Abstract
Background
Activating BRAF V600E mutations are found in approximately 1–2% of adenocarcinomas of the lung offering an opportunity to test targeted therapy for this disease. Dabrafenib is an oral selective inhibitor of the BRAF kinase. The aim of this study was to assess the clinical activity of dabrafenib in patients with advanced BRAF V600E-mutant non-small cell lung cancer (NSCLC).
Methods
In this phase 2, multicenter, nonrandomized, open-label study of previously treated and untreated patients with stage IV, metastatic NSCLC and BRAF V600E mutation, we evaluated the antitumor activity and safety of oral dabrafenib (150 mg twice daily). The primary endpoint was investigator-assessed overall response rate (ORR) in patients receiving ≥ 1 dose of study drug. Safety analysis was performed on the all-treated population (all previously treated and untreated patients receiving ≥ 1 dose of study drug). The study is ongoing but not enrolling participants in this cohort. This trial is registered with ClinicalTrials.gov, number NCT01336634.
Findings
Between August 2011 and February 2014 a total of 84 previously treated and untreated patients were enrolled. Investigator-assessed ORR for 78 pretreated patients was 33% (95% confidence interval [CI], 23·1 to 44·9). Independent review committee assessment of ORR was consistent with investigator-based assessment. Four of the six previously untreated patients had an objective response. One patient died on study due to intracranial hemorrhage that was considered by the investigator to be due to study drug. Serious adverse events were reported in 35 (42%) of 84 patients. The most frequent grade 3 or higher adverse events were cutaneous squamous cell carcinoma (10 [12%] of 84 patients), asthenia (4 [5%] of 84 patients), and basal cell carcinoma (4 [5%] of 84 patients).
Interpretation
This is, to our knowledge, the first prospective trial focusing on BRAF V600E-mutant NSCLC to show clinical activity of a BRAF inhibitor. The results presented here suggest that dabrafenib may represent a future treatment option for patients with BRAF V600E-mutant NSCLC, a population with limited therapeutic options.
Funding
This trial was funded by GlaxoSmithKline. Dabrafenib is an asset of Novartis AG as of March 2, 2015.
Introduction
Non-small cell lung cancer (NSCLC) accounts for approximately 85% of lung cancers and remains a major cause of cancer-related deaths globally.1 In the past few decades, significant strides have been made in defining the molecular pathogenesis of lung cancers—particularly in detection of critical oncogenic drivers—leading to accelerated development of specific targeted agents. Constitutively activating mutations in the BRAF gene, first described in lung cancers in 2002,2,3 drive growth and survival of cancer cells that harbor them and are extremely sensitive to selective BRAF inhibitor therapy across multiple tumor types.4 Moreover, BRAF V600E behaves as an oncogenic driver in a transgenic murine lung cancer model.5
BRAF mutations are present in approximately 2–4% of lung adenocarcinomas, and approximately one-half are V600E mutations.2,6–8 The clinical outcome in patients with BRAF V600E mutations is associated with shorter overall survival (OS) and lower response rates to platinum-based chemotherapy than in patients with wild-type BRAF.9,10 A high unmet need remains for novel therapeutic strategies in this population with limited treatment options and poor prognosis. Importantly, BRAF mutations and other oncogenic drivers, including epidermal growth factor receptor (EGFR) and RAS mutations as well as anaplastic lymphoma kinase (ALK) rearrangements, are typically mutually exclusive; this is consistent with the notion that BRAF mutation defines a unique molecular subset of patients with NSCLC who may benefit from treatment with a BRAF inhibitor.
To date, the clinical experience with BRAF inhibitors in BRAF V600E-mutant NSCLC has been primarily limited to isolated cases and a retrospective case series.11–14 A recent basket study examining the activity of vemurafenib in patients with a variety of solid tumors and hematologic malignancies, enrolled a cohort of 19 patients with BRAF V600E NSCLC and demonstrated a promising overall response rate (ORR) of 42%.15 Here we report the first prospective trial examining the clinical activity and safety of a BRAF inhibitor for the treatment of patients with BRAF V600E-mutant NSCLC. Dabrafenib, a potent adenosine triphosphate–competitive inhibitor of BRAF kinase selective for the BRAF V600E mutant in kinase panel screening, cell lines, and xenografts16 is approved globally for the treatment of unresectable or metastatic BRAF V600–mutated melanoma. This study investigated the therapeutic effects of dabrafenib administered at the approved dose for melanoma (150 mg twice daily)13 to patients with previously treated advanced or metastatic NSCLC whose tumors carry a BRAF V600E mutation.
