Abstract
Background. The use of mold-active azoles for antifungal prophylaxis after allogeneic stem cell transplantation (SCT) is hindered by adverse events and drug–drug interactions. Higher doses of echinocandins administered intermittently may be an alternative in this setting.
Methods. This was a single-center, observational 5-year study to characterize the safety and efficacy of intermittent administration of high-dose intravenous micafungin (≥5 doses of ≥300 mg micafungin 2–3 times weekly) in patients with acute leukemia and allogeneic SCT recipients.
Results. A total of 104 patients (84 allogeneic SCT recipients and 20 patients with leukemia) received intermittent high-dose intravenous micafungin, 83 (79.8%) as prophylaxis. Large variability in the micafungin dosing regimen was observed; 78 (75%) patients received >75% of their course as 300 mg micafungin 3 times weekly. Liver function tests decreased from baseline to end of treatment (EOT; P < .001). Patients with normal baseline liver function (n = 55 [52%]) maintained similar enzyme levels throughout the study. For patients with abnormal baseline liver function (n = 49 [47%]), liver function tests significantly improved from baseline to EOT (P ≤ .005). Duration and/or micafungin dosing algorithms were not associated with liver toxicity at EOT. There were no significant changes in renal function, and infusion-related reactions or deaths were not observed. Five of 83 (6.0%) patients in the prophylaxis group developed a breakthrough fungal infection.
Conclusions. In this largest cohort of patients to date, intermittent administration of high-dose micafungin was well tolerated, without any associated liver or renal function abnormalities, and may be considered an alternative antifungal prophylactic strategy. Prospective studies are needed to further validate these findings.
Keywords: micafungin, high-dose, intermittent, transplant
Mold-active azoles are widely used for antifungal prophylaxis after allogeneic stem cell transplantation (SCT) [1–3]. However, associated adverse events, drug–drug interactions, pill burden, and cost may limit the use of these agents for primary antifungal prophylaxis, particularly during conditioning. Therefore, alternative antifungal prophylactic options are needed. Echinocandins are well-tolerated agents, with minimal side effects and drug–drug interactions, and in vitro activity against Candida and Aspergillus species [4]. However, the need for daily intravenous administration significantly limits their use as prophylactic agents. Higher doses of echinocandins administered intermittently could be a desirable alternative for antifungal prophylaxis and treatment in specific patient populations.
Limited data exist on the safety and efficacy of micafungin administered at doses of ≥150 mg. In a nonrandomized study of 53 patients with hematologic malignancy treated for neutropenic fever with micafungin 150 mg intravenously daily, 6 (11%) patients developed liver dysfunction [5]. When higher doses of micafungin (300 mg intravenously daily) were reviewed in a retrospective study of 26 patients with hematologic malignancy compared with 58 patients who received standard doses (150 mg intravenously daily), hepatotoxicity was noted in 48% and 58% of patients receiving high-dose and standard dose, respectively (P = .48) [6]. Goldberg et al previously reported on 19 allogeneic SCT recipients from our institution, who received intermittent micafungin dosing (150 mg 3 times weekly) between 2006 and 2008 [7]. Although micafungin was well tolerated without any significant adverse events, 2 patients developed a possible invasive fungal infection (IFI), and another 2 patients developed central line-associated bloodstream bacterial infections.
Since then, intermittent administration of micafungin (300 mg 2–3 times weekly) has been more frequently used at our institution for patients requiring mold-active antifungal prophylaxis and with contraindications to azoles. Herein, we report on the safety and efficacy of high-dose intermittent micafungin in a cohort of high-risk patients.
METHODS
This is a single-center, retrospective observational study to characterize the safety and efficacy of intermittent administration of high-dose intravenous micafungin. The study was approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center (MSKCC) and was granted a waiver of authorization. All patients with acute leukemia and allogeneic SCT recipients who received ≥5 doses of intravenous micafungin ≥300 mg 2–3 times weekly for prophylaxis or treatment between 1 January 2009 and 31 July 2014 were included in this cohort.
Collected Data
Patients were identified using the MSKCC pharmacy database. Information on patient demographics, underlying disease, SCT characteristics, graft-vs-host disease (GVHD), and associated treatment, prior and concomitant medications, and concurrent medical events was collected after retrospective chart review of the patients' medical records and recorded on case report forms. Laboratory data including aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total bilirubin, and creatinine were collected at baseline (week 0) and weekly thereafter until the end of treatment (EOT) with micafungin. The timing, indication (prophylaxis vs treatment), dose, frequency, and duration of micafungin administration were transcribed onto the case report form. Detailed data were collected on IFIs, including site of infection, pathogen, and treatment.
Definitions
Intermittent administration of high-dose intravenous micafungin was defined as 300 mg intravenous micafungin administered 2–3 times weekly. Liver impairment was defined as AST or ALT ≥3 times the upper limit of normal (ULN), and/or ALP or total bilirubin ≥2 times the ULN. Renal impairment was defined as creatinine clearance ≤50 mL/minute, calculated based on published guidelines [8]. A decrease in creatinine clearance by ≥25% was considered a significant change in renal function (adjusted based on the RIFLE [risk, injury, failure, loss of kidney function, and end-stage kidney disease] criteria) [9]. Consensus guidelines were used for the definition of IFIs [10].
