Abstract
Background:
Recent study of genome-wide DNA methylation profiling in the postmortem brain of suicidal and nonsuicidal subjects found that gene expression of spindle and kinetochore associated complex subunit 2 (SKA2) is decreased in the postmortem brain of suicide victims compared with nonsuicidal, nonpsychiatric control subjects.
Methods:
To determine if decreased SKA2 is specific to suicide and independent of diagnosis, we determined gene and protein expression of SKA2 in the prefrontal cortex obtained from suicide victims (n= 52), nonsuicidal psychiatric subjects (n= 27), and normal controls (n= 24). We determined gene expression by quantitative PCR technique and protein expression by Western blot. The postmortem brain samples were obtained from the Maryland Psychiatric Research Center.
Results:
We found that protein and gene expression of SKA2 was significantly reduced in the prefrontal cortex of suicide victims compared with normal control subjects and nonsuicidal patients. We also found that SKA2 protein and gene expression in depressed suicide victims, schizophrenic suicide victims, and suicide victims with substance abuse and/or conduct disorders was significantly decreased compared with normal control subjects and also with nonsuicidal depressed or schizophrenic subjects.
Conclusions:
This study shows that decreased gene and protein expression of SKA2 observed in the prefrontal cortex of suicide victims is specific to suicide, which was not observed in the brain of nonsuicidal patients. It also indicates reduced SKA2 expression in suicide is independent of psychiatric diagnosis, since it is observed in all diagnostic groups studied. Therefore, SKA2 may be a potential biomarker for suicide.
Keywords: SKA2, suicide, postmortem brain, schizophrenia, depression
Introduction
Suicide is a major public health concern. About 35000 people die per year of suicide in the United States alone (Center for Disease Control, 2002; Goldsmith et al., 2002a, 2002b; CDC Prevention, 2007; CDC, 2009). Significant progress has been made in understanding the neurobiology of suicide, primarily based on studies of postmortem brain samples. These studies suggested several abnormalities in the brain of suicide victims [for review, see Turecki (2014) and Pandey (2013)]. In a recent report, Guintivano et al. (2014) studied a genome-wide DNA methylation profiling in the postmortem brain from suicidal and nonsuicidal subjects and found that the DNA methylation scan identified an additive epigenetic and genetic association with suicide at rs720805 within the 3′untranslated region of the spindle and kinetochore associated complex subunit 2 (SKA2) gene in 3 brain cohorts. This finding was also replicated in blood from live cohorts. Whereas the major focus of this study was on SKA2 DNA methylation, they also studied the gene expression of SKA2 in suicide victims. They found that the gene expression of SKA2 in the postmortem brain of suicidal and nonsuicidal subjects was significantly reduced in suicidal subjects compared with controls. This study thus showed an abnormality of SKA2 gene in suicide pathogenesis. More recently, Niculescu et al. (2015) determined the expression of SKA2 in blood samples of suicidal patients and the postmortem brain of suicide victims. They observed decreased SKA2 gene expression in the blood of depressed patients and the brain of violent suicide completers. Studies of Guintivano et al. (2014) and Niculescu et al. (2015) thus strongly suggest dysregulation of SKA2 in suicide completers and its essential use as a blood marker for suicidality. It is not clear from their findings if decreased SKA2 gene expression in postmortem brain of suicide victims is independent of diagnosis (ie, whether suicide victims across all diagnostic groups have reduced SKA2 gene expression)—namely, if decreased SKA2 gene expression is specific for suicide and is not observed in the postmortem brain of nonsuicidal patients and if the protein expression of this gene is also altered in the postmortem brain of suicide victims.
To further extend the finding of decreased gene expression of SKA2 in suicide and to examine if this decrease is independent of psychiatric diagnosis and specific to suicide and is also associated with decreased protein expression, we have determined the mRNA and protein expression of SKA2 in the prefrontal cortex (PFC) obtained from suicide victims from different diagnostic groups, such as depression, schizophrenia, and other suicide groups (ie, substance abuse and/or conduct disorders) as well as from subjects from different diagnostic groups who died of natural causes (nonsuicidal patients) and from normal control subjects.
Methods
Subjects and Diagnoses
The study was performed in the PFC (Brodmann area 9) of 52 suicide victims (consisting of 24 depressed suicide victims, 16 schizophrenic suicide victims, and 12 other suicide victims with either substance abuse and/or conduct disorder), 27 nonsuicide patients (consisting of 12 depressed nonsuicide patients and 15 schizophrenic nonsuicidal patients), and 24 normal control subjects. Brain tissues were obtained from the Maryland Brain Collection at the Maryland Psychiatric Research Center, Baltimore, Maryland. All procedures were approved by the University of Maryland Institutional Review Board and by the University of Illinois Institutional Review Board.
Diagnostic Method
Subject diagnosis was based on the Structured Clinical Interview for DSM-IV (Spitzer et al., 1992). At least one family member and/or a friend, after giving written informed consent, underwent an interview. Diagnoses were made by a consensus of 2 psychiatrists from the data obtained in this interview, medical records from the case, and records obtained from the Medical Examiner’s office. Normal control subjects were verified as free from mental illnesses using these consensus diagnostic procedures.
Determination of mRNA Levels
RNA Extraction and Reverse Transcription
Total RNA was extracted from 100mg of tissue using the TRIZOL reagent (Invitrogen) as per the manufacturer’s instructions and treated with DNAse 1 (Invitrogen). The RNA yield was determined by absorbance at 260nm using NanoDrop ND-1000 (NanoDrop Technologies, Montchanin, DE). RNA quality was assessed using Agilent Bioanalyzer 2100 (Agilent). All samples had 28S/18S ratios >1.2 and RNA integrity number >6.6. The mean RNA integrity number was 7.2±0.6.
Expression levels of mRNA were determined using a 2-step real-time RT-PCR method. One microgram of total RNA was reverse transcribed using 50ng random hexamers, 2mM dNTP mix, 10 units ribonuclease inhibitor, and 200 units Moloney murine leukemia virus (MMLV)-reverse transcriptase enzyme in a final reaction volume of 20 μL.
Relative Real-Time PCR
Real-time PCR was performed using Pre-designed Taqman gene expression assays (Applied Biosystems, Foster City, CA) for all target and housekeeping genes on MX3005p sequence detection system (Agilent). The TaqMan assay IDs are in Table 1. To determine the stability and optimal number of housekeeping genes, we used geNORM version 3.4 (PrimerDesign) according to the manufacturer’s instructions (Vandesompele et al., 2002) and tested 12 commonly used reference genes of different functional classes in 10 samples from each test group. The average gene-stability measure (M) ranked β-actin and GAPDH as the most stable genes in our samples. PCR efficiency was tested over 5-log dilution series and confirmed that β-actin, GAPDH, and SKA2 had similar amplification efficiencies. For each primer/probe set, the PCR reaction was carried out using 10 µL of cDNA diluted 1:10-fold. Each quantitative PCR plate included a “no reverse transcriptase” and “no template” control to eliminate nonspecific amplification, one sample was run on a gel to confirm specificity, and samples were run in triplicates. Target gene quantitative PCR data was normalized to the geometic mean of β-actin and GAPDH and was expressed relative to the control samples using 2−(ΔΔCt) method.
