Abstract
Background:
The C-terminal domain of the heavy chain of tetanus toxin (Hc-TeTx) is a nontoxic peptide with demonstrated in vitro and in vivo neuroprotective effects against striatal dopaminergic damage induced by 1-methyl-4-phenylpyridinium and 6-hydoxydopamine, suggesting its possible therapeutic potential in Parkinson’s disease. Methamphetamine, a widely abused psychostimulant, has selective dopaminergic neurotoxicity in rodents, monkeys, and humans. This study was undertaken to determine whether Hc-TeTx might also protect against methamphetamine-induced dopaminergic neurotoxicity and the consequent motor impairment.
Methods:
For this purpose, we treated mice with a toxic regimen of methamphetamine (4mg/kg, 3 consecutive i.p. injections, 3 hours apart) followed by 3 injections of 40 ug/kg of Hc-TeTx into grastrocnemius muscle at 1, 24, and 48 hours post methamphetamine treatment.
Results:
We found that Hc-TeTx significantly reduced the loss of dopaminergic markers tyrosine hydroxylase and dopamine transporter and the increases in silver staining (a well stablished degeneration marker) induced by methamphetamine in the striatum. Moreover, Hc-TeTx prevented the increase of neuronal nitric oxide synthase but did not affect microglia activation induced by methamphetamine. Stereological neuronal count in the substantia nigra indicated loss of tyrosine hydroxylase-positive neurons after methamphetamine that was partially prevented by Hc-TeTx. Importantly, impairment in motor behaviors post methamphetamine treatment were significantly reduced by Hc-TeTx.
Conclusions:
Here we demonstrate that Hc-TeTx can provide significant protection against acute methamphetamine-induced neurotoxicity and motor impairment, suggesting its therapeutic potential in methamphetamine abusers.
Keywords: fragment C tetanus toxin, methamphetamine, neuroprotection, neurotoxicity, neuroinflammation
Introduction
The C-terminal domain of heavy chain tetanus toxin (Hc-TeTx) is a nontoxic fragment with demonstrated capacity to protect against cell death induced by 1-methyl-4-phenylpyridinium (MPP+) in vitro (Chaïb-Oukadour et al., 2009). The efficacy of the recombinant Hc-TeTx fragment in vivo was later confirmed by using the Hc-TeTx against dopaminergic lesion induced by MPP+ (Mendieta et al., 2009) or 6-hydroxydopamine (6-OHDA) in rats (Mendieta et al., 2012; Sánchez-González et al., 2014). Hc-TeTx has also been used as a neurotrophic agent to prevent damage in neurodegenerative models such as amyotrophic lateral sclerosis and ischemia (Moreno-Igoa et al., 2010; Toivonen et al., 2010; Calvo et al., 2011; Radenovic et al., 2014). Moreover, Hc-TeTx fragment can be retrogradely transported to the CNS; hence, it shares the same machinery of retrograde transport with neurotrophin receptors, tropomyosin-related kinases and p75NTR, nerve growth factor and brain-derived neurotrophic factor (Deinhardt et al., 2006). Indeed, several studies have used Hc-TeTx fragment injected i.m. as a carrier to deliver neurotrophic molecules into the brain (Larsen et al., 2006; Payne et al., 2006; Ciriza et al., 2008; Li et al., 2009).
Methamphetamine (METH) is an illicit but popular amphetamine-type stimulant with high addictive potential (UNODC, 2014). However, its prolonged use can lead to severe behavioral, motor, and cognitive deficits in drug abusers (Volkow et al., 2001; Barr et al., 2006; Scott et al., 2007; Darke et al., 2008; Hadamitzky et al., 2011; Rusyniak, 2011; Dean et al., 2013; Granado et al., 2013). Neuroimaging studies on human addicts have revealed that abuse of METH can induce neurodegenerative changes (Volkow et al., 2001; Chang et al., 2007). Brain analyses of chronic METH users have provided evidence of dopaminergic system alterations such as decreases in dopamine (DA), dopamine transporter (DAT), and the tyrosine hydroxylase (TH) in several brain regions, including the amygdala, prefrontal cortex, caudate-putamen, and the nucleus accumbens (Wilson et al., 1996; Moszczynska et al., 2004; Kitamura et al., 2007; McCann et al., 2008). In agreement with persistent neurotoxic effects of METH on nigrostriatal pathway, individuals with a history of METH use are twice to three times as likely to develop Parkinson’s disease (PD) (Callaghan et al., 2012; Curtin et al., 2015). In addition, in the striatum of rodents, METH causes degeneration of dopaminergic fibers (Ricaurte et al., 1984; Ricaurte and McCann 1992; Ares-Santos et al., 2014) and kills some of its corresponding cell bodies in the substantia nigra pars compacta (SNpc) (Ares-Santos et al., 2014). The toxicity of METH involves mitochondrial dysfunctions, glial activation (Thomas et al., 2004; Thomas and Kuhn, 2005; Xu et al., 2005), excitotoxicity, oxidative and nitrosative stress, and activation of apoptotic and necrotic pathways (Wang et al., 2008; Yamamoto et al., 2010; Granado et al., 2011b; Downey and Loftis, 2014).
Our aim in this study was to investigate whether Hc-TeTx might also be effective in ameliorating the neurotoxic and/or motor impairing effects of acute METH treatment. Based on previous animal studies (Mendieta et al., 2012; Sánchez-González et al., 2014), we administered i.m. the recombinant fragment of Hc-TeTx for 3 consecutive days after acute METH treatment in mice and examined DA degeneration by evaluating TH and DAT fiber loss in the striatum and stereological neuronal count in the substantia nigra. The inflammatory and oxidative processes induced by METH were also examined in these mice. The findings indicate the efficiency of Hc-TeTx in ameliorating acute METH-induced striatal terminal degeneration and motor impairments.
Materials and Methods
Animals and Treatment
Adult male C57BL/6J mice (20–25g, Harlan Iberica, Barcelona, Spain) were housed in groups of 4 to 6 per cage at the Cajal Institute in conditions of constant temperature at 21ºC ± 2ºC in a 12-h-light/-dark cycle (lights on at 8:00 am) with free access to food and water. All experimental procedures conformed to European Community guidelines (2003/65/CE) and were approved by Cajal Institute’s Bioethics Committee (following DC86/609/EU).
Mice received 3 injections of METH (4mg/kg, i.p.) with a 3-hour interval between injections as described in detail previously and shown to cause significant striatal toxicity (Granado et al., 2010, 2011a; Ares-Santos et al., 2012, 2014; ElAli et al., 2012; Urrutia et al., 2013, 2014). This regimen is very close to other studies where 4 doses of 5mg/kg at 2-hour intervals were used to induce toxicity (Good et al., 2011; Chiu et al., 2014; Raineri et al., 2015). Control mice were given saline. Doses are expressed as free base. METH was obtained from Sigma-Aldrich (Madrid, Spain).
The animals were administered Hc-TeTx fragment (40 μg/kg) in the gastrocnemius muscle for 3 consecutive days, with the first 1 hour following the last METH injection and the other 2 at 24 and 48 hours after last METH injection as described previously (Mendieta et al., 2012). Controls received the vehicle (SSI, saline solution). The Hc-TeTx fragment was synthetized in accordance with Herrando-Grabulosa et al. (2013). Animals were sacrificed 1, 3, or 7 days posttreatment with METH.
Measurement of Rectal Temperature
Rectal temperature was measured using a digital readout thermocouple (BAT-12 thermometer, Physitemp Instruments; Clifton, NJ) with a resolution of 0.1ºC and accuracy of ±0.1°C attached to a RET-3 Rodent Sensor. The sensor was inserted 2cm into the mouse rectum, while the mouse was lightly restrained by holding in the hand. A steady readout was obtained within 10 seonds of probe insertion. Temperature readings were taken every 30 minutes immediately before and after METH injections and hourly thereafter. The purpose of measuring the temperature was to verify that indeed hyperthermia was induced by the administered dose of METH. Animals that did not show hyperthermia (only 2) were excluded from the study to ensure uniform experimental groups.
