Abstract
Objective:
Oxidative stress and mitochondrial dysfunction are 2 closely integrated processes implicated in the physiopathology of bipolar disorder. Advanced proton magnetic resonance spectroscopy techniques enable the measurement of levels of lactate, the main marker of mitochondrial dysfunction, and glutathione, the predominant brain antioxidant. The objective of this study was to measure brain lactate and glutathione levels in bipolar disorder and healthy controls.
Methods:
Eighty-eight individuals (50 bipolar disorder and 38 healthy controls) underwent 3T proton magnetic resonance spectroscopy in the dorsal anterior cingulate cortex (2x2x4.5cm3) using a 2-D JPRESS sequence. Lactate and glutathione were quantified using the ProFit software program.
Results:
Bipolar disorder patients had higher dorsal anterior cingulate cortex lactate levels compared with controls. Glutathione levels did not differ between euthymic bipolar disorder and controls. There was a positive correlation between lactate and glutathione levels specific to bipolar disorder. No influence of medications on metabolites was observed.
Conclusion:
This is the most extensive magnetic resonance spectroscopy study of lactate and glutathione in bipolar disorder to date, and results indicated that euthymic bipolar disorder patients had higher levels of lactate, which might be an indication of altered mitochondrial function. Moreover, lactate levels correlated with glutathione levels, indicating a compensatory mechanism regardless of bipolar disorder diagnosis.
Keywords: glutathione, lactate, bipolar disorder, mitochondrial disease, oxidative stress
Introduction
Multiple neurobiological pathways have been implicated in the physiopathology of bipolar disorder (BD). Oxidative stress (Berk et al., 2011; Soeiro-de-Souza et al., 2013) and mitochondrial dysfunction number amongst these pathways (Stork and Renshaw, 2005). Although these 2 processes are closely integrated, published reports have generally focused on only one of these abnormalities at a time. Proton magnetic resonance spectroscopy (1H-MRS) is a noninvasive method that allows in vivo detection of metabolic alterations in localized brain areas (voxels). With regard to BD, previous studies based on standard 1-dimensional 1H-MRS protocols report a possible glycolytic shift evidenced by abnormalities in the glutamate to glutamine ratio (Stork and Renshaw, 2005; Soeiro de Souza et al., 2013, 2015). Two-dimensional (2D) J-resolved 1H-MRS techniques allow the measurement of small signal metabolites such as lactate (Lac), the main metabolic marker of mitochondrial dysfunction, and glutathione (GSH), the predominant brain antioxidant. Currently, there are no 2D J-resolved 1H-MRS studies reporting measures of both Lac and GSH concomitantly in BD. Measuring these 2 metabolites at the same time in vivo provides a unique opportunity to test the hypotheses of altered redox state and its association with mitochondrial dysfunction in BD.
Mitochondria play a crucial role in ATP production through oxidative phosphorylation, a process carried out by the respiratory chain complexes I, II, III, and V (Orth and Schapira, 2001; Shanske et al., 2001; Chinnery and Schon, 2003). When mitochondrial function is inhibited or insufficient, anaerobic glycolysis is activated, leading to higher production of Lac. Thus, the accumulation of Lac occurs when oxidative phosphorylation is unable to meet energy requirements and the cell is forced to rely on the glycolytic process (Rudkin and Arnold, 1999). In general, mitochondrial dysfunction contributes to neurodegeneration either by apoptosis or generation of reactive oxygen species (ROS). ROS such as hydrogen peroxide, superoxide, and hydroxyl radicals are produced as by-products of mitochondrial phosphorylation (Gutteridge and Halliwell, 2000; Cavanagh et al., 2002; Clark et al., 2002; Ferrier and Thompson, 2002). Under these circumstances of elevated levels of Lac and ROS, the GSH system, as the major brain antioxidant, has a fundamental role.
The GSH system is especially important for cellular defense against ROS, as GSH is the major antioxidant in the brain. This system comprises the enzymes that synthesize GSH within cells as well as dedicated enzymes that use GSH as the means to exert antioxidant effects (Dringen, 2000). GSH reacts directly with radicals in nonenzymatic reactions and is the electron donor in the reduction of peroxides catalyzed by GSH peroxidase. Astrocytes appear to contain higher GSH levels than neurons both in vivo and in culture (Dringen, 2000). There are few reports about abnormalities of the GSH system in BD regarding altered enzymes that use GSH as a cofactor (GSH reductase, GSH S-transferase, GSH peroxidase), but the interpretation of findings is limited by the heterogeneous methodologies used (Brown et al., 2014). Studies conducted on peripheral blood cells have shown that BD is associated with increased levels of GSH reductase and GSH S-transferase in the late stage of the illness (Andreazza et al., 2009). Moreover, low GSH S-transferase levels have been reported in postmortem prefrontal cortex from patients with BD, major depressive disorder, and schizophrenia (Gawryluk et al., 2011). Additionally, elevated GSH peroxidase in peripheral blood has been reported in BD depressive episodes (Andreazza et al., 2007; de Sousa et al., 2014) but not in mania or euthymia (Abdalla et al., 1986; Kuloglu et al., 2002; Andreazza et al., 2009; Raffa et al., 2012).