Table 1.
Taqman Primers/Probes Used for qPCR Analysis
| Taqman Accession | Probe Location (Exon Boundary) | Assay Function | |
|---|---|---|---|
| ACTB | Hs99999903_m1 | 1-1 | Housekeeping (HK) |
| GAPDH | Hs99999905_m1 | 3-3 | HK |
| SKA2 | Hs00735057_m1 | 3–4 | Target gene |
Determination of Protein Expression of SKA2 by Western Blot
Gel electrophoresis and immunolabeling of SKA2 proteins were performed by Western blot as previously reported (Dwivedi and Pandey, 2000). Equal amounts of protein samples (20 μg protein in each lane) obtained from membrane fraction were loaded onto 7.5% (w/v) acrylamide gel and subsequently transferred electrophoretically to enhanced chemiluminescent (ECL) nitrocellulose membranes (Amersham Pharmacia, Piscataway, NJ). The blots were incubated overnight at 4°C with primary antibody for SKA2 (Santa Cruz Biotechnology, Inc., catalog no. sc-136868) at a dilution of 1:3000 and with horseradish-peroxidase-linked secondary anti-rabbit antibody at a dilution of 1:3000 (Amersham Pharmacia) for 3 to 5 hours at room temperature. The signals were detected with the ECL Western-blot detection system (Amersham) followed by exposure to ECL-autoradiographic film (Amersham). The membranes were stripped using stripping solution (Chemicon International, Temecula, CA) and probed with β-actin monoclonal primary (1:5000 for 2 hours; Sigma Chemical Co., St. Louis, MO) and anti-mouse secondary antibody (1:5000 for 2 hours). The bands on the autoradiograms were quantified using the Loats Image Analysis System (Westminister, MD). A ratio of the optical density of SKA2 over the optical density of the corresponding β-actin band was calculated.
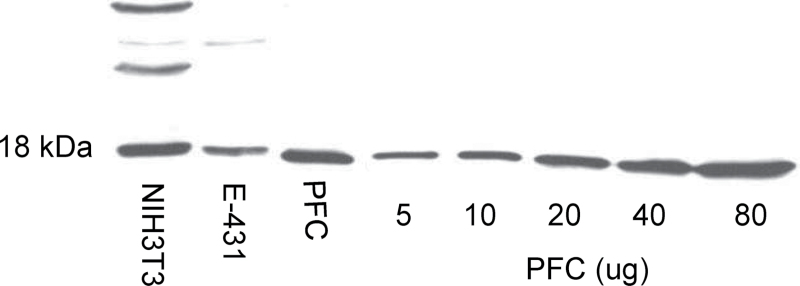
Before starting the experiment, immunolabeling of SKA2 proteins was characterized (Figure 1). The specificity of SKA2 proteins was checked by running NIH3T3 cells and E431 immune lines along with membrane fraction of PFC from one control subject. It was observed that extracts from different cell lines as well as membrane fraction migrated to 18kDa (Figure 1). The appropriate concentration of proteins was selected by running 5 different concentrations (5–80 μg) of protein from membrane fraction of PFC. The optical density was linear between these concentrations of protein. We therefore used 20 μg of protein in subsequent experiments. Similarly, the antibody concentration and duration of exposure of the nitrocellulose membrane onto autoradiographic film were also characterized.
Figure 1.
The specificity of spindle and kinetochore associated complex subunit 2 (SKA2) proteins was checked by running NIH3T3 cells and E431 immune lines along with membrane fraction of prefrontal cortext (PFC) from one control subject. Extracts from different cell lines as well as membrane fraction migrated to 18kDa.
Statistical Analysis and Effect of Confounding Variables
The data analyses were performed using the SAS 9.2 statistical software package. Data normality was assessed by Shapiro-Wilk test, and all analyses were 2-tailed with a level of significance of P<.05. Linear regression was performed to compare the effects of 3 groups: normal control subjects, suicide victims, and nonsuicidal subjects for outcome measures of SKA2 on protein and gene expression by adjusting the effects of age, gender, postmortem interval (PMI), and brain pH. For multiple comparisons, we used t tests with Bonferroni correction to adjust the type I error rates. We also performed posthoc t tests for pairwise comparisons.
To examine if the observed decrease in SKA2 expression in the PFC of suicide victims is related to the covariates, we examined the effects of age, gender, PMI, and brain pH on these parameters. There was no significant effect of age, gender, PMI, or brain pH on the protein or mRNA expression levels of SKA2 in any of the diagnostic groups.
Results
The demographic and clinical characteristics of normal controls, suicide victims, and nonsuicidal patients are shown in Table 2. Although the groups were well matched, there were some significant differences. There was a significant difference in age between normal controls with schizophrenic suicide (P=.05). There was a significant difference in PMI between normal controls and other suicide group (P <.01). There were significant differences in brain pH between normal controls vs schizophrenic suicide (P <.01), vs other suicide (P <.01), vs depressed nonsuicide (P <.01), and vs schizophrenic nonsuicide (P <.01) groups. However, these variables were used as covariates when analyzing the protein and mRNA expression of SKA2 in these groups.
Table 2.