Immunohistochemistry
Immunostaining was carried out in free-floating brain sections (50 μm) with standard avidin-biotin immunohistochemical protocols (Granado et al., 2010; Suarez et al., 2014; Ruiz-DeDiego et al., 2015). The sections were incubated overnight with specific primary antisera (Ab-I), a rabbit TH antiserum (Chemicon International, Temecula, CA) diluted 1:1000; rat monoclonal antibody against DAT (Chemicon International), diluted 1:1000; a rabbit anti glial fibrillary acidic protein antibody (GFAP) (DakoCytomation, Denmark) diluted 1:1000 and rabbit polyclonal anti ionized calcium binding adaptor molecule 1 (Iba1), a microglia/macrophage-specific calcium-binding protein (1:1000), Wako Pure Chemical Industries, Ltd Osaka, Japan) in phosphate buffered saline with Tween solutions containing normal goat serum. After careful washing, the sections were incubated with the secondary biotinylated secondary antisera (Vector) at room temperature and developed using diaminobenzidine. The reaction was monitored every 5 minutes using an optical microscope (Leica). After washing, the sections were mounted on gelatin-coated slides, air dried, and dehydrated in ascending concentrations of ethanol, cleared with xylene, and coverslipped under Permount. For immunofluorescence experiments, we used Alexa fluor 488 and Alexa fluor 594 conjugated secondary antibodies (1:500; Invitrogen, Eugene, OR). The sections were mounted in Mowiol solution (Calbiochem, San Diego, CA). Controls were performed to confirm the specificity of the primary and secondary antibodies.
Quantification of expression of TH, DAT, GFAP, and Iba-1 was performed with the aid of an image analysis system (Analytical Imaging Station, Imaging Research Inc., Linton, UK) using a 4x lens for TH and DAT and 20x for GFAP and Iba-1, converting color intensities into a gray scale and quantifying the area of staining as a percentage of the total striatal area. Thus, the total area of striatum in the controls as represented in the figures is not 100% due to the fact that the staining does not cover the entire striatal surface (Darmopil et al., 2008; Granado et al., 2010, 2011a; Ortiz et al., 2010; Ares-Santos et al., 2012, 2014). Measurements were carried out in 6 to 8 animals/treatment and using 4 to 5 sections/animal. For TH and DAT, only matched rostrocaudal sections in all animals were quantified. It should be noted that the microglia undergo a dramatic transformation from their resting ramified state into an activated amoeboid morphology that express Iba1 (Eyo and Dailey, 2013). Thus, Iba1 staining reflects activated microglia (Buchanan et al., 2010; Granado et al., 2011b).
Amino-Cupric-Silver Staining
Animals were anaesthetized with sodium pentobarbital (50mg/kg, i.p.) and perfused transcardially with 4% paraformaldehyde in 0.2M borate buffer (pH 7.4). Brains were left overnight in the skull and afterwards removed. Brain sections of 50 μm were obtained in a vibratome and stored in 4% paraformaldehyde for amino-cupric-silver technique (A–Cu–Ag) or immunohistochemistry. Neuronal degeneration was analyzed by the A–Cu–Ag stain, which stains degenerating perikarya, dendrites, stem axons, and their terminal ramifications (synaptic endings) (de Olmos et al., 1994; Switzer, 2000; Ares-Santos et al., 2014).
Stereological Quantification in SNpc
The degeneration of neurons in the SNpc was assessed by counting TH-immunoractive (TH-ir) neurons, (stained neurons) unilaterally in every 4th section of the SNpc of all experimental groups (n=4–9/group) in which TH immunostaining was performed. The optical fractionator, Stereoinvestigator program (Microbrightfield Bioscience, Colchester, VT), was used by an experienced observer unaware of treatment conditions as described previously (Ares-Santos et al., 2012; Espadas et al., 2012). The outlines of the striatum and SNpc (including SN pars lateralis) were drawn at low power (2x) using defined anatomic landmarks (Granado et al., 2008a, 2008b; Baquet et al., 2009; Ares-Santos et al., 2012; Urrutia et al., 2014) and the numbers of cells were counted at higher power (100x for the SNpc). To avoid double counting, neurons were counted when their nuclei were optimally visualized, which occurred in only one focal plane. Some neurons with extremely faint signs of TH expression were not counted as TH expressing neurons. Results are expressed as bilateral estimations.
Locomotor Activity and Motor Coordination
Basal horizontal movements were recorded using a multicage activity meter (Columbus Instruments, Columbus, OH) with a set of 8 individual cages measuring 20×20×28cm. Horizontal movement was detected by 2 arrays of 16 infrared beams. The software allowed a distinction to be made between repetitive interruptions of the same photobeam and interruptions of adjacent photobeams. This latter measure was used as an index of ambulatory activity. Mice were habituated to the cages in 30-minute sessions for 3 days prior to the experiment. We tested the horizontal movements, time in movements, and total distance travelled on days 1, 3, and 7 after treatment with METH. Motor coordination was measured right after the locomotor activity evaluation using an accelerating Rotarod apparatus (Hugo Basile, Rome, Italy). Mice underwent 3 consecutive trials separated by 15-minute inter-trial intervals. The Rotarod apparatus accelerate from 4 to 40rpm in 300 seconds (Gonzalez-Aparicio and Moratalla, 2014). The time it took the mouse to fall off the Rotarod was recorded as measure of latency. Mice were habituated 1 day prior to the experiment and were tested at 1, 3, and 7 days after METH treatment.
Statistics
Data are presented as mean ± SEM. The results of rectal temperature measurements were analyzed using 2-way ANOVA for repeated measures. Data from motor behavior and striatal image analysis were analyzed using 1-way ANOVA. Analysis of stereological quantification of TH-positive cells for each group was compared using Student’s t test. Relevant differences post-ANOVA were analyzed pair-wise using Student-Newman-Keuls test to determine specific group differences. The criterion for significance was P<.05.
Results
Hc-TeTx Fragment Attenuates METH-Induced Decreases in TH and DAT Expression in the Striatum
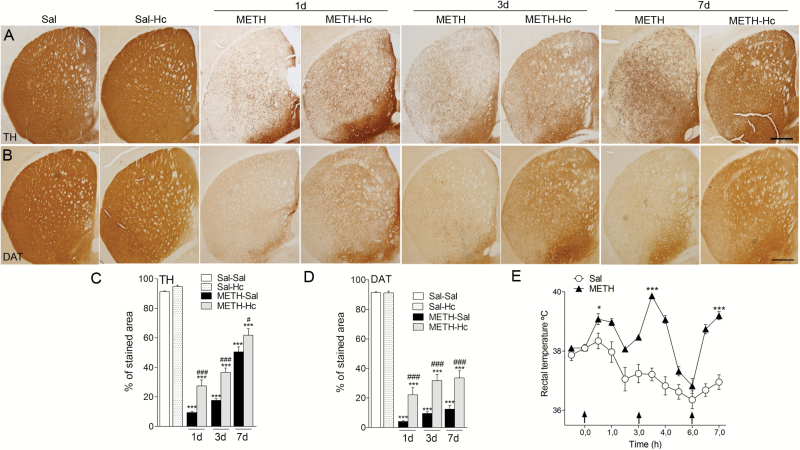
Previous studies showed that METH decreases TH-fiber density in the mouse striatum and that this decrease lasts for more than 30 days (Granado et al., 2010, 2011a, 2011b; Ares-Santos et al., 2014). In the present work, we found that Hc-TeTx treatment attenuated METH-induced reduction in TH. Thus, METH induced an approximately 90% decrease at day 1, 80% at day 3, and a 44% decrease in TH at 7 days posttreatment compared with saline-treated animals (Figure 1A,C). Hc-TeTx significantly attenuated this reduction to approximately 70% on day 1, 60% on day 3 (P<.001), and 32% at day 7 (P<.05) (Figure 1A,C). Although this attenuation took place in all animals treated with Hc-TeTx, the protection was only partial. Hc-TeTx alone had no effect of its own on TH levels (Figure 1A,C). Similarly, we found that Hc-TeTx was able to significantly attenuate DAT reduction to approximately 75%, 65%, and 63% at 1, 3, and 7 days (P<.001), respectively, compared with 95%, 89%, and 86% striatal DAT loss at 1, 3, and 7 days posttreatment with METH alone (Figure 1B,D). Again, these protections were only partial but were seen in all animals. Hc-TeTx alone had no effect of its own on DAT levels (Figure 1B,D).
