Abstract
Background:
Filorexant (MK-6096) is an orexin receptor antagonist; here, we evaluate the efficacy of filorexant in the treatment of insomnia in adults.
Methods:
A double-blind, placebo-controlled, randomized, two 4-week–period, adaptive crossover polysomnography study was conducted at 51 sites worldwide. Patients (18 to <65 years) with insomnia received 1 of 4 doses of oral filorexant (2.5, 5, 10, 20mg) once daily at bedtime during one period and matching placebo in the other period in 1 of 8 possible treatment sequences. Polysomnography was performed on night 1 and end of week 4 of each period. The primary endpoint was sleep efficiency at night 1 and end of week 4. Secondary endpoints included wakefulness after persistent sleep onset and latency to onset of persistent sleep.
Results:
A total of 324 patients received study treatment, 315 received ≥1 dose of placebo, and 318 ≥1 dose of filorexant (2.5mg, n=79; 5mg, n=78; 10mg, n=80; 20mg, n=81). All filorexant doses (2.5/5/10/20mg) were significantly superior to placebo in improving sleep among patients with insomnia as measured by sleep efficiency and wakefulness after persistent sleep onset on night 1 and end of week 4. The 2 higher filorexant doses (10/20mg) were also significantly more effective than placebo in improving sleep onset as measured by latency to onset of persistent sleep at night 1 and end of week 4. Filorexant was generally well tolerated.
Conclusions:
Orexin receptor antagonism by filorexant significantly improved sleep efficiency in nonelderly patients with insomnia. Dose-related improvements in sleep onset and maintenance outcomes were also observed with filorexant.
Keywords: dose-ranging study, filorexant, insomnia, orexin receptor antagonist, sleep efficiency
Clinical Trial Registry: clinicaltrials.gov: NCT01021852.
Introduction
Insomnia, defined as difficulty in initiating or maintaining sleep despite adequate opportunity for sleep, affects between 10% and 30% of the population overall and is associated with significant impairments in health, productivity, and quality of life (Mai and Buysse, 2008; Schutte-Rodin et al., 2008; Sarsour et al., 2011; Ishak et al., 2012; Morin and Benca, 2012). Recent data also suggest that patients with chronic insomnia may be hyperaroused, experiencing increased metabolic rate, body temperature, and heart rate and elevated levels of norepinephrine and catecholamines (Bonnet and Arand, 2010). For several decades, the pharmacological treatment of insomnia has been dominated by benzodiazepine receptor agonists; however, although effective at promoting sleep onset and less frequently sleep maintenance, these agents have limitations for many patients, including next-day residual effects and dependence in at-risk populations. Furthermore, for a sizeable proportion of patients with insomnia, the condition often goes unrecognized and untreated (Morin and Benca, 2012).
The orexin (hypocretin) signaling system originates in the lateral hypothalamus and is a central promoter of wakefulness and arousal. In preclinical studies, genetic depletion of orexin signaling resulted in the development of narcoleptic phenotypes (Lin et al., 1999; Sakurai et al., 2010; Gotter et al., 2012), and specific pharmacological blockade of orexin OX1 and OX2 receptors was associated with the development of somnolence (Brisbare-Roch et al., 2007; Winrow et al., 2011). Several orexin receptor antagonists (ORAs) have been developed, with similar affinities for the 2 orexin receptors, OX1 and OX2 (Winrow and Renger, 2014). In proof of concept studies, the ORAs suvorexant (MK-4305, Belsomra) administered daily for 4 weeks (Herring et al., 2012), almorexant (singe-dose) (Hoever et al., 2012), and SB649868 (single-dose) (Bettica et al., 2012) were shown to be effective and well tolerated for the treatment of sleep disturbances in adult patients with insomnia. Suvorexant also significantly improved sleep onset and maintenance in phase 3 studies in patients with insomnia and has recently been approved in the US and Japan for the treatment of insomnia characterized by difficulties with sleep onset and/or maintenance (Belsomra (suvorexant) Package Insert 2014; Michelson et al., 2014; Herring et al., 2016).
Filorexant (MK-6096) is a dual ORA with a relatively short half-life (t½; 3–6 hours). In preclinical studies in rodents and dogs, filorexant increased nonrapid eye movement (REM) and REM sleep (Coleman et al., 2012; Winrow et al., 2012). Filorexant has also demonstrated sleep-promoting effects in a polysomnography (PSG) study in healthy subjects (Sun et al., 2011a). Based on these findings and its relatively short t½, which suggests potential for a low incidence of next-day residual effects compared with placebo, filorexant was considered a suitable candidate for further clinical evaluation in the treatment of insomnia. Here, we report the results of a phase 2b dose-ranging study evaluating filorexant for the treatment of insomnia in adults.
Methods
Study Design and Treatment
This was a double-blind, placebo-controlled, randomized, 2-period, adaptive crossover PSG study conducted between December 2009 and February 2011 at 51 sites within the US, Germany, United Kingdom, Spain, Finland, and Japan ( clinicaltrials.gov: NCT01021852; Merck protocol 6096-011). The study comprised a 3-week screening period and two 4-week, double-blind treatment periods separated by an intervening 2-week washout period (comprising 3 days of single-blind placebo followed by 11 days off study treatment) (supplementary Figure 1).
Each patient received 1 of 4 doses of oral filorexant (2.5, 5, 10, or 20mg) once daily at bedtime during one period and matching placebo in the other period in 1 of 8 possible treatment sequences. Each treatment period comprised 29±3 days of treatment and included an overnight PSG visit on the first and last nights of the treatment period, with a clinic office visit on day 15. Filorexant or matching placebo was administered approximately 30 minutes before bedtime on PSG nights and immediately (within 5 to 10 minutes) before bedtime on non-PSG nights. On the day following a PSG night, patients were awakened (or allowed to get out of bed if already awake) after 8 hours of PSG recording. Patients completed an electronic sleep diary each morning and evening.
Patients
Men and women (aged 18 to <65 years) were eligible for inclusion in the study if they had a diagnosis of primary insomnia (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision criteria) and reported, on at least 3 of 7 nights each week during the 4 weeks prior to the start of screening (day -21): a total sleep time (TST) of ≤6.5 hours, sleep latency of ≥30 minutes, ≥1 hour of wakefulness after sleep onset (WASO), and spending 6.5 to 9 hours in bed each night. Patients were also required to have a regular bedtime between 9 pm and 12 am and be willing to refrain from napping during the study, limit their alcohol consumption to 2 drinks/d (at least 3 hours before going to bed) on non-PSG visit days, refrain from drinking alcohol at least 24 hours before a PSG visit, and limit their caffeine consumption to ≤600mg caffeine/d. In addition, patients were required to have latency to onset of persistent sleep (LPS) >20 minutes on both the screening PSG (day -14) and baseline PSG (day -7) nights, and mean WASO ≥60 minutes on the combined screening and baseline PSG nights where neither night is ≤45 minutes. Patients were excluded from the study if they had evidence of ongoing depression, history of bipolar disorder or a psychotic disorder, or other concomitant medical conditions, including a history or diagnosis of narcolepsy, idiopathic cataplexy, circadian rhythm sleep disorder, parasomnia, or sleep-related breathing disorder.
All patients were required to give written informed consent prior to inclusion in the study. The study protocol was approved by the relevant International Review Board/Independent Ethics Committee at each participating center and was conducted in accordance with the standards established by the Declaration of Helsinki and in compliance with all local and/or national regulations and directives.
Investigational medications, fluoxetine, and specific moderate and strong cytochrome P450 3A inhibitors and specific strong cytochrome P450 3A inducers (supplementary Table 1) were withdrawn at least 4 weeks before the screening visit.