Objectives
The primary objective of this study was to assess the safety of intermittent administration of high-dose intravenous micafungin. As liver toxicity is the primary concern in patients treated with micafungin, we specifically focused on the levels of liver enzymes between week 0 and EOT. Additionally, safety was assessed by monitoring changes in renal function (as reflected by creatinine clearance levels) and the rate of micafungin discontinuation due to associated adverse events (as assessed by the treating physician). The efficacy of intermittent administration of high-dose intravenous micafungin was studied as a secondary objective among those patients who received micafungin as prophylaxis. Clinical failure was defined as discontinuation of micafungin due to breakthrough IFIs or death. In the treatment group, efficacy analyses were not performed due to the small number of patients and heterogeneity of IFIs.
Statistical Analysis
The median (95% confidence interval) for AST, ALT, ALP, total bilirubin, creatinine, and creatinine clearance for each patient was calculated and compared between baseline (week 0) and week 1, 2, 4, 8, and EOT. Liver function between baseline and EOT was also compared separately for patients with baseline normal and impaired liver function. The Wilcoxon signed-rank test was used to assess the difference between the paired samples. Univariate and multivariable logistic regression analyses were performed to identify potential risk factors for liver toxicity at EOT. Additionally, we sought to identify potential predictors of clinical failure in the patient group that received micafungin as prophylaxis. A stepwise forward selection approach was used to establish a multivariate model for risk factors. Variables with a significant level of 0.3 in the univariate analyses were introduced into stepwise multivariate logistic regression models to assess risk factors for liver dysfunction progression adjusted for the other risk factors in the models, with a significance level of 0.1 to remain stay in the model. A P value of <.05 was used to define statistical significance. Statistical Analysis Software (SAS) version 9.4 (SAS Institute, Cary, North Carolina) was used for statistical analyses.
RESULTS
A total of 104 patients who received ≥5 doses of intravenous micafungin 300 mg 2–3 times weekly were included in this study (Table 1). All patients received an allogeneic SCT, although 20 (19%) patients with acute leukemia completed their course of high-dose intermittent administration of micafungin prior to their SCT. Micafungin was administered as primary antifungal prophylaxis in 83 (79.8%) patients.
Table 1.
Baseline Patient Characteristics
| Characteristic | All Patients (N = 104), No. (%) | Micafungin Prophylaxis (n = 83), No. (%) | Micafungin Treatment (n = 21), No. (%) | P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, y, median (range) | 54.3 (21.6–70.1) | 54.2 (21.6–70.1) | 59.0 (26.7–69.0) | .51 |
| Sex, female | 54 (52) | 44 (53) | 10 (48) | .66 |
| Race | .74 | |||
| White | 76 (73) | 58 (70) | 18 (86) | |
| African American | 13 (13) | 11 (13) | 2 (10) | |
| Asian | 9 (9) | 8 (10) | 1 (5) | |
| Underlying disease | .15 | |||
| Acute leukemia | 57 (55) | 42 (51) | 15 (71) | |
| Chronic leukemia | 3 (3) | 1 (1) | 2 (10) | |
| Myelodysplastic syndrome | 19 (18) | 18 (22) | 1 (5) | |
| Multiple myeloma | 2 (2) | 2 (2) | 0 | |
| Lymphoma | 22 (21) | 19 (23) | 3 (14) | |
| Stem cell transplant characteristicsa | 84 (81) | 78 (94) | 6 (29) | |
| Stem cell source | .23 | |||
| Cord blood | 28 (33) | 24 (31) | 4 (67) | |
| Peripheral blood stem cells | 52 (62) | 50 (64) | 2 (33) | |
| Bone marrow | 4 (5) | 4 (5) | 0 | |
| Conditioning regimen, myeloablativeb | 73 (87) | 68 (87) | 5 (83) | .58 |
| Donor–recipient matching | .41 | |||
| Matched related | 23 (27) | 21 (27) | 2 (33) | |
| Matched unrelated | 21 (25) | 21 (27) | 0 | |
| Mismatched unrelated | 40 (48) | 36 (46) | 4 (67) | |
| Stem cell manipulation, T-cell depletion | 25 (30) | 24 (31) | 1 (17) | .31 |
| Acute graft-vs-host disease, grade 2 or higher | 44 (52) | 42 (54) | 2 (33) | .66 |
| Reason(s) for micafungin selectionc | ||||
| Abnormal liver function testsd | 69 (66) | 67 (81) | 2 (10) | <.0001 |
| Abnormal renal function | 5 (5) | 3 (4) | 2 (10) | .26 |
| Central nervous system adverse event | 4 (4) | 2 (2) | 2 (10) | .18 |
| Drug–drug interactions | 10 (10) | 9 (11) | 1 (5) | .68 |
| Othere | 23 (22) | 6 (7) | 17 (81) | <.0001 |
| Baseline abnormal liver functionf | ||||
| Aspartate aminotransferase | 14 (13) | 0 | 14 (17) | .07 |
| Alanine aminotransferase | 32 (31) | 30 (36) | 2 (10) | .02 |
| Alkaline phosphatase | 26 (25) | 24 (29) | 2 (10) | .09 |
| Total bilirubin | 12 (12) | 11 (13) | 1 (5) | .45 |
a Although all patients included in the study received an allogeneic stem cell transplant (SCT), intermittent high-dose micafungin was started posttransplant in 84 of 104 (80.8%). Hence, baseline SCT characteristics are described only for these patients.
b Thirteen and 22 patients received high and low total body irradiation, respectively. The remaining 38 patients were conditioned with busulfan/fludarabine/melphalan (n = 20), melphalan/fludarabine (n = 10), busulfan/fludarabine (n = 7), and other (n = 1).
c Patients might have had >1 reason to be started on micafungin.
d Abnormal baseline liver function as a reason to use micafungin was based on the treating clinicians' assessment and not on the definition of liver impairment used in this study.
e Micafungin was used in addition to another antifungal agent as combination therapy (n = 13), lack of insurance coverage for azoles (n = 4), baseline prolonged QT interval (n = 1), underlying hepatitis C infection (n = 1), and other (n = 4).
f Abnormal baseline liver function was defined as ≥1 of the following: aspartate and alanine aminotransferase >3 times the upper limit of normal (ULN) and alkaline phosphatase and total bilirubin >2 times the ULN.