Figure 1.
C-terminal domain of the heavy chain of tetanus toxin (Hc-TeTx) attenuated methamphetamine (METH)-induced decreases in tyrosine hydroxylase (TH) and dopamine transporter (DAT) expression in the striatum. Hc-TeTx (Hc) prevents the striatal TH and DAT decrease induced by METH. Photomicrographs of striatal sections stained for TH (A) and DAT (B) from mice at 1, 3, and 7 days after METH with and without Hc-TeTx treatment. Histograms show the percentage of striatal stained area of TH-immunoractive (TH-ir) (C) and DAT-ir (D) in the striatum. (E) METH (4mg/kg, 3 consecutive administrations each 3 hours apart) produced hyperthermia in mice after the injections Arrows indicate drug injections. Data represent mean±SEM, n=6–8/group, using 4–5 sections/animal. *P<.05, ***P<.001 vs saline group, #P<.05, ###P<.001 vs METH only. Bar indicates 500 µm.
METH Induces Hyperthermia
METH treatment resulted in robust hyperthermia (Figure 1E). The purpose of this measurement was to validate effectiveness of METH and establish uniform experimental groups. It should be noted, however, that although hyperthermia in general may contribute to neuronal damage, it is not a requisite for METHinduced dopaminergic neurotoxicity. This contention is based on a large body of evidence including the report that reserpine, which strongly potentiates METH toxicity, actually blocks METHinduced hyperthermia (Albers and Sonsalla, 1995; Thomas et al., 2008; Granado et al., 2011a, 2011b; Ares-Santos et al., 2012).
It is worth noting that both groups of mice (METH alone and METH+Hc-TeTx) had similar hyperthermic response and that Hc-TeTx treatment was initiated 1 hour after the last METH injection (Figure 1E). As indicated in the Methods section, the METH group was divided in 2 groups with similar hyperthermia. One of these groups was further treated with Hc-TeTx (3 x 40 μg/kg i.m.), while the other received saline at the same time points. In summary, both DA markers (TH and DAT) indicate that Hc-TeTx protects against the loss of DA fibers induced by METH in the striatum despite equal hyperthermic response.
Hc-TeTx Fragment Attenuates METH-Induced DA Terminal Degeneration in the Striatum as Assessed by A–Cu–Ag Staining
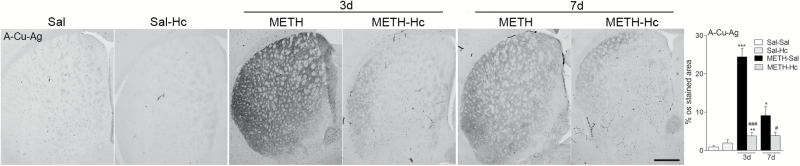
To further confirm that Hc-TeTx protects striatal terminal loss induced by acute METH treatment, we examined the striatum by A-Cu-Ag staining, which specifically stains somatodendritic and terminal degeneration (de Olmos et al., 1994; Ares-Santos et al., 2014). Three days after METH treatment, there was a dramatic increase in striatal silver staining (24% of A-Cu-Ag staining area, P<.001 vs saline). This staining was considerably reduced by Hc-TeTx treatment, as the increase in amino-cupric silver signal was only 4% of A-Cu-Ag staining (P<.001) (Figure 2). As in previous studies by our laboratory (Ares-Santos et al., 2014), there was a time-dependent decrease of silver staining, suggesting removal of debris by macrophages after 7 days. Accordingly, 7 days post-METH we found an increase of only 9% of A-Cu-Ag staining area (P<.05), while in the Hc-TeTx group this increase remained at 4% (P<.05) (Figure 2). The degeneration in both groups was still evident by day 7, and again, Hc-TeTx treatment, while having no effect of its own, significantly attenuated METH-induced damage (Figure 2). In all cases, however, the recovery was partial.
Figure 2.
C-terminal domain of the heavy chain of tetanus toxin (Hc-TeTx) attenuated methamphetamine (METH)-induced increases in amino-cupric-silver technique (A–Cu–Ag) staining in the striatum. METH induced an increase in A–Cu–Ag staining indicative of cell damage that was mitigated by treatment with Hc-TeTx (Hc). Photomicrographs of A–Cu–Ag-stained sections of the striatum of mice at 3 and 7 days after METH with and without Hc treatment. Histograms show the proportional stained area in the striatum. Data represent mean±SEM, n=6–8/group. *P<.05, **P<.01, ***P<.001 vs saline group; #P<.05, ###P<.001 vs METH group. Bar indicates 500 μm.
Hc-TeTx Fragment Has Differential Effects on METH-Induced Microgliosis or Astrogliosis in the Striatum
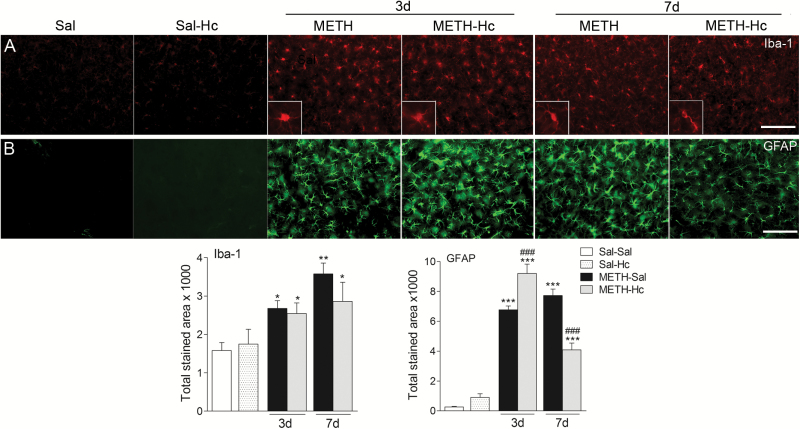
Microgliosis and astrogliosis are important hallmarks of inflammatory effects of toxic doses of METH (Halpin et al., 2014). Here, we found that METH treatment resulted in 69% and 126% increase (P<.05) in Iba-1 staining, reflective of an increase in microgliosis, by day 3 and 7, respectively, compared with the saline-treated mice (Figure 3A). Hc-TeTx did not affect the increase in METH-induced microgliosis at either time point. In contrast, the increase in GFAP staining, reflective of astrogliosis induced by METH on day 3 (approximately 25-fold over baseline, P<.001), was further enhanced by approximately 33% (P<.05) by Hc-TeTx (Figure 3B). However, the increase in GFAP staining by day 7 following METH treatment was significantly reduced by Hc-TeTx by approximately 48% (P<.001) (Figure 3B). Hc-TeTx alone did not affect either Iba-1 or GFAP staining (Figure 3A-B).
Figure 3.
C-terminal domain of the heavy chain of tetanus toxin (Hc-TeTx) had a differential effect on methamphetamine (METH)-induced microgliosis vs astrogliosis in the striatum. Although METH had a consistent effect on both micro- and astrogliosis, Hc-TeTx (Hc) had varied interactions with METH vis-a-vis these markers of central inflammatory responses. Photomicrographs of ionized calcium binding adaptor molecule 1 (Iba-1) (A) and glial fibrillary acidic protein antibody (GFAP)-stained (B) sections of the striatum of mice at 3 and 7 days after METH with and without Hc-TeTx (Hc) treatment. Histograms show the proportional stained area in the striatum. Data represent mean ± SEM, n = 6–8/group. *P<.05, **P<.01, ***P<.001 vs saline group, ###P<.001 vs METH group. Bar indicates 50 μm.