Demographic Characteristics of Subjects
| Group | Age (y) | Race | Gender | PMI (h) |
Brain
pH |
Cause of Death |
Psychotropic Drugs
(at Time of Death) |
Psychiatric Diagnosis | |
|---|---|---|---|---|---|---|---|---|---|
| Normal Control Subjects a | |||||||||
| 1. | CONTROL | 19 | Black | Male | 11 | 6.9 | GSW | None | Normal |
| 2. | CONTROL | 22 | Black | Male | 19 | 6.9 | GSW | None | Normal |
| 3. | CONTROL | 42 | White | Female | 23 | 7.2 | Pneumonia | None | Normal |
| 4. | CONTROL | 37 | Black | Male | 5 | 7.1 | ASCVD | None | Normal |
| 5. | CONTROL | 31 | Black | Male | 8 | 7.2 | GSW | None | Normal |
| 6. | CONTROL | 46 | Black | Male | 9 | 7.1 | Multiple injuries | None | Normal |
| 7. | CONTROL | 33 | White | Male | 15 | 7.0 | GSW | None | Normal |
| 8. | CONTROL | 48 | White | Male | 26 | 6.9 | ASCVD | None | Normal |
| 9. | CONTROL | 40 | White | Female | 7 | 7.0 | ASCVD | None | Normal |
| 10. | CONTROL | 23 | Black | Male | 15 | 6.8 | GSW | None | Normal |
| 11. | CONTROL | 83 | White | Male | 20 | 7.1 | ASCVD | None | Normal |
| 12. | CONTROL | 65 | Black | Female | 23 | 6.9 | ASCVD | None | Normal |
| 13. | CONTROL | 35 | White | Male | 24 | 6.9 | Crush injury to abdomen and chest | None | Normal |
| 14. | CONTROL | 52 | White | Male | 30 | 7.3 | ASCVD | None | Normal |
| 15. | CONTROL | 37 | White | Male | 24 | 7.0 | ASCVD | None | Normal |
| 16. | CONTROL | 45 | White | Male | 22 | 7.3 | ASCVD | None | Normal |
| 17. | CONTROL | 26 | White | Male | 12 | 6.9 | Arrhythmia | None | Normal |
| 18. | CONTROL | 47 | White | Male | 10 | 7.0 | ASCVD | None | Normal |
| 19. | CONTROL | 31 | White | Male | 16 | 7.2 | MVA | None | Normal |
| 20. | CONTROL | 60 | White | Male | 15 | 7.1 | Accidental drowning | None | Normal |
| 21. | CONTROL | 28 | White | Male | 13 | 6.8 | Electrocution | None | Normal |
| 22. | CONTROL | 45 | White | Female | 16 | 6.9 | Cardiac arrhythmia | None | Normal |
| 23. | CONTROL | 62 | White | Male | 19 | 7.0 | Cardiac arrest | None | Normal |
| 24. | CONTROL | 53 | White | Male | 15 | 6.9 | ASCVD | None | Normal |
| Depressed Suicide Victims b | |||||||||
| 1. | SUICIDE | 27 | White | Male | 24 | 7.0 | GSW | None | MDD, ethanol abuse |
| 2. | SUICIDE | 44 | White | Female | 11 | 7.2 | Drug overdose | Nortriptyline | MDD, ethanol abuse |
| 3. | SUICIDE | 36 | White | Female | 10 | 7.1 | GSW | None | MDD |
| 4. | SUICIDE | 24 | White | Male | 7 | 7.1 | GSW | Ethanol | MDD |
| 5. | SUICIDE | 43 | White | Male | 12 | 7.0 | Drug Overdose | None | MDD, polysubstance abuse |
| 6. | SUICIDE | 53 | White | Male | 23 | 6.9 | Jumped from height | None | MDD |
| 7. | SUICIDE | 41 | White | Female | 27 | 7.1 | Drug Overdose | Amitriptyline, desipramine, nortriptyline, ethanol | MDD, ethanol abuse |
| 8. | SUICIDE | 22 | Black | Female | 16 | 7.3 | Drug overdose | None | MDD |
| 9. | SUICIDE | 46 | White | Female | 21 | 6.9 | Drug overdose | Amitriptyline, desipramine, ethanol | MDD |
| 10. | SUICIDE | 36 | White | Female | 18 | 7.2 | GSW | None | MDD |
| 11. | SUICIDE | 38 | White | Male | 24 | 7.0 | Drug overdose & Ethanol overdose | Ethanol | MDD, ethanol abuse |
| 12. | SUICIDE | 46 | White | Female | 16 | 6.8 | Drug overdose / Nortryptyline Intoxication |
Nortriptyline | MDD, panic disorder with agoraphobia |
| 13. | SUICIDE | 23 | White | Male | 12 | 7.0 | Hanging | Paroxetine | MDD |
| 14. | SUICIDE | 18 | White | Male | 17 | 6.3 | Hanging | None | MDD |
| 15. | SUICIDE | 30 | White | Male | 17 | 7.1 | Hanging | Venlafaxine | MDD |
| 16. | SUICIDE | 19 | White | Male | 18 | 6.2 | CO intoxication | Ethanol, CO | MDD, ethanol abuse, polysubstance abuse |
| 17. | SUICIDE | 44 | White | Female | 30 | 7.2 | Drug overdose, Ethanol intoxication | Fluoxetine, ethanol | MDD, ethanol abuse, opioid abuse |
| 18. | SUICIDE | 74 | White | Female | 27 | 7.0 | Venlafaxine overdose | Venlafaxine, ethanol | MDD, ethanol abuse |
| 19. | SUICIDE | 25 | White | Male | 14 | 6.8 | Hanging | Ethanol | MDD |
| 20. | SUICIDE | 23 | Black | Male | 23 | 6.9 | Hanging | None | MDD |
| 21. | SUICIDE | 63 | White | Male | 19 | 6.9 | Drug overdose, Ethanol intoxication | Ethanol | MDD |
| 22. | SUICIDE | 67 | White | Male | 22 | 7.0 | GSW | Fluoxetine, venlafaxine | MDD |
| 23. | SUICIDE | 40 | White | Female | 20 | 7.0 | Drug overdose | Alprazolam | MDD |
| 24. | SUICIDE | 53 | White | Male | 26 | 7.1 | Suicide by stab wound | Sertraline | MDD |
| Schizophrenia Suicide Victims c | |||||||||
| 1. | SUICIDE | 45 | Black | Male | 10 | 6.8 | Suicide, stab wound | Haloperidol | Schizophrenia |
| 2. | SUICIDE | 20 | White | Female | 11 | 6.5 | Jump from height, multiple injuries | Haloperidol | Schizophrenia |
| 3. | SUICIDE | 54 | White | Male | 12 | 6.6 | Suicide, drowning | Haloperidol | Schizophrenia |
| 4. | SUICIDE | 20 | White | Male | 23 | 6.4 | Drug overdose | Fluphenazine | Schizophrenia, ethanol abuse |
| 5. | SUICIDE | 40 | White | Male | 17 | 6.8 | Jump from height, multiple injuries | Trifluoperazine | Schizophrenia |
| 6. | SUICIDE | 28 | White | Male | 13 | 7.1 | Suicide, hanging | Thioridazine | Schizophrenia, ethanol abuse |
| 7. | SUICIDE | 35 | White | Female | 7 | 6.9 | Suicide, multiple drugs intoxication | Amitriptyline, amoxapine, loxapine, nortriptyline | Schizophrenia |
| 8. | SUICIDE | 37 | White | Male | 20 | 6.7 | Suicide, drowning | Haldol | Schizophrenia, ethanol abuse |
| 9. | SUICIDE | 37 | White | Male | 22 | 7.1 | Suicide, GSW to chest | None | Schizophrenia, ethanol abuse |
| 10. | SUICIDE | 51 | White | Female | 21 | 6.5 | Suicide, overdose | None | Schizophrenia |
