Abstract
Laboratory based studies show that acute aerobic and isometric exercise reduces sensitivity to painful stimuli in young healthy individuals, indicative of a hypoalgesic response. However, little is known regarding the effect of aging on exercise-induced hypoalgesia (EIH). The purpose of this study was to examine age differences in EIH following submaximal isometric exercise, and moderate and vigorous aerobic exercise. Healthy older and younger adults completed one training session and four testing sessions consisting of either a submaximal isometric handgrip exercise, vigorous or moderate intensity stationary cycling, or quiet rest (control). The following measures were taken pre and post exercise/quiet rest: 1) pressure pain thresholds (PPTs), 2) suprathreshold pressure pain ratings, 3) pain ratings during 30-s of prolonged noxious heat stimulation, and 3) temporal summation of heat pain. The results revealed age differences in EIH following isometric and aerobic exercise, with younger adults experiencing greater EIH compared to older adults. The age differences in EIH varied across pain induction techniques and exercise type. These results provide evidence for abnormal pain modulation following acute exercise in older adults.
PERSPECTIVE
This article enhances our understanding of the influence of a single bout of exercise on pain sensitivity and perception in healthy older compared to younger adults. This knowledge could potentially help clinicians optimize exercise as a method of pain management.
Keywords: Exercise-induced hypoalgesia, Aging, Pain modulation, Acute exercise, Exercise analgesia
INTRODUCTION
The burden of chronic pain among older adults is substantial with up to 60% of older adults reporting chronic pain in large community based samples.34,40 Pain is one of the primary causes of physical disability17, significantly impacts quality of life39, and dramatically increases individual and national health care costs.8 Substantial evidence supports the use of exercise as an effective tool to reduce pain and it is often recommended as an adjunct therapy in the treatment of chronic pain.7,13,14,19,45 Indeed, evidence from clinical trials suggests regular exercise can reduce pain symptoms in chronic pain conditions affecting older adults.7,19,45 Additionally, a single bout of exercise influences the experience of pain. In healthy young adults, acute aerobic and isometric exercise temporarily reduces pain sensitivity, a phenomenon termed exercise-induced hypoalgesia (EIH).30 However, many individuals with chronic pain (i.e., fibromyalgia, neuropathic pain) don’t experience EIH, and pain sensitivity and perception is often temporarily exacerbated following acute exercise.16,20,23,44,47 As regular exercise becomes an important component of the multidisciplinary treatment recommended for persistent pain in older adults, a comprehensive understanding of how acute exercise influences pain perception in this age group is important to optimize exercise as a method of pain management.
The experience of pain is modulated by complex endogenous systems that both facilitate and inhibit pain.43 Substantial evidence from psychophysical tests (i.e., condition pain modulation (CPM), offset analgesia) indicates that dysfunction of pain inhibitory systems increases with age.5,24,28,38 A recent study revealed that endogenous pain inhibitory capacity, as evidenced on the CPM test, predicted the magnitude of pain reduction following acute isometric exercise.26 Thus, participants that demonstrated a poor pain inhibitory capacity were more likely to experience a hyperalgesic response following isometric exercise. Despite this evidence, little research has explored changes in pain sensitivity and perception following acute exercise in healthy older adults, a cohort that typically exhibits poor pain inhibitory capacity. One of the only studies to date addressing this topic, investigated the effect of isometric contractions that varied in intensity and duration on pressure pain perception in older and younger adults.25 Both older and younger adults exhibited reductions in pain following the isometric contractions; however, the EIH effect was smaller in older adults. To the best of our knowledge, no studies have examined EIH following aerobic exercise in older adults.
The primary purpose of the current study was to examine age differences in EIH following submaximal isometric exercise, and moderate and vigorous aerobic exercise. Several studies have shown that experimental pain measures correlate only moderately across stimulus modalities and tests11,33; thus, we tested for EIH using a multimodal pain assessment. We assessed changes in threshold and suprathreshold pressure pain, temporal summation of pain, and pain perception during a prolonged heat pain test before and immediately after exercise. These pain measures likely represent distinct dimensions of pain perception that may be under the influence of different mechanisms.11 We hypothesized that EIH would be reduced in older compared to younger adults across all forms of acute exercise and pain tests.
METHODS
Participants
Participants were twenty-five (age range: 19–30; average age=21.7±4.1 years; 14 females) healthy young adults and eighteen (age range: 55–74; average age=63.7±6.6; 9 females) older adults. Studies show that adults 55 years and older have reduced capacity for pain inhibition29,38; therefore, we are including adults 55 years and older in our older adult group. The younger group included 16 Caucasians, 3 Asian Americans, and 6 Hispanic Americans. The older group included 15 Caucasians, 1 Asian American, and 2 Hispanic Americans. Detailed results of the data on the younger adults has been previously published.31,32 A power analysis using G Power 3.1.5 was used to estimate the sample size needed for detecting a session by age group interaction for the outcome measures in a mixed model design. With the significance level set at 0.05, power at 0.80, a 0.5 correlation among repeated measures, and the effect size for differences between groups estimated to be moderate, the power analyses determined that a total of 34 participants (17 per group) would yield a power of 0.81.
Participants were recruited through posted advertisements in the local community. Exclusion criteria included: 1) current use of narcotics or tobacco products, 2) uncontrolled hypertension, 3) neurological disease with significant changes in somatosensory and pain perception at intended stimulation sites, 4) the known presence of or any signs or symptoms of cardiovascular disease, pulmonary disease, or metabolic disease, 5) serious psychiatric conditions (e.g., schizophrenia and bipolar disorder), and 6) current use of opioids. Younger adults were excluded if they were not physically ready to exercise without a medical exam as indicated by the Physical Activity Readiness Questionnaire (PAR-Q).41 Older adults had to obtain physician approval from their primary care physician to participate in the study. During the health history interview, no participants indicated they were regularly taking pain medications or reported chronic pain. Session exclusion criteria included active infectious disease or febrile condition (e.g., sinusitis, influenza), severe uncontrolled hypertension, use of caffeinated drinks, or any pain medications prior to the experimental sessions.
Procedures
The University’s Institutional Review Board (IRB) approved all procedures and participants signed an IRB-approved informed consent form. Participants completed a screening/training session followed by four randomized experimental sessions. All sessions were separated by a minimum of 48 hours and conducted at approximately the same time of day (± 2 hours). All sessions began after two stable blood pressure readings separated by 5 minutes.