Hc-TeTx Fragment Attenuates METH-Induced Increases in nNOS
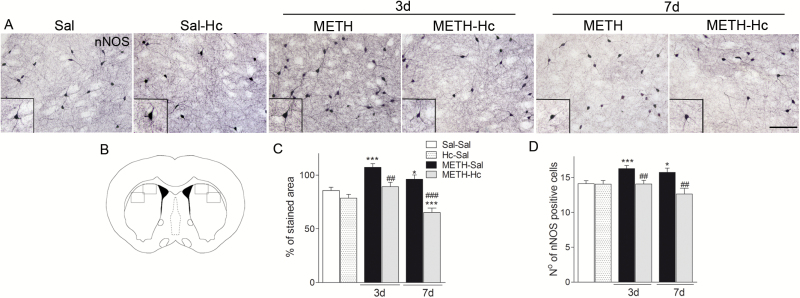
Neuronal nitric oxide synthase (nNOS) is an important indicator of nitric oxide production, which is implicated in METH-induced neurotoxicity in the striatum (Deng and Cadet et al., 1999; Friend et al., 2014). Our results on nNOS immunohistochemistry indicate that Hc-TeTx prevents the increase of nNOS by METH treatment (Figure 4). Quantification studies of nNOS-ir were carried out evaluating the stained area and the number of nNOS-positive neurons in the 2 quadrants depicted in Figure 4B, 2 hemispheres per section, 4 to 6 sections/animal. METH administration increased the stained area of nNOS on day 3 (approximately 29%, P<.001), which was normalized by Hc-TeTx (P<.01) (Figure 4A,C). There was less increase in nNOS-ir on day 7 (approximately 12%, P<.05), which was further reduced by Hc-TeTx below the baseline (approximately 17%, P<.001) (Figure 4C). A similar pattern of Hc-TeTx effect was observed for nNOS-positive cells in the striatum, where Hc-TeTx prevented the increase of nNOS at both 3 and 7 days post METH treatment (Figure 4D). Hc-TeTx by itself had no effect on nNOS (Figure 4).
Figure 4.
C-terminal domain of the heavy chain of tetanus toxin (Hc-TeTx) blocked methamphetamine (METH)-induced increases in neuronal nitric oxide synthase (nNOS) expression in the striatum. METH caused an in increase in the expression of nNOS in the striatum, which was totally blocked by Hc-TeTx (Hc). Photomicrographs of nNOS-stained sections of the striatum of mice at 3 and 7 days after METH with and without Hc-TeTx treatment (A). Schematic representation of mouse striatum (B). Histograms of nNOS-stained area (C) and nNOS-positive cells (D). Data represent mean ± SEM, n = 6–8/group. *P<.05, ***P<.001 vs saline; ##P<.01, ###P<.001 vs METH. Bar indicates 100 μm.
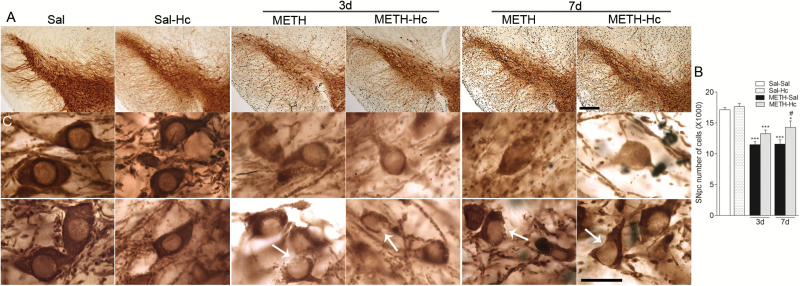
Hc-TeTx Fragment Mitigates METH-Induced Reductions in TH-ir Neurons in SNpc at 7 Days Posttreatment
As we showed previously (Ares-Santos et al., 2014), METH treatment resulted in significant reduction in the number of TH-ir neurons in SNpc at day 3 (11,476±471) and day 7 (11,563±715) compared with saline-treated animals (17,126±377) (P<.01, P<.001) (Figure 5). These reductions represent approximately 33% neuronal loss as demonstrated by stereological counts. Although Hc-TeTx treatment appears to prevent some of these losses at both time points, only at day 7 posttreatment was the mitigating effect of Hc-TeTx (approximately 20%) statistically significant (P<.05) (Figure 5B). Hc-TeTx by itself had no effects on TH-ir in SNpc (Figure 5). Close microscopic observations of TH-positive neurons in the SNpc revealed neurons with less TH-ir signal and neurons with citoarchitecture damage in both groups of mice (METH and METH + Hc-TeTx) compared with the saline group (Figure 5C). Citoarchitecture damage was evident by broken cell membranes in both nuclei and cytoplasm (see examples in Figure 5). Overall, the data suggest that TH-positive cells of both groups undergo a similar damaging process with some protection afforded at a later time point by Hc-TeTx.
Figure 5.
C-terminal domain of the heavy chain of tetanus toxin (Hc-TeTx) mitigated methamphetamine (METH)-induced dopaminergic neuron loss in substantia nigra pars compacta (SNpc) at 7 days posttreatment. METH induced loss of tyrosine hydroxylase (TH)-ir neurons in substantia nigra. (A) Left, high magnification photomicrographs of nigral sections stained for TH in saline, METH, and Hc-TeTx + METH-treated animals after 3 and 7 days. Bar indicates 200 μm. (B) Histogram shows number of TH-positive neurons in SNpc of mice at 3 and 7 days after METH with and without Hc-TeTx (Hc) treatment. Hc-TeTx reduced METH-induced neuronal loss at 7 days posttreatment. Cells were counted by unbiased stereology in midbrain sections. Data represent mean ± SEM, n=4–9/group. *P<.05, ***P<.001 vs saline, #P<.05 vs METH. (C) High magnification photomicrographs show examples of TH-ir neurons for saline, METH, and Hc-TeTx+METH-treated groups. METH (3 x 4mg/kg) caused loss of TH-ir neurons as observed at 3 and 7 days posttreatment. The toxic effects by METH were found in both groups as evidenced by lighter TH staining and broken membrane cells. Bar indicates 10 μm.
Hc-TeTx Fragment Prevents the Reduction of Motor Behavior Post-METH Treatment
Consistent with previous reports (Ares-Santos et al., 2014), 1 day after METH treatment there was a significant decrease in locomotor activity as assessed by horizontal activity (53% decrease, P<.001; Figure 6A), time in movement (68% decrease, P<.001; Figure 6B), and total distance travelled (68% decrease, P<.01; Figure 6C) compared with saline. All these effects were significantly reduced by Hc-TeTx treatment (24% decrease in horizontal activity, P<.001; 20% decrease in movement time, P<.001; and 22% decrease in total distance, P<.01) compared with the saline-treated group. Similarly, the decrease in Rotarod motor coordination induced by METH on days 1 (approximately 49%, P<.001) and 3 (approximately 35%, P<.001) posttreatment were also reduced by Hc-TeTx to approximately 14% on day 1 and 5% on day 3, practically normalizing the effect on Rotarod performance (Figure 6D). Hc-TeTx by itself had no effect on any behavioral parameter. At day 7, the motor coordination was recovered and no differences were found between groups.
Figure 6.
C-terminal domain of the heavy chain of tetanus toxin (Hc-TeTx) prevented impairment of motor behavior induced by methamphetamine (METH). METH induced a decrease in all measures of locomotor activity [(A) horizontal; (B) time in movement; (C) total distance travelled]. All these effects were almost completely blocked by Hc-TeTx (Hc) treatment. Similarly, the reduction in Rotarod induced by METH on days 3 and 7 were also totally blocked by Hc-TeTx (D). Data represent mean ± SEM, n=6–8/group. *P<.05, ***P<.001 vs saline; #P<.05, ##P<.01, ###P<.001 vs METH.
