). On the other hand, coinhibitory signals suppress antigen-specific T-cell responses. The costimulatory receptor and coinhibitory receptors control the balance of T-cell activation and tolerance (Figure 3). Therefore, autoimmune conditions may occur when this balance is disturbed. Programmed death (PD)-1 and cytotoxic T-lymphocyte-associated antigen (CTLA)-4 are the coinhibitory receptors and are mainly expressed on T cells. Although both PD-1 and CTLA-4 function as negative regulators, they play a non-redundant role in inhibition of immune responses. Interaction of PD-1 with its ligands, PD-L1 and PD-L2, inhibits effector T-cell activation and proliferation. Although both CTLA-4 and CD28 bind to same ligands, CD80 (B7-1) and CD86 (B7-2), which are expressed in antigen-presenting cells (APCs), CTLA-4 has higher affinity and avidity for B7-1 and B7-2 than CD28. Therefore, binding of CTLA-4 with its ligands, B7-1 and B7-2, suppresses the early activation and survival of naive and memory T cells by competing with CD28 binding.
Figure 3.
The role of checkpoint pathways in chronic inflammation and cancer.
Data from animal studies suggest that the coinhibitory signals play a key role in controlling the progress of immune response and reducing the risk for development of chronic inflammation (Figure 3). Deletion of PD-1 in neonatal thymectomized mice led to autoimmune gastritis and hepatitis (49,50). Blockage PD-1/PD-L1 signaling resulted in CD8+ T-cell-mediated intestinal inflammation by elimination of CD8+ T-cell tolerance to intestinal self-antigens (51). Cytokines such as IL-2, IL-7, IL-15 and IL-21 induce the expression of PD-1 in peripheral T cells and its ligands in peripheral monocytes/macrophages (52). The role of PD-1 signaling in connecting inflammation to cancer is unknown. One observation that elevation of PD-L1 expression in intestinal epithelial cells of IBD patients and in gastric epithelial cells of H.pylori-infected patients (53,54) may support the idea that PD-1 signaling may mediate the contribution of chronic inflammation to carcinogenesis by preventing transformed epithelial cells from CD8+ T-cell attack. Further studies are needed to test this hypothesis.
CTLA-4 is minimally expressed on resting T cells and is induced after T-cell activation. In acute infections, CTLA-4 is transiently induced and binds to B7-1 and B7-2, competing with CD28 binding following T-cell activation, which in turn attenuates the T-cell response by counteracting CD28-mediated costimulatory signals. By contrast, CTLA-4 is constitutively expressed in T cells during chronic infections and cancer because of chronic antigen exposure. CTLA-4 is also constitutively expressed on antigen-experienced memory CD4+ and CD8+ T cells as well as Tregs. Similarly, B7-1 is not expressed on resting APCs and is induced after APC activation. By contrast, B7-2 is constitutively expressed on resting APCs and its expression is further induced after APC activation. Inhibition of CTLA-4 signaling by its antibody, ipilimumab, induces bowel inflammation in patients with melanoma (55), suggesting that this signaling is important for maintenance of immune homeostasis in gut (Figure 3). However, the role of CTLA-4 in H.pylori-associated gastritis is not clear because the results from mouse models of H.pylori-associated gastritis are controversial. One report showed that blockage of CTLA-4 accelerated gastric inflammation induced by H.pylori infection (56), whereas another study showed that CTLA-4 blockage reduced H.pylori-induced gastric inflammation (57). The reason for this discrepancy is not known. Further work is necessary to clarify the role of CTLA-4 in autoimmune gastritis and H.pylori-induced gastritis.
Since Tregs constitutively express CTLA-4 (58,59), loss of CTLA-4 in Tregs led to fatal systemic autoimmune disease (60). Moreover, deletion of B7 in mice expressing a soluble B7-2 Ig Fc chimeric protein resulted in more severe colitis with reduction of Tregs (61). Blockage of CTLA-4 by its antibody abrogated the effect of Tregs on prevention of colitis in vivo (58,59). These results demonstrate that CTLA-4 is required for Treg development and function in control of colitis. Collectively, these results suggest that checkpoint signaling is important for prevention and control of chronic inflammation. The question is whether the checkpoint pathways are involved in contribution of chronic inflammation to carcinogenesis.
Tumor microenvironment
Similar to chronic inflammation, malignant cells secrete proinflammatory mediators such as cytokines, chemokines and eicosanoids that recruit and reprogram various types of proinflammatory leukocytes and other cells to establish a more tumor-supportive microenvironment. The tumor microenvironment not only allows tumor cells to evade from host immunosurveillance but also supports tumor growth, progression and spread by inducing angiogenesis and formation of cancer-like stem cells. Cancer immune evasion involves a shift from Th1 to Th2 immune responses, a defective APC function, impaired cytotoxic activity of CD8+ T cells and natural killer (NK) cells and enhancement of immunosuppressive cells such as MDSCs and Tregs. Here, we focus on the multipronged immunosuppressive network that develops in the tumor microenvironment.