Screening and training session
To determine eligibility, participants completed the PAR-Q, a health history questionnaire, supplemented by clarification by interview, height and weight measurement, and a resting heart rate (HR) and blood pressure measurement. Older adults were also given a letter that had to be signed by a physician, which granted the participant medical clearance to participate in the study. The experimental sessions were not scheduled until medical clearance had been obtained. Once eligibility was determined, participants completed a training session designed to 1) teach them the continuous pain rating system, and 2) determine the individualized temperatures of the thermal stimuli for the heat pain testing protocols such that participants would experience moderate pain (i.e., 50/100 on a 0–100 visual analogue scale). For this purpose, trains of increasing heat stimuli were applied to the forearm until participants experienced a moderate level of pain (40–60 on a 0–100 visual analogue scale). Additionally, the experimental pain testing procedures were conducted once during the training session to ensure familiarity with the testing protocols. During this session participants also completed the International Physical Activity Questionnaire which assess the amount of time during the past week spent on vigorous activity, moderate activity, and walking.4
During the training session, maximal voluntary contraction (MVC) of handgrip muscles was also determined using a hand dynamometer (Jamar Hydraulic Hand Dynamometer). The dynamometer handle was adjusted according to manufacture guidelines for each participant. Participants placed their dominant arm on a table surface with the elbow at a 90° angle. Participants were asked to squeeze a hand dynamometer as hard as possible for 5 seconds. This procedure was repeated three times with a one-minute rest between trials. The maximum of the three MVC’s was used to calculate the percent of MVC used for the isometric hand grip exercise.
Experimental sessions
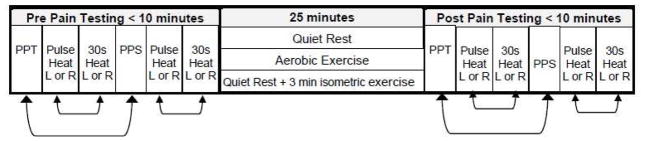
Participants completed four experimental sessions in randomized order consisting of one of the following conditions: vigorous intensity aerobic exercise, moderate intensity aerobic exercise, submaximal isometric exercise, and quiet rest. At the beginning of each session, participants were fitted with a Polar Heart Rate monitor (FT7) (Polar Electro, Lake Success, NY), which monitored and collected heart rate (HR) at rest (sitting) and during exercise. Heart rate was similarly measured during all experimental sessions. During each session, four different pain tests were administered on each forearm followed by a 25 minute interval of either aerobic exercise, quiet rest, or 22 minutes of quiet rest and a 3 minute isometric handgrip. Blood pressure was immediately taken upon completion of exercise or quiet rest followed by the administration of the same 4 pain tests in the same order as the pre-exercise pain tests. The administration of the 4 pain tests pre and post exercise took under 10 minutes to complete. Figure 1 shows a timeline of an experimental session.
Figure 1.
Timeline of procedures during the experimental sessions. The bidirectional arrows between the pressure and heat pain tests indicate that these tests were conducted in counterbalanced order. The site of pain testing alternated between left and right forearms, so that one arm was never tested consecutively. Participants maintained the same pain testing order for each session pre and post exercise and quiet rest. The 25 minute period between the pre pain testing and post pain testing included one of the following conditions: 1) quiet rest, 2) moderate intensity aerobic exercise, 3) vigorous intensity aerobic exercise, or 4) 22 minutes of quiet rest followed by a 3 minute submaximal isometric handgrip. PPT=pressure pain threshold; PPS= pressure pain suprathreshold test; R=right forearm; L=left forearm.
Acute bout of vigorous aerobic exercise session
This session tested for changes in pain sensitivity and perception (as described under experimental pain measures) following 25 minutes of vigorous stationary cycling. Participants cycled the first 5 minutes at an intensity of up to 50% heart rate reserve (warm-up period), followed by 20 minutes at 70% heart rate reserve (HRR). The speed and/or resistance of the cycle ergometer were adjusted to meet the prescribed intensity (target HR zone) throughout the exercise bout. The following data were recorded every five minutes during exercise: 1) ratings of perceived exertion (RPE) using Borg’s 6–20 scale2, and 2) heart rate with a heart rate monitor. A target HR was determined for each participant using the Karvonen formula.18 The Karvonen formula is related to the percent of age-predicted maximal heart rate but allows for differences in resting HR with the following formula: Target heart rate = [(age-predicted maximal heart rate – resting HR) × %Intensity] + resting HR. Age predicted maximal heart rate = 220-age.
Acute bout of moderate intensity aerobic exercise session
This session tested for changes in pain sensitivity following 25 minutes of moderate intensity stationary cycling. This session was identical to the vigorous exercise session, however, following the 5 minute warm-up period participants cycled for 20 minutes at an intensity of 50–55% HRR.
Submaximal isometric exercise session (isometric)
This session tested for changes in pain sensitivity following a 3-minute trial of submaximal isometric handgrip exercise at 25% of MVC. We chose a duration of 3 minutes for the handgrip task because prior research has shown handgrips of this duration produce the largest EIH effects (as opposed to 1 and 5 minute handgrips).46 Twenty-two minutes separated the pre-exercise pain assessments and the initiation of the isometric contraction, during which subjects sat quietly. The isometric hand grip exercise was performed with the dominant arm resting on the table surface with the elbow at a 90° angle. Participants were able to see the dynamometer read-out and adjust their effort as necessary to maintain a level of force production at 25% of their MVC. Ratings of perceived exertion (RPE) using Borg’s 6–20 RPE scale and HR were assessed every 20 s during the isometric exercise trial.
Experimental session 3: Quiet Rest – control condition
This session tested for changes in pain sensitivity and perception following 25 minutes of quiet rest. Participants remained in a seated position for the entire 25 minutes and were allowed to read. Heart rate was recorded every five minutes.
Psychophysical Pain Testing
Participants were administered 4 different pain tests to each forearm pre and post exercise or quiet rest, including: 1) pressure pain thresholds, 2) suprathreshold pressure pain trial, 2) prolonged static heat pain trial, and 4) temporal summation (TS) of heat pain trial. The order of the pain tests are shown in Figure 1 and were conducted as follows: 1) a pressure pain test administered to both forearms, 2) a prolonged or TS heat pain test administered to one forearm, 3) a prolonged or TS heat pain test administered to the other forearm, 4) a pressure pain test administered to both forearms 5) a prolonged or TS heat pain test administered to one forearm, 6) a prolonged or TS heat pain test administered to the other forearm. The site of pain testing alternated between left and right arms, so that one arm was never tested consecutively. Additionally, the order of the pressure and heat pain tests, as well as the bodily site (right v. left arm) was counterbalanced among participants. Participants maintained the same pain testing order for every session pre and post exercise and quiet rest.
Pressure pain thresholds (PPT)
Pressure pain threshold was assessed with a handheld algometer (Jtech, Heber City, Utah) on the right and left ventral forearm, approximately 8 cm distal to the elbow. The tip of the algometer consisted of a rubber flat 1.0 cm2 probe. Pressure stimuli were delivered to the forearm at an approximate rate of 0.5 kg/s. Participants were instructed to respond verbally when the pressure sensation first became painful, at which the algometer was removed. The amount of pressure applied to the forearm did not exceed 5 kg. Pressure pain threshold was defined as the amount of pressure in kilograms at which the participant first reported experiencing pain.