Previous 1H-MRS studies investigating GSH are scarce in BD, because standard techniques are not sufficiently sensitive to detect metabolites present in low concentrations, such as GSH. Our review identified 5 previous MRS studies on GSH in BD, all of which included a mixed sample of BD patients (type I, I, or NOS) in mania, depression, or euthymia (Chitty et al., 2013, 2014, 2015; Lagopoulos et al., 2013; Godlewska et al., 2014). All of these studies reported no differences in GSH levels between BD and controls in anterior cingulate (ACC) (Chitty et al., 2013, 2014; Lagopoulos et al., 2013), prefrontal/occipital voxels (Godlewska et al., 2014), or hippocampus (Chitty et al., 2015). With the objective of confirming an absence of differences in GSH between BD patients and HC we, studied a large and homogeneous sample of euthymic BD type I patients. Furthermore, the 2D-J resolved-PRESS 1H-MRS technique was employed for its greater sensitivity in detecting small GSH signals compared with the conventional 1H-MRS technique used in previous studies.
Previous evidence on oxidative stress in BD has shown alterations in antioxidant enzymes and lipid peroxidation in different states and stages of BD (Berk et al., 2011). Superoxide dismutase and catalase activity have been reported to be altered in BD mood episodes (Andreazza et al., 2007; Machado-Vieira et al., 2007). Evidence for oxidative stress in BD has been found in the form of lipid peroxidation and reduced Na+-K+-ATPase activity, alterations that can be counteracted by lithium treatment (Banerjee et al., 2012). Moreover, decreased plasma levels of total GSH, together with lower catalase expression, increased protein carbonyls, 4-HNE, and 3-NT, were found in BD patients (Raffa et al., 2012; Andreazza et al., 2013). Mitochondrial abnormalities in BD patients displayed decreased attachment of hexokinase 1 to outer mitochondrial membrane and decreased Complex I levels (Andreazza et al., 2013).Moreover, there is some evidence indicating that the lifetime number of manic episodes increases oxidative damage to guanosine in BD, where our group previously reported an association between elevated levels of 8-OHdG and number of manic episodes (Soeiro-de-Souza et al., 2013).
Elevated Lac has been considered a marker of mitochondrial dysfunction in BD (Stork and Renshaw, 2005). Brain Lac, a metabolic product of glycolysis, plays an integral role in neuronal energy metabolism (Schurr, 2006). Lac exists in the healthy brain at low basal concentrations, and elevations can indicate transient changes in physiological state (Dager et al., 1999) or neural activation (Frahm et al., 1996). Lac is a metabolite that is hard to measure due to its low concentration as well as to difficulties distinguishing the Lac signal from that of overlapping lipids and macromolecules, where specific 1H-MRS methods are required to reliably detect Lac (Rudkin and Arnold, 1999). The 4 previous 1H-MRS studies on Lac comparing BD patients with controls have reported increased levels in patients with BD (Dager et al., 2004; Brady et al., 2012; Chu et al., 2013; Xu et al., 2013), but none have exclusively investigated BD type I euthymic patients. These studies have included a mixture of BD subjects in different mood episodes, where this might be problematic when studying Lac in BD, as far as antioxidative enzymes have been reported to be altered during mood episodes (Andreazza et al., 2007; Machado-Vieira et al., 2007). Moreover, the majority of previous Lac 1H-MRS studies used a short echo-time (TE), which can obscure Lac detection because of an important overlap with lipids’ signal in this region (Rudkin and Arnold, 1999).
Aims of the Study
The aim of this study was to measure simultaneously dorsal ACC (dACC) levels of Lac and GSH in euthymic BD type I and healthy controls (HC), using in vivo 2D 1H-MRS. We hypothesized that BD patients present higher levels of Lac and lower levels of GSH based on the assumption of increased redox state and mitochondrial dysfunction in BD.
Methods
Eighty-eight subjects were included in this study. Of these, 50 (31 F, 18–45 years old) were euthymic BD I subjects and 38 (15 F, 18–45 years old) were HC. Diagnoses were made by trained psychiatrists based on the Structured Clinical Interview (First et al., 1996) for DSM-IV TR (DSM-IV, 2000). The subjects had been on stable medication regimens for at least 2 months prior to the scanning session. Subjects with neurological disorders or medical disorders, head trauma, or current/past (3 months) substance abuse, as well as those who had been treated with electroconvulsive therapy in the last 6 months were excluded. Moreover, subjects reporting heavy episodic drinking (consuming 5 or more standard drinks [male], or 4 or more drinks [female] over a 2-hour period) (Moreira et al., 2009) over the past 3 months were excluded. The Young Mania Rating Scale (Young et al., 1978) and the Hamilton Depression Rating Scale (Hamilton, 1960) were used to assess residual subthreshold depressive and manic symptoms. Euthymia was defined as <7 Young Mania Rating Scale and <7 Hamilton Depression Rating Scale. The patients also fulfilled the DSM-IV criteria for remission.