| 11. | SUICIDE | 34 | White | Male | 16 | 6.60 | Suicide, jumped from height, multiple injuries | Mesoridazine | Schizophrenia |
| 12. | SUICIDE | 21 | White | Male | 26 | 6.4 | Jumped from height, multiple injuries | Fluphenazine | Schizophrenia |
| 13. | SUICIDE | 23 | White | Male | 20 | 6.6 | Jumped from height, multiple injuries | None | Schizophrenia, hallucinogen abuse |
| 14. | SUICIDE | 45 | Black | Male | 8 | 6.6 | Suicide, hanging | Olanzapine | Schizophrenia |
| 15. | SUICIDE | 37 | White | Male | 14 | 6.7 | Suicide, electrocution | Risperidone, fluphenazine | Schizophrenia |
| 16. | SUICIDE | 54 | White | Male | 19 | 6.6 | Suicide, bleeding | None | Schizophrenia |
| Other Suicide Victims d | |||||||||
| 1. | SUICIDE | 34 | White | Male | 16 | 6.4 | GSW | Ethanol | Ethanol abuse |
| 2. | SUICIDE | 21 | White | Male | 17 | 6.8 | GSW | None | Adjustment disorder, mixed |
| 3. | SUICIDE | 75 | White | Male | 18 | 6.4 | GSW | None | Adjustment disorder, conduct disorder |
| 4. | SUICIDE | 87 | White | Male | 16 | 6.6 | GSW | None | Adjustment disorder, conduct disorder |
| 5. | SUICIDE | 39 | White | Male | 30 | 6.7 | Asphyxia | Freon, cocaine, and metabolites | Cocaine abuse |
| 6. | SUICIDE | 30 | White | Male | 32 | 6.5 | Hanging | Cocaine, ethanol | Ethanol abuse, cocaine abuse, drug abuse |
| 7. | SUICIDE | 40 | White | Male | 26 | 6.7 | GSW | Ethanol | Adjustment disorder |
| 8. | SUICIDE | 20 | White | Male | 32 | 6.9 | Hanging | Ethanol | Ethanol abuse |
| 9. | SUICIDE | 71 | White | Female | 24 | 6.5 | Drug overdose | None | Adjustment disorder, mixed |
| 10. | SUICIDE | 24 | White | Male | 22 | 6.8 | Hanging | None | Schizoaffective disorder |
| 11. | SUICIDE | 21 | White | Male | 23 | 6.8 | Hanging | None | Adjustment disorder, conduct disorder |
| 12. | SUICID | 19 | White | Male | 15 | 6.9 | GSW to chest | Fluoxetine | Dissociative disorder, substance abuse, PTSD |
| Depressed Nonsuicide Subjects e | |||||||||
| 1. | Nonsuicide depressed | 65 | White | Male | 14 | 6.9 | ASCVD | None | MDD |
| 2. | Nonsuicide depressed | 55 | Black | Female | 8 | 6.4 | ASCVD | Fluoxetine, ethanol | MDD, polysubstance abuse |
| 3. | Nonsuicide depressed | 71 | White | Male | 4 | 6.3 | ASCVD | Bupropion | MDD |
| 4. | Nonsuicide depressed | 74 | Black | Female | 7 | 6.7 | ASCVD | Paroxetine, thioridazine | MDD |
| 5. | Nonsuicide depressed | 14 | White | Male | 11 | 7.0 | MVA | Sertraline | MDD, polysubstance abuse |
| 6. | Nonsuicide depressed | 39 | White | Male | 36 | 6.8 | Fatty Liver | Thioridazine | MDD |
| 7. | Nonsuicide depressed | 46 | Black | Male | 20 | 7.1 | Seizure d/o | Fluoxetine, risperidone | MDD |
| 8. | Nonsuicide depressed | 59 | White | Male | 20 | 7.0 | ASCVD | Sertraline | MDD, ethanol dependence |
| 9. | Nonsuicide depressed | 46 | White | Female | 23 | 6.9 | Mixed Drug intoxication | Bupropion, lamotrigine | MDD, ethanol abuse, polysubstance abuse |
| 10. | Nonsuicide depressed | 29 | White | Female | 22 | 6.9 | Obesity, Cardiomegaly |
Fluoxetine, norfluoxetine | MDD |
| 11. | Nonsuicide depressed | 49 | White | Male | 24 | 7.1 | ASCVD | Desmethylsertraline | MDD |
| 12. | Nonsuicide depressed | 47 | White | Female | 26 | 6.5 | Diabetic ketoacidosis | Fluoxetine | MDD |
| Schizophrenia Nonsuicide Subjects f | |||||||||
| 1. | Nonsuicide | 71 | White | Female | 12 | 6.8 | ASCVD | None | Schizophrenia |
| 2. | Nonsuicide | 41 | Black | Female | 16 | 6.6 | Morbidly obese, dilated cardiomyopathy | Perphenazine | Schizophrenia |
| 3. | Nonsuicide | 50 | Black | Female | 11 | 6.9 | Environmental hyperthermia, complication from schizophrenia | None | Schizophrenia |
| 4. | Nonsuicide | 24 | Black | Male | 23 | 6.7 | ASCVD | Olanzapine | Schizophrenia |
| 5. | Nonsuicide | 77 | White | Male | 17 | 6.8 | ASCVD | Risperidone | Schizophrenia |
| 6. | Nonsuicide | 45 | Black | Female | 17 | 6.6 | Diabetic ketoacidosis | Haloperidol | Schizophrenia |
| 7. | Nonsuicide | 47 | Black | Male | 20 | 7.0 | ASCVD | Fluphenazine | Schizophrenia, ethanol abuse, polysubstance abuse |
| 8. | Nonsuicide | 55 | White | Male | 12 | 6.4 | ASCVD | Olanzapine | Schizophrenia, ethanol abuse |
| 9. | Nonsuicide | 41 | Black | Male | 19 | 6.5 | ASCVD | Haloperidol | Schizophrenia, ethanol abuse |
| 10. | Nonsuicide | 40 | White | Male | 14 | 6.6 | MVA | None | Schizophrenia, ethanol abuse, cannabis abuse, cocaine abuse |
| 11. | Nonsuicide | 33 | Black | Male | 12 | 6.2 | Appendicitis/ Peritonitis | Olanzapine | Schizophrenia |
| 12. | Nonsuicide | 42 | White | Female | 14 | 6.8 | Liver Cirrhosis | None | Schizophrenia, cocaine abuse, polysubstance abuse |
| 13. | Nonsuicide | 53 | White | Male | 14 | 6.1 | ASCVD | None | Schizophrenia |
| 14. | Nonsuicide | 83 | White | Male | 18 | 7.0 | Electrocution, accidental | None | Schizophrenia |
| 15. | Nonsuicide | 57 | White | Male | 11 | 6.2 | Allergic reaction | Haloperidol | Schizophrenia |
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; CO, carbon monoxide; GSW, gunshot wound; MDD, major depressive disorder; MVA, motor vehicle accident.
a Normal controls (mean ± SD) age is 42.08±15.35 years; PMI is 16.54±6.56 hours; brain pH is 7.02±0.15; 7 Black, 17 White; 20 Males, 4 Females.
b Depressed suicide victims (mean ± SD) age is 38.96±15.40 years; PMI is 18.92±6.02 hours; brain pH is 6.96±0.25; 2 Black, 22 White; 14 Males, 10 Females.
c Schizophrenia suicide victims (mean ± SD) age is 36.31±11.63 years; PMI is 16.19±5.69 hours; brain pH is 6.68±0.21; 2 Black, 14 White; 13 Males, 3 Females.