Discussion
In recent years, the efficacy of the recombinant Hc-TeTx as neuroprotective agent against toxins has been demonstrated in different animal models of PD. These studies show that Hc-TeTx is able to decrease dopaminergic lesions induced by MPP+ and 6-OHDA (Mendieta et al., 2009, 2012). In this study, we provide the first evidence that Hc-TeTx fragment can also protect striatal terminal loss and motor impairments induced by acute METH administration. Numerous studies have investigated the mechanism of METH-induced neuronal toxicity using various paradigms and species. Most of these studies, as discussed above, have concentrated on the dopaminergic system, since the primary target of METH appears to be this neurotransmitter. Thus, it has been demonstrated that METH causes degeneration of dopaminergic fibers in the striatum (Ricaurte et al., 1982, 1984; Ricaurte and McCann, 1992; Ares-Santos et al., 2014) and destruction of some of its corresponding cell bodies in the SNpc (Ares-Santos et al., 2014), which might lead to PD-like symptoms (Callaghan et al., 2012; Curtin et al., 2015). However, it should be noted that acute effects of METH on dopaminergic system may not be associated or correlated with changes in motor activity (Krasnova et al., 2009) or as seen in this study, with impairments in Rotarod performance. Nonetheless, our study highlights the protective action of the protein fragment Hc-TeTx that, when given i.m., mitigates METH-induced dopaminergic degeneration as well as the motor impairments, suggesting potential usefulness of Hc-TeTx effects against METH-induced damages. In this regard, it would be of considerable interest to investigate long term effects of METH as well as Hc-TeTx fragment.
In addition to the dopaminergic system, METH may also influence the function of several other neurotransmitters systems such as the serotonergic (Ali and Itzhak, 1998; Krasnova and Cadet, 2009; Silva et al., 2014), glutamatergic (Simões et al., 2007; Kerdsan et al., 2012; Miyazaki et al., 2013; Zhang et al., 2014), GABAergic (Zhang et al., 2006; Mizoguchi and Yamada, 2011; Padgett et al., 2012; Shen et al., 2013), and the cholinergic system (Lim et al., 2014). However, these interactions may be more related to nonmotor effects of METH. For example, its serotonergic interactions may be related to its mood-altering effects (Silva et al., 2014); its glutamatergic interactions may be more relevant to its dependency, memory impairments, and psychotic-like effects (Simões et al., 2007; Miyazaki et al., 2013); and its GABAergic interactions may be more relevant to its cognitive dysfunctions and anxiety-like symptoms during withdrawal (Mizoguchi and Yamada, 2011; Shen et al., 2013). METH interaction with the striatal cholinergic system, however, may also be related to its motor impairments (Lim et al., 2014). In this context, it is important to note that interactions of these neurotransmitter systems with the dopaminergic systems can also influence the final behavioral and neuronal effects of METH (Miyazaki et al., 2013). Moreover, as mentioned above (see Results) hyperthermia may contribute to neuronal damage, although it is not a requisite for METH-induced dopaminergic neurotoxicity. Some common denominators of METH influence on all transmitter systems may involve its interaction with the neurotrophic, inflammatory, or oxidative stress pathways (Krasnova and Cadet, 2009; Moratalla et al., 2015). Our findings suggest protective effects of Hc-TeTx on dopaminergic system, TH, and DAT, disrupted by acute METH administration. In this regard, it is of relevance to note that some in vitro studies have shown stimulatory effects of Hc-TeTx on TrkB pathways (Gil et al., 2003; Chaïb-Oukadour et al., 2004), which can promote not only TH expression (Salvatore et al., 2014) but might also act a general trophic mechanism, hence maintaining other transmitter systems as well. Collectively, the data indicate that Hc-TeTx has protective effects against METH-induced damage on the striatal dopaminergic system as well as the motor impairment. Furthermore, mitigation of the neuronal damage in SNpc, albeit at 7 days posttreatment, can be an additional beneficial effect of Hc-TeTx.
Previous studies with Hc-TeTx fragment suggest its protection against excitotoxicity and oxidative stress (Herrando-Grabulosa et al., 2013; Radenovic et al., 2014; Patricio-Martínez et al., 2016). In our paradigm, we concentrated on the involvement of inflammatory or oxidative stress in METH-induced toxicity and possible interaction of Hc-TeTx with these mechanisms. The results suggest that the protective effects of Hc-TeTx are more likely to be mediated through inhibition of METH-induced oxidative stress. The reason for this contention is that METH increases nNOS, which may lead to nitric oxide production (Deng and Cadet, 1999; Solís et al., 2015), and this increase was completely abolished by Hc-TeTx. Moreover, substances that act as inhibitors of nNOS might also have protective effects against METH-induced neurotoxicity (Eyerman and Yamamoto, 2007). We did not detect any robust antiinflammatory effect of Hc-TeTx. This was evident in the lack of Hc-TeTx on METH-induced microgliosis at either 3 or 7 days posttreatment. On the contrary, Hc-TeTx caused a further increase in astrogliosis on day 3 post METH treatment. It is important to note that microgliosis and astrogliosis are hallmarks of inflammatory response to toxic substances such as METH (Granado et al., 2011a, 2011b; Ares-Santos et al., 2012; Halpin et al., 2014). The increase in astrogliosis shortly after Hc-TeTx administration might actually signal a compensatory and protective effect of initial inflammatory response by Hc-TeTx (O’Callaghan et al., 2008; Miyazaki et al., 2011; Colangelo et al., 2014; Pekny et al., 2014). In this regard, it should be noted that the immune response to an insult is to provide adequate opportunity for repair mechanism to occur. However, overactivation of inflammatory mechanism can be a source of damage by itself (Hurley and Tizabi, 2013; Tizabi et al., 2014; Moratalla et al., 2015). Hence, the significant decrease in astrogliosis on day 7 post METH treatments might be indicative of a delayed antiinflammatory effect of Hc-TeTx similar to what was seen previously against 6-OHDA (Mendieta et al., 2012). However, further studies on possible antiinflammatory effects of Hc-TeTx and interaction between oxidative stress and immune/inflammatory system need to be carried out. Moreover, as mentioned above, part of Hc-TeTx effect may be through its interaction with neurotrophic pathway, as several studies demonstrate that neurotrophic factors such as GDNF or brain-derived neurotrophic factor prevent dopaminergic neurodegeneration (Rosenblad et al., 2000; Singh et al., 2006; Boger et al., 2007). Since neurotrophic factors may interact and influence both inflammatory and oxidative stress pathways (Hurley and Tizabi, 2013; Beardsley and Hauser, 2014), it is very likely that multiple mechanisms may contribute to a beneficial effect of Hc-TeTx. A major advantage of Hc-TeTx is that it can be administered i.m. at very low (micromolar) doses and reach the CNS, while neurotrophic factors need to be injected directly into the brain or delivered via viral vectors (Kordower and Bjorklund, 2013).
In summary, we report for the first time that Hc-TeTx reduces the neurotoxic effects of acute METH in striatal terminals and also mitigates METH-induced locomotor impairment. These findings lead us to suggest therapeutic potential for Hc-TeTx fragment against the damage induced by METH or possibly other dopaminergic neurotoxins.
Statement of Interest
None.
Acknowledgments
We thank Emilia Rubio, Beatriz Pro, Irene Ruíz De Diego, and Ana Candalija for their expert technical assistance.
This work was supported by grants from the Spanish Ministerios Economía y Competitividad (SAF2013-48532-R) and Sanidad Política Social e Igualdad (PNSD #2012/071, and ISCIII, CIBERNED CB06/05/0055) and from Comunidad de Madrid ref. S2011/BMD-2336. Liliana Martínez Mendieta has a CONACYT-Mexico postdoctoral scholarship (208063).