Methods
Study design and participants
In this phase 2, multicenter, nonrandomized, open-label study, patients were recruited from 34 centers in 10 countries within North America, Europe, and Asia (appendix pp 3–4). Eligible patients, ≥ 18 years of age, had histologically confirmed stage IV NSCLC that had progressed after receiving ≥ one systemic treatment for metastatic disease. BRAF V600E mutational status was required based on local testing in Clinical Laboratory Improvement Amendments–approved (or equivalent) laboratories as well as measurable disease per Response Evaluation Criteria In Solid Tumors (RECIST) v1.1. The authors and sponsor believe that an FDA approved next-generation sequencing platform will improve patient identification, however, as this platform is not yet clinically validated, central screening has not yet been carried out. Other key eligibility criteria included an Eastern Cooperative Oncology Group performance status of ≤ 2, a tumor sample adequate for central confirmation of BRAF V600E mutation, and an anticipated life expectancy > 3 months. Adequate amount of tumor tissue for central BRAF V600E confirmation testing was defined as at least 10–15 unstained slides with no less than 50% tumor content per slide. All enrolled subjects were required to either provide archival tumor tissue or, if archival sample was not available, a pre-dose biopsy was required to collect an adequate amount of fresh tumor tissue. The central BRAF V600E confirmation has not yet been completed at the time of the submission. Patients with inadequate tumor sample after enrollment were permitted to stay on study; additional patients were enrolled to ensure an adequate number with centrally confirmed mutation for the analysis of clinical activity. Laboratory assessments for eligibility included hematology (ANC ≥ 1.5 × 109/L; hemoglobin ≥ 9 g/dL; platelet count ≥ 100 × 109/L), chemistry (total bilirubin ≤ 1.5 × upper limit of normal [ULN]; alanine aminotransferase and aspartate aminotransferase ≤ 2.5 × ULN; serum creatinine ≤ 1.5 mg/dL or creatinine clearance ≥ 50 mL/min), and coagulation (prothrombin time/international normalized ratio and partial thromboplastin time ≤ 1.5 × ULN). Patients with EGFR mutations or ALK rearrangement were eligible if they had previously received EGFR or ALK inhibitors, respectively. Key exclusion criteria were previous therapy with a BRAF or MEK inhibitor and symptomatic or unstable brain metastases. Patients were excluded if they had received anticancer therapy (including chemotherapy, radiation therapy, immunotherapy, biological therapy, or major surgery) within 14 days of the start of therapy or if they had received an investigational anticancer drug within 14 days or 5 half-lives of start of therapy (minimum 14 days). Women who are pregnant, patients with known hepatitis B or C virus, or those with a history or signs of cardiovascular risk (left ventricular ejection fraction ≥ lower limit of normal by ECHO) were excluded. Patients with asymptomatic, untreated brain metastases < 1 cm were allowed to enroll. The rate and duration of response for dabrafenib in ≥ second-line patients at the interim analysis and the preferential safety profile supported the use of dabrafenib prior to chemotherapy in first-line patients. Therefore, patients with no prior systemic anticancer therapy for metastatic disease were enrolled in the expansion cohort under a protocol amendment in April 2013. Following discussions with regulatory agencies, a decision was made to delay further enrollment for first-line patients until the enrollment of the dabrafenib plus trametinib combination cohort due to an expectation of increased response rates with combination therapy. Therefore, first-line enrollment in this cohort was stopped at 6 patients. This study was conducted in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines. The protocol was approved by the institutional review board at each participating institution. All patients gave written informed consent.
Procedures
Patients were treated with dabrafenib (Tafinlar; Novartis AG) 150 mg orally twice daily until disease progression (PD), unacceptable adverse events (AEs), withdrawal of consent, or death. Study treatment could also be discontinued for any of the following reasons: protocol deviation, patient request, investigator discretion, patient is lost to follow-up, or in the event that the study is closed or terminated. Dose interruptions and/or modifications were used to manage intolerable ≥ grade 2 AEs. Doses were sequentially reduced to 100, 75, or 50 mg twice daily, depending on event severity. Treatment was discontinued in patients not tolerating 50 mg twice daily. Patients with PD were permitted to continue dabrafenib if they had a confirmed response (complete response [CR] or partial response [PR]) or stable disease (SD) lasting ≥ 12 weeks while taking dabrafenib and the investigator believed that the patient was clinically benefiting from therapy. Baseline disease assessment included computed tomography (CT) with contrast material of the chest and abdomen and clinical disease assessment for palpable lesions. In patients with known brain metastases, contrast-enhanced brain magnetic resonance imaging or head CT was conducted at baseline and repeated during each disease assessment. All baseline medical history, physical examination, laboratory, demographic, cardiac, and radiological tumor assessments were performed within 28 days before the first dose of dabrafenib. Patients were evaluated for safety at least once every three weeks. AEs, laboratory values (hematology and clinical chemistry), and vital signs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v4.0. Cardiac echocardiograms and electrocardiograms were performed at baseline, week 6, week 15, and every 9 weeks thereafter. Radiological disease assessments by CT using RECIST 1.1 were performed every 6 weeks until week 36, then every 12 weeks, with any responses confirmed by repeat assessment ≥ 28 days after the initial response. RECIST scans to determine the primary endpoint, and all time-to-event endpoints, except for OS, were reviewed by an independent review committee as a sensitivity analysis. All patients who discontinued study medication were followed for subsequent treatment(s) and survival every 12 weeks, until death or study completion. Safety data were evaluated three times per year by an independent data monitoring committee.
Outcomes
The primary endpoint was investigator-assessed overall response rate (ORR) which is defined as the percentage of subjects with a confirmed CR or PR by investigator assessment as per RECIST v1.1 criteria. Tumor response was also assessed by independent review. Secondary endpoints included PFS (defined as the interval between first dose and the earliest date of disease progression or death due to any cause), duration of response (DOR; defined as the time from first documented evidence of CR or PR until time of first documented disease progression or death due to any cause, whichever occurs earlier), disease control rate (DCR; defined as the percentage of patients with ≥ SD) for > 12 weeks, OS (defined as the time from first dose until death due to any cause), pharmacokinetic assessment and safety and tolerability of dabrafenib.
Statistical analysis
The anticipated ORR based on prior literature in patients with advanced unselected NSCLC receiving single-agent chemotherapy or erlotinib in the second- or third-line setting was estimated to be 7–10%.17,18 The null hypothesis was that the ORR was not clinically meaningful (≤ 10%). The alternative hypothesis was that the ORR was clinically meaningful (≥ 30%) and therefore, the compound warrants further development. To allow early termination of the trial due to lack of activity, ORR was assessed at an interim time point based on a 2-stage Green-Dahlberg19 design for phase 2 cancer trials with a planned enrollment of 40 patients (20 patients in each stage; criteria for study continuation are provided in the Appendix p. 5). The above design corresponded to a type I error of 0·038 and power of 92·6%. To further refine the 95% confidence interval for ORR in this treatment setting and to allow treatment-naive patients, an expansion cohort was added with a planned enrollment of 20 patients in the ≥ second line therapy and first-line patients.