Efficacy Endpoints
The primary endpoint was mean change from baseline in sleep efficiency (SE), derived from TST as a percentage of total time in bed (fixed at 8 hours for this study), on both night 1 and end of week 4, as measured by PSG. Secondary endpoints (measured by PSG) were mean change from baseline in WASO and LPS both on night 1 and end of week 4. Sleep stage scoring was performed visually in 30-second epochs by a central reader (Rechtschaffen A, Kales A, eds., 1968; Iber et al., 2007). Exploratory endpoints evaluated other PSG sleep parameters (TST, number of awakenings) and the sleep architecture of filorexant compared with placebo, as measured by duration and percentages in stages 1, 2, 3, 4 (stages 3 and 4 were combined as slow wave sleep) and REM sleep on night 1 and week 4.
Exploratory subjective sleep measures included subjective TST (sTST) and subjective time to sleep onset (sTSO), as reported by patients in electronic morning diaries; and patient-reported insomnia, as measured by the Insomnia Severity Index (ISI), freshness (sFresh), and quality of sleep (sQual) visual analog scales. Next-day function measures included the patient-reported Sheehan Disability Scale (SDS).
Safety and Tolerability
Safety was assessed through the collection of adverse event (AE) data and routine laboratory tests performed at clinic visits (including hematology, chemistry, and urinalysis), electrocardiography, and vital signs assessments. AEs of clinical interest included cataplexy, hypnagogic/hypnopompic hallucinations, sleep paralysis, sleep-onset paralysis, falls, excessive daytime sleepiness, complex sleep-related behaviors, selected events associated with potential for abuse, and suicidal ideation and/or behaviors.
Next-day residual effects were assessed using the Digit Symbol Substitution Test (DSST) performed in the morning (within 30 minutes to 1 hour following lights on) after each PSG assessment (night 1 and week 4). Acute withdrawal effects were measured based on analysis of patients reporting 3 or more emergent or worsening symptoms of 20 in a single day on the Tyrer Withdrawal Symptom Questionnaire (WSQ) (Tyrer et al., 1990) completed for the first 3 nights of the single-blind washout period, or 3 or more symptoms across the first 3 days, after the end of treatment period 1. Rebound insomnia was measured using sTSO and sTST assessments for each of the first 3 nights of the washout period. The Columbia Suicide Severity Rating Scale was administered by trained raters at baseline prior to treatment period 1 and at each visit during periods 1 and 2 to provide a detailed assessment of suicidal ideation and behaviors.
Statistical Methods
Based on a planned sample size of 272 patients (68 in each filorexant dose group) completing Periods 1 and 2, the study had approximately 99% power to detect a difference of 8.33 percentage points in SE at both time points (night 1 and end of week 4) for a particular filorexant dose (a difference of 8.33 percentage points in SE corresponds to a 40-minute difference in TST when time in bed is fixed at 8 hours).
Efficacy analyses were conducted using the full analysis set population (all randomized patients who received at least one dose of study treatment and had a post-dose assessment of the primary efficacy measure in either treatment period). Patients were analyzed according to the treatment sequence to which they were randomized for the efficacy analyses.
The primary (SE) and secondary endpoints (WASO and LPS) at night 1 and the end of 4 weeks of treatment were compared between each dose of filorexant and placebo using a fixed-effects repeated-measures model including terms for baseline value (prerandomization), geographic region (Japan vs ex-Japan), gender, treatment, sequence, period, time (as a categorical variable), and treatment-by-time and period-by-time interactions for each dose. An unstructured covariance matrix was used for within-subject correlation assuming independence for between-subject correlation. The model was used to provide an estimate of treatment effect for the comparison of each filorexant dose with placebo. Least-squares mean differences between filorexant and placebo with 95% CI and P values (based upon a normal approximation) were computed. Exploratory efficacy analyses for continuous endpoints were evaluated using a similar model. To account for the multiple dose comparisons to placebo for the primary efficacy hypothesis, a fixed sequential testing procedure was used to assess statistical significance at both timepoints (night 1 and week 4), starting with the highest filorexant dose. Since both night 1 and week 4 results had to be positive for each endpoint and filorexant dose comparison with placebo, no adjustment was required for multiple timepoints. Filorexant doses that were statistically significant for the primary endpoint (at both timepoints) were tested in a similar fashion for the first secondary endpoint (WASO), and filorexant doses that were statistically significant for both SE and WASO (at both timepoints) were tested in a similar fashion for the second secondary endpoint (LPS). The same mixed effects model used to evaluate primary and secondary endpoints was used to evaluate DSST. WSQ was assessed via point estimates with 95% CIs provided for comparisons of treatment vs placebo.
A prespecified interim analysis was conducted when approximately 50% of patients had completed the study to evaluate for futility and also to determine if evaluation of a lower (1mg) or higher (40mg) dose of filorexant was warranted.
The all-patients-as-treated population was used for safety analyses (all randomized patients who received at least one dose of study treatment).
Results
Patients
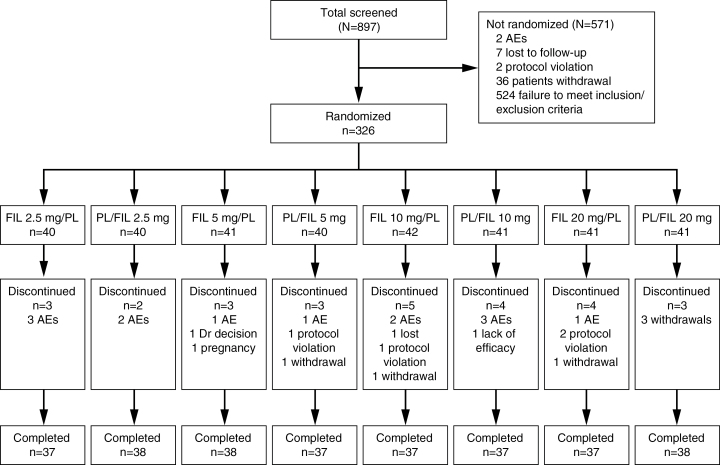
A total of 326 patients were randomized to treatment, and 2 patients discontinued from the study without taking study drug. Of the 324 patients who received study drug, 315 received at least 1 dose of placebo and 318 received at least 1 dose of filorexant (2.5mg, n=79; 5mg, n=78; 10mg, n=80; 20mg, n=81). Figure 1 shows patient disposition by treatment sequence. A total of 299 patients completed the study and 27 discontinued, primarily due to AEs (n=13).
Figure 1.
Patient disposition. AE, adverse event; FIL, filorexant; PL, placebo.
Patient baseline characteristics were similar across treatment groups (Table 1). Of the 324 patients treated, 62.3% were female, 68.8% were white, and mean age was 46.9 years.
Table 1.