Suprathreshold pressure pain test
Ratings of suprathreshold pressure stimuli were also assessed on the right and left ventral forearm with the same handheld algometer used for PPTs. The site of the pressure pain threshold and suprathreshold assessments were always separated by a minimum of 1 cm on the forearm and the same sites were used for pre and post assessments. Pressure stimuli were delivered to the forearm at an approximate rate of 0.5 kg/s, until 5 kg was applied. This level of pressure was chosen to assess stimulus intensity without producing excessive discomfort. Immediately after each trial, participants rated the intensity of the stimulus on a 0 to 100 numeric rating scale (NRS), with “0” indicating “no pain” and “100” indicating “intolerable pain”.
Prolonged static heat pain test
Contact heat stimuli were delivered by a computer controlled Peltier-based thermode (32 mm x 32 mm; TSA-2001, Ramat Yishai, Israel) to the right and left ventral forearms. For each 30-second continuous heat pain trial, the thermode was first brought to a neutral temperature (32°C) and then ramped (2.0°C/s) to the individualized temperature (44–49°C) determined during the training session and maintained at that temperature for 30 s. The thermode position was altered slightly between each heat trial (i.e., continuous and TS heat pain trials). The intensity of the pain produced by the contact thermode was rated every 5 s on a 0 to 100 NRS. As done in previous studies using prolonged static heat tests, area under the curve (AUC) was calculated for each trial by summing the recorded pain ratings.29,38 AUC was chosen as the primary dependent variable for this test because it has been used in past research as an outcome variable for prolonged heat pain ratings and is an effective way to simply quantify the amount of pain experienced over time.29,38
Temporal summation of heat pain
Temporal summation refers to the increased perception of pain in response to repetitive noxious stimuli delivered at frequencies above 0.3 Hz.35,36 Brief repetitive suprathreshold heat pulses were delivered to the right and left ventral forearms. Each trial consisted of a series of 10 heat pulses, with each pulse delivered at a rate of 10°C/s. The peak to peak inter-pulse interval was approximately 2.5 seconds. The baseline temperature was 38 °C and the target temperature was the individualized temperature determined during the training session (45°C – 51.5 °C). Participants were instructed to rate the intensity of the late pain sensations experienced after each pulse (i.e., pain felt between the pulses not during each pulse, termed second pain) with a 0–100 scale. This pain test permitted the assessment of the effect of exercise on the temporal summation (TS) of C-fiber mediated heat pain (i.e., late heat pain sensations, often termed “second pain”).36 A temporal summation score was calculated by subtracting the pain rating following the first pulse from the highest inter-pulse pain rating. This score captures the maximum amount of temporal summation across the 10 pulses.
Reliability of Pain Measures
Because we only administered one trial at each body site for each pain test at pretest and posttest, we conducted Interclass Correlations Coefficients (ICCs) on the pretests for each pain measure to determine the reliability of these measures. The ICCs were conducted separately for older and younger adults. These analyses indicated excellent reliability for all pain measures for younger adults (ICC’s ranged from 0.80 to 0.93) and older adults (ICC’s ranged from 0.82 to 0.92).
Data Analysis
Descriptive statistics were calculated for average percentage of HRR, and RPE for each exercise session. One younger participant’s average HRR% for the vigorous aerobic exercise was only 55%; therefore, this participant’s data were not included in the statistical analyses. During the suprathreshold pressure pain test, one younger participant did not report experiencing any pressure pain after the target pressure of 5 kg was reached; thus this participant’s data was removed from the suprathreshold pressure pain analyses. During the PPT test, two younger participants did not report experiencing pain before the upper limit of pressure for the test was reached; therefore their data were not included in the PPT analyses.
The primary purpose of this paper was to determine whether exercise-induced hypoalgesia differed by age. Thus, we created an index of EIH for each pain test, similar to what has been used in other pain inhibitory tests such as CPM.29 First, change scores for each dependent variable were calculated for each session by subtracting the pretest value from the posttest value. Then, the control change score was subtracted from the exercise session (isometric, moderate AE or vigorous AE) change scores [EIH index = (posttest scoreexercise − pretest scoreexercise) − (posttest scorecontrol − pretest scorecontrol)]. The adjusted change score (EIH index) provides a controlled measure of the degree to which pain perception changed as a function of the exercise. A positive number for the PPTs indicates that pain sensitivity was reduced following exercise compared to the control condition. For the other three measures, a negative value indicates that pain was reduced following exercise compared to the control condition. Shapiro-Wilk’s test of normality was used to test for normal distribution of each measure in each age group.
To determine age differences in EIH during the isometric exercise session, the EIH index for each pain test was analyzed with an Age Group × Sex × Forearm (active v. inactive) mixed model ANCOVA with target force level added as a covariate. Thermode temperature was added as a covariate for the heat pain tests. Additionally, preliminary analyses showed that PPT’s differed between age groups at baseline; therefore, the average pretest PPT score was also added as a covariate for the PPT analyses. Sex was included as a factor because prior research has shown sex differences in EIH.25,31 Forearm was included as a variable to determine whether EIH differed between the exercised and non-exercised forearm.
For the aerobic exercise sessions, data for each pain test was averaged between the two forearms. To determine age differences in EIH during aerobic exercise, the EIH index for each pain test was analyzed with an Age group × Sex × Session (moderate v. vigorous) mixed model ANOVA. Thermode temperature was added as a covariate for the heat pain tests. Preliminary analyses showed that PPT’s differed between age groups at baseline; therefore, the average pretest PPT score was also added as a covariate for the PPT analyses. If the sphericity assumption was violated, then Greenhouse-Geisser degrees of freedom corrections were applied to obtain the critical p-value. Post-hoc comparisons were made with Tukey’s HSD procedure. A level of p ≤ .05 was used for all statistical analyses.
To determine the magnitude of age differences in EIH, effect sizes (ES) were calculated using Cohen’s d. Cohen’s d was defined as the mean for the young adults minus the mean for the older adults, divided by the pooled within group standard deviation (d=[Xyoung – Xold]/pooled standard deviation). Effect sizes were calculated for men and women separately. A positive effect size reflects greater EIH for the younger adults. These effect sizes are presented in Table 1.
Table 1.