d Other suicide victims (mean ± SD) age is 40.08±24.03 years; PMI is 22.58±6.35 hours; brain pH is 6.67±0.18; 12 White; 11 Males, 1 Females.
e Depressed nonsuicide subjects (mean ± SD) age is 49.50±17.18 years; PMI is 17.92±9.32 hours; brain pH is 6.80±0.27; 3 Black, 9 White; 7 Males, 5 Females.
f Schizophrenia nonsuicide subjects (mean ± SD) age is 50.60±16.17 years; PMI is 15.33±3.62 hours; brain pH is 6.61±0.29; 7 Black, 8 White; 10 Males, 5 Females.
mRNA Expression of SKA2 in Normal Control Subjects, Suicide Victims, and Nonsuicidal Patients
We determined the mRNA expression of SKA2 in the PFC (Brodmann area 9) of 24 normal control subjects, 52 suicide victims with different diagnoses, and 27 nonsuicidal subjects. The mean mRNA expression of SKA2 in these 3 groups is shown in Figure 2A. One-way ANOVA showed significant differences between the groups (F= 6447.06, P <.001), and when we compared the mRNA expression of SKA2 between these 3 groups, we found that the mRNA expression of SKA2 was significantly decreased [t= -5.40, P <.001, CI (-.99,-.37), ES = .58] in suicide victims compared with normal control subjects (Figure 2A). The SKA2 expression in suicide victims was also significantly decreased (t=-5.70, P <.001, ES =. 58) in the PFC of suicide victims compared with nonsuicidal patients (Figure 2A). When we compared the SKA2 expression between nonsuicidal patients and normal control subjects, we found that there was no significant difference (t=-.256, P =1.0, ES = .04) in the expression of SKA2 between normal control subjects and nonsuicidal patients (Figure 2A). These results suggested that decreased SKA2 expression was specific to suicide.
Figure 2.
Mean mRNA expression levels of spindle and kinetochore associated complex subunit 2 (SKA2) in the prefrontal cortext (PFC) of normal controls, suicide victims, and nonsuicidal subjects from different diagnostic groups. The data are shown as fold change in mRNA levels and values are fold change ± SEM. (A) Mean mRNA expression levels of SKA2 in normal controls, all suicide victims, and all nonsuicidal patients. (a) Normal controls vs all suicides: P< .0001. (b) All nonsuicidal vs all suicides: P< .0001. (B) Mean mRNA expression levels of SKA2 in normal controls, depressed suicide victims, nonsuicidal depressed subjects, schizophrenic suicide victims, nonsuicidal schizophrenic subjects, and other (mainly substance abuse and/or conduct disorders) suicide victims. *P < .05.
To examine the diagnostic specificity, that is, if decreased mRNA expression of SKA2 in suicide victims was independent of diagnosis, we divided the suicide victims into different diagnostic groups. The suicide group consisted of 24 depressed suicide victims, 16 schizophrenic suicide victims, and 12 suicide victims with other diagnoses, primarily substance abuse and/or conduct disorders. We found that mean mRNA expression of SKA2 in depressed suicide victims (t = 5.94, P <.001, ES = .66), schizophrenic suicide victims (t = 2.23, P <.03, ES = .37), and other suicide victims (t = 4.52, P <.001, ES = .58) was significantly different compared with normal control subjects, as shown in Figure 2B and Table 3. However, there was no significant difference between the 3 suicide groups compared against each other (Table 3).
Table 3.
Statistical Summary for mRNA Expression of SKA2
| Comparisons | T STAT | P value | Effect Size | |
|---|---|---|---|---|
| Normal controls | Depressed suicide | -5.949763 | <.0001 | -.66 |
| Normal controls | Other suicide | -4.52416 | .0001 | -.58 |
| Normal controls | Schizophrenic suicide | -2.23897 | 0.3 | -.37 |
| Normal controls | Depressed nonsuicide |
0.462002 | 1.0000 | .07 |
| Normal controls | Schizophrenic nonsuicide | 0.760534 | 1.0000 | .15 |
| Depressed suicide | Other suicide | 0.686473 | 1.0000 | .104 |
| Depressed suicide | Schizophrenic suicide | 3.043836 | .0196 | .48 |
| Other suicide | Schizophrenic suicide | 2.111131 | .2297 | .37 |
| Depressed nonsuicide |
Schizophrenic nonsuicide | 1.037718 | .9148 | .20 |
On the other hand, the SKA2 mRNA expression was not significantly different in nonsuicidal depressed subjects and nonsuicidal schizophrenic subjects compared with normal control subjects (Figure 2B; Table 3), showing that decreased SKA2 mRNA expression was specific to suicide and independent of diagnosis.
We also compared the mRNA expression of SKA2 between depressed suicide victims and nonsuicidal depressed subjects and between schizophrenic suicide victims and nonsuicidal schizophrenic subjects (Table 3). We found that the mRNA expression of SKA2 was again significantly lower (t = -3.68, P =.009, ES = .56) in depressed suicide victims compared with nonsuicidal depressed subjects, and it was also significantly lower in schizophrenic suicide victims compared with nonsuicidal schizophrenic subjects (t = -3.61, P =.0012, ES = .57) (Figure 2B; Table 3).
Protein Expression of SKA2 in Normal Controls, Suicide Victims, and Nonsuicidal Patients
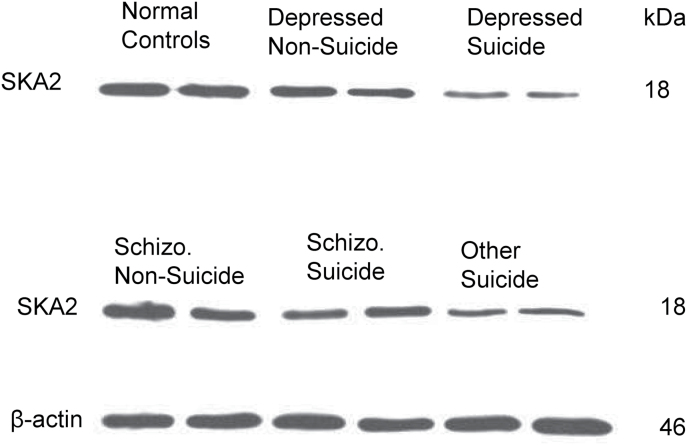
Representative immunoblots of 2 subjects from the following groups—normal controls, depressed suicide, nonsuicidal depressed, schizophrenic suicide, nonsuicidal schizophrenic, and other suicide—are shown in the Figure 3. As can be seen, SKA2 protein expression appears to be decreased in suicide (ie, depressed suicide, schizophrenic suicide, and other suicide) victims, but not in nonsuicidal (ie, nonsuicidal depressed and nonsuicidal schizophrenic) subjects compared with normal control subjects.
Figure 3.
Representative Western blots showing the immunolabeling of spindle and kinetochore associated complex subunit 2 (SKA2) and beta-actin in the prefrontal cortex (PFC) membrane fraction of 2 subjects from the following groups: normal controls, depressed suicide, nonsuicidal depressed, schizophrenic suicide, nonsuicidal schizophrenic, and other suicide (consisting mainly of substance abuse and/or conduct disorders).