Myeloid-derived suppressor cells
In healthy individuals, immature myeloid cells differentiate into mature myeloid cells including macrophages, DCs and granulocytes. However, this normal physiological process is interrupted in cancer patients (Figure 1). In general, there are small numbers of MDSCs in the circulation (3–5%) of healthy individuals, but their numbers are significantly increased in blood and tumor tissues of patients with cancer (Figure 1). The levels of MDSCs in the blood and/or tumor tissue are positively correlated with clinical cancer stage, metastatic tumor burden or poor survival in patients with colon, esophageal, gastric or pancreatic cancers (62–68).
MDSCs have been shown to contribute to cancer immune evasion by suppressing effector T-cell activation, proliferation, trafficking and viability; inhibiting NKs; and promoting activation and expansion of Treg cells (69). In addition, new evidence reveals novel mechanisms by which MDSCs promote cancer progression and metastasis by directly targeting cancer stem-like cells and tumor cells (Figure 1). MDSCs directly enhanced cancer stem-like cell formation and protected proliferating tumor cells from senescence without involvement of T cells and NKs in vivo (70,71). In tumor implantation models, inhibition of CXCR2 by its antagonist reduced MDSCs abundance in breast tumors (72). Similarly, knockdown of CXCL1/2, ligands of CXCR2, in a breast cancer cell line is associated with reduction of myeloid cells in the tumor (73). These results suggest that CXCR2 is required for infiltration of MDSCs into tumor sites.
Proinflammatory proteins S100A8/9 have been shown to promote tumor growth by induction of MDSC accumulation and inhibition of MDSC differentiation (74). In addition, IL-1β, IL-6 and PGE2 secreted from tumor cells and/or their stromal cells also induce MDSC accumulation and/or activation in the tumor microenvironment (32,33) (Figure 1). IL-1β and IL-6 promote the expansion of MDSCs by induction of myelopoiesis and inhibition of the differentiation of mature myeloid cells via STAT3 (75). Furthermore, IL-1β activated MDSCs via an IL-1R1–NF-κB pathway in gastric inflammation and cancer (35), whereas IL-6 activated breast cancer-infiltrating MDSCs via a STAT3–NF-κB–IDO pathway (76). Other cytokines and growth factors such as interferon γ, IL-4, IL-13 and TGF-β mainly secreted following tumor cell death, and T cells have been shown to activate MDSCs via STAT3 (75). In addition, miR-155 and miR-21 may mediate the effects of granulocyte–macrophage colony-stimulating factor and IL-6 on induction of MDSCs from mouse bone marrow cells (77).
In addition to proinflammatory cytokines, inflammatory PGE2 also plays a central role in regulation of MDSC accumulation and activation (Figure 1). PGE2 promoted tumor growth via inducing the differentiation of MDSCs from bone marrow myeloid progenitor cells, whereas inhibition of PGE2 signaling by deletion of prostaglandin E2 receptor (EP2) or its antagonists blocked this differentiation in mice implanted with 4T1 mammary carcinoma (78). PGE2 has been shown to convert DCs to MDSCs in vitro (79). PGE2 directly activated MDSC-mediated T-cell suppression by induction of arginase I expression via the EP4 receptor (80). Reduction of PGE2 levels in mesothelioma-bearing mice by celecoxib treatment suppressed MDSC accumulation and activation (81). Inhibition of tumor-derived PGE2 by silencing COX-2 in 4T1 cancer cells reduced the accumulation of MDSCs in the spleen (82). One potential mechanism responsible for PGE2 induction of MDSC accumulation could be that PGE2 induces chemokines that attract MDSCs into the tumor microenvironment from the circulation. Indeed, deletion of COX-2 or treatment with non-steroidal anti-inflammatory drugs inhibits gliomagenesis by reducing infiltration of MDSCs into tumor microenvironment via CCL2 in vivo (83). PGE2 has also shown to induce CXCR2 ligand expression in inflamed colonic mucosa and colitis-associated tumors from azoxymethane/dextran sodium sulfate-treated mice as well as intestinal mucosa and tumors from Apc min/+ mice (18), suggesting that PGE2 induces an infiltration of MDSCs into sites of inflammation and in solid tumors through induction of CXCR2 ligands. Further studies are needed to test this hypothesis.