EIH index means and standard error and effect sizes for each pain test and exercise session
| Exercise | Isometric
|
Moderate AE
|
Vigorous AE
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Age group | Young | Older | ES | Young | Older | ES | Young | Older | ES |
| PPT | |||||||||
| Male | 0.27±.17 | −0.30±0.14 | 1.08 | 0.21±0.15 | −0.03±0.18 | 0.51 | 0.36±.15 | −0.15±0.19 | 1.07 |
| Female | 0.21±.11 | 0.06±0.19 | 0.26 | 0.20±0.14 | −0.11±0.20 | 0.65 | 0.37±.15 | 0.07±0.20 | 0.61 |
| PPS | |||||||||
| Male | −5.47±5.07 | −2.41±4.30 | 0.21 | −5.63±6.0 | 0.63±7.37 | 0.93 | −5.75±5.54 | −3.13±6.79 | 0.11 |
| Female | −5.51±3.48 | 5.50±5.62 | 0.77 | −6.53±5.38 | 1.33±6.78 | 0.41 | −2.43±4.96 | −9.33±6.40 | −0.38 |
| TS max | |||||||||
| Male | −5.09±3.50 | −0.17±2.93 | 0.50 | −3.54±2.24 | 0.22±3.16 | 0.43 | −1.20±2.41 | 1.56±3.39 | 0.35 |
| Female | −4.98±2.30 | 5.72±3.69 | 1.09 | −2.55±2.00 | −0.17±2.57 | 0.32 | 1.69±2.15 | −4.37±2.76 | −0.37 |
| AUC heat pain | |||||||||
| Male | 35.73±27.0 | 45.25±23.0 | 0.12 | −3.45±24.2 | 20.19±25.2 | 0.30 | −27.15±23.5 | 39.30±24.5 | 0.88 |
| Female | −39.29±17.7 | −17.56±27.6 | 0.30 | −21.5±19.0 | 50.67±26.9 | 0.98 | −27.58±18.4 | 56.55±26.2 | 1.17 |
Note. EIH=exercise-induced hypoalgesia. AE=aerobic exercise. PPT=pressure pain threshold. PPS=pressure pain suprathreshold test. TS=temporal summation. AUC=area under the curve. A positive number for the PPTs indicates that pain sensitivity was reduced following exercise compared to the control condition. For the other three measures, a negative value indicates that pain was reduced following exercise compared to the control condition. The effect sizes (ES) represent the magnitude of difference between older and younger adults. A positive effect size indicates that EIH was greater for younger adults compared to older adults.
Effect sizes were also calculated to determine the magnitude of pain reduction after exercise. Because we didn’t find differences between forearms, data for each pain test was averaged between the two forearms. Cohen’s d was defined as the mean for trial 1 minus the mean for trial 2, divided by the pooled within group standard deviation (d=[Xtrial1 – Xtrial2]/pooled standard deviation). Due to the within subjects design, the effect sizes were adjusted as recommended by Portney and Watkins.35 Effect sizes were calculated for each age group separately. Reductions in pain sensitivity are reflected by positive effect sizes. These effect sizes are presented in Table 2.
Table 2.
Means and standard errors and effect sizes for the pre- and post-tests for each pain test and condition
| Condition | Control
|
Isometric*
|
Moderate AE
|
Vigorous AE
|
||||
|---|---|---|---|---|---|---|---|---|
| Age group | Young | Older | Young | Older | Young | Older | Young | Older |
| PPT (kg) | ||||||||
| Pre | 2.91±0.23 | 3.62±0.29 | 2.77±0.27 | 3.90±0.32 | 2.97±0.22 | 3.39±0.27 | 2.72±0.21 | 3.45±0.26 |
| Post | 2.90±0.22 | 3.80±0.27 | 3.00±0.26 | 3.99±0.30 | 3.11±0.22 | 3.51±0.28 | 3.10±0.23 | 3.55±0.29 |
| ES | −0.01 | 0.21 | 0.27 | 0.09 | 0.18 | 0.14 | 0.49 | 0.12 |
| PPS (0–100 NRS) | ||||||||
| Pre | 57.15±6.1 | 44.48±7.3 | 59.79±5.5 | 45.65±6.6 | 54.51±5.2 | 44.49±6.6 | 56.35±5.2 | 50.84±6.7 |
| Post | 60.59±6.2 | 42.58±7.5 | 57.23±5.6 | 45.29±6.7 | 51.53±5.5 | 50.63±7.1 | 55.35±5.8 | 48.03±7.5 |
| ES | −0.23 | 0.08 | 0.14 | 0.01 | 0.16 | −0.28 | 0.06 | 0.13 |
| TS max | ||||||||
| Pre | 7.82±2.0 | 10.20±2.4 | 13.70±2.6 | 9.09±3.0 | 11.47±2.4 | 8.07±3.2 | 11.95±2.5 | 9.78±3.4 |
| Post | 9.29±2.1 | 9.04±2.4 | 10.18±2.4 | 10.70±2.7 | 8.99±1.9 | 6.72±2.6 | 12.79±2.3 | 7.00±3.1 |
| ES | −0.23 | 0.15 | 0.45 | −0.18 | 0.37 | 0.14 | −0.11 | 0.27 |
| AUC heat pain | ||||||||
| Pre | 215.87±16.4 | 201.97±20.3 | 213.36±19.0 | 203.78±20.9 | 235.39±17.0 | 180.88±21.1 | 242.07±19.5 | 179.63±24.2 |
| Post | 204.44±19.6 | 143.88±23.6 | 190.62±18.6 | 166.06±20.5 | 211.76±17.6 | 152.19±21.7 | 202.73±18.1 | 155.93±22.4 |
| ES | 0.20 | 0.83 | 0.38 | 0.58 | 0.44 | 0.42 | 0.66 | 0.34 |
Note. AE=aerobic exercise. PPT=pressure pain threshold. PPS=pressure pain suprathreshold test. TS=temporal summation. AUC=area under the curve. Effect sizes (ES) represent the magnitude of change from pretest to posttest. A positive ES indicates pain was reduced following exercise or quiet rest.
AUC values by sex for Isometric condition: Young Male pre=183.10±22.7 post=183.12±25.4; Young female pre=205.16±24.3 post=165.87±23.8; Older male pre=148.86±31.6 post=136.93±31.0; Older female pre=258.69±37.9 post=195.19±37.8.
Finally, 2-way ANOVAs determined whether differences existed between age groups and sex on thermode test temperatures and target force production. Shapiro-Wilk’s test of normality indicated that the IPAQ data were not normally distributed; thus Mann-Whitney U tests were conducted to determine if the IPAQ scores differed by age. Average heart rate reserve percentage during the aerobic exercise sessions was analyzed with a 3-way ANOVA with sex and age group as between subjects factors and session as the within subject factor. Average RPE during all exercise sessions was also analyzed with an Age group × Sex × Session ANOVA. All data presented in the text are presented as means±standard error.
RESULTS
The EIH index means and standard errors (SE) for each pain test and exercise session by sex and age group are presented in Table 1. Additionally, the means and SE’s for the pre-test and post-test values for each pain test and condition by age group are presented in Table 2. See Table 3 for a summary of significant age and sex differences in EIH.
Table 3.