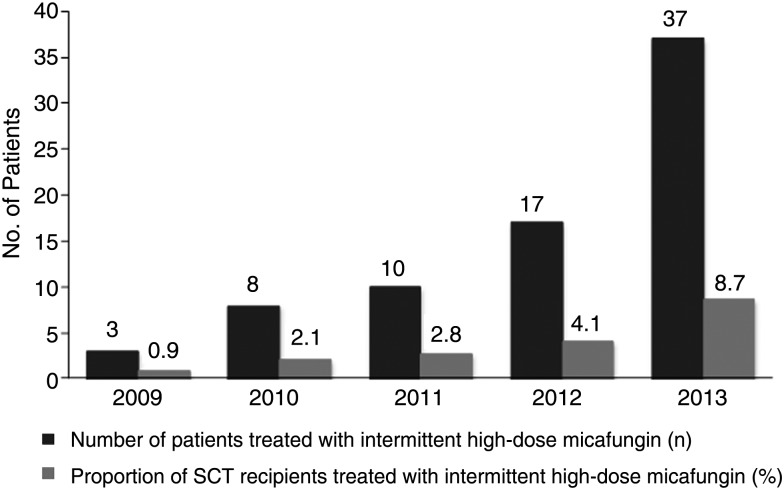
Micafungin was administered for ≤4 weeks (median, 17 days; interquartile range [IQR], 15–22 days), 5–8 weeks (median, 41 days; IQR, 33–46 days), 9–12 weeks (median, 67 days; IQR, 60–78 days), and >12 weeks (median, 124 days; IQR, 102–159 days) in 41, 28, 20, and 15 patients, respectively. The use of intermittent administration of high-dose micafungin increased during the study period (P < .05 between 2013 and prior years; Figure 1). The amount of micafungin administered as mg/kg/day was calculated by dividing the total micafungin dose administered per patient by the patient's baseline weight, divided by the total number of days of micafungin administration. The average and maximal daily doses administered in this study were 1.78 mg/kg (IQR, 1.39–2.11 mg/kg) and 3.59 mg/kg, respectively.
Figure 1.
Number of allogeneic stem cell transplant (SCT) recipients who received intermittent administration of high-dose micafungin between 2009 and 2014, presented as absolute numbers and proportion of allogeneic SCT recipients performed per year. P values between 2013 and 2012, 2011, 2010, and 2009 were .0075, .0005, .0001, and .0001, respectively. Fisher exact test was used to assess the difference between 2013 and prior years. Analyses were not performed for 2014 due to data collection reflecting only the first part of the year.
Large variability in the micafungin dosing regimen was observed: (i) 63 (60.6%) patients received 300 mg 3 times weekly (median, 24 days; range, 8–159 days); (ii) 15 (14.4%) patients received 300 mg 3 times weekly, representing >75% of the administered drug (median, 61 days; range, 41–191 days); (iii) 6 (5.8%) patients received 300 mg twice weekly (median, 32.5 days; range, 22–113 days); and (iv) 20 (19.2%) patients received 300 mg variable frequency (median, 41.5 days; range, 13–159 days) (Table 2). Therefore, patients were divided into 3 groups: (i) group 1 (n = 63 + 15 = 78), representing those patients who received >75% of their course as 300 mg 3 times weekly; (ii) group 2 (n = 6) including patients on 300 mg of micafungin twice weekly; and (iii) group 3 (n = 20) including the remaining patients with variable dosing regimen and frequency. Safety analyses were performed for the overall patient population (n = 104) and for group 1 (n = 78), to specifically study the effect of 300 mg of micafungin administered 3 times weekly. Due to the small number of patients and large variability in dosing regimen, separate analyses were not performed for groups 2 and 3, respectively.
Table 2.
Detailed Description of Micafungin Dosing Regimens Included in This Study
| Micafungin Dose and Frequency | Patients (N = 104), No. (%) |
|---|---|
| Group 1 | 78 (75) |
| 300 mg 3 times weekly only | 63 (60.6) |
| 300 mg 3 times weekly (>75% of the course)a | 15 (14.4) |
| Group 2 | 6 (5.8) |
| 300 mg twice weekly only | |
| Group 3 | 20 (19.2) |
| 300 mg 3 times weekly intermittentlyb | |
| 300 mg 3 times weekly ×1 wk | 7 |
| 300 mg 3 times weekly ×2 wk | 4 |
| 300 mg 3 times weekly ×3 wk | 3 |
| 300 mg 3 times weekly ×4 wk | 2 |
| 300 mg 3 times weekly ×5 wk | 3 |
| 300 mg 3 times weekly ×6 wk | 1 |
a Eleven patients received 300 mg of micafungin twice and/or once weekly before, after, or during the administration of 300 mg 3 times weekly: 5, 1, 1, and 1 patients received 300 mg of micafungin twice weekly for 1, 2, 3, and 4 weeks, respectively; 1 patient received 1 and 2 weeks of 300 mg of micafungin administered twice and once weekly, respectively. Six patients received micafungin daily at 150 mg before, after, or during the administration of 300 mg 3 times weekly: 4, 1, and 1 patients received 1, 2, and 3 weeks of daily 150 mg of micafungin, respectively.
b In addition to 300 mg 3 times weekly, these patients also received 300 mg once (n = 11) and/or twice (n = 19) weekly, some (n = 6) with intermittently administered daily micafungin at 150 mg.