References
- Albers DS, Sonsalla PK. (1995) Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: pharmacological profile of protective and nonprotective agents. J Pharmacol Exp Ther 275:1104–1114. [PubMed] [Google Scholar]
- Ali SF, Itzhak Y. (1998) Effects of 7-nitroindazole, an NOS inhibitor on methamphetamine-induced dopaminergic and serotonergic neurotoxicity in mice. Ann N Y Acad Sci 844:122–130. [PubMed] [Google Scholar]
- Ares-Santos S, Granado N, Oliva I, O’Shea E, Martin ED, Colado MI, Moratalla R. (2012) Dopamine D(1) receptor deletion strongly reduces neurotoxic effects of methamphetamine. Neurobiol Dis 45:810–820. [DOI] [PubMed] [Google Scholar]
- Ares-Santos S, Granado N, Espadas I, Martinez-Murillo R, Moratalla R. (2014) Methamphetamine causes degeneration of dopamine cell bodies and terminals of the nigrostriatal pathway evidenced by silver staining. Neuropsychopharmacology 39:1066–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baquet ZC, Williams D, Brody J, Smeyne RJ. (2009) A comparison of model-based (2D) and design-based (3D) stereological methods for estimating cell number in the substantia nigra pars compacta (SNpc) of the C57BL/6J mouse. Neuroscience 161:1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AM, Panenka WJ, MacEwan GW, Thornton AE, Lang DJ, Honer WG, Lecomte T. (2006) The need for speed: an update on methamphetamine addiction. J Psychiatry Neurosci 31:301–313. [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Hauser KF. (2014) Glial modulators as potential treatments of psychostimulant abuse. Adv Pharmacol 69:1–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boger HA, Middaugh LD, Patrick KS, Ramamoorthy S, Denehy ED, Zhu H, Pacchioni AM, Granholm AC, McGinty JF. (2007) Long-term consequences of methamphetamine exposure in young adults are exacerbated inglial cell line-derived neurotrophic factor heterozygous mice. J Neurosci 27:8816–8825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan JB, Sparkman NL, Johnson RW. (2010) A neurotoxic regimen of methamphetamine exacerbates the febrile and neuroinflammatory response to a subsequent peripheral immune stimulus. J Neuroinflammation 7:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan RC, Cunningham JK, Sykes J, Kish SJ. (2012) Increased risk of Parkinson’s disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs. Drug Alcohol Depend 120:35–40. [DOI] [PubMed] [Google Scholar]
- Calvo AC, Moreno-Igoa M, Mancuso R, Manzano R, Oliván S, Muñoz MJ, Penas C, Zaragoza P, Navarro X, Osta R. (2011) Lack of a synergistic effect of a non-viral ALS gene therapy based on BDNF and a TTC fusion molecule. Orphanet J Rare Dis 6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaïb-Oukadour I, Gil C, Aguilera J. (2004) The C-terminal domain of the heavy chain of tetanus toxin rescues cerebellar granule neurones from apoptotic death: involvement of phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathways. J Neurochem 90:1227–1236. [DOI] [PubMed] [Google Scholar]
- Chaïb-Oukadour I, Gil C, Rodríguez-Alvarez J, Ortega A, Aguilera J. (2009) Tetanus toxin H(C) fragment reduces neuronal MPP+ toxicity. Mol Cell Neurosci 41:297–303. [DOI] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. (2007) Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction 102:16–32. [DOI] [PubMed] [Google Scholar]
- Chiu HY, Chan MH, Lee MY, Chen ST, Zhan ZY, Chen HH. (2014) Long-lasting alterations in 5-HT2A receptor after a binge regimen of methamphetamine in mice. Int J Neuropsychopharmacol. 17:1647–1658. [DOI] [PubMed] [Google Scholar]
- Ciriza J, Moreno-Igoa M, Calvo AC, Yague G, Palacio J, Miana-Mena FJ, Muñoz MJ, Zaragoza P, Brûlet P, Osta R. (2008) A genetic fusion GDNF-C fragment of tetanus toxin prolongs survival in a symptomatic mouse ALS model. Restor Neurol Neurosci 26:459–465. [PubMed] [Google Scholar]
- Colangelo AM, Alberghina L, Papa M. (2014) Astrogliosis as a therapeutic target for neurodegenerative diseases. Neurosci Lett 565:59–64. [DOI] [PubMed] [Google Scholar]
- Curtin K, Fleckenstein AE, Robison RJ, Crookston MJ, Smith KR, Hanson GR. (2015) Methamphetamine/amphetamine abuse and risk of Parkinson’s disease in Utah: a population-based assessment. Drug Alcohol Depend 146:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke S, Kaye S, McKetin R, Duflou J. (2008) Major physical and psychological harms of methamphetamine use. Drug Alcohol Rev 27:253–262. [DOI] [PubMed] [Google Scholar]
- Darmopil S, Muñetón-Gómez VC, de Ceballos ML, Bernson M, Moratalla R. (2008) Tyrosine hydroxylase cells appearing in the mouse striatum after dopamine denervation are likely to be projection neurones regulated by L-DOPA. Eur J Neurosci 27:580–592. [DOI] [PubMed] [Google Scholar]
- Dean AC, Groman SM, Morales AM, London ED. (2013) An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology 38:259–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deinhardt K1, Salinas S, Verastegui C, Watson R, Worth D, Hanrahan S, Bucci C, Schiavo G. (2006) Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron 52:293–305. [DOI] [PubMed] [Google Scholar]
- Deng X, Cadet JL. (1999) Methamphetamine administration causes overexpression of nNOS in the mouse striatum. Brain Res. 851:254–257. [DOI] [PubMed] [Google Scholar]
- de Olmos JS, Beltramino CA, de Olmos de Lorenzo S. (1994) Use of an amino-cupric-silver technique for the detection of early and semiacute neuronal degeneration caused by neurotoxicants, hypoxia, and physical trauma. Neurotoxicol Teratol 16:545–561. [DOI] [PubMed] [Google Scholar]
- Downey LA, Loftis JM. (2014) Altered energy production, lowered antioxidant potential, and inflammatory processes mediate CNS damage associated with abuse of the psychostimulants MDMA and methamphetamine. Eur J Pharmacol 727:125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElAli A, Urrutia A, Rubio-Araiz A, Hernandez-Jimenez M, Colado MI, Doeppner TR, Hermann DM. (2012) Apolipoprotein-E controls adenosine triphosphate-binding cassette transporters ABCB1 and ABCC1 on cerebral microvessels after methamphetamine intoxication. Stroke. 43:1647–1653. [DOI] [PubMed] [Google Scholar]
- Espadas I, Darmopil S, Vergaño-Vera E, Ortiz O, Oliva I, Vicario-Abejón C, Martín ED, Moratalla R. (2012) L-DOPA-induced increase in TH-immunoreactive striatal neurons in parkinsonian mice: insights into regulation and function. Neurobiol Dis 48:271–281. [DOI] [PubMed] [Google Scholar]
- Eyerman DJ, Yamamoto BK. (2007) A rapid oxidation and persistent decrease in the vesicular monoamine transporter 2 after methamphetamine. J Neurochem 103:1219–1227. [DOI] [PubMed] [Google Scholar]
- Eyo UB, Dailey ME. (2013) Microglia: key elements in neural development, plasticity, and pathology. J Neuroimmune Pharmacol 8:494–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend DM, Fricks-Gleason AN, Keefe KA. (2014) Is there a role for nitric oxide in methamphetamine-induced dopamine terminal degeneration? Neurotox Res 25:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil C, Chaïb-Oukadour I, Aguilera J. (2003) C-terminal fragment of tetanus toxin heavy chain activates Akt and MEK/ERK signalling pathways in a Trk receptor-dependent manner in cultured cortical neurons. Biochem J 373:613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Aparicio R, Moratalla R. (2014) Oleoylethanolamide reduces L-Dopa-induced dyskinesias via TRPV1 receptor in a mouse model of Parkinson′s Disease. Neurobiol Dis 62:416–425. [DOI] [PubMed] [Google Scholar]