All HC had no current or past history of psychiatric disorders according to the evaluation conducted by trained psychiatrists using the Mini International Neuropsychiatric Interview (Sheehan et al., 1998). In addition, HC subjects had no family history of mood or psychotic disorders among first-degree relatives based on a semistructured interview.
The research ethics committee CEP CAPPesq from the University of Sao Paulo approved the study. Written informed consent was obtained from all study participants.
Image Acquisition
All MRI exams were performed on a Philips 3T Achieva scanner (Philips Healthcare, Best, The Netherlands) using an 8-channel head coil. Spectroscopy measurements were performed using the maximum echo sampled JPRESS sequence proposed by Schulte and Bosiger (2006). The JPRESS sequence is based on the conventional PRESS spin-echo technique used for selection of a single voxel. By varying the echo time of the acquisition, the J coupling evolution is encoded in an additional dimension. This technique is therefore also known as 2-dimensional spectroscopy, whereby the signal is measured as a function of chemical shift expressed by the Larmor frequency (as in conventional 1-dimensional spectroscopy) but also as a function of the coupling constant J in Hz. With the information of the coupling constant J, it is possible to resolve the signals from overlapping multiplets, such as Lac and GSH. In this study, the JPRESS sequence was used to evaluate a voxel of 20mm (L-R) x 20mm (I-S) x 45mm (A-P) (total voxel size 18cm3) in the dACC region, as shown in Figure 1. The minimum TE used was 31ms, and TE was incremented in 100 steps of 2ms each. For each time increment ΔTE, the maximum-echo sampling started the acquisition ΔTE/2 earlier with respect to the echo top (Schulte et al., 2006). The repetition time (TR) was 1600ms, and 8 averages were acquired for each TE step. One non-water suppressed spectrum was also acquired at each TE. The number of points per spectrum was 1024, and the spectral bandwidth was 2000 Hz. An automatic second-order B0 shimming routine was used and water suppression was achieved by VAPOR (Tkác et al., 1999). Spectroscopy acquisition took 24 minutes, and the total exam duration, including volumetric imaging and voxel planning, was about 45 minutes. Metabolite quantification was obtained using ProFit (PRiOr knowledge FITting) version 2.0 running on Matlab R2011b (Fuchs et al., 2013). The first version of ProFit was developed by Schulte et al. (Schulte and Boesiger, 2006) to fit 2D JPRESS data by extending LCModel (Provencher, 1993) principles to 2D data sets. In ProFit, as in the LCModel approach, the prior knowledge comes from a known metabolite basis set (experimentally acquired or calculated) used in the fitting process, and the VARPRO approach (van der Veen et al., 1988) is used to separate the optimization of nonlinear and linear parameters for faster convergence. Fuchs et al. (2013) improved the quantification program (ProFit version 2.0) by introducing an experimentally acquired 2D macromolecular baseline into the fitting model and allowing for a more accurate and precise fit by accounting for the actual line shape and additional baseline distortions by self-deconvolution and spline modeling approaches.
Figure 1.
Magnetic resonance spectroscopy (MRS) voxel location in the sagittal plane. Size 20 x 20 x 45mm3.
The metabolite basis set used by ProFit includes spectra from a total of 18 brain metabolites including the metabolite of interest in this study: Lac and GSH. Basis set metabolite spectra were calculated with the GAMMA library (Smith et al., 1994) using the chemical shift and J-coupling values from the literature (Fan, 1996; Govindaraju et al., 2000). Quantitative results in ProFit are given in the form of ratios to Cr signal (met/Cr). These ratios are already corrected for T2 relaxation effects, since ProFit automatically calculates T2 relaxation times for each metabolite from the signal obtained at the different TEs. “Pseudo” absolute metabolite values [met] were obtained by assuming a white matter (WM) Cre concentration of 4.83mM (mmol/L) and a grey matter (GM) Cre concentration of 9.59mM, as expressed in the equation below. These Cr values in mM were calculated from previously reported Cr concentrations in units of mmol/kg (Gasparovic et al., 2006), as used previously (Soeiro de Souza et al., 2015; Zoelch et al., 2015) and expressed in the following equation:
where
T1 relaxation effects were corrected for, assuming a mono-exponential T1 relaxation with GM T1 of 1.46s and a WM T1 of 1.24s (Mlynárik et al., 2001).
GM% and WM% represent GM and WM volume percentages, respectively, in the selected MRS voxel, while fGM and fWM represent the fractions of Cr signal attributable to GM and WM, respectively. To determine the brain tissue composition contained in the MRS voxel of interest, 3-dimensional volumetric images were obtained using the 3D-T1- FFE (fast field echo) technique (FA=8°; TE/TR/TI=3.2/7/900ms) with an isotropic voxel size of 1mm3. Briefly, the brain tissue was extracted using the brain extraction tool, and segmentation into WM, GM, and CSF was achieved using the automated brain segmentation tool FAST (Zhang et al., 2001). Both tools are part of the FSL suite (http://www.fmrib.ox.ac.uk/fsl). Finally, the MRS voxel was overlaid on the segmented image using a Python-based script developed in-house, and percentages of WM, GM, and CSF were calculated for each voxel. The ProFit program also provides a Cramér-Rao lower bound (CRLB) (Cavassila et al., 2001), a measure of the quality of the metabolite quantification, for each metabolite. CRLBs were noted for each metabolite. Excluding spectra with Lac CRLBs above a specific threshold (30%) is a common practice in the literature on Lac. However, it was recently shown that to do so might prevent the observation of real differences between groups (Kreis, 2016), so we decided not to set a CRLB cutoff point in this study to avoid a misinterpretation of our Lac results. Only data with a CRLB of 999% were discarded from the analysis, since this specific CRLB output denotes that the program failed to calculate a reliable CRLB.