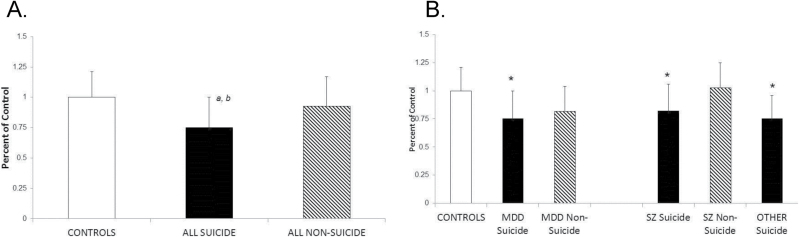
The mean protein expression of SKA2 in suicide victims, nonsuicidal subjects, and normal controls are shown in Figure 4A. We examined if protein expression of SKA2, similar to its mRNA expression, was also altered in the PFC of suicide victims by comparing SKA2 protein expression between suicide victims, nonsuicidal subjects, and normal controls. The regression analysis showed differences between normal controls, total suicide victims, and total nonsuicidal subjects (F = 460.1, P <.0001). We then examined if there was a significant difference between the groups by posthoc t test with Bonferroni correction. We found that the protein expression of SKA2 was significantly decreased in suicide victims (t = 3.98, P =.0004, ES = .46) compared with normal control subjects and nonsuicidal patients (Figure 4A). We also found that there was no significant difference (t =1.10, P =.82, ES = .15) in the protein expression levels of nonsuicidal patients compared with normal control subjects.
Figure 4.
Protein expression levels of spindle and kinetochore associated complex subunit 2 (SKA2) in the prefrontal cortext (PFC) of normal controls, and suicide victims and nonsuicidal subjects from different diagnostic groups. The data are shown as percent of controls. Values are mean ± SD. (A) Mean protein expression levels of SKA2 in normal controls, depressed suicide victims, nonsuicidal depressed subjects, schizophrenic suicide victims, nonsuicidal schizophrenic subjects, and other (mainly substance abuse and/or conduct disorders) suicide victims. (a) Normal controls vs all suicides: P < .001. (b) All nonsuicidal vs all suicides: P < .05. (B)
Mean protein expression levels of SKA2 in normal controls, depressed suicide victims, nonsuicidal depressed subjects, schizophrenic suicide victims, nonsuicidal schizophrenic subjects, and other (mainly substance abuse and/or conduct disorders) suicide victims. *P < .05.
To examine if SKA2 protein expression was related to diagnosis or was independent of diagnosis and specific to suicide, we compared each diagnostic group separately with each other and also with normal control subjects as well as with each diagnostic group in the nonsuicidal patient group (Table 4). Suicide groups consisted of depressed suicide victims (n = 24), schizophrenic suicide victims (n=16), and other suicide victims (n=12), consisting primarily the subjects with substance abuse and/or conduct disorders.
Table 4.
Statistical Summary for Protein Expression of SKA2
| Comparisons | T STAT | P value | Effect Size | |
|---|---|---|---|---|
| Normal controls | Depressed suicide | 3.67543 | .0028 | .47 |
| Normal controls | Other suicide | 3.144044 | .0148 | .51 |
| Normal controls | Schizophrenic suicide | 2.275038 | .3 | .37 |
| Normal controls | Depressed nonsuicide |
-2.40996 | .0603 | -.39 |
| Normal controls | Schizophrenic nonsuicide | -0.4643 | 1.0000 | -.08 |
| Depressed suicide | Other suicide | -0.00612 | 1.0000 | -.001 |
| Depressed suicide | Schizophrenic suicide | -0.86157 | 1.0000 | -.14 |
| Other suicide | Schizophrenic suicide | -0.76296 | 1.0000 | -.15 |
| Depressed nonsuicide |
Schizophrenic nonsuicide | -2.48918 | .0497 | -.45 |
The mean protein expression of SKA2 in each group is shown in Figure 4B. When we compared different groups against the normal controls, we found that the protein expression of SKA2 in depressed suicide (t = 3.67, P =.003, ES = .47), schizophrenic suicide (t = 2.27, P <.03, ES=.37), and other suicide groups (t = 3.14, P =.01, ES = .51) was significantly decreased compared with normal control subjects (Figure 4B; Table 4).There was no significant difference between any of the suicide groups compared against each other. When we compared the SKA2 protein expression in nonsuicidal subjects, consisting of 12 nonsuicidal depressed (t = 2.41, P =.06, ES = .38) and 15 nonsuicidal schizophrenic subjects (t = -.46, P =1.0, ES = .07), we found that the mean SKA2 protein expression in these subjects was not significantly different from that of the normal control subjects (Figure 4B; Table 4). These results therefore suggested that decreased SKA2 protein expression in suicide is independent of psychiatric diagnosis, since suicide victims from different diagnostic groups still showed a significant decrease in SKA2 protein expression, whereas nonsuicidal subjects did not show any significant difference compared with normal control subjects. These results also suggested that decreased SKA2 protein expression was specific to suicide.
Discussion
In this study, we found that the gene and protein expression of SKA2, a gene recently implicated in suicidal behavior (Guintivano et al., 2014), is significantly decreased in the PFC of suicide victims compared with normal controls. The protein and gene expression of SKA2 in the PFC of nonsuicidal patients was not significantly different from normal controls. Also, the gene and protein expression of SKA2 in the PFC was significantly lower in suicide victims compared with nonsuicidal patients. This observation suggests that decreased protein and mRNA expression of SKA2 is specific to suicide, as this decrease was not observed in nonsuicidal subjects.
We then examined if the decrease of SKA2 in the PFC of suicide victims is independent of diagnosis. We observed that SKA2 protein and gene expression was decreased in depressed suicide, schizophrenic suicide, and other suicide groups compared with normal controls and also compared with corresponding nonsuicidal groups, for example, depressed suicide vs depressed nonsuicide, suggesting that decreased SKA2 expression is not related to diagnosis (ie, it is independent of diagnosis, since the decrease was observed only in suicide victims).
In a recent study, Guintivano et al. (2014) reported altered DNA methylation of SKA2 in the blood of suicide patients and the brain of suicide victims. They also found decreased mRNA SKA2 expression in the PFC of suicide victims. However, it was not clear if the observed decrease in SKA2 mRNA expression was independent of diagnosis.
Our observation that mRNA expression of SKA2 is decreased in the PFC of suicide victims compared with controls is similar to that of Guintivano et al. (2014). Although the studies of Guintivano et al. (2014) focused more on DNA methylation in the brain and also in the blood, we focused on SKA2 abnormalities in suicide brain. In addition to mRNA expression, we have determined SKA2 protein expression in our cohort, and we also examined the diagnostic specificity of decreased SKA2 expression by determining SKA2 in suicide victims and nonsuicidal psychiatric subjects belonging to different diagnostic groups. We examined if decreased SKA2 expression was specific to suicide and not related to diagnosis. Our results suggest decreased gene and protein expression of SKA2 in suicide. The reasons, mechanisms, or functional consequences of this decrease are unclear, since the role of SKA2 in suicide and psychiatric disorders is not well known.