Primary analyses of clinical activity were performed in patients who received ≥ 1 dose of dabrafenib and had prior systemic therapy for metastatic NSCLC (≥ second-line patients). The DOR, PFS, and OS were estimated by medians calculated using the Kaplan-Meier method, with corresponding two-sided 95% confidence intervals calculated using the Brookmeyer-Crowley method.20 Two-sided 95% confidence intervals for ORR were determined using the Clopper-Pearson method.21 Six patients who did not have prior systemic therapy for metastatic disease were included in an exploratory activity analysis population (first-line all-treated population). All patients (both pretreated and previously untreated) who received ≥ 1 dose of study drug were included in the safety analysis. Exploratory analyses utilized the same methodology as primary and secondary analyses. A protocol-mandated analysis was performed when the investigators and sponsor believed that enrollment was sufficient to include 60 previously treated patients with measurable disease by independent reviewer assessment and is presented in the appendix (p 5, 8, 10). An updated analysis was performed to obtain more mature DOR data and is presented in the main text of the manuscript. SAS version 9.4 was used for statistical analyses. This study is registered with ClinicalTrials.gov, number NCT01336634.
Role of the funding source
This study was sponsored by GlaxoSmithKline; dabrafenib is an asset of Novartis AG as of March 2, 2015. The study was designed by the academic authors in conjunction with representatives of the sponsor. The data were collected by the sponsor and analyzed in collaboration with the authors. LP, CN, B Ma, AD, BM, and MC had access to the raw data. The first and last authors wrote the initial draft of the manuscript, and all authors contributed to subsequent drafts and made the decision to submit the manuscript for publication. The authors vouch for the accuracy of the data and the fidelity of the study to the protocol. Editorial support that did not involve writing was provided by Articulate Science and funded by the sponsor.
Results
Patients
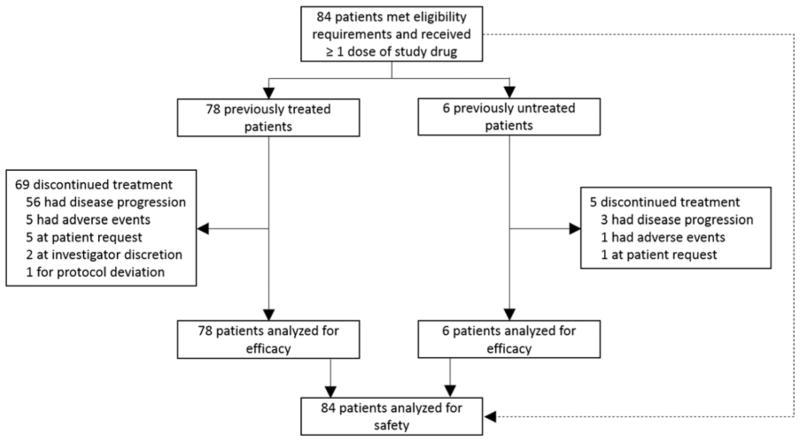
Between 3 August, 2011 and 25 February, 2014, 84 patients (44 female), with a median age of 66 years were enrolled. A total of 78 patients received dabrafenib after ≥ one prior chemotherapy regimen for metastatic disease due to over-recruitment designed to offset any potential issues with central confirmation of mutation status and central review of responses, and six patients received dabrafenib as first-line treatment for metastatic disease. Central confirmation of BRAF mutation status has not yet been completed. The median duration of exposure to dabrafenib monotherapy was 4·6 months (IQR, 1·79 to 11·07 months; appendix p 11).
Baseline characteristics of the 78 ≥ second-line patients are provided in Table 1. One-half of the patients were women; a large majority of patients had adenocarcinoma of the lung and were former (46 [59%] of 78) or current (3 [4%] of 78) smokers. As of November 21, 2014, nine (12%) of 78 patients remain on therapy, 69 (88%) discontinued therapy, and 46 (59%) died (Fig. 1).
Table 1.
Baseline demographic and clinical characteristics of the 78 ≥ second-line patients
| Characteristic | Classification | ≥ Second-Line Patients (N = 78) |
|---|---|---|
| Age, years | Median (range) | 66 (28–85) |
| Sex, n (%) | Female/male | 39 (50)/39 (50) |
| Race, n (%) | White | 59 (76) |
| Asian | 17 (22) | |
| African American | 2 (3) | |
| ECOG PS at baseline, n (%) | 0 | 16 (21) |
| 1 | 50 (64) | |
| 2 | 12 (15) | |
| Smoking history, n (%) | Never smokera | 29 (37) |
| Smoker ≤ 30 pack-yearsb | 25 (32) | |
| Smoker > 30 pack-yearsb | 24 (31) | |
| Histology at initial diagnosis, n (%) | Adenocarcinoma | 75 (96) |
| Otherc | 3 (4) | |
| Number of prior systemic regimens for metastatic disease, n (%) | 1 | 40 (51) |
| 2 | 14 (18) | |
| 3 | 24 (31) | |
| Time since last progression, months (n = 71) | Median (IQR) | 1·1 (0·7–2·1) |
ECOG denotes Eastern Cooperative Oncology Group, PS performance status.
The definition of never-smokers was at the discretion of the local sites.
Among 49 smokers, 3 current smokers, and 46 former smokers.
“Other” includes 1 patient with adenosquamous carcinoma, predominately squamous cell carcinoma; 1 patient with bronchioalveolar carcinoma, mucinous type; and 1 patient with large cell carcinoma, adenocarcinoma.
Figure 1.
Trial profile
Clinical Activity
Analyses based on data generated from independent review of disease assessment scans were performed to validate response data based on the investigator’s assessments. For the ≥ second-line population, this consisted of 64 patients with measurable disease at baseline as determined by an independent review committee (IRC). At the time of the protocol-mandated activity analysis, results for DOR were immature and thus an updated, mature activity analysis, was performed and is presented in this section (protocol-mandated activity results are presented the appendix, p 5, 8, 10).