Summary of significant age and sex differences for each exercise condition and pain test
| Exercise condition | Isometric | Moderate AE | Vigorous AE |
|---|---|---|---|
| PPT | Age | Age | Age |
| PPS | None | Age | None |
| TS max | Age | None | None |
| AUC heat pain | Sex | Age | Age |
Note. AE=aerobic exercise. PPT=pressure pain threshold. PPS=pressure pain suprathreshold test. TS=temporal summation. AUC=area under the curve. Significance p < .05. Age=significant age differences. Sex=significant sex differences. None=no significant differences between groups.
Isometric Exercise
Pressure pain test results
The 3-way ANCOVA conducted on the EIH index for PPTs revealed a main effect of age group, p=.030. Younger adults experienced greater EIH compared to the older adults (younger adults= 0.24±0.09, older adults= −0.12±0.12). The effect sizes indicated a small age group difference for females and a large difference for males. No other main effects or interactions were significant, p’s > 0.05. The effect sizes presented in Table 2 showed that the magnitude of pain reduction was moderate to small for younger adults and very small for older adults.
The ANCOVA conducted on the suprathreshold pressure pain EIH index showed no significant results, p’s > 0.05. Effects sizes indicated that the magnitude of pain reduction following isometric exercise was small for younger adults and virtually non-existent for older adults.
Heat pain tests results
The analysis on the EIH index for temporal summation of pain revealed significant age differences (p=0.01), with younger adults exhibiting greater EIH compared to older adults (younger adults=−4.99±1.80, older adults=2.78±2.06). No other main effects or interactions were significant, p’s > 0.05. The effect sizes indicated a moderate age group difference for males and a large difference for females. The magnitude of pain reduction for younger adults was moderate, while the effect size for older adults revealed a small increase in temporal summation of pain following exercise.
The ANCOVA conducted on the EIH index for AUC on the continuous heat pain test revealed a significant main effect of sex, p=0.043. Females showed greater EIH compared to males (females= −28.43±18.20, males= 40.49±20.00), regardless of age. In regards to magnitude of pain reduction following isometric exercise, the effect sizes were as follows: younger males ES=0.0004, older males ES=0.21, younger females ES=0.63, older females ES=0.84. No other main effects or interactions were significant, p’s > 0.05.
Aerobic Exercise
Pressure pain test results
Similar to the isometric exercise results, the 3-way ANOVA conducted on the EIH index for PPT showed a significant effect of age group, p=0.046. Younger adults exhibited greater EIH following aerobic exercise compared to older adults (younger adults=0.29±0.09, older adults= −0.06±0.11). No significant differences were found as a function of session (moderate v. vigorous exercise) or sex. The magnitude of age differences (Table 1) were moderate for the moderate intensity aerobic exercise and large for vigorous aerobic exercise. The magnitude of pain reduction following moderate aerobic exercise (Table 2) was small for younger and older adults. For vigorous aerobic exercise, the magnitude of pain reduction was moderate for young adults and small for older adults. Importantly, effects sizes showed that the magnitude of pain reduction for older adults was greater during the control condition compared to the exercise sessions.
A significant age group × session interaction (p=.028) was found for the EIH index for suprathreshold pressure pain. Older adults exhibited reduced EIH following moderate aerobic exercise compared to vigorous aerobic exercise and compared to younger adults following moderate aerobic exercise (moderate exercise, younger adults= −6.10±4.03; vigorous exercise, younger adults= −4.09±3.70; moderate exercise, older adults= 0.97±5.06; vigorous exercise, older adults= −6.23±4.66). Accordingly, the effect sizes showed that the magnitude of age differences was moderate to large for the moderate aerobic exercise and small to non-existent for the vigorous aerobic exercise. However, the magnitude of pain reduction on the suprathreshold pain test following aerobic exercise was small for both age groups, ranging from −0.28 to 0.16 (Table 2). No other main effects or interactions were significant, p’s > 0.05.
Heat pain tests results
The analysis on the EIH index for temporal summation of pain revealed no significant results, p’s > 0.05. The EIH index for younger and older adults by session were as follows: moderate exercise, younger adults= −3.05±1.50; vigorous exercise, younger adults= 0.25±1.60; moderate exercise, older adults= 0.03±2.03; vigorous exercise, older adults= −1.4±2.18. Generally, the effect sizes in Table 2 indicate that the magnitude of reduction in temporal summation of pain following aerobic exercise was small for both groups, ranging from 0.37 to −0.11.
The 3-way ANOVA conducted on the EIH index for AUC for the continuous heat pain test showed a significant effect of age group (p=0.001), with younger adults exhibiting greater EIH compared to older adults, regardless of the session (younger adults= −19.90±11.0, older adults= 41.67±13.83). The magnitude of the age differences was large for males and females following vigorous exercise and for females following moderate exercise, but small for males following moderate aerobic exercise. Importantly, while the older adults revealed a moderate level of pain reduction following aerobic exercise (Table 2), the magnitude of pain reduction during the control session was greater.
Characteristics of exercise and pain tests
Table 4 presents the means and SE for each age group by sex for target force during the isometric handgrip, average HRR% during the aerobic exercise sessions, average RPE during all exercise sessions, and average thermode temperature for the heat pain tests. The analysis conducted on target force during the isometric handgrip showed a main effect of age group (p=0.002) and sex (p<0.001). Males had greater force production than females during the handgrip and younger adults produced greater force than older adults. The analyses on HRR% confirmed that participants exercised at a greater HRR% during the vigorous aerobic exercise (M=70.84±1.28) compared to the moderate aerobic exercise (M=53.35±0.71). HRR% did not differ as a function of sex or age group, p’s > 0.05. The ANOVA conducted on RPE revealed a main effect of exercise session, p<0.001. For all participants, RPE was greater during the vigorous aerobic exercise compared to moderate aerobic exercise and the isometric exercise. The 2-way ANOVAs conducted on thermode temperature for the heat pain tests revealed no significant differences between age groups or sex, p’s >0.05. Additionally, results of the Mann-Whitney U test indicated no differences between age groups in total physical activity (young = 3428±494 METS-min/week; older=5368±1330 METS-min/week), vigorous physical activity (young = 1393±248 METS-min/week; older=1910±555 METS-min/week), moderate physical activity (young = 852±1030 METS-min/week; older=1927±654 METS-min/week) and walking (young = 1182±279 METS-min/week; older=1430±512 METS-min/week) on the IPAQ.
Table 4.