In a more recent study, Niculescu et al. (2015) found decreased SKA2 gene expression in violent suicide completers. We therefore also compared SKA2 expression between violent and nonviolent suicide. Although the number of violent suicide completers was small, we did not find differences in SKA2 protein or mRNA expression between violent and nonviolent suicide completers.
SKA2 belongs to the SKA complex consisting of the proteins SKA1 and SKA2 (Hanisch et al., 2006). This complex was originally identified in a proteomic survey of the human mitotic spindle apparatus (Hanisch et al., 2006). A novel spindle and kinetochore associated (SKA) complex required for a timely anaphase onset consists of both SKA1 and SKA2. A third component of the SKA complex, known as SKA3, has also been identified (Raaijmakers et al., 2009), and the depletion of this gene (SKA3) from the complex results in mitotic arrest (Guimaraes and Deluca, 2009; Welburn et al., 2009). It is believed that this complex is required to generate stable kinetochore-microtubule attachments during mitosis in humans (Hanisch et al., 2006).
This SKA complex in general, and SKA2 in particular, may be associated with suicide/suicidal behavior through its interaction with glucocorticoid receptor (GR), which is involved in the feedback inhibition of hypothalamic-pituitary-adrenal (HPA) axis (Pariante and Lightman, 2008; Rice et al., 2008). Abnormal HPA axis function has been implicated in depression and completed suicide (Coryell and Schlesser, 2001). One of the tests by which HPA axis abnormalities have been assessed is the dexamethasone (DEX) suppression test (DST). DST was found to be abnormal in both depression and suicidal behavior (Plotsky et al., 1998; Coryell and Schlesser, 2001; Pariante and Lightman, 2008). In addition, some studies suggest that abnormal DST may be a risk factor and even a predictor of completed suicide (Coryell and Schlesser, 2001; Yerevanian et al., 2004; Jokinen et al., 2007). For example, Yerevanian et al. (2004) found that DST nonsuppressors were significantly more likely to commit and complete suicide than DST suppressors. Other investigators have also found an association between DST nonsuppression and suicide (Coryell and Schlesser, 2001; Yerevanian et al., 2004; Jokinen et al., 2007; Le-Niculescu et al., 2013).
The DST nonsuppression (ie, abnormal DST) or the HPA axis hyperactivity in depressed and suicidal patients has been related to a deficiency in feedback mechanisms, primarily to altered GR in the brain (Nemeroff, 1996; Pariante and Lightman, 2008). Thus, GR is a key component regulating the HPA axis and it may be abnormal in suicide. Abnormality of this receptor in the postmortem brain has been implicated in suicide (McGowan et al., 2009; Alt et al., 2010; Pandey et al., 2013). For example, it has been found that the expression of GR and its target gene, GILZ, is decreased in the PFC and amygdala of suicide victims (Alt et al., 2010; Pandey et al., 2013). Abnormalities of DNA methylation of GR have also been reported in suicide subjects with early life trauma (McGowan et al., 2009). Thus, it is quite possible that abnormal DST suppression and GR expression observed in suicide (Raison and Miller, 2003; Alt et al., 2010; Pandey et al., 2013) may be related to a functional interaction between GR and SKA2, as suggested by some studies (Rice et al., 2008). Rice et al. (2008) studied in great detail the interaction of SKA2 with GR and found that SKA2 is colocalized with GR in the cytoplasm and moves to the nucleus in DEX-treated cells. They also found that in cells overexpressing a GR DNA construct, there was a partial translocation of SKA2 to the nucleus following glucocorticoid treatment. Since SKA2 has no nuclear localization, it may interact with GR in the cytoplasm to facilitate its movement to the nucleus. The main requirement for this process to occur is the overexpression of GR. Since there is some evidence to suggest that GR may be underexpressed in suicide brain (Alt et al., 2010; Pandey et al., 2013), this may lead to decreased SKA2 translocation to the nucleus. Similarly, underexpression of SKA2 in suicide may alter the translocation of GR to the nucleus (Rice et al., 2008).
Another possible mechanism of GR-SKA2 interaction may be the SKA2 effect on GR transactivation function. Rice et al. (2008) found that overexpression of SKA2 results in modest enhancement of GR transactivation, while knockdown of SKA2 markedly inhibits GR transactivation (Rice et al., 2008). Since we find decreased SKA2 expression in suicide, it may suggest decreased GR transactivation.
The strong interaction between GR and SKA2 suggests that abnormalities in HPA function in suicide may also be related to GR-SKA2 interaction. This assumption is further substantiated by the observation of Guintivano et al. (2014) that SKA2 genetic and epigenetic differences were associated with reduced suppression of salivary cortisol.
It has been shown that SKA2 may play a role in cell proliferation and apoptosis, as SKA2 knockdown prevented cell proliferation (Rice et al., 2008). It was also found that DEX has a profound inhibitory effect on SKA2 expression, suggesting that SKA2 may play a role in antiproliferation or proapoptotic activity.
In addition to DEX, the levels of SKA2 are also regulated by Staurosporine, phorbol ester, and trichostatin A (Rice et al., 2008). All of these either induce apoptosis or inhibit cell proliferation. These findings may also suggest that abnormalities of SKA2 in suicide may either cause or be related to the observed abnormalities of protein kinase C (PKC) in suicide (Pandey et al., 2004). Since phorbol ester binds and activates PKC and since abnormalities of PKC enzymes have been reported in the suicide brain, it is possible that underexpression of SKA2 in suicide may be related to the reported abnormalities of PKC in suicide.
It therefore appears that the main factors regulating the expression of SKA2 are GR, PKC, and DEX. Abnormal expression of these factors—PKC and GR—has been reported in the suicide brain (Pandey et al., 2004, 2013).
What may be the consequences of SKA2 abnormalities in suicide? Decreased SKA2 expression may cause apoptosis and/or decreased cell proliferation through its interaction with GR and/or PKC. Reported volume and structural changes in the PFC of suicidal patients (Jollant et al., 2011) may be a consequence of SKA2 underexpression in the suicidal brain.
Identification of biomarkers with high sensitivity and specificity for suicide and/or suicidal behavior and genes that represent a risk factor for suicide may be important in the prevention and treatment of suicidal behavior. Recent studies have suggested that some genes and their DNA methylation such as SKA2 predict suicidal behavior with high specificity in patients with suicidal behavior. Also, Le-Niculescu et al. (2013) have identified SAT-1 gene to be specific to suicidal behavior. They also recently reported that SKA2 is indeed also a blood biomarker based on gene expression studies in blood samples of psychiatric patients and suicide completers (Niculescu et al., 2015). Gene and protein expression studies of SKA2 show promise as a biomarker for suicide, since it appears that it is not only specific to suicide but the SKA2 decrease observed in suicide victims is independent of diagnosis and not present in nonsuicidal psychiatric patients. Studies of this gene in blood of suicidal patients are needed to further validate the usefulness of this gene as a biomarker or vulnerability marker for suicide.