Patient Demography and Baseline Characteristics
| Filorexant 2.5mg (n=79)a | Filorexant 5mg (n=78)a | Filorexant 10mg (n=80)a | Filorexant 20mg (n=81)a | Placebo (n=315)a | |
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Male | 30 (38.0) | 29 (37.2) | 30 (37.5) | 31 (38.3) | 117 (37.1) |
| Female | 49 (62.0) | 49 (62.8) | 50 (62.5) | 50 (61.7) | 198 (62.9) |
| Age, mean (SD), years | 47.3 (11.8) | 46.4 (10.6) | 45.4 (10.5) | 48.3 (11.2) | 46.9 (10.8) |
| BMI, mean (SD), kg/m2 | 25.5 (4.6) | 26.1 (3.8) | 25.9 (4.5) | 25.6 (4.0) | 25.7 (4.2) |
| Race | |||||
| White | 54 (68.4) | 54 (69.2) | 51(63.8) | 62 (76.5) | 216 (68.6) |
| Black | 10 (12.7) | 8 (10.3) | 9 (11.3) | 5 (6.2) | 33 (10.5) |
| Asian | 14 (17.7) | 14 (17.9) | 18 (22.5) | 12 (14.8) | 58 (18.4) |
| Otherb | 1 (1.3) | 2 (2.6) | 2 (2.5) | 2 (2.5) | 8 (2.5) |
| ISI | |||||
| Total score | 15.7 (4.7) | 15.7 (4.9) | 14.9 (4.5) | 15.1 (3.7) | 15.3 (4.5) |
| PSG sleep | |||||
| SE, % | 64.8 (11.0) | 65.5 (13.4) | 67.6 (13.1) | 65.0 (15.1) | 65.9 (12.8) |
| WASO, min | 112.1 (42.7) | 105.1 (47.1) | 101.1 (50.4) | 114.1 (54.0) | 106.8 (46.3) |
| LPS, min | 64.1 (33.7) | 67.0 (46.6) | 63.0 (37.0) | 61.2 (43.1) | 64.3 (40.3) |
| TST, min | 310.9 (52.8) | 314.3 (64.1) | 324.4 (63.0) | 312.0 (72.3) | 316.1 (61.6) |
| NAW | 13.8 (6.1) | 13.3 (7.0) | 14.0 (5.8) | 13.7 (6.6) | 13.8 (6.4) |
| Sleep architecture, min | |||||
| Stage 1 | 38.7 (20.5) | 38.3 (19.8) | 44.6 (20.7) | 41.6 (22.8) | 40.9 (21.2) |
| Stage 2 | 183.1 (44.4) | 192.4 (50.3) | 188.7 (48.0) | 182.3 (52.0) | 186.9 (48.4) |
| Stages 3+4 (SWS) | 41.5 (26.1) | 32.7 (25.4) | 38.5 (29.4) | 41.2 (35.4) | 38.6 (29.4) |
| REM | 55.1 (21.3) | 56.1 (25.3) | 59.5 (23.7) | 54.8 (24.1) | 56.6 (23.3) |
| Latency to REM | 90.7 (63.8) | 80.0 (55.1) | 87.3 (61.6) | 87.1 (60.8) | 84.7 (58.3) |
| Subjective sleep, min | |||||
| sTSO | 64.1 (31.5) | 67.5 (36.5) | 63.6 (32.0) | 63.1 (40.7) | 64.4 (34.1) |
| sTST | 322.2 (58.3) | 326.1 (56.8) | 330.8 (57.1) | 318.1 (59.0) | 323.7 (59.0) |
| SDS | |||||
| Total score | 9.8 (8.1) | 9.4 (8.5) | 10.1 (7.7) | 8.8 (6.4) | 9.5 (7.7) |
| Work | 3.2 (2.8) | 3.1 (2.7) | 3.3 (2.5) | 3.1 (2.3) | 3.2 (2.6) |
| Social | 2.7 (2.5) | 2.7 (2.8) | 3.1 (2.6) | 2.8 (2.3) | 2.8 (2.5) |
| Family | 3.0 (2.7) | 2.9 (2.7) | 3.4 (2.7) | 2.9 (2.4) | 3.0 (2.6) |
| Residual effects | |||||
| DSST, correct | 57.7 (14.2) | 56.3 (14.8) | 59.3 (13.7) | 54.7 (13.5) | 57.3 (14.3) |
Abbreviations: BMI, body mass index; DSST, Digit Symbol Substitution Test; ISI, Insomnia Severity Index; LPS, latency to persistent sleep; NAW, number of awakenings; PSG, polysomnography; REM, rapid eye movement; SDS, Sheehan Disability Scale; SE, sleep efficiency; sTSO, subjective time to sleep onset; sTST, subjective total sleep time; SWS, slow-wave sleep; TSO, time to sleep onset; TST, total sleep time; WASO, wakefulness after sleep onset.
an = all patients treated; baseline means for efficacy measures based upon patients included in analyses at night 1 for PSG and DSST endpoints, Week 1 for subjective sleep endpoints, and week 4 for ISI and SDS.
bMultiracial or American Indian/Alaskan native.
Efficacy
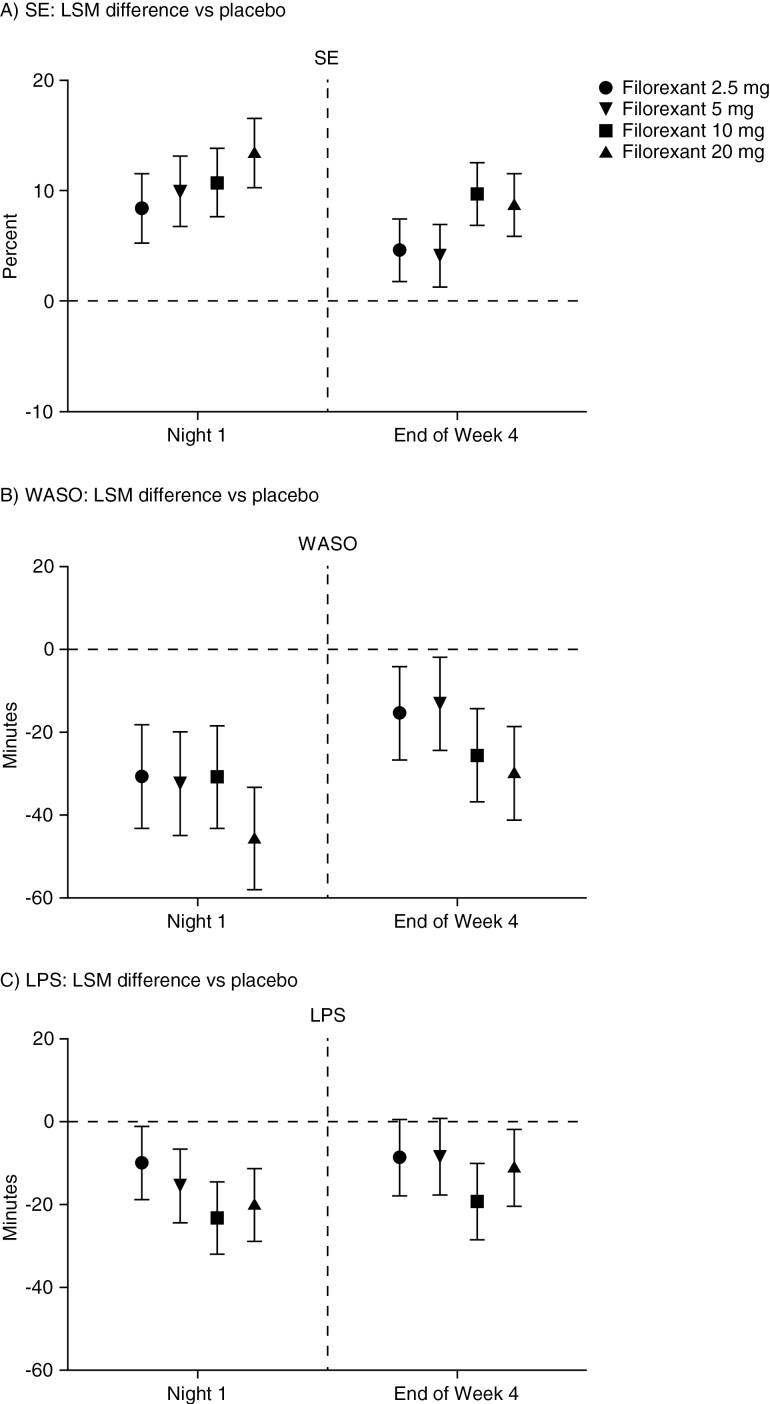
All doses of filorexant (2.5, 5, 10, and 20mg) were significantly more effective than placebo in improving sleep as measured by the primary endpoint of SE both on night 1 and at the end of week 4. Mean change in SE from baseline was 18.3% to 25.0% with filorexant vs 10.2% with placebo on night 1, and 16.8% to 22.4% with filorexant vs 12.5% with placebo at week 4 (Table 2). The differences in least-squares means for SE between filorexant and placebo at night 1 and week 4 were statistically significantly in favor of filorexant for all doses (night 1, P<.001; week 4, P≤.004), with an indication of a dose-response effect at night 1 (Figure 2).