Regulatory T cells
In contrast to autoimmune diseases, Tregs are thought to contribute to cancer-induced immunosuppression by suppressing effector T cells, NKs and DCs. The frequency of Tregs is elevated in the peripheral blood and at the tumor sites of patients with esophageal cancer, gastric cancer or CRC (84–86) and tumor-infiltrating Tregs correlate with poor prognosis in esophageal, gastric and ovarian cancers (87,88). In tumor-bearing mice, depletion of Tregs resulted in regression of many tumors, including CRC, by evoking immunosurveillance, whereas adoptive transfer of Tregs suppressed CD8+ T-cell cytotoxicity against tumor (89–93). These findings indicate that Tregs promote tumor escape from cytotoxic immune responses (Figure 2).
Tumor-infiltrated Tregs include infiltration of thymus-derived CD4+CD25+FoxP3+ T cells, local expansion of Tregs and local differentiation from CD4+ T cells. CCL22 has been shown to recruit Tregs into tumors via its receptor, CCR4 (87,94). Treatment with CCR4 antagonists enhanced the efficacy of cancer vaccines against tumor growth by reduction of infiltration of Tregs (95). Moreover, CCL17 and CCL22 are correlated with Treg infiltration in gastric cancer (85). In addition, CCL5 secreted from CRC is not only required for infiltration of Tregs into tumors but also enhances cytotoxicity of Tregs against CD8+ T cells via induction of TGF-β in vivo (96). Neutralization of CCL5 inhibited the infiltration of Tregs into tumors (97). These studies suggest that chemokines such as CCL5, CCL17 and/or CCL22 mediate the trafficking of Tregs into tumor microenvironment (Figure 2). In addition to infiltration of Tregs into tumors, TGF-β-secreting DCs in tumor microenvironment induces Tregs proliferation (98). In vitro studies further revealed that TGF-β secreted from tumor cells converts CD4+CD25− T cells into Tregs (99) (Figure 2). Treatment of patients with melanoma or renal cell carcinoma (RCC) with IL-2 increased Tregs in blood and/or tumors (100,101). By contrast, anti-VEGF-A treatment reduced peripheral Treg proliferation in CRC patients and CRC-bearing mice (102). Similarly, PGE2 secreted from mature DCs attracted Tregs via CCL22 (103). PGE2 can also directly enhance the differentiation of naive CD4+ T cells into Tregs in vitro and induce Treg activation in lung cancer in vivo (104,105) (Figure 2). Deletion of mPges-1 gene suppressed AOM-induced colon carcinogenesis accompanied with reduced frequency of Tregs in the draining mesenteric lymph nodes and lowered serum PGE2 levels (106). In addition, treatment with an EP4 antagonist resulted in a decreased number of Tregs in lymph nodes and the skin after ultraviolet irradiation (107). These results indicate that PGE2 may enhance tumor growth via Tregs. Although the role of Tregs in the tumor microenvironment is well established, their role in connecting chronic inflammation to cancer is not known.
Immune checkpoint molecules
Besides the role of MDSCs and Tregs in tumor-induced immunosuppression, malignant cells can also escape from immunosurveillance by directly impairing cytotoxic activity and proliferation of CD8+ T cells through PD-1 and CTLA-4 receptors (Figure 3). PD-1 is transiently induced in activated T cells (108) and its expression is maintained in tumor-infiltrating effector T cells (109–111). PD-L1 expression is elevated in various human cancers, including lung, colon, head and neck, and ovarian cancers as well as melanomas (112–114), and its expression is associated with poor prognosis among patients with esophageal, colon, ovarian or RCC (115–118). Although transgenic mice with PD-L1 expression driven by the keratin-14 promoter did not develop skin cancer spontaneously, these mice were much more sensitive to carcinogen-induced skin cancer formation (119). These findings suggest that PD-1 signaling plays an important role in tumor-induced immune evasion and that PD-1 and PD-L1 are promising immunotherapeutic targets for cancer patients (Figure 3). Indeed, recent clinical trials have demonstrated that blockage of PD-1 signaling by anti-PD-1 or anti-PD-L1 antibodies are benefits for patients with advanced melanoma, RCC or non-small cell lung cancer (NSCLC) (120).
Emerging evidence further revealed that oncogenes, tumor suppressive genes and proinflammatory cytokines regulate PD-L1 expression. Elevated PD-L1 expression is associated with epidermal growth factor receptor mutations in both human and mouse NSCLC (121,122). Although previous studies reveal that PD-L1 is expressed in immune cells, recent studies show that it is also aberrantly expressed in cancer cells. High levels of PD-L1 on cancer cells are one potential mechanism underlying tumor evasion from immunosurveillance. In vitro studies demonstrated that loss of phosphatase and tensin homolog resulted in induction of PD-L1 expression in glioma and CRC cells (117,123) and overexpression of mutated epidermal growth factor receptor led to induction of PD-L1 in immortalized bronchial epithelial cells (121). Interferon γ is also able to induce PD-L1 expression in lung, ovarian and colon cancer cell lines (112). In addition, Toll-like receptor 4 signaling induced PD-L1 expression via mitogen-activated protein kinase pathways in bladder cells (124). However, little information of PD-L2 expression in human cancer is available.