Statistical Analysis
The sample was first tested for homogeneity. Categorical variables were analyzed using χ2 tests, whereas continuous variables were analyzed using t tests. Normality was checked using the Kolmogorov-Smirnov test. Significant differences in age and gender were observed in the sample and to prevent this potential bias from influencing results, age and gender correction was performed in all analyses. Normally distributed variables were compared between the 2 groups using ANCOVAs in which Lac or GSH were entered as a dependent variable, while age, gender, group, CSF (only for Lac), and fGM were entered as covariates. To investigate the influence of medication use on Lac and GSH, we performed an ANCOVA test in which Lac or GSH were entered as a dependent variable and medication type (lithium, atypical antipsychotics, or anticonvulsants), age, gender, CSF (only for Lac), and fGM were entered as covariates. To investigate the influence of illness duration or lifetime psychotic symptoms on metabolites, we used an ANCOVA test in which Lac or GSH was entered as dependent variable and illness duration or lifetime psychotic symptoms, age, gender, CSF (only for Lac), and fGM were entered as covariates. Finally, to investigate the correlation between GSH and Lac, we performed a regression analysis controlled by age and gender. All statistical analyses were carried out using IBM SPSS version 20.
Results
After controlling for age and gender, significant differences were observed between BD patients and HC in voxel content for GM% (BD 52% vs HC 54%) (F=5.5, df=88, P=.02) and CSF% (BD 25% vs HC 23%) (F=6.2, df=88, P=.01), but no differences were observed in WM content (BD 23% vs HC 23%) (F=0.4, df=88, p P=.48). Given the observed differences between HC and BD in voxel composition, we also performed a more detailed segmentation analysis of the brain tissue contributing to the spectrum by overlaying the 1H-MRS voxel on to structural maps segmented by FreeSurfer software (https://surfer.nmr.mgh.harvard.edu). This volumetric analysis is part of a separate publication including a larger cohort of BD patients (M. G. Soeiro-de-Souza and M. C. Garcia Otaduy, unpublished observations). Results of the MRS voxel overlay onto the segmented volumetric images indicate that the cortical structure driving this difference in voxel brain tissue composition was the caudal ACC, which was reduced in BD compared with HC (HC=16.5±3.5% vs BD=14.1±3.8%; P=.005). As a result, BD subjects had lower GM volume and higher CSF volume within the MRS voxel. These differences were compensated for in the statistical analysis by inclusion of fGM (as described above in Image acquisition) as a variable in the statistical model. When comparing Lac between groups, CSF% was also considered as a covariate, since Lac can be present in CSF. GSH was present and quantifiable for all patients with a mean CRLB of 3.98% (1.37%-5.71%). Group characteristics for the GSH analysis are described in Table 1. There was no statistically significant difference in GSH levels between groups, although the BD group had higher mean levels (f=2.9, df=88, P=.08) (BD mean 1.32±0.31; HC mean 1.24±0.14).
Table 1.
Subject Demographic and Clinical Information for GSH MRS
| Healthy Controls | Bipolar I Disorder | |
|---|---|---|
| n=38 | n=50 | |
| Age (y), mean ±SD | 25.7 ±5.7 | 31.7 ±9.1 |
| Gender (male/female) | 23/15 | 19/31 |
| HDRS, mean ±SD | 3.7 ±2.1 | |
| YMRS, mean ±SD | 2.4 ±2.1 | |
| Illness duration, mean ±SD | 9.1±7.6 | |
| Lifetime psychotic symptoms (yes/no) | 15/35 | |
| Mean GSH 1.24 ±0.14 | 1.32 ±0.31 | |
| Mean GSH CRLB 3.94 ±0.33 | 4.00 ±0.67 | |
| Anticonvulsants (valproate or carbamazepine) | n=23 | |
| Lithium | n=29 | |
| Atypical antipsychotics | n=23 |
Abbreviations: HDRS, Hamilton Depression Rating Scale; YMRS, Young Mania Rating Scale; y, years; SD, standard deviation; Lac, lactate; CRLB, Cramér-Rao Lower Bound.