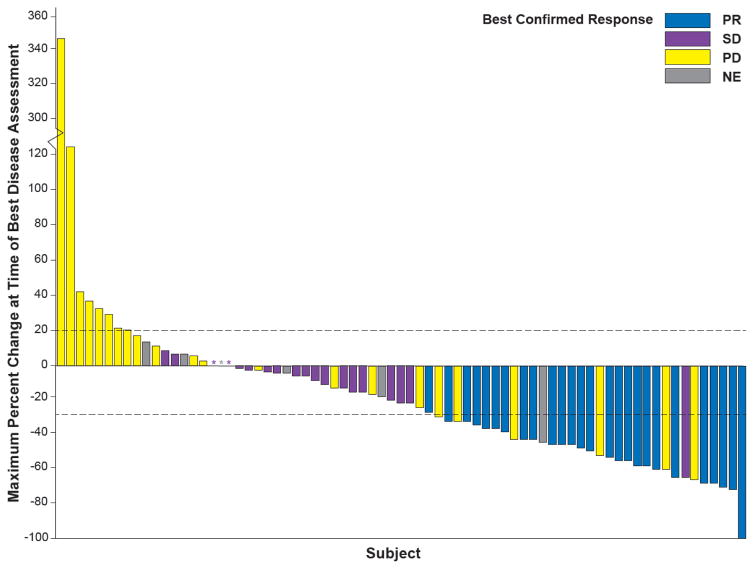
With a median follow-up of 10·7 months (IQR, 4·5–16·2 months), 26 of 78 ≥ second-line patients receiving dabrafenib monotherapy (33%; 95% CI, 23 to 45; Fig. 1 and Table 2) had a confirmed ORR by the investigator. Most initial objective responses were observed at first postbaseline disease assessment (19 patients with PR). Seven patients had a PR after the first postbaseline assessment (three at week 12; two at week 18; one at week 24; and one at week 36). The DCR (≥ SD) was 58% (45 of 78 patients [95% CI, 46 to 67]; Fig. 2, Table 2). Of the 78 ≥ second-line patients, 23 (29%) had PD as best response. Ten (13%) of 78 patients were not evaluable for response due to lack of post-baseline assessment or discontinuation prior to 12-weeks without PD according to RECIST (n=6 had SD < 12 weeks [< 2 planned post-baseline assessments] without PD; n=4 had no post-baseline assessment [n=3 due to AEs; n=1 due to patient or proxy decision to transfer to palliative care]). The IRC ORR and DCR were 33% (21 of 64 patients [95% CI, 22 to 46]; PR, 20 [31%] of 64 patients; SD, 13 [20%] of 64 patients; PD, 23 [36%] of 64 patients) and 53% (34 of 64 patients [95% CI, 40 to 66]), respectively. A post-hoc analysis demonstrated an ORR of 38% (≥ PR in 15 of 40 patients) and DCR of 65% (≥ SD in 26 of 40 patients) in patients with one prior line of therapy compared with an ORR of 29% (≥ PR in 11 of 38 patients) and DCR of 50% (≥ SD in 19 of 38 patients) in patients who had received ≥ 2 prior lines of therapy (appendix p 6). In a post-hoc analysis of response based upon prior smoking history, the ORR of patients with no prior history of smoking was 52% (≥ PR in 15 of 29 patients) vs 24% (≥ PR in 6 of 25 patients) in patients with a history of < 30 pack-years and 21% (≥ PR in 5 of 24 patients) among patients with a history of ≥ 30 pack-years (appendix p 7).
Table 2.
Clinical activity endpoints as assessed in patients with measurable disease at baseline (≥ second-line patients) at the time of mature activity analyses
| Clinical Activity Endpoint: | Investigator assessment (N= 78) | Independent review committee (n = 64) |
|---|---|---|
| Best Response | ||
| Response rate, n (%), ≥ confirmed PR [95% CI] | 26 (33) [23–45] | 21 (33) [22–46]d |
| Stable disease, n (%), confirmed SD [95% CI]a | 19 (24) [15–35] | 13 (20) [11–32] |
| Disease control rate, n (%), CR + PR + SD [95% CI]b | 45 (58) [46–67] | 34 (53) [40–66] |
| PD, n (%) | 23 (29) | 23 (36) |
| Not evaluable, n (%)c | 10 (13) | 7 (11) |
| Progression-free survival, median (95% CI), months | 5·5 (3.4–7.3) | 5.5 (2.8 – 6.9) |
| Duration of response, median (95% CI) | 9·6 (5.4–15.2) | 9.9 (4.2 – ND) |
CR, complete response; ND, not defined; PD, progressive disease; PR, partial response; SD stable disease.
Defined as SD ≥ 12 weeks (planned time for the second postbaseline disease assessment).
Disease control rate was the percentage of patients with a confirmed response or stable disease for at least 12 weeks after initiation of therapy.
Not evaluable patients were those lacking post-baseline assessment or discontinuing prior to 12-weeks without PD according to RECIST (n=6 had SD < 12 weeks [planned 2 post-baseline assessments]; n=4 had no post-baseline assessment [n=3 due to AEs; n=1 due to patient or proxy decision to transfer to palliative care])
One patient reviewed by independent review committee had a best response of complete response
Figure 2. Response to dabrafenib in BRAF V600E–mutant NSCLC.
Maximum change in the sum of lesion diameters by best confirmed response in ≥ second-line patients treated with dabrafenib (N = 78) at the time of the clinical activity analyses. The dashed line at 20 represents the RECIST 1.1 definition for progressive disease, while the dashed line at −30 represents the definition for partial response. Asterisks represent patients with no change from baseline at the time of assessment. NE denotes not estimable, NSCLC non-small cell lung cancer, PD progressive disease, PR partial response, RECIST Response Evaluation Criteria In Solid Tumors, SD stable disease.