Safety Data
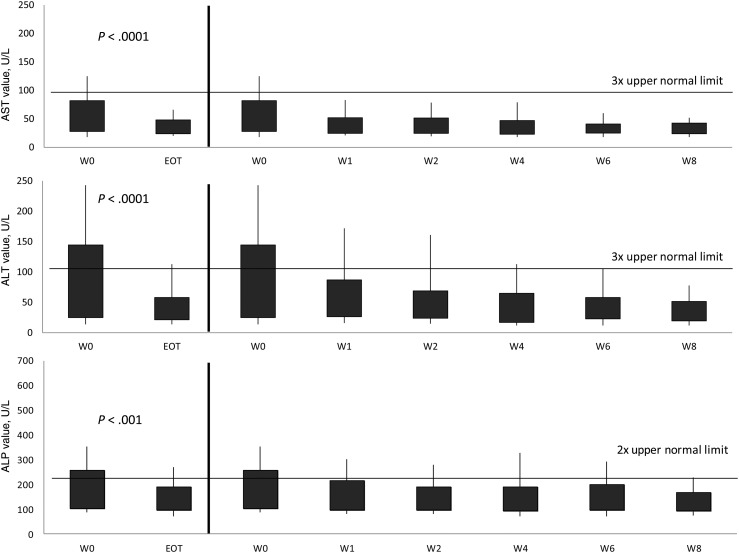
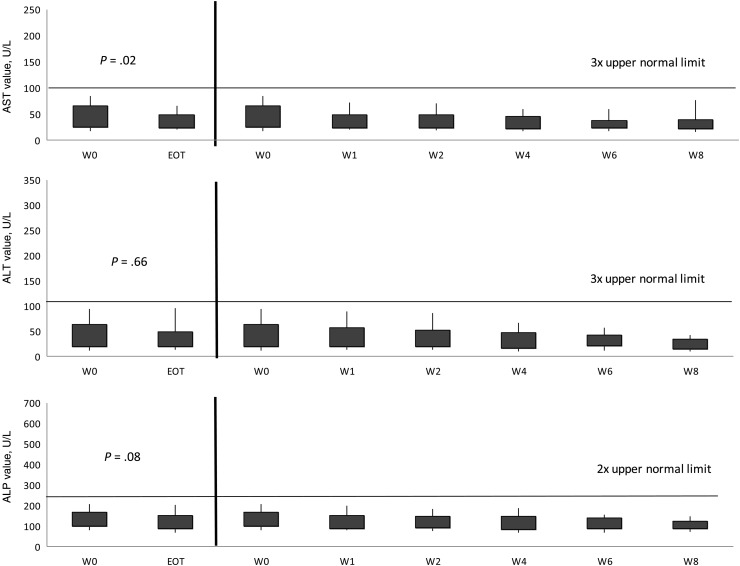
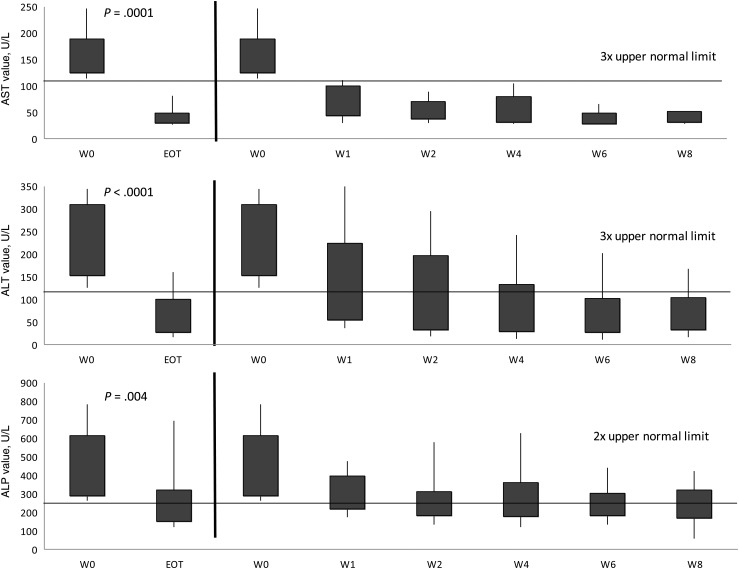
The total number of tests for AST, ALT, ALP, and total bilirubin at weeks 0, 2, 4, and 8, and EOT were 104, 100, 65, 32, and 104 for AST; 104, 100, 65, 32, and 104 for ALT; 104, 100, 65, 32, and 104 for ALP; and 104, 99, 65, 31, and 104 for bilirubin. All liver function tests (LFTs) decreased from week 0 to EOT (P < .001; Figure 2). As 55 (52%) patients had impaired liver function at baseline, separate analyses were performed based on the patients' baseline liver function profile. Patients with normal baseline liver function maintained similar enzyme levels throughout the study, with trends for lower values to the EOT (Figure 3). For patients with abnormal baseline liver function, all enzyme values significantly improved from baseline to EOT (P ≤ .005; Figure 4).