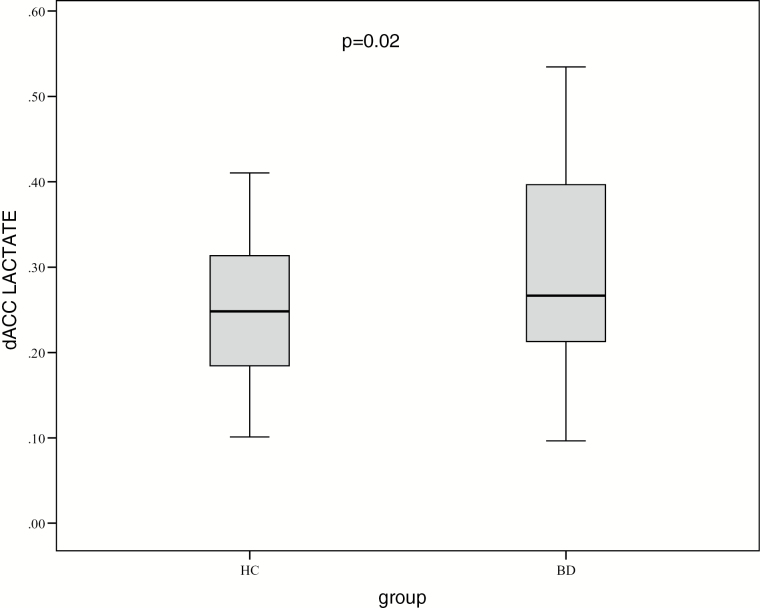
Regarding the Lac MRS, 16 individuals (3 HC and 13 BD) were excluded for having a CRLB of 999%, indicating lipid contamination or other artifacts (supplementary Table 1). Therefore, the sample used for Lac analysis consisted of 37 BD and 35 HC (Table 2). A statistically significant difference in Lac level was observed between the groups; the BD group had higher mean levels (F=5.49, df=72, P=.02) (BD mean 0.31±0.15; HC mean 0.24±0.09) compared with the HC group (Figure 2). As supplementary material, an analysis was performed considering CRLB<30% (sample size BD=26 x HC=26), whose outcome supported the result of higher Lac in BD (F=5.0, df=52, P=.02) (supplementary Table 2).
Table 2.
Subject Demographic and Clinical Information for Lactate MRS (n=72)
| Healthy Controls | Bipolar I Disorder | |
|---|---|---|
| n=35 | n=37 | |
| Age (y), mean ±SD | 25 ±4.5 | 31.1 ±9.3 |
| Gender (male/female) | 23/12 | 13/24 |
| HDRS, mean ±SD | 3.1 ±1.9 | |
| YMRS, mean ±SD | 2.7 ±2 | |
| Illness duration, mean ±SD | 8.8±7.2 | |
| Lifetime psychotic symptoms (yes/no) | 11/26 | |
| Mean Lac 0.24 ±0.09 | 0.31 ±0.15 | |
| Mean Lac CRLB 28.8 ±15.6 | 32.7±36.3 | |
| Anticonvulsants (valproate or carbamazepine) | n=16 | |
| Lithium | n=22 | |
| Atypical antipsychotics | n=18 |
Abbreviations: HDRS, Hamilton Depression Rating Scale; YMRS, Young Mania Rating Scale; y, years; SD, standard deviation; Lac, lactate; CRLB, Cramér-Rao Lower Bound.
Figure 2.
Mean dorsal anterior cingulate cortex (dACC) levels of lactate (Lac) in bipolar disorder (BD) type I compared with healthy controls (HC).
We observed a positive correlation between Lac and GSH levels in BD (B=0.20, t=3.2, P=.003, CI=0.07–0.33) but not in HC (B=0.17, t=1.64, P=.11, CI=-0.04–0.39) (Figure 3). When we perform this analysis without correcting for age, gender, or fGM, we observed the same results: in BD, Lac was correlated with GSH (B=0.21, t=3.44, P=.002, CI=0.08–0.33), while in HC there was no correlation between these 2 metabolites (B=0.19, t=1.8, P=.07, CI=-0.01–0.41).
Figure 3.
Correlation between dorsal anterior cingulate cortex (dACC) levels of lactate (Lac) and glutathione (GSH) adjusted for age and gender.
Neither illness duration nor the presence of lifetime psychotic symptoms demonstrated to influence any of the metabolite measures (P<.05). There was no influence of lithium, atypical antipsychotics, or anticonvulsants on Lac or GSH levels.
Discussion
In the present study, higher dACC Lac levels were found in euthymic BD type I patients compared with HC. No significant differences in GSH were observed, but a positive correlation between Lac and GSH level was detected. Moreover, no influence of medications on metabolite levels was found.
Our results revealed increased Lac in BD regardless of medication use, supporting the hypothesis of mitochondrial dysfunction in BD (Stork and Renshaw, 2005). Mitochondrial dysfunction occurs when oxidative phosphorylation is unable to meet energy requirements and the cell is forced to rely on the glycolytic process, which increases the production of Lac (Moore and Galloway, 2002). Some studies have reported higher Lac concentrations in the CSF of BD patients (Regenold et al., 2009). Furthermore, postmortem studies have found decreased expression of mitochondrial genes encoding the electron transport chain (Konradi et al., 2004; Sun et al., 2006) and abnormal mitochondrial complex I activity (Andreazza et al., 2010) in brain of BD individuals. Moreover, the presented data are reinforced by other MRS studies reporting lower intracellular pH (Kato et al., 1992; 1993), decreased N-acetyl aspartate (Cecil et al., 2002; Chang et al., 2003), and higher Glx (Yüksel and Ongur, 2010; Soeiro de Souza et al., 2013). Findings of increased Glx in bipolar subjects suggest that the hypothesized glycolytic shift underlying the pathology of BD may be linked to some degree of glutamate-induced neuronal hyperactivation (Stork and Renshaw, 2005).