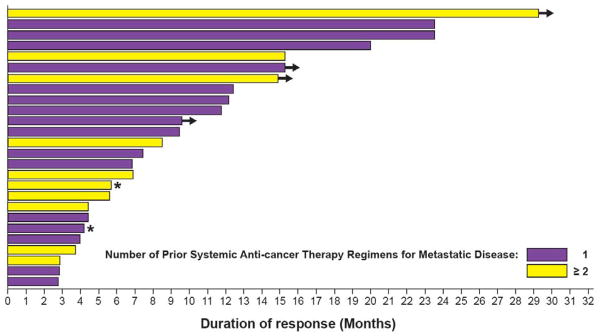
Investigator-assessed median DOR for ≥ second-line patients was 9·6 months (95% CI, 5·4 to 15·2; Fig. 3). DOR was > 6 months in 16 patients, > 9 months in 12 patients, and > 12 months in 9 patients. Median PFS was 5·5 months (95% CI, 3·4 to 7·3); 59 (76%) of 78 patients progressed or died at the time of updated analyses (Table 2; appendix pp 12–13). Based on IRC assessment, median DOR was 9·9 months (95% CI, 4·2 to not defined) and PFS was 5·5 months (95% CI, 2·8 to 6·9). The preliminary median OS was 12·7 months (95% CI, 7·3 to 16·9; appendix p 14); 46 (59%) of 78 patients had died at the time of the analysis. Results of a protocol-mandated clinical activity analysis performed prior to full maturation of DOR data were similar and are included in the appendix p 5, 8, & 10.
Figure 3. Duration of investigator-assessed response in ≥ second-line patients at time of mature activity analyses.
Duration of response in patients with one prior therapy (purple bars) or ≥ two prior therapies (yellow bars). Arrows indicate patients remaining on therapy. Asterisks represent patients censored/lost to follow-up.
Four of six patients in first-line treatment had a PR by investigator assessment. The four patients with PR had PFS of 4·5, 8·6, 11·0, and 16·6 months, corresponding to DORs of 3·2, 7·2, 9·6, and 12·5 months, respectively. The two patients without a response had PFS of 4·0 and 8·1 months.
Safety and adverse events
As of April 30, 2014, almost all patients had ≥ one AE (83 of 84 patients [99%]), with 45 (54%) of 84 patients having maximum-grade AEs ≤ grade 2 (appendix p 9). Maximum AE grades of 3, 4, and 5 were reported in 33 (39%), 4 (5%), and 1 (1%) of the 84 patients, respectively. Seventy-seven (92%) of 84 patients had AEs related to study treatment. Five (6%) of 84 patients had AEs that led to dabrafenib discontinuation (blister, general health deterioration, intracranial hemorrhage, malaise, and palmar-plantar erythrodysaesthesia syndrome; n=1 each). Thirty-six (43%) and 15 (18%) of the 84 patients had AEs that led to dose interruptions and reductions, respectively. The most common AEs leading to dose interruption were pyrexia (9 [11%] of 84 patients), chills (5 [6%] of 84 patients), and vomiting (4 [5%] of 84 patients). The most common AEs leading to dose reduction included palmar-plantar erythrodysaesthesia (3 [4%] of 84 patients) and pyrexia (3 [4%] of 84 patients). Patients were exposed to a median dose of 296·2 mg per day, representing 98·7% of the intended dose of 300 mg per day. The most common AEs (all grades, > 20%) were pyrexia (36% [30 of 84 patients]), asthenia (30% [25 of 84 patients])), hyperkeratosis (30% [25 of 84 patients]), decreased appetite (29% [24 of 84 patients]), nausea (27% [23 of 84 patients]), cough (26% [22 of 84 patients]), fatigue (26% [22 of 84 patients]), skin papillomas (26% [22 of 84 patients]), dry skin (23% [19 of 84 patients]), and alopecia (21% [18 of 84 patients]; Table 3). Ten (12%) of 84 patients had cutaneous squamous cell carcinomas (SCCs) and four (5%) had basal cell carcinomas (all grade 3). The median time to development of cutaneous SCC was 13·1 weeks, and dose modification/interruption was not required. No SCCs at other organ sites were observed. One patient with asymptomatic brain metastasis at baseline did not have visible brain lesion on 6-week or 12-week tumor assessment prior to study discontinuation due to non-compliance. A total of 4 patients developed new brain metastases during the course of the study. One patient died on study due to intracranial hemorrhage that was reported within 2 weeks of starting dabrafenib and was considered related to study treatment. Serious AEs were pyrexia (five of 84 patients [6%]) and ejection fraction decrease and pneumonias (two of 84 patients each [2%]).
Table 3.