Little is known about how oncogenes, tumor suppressive genes and proinflammatory mediators regulate CTLA-4 and its ligands. Similar to antibodies against PD-1 and its ligands, the antibodies against CTLA-4, including ipilimumab and tremelimumab, have been evaluated in multiple clinical trials and demonstrated significant promise in treatment of advanced melanoma, NSCLC, RCC and prostate cancer (120) (Figure 3). Ipilimumab is the first immune checkpoint-blocking antibody approved by Food and Drug Administration in 2011 for patients with metastatic melanoma. Although immunotherapies with these immune checkpoint antibodies against PD-1, PD-L1 and CTLA-4 offer great promise for treatment of many malignancies, the objective response rate of these antibodies is less than 30% in patients with melanoma, RCC and NSCLC and few responses observed in patients with colorectal, pancreatic, gastric or breast cancer (120). These agents seem more effective when the mutational burden is high in the primary cancer or when T cells have infiltrated into the tumor microenvironment.
Conclusion and perspective
The standard treatment such as chemotherapy and radiotherapy for advanced malignancies has improved over the past decades, but clinical outcomes have not improved as much as we would like because these therapies only target tumor cells and are associated with numerous side effects. Immunotherapy with immune checkpoint inhibitors is a promising approach for cancer treatment. However, the success rates are somewhat limited because of an immunosppressive tumor microenvironment. A growing body of evidence supports the hypothesis that effective therapies should include elimination of tumor cells, subverting tumor-induced immunosuppression by targeting immunosuppressive cells and reactivation of tumor-inhibited effector T cells by checkpoint inhibitors. Inhibition of an immunosuppressive tumor microenvironment by targeting immunosuppressive cells would facilitate antitumor immune responses and enhance antitumor effects of chemotherapeutic agents. Targeting MDSCs and Tregs by inactivation or depletion is not feasible now because we lack specific surface markers of these cells. However, recent in vivo studies showed that inhibition of tumor-infiltrating MDSCs by targeting CXCR2 enhanced anti-PD-1 efficacy in rhabdomyosarcoma in vivo (125) and improved the efficacy of chemotherapy in a spontaneous mouse model of prostate cancer (71). These findings suggest that inhibition of MDSC trafficking into the tumor microenvironment by targeting CXCR2 should not only enhance efficacy of immune checkpoint inhibitors or chemotherapeutic agents but also have benefits for cancer patients who did not orginally respond to checkpoint inhibitor treatment. Finally, rational design of combination strategies relies on better understanding of the complexity of the immunosuppressive mechanisms in each cancer type and an accounting for individual variation.
Funding
NIH R01 DK47297; NCI R01 CA184820 and P01 CA77839; National Colorectal Cancer Research Alliance (NCCRA) (to R.N.D.).
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- APC
antigen-presenting cell
- CCL
C–C motif chemokine ligand
- COX
cyclooxygenase
- CRC
colitis-associated colorectal cancer
- CTLA
cytotoxic T-lymphocyte-associated antigen
- CXCL
C–X–C motif chemokine ligand
- DCs
dendritic cells
- IBD
inflammatory bowel diseases
- IL
interleukin
- MDSCs
myeloid-derived suppressor cells
- NF-κB
nuclear factor-kappaB
- NK
natural killer
- NSCLC
non-small cell lung cancer
- PD
programmed death
- PGE2
prostaglandin E2
- RCC
renal cell carcinoma
- TGF
transforming growth factor
- Tregs
regulatory T cells
References
- 1. Lakatos P.L., et al. (2008) Risk for colorectal cancer in ulcerative colitis: changes, causes and management strategies. World J. Gastroenterol., 14, 3937–3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kuper H., et al. (2000) Infections as a major preventable cause of human cancer. J. Intern. Med., 248, 171–183. [DOI] [PubMed] [Google Scholar]
- 3. Algra A.M., et al. (2012) Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol., 13, 518–527. [DOI] [PubMed] [Google Scholar]
- 4. Harris R.E. (2009) Cyclooxygenase-2 (cox-2) blockade in the chemoprevention of cancers of the colon, breast, prostate, and lung. Inflammopharmacology, 17, 55–67. [DOI] [PubMed] [Google Scholar]
- 5. Rothwell P.M., et al. (2012) Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet, 379, 1602–1612. [DOI] [PubMed] [Google Scholar]
- 6. Rothwell P.M., et al. (2012) Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet, 379, 1591–1601. [DOI] [PubMed] [Google Scholar]
- 7. Neuman M.G. (2007) Immune dysfunction in inflammatory bowel disease. Transl. Res., 149, 173–186. [DOI] [PubMed] [Google Scholar]
- 8. Sands B.E. (2007) Inflammatory bowel disease: past, present, and future. J. Gastroenterol., 42, 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Strober W., et al. (2007) The fundamental basis of inflammatory bowel disease. J. Clin. Invest., 117, 514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Noonan D.M., et al. (2008) Inflammation, inflammatory cells and angiogenesis: decisions and indecisions. Cancer Metastasis Rev., 27, 31–40. [DOI] [PubMed] [Google Scholar]