Our data evidencing increased Lac in BD is in agreement with the majority of previous Lac 1H-MRS studies involving mania, depression, and euthymia (Dager et al., 2004; Chu et al., 2013; Xu et al., 2013). Only one study, by Brady et al. (2012) reported lower Lac levels in BD patients (n=7) compared with HC. Brady et al. (2012) reported that Lac levels in mania (n=7) were increased compared with HC in the parietal occipital cortex and ACC. However, when these same patients were in euthymia after treatment, their Lac levels were lower (n=7) than those of HC (n=6) (Brady et al., 2012). Chu et al. investigated Lac levels (4T scanner) in a sample of 21 BD patients in euthymia and mixed episode compared with 10 HC. The group reported increased Lac levels in BD patients, symptomatic or otherwise (Chu et al., 2013). The other 2 studies comparing Lac levels in BD patients with HC reported increased levels of Lac in the ACC of BD patients during mania, depression, and mixed state (Dager et al., 2004; Xu et al., 2013). Three of the 4 Lac MRS studies described CRLB levels and used a CRLB cutoff for inclusion of subjects in the study. Brady et al. (2012) included individuals with CRLB<26%, while Xu et al. (2013) and Chu et al. (2013) included those with CRLB≤30%. A known problem with measuring Lac is systematic overestimation when using linear combination fitting algorithms to estimate concentrations (Kreis, 2004). Excluding spectra with Lac CRLBs above a specific threshold (30%) is a common practice in the literature on Lac, since CRLBs are linked to chemical estimation reliability. However, due to the difficulty discriminating the Lac signal from background noise, the meaning of the CRLBs produced by LCModel for this metabolite is less clear. Kreis et al. (Kreis and Kyathanally, 2015; Kreis, 2016) reported that using CRLB threshold as an exclusion criterion can lead to false conclusions and suggested that quality checks should be based on metabolites present at higher concentrations. Therefore, we decided not to set a CRLB cutoff point in this study to avoid misinterpretation of our Lac data, and by doing so, the mean CRLB of Lac in this study was 30.8%. Supplementary Table 2 demonstrates that inclusion of only individuals with CRLB<30% would have yielded a smaller sample size (BD, n=26; HC, n=26) with similar results.
Our GSH data are in agreement with the 5 previous MRS studies on GSH in BD (Chitty et al., 2013, 2014, 2015; Lagopoulos et al., 2013; Godlewska et al., 2014). All studies reported no differences in GSH levels between BD and controls within different voxels but included BD type I, II, and BD spectrum during all mood episodes. Two of these studies reported a negative association between alcohol and tobacco use with GSH levels in the ACC that was specific to BD patients (Chitty et al., 2013, 2014). A longitudinal study reported that elevated GSH levels in the hippocampus were associated with lower alcohol consumption and frequency of tobacco use (Chitty et al., 2015). Based on our data, from a sample that included exclusively euthymic patients, taken together with previous GSH 1H-MRS studies, it can be concluded that the levels of GSH measured by 1H-MRS do not differ between BD patients and HC regardless of mood state. In BD patients, we found a correlation between Lac and GSH, where higher Lac was associated with higher GSH levels. We hypothesize that the positive correlation found between GSH and Lac could be related to the presence of a physiological compensatory mechanism, in which there is an increase in GSH levels as Lac levels increase. Similar results have been observed in early-stage schizophrenia and posttraumatic stress disorder, where a higher GSH was noted in subjects with greater oxidative stress (Michels et al., 2014; Wijtenburg et al., 2015). We hypothesize that both BD and HC groups had different patterns of correlation between Lac and GSH, because the patients were medicated and stable, allowing a compensatory response of GSH to increased mitochondrial dysfunction. Therefore, we speculate that the association between Lac and GSH is impaired during acute mood episodes.
A limitation to studying euthymic BD type I patients is the difficulty finding subjects without symptoms who are not under medication treatment. Therefore, the use of medications should always be controlled as a cofactor in this type of study. Consequently, we can only state that the findings reported are the result of an interaction among all the factors and probably a metabolic feature of euthymic medicated BD type I patients. Moreover, the present sample of BD patients differed from HC for age and gender, although these variables were controlled for in all analysis. Furthermore, the differences in tissue voxel composition observed between BD patients and HC are probably explained by cortical thinning, previously reported for BD (Lyoo et al., 2006) and major depressive disorder (Li M et al., 2014). In congruence with this phenomenon, we found a smaller contribution of the caudal ACC cortex to the total brain tissue in the MRS voxel among BD patients compared with HC. To compensate for this variation, the fGM and CSF% were considered in the statistical analysis.