Adverse events in the 84 dabrafenib-treated patients
| Common adverse events (preferred term) | Grade 1–2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|
| Pyrexia | 28 (33) | 2 (2) | 0 | 0 |
| Asthenia | 21 (25) | 3 (4) | 1 (1) | 0 |
| Hyperkeratosis | 24 (29) | 1 (1) | 0 | 0 |
| Decreased appetite | 23 (27) | 1 (1) | 0 | 0 |
| Nausea | 22 (26) | 1 (1) | 0 | 0 |
| Cough | 22 (26) | 0 | 0 | 0 |
| Fatigue | 21 (25) | 1 (1) | 0 | 0 |
| Skin papilloma | 22 (26) | 0 | 0 | 0 |
| Dry skin | 19 (23) | 0 | 0 | 0 |
| Alopecia | 18 (21) | 0 | 0 | 0 |
| Palmar-plantar erythrodysaesthesia syndrome | 15 (18) | 2 (2) | 0 | 0 |
| Rash | 16 (19) | 1 (1) | 0 | 0 |
| Vomiting | 16 (19) | 1 (1) | 0 | 0 |
| Dyspnea | 14 (17) | 2 (2) | 0 | 0 |
| Headache | 13 (15) | 2 (2) | 0 | 0 |
| Arthralgia | 13 (15) | 1 (1) | 0 | 0 |
| Diarrhea | 13 (15) | 1 (1) | 0 | 0 |
| Pain in extremity | 14 (17) | 0 | 0 | 0 |
| Chills | 12 (14) | 1 (1) | 0 | 0 |
| Weight decreased | 13 (15) | 0 | 0 | 0 |
| Pruritus | 12 (14) | 0 | 0 | 0 |
| Myalgia | 11 (13) | 0 | 0 | 0 |
| Papule | 11 (13) | 0 | 0 | 0 |
| Squamous cell carcinoma | 0 | 10 (12) | 0 | 0 |
| Back pain | 10 (12) | 0 | 0 | 0 |
| Anemia | 7 (8) | 2 (2) | 0 | 0 |
| Constipation | 8 (10) | 1 (1) | 0 | 0 |
| Melanocytic naevus | 9 (11) | 0 | 0 | 0 |
| Seborrheic keratosis | 9 (11) | 0 | 0 | 0 |
| Actinic keratosis | 8 (10) | 0 | 0 | 0 |
| Dysphonia | 8 (10) | 0 | 0 | 0 |
| Nasopharyngitis | 8 (10) | 0 | 0 | 0 |
| Muscular weakness | 5 (6) | 1 (1) | 0 | 0 |
| Hypophosphatemia | 2 (2) | 3 (4) | 0 | 0 |
| Hypotension | 4 (5) | 1 (1) | 0 | 0 |
| Anxiety | 2 (2) | 2 (2) | 0 | 0 |
| Basal cell carcinoma | 0 | 4 (5) | 0 | 0 |
| Hyperglycemia | 3 (4) | 0 | 1 (1) | 0 |
| Hypokalemia | 3 (4) | 1 (1) | 0 | 0 |
| Lymphopenia | 3 (4) | 1 (1) | 0 | 0 |
| White blood cell count increased | 2 (2) | 2 (2) | 0 | 0 |
| Confusional state | 2 (2) | 1 (1) | 0 | 0 |
| Depression | 2 (2) | 1 (1) | 0 | 0 |
| Hypertension | 2 (2) | 1 (1) | 0 | 0 |
| Hyponatremia | 1 (1) | 2 (2) | 0 | 0 |
| Leukopenia | 2 (2) | 0 | 1 (1) | 0 |
| Thrombocytopenia | 2 (2) | 1 (1) | 0 | 0 |
| Blood creatinine increased | 1 (1) | 1 (1) | 0 | 0 |
| Gastritis | 1 (1) | 1 (1) | 0 | 0 |
| Pericardial effusion | 1 (1) | 1 (1) | 0 | 0 |
| Respiratory tract infection | 1 (1) | 1 (1) | 0 | 0 |
| Cardiac ventricular thrombosis | 0 | 1 (1) | 0 | 0 |
| Colitis | 0 | 1 (1) | 0 | 0 |
| Ischemic colitis | 0 | 1 (1) | 0 | 0 |
| Intracranial hemorrhage | 0 | 0 | 0 | 1 (1) |
| Lip squamous cell carcinoma | 0 | 1 (1) | 0 | 0 |
| Malnutrition | 0 | 1 (1) | 0 | 0 |
| Bacterial peritonitis | 0 | 0 | 1 (1) | 0 |
| Pleuritic pain | 0 | 1 (1) | 0 | 0 |
| Pneumonia aspiration | 0 | 1 (1) | 0 | 0 |
| Prostatic obstruction | 0 | 1 (1) | 0 | 0 |
| Radiation injury | 0 | 1 (1) | 0 | 0 |
| Uveitis | 0 | 1 (1) | 0 | 0 |
Data are n (%). Adverse events (preferred terms) of grades 1–2 occurring in at least 10% of patients and all grade 3 or higher events are listed
PK assessment is a secondary outcome. The assessments are ongoing so we do not yet have the full dataset. Therefore, the investigators have not yet analyzed the PK data and are not yet able to report it.
Discussion
This phase 2 study demonstrates antitumor activity of dabrafenib monotherapy in patients with BRAF V600–mutated NSCLC. We report a confirmed ORR of 33% with a DCR of 58% for dabrafenib monotherapy in 78 previously treated metastatic BRAF V600–mutated NSCLC. Median PFS and DOR were 5·5 and 9·6 months, respectively. The responses were durable and had rapid onset, with 73% of responses initially observed at first postbaseline assessment at week 6. Results from an independent review of clinical activity data were consistent with investigator-assessed responses. The preliminary survival data for these patients with one to three previous lines of therapy shows a median OS of 12·7 months. Targeted treatment options for patients with advanced NSCLC are limited thus far except for the subset of patients with cancers harboring activating mutations in the EGFR gene or ALK rearrangements.22,23 The antitumor activity with BRAF inhibitors in metastatic BRAF V600E–mutant lung cancers has been primarily reported in isolated clinical cases, one retrospective case series of 35 patients, and a basket trial of 19 patients.11–14 In the recently reported phase 2 basket study, a cohort of 19 patients with BRAF V600–mutant NSCLC treated with the BRAF inhibitor vemurafenib had an ORR of 42% and a median PFS of 7·3 months, similar to the clinical activity observed here in a much larger cohort.15 The current results also compare favorably with those observed in BRAF V600E metastatic melanoma (median PFS and DOR of 5·1 and 5·5 months, respectively).24 However, cross-trial comparison should be interpreted with caution. The BRAF V600E mutant kinase is considered a promising therapeutic target for different cancers, and targeting the mutant kinase is a standard approach in malignant BRAF V600-mutated melanoma.2,25