Table 2.
Summary of Primary and Secondary Efficacy Endpoints: SE, WASO, and LPS (Full Analysis Set)
| Endpoint | n | Baseline | Treatment | Change from Baseline | |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | 95% CI | ||
| SE (%) | |||||
| Night 1 | |||||
| Placebo | 313 | 65.9 (12.8) | 76.1 (13.3) | 10.2 (15.1) | 8.6, 11.9 |
| Filorexant 2.5 mg | 79 | 64.8 (11.0) | 83.1 (9.9) | 18.3 (11.9) | 15.6, 21.0 |
| Filorexant 5 mg | 78 | 65.5 (13.4) | 85.8 (9.0) | 20.3 (16.3) | 16.6, 24.0 |
| Filorexant 10 mg | 80 | 67.6 (13.1) | 87.5 (7.7) | 19.9 (12.1) | 17.2, 22.6 |
| Filorexant 20 mg | 80 | 65.0 (15.1) | 90.0 (8.6) | 25.0 (14.0) | 21.9, 28.1 |
| Week 4 | |||||
| Placebo | 300 | 65.7 (12.9) | 78.2 (12.6) | 12.5 (15.3) | 10.7, 14.2 |
| Filorexant 2.5 mg | 76 | 64.5 (11.1) | 81.3 (11.5) | 16.8 (11.2) | 14.2, 19.4 |
| Filorexant 5 mg | 76 | 65.6 (13.5) | 84.5 (8.9) | 19.0 (15.7) | 15.4, 22.5 |
| Filorexant 10 mg | 76 | 68.0 (12.1) | 85.9 (9.0) | 17.9 (13.2) | 14.9, 20.9 |
| Filorexant 20 mg | 75 | 65.7 (14.7) | 88.1 (7.3) | 22.4 (14.8) | 19.0, 25.9 |
| WASO (mins) | |||||
| Night 1 | |||||
| Placebo | 313 | 106.8 (46.3) | 78.9 (53.3) | -27.9 (57.3) | -34.3, -21.6 |
| Filorexant 2.5 mg | 79 | 112.1 (42.7) | 54.8 (40.7) | -57.3 (44.6) | -67.3, -47.3 |
| Filorexant 5 mg | 78 | 105.1 (47.1) | 47.4 (34.5) | -57.6 (54.7) | -70.0, -45.3 |
| Filorexant 10 mg | 80 | 101.1 (50.4) | 40.7 (28.7) | -60.5 (43.4) | -70.1, -50.8 |
| Filorexant 20 mg | 80 | 114.1 (54.0) | 35.0 (31.0) | -79.1 (55.9) | -91.6, -66.7 |
| Week 4 | |||||
| Placebo | 300 | 107.7 (46.5) | 72.1 (46.8) | -35.6 (56.9) | -42.1, -29.1 |
| Filorexant 2.5 mg | 76 | 112.7 (43.2) | 62.2 (49.7) | -50.5 (45.1) | -60.8, -40.2 |
| Filorexant 5 mg | 76 | 104.0 (46.7) | 52.6 (36.8) | -51.5 (51.1) | -63.1, -39.8 |
| Filorexant 10 mg | 76 | 98.7 (40.6) | 47.9 (38.4) | -50.8 (47.8) | -61.7, -39.9 |
| Filorexant 20 mg | 75 | 114.3 (55.2) | 41.2 (27.8) | -73.2 (53.6) | -85.5, -60.8 |
| LPS (mins) | |||||
| Night 1 | |||||
| Placebo | 313 | 64.3 (40.3) | 41.5 (38.2) | -22.8 (48.4) | -28.2, -17.4 |
| Filorexant 2.5 mg | 79 | 64.1 (33.7) | 30.6 (25.7) | -33.5 (39.1) | -42.3, -24.8 |
| Filorexant 5 mg | 78 | 67.0 (46.6) | 26.1 (24.0) | -40.9 (44.4) | -50.9, -30.9 |
| Filorexant 10 mg | 80 | 63.0 (37.0) | 22.7 (23.2) | -40.3 (39.5) | -49.1, -31.5 |
| Filorexant 20 mg | 80 | 61.2 (43.1) | 17.2 (20.2) | -44.0 (39.1) | -52.7, -35.3 |
| Week 4 | |||||
| Placebo | 300 | 63.9 (40.6) | 37.3 (41.1) | -26.6 (51.7) | -32.5, -20.7 |
| Filorexant 2.5 mg | 76 | 65.0 (34.0) | 33.0 (26.6) | -32.0 (38.1) | -40.7, -23.3 |
| Filorexant 5 mg | 76 | 67.3 (47.2) | 25.3 (23.7) | -42.0 (51.1) | -53.7, -30.3 |
| Filorexant 10 mg | 76 | 63.6 (37.6) | 24.1 (19.2) | -39.6 (38.6) | -48.4, -30.7 |
| Filorexant 20 mg | 75 | 57.7 (36.5) | 20.9 (27.5) | -36.8 (42.5) | -46.6, -27.1 |
Abbreviations: CI, confidence interval; LPS, latency to onset of persistent sleep; SD, standard deviation; SE, sleep efficiency; WASO, wakefulness after persistent sleep onset.
Figure 2.
Difference in least-squares means between filorexant and placebo at night 1 and week 4 for (A) sleep efficiency (SE), (B) wakefulness after sleep onset (WASO), and (C) latency to persistent sleep (LPS). Footnote (A): night 1, P<.001 for all doses; week 4, P≤.001 for filorexant 2.5, 10, and 20mg and P=.004 for filorexant 5mg. Footnote (B): night 1, P<.001 for all doses; week 4, P=.006 for filorexant 2.5mg, P=.020 for filorexant 5mg, P<.001 for filorexant 10 and 20mg. Footnote (C): night 1, P=.022 for filorexant 2.5mg, P<.001 for filorexant 5, 10, and 20mg; week 4, P=.055 for filorexant 2.5mg, P=.060 for filorexant 5mg, P<.001 for filorexant 10mg, P=.015 for filorexant 20mg. LPS, latency to persistent sleep; LSM, least-squares mean; SE, sleep efficiency; WASO, wakefulness after sleep onset.
All doses of filorexant (2.5, 5, 10, and 20mg) were also significantly more effective than placebo in improving sleep maintenance as measured by the secondary endpoint of WASO on night 1 and at the end of week 4. Results for the secondary efficacy endpoint of LPS were also statistically significantly in favor of the 10- and 20-mg filorexant doses on night 1 and at the end of week 4. While results for the lower filorexant doses (2.5 and 5mg) suggested beneficial effects at night 1 and week 4 for LPS (nominal P values ≤ .06), these were not considered statistically significant according to the multiplicity strategy (Table 2; Figure 2).