Statement of Interest
None.
Acknowledgments
This study was supported by grant RO1-MH-98554 (Dr. Pandey) from the National Institute of Mental Health, Rockville, MD. The funding source had no involvement in the conduct of the research and/or preparation of the article.
References
- Alt SR, Turner JD, Klok MD, Meijer OC, Lakke EA, Derijk RH, Muller CP. (2010) Differential expression of glucocorticoid receptor transcripts in major depressive disorder is not epigenetically programmed. Psychoneuroendocrinology 35:544–556. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2009) National Center for Injury Prevention and Control. Web-based Injury Statistics Query and Reporting System (WISQARS) [online] [Last accessed, 19 Jun 2009]. Source of data from WISQARS is the National Vital Statistics System from the National Center for Health Statistics. Available from URL:www.cdc.gov/ncipc/wisqars. In.
- CDCPrevention (2007) Web-based injury statistics query and reporting system.[online] [Last accessed, 15 Oct 2007] Available from URL: http://www.who.int/mental_health/prevention/suicide/suicideprevent/en .
- Center for Disease Control (2004) Deaths: Final Data for 2002. National Vital Statistics Reports, Volume 53, Number 5, 12 Oct 2004. Available from URL: http://www.cdc.gov/nchs/data/nvsr/nvsr53/nvsr53_05acc.pdf. [PubMed]
- Coryell W, Schlesser M. (2001) The dexamethasone suppression test and suicide prediction. Am J Psychiatry 158:748–753. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Pandey GN. (2000) Adrenal glucocorticoids modulate [3H]cyclic AMP binding to protein kinase A (PKA), cyclic AMP-dependent PKA activity, and protein levels of selective regulatory and catalytic subunit isoforms of PKA in rat brain. J Pharmacol Exp Ther 294:103–116. [PubMed] [Google Scholar]
- Goldsmith SK, Pellmar TC, Kleinman AM, Bunney WE. (2002. a) Reducing suicide, a national imperative. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- Goldsmith SK, Pellmar TC, Kleinman J, Bunney WE, eds (2002. b) Committee on Pathophysiology and Prevention of Adolescent and Adult Suicide; Board on Neuroscience and Behavioral Health; Institute of Medicine of the National Academies: reducing suicide, a national imperative. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- Guimaraes GJ, Deluca JG. (2009) Connecting with Ska, a key complex at the kinetochore-microtubule interface. EMBO J 28:1375–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guintivano J, Brown T, Newcomer A, Jones M, Cox O, Maher BS, Eaton WW, Payne JL, Wilcox HC, Kaminsky ZA. (2014) Identification and replication of a combined epigenetic and genetic biomarker predicting suicide and suicidal behaviors. Am J Psychiatry 171:1287–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch A, Sillje HH, Nigg EA. (2006) Timely anaphase onset requires a novel spindle and kinetochore complex comprising Ska1 and Ska2. EMBO J 25:5504–5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokinen J, Carlborg A, Martensson B, Forslund K, Nordstrom AL, Nordstrom P. (2007) DST non-suppression predicts suicide after attempted suicide. Psychiatry Res 150:297–303. [DOI] [PubMed] [Google Scholar]
- Jollant F, Lawrence NL, Olie E, Guillaume S, Courtet P. (2011) The suicidal mind and brain: a review of neuropsychological and neuroimaging studies. World J Biol Psychiatry 12:319–339. [DOI] [PubMed] [Google Scholar]
- Le-Niculescu H, Levey DF, Ayalew M, Palmer L, Gavrin LM, Jain N, Winiger E, Bhosrekar S, Shankar G, Radel M, Bellanger E, Duckworth H, Olesek K, Vergo J, Schweitzer R, Yard M, Ballew A, Shekhar A, Sandusky GE, Schork NJ, Kurian SM, Salomon DR, Niculescu AB (2013) Discovery and validation of blood biomarkers for suicidality. Mol Psychiatry 18:1249–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. (2009) Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci 12:342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB. (1996) The corticotropin-releasing factor (CRF) hypothesis of depression: new findings and new directions. Mol Psychiatry 1:336–342. [PubMed] [Google Scholar]
- Niculescu AB, Levey D, Le-Niculescu H, Niculescu E, Kurian SM, Salomon D. (2015) Psychiatric blood biomarkers: avoiding jumping to premature negative or positive conclusions. Mol Psychiatry 20:286–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey GN. (2013) Biological basis of suicide and suicidal behavior. Bipolar Disord 15:524–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Rizavi HS, Ren X, Conley RR. (2004) Decreased catalytic activity and expression of protein kinase C isozymes in teenage suicide victims: a postmortem brain study. Arch Gen Psychiatry 61:685–693. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Ren X, Dwivedi Y, Palkovits M. (2013) Region-specific alterations in glucocorticoid receptor expression in the postmortem brain of teenage suicide victims. Psychoneuroendocrinology 38:2628–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. (2008) The HPA axis in major depression: classical theories and new developments. Trends Neurosci 31:464–468. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Owens MJ, Nemeroff CB. (1998) Psychoneuroendocrinology of depression. Hypothalamic-pituitary-adrenal axis. Psychiatr Clin North Am 21:293–307. [DOI] [PubMed] [Google Scholar]
- Raaijmakers JA, Tanenbaum ME, Maia AF, Medema RH. (2009) RAMA1 is a novel kinetochore protein involved in kinetochore-microtubule attachment. J Cell Sci 122:2436–2445. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. (2003) When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry 160:1554–1565. [DOI] [PubMed] [Google Scholar]
- Rice L, Waters CE, Eccles J, Garside H, Sommer P, Kay P, Blackhall FH, Zeef L, Telfer B, Stratford I, Clarke R, Singh D, Stevens A, White A, Ray DW. (2008) Identification and functional analysis of SKA2 interaction with the glucocorticoid receptor. J Endocrinol 198:499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. (1992) The Structured Clinical Interview for DSM-III-R (SCID). I: history, rationale, and description. Arch Gen Psychiatry 49:624–629. [DOI] [PubMed] [Google Scholar]
- Turecki G. (2014) The molecular bases of the suicidal brain. Nat Rev Neurosci 15:802–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welburn JP, Grishchuk EL, Backer CB, Wilson-Kubalek EM, Yates JR, 3rd, Cheeseman IM. (2009) The human kinetochore Ska1 complex facilitates microtubule depolymerization-coupled motility. Dev Cell 16:374–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerevanian BI, Feusner JD, Koek RJ, Mintz J. (2004) The dexamethasone suppression test as a predictor of suicidal behavior in unipolar depression. J Affect Disord 83:103–108. [DOI] [PubMed] [Google Scholar]