Figure 2.
Trends in aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP), from baseline to end of treatment (EOT) of intermittent administration of high-dose micafungin for the overall study population. Shaded regions represent the interquartile range. Capped whiskers represent the 10th and 90th percentiles. Values of AST, ALT, and ALP were compared between baseline (week [W] 0) and W1, W2, W4, W6, W8, and EOT. The Wilcoxon signed-rank test was used to assess the difference between the paired samples. P values between W0 and W1, W2, W4, W6, and W8 for AST, ALT, and ALP were all <.01.
Figure 3.
Trends in aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP), from baseline to end of treatment (EOT) of intermittent administration of high-dose micafungin for patients with normal liver function at baseline. Normal liver function was defined as AST or ALT <3 times the upper limit of normal (ULN) and/or ALP <2 times the ULN. Shaded regions represent the interquartile range. Capped whiskers represent the 10th and 90th percentiles. Values of ALT were compared between baseline (week [W] 0) and W1, W2, W4, W6, W8, and EOT. The Wilcoxon signed-rank test was used to assess the difference between the paired samples. P values between W0 and W1, W2, W4, W6, and W8 were .04, .12, .29, .03, and .02, respectively, for AST; .52, .86, .14, .43, and .09, respectively, for ALT; and .006, .03, .01, .005, and .002, respectively, for ALP.
Figure 4.
Trends in aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP), from baseline to end of treatment (EOT) of intermittent administration of high-dose micafungin for patients with liver impairment at baseline. Liver impairment was defined as AST or ALT ≥3 times the upper limit of normal (ULN) and/or ALP ≥2 times the ULN. Shaded regions represent the interquartile range. Capped whiskers represent the 10th and 90th percentiles. Values of AST, ALT, and ALP were compared between baseline (week [W] 0) and W1, W2, W4, W6, W8, and EOT. The Wilcoxon signed-rank test was used to assess the difference between the paired samples. P values between W0 and W1, W2, W4, W6, and W8 were all <.01 for AST; <.005 for ALT; and <.01 for ALP.
Univariate analyses were performed to identify potential risk factors for liver toxicity at EOT (Table 3), including the following independent variables: age (<50 or ≥50 years), sex, ethnicity (white vs other), underlying disease (acute leukemia vs other), baseline liver and renal values. Micafungin-related independent variables were also studied, including duration of administration (<4, 5–8, 9–12, and >12 weeks) and patient groups, as defined above (group 1 vs group 2 vs group 3). There was a trend for baseline liver impairment to predict hepatotoxicity at EOT (odds ratio, 2.36; 95% confidence interval, .87–6.4; P = .09) in univariate analyses. However, no variable was found to be significant in multivariable analyses. Additional analyses were performed in the 84 SCT recipients, using the following SCT characteristics as independent variables: matched related vs other, T-cell depletion, SCT source, type of conditioning, and presence of acute grade ≥2 GVHD. None of these variables were significant predictors for liver impairment at EOT.
Table 3.
Risk Factor Analysis to Identify Independent Predictors for Abnormal Liver Function at the End of Treatment in the Overall Patient Population and in Patients Who Received ≥75% of Their Course With 300 mg Micafungin 3 Times Weekly
| Variable | Overall Patient Population (N = 104) |
Micafungin 300 mg 3×/wk (N = 78) |
||||
|---|---|---|---|---|---|---|
| Univariate Analysisa |
Univariate Analysisa |
|||||
| OR | (95% CI) | P Value | OR | (95% CI) | P Value | |
| Demographics | ||||||
| Age, ≥50 y vs <50 y | 0.97 | (.37–2.54) | .95 | 0.86 | (.28–2.66) | .79 |
| Sex, female | 1.14 | (.45–2.94) | .78 | 0.90 | (.29–2.79) | .86 |
| Race, white | 0.57 | (.21–1.54) | .26 | 0.68 | (.20–2.29) | .53 |
| Underlying disease | ||||||
| Acute leukemia vs other | 1.59 | (.60–4.19) | .35 | 1.45 | (.46–4.57) | .52 |
| Laboratory variables | ||||||
| Baseline abnormal liver function vs normalb | 2.36 | (.87–6.40) | .09 | 1.60 | (.49–5.22) | .44 |
| Baseline abnormal renal function vs normalc | 0.92 | (.24–3.60) | .91 | 0.49 | (.06–4.26) | .52 |
| Micafungin-related variables | ||||||
| Duration of micafungin administration | ||||||
| ≤4 wk | 1 | 1 | ||||
| 5–8 wk | 1.05 | (.35–3.17) | .53 | 0.83 | (.21–3.24) | .91 |
| 9–12 wk | 0.37 | (.07–1.86) | .19 | 0.57 | (.10–3.15) | .50 |
| >12 wk | 1.10 | (.25–4.86) | .57 | 1.24 | (.20–7.53) | .60 |
| Group 1 vs all othersd | 0.65 | (.23–1.82) | .41 | NA | NA | NA |
| Micafungin daily dose estimate | ||||||
| <1.5 mg/kg | NA | 1 | ||||
| 1.5–2 mg/kg | NA | 0.53 | (.12–2.29) | .40 | ||
| >2 mg/kg | NA | 0.84 | (.22–3.21) | .82 | ||
Liver impairment was defined as any of the following: aspartate and alanine aminotransferase >3 times the upper limit of normal (ULN) and alkaline phosphatase and total bilirubin >2 times the ULN.