- 11. Greten F.R., et al. (2004) IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell, 118, 285–296. [DOI] [PubMed] [Google Scholar]
- 12. Grivennikov S., et al. (2009) IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell, 15, 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang K., et al. (2013). Implications of anti-cytokine therapy in colorectal cancer and autoimmune diseases. Ann. Rheum. Dis., 72 (suppl. 2), ii100–ii103. [DOI] [PubMed] [Google Scholar]
- 14. Chung H.W., et al. (2014). Role of the tumor microenvironment in the pathogenesis of gastric carcinoma. World J. Gastroenterol., 20, 1667–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang D., et al. (2014) Peroxisome proliferator-activated receptor d promotes colonic inflammation and tumor growth. Proc. Natl Acad. Sci. USA, 111, 7084–7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang D., et al. (2009) The role of chemokines in intestinal inflammation and cancer. Curr. Opin. Pharmacol., 9, 688–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andres P.G., et al. (2000) Mice with a selective deletion of the CC chemokine receptors 5 or 2 are protected from dextran sodium sulfate-mediated colitis: lack of CC chemokine receptor 5 expression results in a NK1.1+ lymphocyte-associated Th2-type immune response in the intestine. J. Immunol., 164, 6303–6312. [DOI] [PubMed] [Google Scholar]
- 18. Katoh H., et al. (2013) CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell, 24, 631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khan W.I., et al. (2006) Critical role of MCP-1 in the pathogenesis of experimental colitis in the context of immune and enterochromaffin cells. Am. J. Physiol. Gastrointest. Liver Physiol., 291, G803–G811. [DOI] [PubMed] [Google Scholar]
- 20. Tokuyama H., et al. (2005) The simultaneous blockade of chemokine receptors CCR2, CCR5 and CXCR3 by a non-peptide chemokine receptor antagonist protects mice from dextran sodium sulfate-mediated colitis. Int. Immunol., 17, 1023–1034. [DOI] [PubMed] [Google Scholar]
- 21. Horiike S., et al. (2005) Accumulation of 8-nitroguanine in the liver of patients with chronic hepatitis C. J. Hepatol., 43, 403–410. [DOI] [PubMed] [Google Scholar]
- 22. Murata M., et al. (2012) Role of nitrative and oxidative DNA damage in inflammation-related carcinogenesis. J. Biomed. Biotechnol., 2012, 623019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rachmilewitz D., et al. (1995) Enhanced colonic nitric oxide generation and nitric oxide synthase activity in ulcerative colitis and Crohn’s disease. Gut, 36, 718–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rieder G., et al. (2003) Up-regulation of inducible nitric oxide synthase in Helicobacter pylori-associated gastritis may represent an increased risk factor to develop gastric carcinoma of the intestinal type. Int. J. Med. Microbiol., 293, 403–412. [DOI] [PubMed] [Google Scholar]
- 25. Hussain S.P., et al. (2000) Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: a cancer-prone chronic inflammatory disease. Cancer Res., 60, 3333–3337. [PubMed] [Google Scholar]
- 26. Gasche J.A., et al. (2011) Interleukin-6 promotes tumorigenesis by altering DNA methylation in oral cancer cells. Int. J. Cancer, 129, 1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xia D., et al. (2012) Prostaglandin E2 promotes intestinal tumor growth via DNA methylation. Nat. Med., 18, 224–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schwitalla S., et al. (2013) Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell, 152, 25–38. [DOI] [PubMed] [Google Scholar]
- 29. Kanterman J., et al. (2012) New insights into chronic inflammation-induced immunosuppression. Semin. Cancer Biol., 22, 307–318. [DOI] [PubMed] [Google Scholar]
- 30. Haile L.A., et al. (2008) Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology, 135, 871–881, 881.e1–881.e5. [DOI] [PubMed] [Google Scholar]
- 31. Poh T.W., et al. (2013) Downregulation of hematopoietic MUC1 during experimental colitis increases tumor-promoting myeloid-derived suppressor cells. Clin. Cancer Res., 19, 5039–5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ostrand-Rosenberg S., et al. (2009) Myeloid-derived suppressor cells: linking inflammation and cancer. J. Immunol., 182, 4499–4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang D., et al. (2013) An inflammatory mediator, prostaglandin E2, in colorectal cancer. Cancer J., 19, 502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Asfaha S., et al. (2013) Mice that express human interleukin-8 have increased mobilization of immature myeloid cells, which exacerbates inflammation and accelerates colon carcinogenesis. Gastroenterology, 144, 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tu S., et al. (2008) Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell, 14, 408–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Izcue A., et al. (2008) Special regulatory T-cell review: Regulatory T cells and the intestinal tract–patrolling the frontier. Immunology, 123, 6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Eastaff-Leung N., et al. (2010) Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J. Clin. Immunol., 30, 80–89. [DOI] [PubMed] [Google Scholar]