Placebo-subtracted results for exploratory objective PSG and sleep architecture measures are summarized in Table 3. All doses of filorexant showed improvements from baseline in TST compared with placebo at night 1 and week 4, ranging from 80.6 to 120.1 minutes (vs 48.6 and 59.3 minutes with placebo). Filorexant generally had no notable effect on number of awakenings after onset of persistent sleep (Table 3). Analysis of sleep architecture endpoints generally showed numerical increases in time spent in most sleep stages (stage 1, stage 2, slow-wave sleep, and REM sleep) with filorexant vs placebo. However, increases in TST compared with placebo were mainly due to increases in the durations of stage 2 and REM sleep. When the percentage of time in each sleep stage (rather than minutes in each stage) was analyzed, only the percentage of REM sleep showed nominally significant increases compared with placebo for most of the filorexant doses (night 1: 2.5% to 3.3%; week 4: -0.8% to 2.1%). In terms of latency to REM sleep, numerically shorter latencies (reductions of 5.5 to 31.7 minutes vs placebo) were observed for most filorexant doses compared with placebo. All differences were nominally significant at night 1 with the exception of the 2.5-mg dose; however, the only difference that was nominally significant at week 4 was 20mg.
Table 3.
Summary of Exploratory Efficacy Endpoints (Full Analysis Set)a
| PSG endpoints a | Night 1 | Week 4 | ||||||
|---|---|---|---|---|---|---|---|---|
| 2.5 mg | 5 mg | 10 mg | 20 mg | 2.5 mg | 5 mg | 10 mg | 20 mg | |
| TST, min | 40.4 (25.4, 55.3)b | 48.1 (33.1, 63.1)b | 53.1 (38.3, 67.9)b | 64.2 (49.4, 78.9)b | 23.4 (9.9, 37.0)b | 19.9 (6.4, 33.5)c | 45.6 (32.1, 59.2)b | 42.2 (28.6. 55.9)b |
| NAW | 0.1 (-1.4, 1.6) | -1.2 (-2.8, 0.3) | -0.6 (-2.0, 0.9) | -3.0 (-4.4, -1.5)b | 1.3 (-0.2, 2.7) | -0.5 (-2.0, 0.9) | -0.5 (-1.9, 0.9) | 0.4 (-1.1, 1.8) |
| Sleep architecture | ||||||||
| Stage 1, min | 1.9 (-1.8, 5.6) | 2.7 (-1.0, 6.5) | 7.1 (3.4, 10.8)b | 1.8 (-1.9, 5.4) | 7.3 (3.3, 11.2)b | -0.2 (-4.1, 3.7) | 3.6 (-0.3, 7.6) | 3.1 (-0.8, 7.1) |
| Stage 1, % | -0.8 (-1.8, 0.3) | -0.7 (-1.7, 0.3) | 0.0 (-1.0, 1.0) | -1.6 (-2.6, -0.6)c | 0.9 (-0.3, 2.0) | -0.5 (-1.7, 0.6) | -0.8 (-1.9, 0.3) | -0.3 (-1.4, 0.8) |
| Stage 2, min | 15.8 (5.1, 26.5)c | 15.5 (4.8, 26.3)c | 20.0 (9.4, 30.6)b | 34.3 (23.7, 44.9)b | 13.8 (3.1, 24.5)d | 7.4 (-3.3, 18.0) | 24.0 (13.3, 34.8)b | 23.5 (12.8, 34.3)b |
| Stage 2, % | -2.0 (-3.7, -0.2)d | -3.3 (-5.1, -1.6)b | -3.2 (-4.9, -1.5)b | -1.2 (-2.9, 0.6) | -0.2 (-1.9, 1.6) | -1.1 (-2.8, 0.7) | -0.8 (-2.6, 0.9) | -0.1 (-1.8, 1.7) |
| SWS, min | 3.8 (-2.5, 10.1) | 11.1 (4.8, 17.3)b | 4.4 (-1.7, 10.5) | 7.8 (1.5, 14.2)d | 0.8 (-5.5, 7.1) | 1.4 (-4.9, 7.7) | 2.5 (-3.8, 8.9) | 3.5 (-3.2, 10.1) |
| SWS, % | -0.5 (-2.0, 1.1) | 1.3 (-0.3, 2.8) | -0.2 (-1.7, 1.3) | 0.0 (-1.5, 1.6) | -0.5 (-2.0, 1.1) | -0.2 (-1.7, 1.4) | -0.7 (-2.2, 0.9) | -0.4 (-2.1, 1.2) |
| REM, min | 19.1 (11.7, 26.5)b | 18.6 (11.2, 26.0) b | 22.3 (15.0, 29.6)b | 21.6 (14.4, 28.9)b | 0.8 (-5.5, 7.2) | 11.3 (4.9, 17.7)b | 16.3 (10.0, 22.7)b | 11.3 (4.9, 17.7)b |
| REM, % | 3.3 (1.7, 4.8)b | 2.5 (1.0, 4.1)b | 3.1 (1.6, 4.6)b | 2.6 (1.1, 4.1)b | -0.8 (-2.2, 0.6) | 1.5 (0.1, 2.9)d | 2.1 (0.7, 3.5)c | 0.6 (-0.8, 2.0) |
| Latency to REM, min | -14.0 (-28.4, 0.5) | -26.1 (-40.6, -11.5)b | -25.4 (-39.7, -11.1)b | -31.7 (-46.0, -17.5)b | -5.5 (-18.3, 7.3) | -10.6 (-23.4, 2.3) | -11.8 (-24.6, 1.0) | -25.6 (-38.4, -12.8)b |
| Patient-reported endpoints a | Week 1 | Week 4 | ||||||
| 2.5 mg | 5 mg | 10 mg | 20 mg | 2.5 mg | 5 mg | 10 mg | 20 mg | |
| Subjective sleep | ||||||||
| sTST, min | 5.3 (-6.1, 16.7) | 19.2 (7.6, 30.7)c | 20.1 (8.7, 31.5)b | 30.1 (19.1, 41.2)b | 14.1 (3.3, 25.0)d | 25.7 (14.7, 36.7)b | 30.7 (19.8, 41.6)b | 38.0 (27.4, 48.6)b |
| sTSO, min | -8.0 (-15.4, -0.6)d | -12.0 (-19.5, -4.5)c | -17.6 (-25.0, -10.2)b | -16.9 (-24.1, -9.7)b | -9.4 (-16.0, -2.7)c | -14.0 (-20.8, -7.3)b | -17.1 (-23.8, -10.5)b | -18.2 (-24.7, -11.7)b |
| sQual, 4-point scalee | 0.1 (0.0, 0.2) | 0.1 (0.0, 0.3)d | 0.2 (0.1, 0.4)b | 0.2 (0.1, 0.3)b | 0.1 (0.0, 0.2) | 0.2 (0.1, 0.4)b | 0.3 (0.2, 0.4)b | 0.3 (0.2, 0.4)b |
| sFresh, 5-point scalef | 0.1 (-0.1, 0.2) | 0.2 (0.0, 0.3)d | 0.2 (0.0, 0.4)d | 0.2 (0.0, 0.3)d | 0.1 (0.0, 0.3) | 0.2 (0.0, 0.4)d | 0.3 (0.1, 0.4)c | 0.3 (0.1, 0.4)c |
| ISI | ||||||||
| Total score | - | - | - | - | -1.5 (-2.6, -0.4)c | -2.3 (-3.3, -1.2)b | -3.0 (-4.1, -2.0)b | -3.4 (-4.4, -2.3)b |
| SDS | ||||||||
| Total score | - | - | - | - | -1.1 (-2.5, 0.2) | -1.4 (-2.7, -0.1)d | -1.8 (-3.1, -0.4)c | -1.8 (-3.1, -0.5)c |
| Work | - | - | - | - | -0.2 (-0.7, 0.3) | -0.5 (-1.0, 0.0)d | -0.4 (-0.9, 0.1) | -0.6 (-1.1, -0.1)d |
| Social | - | - | - | - | -0.4 (-0.9, 0.1) | -0.3 (-0.8, 0.1) | -0.6 (-1.0, -0.1)d | -0.6 (-1.1, -0.1)d |
| Family | - | - | - | - | -0.1 (-0.6, 0.4) | -0.6 (-1.1, -0.1)d | -0.7 (-1.2, -0.2)c | -0.5 (-1.0, 0.0)d |
Abbreviations: CI, confidence interval; ISI, Insomnia Severity Index; NAW, number of awakenings after onset of persistent sleep; REM, rapid eye movement; SDS, Sheehan Disability Scale; sFresh, patient-reported freshness of sleep; sQual, patient-reported quality of sleep; sTSO, subjective time to sleep onset; sTST, subjective total sleep time; SWS, slow-wave sleep (stage 3 and 4); TSO, time to sleep onset; TST, total sleep time; VAS, visual analog scale.
aData presented are difference (95% CI) between filorexant and placebo in least-squares mean changes from baseline.
b P≤.001.
c P≤.01.
d P≤.05 vs placebo.
eResponse options were: 1=poor, 2=fair, 3=good, 4=excellent, in response to the question, “How would you describe the quality of your sleep last night?”
fResponse options were: 0=not at all, 1=a little, 2=moderately, 3=quite a bit, 4=extremely, in response to the question, “How refreshed do you feel this morning?”