Abbreviations: CI, confidence interval; NA, not applicable; OR, odds ratio.
a No variable was eligible to stay in the stepwise selection multivariate model.
b Abnormal baseline liver function was defined as ≥1 of the following: aspartate and alanine aminotransferase >3 times the ULN and alkaline phosphatase and total bilirubin >2 times the ULN.
c Abnormal baseline renal function was defined as creatinine clearance <50 mL/minute.
d Group 1 included 78 patients who received 300 mg micafungin 3 times weekly for ≥75% of their course.
There were no significant changes in renal function during the study period. Creatinine clearance remained stable between baseline (median, 85.6 mL/minute) and EOT (78.2 mL/minute) (P = .68). Micafungin was discontinued in 3 (2.8%) patients because of persistent LFT abnormality (n = 2) and rash (n = 1). However, associations of these events with micafungin administration were not established. Moreover, there were no infusion-related reactions, cardiac events, or deaths observed during the study period.
Safety Data: Subgroup Analysis
In the group of 78 patients who received 300 mg intravenous micafungin 3 times weekly for >75% of their course, all LFTs decreased from week 0 to EOT (P < .0001; data not shown). Patients with normal baseline LFTs (n = 33 [42%]) demonstrated lower values to the EOT for all variables (P < .05), except for ALT, which remained relatively stable (P = .29). For patients with abnormal baseline LFTs (n = 45 [52%]), all enzyme values significantly improved from baseline to EOT (P < .005). Univariate analyses were performed to identify potential risk factors for liver toxicity at EOT for group 1 patients using the same independent variables listed above (Table 3). The following micafungin-related variables were studied as independent predictors: (i) duration of 300 mg 3 times weekly (<4, 4–8, 9–12, >12 weeks) and (ii) micafungin dosing: <1.5 mg/kg/day (n = 21), 1.5–2 mg/kg/day (n = 29), and >2 mg/kg/day (n = 27); in the latter group, only 1 patient received >3 mg/kg/day of micafungin (3.59 mg/kg/day). None of these variables were eligible to remain in the stepwise selection multivariate model.
Efficacy Data
Five of 83 (6.0%) patients in the prophylaxis group developed a breakthrough IFI: 3 with probable invasive aspergillosis (1 of them mixed infection with Mucor species), 1 with proven invasive aspergillosis, and 1 with Rhodotorula species bloodstream infection. Bivariate exploratory analyses were performed to identify potential associations between breakthrough IFI and micafungin-related variables. There was no significant association between clinical failure and micafungin (i) groups (group 1 vs 2 vs 3; P > .51) and (ii) dosing (<1.5 vs 1.5–2 mg/kg/day vs >2 mg/kg/day; P = .61).
DISCUSSION
This is, to our knowledge, the largest cohort of patients to receive intermittently administered high-dose micafungin. Our findings demonstrate that 300 mg of micafungin administered 2–3 times weekly was well tolerated, without any associated significant liver or other abnormalities, and a relatively effective antifungal prophylactic strategy in a large cohort of high-risk patients. These findings should be taken with caution considering a number of limitations outlined in detail herein.
Liver toxicity is the major concern in patients treated with echinocandins. We performed a rigorous investigation of LFT changes between baseline and EOT to identify potential associations between hepatotoxicity and intermittent administration of high-dose micafungin. Our observations suggest that micafungin administered at 300 mg multiple times weekly for as long as 20 weeks may be well tolerated, even in patients with baseline liver impairment. Indeed, when patients were examined separately based on their baseline liver function (normal vs abnormal baseline liver function), there was no deterioration of liver function in any of the 2 groups from baseline to EOT, consistent with previously reported data [11]. Due to the large variability in the way micafungin was administered, we specifically investigated a subgroup of patients who received >75% of their course as 300 mg micafungin 3 times weekly. Our findings on liver toxicity were the same as for the overall patient population. However, micafungin dosing variability and limited available data on concomitant hepatotoxic drugs (eg, sirolimus) or conditions (eg, liver GVHD, viral hepatitis) might have limited our ability to attribute causality in hepatotoxicity observed during the study period. In addition, it is likely that some patients may be able to tolerate micafungin better and therefore were maintained on this drug for a longer duration. Although animal studies have raised concerns for higher incidence of liver tumors associated with micafungin, there are no human data supporting this hypothesis [6, 12]. Due to the retrospective nature of this study and limited follow-up, we were not able to make any relevant observations.
In addition, intermittent administration of high-dose micafungin did not impact renal function and was not associated with any significant infusion-related reactions in this study. Concerns have been raised about the potential effect of anidulafungin and caspofungin on cardiac contractility, particularly when administered via central venous lines in close proximity to the heart and in patients with baseline left ventricular dysfunction [13, 14]. Notably, micafungin did not appear to suppress cardiac contractility when studied in animal models at concentrations as high as 10 times the therapeutic concentrations [13]. We did not observe any major cardiac events in any of the patients studied. All SCT recipients at our institution undergo cardiac function assessment with an echocardiogram prior to their SCT. Typically, an ejection fraction >45% is required to meet eligibility criteria for transplant protocols. The median ejection fraction in this study was 63% (range, 42%–75%). This fairly “normal” baseline cardiac profile and the retrospective design of the study might have, in part, limited our ability to assess the effect of higher micafungin doses on cardiac function.