To the best of our knowledge, this investigation had the largest sample size for an MRS study of Lac and GSH in euthymic BD type I patients. Our data showed that BD type I patients had higher levels of Lac in the dACC, which could be an indication of altered mitochondrial function and a glycolytic shift in BD even during euthymia. Moreover, Lac correlated with GSH levels regardless of BD diagnosis, indicating a physiological association between the antioxidative system and mitochondrial dysfunction.
Statement of Interest
None.
Supplementary Material
Acknowledgments
This study was sponsored by Sao Paulo research Foundation grant numbers 2012/23796-2 and 2010/18672–7. This trial is registered at ClincalTrials.gov: NCT01237158.
References
- Abdalla DS, Monteiro HP, Oliveira JA, Bechara EJ. (1986) Activities of superoxide dismutase and glutathione peroxidase in schizophrenic and manic-depressive patients. Clin Chem 32:805–807. [PubMed] [Google Scholar]
- Andreazza AC, Cassini C, Rosa AR, Leite MC, de Almeida LMV, Nardin P, Cunha ABN, Ceresér KM, Santin A, Gottfried C, Salvador M, Kapczinski F, Gonçalves CA. (2007) Serum S100B and antioxidant enzymes in bipolar patients. J Psychiatr Res 41:523–529. [DOI] [PubMed] [Google Scholar]
- Andreazza AC, Kapczinski F, Kauer-Sant’Anna M, Walz JC, Bond DJ, Gonçalves CA, Young LT, Yatham LN. (2009) 3-Nitrotyrosine and glutathione antioxidant system in patients in the early and late stages of bipolar disorder. J Psychiatry Neurosci 34:263–271. [PMC free article] [PubMed] [Google Scholar]
- Andreazza AC, Shao L, Wang J-F, Young LT. (2010) Mitochondrial complex I activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Arch Gen Psychiatry 67:360–368. [DOI] [PubMed] [Google Scholar]
- Andreazza AC, Wang J-F, Salmasi F, Shao L, Young LT. (2013) Specific subcellular changes in oxidative stress in prefrontal cortex from patients with bipolar disorder. J Neurochem 127:552–561. [DOI] [PubMed] [Google Scholar]
- Banerjee U, Dasgupta A, Rout JK, Singh OP. (2012) Effects of lithium therapy on Na+-K+-ATPase activity and lipid peroxidation in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry 37:56–61. [DOI] [PubMed] [Google Scholar]
- Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, Yücel M, Gama CS, Dodd S, Dean B, Magalhães PVS, Amminger P, McGorry P, Malhi GS. (2011) Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev 35:804–817. [DOI] [PubMed] [Google Scholar]
- Brady RO, Cooper A, Jensen JE, Tandon N, Cohen B, Renshaw P, Keshavan M, Ongür D. (2012) A longitudinal pilot proton MRS investigation of the manic and euthymic states of bipolar disorder. Transl Psychiatry 2:e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NC, Andreazza AC, Young LT. (2014) An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res 218:61–68. [DOI] [PubMed] [Google Scholar]
- Cavanagh JTO, Van Beck M, Muir W, Blackwood DHR. (2002) Case-control study of neurocognitive function in euthymic patients with bipolar disorder: an association with mania. Br J Psychiatry 180:320–326. [DOI] [PubMed] [Google Scholar]
- Cavassila S, Deval S, Huegen C, van Ormondt D, Graveron-Demilly D. (2001) Cramér-Rao bounds: an evaluation tool for quantitation. NMR Biomed 14:278–283. [DOI] [PubMed] [Google Scholar]
- Cecil KM, DelBello MP, Morey R, Strakowski SM. (2002) Frontal lobe differences in bipolar disorder as determined by proton MR spectroscopy. Bipolar Disord 4:357–365. [DOI] [PubMed] [Google Scholar]
- Chang K, Adleman N, Dienes K, Barnea-Goraly N, Reiss A, Ketter T. (2003) Decreased N-acetylaspartate in children with familial bipolar disorder. Biol Psychiatry 53:1059–1065. [DOI] [PubMed] [Google Scholar]
- Chinnery PF, Schon EA. (2003) Mitochondria. J Neurol Neurosurg Psychiatr 74:1188–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitty KM, Lagopoulos J, Hickie IB, Hermens DF. (2013) Risky alcohol use in young persons with emerging bipolar disorder is associated with increased oxidative stress. J Affect Disord 150:1238–1241. [DOI] [PubMed] [Google Scholar]
- Chitty KM, Lagopoulos J, Hickie IB, Hermens DF. (2014) The impact of alcohol and tobacco use on in vivo glutathione in youth with bipolar disorder: an exploratory study. J Psychiatr Res 55:59–67. [DOI] [PubMed] [Google Scholar]
- Chitty KM, Lagopoulos J, Hickie IB, Hermens DF. (2015) A longitudinal proton magnetic resonance spectroscopy study investigating oxidative stress as a result of alcohol and tobacco use in youth with bipolar disorder. J Affect Disord 175:481–487. [DOI] [PubMed] [Google Scholar]