In terms of exploratory patient-reported outcome measures, filorexant doses of 5, 10, and 20mg provided improvements compared with placebo at week 1 and week 4 for sTSO, sTST, sQual, and sFresh measures. Results for sTST and sTSO were suggestive of a dose response, with higher doses of filorexant generally achieving greater benefit. Improvement in insomnia, as assessed by ISI, was observed with all doses of filorexant compared with placebo, showing greatest benefit with the 2 highest doses (10 and 20mg). Except for filorexant 2.5mg, all doses suggested improvement compared with placebo in the total score of the SDS, with associated improvements in the individual domains of work, social, and family life/home reported (Table 3).
Safety and Tolerability
Filorexant was generally well tolerated in patients with insomnia treated for up to 4 weeks (Table 4). A dose-related increase in the overall rate of AEs was observed with filorexant; the AE rate for filorexant 2.5 and 5mg was comparable with placebo, whereas the higher doses of filorexant (10 and 20mg) were associated with an increased incidence of AEs.
Table 4.
Summary of AEs (All Patients as Treated)
| Placebo (n=315) | Filorexant | |||||
|---|---|---|---|---|---|---|
| 2.5mg (n=79) | 5mg (n=78) | 10mg (n=80) | 20mg (n=81) | Total (n=318) | ||
| Number (%) of patients | ||||||
| With ≥1 AE | 82 (26.0) | 21 (26.6) | 20 (25.6) | 26 (32.5) | 28 (34.6) | 95 (29.9) |
| With drug-related AEs | 29 (9.2) | 9 (11.4) | 8 (10.3) | 14 (17.5) | 20 (24.7) | 51 (16.0) |
| With SAEs | 0 (0) | 1 (1.3) | 1 (1.3) | 1 (1.3) | 0 (0) | 3 (0.9) |
| With drug-related SAEs | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Discontinued due to AEs | 7 (2.2) | 4 (5.1) | 1 (1.3) | 2 (2.5) | 0 (0) | 7 (2.2) |
| Common AEs (≥3% incidence in any treatment group) | ||||||
| Somnolence | 9 (2.9) | 1 (1.3) | 2 (2.6) | 5 (6.3) | 11 (13.6) | 19 (6.0) |
| Headache | 12 (3.8) | 3 (3.8) | 3 (3.8) | 5 (6.3) | 3 (3.7) | 14 (4.4) |
| Fatigue | 1 (0.3) | 2 (2.5) | 1 (1.3) | 0 (0) | 3 (3.7) | 6 (1.9) |
| Diarrhea | 3 (1.0) | 0 (0) | 1 (1.3) | 3 (3.8) | 0 (0) | 4 (1.3) |
| Common drug-related AEs (≥2% incidence in any treatment group) | ||||||
| Somnolence | 9 (2.9) | 1 (1.3) | 2 (2.6) | 5 (6.3) | 11 (13.6) | 19 (6.0) |
| Headache | 6 (1.9) | 2 (2.5) | 1 (1.3) | 2 (2.5) | 1 (1.2) | 6 (1.9) |
| Fatigue | 1 (0.3) | 2 (2.5) | 1 (1.3) | 0 (0) | 2 (2.5) | 5 (1.6) |
| Irritability | 0 (0) | 0 (0) | 2 (2.6) | 2 (2.5) | 1 (1.2) | 5 (1.6) |
| Abnormal dreams | 1 (0.3) | 0 (0) | 1 (1.3) | 1 (1.3) | 2 (2.5) | 4 (1.3) |
AE, adverse event; SAE, serious adverse event.
The most common AE among patients treated with filorexant compared with placebo was somnolence, which was also the most commonly reported drug-related AE (ie, considered by the investigator to be related to study medication). Fourteen patients discontinued treatment due to an AE and of these patients, 7 were in the filorexant treatment group (2.5 mg: headache n=2, fatigue n=1, periorbital cellulitis n=1; 5 mg: atrial fibrillation n=1; 10 mg: upper respiratory tract infection n=1, acute cholecystitis n=1). All patients recovered following discontinuation of study medication.
Three patients reported serious AEs occurring during treatment with filorexant (2.5 mg: periorbital cellulitis; 5 mg: atrial fibrillation and syncope; 10 mg: acute cholecystitis); none of these serious AEs were considered by the investigator to be related to filorexant. Two serious AEs occurred posttreatment. One case of acute cholecystitis occurred 10 days after the last dose of placebo in treatment period 2, and one case of completed suicide (death) occurred during the washout period, 8 days after the last dose of placebo during treatment period 1. This was the only death reported during the study, and it was not considered by the investigator to be related to study drug. No suicidal ideation and/or behavior were reported during the treatment phase based on reported AEs or Columbia Suicide Severity Rating Scale assessments.
AEs that were prespecified as events of clinical interest occurred in 7 patients. One patient reported 3 episodes of sleep-onset paralysis, which were confirmed by the adjudication committee (1 episode on filorexant 10mg and 2 on placebo). Two patients reported excessive daytime sleepiness (filorexant 20mg and placebo), 2 patients reported falls (both on placebo), and 2 cases of drug administration error/events associated with potential drug abuse (filorexant 2.5mg and 5mg) were reported.
By objective assessment, no pattern was observed indicative of next-day residual effects as measured by the number of correct responses on the DSST (Table 5). No statistically significant differences were observed between the filorexant and placebo groups in terms of baseline-adjusted number of correct responses at either night 1 or week 4.
Table 5.
Digit Symbol Substitution Test: Number of Correct Responses
| Differences between Filorexant and Placebo in LSM a | 95% CI | P Value | |
|---|---|---|---|
| Night 1 | |||
| Filorexant | |||
| 2.5 mg | 0.2 | (-2.2, 2.6) | 0.865 |
| 5 mg | 1.6 | (-0.7, 4.0) | 0.177 |
| 10 mg | 0.9 | (-1.4, 3.3) | 0.435 |
| 20 mg | -1.7 | (-4.1, 0.6) | 0.143 |
| Week 4 | |||
| Filorexant | |||
| 2.5 mg | -1.2 | (-3.6, 1.2) | 0.337 |
| 5 mg | 0.2 | (-2.2, 2.6) | 0.852 |
| 10 mg | 1.1 | (-1.3, 3.5) | 0.387 |
| 20 mg | 0.0 | (-2.3, 2.4) | 0.980 |
Abbreviation: CI, confidence interval; LSM, least-squares mean.
aData presented are difference (95% CI) between filorexant and placebo LSM changes from baseline.