Although this study was not powered to assess efficacy, intermittent administration of 300 mg micafungin appeared to be a promising alternative for primary antifungal prophylaxis, with only a handful of patients (6%) developing a breakthrough IFI. This was consistent with breakthrough rates ranging between 5.3% and 7.3% reported in antifungal prophylactic clinical trials [1, 2, 15–17]. One breakthrough bloodstream infection with Rhodotorula species was identified, which was consistent with the susceptibility profile of this organism [18]. Concerns for selection of resistant organisms and the potential paradoxical effect (increased organism burden due to higher drug concentrations) need to be considered when opting for infrequent administration of micafungin [19, 20]. Notably, there were no breakthrough infections with Candida species. Considering that the gut remains the major reservoir for Candida species, tissue concentrations in association with plasma levels of micafungin when administered intermittently need to be further studied; this would prevent the emergence of echinocandin-resistant Candida species (ie, C. parapsilosis, C. kefyr) due to (potentially) lower than the minimum inhibitory concentration (MIC) drug concentrations in the gut epithelium toward the end of dosing intervals.
Overall, our findings support the concept that higher doses of micafungin, less frequently administered, may be well tolerated and effective. The maximal daily and weekly doses of micafungin administered in this cohort were 1.78 mg/kg and 900 mg, respectively. That was within the range of a weekly dose of 700–1050 mg achieved when administering 100–150 mg of the drug daily. Based on their pharmacodynamic profile, it is the total amount of echinocandins, rather than the dosing frequency, that impacts results, with the area under the curve/MIC ratio being the best outcome predictor [21]. Considering the concentration-dependent killing, linear pharmacokinetics, high tissue concentrations, and postulated prolonged postantifungal effect, higher doses of micafungin administered several times or once weekly may be a viable, if not better, alternative to daily dosing [20–24]. Administration of micafungin at 300 mg every 48 or 72 hours has been associated with successful clinical outcomes [25]. In addition, initial administration of >2.25 mg/kg of micafungin has been associated with accelerated clinical response [26]. Moreover, micafungin doses as high as 896 mg daily for 7 days, 3 mg/kg every other day, and 300 mg or 8 mg/kg daily have been well tolerated without significant dose-related toxicities [6, 27, 28]. However, the maximally tolerated dose and minimal dosing intervals for micafungin to achieve maximal efficacy, without compromising safety, remain to be defined.
Major limitations of this study include its retrospective observational design, small number of patients, and suboptimal follow-up. The large variability in the way micafungin was administered might have biased our findings. Notably, a number of patients received a combination of 300 mg of micafungin several times weekly alternating with 150 mg daily for several weeks. However, liver and renal toxicity in subgroup analyses did not significantly differ from the overall study population. Detailed recording of cardiac events or hypertension was not performed. Finally, our findings may not apply in patients at the extremes of the weight scale and other patient categories, including liver transplant recipients.
In conclusion, intermittent administration of 300 mg of micafungin several times weekly was safe and relatively effective in a large cohort of patients with acute leukemia and allogeneic SCT recipients. While taken with caution, our findings could ignite interest in intermittent use of higher doses of echinocandins, in an effort to decrease pill burden, increase compliance, and limit potential side effects and drug–drug interactions associated with other classes of antifungal agents in select patient populations. Multiple questions remain to be answered, including the maximally tolerated dose, longest dosing intervals, and long-term outcomes (eg, resistance development, paradoxical effect, liver tumors). For that, animal modeling data followed by carefully designed clinical trials would be required.
Notes
Supplement sponsorship. This article appears as part of the supplement “Advances and New Directions for Echinocandins,” sponsored by Astellas Pharma Global Development, Inc.