Descriptors of exercise and thermode test temperature (means ± standard error)
| Younger Adults
|
Older Adults
|
|||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Isometric target force (kg) | 10.81±0.51 | 6.19±0.44 | 9.06±0.57 | 4.44±0.57 |
| Heart rate reserve % | ||||
| Vigorous AE | 69.81±2.51 | 69.45±2.24 | 71.04±3.20 | 73.06±2.61 |
| Moderate AE | 52.71±1.33 | 52.56±1.19 | 54.34±1.76 | 53.77±1.44 |
| RPE (scale 6–20) | ||||
| Vigorous AE | 14.70±0.53 | 14.20±0.44 | 14.97±0.60 | 15.25±0.56 |
| Moderate AE | 12.23±0.60 | 11.55±0.49 | 13.09±0.68 | 13.64±0.64 |
| Isometric exercise | 13.68±0.67 | 12.82±0.55 | 12.92±0.75 | 13.01±0.71 |
| TS thermode test temp (°C) | 48.92±0.57 | 48.13±0.51 | 48.33±0.67 | 48.22±0.69 |
| Continuous heat | ||||
| thermode test temp (°C) | 47.33±0.48 | 46.37±0.43 | 46.67±0.55 | 46.83±0.55 |
Note. AE=aerobic exercise. TS=temporal summation. Temp=temperature.
DISCUSSION
The current study investigated age differences in EIH following isometric and aerobic exercise using heat and pressure psychophysical testing. Three key findings emerged from this study: 1) age differences in EIH emerged following isometric and aerobic exercise, with younger adults experiencing greater EIH compared to older adults, 2) the age differences in EIH varied across pain induction methods, and 3) despite the observed reduction in EIH for older adults, these participants generally did not demonstrate increased pain perception or sensitivity following acute exercise.
Age Differences in EIH following Isometric Exercise
Supporting our hypothesis, we found age differences in EIH during the submaximal isometric handgrip condition with the PPT test. Pressure pain thresholds signify the lowest boundary of painful sensations in musculoskeletal structures.3 The degree to which isometric exercise increased the pain threshold boundary was greater for younger compared to older adults. This finding is in partial support of Lemley and Colleagues, who found increased PPTs following isometric exercise for older and younger adults; however, the magnitude of EIH was greater in younger adults.35 In contrast to the PPT data, our results did not show age differences in EIH on the suprathreshold pressure pain test. The lack of age differences was likely due to the negligible effect of the isometric handgrip on pain ratings during the pressure suprathreshold test in all participants. Importantly, the isometric exercise did not cause a hyperalgesic response in older adults on either pressure pain test.
The current study was the first to examine EIH in older adults using temporal summation of pain and prolonged heat pain. Temporal summation of pain is reduced in younger adults following isometric exercise22, but amplified following acute exercise in chronic pain patients.44 Our findings revealed that an isometric handgrip induces greater EIH on the temporal summation test in younger compared to older adults. Effects sizes revealed a moderate reduction in temporal summation in younger adults and a minimal to small increase in older adults.
Using the prolonged heat pain test, we discovered sex rather than age differences in EIH with females experiencing greater EIH compared to males. Excluding the difference in modality (pressure v. heat), the prolonged suprathreshold heat test used in the current study was very similar to the prolonged suprathrehold pressure pain test used in the Lemley et al. study, which also found sex rather than age differences in EIH.25 The reason for the sex differences in EIH during prolonged static pain tests remains unclear. Hashmi and Davis suggested that effective central inhibitory mechanisms to attenuate sustained pain would be more biologically advantageous for women to cope with natural pain including childbirth pain.10 Nonetheless, the mechanism enabling women effective isometric EIH for prolonged suprathreshold pain does not appear to deteriorate with age.
Age Differences in EIH following Aerobic Exercise
To the best of our knowledge, this is the first study to investigate age differences in EIH following aerobic exercise. Prior research has shown that aerobic exercise reduces pain perception in healthy adults, but can have no effect or even a hyperalgesic effect on experimentally-induced pain in chronic pain patients.30 In the current study, moderate and vigorous aerobic exercise elicited greater EIH for the PPT test in younger compared to older adults. The data also demonstrated age differences in EIH for the suprathreshold pressure pain test. Older adults exhibited less EIH compared to younger adults only during the moderate aerobic exercise condition. Notably, the magnitude of change in pain ratings on the supratheshold pressure pain test was minimal to small for all exercise conditions and groups (i.e., effect sizes range from 0.16 to −0.28). The small effects of exercise on the suprathreshold pressure pain test may have been caused by the large between-subject variability in pre-exercise pain ratings during this test (i.e., ranging from 5 to 99), increasing the likelihood of ceiling and floor effects.
We also found age differences in aerobic EIH with the prolonged heat pain test. While older men and women demonstrated a reduction in pain ratings following aerobic exercise on this test, the reduction in pain ratings from pre- to post-test was greater during the control session. Thus, while aerobic exercise did not induce a hypoalgesic response compared to quiet rest, heat pain perception was also not enhanced by exercise as found in some chronic pain patients.47
In contrast to our hypothesis, we observed no age differences in aerobic EIH using the temporal summation test. The small magnitude of change in temporal summation of pain following aerobic exercise for both age groups may have contributed to the lack of age differences using this measure. In a prior study using the same repetitive pulse heat pain test as the current study, Naugle et al. found that aerobic exercise decreased pain ratings on pulses in the latter end of the temporal summation trial (i.e., pulses 6–10) in younger adults.32 However, the Naugle et al. study did not look at the effect of aerobic exercise on the magnitude of summation (increase in pain ratings from 1st pulse to max pain rating) during the temporal summation trial. Perhaps, aerobic exercise temporarily reduces heat pain sensitivity in healthy younger adults but does not reduce the hyperexcitability of the central nervous system (CNS).
Several different mechanisms have been proposed to underlie EIH and could underlie the deterioration of this phenomenon with age. The most widely ascribed mechanism for EIH involves the activation of the endogenous opioid system during exercise. Animal studies indicate that exercise of sufficient intensity and duration causes the release of central and peripheral beta-endorphins, which have been linked with decreased pain sensitivity.9,15,42 Furthermore, rodent studies suggest that aging is associated with decreased opioid peptide and receptor levels in the CNS.27 However, human studies show no age-related differences in circulating levels of beta-endorphins in response to exercise.12 Furthermore, a recent human study suggested the involvement of a non-opioid vs. opioid mechanism in isometric EIH using temporal summation and PPTs as the experimental pain tests.21 Greater EIH was associated with increased circulating levels of the endocannabinoid docosahexaenoylethanolamine (DHA) following isometric exercise. Interestingly, DHA deficiency is associated with aging1, however age-related differences in the DHA response to exercise is not known. Another potential mechanism involves the activation of endogenous pain inhibitory mechanisms, such as CPM. CPM refers to the phenomenon whereby a noxious stimulus at one body part results in reduced pain perception to another noxious stimulus at a distant body part.48 Ellingson and colleagues recently tested this hypothesis by examining EIH via painful exercise, nonpainful exercise, and quit rest.6 The results suggested that while exercise-induced muscle pain may contribute to the magnitude of pain reduction following acute exercise, CPM is likely not the primary mechanism of EIH. Lemely et al. tested whether CPM predicts isometric EIH in healthy older and younger adults.26 Findings revealed that individuals exhibiting a greater ability to activate descending inhibitory pathways in the CPM paradigm also demonstrated greater EIH. Thus, while multiple factors likely contribute, abnormal descending pain inhibition may play a role in the diminished hypoalgesic effect of acute exercise in older adults. While the mechanisms underlying age differences in EIH were not tested in this study, our results suggest that age related reductions in EIH following isometric and aerobic exercise are likely caused by unique and shared mechanisms. For example, with the prolonged heat test used as the test stimulus, we observed age differences in EIH during the aerobic conditions, but sex differences in EIH during the isometric condition. These findings illustrate the complexity of the EIH phenomenon which is likely produced by multiple analgesic systems, each of which may preferentially alter different types of nociceptive input.