Although comparison between trials should be viewed cautiously, in the current study, dabrafenib demonstrated clinically evident antitumor activity with increased response rates and prolonged PFS and OS compared with previously used treatments in unselected patients (10% response rate, 2- to 3-month PFS, and OS of 7 to 10.5 months in patients with EGFR and ALK wild-type tumors treated with docetaxel and EGFR-TKI).17,18,26,26 The AEs are also tolerable for those patients treated with dabrafenib when compared with approved therapies for second- and third-line NSCLC. Thirty-five of the patients treated with dabrafenib (42%) had a SAE compared to 42% of those treated with docetaxel and 37% for erlotinib.17,27 Therefore, the increased response rates, longer PFS, and promising survival with acceptable toxicity makes this a reasonable treatment option for patients with BRAF V600E NSCLC. However, the response rates for the patients with BRAF V600E mutations treated with dabrafenib were indeed lower when compared with the > 50% response rates observed with other targeted therapies in oncogene-driven NSCLCs including responses to EGFR-TKIs in patients with EGFR activating mutations28,29 and responses to ALK inhibitors in patients with ALK rearrangement.30
AEs in this study were common but generally not severe or life-threatening. While being treated with dabrafenib, one patient on a factor Xa inhibitor died from an intracranial hemorrhage that was considered by the investigator to be related to dabrafenib treatment. Although some patients required dose interruptions or reductions, most patients were treated with their intended daily dose. AEs were largely related to the skin (hyperkeratosis, skin papilloma, dry skin); other common AEs included pyrexia, asthenia, decreased appetite, nausea, cough, fatigue, and alopecia. Dabrafenib, as with other BRAF inhibitors, was associated with development of cutaneous SCC (12%) or keratoacanthoma (8%), which was similar to that observed in the treatment of melanoma.13,31,32 Lesions usually appeared in the first months of therapy and were effectively managed with simple resection without discontinuation of dabrafenib. No further prospective information was collected on SCC and KAs because the protocol mandated that they be removed surgically by the institution according to institutional (not protocol mandated) practices. The AE profile appears comparable to that in melanoma studies except for rates of asthenia, decreased appetite, dry skin, and cough, which were higher in this study.13,24,33
The need for more systematic profiling of gene mutations to ensure that patients receive the most appropriate treatments in NSCLC is well-accepted but this remains a challenge for rare genomic changes (those less than 1–2%). The ability to molecularly prescreen large numbers of patients with NSCLC was crucial in this study given the low frequency of BRAF V600E in NSCLC (1·5%).6,8 Studies have shown that BRAF-mutated melanomas harbor a V600E mutation in > 80% of cases in contrast to BRAF-mutated NSCLCs which harbor a V600E mutation in only approximately 50% of cases.7,9,10,34 As BRAF screening is widely available for melanomas in most molecular platforms, local testing should be available in real time and should be reproducible in most oncology settings. The clinical characteristics of the patients reported in this study demonstrate the importance of screening all patients for oncogenic drivers and not selecting them solely by using clinical characteristics (non-smoking women) for multiplex genomic testing. The frequency of BRAF mutations in former and current smokers is striking when compared with patients with EGFR mutations and ALK rearrangements, in whom never smokers are more frequent, however these alterations are almost exclusively present in adenocarcinomas. Selection of patients on the basis of clinicopathological characteristics (aside from adenocarcinoma histology) is probably limited in the subset of BRAF V600E NSCLC indicating that molecular genetic identification is critical to guide the selection of patients and should include patients with a history of smoking.
In conclusion, this study is, to our knowledge, the first trial of BRAF inhibition to focus on BRAF V600E–mutant NSCLC. Dabrafenib induced durable clinical responses in a significant number of patients and had an acceptable safety profile. This study defines a new molecular subgroup of metastatic NSCLC in which dabrafenib demonstrates substantial antitumor activity. These results highlight the importance of screening for BRAF genetic alteration in patients with advanced NSCLC, notably in EGFR and ALK negative patients. Potential limitations of the current study are the inclusion of only BRAF V600E-mutant patients precluding the analysis of dabrafenib activity in other BRAF-mutant and wild-type NSCLC and the lack of systematic tumor biopsy upon progression to assess mechanisms of resistance to BRAF inhibition. Another potential limitation with regard to BRAF inhibitor therapy is the lower response rate in comparison to targeted therapies in patients harboring mutations in EGFR or ALK rearrangements. However, upfront inhibition of both MEK and mutant BRAF kinases may be a strategy for obtaining a higher number of and more durable responses than BRAF inhibition alone, as observed in melanoma studies.33,35 Two additional cohorts in this study involving combination of dabrafenib and trametinib are ongoing. The first combination cohort enrolled second- to fourth-line patients with metastatic BRAF V600E–mutant NSCLC,36 and the second cohort is enrolling first-line patients.
Supplementary Material
Acknowledgments
This study is/remains sponsored by GlaxoSmithKline; however, as of March 2, 2015, dabrafenib and trametinib became an asset of Novartis AG. The authors thank Michael Demars, PhD, Articulate Science, for editorial assistance with this manuscript funded by Novartis Pharmaceuticals.