- Granado N, Escobedo I, O’Shea E, Colado I, Moratalla R. (2008. a) Early loss of dopaminergic terminals in striosomes after MDMA administration to mice. Synapse 62:80–84. [DOI] [PubMed] [Google Scholar]
- Granado N, O’Shea E, Bove J, Vila M, Colado MI, Moratalla R. (2008. b) Persistent MDMA-induced dopaminergic neurotoxicity in the striatum and substantia nigra of mice. J Neurochem 107:1102–1112. [DOI] [PubMed] [Google Scholar]
- Granado N, Ares-Santos S, O’Shea E, Vicario-Abejón C, Colado MI, Moratalla R. (2010) Selective vulnerability in striosomes and in the nigrostriatal dopaminergic pathway after methamphetamine administration: early loss of TH in striosomes after methamphetamine. Neurotox Res 18:48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granado N, Ares-Santos S, Oliva I, O’Shea E, Martin ED, Colado MI, Moratalla R. (2011. a) Dopamine D2-receptor knockout mice are protected against dopaminergic neurotoxicity induced by methamphetamine or MDMA. Neurobiol Dis 42:391–403. [DOI] [PubMed] [Google Scholar]
- Granado N, Lastres-Becker I, Ares-Santos S, Oliva I, Martin E, Cuadrado A, Moratalla R. (2011. b) Nrf2 deficiency potentiates methamphetamine-induced dopaminergic axonal damage and gliosis in the striatum. Glia 59:1850–1863. [DOI] [PubMed] [Google Scholar]
- Granado N, Ares-Santos S, Moratalla R. (2013) Methamphetamine and Parkinson’s disease. Parkinson′s Dis. 2013:308052. doi: 10.1155/2013/308052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good RL, Liang LP, Patel M, Radcliffe RA. (2011) Mouse strain- and age-dependent effects of binge methamphetamine on dopaminergic signaling. Neurotoxicology 32:751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadamitzky M, Markou A, Kuczenski R. (2011) Extended access to methamphetamine self-administration affects sensorimotor gating in rats. Behav Brain Res 217:386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin LE, Collins SA, Yamamoto BK. (2014) Neurotoxicity of methamphetamine and 3,4-methylenedioxymethamphetamine. Life Sci 97:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrando-Grabulosa M, Casas C, Aguilera J. (2013) The C-terminal domain of tetanus toxin protects motoneurons against acute excitotoxic damage on spinal cord organotypic cultures. J Neurochem 124:36–44. [DOI] [PubMed] [Google Scholar]
- Hurley LL, Tizabi Y. (2013) Neuroinflammation, neurodegeneration, and depression. Neurotox Res 23:131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerdsan W, Thanoi S, Nudmamud-Thanoi S. (2012) Changes in the neuronal glutamate transporter EAAT3 in rat brain after exposure to methamphetamine. Basic Clin Pharmacol Toxicol 111:275–278. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Tokunaga I, Gotohda T, Kubo S. (2007) Immunohistochemical investigation of dopaminergic terminal markers and caspase-3 activation in the striatum of human methamphetamine users. Int J Legal Med 121:163–168. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Bjorklund A. (2013) Trophic factor gene therapy for Parkinson’s disease. Mov Disord 28:96–109. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. (2009) Methamphetamine toxicity and messengers of death. Brain Res Rev. 60:379–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Hodges AB, Ladenheim B, Rhoades R, Phillip CG, Cesena A, Ivanova E, Hohmann CF, Cadet JL. (2009) Methamphetamine treatment causes delayed decrease in novelty-induced locomotor activity in mice. Neurosci Res 65:160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen KE, Benn SC, Ay I, Chian RJ, Celia SA, Remington MP, Bejarano M, Liu M, Ross J, Carmillo P, et al. (2006) A glial cell line-derived neurotrophic factor (GDNF): Tetanus toxin fragment C protein conjugate improves delivery of GDNF to spinal cord motor neurons in mice. Brain Res 1120:1–12. [DOI] [PubMed] [Google Scholar]
- Li J, Chian RJ, Ay I, Kashi BB, Celia SA, Tamrazian E, Pepinsky RB, Fishman PS, Brown RH, Jr, Francis JW. (2009) Insect GDNF:TTC fusion protein improves delivery of GDNF to mouse CNS. Biochem Biophys Res Commun 390:947–951. [DOI] [PubMed] [Google Scholar]
- Lim SA, Kang UJ, McGehee DS. (2014) Striatal cholinergic interneuron regulation and circuit effects. Front Synaptic Neurosci 6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J, Ye W, Alexander M, Dannals RF, Wong DF, Ricaurte GA. (2008) Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse 62:91–100. [DOI] [PubMed] [Google Scholar]
- Mendieta L, Venegas B, Moreno N, Patricio A, Martínez I, Aguilera J, Limón ID. (2009) The carboxyl-terminal domain of the heavy chain of tetanus toxin prevents dopaminergic degeneration and improves motor behavior in rats with striatal MPP(+)-lesions. Neurosci Res 65:98–106. [DOI] [PubMed] [Google Scholar]
- Mendieta L, Bautista E, Sánchez A, Guevara J, Herrando-Grabulosa M, Moran J, Martínez R, Aguilera J, Limón ID. (2012) The C-terminal domain of the heavy chain of tetanus toxin given by intramuscular injection causes neuroprotection and improves the motor behavior in rats treated with 6-hydroxydopamine. Neurosci Res 74:156–167. [DOI] [PubMed] [Google Scholar]
- Miyazaki I, Asanuma M, Kikkawa Y, Takeshima M, Murakami S, Miyoshi K, Sogawa N, Kita T. (2011) Astrocyte-derived metallothionein protects dopaminergic neurons from dopamine quinone toxicity. Glia 59:435–451. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Yamada K. (2011) Pharmacologic treatment with GABA(B) receptor agonist of methamphetamine-induced cognitive impairment in mice. Curr Neuropharmacol 9:109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratalla R, Khairnar A, Simola N, Granado N, García-Montes JR, Porceddu FP, Tizabi Y, Costa Y, Morelli M (2015) Amphetamine-related drugs neurotoxicity in humans and in experimental animals: Main mechanisms. Prog Neurobiol [Epub ahead of print]. doi:10.1016/j.pneurobio.2015.09.011. [DOI] [PubMed] [Google Scholar]
- Moreno-Igoa M, Calvo AC, Penas C, Manzano R, Oliván S, Muñoz MJ, Mancuso R, Zaragoza P, Aguilera J, Navarro X, Osta Pinzolas R. (2010) Fragment C of tetanus toxin, more than a carrier. Novel perspectives in non-viral ALS gene therapy. J Mol Med (Berl) 88:297–308. [DOI] [PubMed] [Google Scholar]
- Moszczynska A, Fitzmaurice P, Ang L, Kalasinsky KS, Schmunk GA, Peretti FJ, Aiken SS, Wickham DJ, Kish SJ. (2004) Why is parkinsonism not a feature of human methamphetamine users? Brain 127:363–370. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP, Sriram K, Miller DB. (2008) Defining “neuroinflammation.” Ann N Y Acad Sci. 1139:318–330. [DOI] [PubMed] [Google Scholar]
- Ortiz O, Delgado-García JM, Espadas I, Bahí A, Trullas R, Dreyer JL, Gruart A, Moratalla R. (2010) Associative learning and CA3-CA1 synaptic plasticity are impaired in D1R null, Drd1a-/- mice and in hippocampal siRNA silenced Drd1a mice. J Neurosci 30:12288–12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett CL, Lalive AL, Tan KR, Terunuma M, Munoz MB, Pangalos MN, Martínez-Hernández J, Watanabe M, Moss SJ, Luján R, Lüscher C, Slesinger PA. (2012) Methamphetamine-evoked depression of GABA(B) receptor signaling in GABA neurons of the VTA. Neuron 73:978–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patricio-Martínez A, Mendieta L, Martínez I, Aguilera J, Limón ID. (2016) The recombinant C-terminal fragment of tetanus toxin protects against cholinotoxicity by intraseptal injection of β-amyloid peptide (25–35) in rats. Neuroscience 315:18–30. [DOI] [PubMed] [Google Scholar]
- Payne AM, Zheng Z, Messi ML, Milligan CE, Gonzalez E, Delbono O. (2006) Motor neurone targeting of IGF-1 prevents specific force decline in ageing mouse muscle. J. Physiol 570:283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Wilhelmsson U, Pekna M. (2014) The dual role of astrocyte activation and reactive gliosis. Neurosci Lett 565:30–38. [DOI] [PubMed] [Google Scholar]