- 38. Kandulski A., et al. (2010) Role of regulatory T-cells in H. pylori-induced gastritis and gastric cancer. Anticancer Res., 30, 1093–1103. [PubMed] [Google Scholar]
- 39. Uhlig H.H., et al. (2006) Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J. Immunol., 177, 5852–5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nguyen T.L., et al. (2014) In vitro induced regulatory T cells are unique from endogenous regulatory T cells and effective at suppressing late stages of ongoing autoimmunity. PLoS One, 9, e104698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rad R., et al. (2006) CD25+/Foxp3+ T cells regulate gastric inflammation and Helicobacter pylori colonization in vivo . Gastroenterology, 131, 525–537. [DOI] [PubMed] [Google Scholar]
- 42. Gounaris E., et al. (2009) T-regulatory cells shift from a protective anti-inflammatory to a cancer-promoting proinflammatory phenotype in polyposis. Cancer Res., 69, 5490–5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hovhannisyan Z., et al. (2011) Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology, 140, 957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kryczek I., et al. (2011) IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J. Immunol., 186, 4388–4395. [DOI] [PubMed] [Google Scholar]
- 45. Li L., et al. (2010) IL-1β-mediated signals preferentially drive conversion of regulatory T cells but not conventional T cells into IL-17-producing cells. J. Immunol., 185, 4148–4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Raffin C., et al. (2011) Ex vivo IL-1 receptor type I expression in human CD4+ T cells identifies an early intermediate in the differentiation of Th17 from FOXP3+ naive regulatory T cells. J. Immunol., 187, 5196–5202. [DOI] [PubMed] [Google Scholar]
- 47. Li L., et al. (2013) The role of IL-17-producing Foxp3+ CD4+ T cells in inflammatory bowel disease and colon cancer. Clin. Immunol., 148, 246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pastille E., et al. (2014) Transient ablation of regulatory T cells improves antitumor immunity in colitis-associated colon cancer. Cancer Res., 74, 4258–4269. [DOI] [PubMed] [Google Scholar]
- 49. Kido M., et al. (2008) Fatal autoimmune hepatitis induced by concurrent loss of naturally arising regulatory T cells and PD-1-mediated signaling. Gastroenterology, 135, 1333–1343. [DOI] [PubMed] [Google Scholar]
- 50. Nishiura H., et al. (2013) Interleukin-21 and tumor necrosis factor-a are critical for the development of autoimmune gastritis in mice. J. Gastroenterol. Hepatol., 28, 982–991. [DOI] [PubMed] [Google Scholar]
- 51. Reynoso E.D., et al. (2009) Intestinal tolerance is converted to autoimmune enteritis upon PD-1 ligand blockade. J. Immunol., 182, 2102–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kinter A.L., et al. (2008) The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J. Immunol., 181, 6738–6746. [DOI] [PubMed] [Google Scholar]
- 53. Nakazawa A., et al. (2004) The expression and function of costimulatory molecules B7H and B7-H1 on colonic epithelial cells. Gastroenterology, 126, 1347–1357. [DOI] [PubMed] [Google Scholar]
- 54. Wu Y.Y., et al. (2010) Increased programmed death-ligand-1 expression in human gastric epithelial cells in Helicobacter pylori infection. Clin. Exp. Immunol., 161, 551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Berman D., et al. (2010) Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun., 10, 11. [PMC free article] [PubMed] [Google Scholar]
- 56. Anderson K.M., et al. (2006) Induction of CTLA-4-mediated anergy contributes to persistent colonization in the murine model of gastric Helicobacter pylori infection. J. Immunol., 176, 5306–5313. [DOI] [PubMed] [Google Scholar]
- 57. Watanabe K., et al. (2004) CTLA-4 blockade inhibits induction of Helicobacter pylori-associated gastritis in mice. Clin. Exp. Immunol., 135, 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Read S., et al. (2000) Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J. Exp. Med., 192, 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Takahashi T., et al. (2000) Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med., 192, 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wing K., et al. (2008) CTLA-4 control over Foxp3+ regulatory T cell function. Science, 322, 271–275. [DOI] [PubMed] [Google Scholar]
- 61. Kim G., et al. (2008) Spontaneous colitis occurrence in transgenic mice with altered B7-mediated costimulation. J. Immunol., 181, 5278–5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Diaz-Montero C.M., et al. (2009) Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol. Immunother., 58, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Duffy A., et al. (2013) Comparative analysis of monocytic and granulocytic myeloid-derived suppressor cell subsets in patients with gastrointestinal malignancies. Cancer Immunol. Immunother., 62, 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gabitass R.F., et al. (2011) Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol. Immunother., 60, 1419–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mandruzzato S., et al. (2009) IL4Ralpha+ myeloid-derived suppressor cell expansion in cancer patients. J. Immunol., 182, 6562–6568. [DOI] [PubMed] [Google Scholar]