Footnotes
Contributions
Conception and design: DP, JM, GR, PJS, MAS, LP, CN, B. Ma, AD, B. Mookerjee, CMC and BEJ
Collection and assembly of data: DP, TMK, JM, EQ, GR, FB, RJK, BCC, MAS, LP, B. Mookerjee and BEJ
Data analysis and interpretation: DP, TMK, JM, GR, FB, PJS, EFS, HJMG, RJK, BCC, MAS, LP, CN, B. Ma, AD, B. Mookerjee, CMC and BEJ
Manuscript writing: DP, JM, EQ, FB, EFS, HJMG, RJK, MAS, LP, AD, B. Mookerjee, CMC, BEJ
Final approval of manuscript: DP, TMK, JM, EQ, GR, FB, PJS, EFS, HJMG, RJK, BCC, MAS, LP, CN, B. Ma, AD, B. Mookerjee, CMC and BEJ
Provision of study material or patients: EFS and HJMG
Declaration of interests
DP reports advisory role for Novartis, Pfizer, Roche, Boehinger, Lilly, Bristol-Myers Squibb, AstraZeneca, MSD and Pierre Fabre. GR reports grants from GlaxoSmithKline and Novartis; consulting with Novartis; and employer receives research funding from Pfizer, Chugai/Roche and Millennium for support of research led by him. FB reports personal fees from GlaxoSmithKline and Novartis. P-J Souquet reports clinical trial for GlaxoSmithKline. HJMG reports payments to his institution from Roche, MSD, Pfizer and GlaxoSmithKline. LP at the time of the study, was an employee of GlaxoSmithKline. CN reports employment at GlaxoSmithKline at the time the study was conducted, former employment at Novartis and current employment at GlaxoSmithKline. B. Ma reports employment at GlaxoSmithKline at the time the study was conducted. AD, at the time of the study, was an employee of GlaxoSmithKline, is now an employee of Novartis. B Mookerjee, at the time of the study, was an employee of GlaxoSmithKline, is now an employee of Novartis; and owns stock in GlaxoSmithKline and Novartis. CMC was an employee of Novartis and GlaxoSmithKline. BEJ reports personal fees from AstraZeneca, Clovis Oncology, Novartis, Merck, Genentech; honoraria from Chugai Pharmaceuticals; received shares of post-market revenue for EGFR Genotyping patent. TMK, JM, EQ, EFS, RJK, BCC, and MAS report nothing to disclose.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 3.Naoki K, Chen TH, Richards WG, Sugarbaker DJ, Meyerson M. Missense mutations of the BRAF gene in human lung adenocarcinoma. Cancer Res. 2002;62:7001–3. [PubMed] [Google Scholar]
- 4.Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–67. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 5.Dankort D, Filenova E, Collado M, Serrano M, Jones K, McMahon M. A new mouse model to explore the initiation, progression, and therapy of BRAFV600E-induced lung tumors. Genes Dev. 2007;21:379–84. doi: 10.1101/gad.1516407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barlesi F, Blons H, Beau-Faller M, et al. Biomarkers (BM) France: Results of routine EGFR, HER2, KRAS, BRAF, PI3KCA mutations detection and EML4-ALK gene fusion assessment on the first 10,000 non-small cell lung cancer (NSCLC) patients (pts) ASCO Meeting Abstracts. 2013;31(15_suppl):8000. [Google Scholar]
- 7.Paik PK, Arcila ME, Fara M, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol. 2011;29:2046–51. doi: 10.1200/JCO.2010.33.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311:1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marchetti A, Felicioni L, Malatesta S, et al. Clinical features and outcome of patients with non-small-cell lung cancer harboring BRAF mutations. J Clin Oncol. 2011;29:3574–9. doi: 10.1200/JCO.2011.35.9638. [DOI] [PubMed] [Google Scholar]
- 10.Cardarella S, Ogino A, Nishino M, et al. Clinical, pathologic, and biologic features associated with BRAF mutations in non-small cell lung cancer. Clin Cancer Res. 2013;19:4532–40. doi: 10.1158/1078-0432.CCR-13-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gautschi O, Pauli C, Strobel K, Hirschmann A, Printzen G, Aebi S, Diebold J. A patient with BRAF V600E lung adenocarcinoma responding to vemurafenib. J Thorac Oncol. 2012;7:e23–4. doi: 10.1097/JTO.0b013e3182629903. [DOI] [PubMed] [Google Scholar]
- 12.Gautschi O, Milia J, Cabarrou B, et al. Targeted Therapy for Patients with BRAF-Mutant Lung Cancer: Results from the European EURAF Cohort. J Thorac Oncol. 2015;10:1451–57. doi: 10.1097/JTO.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 13.Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379:1893–901. doi: 10.1016/S0140-6736(12)60398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudin CM, Hong K, Streit M. Molecular characterization of acquired resistance to the BRAF inhibitor dabrafenib in a patient with BRAF-mutant non-small-cell lung cancer. J Thorac Oncol. 2013;8:e41–2. doi: 10.1097/JTO.0b013e31828bb1b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N Engl J Med. 2015;373:726–36. doi: 10.1056/NEJMoa1502309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King AJ, Arnone MR, Bleam MR, et al. Dabrafenib; preclinical characterization, increased efficacy when combined with trametinib, while BRAF/MEK tool combination reduced skin lesions. PLoS One. 2013;8:e67583. doi: 10.1371/journal.pone.0067583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–97. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 18.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 19.Green SJ, Dahlberg S. Planned versus attained design in phase II clinical trials. Stat Med. 1992;11:853–62. doi: 10.1002/sim.4780110703. [DOI] [PubMed] [Google Scholar]
- 20.Brookmeyer R, Crowley J. A confidence interval for the mean survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 21.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–13. [Google Scholar]
- 22.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 24.Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–65. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 25.Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867–76. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 26.Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384:665–73. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- 27.Scagliotti G, von Pawel J, Novello S, et al. Phase III Multinational, Randomized, Double-Blind, Placebo-Controlled Study of Tivantinib (ARQ 197) Plus Erlotinib Versus Erlotinib Alone in Previously Treated Patients With Locally Advanced or Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol. 2015;33:2667–74. doi: 10.1200/JCO.2014.60.7317. [DOI] [PubMed] [Google Scholar]
- 28.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 29.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 30.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–94. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 31.Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–14. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larkin J, Del Vecchio M, Ascierto PA, et al. Vemurafenib in patients with BRAF(V600) mutated metastatic melanoma: an open-label, multicentre, safety study. Lancet Oncol. 2014;15:436–44. doi: 10.1016/S1470-2045(14)70051-8. [DOI] [PubMed] [Google Scholar]
- 33.Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371:1877–88. doi: 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]
- 34.Villaruz LC, Socinski MA, Abberbock S, et al. Clinicopathologic features and outcomes of patients with lung adenocarcinomas harboring BRAF mutations in the Lung Cancer Mutation Consortium. Cancer. 2015;121:448–56. doi: 10.1002/cncr.29042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Planchard D, Groen HJM, Kim TM, et al. Interim results of a phase II study of the BRAF inhibitor (BRAFi) dabrafenib (D) in combination with the MEK inhibitor trametinib (T) in patients (pts) with BRAF V600E mutated (mut) metastatic non-small cell lung cancer (NSCLC) ASCO Meeting Abstracts. 2015;33(15_suppl):8006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.