- Radenovic L, Selakovic V, Olivan S, Calvo AC, Rando A, Janac B, Osta R. (2014) Neuroprotective efficiency of tetanus toxin C fragment in model of global cerebral ischemia in Mongolian gerbils. Brain Res Bull 101:37–44. [DOI] [PubMed] [Google Scholar]
- Raineri M, González B, Rivero-Echeto C, Muñiz JA, Gutiérrez ML, Ghanem CI, Cadet JL, García-Rill E, Urbano FJ, Bisagno V. (2015) Differential effects of environment-induced changes in body temperature on modafinil’s actions against methamphetamine-induced striatal toxicity in mice. Neurotox Res 27:71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricaurte GA, Guillery RW, Seiden LS, Schuster CR, Moore RY. (1982) Dopamine nerve terminal degeneration produced by high doses of methylamphetamine in the rat brain. Brain Res 235:93–103. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Seiden LS, Schuster CR. (1984) Further evidence that amphetamines produce long-lasting dopamine neurochemical deficits by destroying dopamine nerve fibers. Brain Res 303:359–364. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, McCann UD. (1992) Neurotoxic amphetamine analogues: effects in monkeys and implications for humans. Ann N Y Acad Sci 11:371–382. [DOI] [PubMed] [Google Scholar]
- Rosenblad C, Kirik D, Björklund A. (2000) Sequential administration of GDNF into the substantia nigra and striatum promotes dopamine neuron survival and axonal sprouting but not striatal reinnervation or functional recovery in the partial 6-OHDA lesion model. Exp Neurol 161:503–516. [DOI] [PubMed] [Google Scholar]
- Ruiz-DeDiego I, Mellstrom B, Vallejo M, Naranjo JR, Moratalla R. (2015) Activation of DREAM (downstream regulatory element antagonistic modulator), a calcium-binding protein, reduces L-DOPA-induced dyskinesias in mice. Biol Psychiatry 77:95–105. [DOI] [PubMed] [Google Scholar]
- Rusyniak DE. (2011) Neurologic manifestations of chronic methamphetamine abuse. Neurologic Clin 29:641–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore MF. (2014) Ser31 tyrosine hydroxylase phosphorylation parallels differences in dopamine recovery in nigrostriatal pathway following 6-OHDA lesion. J. Neurochem 129:548–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-González A, Mendieta L, Palafox V, Candalija A, Luna F, Aguilera J, Limón ID. (2014) The restorative effect of intramuscular injection of tetanus toxin C-fragment in hemiparkinsonian rats. Neurosci Res 84:1–9. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. (2007) Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev 17:275–297. [DOI] [PubMed] [Google Scholar]
- Shen H, Mohammad A, Ramroop J, Smith SS. (2013) A stress steroid triggers anxiety via increased expression of α4βδ GABAA receptors in methamphetamine dependence. Neuroscience 254:452–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva CD, Neves AF, Dias AI, Freitas HJ, Mendes SM, Pita I, Viana SD, de Oliveira PA, Cunha RA, Fontes Ribeiro CA, Prediger RD, Pereira FC. (2014) A single neurotoxic dose of methamphetamine induces a long-lasting depressive-like behaviour in mice. Neurotox Res 25:295–304. [DOI] [PubMed] [Google Scholar]
- Simões PF, Silva AP, Pereira FC, Marques E, Grade S, Milhazes N, Borges F, Ribeiro CF, Macedo TR. (2007) Methamphetamine induces alterations on hippocampal NMDA and AMPA receptor subunit levels and impairs spatial working memory. Neuroscience 150:433–441. [DOI] [PubMed] [Google Scholar]
- Singh S, Ahmad R, Mathur D, Kumar Sagar R, Krishana B. (2006) Neuroprotective effect of BDNF in young and aged 6-OHDA treated rat model of Parkinson disease. Ind J Exp Biol 44:699–704. [PubMed] [Google Scholar]
- Solís O, Espadas I, Del-Bel EA, Moratalla R. (2015) Nitric oxide synthase inhibition decreases l-DOPA-induced dyskinesia and the expression of striatal molecular markers in Pitx3(-/-) aphakia mice. Neurobiol Dis 73:49–59. [DOI] [PubMed] [Google Scholar]
- Suárez LM, Solís O, Caramés JM, Taravini IR, Solís JM, Murer MG, Moratalla R. (2014) L-DOPA treatment selectively restores spine density in dopamine receptor D2-expressing projection neurons in dyskinetic mice. Biol Psychiatry 75:711–722. [DOI] [PubMed] [Google Scholar]
- Switzer RC., III (2000) Application of silver degeneration stains for neurotoxicity testing. Toxicol Pathol 28:70–83. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Dowgiert J, Geddes TJ, Francescutti-Verbeem D, Liu X, Kuhn DM. (2004) Microglial activation is a pharmacologically specific marker for the neurotoxic amphetamines. Neurosci Lett 367:349–354. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM. (2008) The newly synthesized pool of dopamine determines the severity of methamphetamine-induced neurotoxicity. J Neurochem 105:605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Kuhn DM. (2005) MK-801 and dextromethorphan block microglial activation and protect against methamphetamine-induced neurotoxicity. Brain Res 1050:190–198. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Hurley LL, Qualls Z, Akinfiresoye L. (2014) Relevance of the anti-inflammatory properties of curcumin in neurodegenerative diseases and depression. Molecules 19:20864–20879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toivonen JM, Oliván S, Osta R. (2010) Tetanus toxin C-fragment: the courier and the cure? Toxins (Basel) 2:2622–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNODC World Drug Report 2014. (United Nations publication, Sales No. E.14.XI.7). Available at http://www.unodc.org/documents/wdr2014/World_Drug_Report_2014_web.pdf.
- Urrutia A, Rubio-Araiz A, Gutierrez-Lopez MD, ElAli A, Hermann DM, O’Shea E, Colado MI. (2013) A study on the effect of JNK inhibitor, SP600125, on the disruption of blood-brain barrier induced by methamphetamine. Neurobiol Dis. 50:49–58. [DOI] [PubMed] [Google Scholar]
- Urrutia A, Granado N, Gutierrez-Lopez MD, Moratalla R, O’Shea E, Colado MI. (2014) The JNK inhibitor, SP600125, potentiates the glial response and cell death induced by methamphetamine in the mouse striatum. Int J Neuropsychopharmacol 17:235–246. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. (2001) Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry 158:377–382. [DOI] [PubMed] [Google Scholar]
- Wang J, Xu W, Ali SF, Angulo JA. (2008) Connection between the striatal neurokinin-1 receptor and nitric oxide formation during methamphetamine exposure. Ann N Y Acad Sci 1139:164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. (1996) Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med 2:699–703. [DOI] [PubMed] [Google Scholar]
- Xu W, Zhu JP, Angulo JA. (2005) Induction of striatal pre- and postsynaptic damage by methamphetamine requires the dopamine receptors. Synapse 58:110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto BK, Moszczynska A, Gudelsky GA. (2010) Amphetamine toxicities: classical and emerging mechanisms. Ann N Y Acad Sci 1187:101–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Jin Y, Liu X, Yang L, Ge Z, Wang H, Li J, Zheng J. (2014) Methamphetamine modulates glutamatergic synaptic transmission in rat primary cultured hippocampal neurons. Brain Res 1582:1–11. [DOI] [PubMed] [Google Scholar]
- Zhang X, Lee TH, Xiong X, Chen Q, Davidson C, Wetsel WC, Ellinwood EH. (2006) Methamphetamine induces long-term changes in GABAA receptor alpha2 subunit and GAD67 expression. Biochem Biophys Res Commun 351:300–305. [DOI] [PubMed] [Google Scholar]