A few limitations of this study should be noted. First, the older adult group in this study was extremely healthy and active. Thus, these results may not generalize to older adults who are less active or present with more health conditions. Second, we did not assess for potential post-exercise pain. Participants that completed sessions separated by only 48-hours may have experienced delayed onset-muscle soreness (DOMS) in the latter session. However, given the activity level of participants, the risk for DOMS was likely low. Finally, based on our power analysis, this study was not powered to detect small effects.
In summary, the present data suggest diminished EIH in older adults compared to younger adults following isometric and aerobic exercise. However, our data in combination with Lemley et al., also suggest that acute exercise generally does not cause a hyperalgesic response in healthy older adults. Our results also demonstrated that EIH varies by age based on the pain test stimulus and type of exercise. Currently, little is known regarding the clinical implications of experiencing diminished EIH and what pain test/modality in testing EIH has the most clinical relevance. Future studies need to investigate the impact of individual differences in EIH on physical activity behavior and clinical pain experiences specifically related to physical activity in older adults. This knowledge could have important implications regarding the identification of high-risk individuals for persistent pain, declining physical activity levels, and functional disability.
Highlights.
Younger compared to older adults showed greater exercise-induced hypoalgesia (EIH).
Age differences in EIH varied across experimental pain induction methods.
Older adults did not exhibit increased pain perception following acute exercise.
Footnotes
Conflicts of Interest: There are no other conflicts of interest to report with regard to this work for any of the authors.
Disclosures: This research was supported by NIH-NIA Grant R01AG039659 (J.L.R.) and NIH Grant T32 T32NS045551-06 (K.M.N.).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bazan NG, Molina MF, Gordon WC. Doxosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases. Annu Rev Nutr. 2011;21:321–51. doi: 10.1146/annurev.nutr.012809.104635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borg G. Borg’s Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998. [Google Scholar]
- 3.Chapman CR, Casey KL, Dubner R, Foley KM, Gracely RH, Reading AE. Pain measurement: an overview. Pain. 1985;22:1–31. doi: 10.1016/0304-3959(85)90145-9. [DOI] [PubMed] [Google Scholar]
- 4.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Opa P. International physical activity questionnaire: 12- country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 5.Edwards RR, Fillingim RB, Ness TJ. Age-related differences in the endogenous pain modulation: a comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain. 2003;101:155–156. doi: 10.1016/s0304-3959(02)00324-x. [DOI] [PubMed] [Google Scholar]
- 6.Ellingson LD, Koltyn KF, Kim J, Cook DB. Does exercise induce hypoalgesia through conditioned pain modulation? Psychophysiology. 2014;51:267–276. doi: 10.1111/psyp.12168. [DOI] [PubMed] [Google Scholar]
- 7.Focht BC. Effectiveness of exercise interventions in reducing pain symptoms among older adults with knee osteoarthritis: A review. Journal of Aging and Physical Activity. 2006;14:212–235. doi: 10.1123/japa.14.2.212. [DOI] [PubMed] [Google Scholar]
- 8.Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2013;13:715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Goldfarb AH, Jamurtas AZ. Beta-endorphin response to exercise. An update. Sports Med. 1997;24:8–16. doi: 10.2165/00007256-199724010-00002. [DOI] [PubMed] [Google Scholar]
- 10.Hashmi JA, Davis KD. Women experience greater heat pain adaptation and habituation than men. Pain. 2009;145:350–357. doi: 10.1016/j.pain.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Hastie BA, Riley JL, III, Robinson ME, Glover T, Campbell CM, Staud R, Fillingim RB. Cluster analysis of multiple experimental pain modalities. Pain. 2005;116:227–237. doi: 10.1016/j.pain.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Hatfield BD, Goldfarb AH, Sforzo GA, Flynn MG. Serum beta-endorphin and effective responses to graded exercise in young and elderly men. J Gerontol. 1987;42:429–31. doi: 10.1093/geronj/42.4.429. [DOI] [PubMed] [Google Scholar]
- 13.Häuser W, Klose P, Langhorst J, Moradi B, Steinbach M, Schiltenwolf M, Busch A. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther. 2010;12:R79. doi: 10.1186/ar3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henchoz Y, Kai-Lik So A. Exercise and nonspecific low back pain: a literature review. Joint Bone Spine. 2008;75:533–539. doi: 10.1016/j.jbspin.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Hoeger Bement M, Sluka KA. Low-intensity exercise reverses chronic muscle pain in the rat in a naloxone-dependent manner. Arch Phys Med Rehabil. 2005;86:1736–1740. doi: 10.1016/j.apmr.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 16.Hoeger Bement M, Wyer A, Hartley S, Drewek B, Harkins AL, Hunter SK. Pain perception after isometric exercise in women with fibromyalgia. Arch Phys Med Rehabil. 2011;92:89–95. doi: 10.1016/j.apmr.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Hopman-Rock M, Odding E, Hofman A, Kraaimaat FW, Bijlsma JW. Physical and psychosocial disability in elderly subjects in relation to pain in the hip and/or knee. J Rheumatol. 1996;23:1037–1044. [PubMed] [Google Scholar]
- 18.Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn. 1957;35:307–315. [PubMed] [Google Scholar]
- 19.Kelley GA, Kelley KS, Hootman JM, Jones DL. Effects of community-deliverable exercise on pain and physical function in adults with arthritis and other rheumatic diseases: A meta-analysis. Arthritis Care & Research. 2011;63:79–93. doi: 10.1002/acr.20347. [DOI] [PubMed] [Google Scholar]