- 66. Sun H.L., et al. (2012) Increased frequency and clinical significance of myeloid-derived suppressor cells in human colorectal carcinoma. World J. Gastroenterol., 18, 3303–3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang L., et al. (2013) Increased myeloid-derived suppressor cells in gastric cancer correlate with cancer stage and plasma S100A8/A9 proinflammatory proteins. J. Immunol., 190, 794–804. [DOI] [PubMed] [Google Scholar]
- 68. Zhang B., et al. (2013) Circulating and tumor-infiltrating myeloid-derived suppressor cells in patients with colorectal carcinoma. PLoS One, 8, e57114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gabrilovich D.I., et al. (2012) Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol., 12, 253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cui T.X., et al. (2013) Myeloid-derived suppressor cells enhance stemness of cancer cells by inducing microRNA101 and suppressing the corepressor CtBP2. Immunity, 39, 611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Di Mitri D., et al. (2014) Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature, 515, 134–137. [DOI] [PubMed] [Google Scholar]
- 72. Yang L., et al. (2008) Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell, 13, 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Acharyya S., et al. (2012) A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell, 150, 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cheng P., et al. (2008) Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J. Exp. Med., 205, 2235–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gabrilovich D.I., et al. (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol., 9, 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yu J., et al. (2014) Noncanonical NF-kappaB activation mediates STAT3-stimulated IDO upregulation in myeloid-derived suppressor cells in breast cancer. J. Immunol., 193, 2574–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Li L., et al. (2014) MicroRNA-155 and MicroRNA-21 promote the expansion of functional myeloid-derived suppressor cells. J. Immunol., 192, 1034–1043. [DOI] [PubMed] [Google Scholar]
- 78. Sinha P., et al. (2007) Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res., 67, 4507–4513. [DOI] [PubMed] [Google Scholar]
- 79. Obermajer N., et al. (2011) Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood, 118, 5498–5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rodriguez P.C., et al. (2005) Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J. Exp. Med., 202, 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Veltman J.D., et al. (2010) COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma. Celecoxib influences MDSC function. BMC Cancer, 10, 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mao Y., et al. (2014) Inhibition of tumor-derived prostaglandin-e2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity. Clin. Cancer Res., 20, 4096–4106. [DOI] [PubMed] [Google Scholar]
- 83. Fujita M., et al. (2011) COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res., 71, 2664–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ichihara F., et al. (2003) Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin. Cancer Res., 9, 4404–4408. [PubMed] [Google Scholar]
- 85. Mizukami Y., et al. (2008) CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int. J. Cancer, 122, 2286–2293. [DOI] [PubMed] [Google Scholar]
- 86. Wolf A.M., et al. (2003) Increase of regulatory T cells in the peripheral blood of cancer patients. Clin. Cancer Res., 9, 606–612. [PubMed] [Google Scholar]
- 87. Curiel T.J., et al. (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med., 10, 942–949. [DOI] [PubMed] [Google Scholar]
- 88. Kono K., et al. (2006) CD4(+)CD25 high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol. Immunother., 55, 1064–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Antony P.A., et al. (2005) CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J. Immunol., 174, 2591–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Casares N., et al. (2003) CD4+/CD25+ regulatory cells inhibit activation of tumor-primed CD4+ T cells with IFN-gamma-dependent antiangiogenic activity, as well as long-lasting tumor immunity elicited by peptide vaccination. J. Immunol., 171, 5931–5939. [DOI] [PubMed] [Google Scholar]
- 91. Onizuka S., et al. (1999) Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res., 59, 3128–3133. [PubMed] [Google Scholar]
- 92. Shimizu J., et al. (1999) Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J. Immunol., 163, 5211–5218. [PubMed] [Google Scholar]
- 93. Turk M.J., et al. (2004) Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J. Exp. Med., 200, 771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gobert M., et al. (2009) Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res., 69, 2000–2009. [DOI] [PubMed] [Google Scholar]
- 95. Pere H., et al. (2011) A CCR4 antagonist combined with vaccines induces antigen-specific CD8+ T cells and tumor immunity against self antigens. Blood, 118, 4853–4862. [DOI] [PubMed] [Google Scholar]