Evaluation of sTST and sTSO for evidence of rebound insomnia upon stopping filorexant showed no clear, consistent pattern across the doses in the proportion of patients reporting any worsening in sTST or sTSO on the first 3 nights of the washout period compared with baseline. The proportion of patients reporting any worsening in sTST during this time was numerically greater for the filorexant 20mg group than for placebo only on night 1 (P=.014; night 2, P=.939; night 3, P=.086), and for either night 1, 2, or 3 (P=.013). The proportion of patients reporting any worsening in sTSO during this time was also greater for filorexant 10mg on nights 1 and 2 (P=.006 and P=.046, respectively) and for filorexant 20mg on night 1 (P<.001).
There was no evidence of acute withdrawal following discontinuation of filorexant, as measured by the Tyrer WSQ, with a similar low proportion of patients demonstrating withdrawal effects in the filorexant (8.1% to 10.8%) and placebo (12.9%) treatment groups across nights 1 to 3 of the washout period (Table 6).
Table 6.
Patients reporting ≥3 of 20 emergent or worsening symptoms on the Tyrer Withdrawal Symptom Questionnaire for the first 3 nights after the end of treatment Period 1
| Across Nights 1–3 after the end of treatment Period 1 | |||
|---|---|---|---|
| Difference in proportion of patients with withdrawal between filorexant and placebo (%) | 95% CI | P Value | |
| Filorexant | |||
| 2.5 mg | -3.18 | (-12.7, 12.8) | 0.687 |
| 5 mg | -3.77 | (-13.0, 11.4) | 0.724 |
| 10 mg | -4.75 | (-13.6, 9.2) | 0.786 |
| 20 mg | -2.05 | (-11.6, 12.6) | 0.631 |
CI, confidence interval.
Discussion
The current dose-ranging study was designed to evaluate filorexant as a treatment for insomnia in adults. All doses of filorexant investigated (2.5, 5, 10, and 20mg) were significantly superior to placebo in improving sleep among patients with insomnia as measured by SE (primary endpoint) and WASO on night 1 and at the end of week 4. The 2 higher doses of filorexant (10 and 20mg) were significantly more effective than placebo in improving sleep onset as measured by LPS at night 1 and at the end of week 4, although the study was not powered to detect minimally clinically meaningful treatment effects on LPS. Filorexant also increased TST, primarily due to increases in the duration of sleep bout duration, with no overall effect on the NAW. Latency to REM sleep was numerically decreased by filorexant in a dose-dependent manner, and the percentage of time spent in REM sleep was minimally increased relative to placebo. Filorexant also had a beneficial effect on subjective assessments of sleep variables, including sTST, sTSO, sQual, and sFresh, improved insomnia as assessed by the ISI, and improved daytime function based on changes in the SDS. Together, these findings provide further validation of the orexin receptor pathway as an important and effective mechanistic target for the treatment of insomnia. These results add to earlier evidence from preclinical studies and a PSG sleep study in healthy volunteers showing that antagonism of orexin receptors by filorexant produces sleep-promoting effects (Sun et al., 2011a; Winrow et al., 2012).
Suvorexant is the first ORA to be approved by the U.S. FDA for the treatment of insomnia, having demonstrated improvements in PSG and patient-reported sleep endpoints on the first night after dosing and after 4 weeks, and 1 year, of treatment in patients with insomnia (Herring et al., 2012, 2016; Michelson et al., 2014). Although the current study was not designed as a comparative study, filorexant generally demonstrated improvements in PSG and patient-reported sleep endpoints that were comparable with those typically achieved with suvorexant in nonelderly patients with insomnia (Herring et al., 2012).
Overall, treatment with filorexant was generally well tolerated with no important safety concerns. The observed safety profile of filorexant was similar to that reported with compounds in the same class, such as suvorexant (Herring et al., 2012, 2016; Michelson et al., 2014), SB649868 (Bettica et al., 2012), and almorexant (Hoever et al., 2012; Cruz et al., 2014) in healthy subjects and patients with insomnia. Somnolence was the most common AE reported with filorexant. Serious AEs were uncommon during treatment and none were considered related to filorexant. Significant residual or rebound effects were not reported.
The current study incorporated some important design considerations. The 4-week dosing duration for each treatment period allowed both an acute (night 1) and more chronic (end of week 4) assessment of filorexant efficacy and tolerability. This design allowed the evaluation of immediate and sustained efficacy, both of which are fundamental requirements for insomnia therapies. Furthermore, the washout duration of 14 days (3 days of single-blind placebo followed by 11 days off study drug) provided an estimated 67 half-lives (based upon a t½ for filorexant of 5 hours) of pharmacokinetic clearance for filorexant. This provided an adequate buffer for the clearance of any potentially associated pharmacodynamic effects prior to night 1 of the subsequent 4-week treatment period (period 2) and minimized the likelihood of a carryover effect of treatment between the 2 treatment periods. There was no evidence of significant sequence effects. Further studies are required to evaluate the long-term safety and efficacy of filorexant and also its safety and efficacy in the elderly.
The relatively short apparent t½ of filorexant (approximately 3 to 6 hours) suggests the possibility of a unique clinical profile compared with that of other known ORA receptor antagonists (Sun et al., 2011b). For instance, a compound with a shorter t½ may offer an improved residual effect profile, but, perhaps, at the expense of maintaining sleep throughout the night. In this trial, while a dose-related effect was observed for somnolence, the incidence of somnolence at the 2 lower doses was similar to placebo, and sustained maintenance effects were noted across the full filorexant dose range. However, without data from a study specifically designed to make cross-compound comparisons between doses ultimately deemed clinically acceptable for regulatory approval (suvorexant is approved in the US for insomnia at a dose of 10 to 20mg once daily and is currently the only FDA-approved ORA), comparisons in this regard would be speculative.
In conclusion, this study has shown that filorexant, a shorter-acting dual ORA, is well tolerated and promotes the initiation and maintenance of sleep in patients with insomnia, achieving consistent improvements in sleep time, as determined by both PSG and patient report.
Supplementary Material
Acknowledgments
Funding for this research was provided by Merck & Co., Inc., Kenilworth, NJ. Medical writing assistance under the direction of the authors was provided by Julie Adkins for Complete Medical Communications and Erin Bekes, PhD, of Complete Medical Communications, Inc, Hackensack, NJ. This assistance was funded by Merck & Co., Inc., Kenilworth, NJ. The authors are entirely responsible for the scientific content of the paper.
Statement of Interest
K.M. Connor, E. Mahoney, S. Jackson, J. Hutzelmann, X. Zhao, N. Jia, E. Snyder, D. Snavely, D. Michelson, and W. J. Herring are current or former employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, and own or owned stock/stock options. T. Roth has received grants/research support from Aventis, Cephalon, GlaxoSmithKline, Neurocrine, Pfizer, Sanofi, Schering-Plough, Sepracor, Somaxon, Syrex, Takeda, TransOral, Wyeth, and Xenoport; has acted as a consultant for Abbott, Acadia, Acoglix, Actelion, Alchemers, Alza, Ancil, Arena, AstraZeneca, Aventis, AVER, BMS, BTG, Cephalon, Cypress, Dove, Elan, Eli Lilly, Evotec, Forest, GlaxoSmithKline, Hypnion, Impax, Intec, Intra-Cellular, Jazz, Johnson & Johnson, King, Lundbeck, McNeil, MediciNova, Merck, Neurim, Neurocrine, Neurogen, Novartis, Orexo, Organon, Prestwick, Procter & Gamble, Pfizer, Purdue, Resteva, Roche, Sanofi, Schering-Plough, Sepracor, Servier, Shire, Somaxon, Syrex, Takeda, TransOral, Vanda, Vivometrics, Wyeth, Yamanuchi, and Xenoport; and has participated in speaking engagements supported by Cephalon, Sanofi, and Takeda.