- 20.Knauf MT, Koltyn KF. Exercise-induced modulation of pain in adults with and without painful diabetic neuropathy. J Pain. 2014;15:656–63. doi: 10.1016/j.jpain.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koltyn KF, Brellenthin AG, Cook DB, Sehgal N, Hillard C. Mechanisms of exercise-induced hypoalgesia. J Pain. 2014;15:1294–1304. doi: 10.1016/j.jpain.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koltyn KF, Knauf MT, Brellenthin AG. Temporal summation of heat pain modulated by isometric exercise. European Journal of Pain. 2013;17:1005–11. doi: 10.1002/j.1532-2149.2012.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lannersten L, Kosek E. Dysfunction of endogenous pain inhibition during exercise with painful muscles in patients with shoulder myalgia and fibromyalgia. Pain. 2010;151:77–86. doi: 10.1016/j.pain.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 24.Lariviere M, Goffaux P, Marchand S, Julien N. Changes in pain perception and descending inhibitory controls start at middle age in healthy adults. Clin J Pain. 2007;23:506–510. doi: 10.1097/AJP.0b013e31806a23e8. [DOI] [PubMed] [Google Scholar]
- 25.Lemley KJ, Drewek B, Hunter SK, Hoeger Bement MK. Pain Relief after isometric exercise is not task-dependent in older men and women. Med Sci Sports Exerc. 2014;46:185–191. doi: 10.1249/MSS.0b013e3182a05de8. [DOI] [PubMed] [Google Scholar]
- 26.Lemley KJ, Hunter SK, Hoeger Bement MK. Conditioned pain modulation predicts exercise-induced hypoalgesia in healthy adults. Med Sci Sports Exerc. 2015;47:176–184. doi: 10.1249/MSS.0000000000000381. [DOI] [PubMed] [Google Scholar]
- 27.Morley JE, Flood JF, Silver AJ. Opioid peptides and aging. Ann NY Acad Sci. 1990;579:123–32. doi: 10.1111/j.1749-6632.1990.tb48355.x. [DOI] [PubMed] [Google Scholar]
- 28.Naugle KM, Cruz-Almeida Y, Fillingim RB, Riley JL., 3rd Offset analgesia is reduced in older adults. Pain. 2013;154:2381–7. doi: 10.1016/j.pain.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naugle KM, Cruz-Almeida Y, Vierck CJ, Mauderli AP, Riley JL., 3rd Age-related differences in conditioned pain modulation of sensitizing and desensitizing trends during response dependent stimulation. Behav Brain Res. 2015;289:61–8. doi: 10.1016/j.bbr.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naugle KM, Fillingim RB, Riley JL., 3rd A meta-analytic review of the hypoalgesic effects of exercise. J Pain. 2012;13:1139–50. doi: 10.1016/j.jpain.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naugle KM, Naugle KE, Fillingim RB, Riley JL., 3rd Isometric exercise as a test of pain modulation: effects of experimental pain test, psychological variables, and sex. Pain Med. 2014;15:692–701. doi: 10.1111/pme.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naugle KM, Naugle KE, Fillingim RB, Samuels B, Riley JL., III Intensity thresholds for aerobic exercise-induced hypoalgesia. Med Sci Sports Exerc. 2014;46:817–25. doi: 10.1249/MSS.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neziri AY, Curatolo M, Nuesch E, Scaramozzino P, Andersen OK, Arendt-Nielsen L, Juni P. Factor analysis of responses to thermal, electrical, and mechanical painful stimuli supports the importance of multi-modal pain assessment. Pain. 2011;152:1146–55. doi: 10.1016/j.pain.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 34.Picavet HS, Hazes JM. Prevalence of self-reported musculoskeletal diseases is high. Ann Rheum Di. 2003;62:644–50. doi: 10.1136/ard.62.7.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Portney LG, Watkins MP. Foundations of clinical research: applications to practice. Appleton and Lang; East Norwalk, CT: 1993. [Google Scholar]
- 36.Price DD. Characteristics of second pain and flexion reflexes indicative of prolonged central summation. Experimental Neurology. 1972;7:371–387. doi: 10.1016/0014-4886(72)90081-7. [DOI] [PubMed] [Google Scholar]
- 37.Price DD, Dubner R. Mechanisms of first and second pain in the peripheral and central nervous systems. Journal of Investigative Dermatology. 1977;69:167–171. doi: 10.1111/1523-1747.ep12497942. [DOI] [PubMed] [Google Scholar]
- 38.Riley JL, King CD, Wong F, Fillingim RB, Mauderli AP. Lack of endogenous modulation and reduced decay of prolonged heat pain in older adults. Pain. 2010;150:153–160. doi: 10.1016/j.pain.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rustoen T, Wahl AK, Hanestad BR, Lerdal A, Paul S, Miaskowski C. Age and the experience of chronic pain: differences in health and quality of life among younger, middle-aged, and older adults. Clin J Pain. 2005;21:513–23. doi: 10.1097/01.ajp.0000146217.31780.ef. [DOI] [PubMed] [Google Scholar]
- 40.Scudds RJ, McD Robertson J. Empirical evidence of the association between the presence of musculoskeletal pain and physical disability in community-dwelling senior citizens. Pain. 1998;75:229–235. doi: 10.1016/s0304-3959(97)00224-8. [DOI] [PubMed] [Google Scholar]
- 41.Shephard R. PAR-Q, Canadian Home Fitness Test and exercise screening alternatives. Sports Med. 1988;5:185–95. doi: 10.2165/00007256-198805030-00005. [DOI] [PubMed] [Google Scholar]
- 42.Stagg NJ, Mata HP, Ibrahim MM, Henriksen EJ, Porreca F, Vanderah TW, Malan PT. Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: role of endogenous opioids. Anesthesiology. 2011;114:940–948. doi: 10.1097/ALN.0b013e318210f880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Staud R. Abnormal endogenous pain modulation is a shared characteristic of many chronic pain conditions. Expert Rev Neurother. 2012;12:577–585. doi: 10.1586/ern.12.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Staud R, Robinson M, Price D. Isometric exercise has opposite effects on central pain mechanisms in fibromyalgia patients compared to normal controls. Pain. 2005;118:176–184. doi: 10.1016/j.pain.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 45.Tse MM, Wan VT, Ho SS. Physical exercise: does it help in relieving pain and increasing mobility among older adults with chronic pain? J Clin Nurs. 2011;20:635–44. doi: 10.1111/j.1365-2702.2010.03548.x. [DOI] [PubMed] [Google Scholar]
- 46.Umeda M, Newcomb L, Ellingson L, Koltyn K. Examination of the dose-response relationship between pain perception and blood pressure elevations induced by isometric exercise. Biol Psychol. 2010;85:90–96. doi: 10.1016/j.biopsycho.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Vierck C, Staud R, Price D, Cannon R, Mauderli A, Martin A. The effect of maximal exercise on temporal summation of second pain (windup) in patients with Fibromyalgia Syndrome. J Pain. 2001;2:334–44. doi: 10.1054/jpai.2001.25533. [DOI] [PubMed] [Google Scholar]
- 48.Yarnitsky D, Arendt-Nielsen L, Bouhassira D, Edwards RR, Fillingim RB, Granot M, Hansson P, Lautenbacher S, Marchand S, Wilder-Smith O. Recommendations on terminology and practice of psychophysical DNIC testing. European Journal of Pain. 2010;14:339. doi: 10.1016/j.ejpain.2010.02.004. [DOI] [PubMed] [Google Scholar]