- 96. Chang L.Y., et al. (2012) Tumor-derived chemokine CCL5 enhances TGF-β-mediated killing of CD8(+) T cells in colon cancer by T-regulatory cells. Cancer Res., 72, 1092–1102. [DOI] [PubMed] [Google Scholar]
- 97. Tan W., et al. (2011) Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature, 470, 548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ghiringhelli F., et al. (2005) Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J. Exp. Med., 202, 919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Liu V.C., et al. (2007) Tumor evasion of the immune system by converting CD4+CD25- T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-beta. J. Immunol., 178, 2883–2892. [DOI] [PubMed] [Google Scholar]
- 100. Ahmadzadeh M., et al. (2006) IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood, 107, 2409–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Jensen H.K., et al. (2009) Increased intratumoral FOXP3-positive regulatory immune cells during interleukin-2 treatment in metastatic renal cell carcinoma. Clin. Cancer Res., 15, 1052–1058. [DOI] [PubMed] [Google Scholar]
- 102. Terme M., et al. (2013) VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res., 73, 539–549. [DOI] [PubMed] [Google Scholar]
- 103. Muthuswamy R., et al. (2008) Ability of mature dendritic cells to interact with regulatory T cells is imprinted during maturation. Cancer Res., 68, 5972–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Baratelli F., et al. (2005) Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J. Immunol., 175, 1483–1490. [DOI] [PubMed] [Google Scholar]
- 105. Sharma S., et al. (2005) Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res., 65, 5211–5220. [DOI] [PubMed] [Google Scholar]
- 106. Nakanishi M., et al. (2011) Selective PGE(2) suppression inhibits colon carcinogenesis and modifies local mucosal immunity. Cancer Prev. Res. (Phila.), 4, 1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Soontrapa K., et al. (2011) Prostaglandin E2-prostaglandin E receptor subtype 4 (EP4) signaling mediates UV irradiation-induced systemic immunosuppression. Proc. Natl Acad. Sci. USA, 108, 6668–6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Barber D.L., et al. (2006) Restoring function in exhausted CD8 T cells during chronic viral infection. Nature, 439, 682–687. [DOI] [PubMed] [Google Scholar]
- 109. Ahmadzadeh M., et al. (2009) Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood, 114, 1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Fourcade J., et al. (2009) PD-1 is a regulator of NY-ESO-1-specific CD8+ T cell expansion in melanoma patients. J. Immunol., 182, 5240–5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mumprecht S., et al. (2009) Programmed death 1 signaling on chronic myeloid leukemia-specific T cells results in T-cell exhaustion and disease progression. Blood, 114, 1528–1536. [DOI] [PubMed] [Google Scholar]
- 112. Dong H., et al. (2002) Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med., 8, 793–800. [DOI] [PubMed] [Google Scholar]
- 113. Strome S.E., et al. (2003) B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res., 63, 6501–6505. [PubMed] [Google Scholar]
- 114. Zhu J., et al. (2014) MiR-20b, -21, and -130b inhibit PTEN expression resulting in B7-H1 over-expression in advanced colorectal cancer. Hum. Immunol., 75, 348–353. [DOI] [PubMed] [Google Scholar]
- 115. Hamanishi J., et al. (2007) Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc. Natl Acad. Sci. USA, 104, 3360–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ohigashi Y., et al. (2005) Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin. Cancer Res., 11, 2947–2953. [DOI] [PubMed] [Google Scholar]
- 117. Song M., et al. (2013) PTEN loss increases PD-L1 protein expression and affects the correlation between PD-L1 expression and clinical parameters in colorectal cancer. PLoS One, 8, e65821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Thompson R.H., et al. (2005) B7-H1 glycoprotein blockade: a novel strategy to enhance immunotherapy in patients with renal cell carcinoma. Urology, 66 (suppl. 5), 10–14. [DOI] [PubMed] [Google Scholar]
- 119. Cao Y., et al. (2011) B7-H1 overexpression regulates epithelial-mesenchymal transition and accelerates carcinogenesis in skin. Cancer Res., 71, 1235–1243. [DOI] [PubMed] [Google Scholar]
- 120. Kyi C., et al. (2014) Checkpoint blocking antibodies in cancer immunotherapy. FEBS Lett., 588, 368–376. [DOI] [PubMed] [Google Scholar]
- 121. Akbay E.A., et al. (2013) Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov., 3, 1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Azuma K., et al. (2014) Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann. Oncol., 25, 1935–1940. [DOI] [PubMed] [Google Scholar]
- 123. Parsa A.T., et al. (2007) Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med., 13, 84–88. [DOI] [PubMed] [Google Scholar]
- 124. Qian Y., et al. (2008) TLR4 signaling induces B7-H1 expression through MAPK pathways in bladder cancer cells. Cancer Invest., 26, 816–821. [DOI] [PubMed] [Google Scholar]
- 125. Highfill S.L., et al. (2014) Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci. Transl. Med., 6, 237ra67. [DOI] [PMC free article] [PubMed] [Google Scholar]