Potential conflicts of interest. D. N. has received research grants from Pfizer and has served as a consultant or advisory board member for Roche and Astellas. M. P. has served as an advisory board member for Astellas and Merck. G. P. has received research grants from Pfizer and Astellas and has served as a consultant or advisory board member for Astellas and Merck. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Ullmann AJ, Lipton JH, Vesole DH et al. . Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med 2007; 356:335–47. [DOI] [PubMed] [Google Scholar]
- 2.Wingard JR, Carter SL, Walsh TJ et al. . Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood 2010; 116:5111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freifeld AG, Bow EJ, Sepkowitz KA et al. . Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2011; 52:e56–93. [DOI] [PubMed] [Google Scholar]
- 4.Denning DW. Echinocandin antifungal drugs. Lancet 2003; 362:1142–51. [DOI] [PubMed] [Google Scholar]
- 5.Goto N, Hara T, Tsurumi H et al. . Efficacy and safety of micafungin for treating febrile neutropenia in hematological malignancies. Am J Hematol 2010; 85:872–6. [DOI] [PubMed] [Google Scholar]
- 6.Yamazaki S, Nakamura F, Yoshimi A, Ichikawa M, Nannya Y, Kurokawa M. Safety of high-dose micafungin for patients with hematological diseases. Leuk Lymphoma 2014; 55:2572–6. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg JD, Barker JN, Castro-Malaspina H et al. . The MSKCC experience with outpatient intermittent dosing of micafungin for antifungal prophylaxis and treatment following allogeneic hematopoietic stem cell transplant. Biol Blood Marrow Transplant 2010; 16:S262–3. [Google Scholar]
- 8.Levey AS, Stevens LA, Schmid CH et al. . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellomo R, Kellum JA, Ronco C. Defining and classifying acute renal failure: from advocacy to consensus and validation of the RIFLE criteria. Intensive Care Med 2007; 33:409–13. [DOI] [PubMed] [Google Scholar]
- 10.De Pauw B, Walsh TJ, Donnelly JP et al. . Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanadate T, Wakasugi M, Sogabe K et al. . Evaluation of the safety and efficacy of micafungin in Japanese patients with deep mycosis: a post-marketing survey report. J Infect Chemother 2011; 17:622–32. [DOI] [PubMed] [Google Scholar]
- 12.Lehrnbecher T, Groll AH. Micafungin: a brief review of pharmacology, safety, and antifungal efficacy in pediatric patients. Pediatr Blood Cancer 2010; 55:229–32. [DOI] [PubMed] [Google Scholar]
- 13.Stover KR, Farley JM, Kyle PB, Cleary JD. Cardiac toxicity of some echinocandin antifungals. Expert Opin Drug Saf 2014; 13:5–14. [DOI] [PubMed] [Google Scholar]
- 14.Stover KR, King ST, Cleary JD. Cardiac toxicity of the echinocandins: chance or cause and effect association? J Clin Pharm Ther 2014; 39:1–3. [DOI] [PubMed] [Google Scholar]
- 15.Wolff SN, Fay J, Stevens D et al. . Fluconazole vs low-dose amphotericin B for the prevention of fungal infections in patients undergoing bone marrow transplantation: a study of the North American Marrow Transplant Group. Bone Marrow Transplant 2000; 25:853–9. [DOI] [PubMed] [Google Scholar]
- 16.Hiramatsu Y, Maeda Y, Fujii N et al. . Use of micafungin versus fluconazole for antifungal prophylaxis in neutropenic patients receiving hematopoietic stem cell transplantation. Int J Hematol 2008; 88:588–95. [DOI] [PubMed] [Google Scholar]
- 17.Chou LS, Lewis RE, Ippoliti C, Champlin RE, Kontoyiannis DP. Caspofungin as primary antifungal prophylaxis in stem cell transplant recipients. Pharmacotherapy 2007; 27:1644–50. [DOI] [PubMed] [Google Scholar]
- 18.Neofytos D, Horn D, De Simone JA Jr. Rhodotorula mucilaginosa catheter-related fungemia in a patient with sickle cell disease: case presentation and literature review. South Med J 2007; 100:198–200. [DOI] [PubMed] [Google Scholar]
- 19.Clemons KV, Stevens DA. Efficacy of micafungin alone or in combination against experimental pulmonary aspergillosis. Med Mycol 2006; 44:69–73. [DOI] [PubMed] [Google Scholar]
- 20.Fleischhacker M, Radecke C, Schulz B, Ruhnke M. Paradoxical growth effects of the echinocandins caspofungin and micafungin, but not of anidulafungin, on clinical isolates of Candida albicans and C. dubliniensis. Eur J Clin Microbiol Infect Dis 2008; 27:127–31. [DOI] [PubMed] [Google Scholar]
- 21.Andes DR, Diekema DJ, Pfaller MA, Marchillo K, Bohrmueller J. In vivo pharmacodynamic target investigation for micafungin against Candida albicans and C. glabrata in a neutropenic murine candidiasis model. Antimicrob Agents Chemother 2008; 52:3497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ernst EJ, Roling EE, Petzold CR, Keele DJ, Klepser ME. In vitro activity of micafungin (FK-463) against Candida spp.: microdilution, time-kill, and postantifungal-effect studies. Antimicrob Agents Chemother 2002; 46:3846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groll AH, Mickiene D, Petraitis V et al. . Compartmental pharmacokinetics and tissue distribution of the antifungal echinocandin lipopeptide micafungin (FK463) in rabbits. Antimicrob Agents Chemother 2001; 45:3322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gumbo T, Drusano GL, Liu W et al. . Once-weekly micafungin therapy is as effective as daily therapy for disseminated candidiasis in mice with persistent neutropenia. Antimicrob Agents Chemother 2007; 51:968–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andes DR, Reynolds DK, Van Wart SA, Lepak AJ, Kovanda LL, Bhavnani SM. Clinical pharmacodynamic index identification for micafungin in esophageal candidiasis: dosing strategy optimization. Antimicrob Agents Chemother 2013; 57:5714–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ota Y, Tatsuno K, Okugawa S et al. . Relationship between the initial dose of micafungin and its efficacy in patients with candidemia. J Infect Chemother 2007; 13:208–12. [DOI] [PubMed] [Google Scholar]
- 27.Mehta PA, Vinks AA, Filipovich A et al. . Alternate-day micafungin antifungal prophylaxis in pediatric patients undergoing hematopoietic stem cell transplantation: a pharmacokinetic study. Biol Blood Marrow Transplant 2010; 16:1458–62. [DOI] [PubMed] [Google Scholar]
- 28.Sirohi B, Powles RL, Chopra R et al. . A study to determine the safety profile and maximum tolerated dose of micafungin (FK463) in patients undergoing haematopoietic stem cell transplantation. Bone Marrow Transplant 2006; 38:47–51. [DOI] [PubMed] [Google Scholar]