References
- Rechtschaffen A, Kales A. eds. A manual of standardized terminology, techniques, and scoring system for sleep states of human subjects (1968). Bethesda, MD: National Institutes of Health. [Google Scholar]
- Belsomra (suvorexant) Package Insert (2014). Available at: http://www.merck.com/product/usa/pi_circulars/b/belsomra/belsomra_pi.pdf.
- Bettica P, Squassante L, Zamuner S, Nucci G, Danker-Hopfe H, Ratti E. (2012) The orexin antagonist SB-649868 promotes and maintains sleep in men with primary insomnia. Sleep 35:1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet MH, Arand DL. (2010) Hyperarousal and insomnia: state of the science. Sleep Med Rev 14:9–15. [DOI] [PubMed] [Google Scholar]
- Brisbare-Roch C, Dingemanse J, Koberstein R, Hoever P, Aissaoui H, Flores S, Mueller C, Nayler O, van Gerven J, de Haas SL, Hess P, Qiu CB, Buchmann S, Scherz M, Weller T, Fischli W, Clozel M, Jenck F. (2007) Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med 13:150–155. [DOI] [PubMed] [Google Scholar]
- Coleman PJ, Schreier JD, Cox CD, Breslin MJ, Whitman DB, Bogusky MJ, McGaughey GB, Bednar RA, Lemaire W, Doran SM, Fox SV, Garson SL, Gotter AL, Harrell CM, Reiss DR, Cabalu TD, Cui D, Prueksaritanont T, Stevens J, Tannenbaum PL, Ball RG, Stellabott J, Young SD, Hartman GD, Winrow CJ, Renger JJ. (2012) Discovery of [(2R,5R)-5-{[(5-fluoropyridin-2-yl)oxy]methyl}-2-methylpiperidin-1-yl][5-methyl-2-(pyrimidin-2-yl)phenyl]methanone (MK-6096): a dual orexin receptor antagonist with potent sleep-promoting properties. ChemMedChem 7:415–24, 337. [DOI] [PubMed] [Google Scholar]
- Cruz HG, Hay JL, Hoever P, Alessi F, Te Beek ET, van Gerven JM, Dingemanse J. (2014) Pharmacokinetic and pharmacodynamic interactions between almorexant, a dual orexin receptor antagonist, and desipramine. Eur Neuropsychopharmacol 24:1257–1268. [DOI] [PubMed] [Google Scholar]
- Gotter AL, Webber AL, Coleman PJ, Renger JJ, Winrow CJ. (2012) International Union of Basic and Clinical Pharmacology. LXXXVI. Orexin receptor function, nomenclature and pharmacology. Pharmacol Rev 64:389–420. [DOI] [PubMed] [Google Scholar]
- Herring WJ, Snyder E, Budd K, Hutzelmann J, Snavely D, Liu K, Lines C, Roth T, Michelson D. (2012) Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology 79:2265–2274. [DOI] [PubMed] [Google Scholar]
- Herring WJ, Connor KM, Ivgy-May N, Snyder E, Liu K, Snavely DB, Krystal AD, Walsh JK, Benca RM, Rosenberg R, Sangal RB, Budd K, Hutzelmann J, Leibensperger H, Froman S, Lines C, Roth T, Michelson D. (2016) Suvorexant in patients with insomnia: results from two 3-month randomized controlled clinical trials. Biol Psychiatry 79:136–148. [DOI] [PubMed] [Google Scholar]
- Hoever P, Dorffner G, Benes H, Penzel T, Danker-Hopfe H, Barbanoj MJ, Pillar G, Saletu B, Polo O, Kunz D, Zeitlhofer J, Berg S, Partinen M, Bassetti CL, Hogl B, Ebrahim IO, Holsboer-Trachsler E, Bengtsson H, Peker Y, Hemmeter UM, Chiossi E, Hajak G, Dingemanse J. (2012) Orexin receptor antagonism, a new sleep-enabling paradigm: a proof-of-concept clinical trial. Clin Pharmacol Ther 91:975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson A, Quan SF, for the American Academy of Sleep Medicine (2007) The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester: American Academy of Sleep Medicine. [Google Scholar]
- Ishak WW, Bagot K, Thomas S, Magakian N, Bedwani D, Larson D, Brownstein A, Zaky C. (2012) Quality of life in patients suffering from insomnia. Innov Clin Neurosci 9:13–26. [PMC free article] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin XY, Qiu XH, de Jong PJ, Nishino S, Mignot E. (1999) The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 98:365–376. [DOI] [PubMed] [Google Scholar]
- Mai E, Buysse DJ. (2008) Insomnia: prevalence, impact, pathogenesis, differential diagnosis, and evaluation. Sleep Med Clin 3:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson D, Snyder E, Paradis E, Chengan-Liu M, Snavely DB, Hutzelmann J, Walsh JK, Krystal AD, Benca RM, Cohn M, Lines C, Roth T, Herring WJ. (2014) Safety and efficacy of suvorexant during 1-year treatment of insomnia with subsequent abrupt treatment discontinuation: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet Neurol 13:461–471. [DOI] [PubMed] [Google Scholar]
- Morin CM, Benca R. (2012) Chronic insomnia. Lancet 379:1129–1141. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Mieda M, Tsujino N. (2010) The orexin system: roles in sleep/wake regulation. Ann N Y Acad Sci 1200:149–161. [DOI] [PubMed] [Google Scholar]
- Sarsour K, Kalsekar A, Swindle R, Foley K, Walsh JK. (2011) The association between insomnia severity and healthcare and productivity costs in a health plan sample. Sleep 34:443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. (2008) Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med 4:487–504. [PMC free article] [PubMed] [Google Scholar]
- Sun H, Brown KM, Calder N, Li X, Yee KL, Perlstein I, Wilbraham D, Rosen L, Chodakewitz J, Wagner JA, Murphy MG. (2011. a) MK-6096, a dual orexin receptor antagonist, enhances sleep onset and maintenance as measured by PSG in healthy male subjects. Sleep Biol Rhythms 9:334. [Google Scholar]
- Sun H, Kennedy WD, Lewis N, Laethem T, Tee K, Li X, Hoon J, van Bortel L, Rosen L, Chodakewitz J, Wagner JA, Murphy MG. (2011. b) The single dose pharmacokinetic (PK) and pharmacodynamic (PD) profiles of suvorexant (MK-4305), a dual orexin receptor antagonist, in healthy male subjects. Sleep Biol Rhythms 9:332, Abstract PO-1-235. [Google Scholar]
- Tyrer P, Murphy S, Riley P. (1990) The benzodiazepine withdrawal symptom questionnaire. J Affect Disord 19:53–61. [DOI] [PubMed] [Google Scholar]
- Winrow CJ, Gotter AL, Cox CD, Doran SM, Tannenbaum PL, Breslin MJ, Garson SL, Fox SV, Harrell CM, Stevens J, Reiss DR, Cui D, Coleman PJ, Renger JJ. (2011) Promotion of sleep by suvorexant - a novel dual orexin receptor antagonist. J Neurogenet 25:52–61. [DOI] [PubMed] [Google Scholar]
- Winrow CJ, Gotter AL, Cox CD, Tannenbaum PL, Garson SL, Doran SM, Breslin MJ, Schreier JD, Fox SV, Harrell CM, Stevens J, Reiss DR, Cui D, Coleman PJ, Renger JJ. (2012) Pharmacological characterization of MK-6096 - a dual orexin receptor antagonist for insomnia. Neuropharmacology 62:978–987. [DOI] [PubMed] [Google Scholar]
- Winrow CJ, Renger JJ. (2014) Discovery and development of orexin receptor antagonists as therapeutics for insomnia. Br J Pharmacol 171:283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.