- Chu W-J, DelBello MP, Jarvis KB, Norris MM, Kim M-J, Weber W, Lee J-H, Strakowski SM, Adler CM. (2013) Magnetic resonance spectroscopy imaging of lactate in patients with bipolar disorder. Psychiatry Res 213:230–234. [DOI] [PubMed] [Google Scholar]
- Clark L, Iversen SD, Goodwin GM. (2002) Sustained attention deficit in bipolar disorder. Br J Psychiatry 180:313–319. [DOI] [PubMed] [Google Scholar]
- Dager SR, Friedman SD, Heide A, Layton ME, Richards T, Artru A, Strauss W, Hayes C, Posse S. (1999) Two-dimensional proton echo-planar spectroscopic imaging of brain metabolic changes during lactate-induced panic. Arch Gen Psychiatry 56:70–77. [DOI] [PubMed] [Google Scholar]
- Dager SR, Friedman SD, Parow A, Demopulos C, Stoll AL, Lyoo IK, Dunner DL, Renshaw PF. (2004) Brain metabolic alterations in medication-free patients with bipolar disorder. Arch Gen Psychiatry 61:450–458. [DOI] [PubMed] [Google Scholar]
- de Sousa RT, Zarate CA, Zanetti MV, Costa AC, Talib LL, Gattaz WF, Machado-Vieira R. (2014) Oxidative stress in early stage bipolar disorder and the association with response to lithium. J Psychiatr Res 50:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R. (2000) Metabolism and functions of glutathione in brain. Prog Neurobiol 62:649–671. [DOI] [PubMed] [Google Scholar]
- DSM-IV PATFO (2000) Diagnostic and statistical manual of mental disorders: DSM-IV-TR. American Psychiatric Publishing, Inc. [Google Scholar]
- Fan T. (1996) Metabolite profiling by one- and two-dimensional NMR analysis of complex mixtures. Prog Nucl Mag Reson Spectrosc:161–219. [Google Scholar]
- Ferrier IN, Thompson JM. (2002) Cognitive impairment in bipolar affective disorder: implications for the bipolar diathesis. Br J Psychiatry 180:293–295. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Williams JB. (1996) Structured clinical interview for DSM-IV axis I disorders SCID-I. Washington, DC: American Psychiatric Press. [Google Scholar]
- Frahm J, Krüger G, Merboldt KD, Kleinschmidt A. (1996) Dynamic uncoupling and recoupling of perfusion and oxidative metabolism during focal brain activation in man. Magn Reson Med 35:143–148. [DOI] [PubMed] [Google Scholar]
- Fuchs A, Boesiger P, Schulte RF, Henning A. (2013) ProFit revisited. Magn Reson Med 71:458–468. [DOI] [PubMed] [Google Scholar]
- Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, Posse S, Jung RE, Morrison LA. (2006) Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med 55:1219–1226. [DOI] [PubMed] [Google Scholar]
- Gawryluk JW, Wang J-F, Andreazza AC, Shao L, Young LT. (2011) Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol 14:123–130. [DOI] [PubMed] [Google Scholar]
- Godlewska BR, Yip SW, Near J, Goodwin GM, Cowen PJ. (2014) Cortical glutathione levels in young people with bipolar disorder: a pilot study using magnetic resonance spectroscopy. Psychopharmacology (Berl) 231:327–332. [DOI] [PubMed] [Google Scholar]
- Govindaraju V, Young K, Maudsley AA. (2000) Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed 13:129–153. [DOI] [PubMed] [Google Scholar]
- Gutteridge JM, Halliwell B. (2000) Free radicals and antioxidants in the year 2000. A historical look to the future. Ann N Y Acad Sci 899:136–147. [DOI] [PubMed] [Google Scholar]
- Hamilton M. (1960) A rating scale for depression. J Neurol Neurosurg Psychiatr 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Takahashi S, Shioiri T, Inubushi T. (1992) Brain phosphorous metabolism in depressive disorders detected by phosphorus-31 magnetic resonance spectroscopy. J Affect Disord 26:223–230. [DOI] [PubMed] [Google Scholar]
- Kato T, Takahashi S, Shioiri T, Inubushi T. (1993) Alterations in brain phosphorous metabolism in bipolar disorder detected by in vivo 31P and 7Li magnetic resonance spectroscopy. J Affect Disord 27:53–59. [DOI] [PubMed] [Google Scholar]
- Konradi C, Eaton M, MacDonald ML, Walsh J, Benes FM, Heckers S. (2004) Molecular evidence for mitochondrial dysfunction in bipolar disorder. Arch Gen Psychiatry 61:300–308. [DOI] [PubMed] [Google Scholar]
- Kreis R. (2004) Issues of spectral quality in clinical 1H-magnetic resonance spectroscopy and a gallery of artifacts. NMR Biomed 17:361–381. [DOI] [PubMed] [Google Scholar]
- Kreis R. (2016) The trouble with quality filtering based on relative Cramér-Rao lower bounds. Magn Reson Med 75:15–18. [DOI] [PubMed] [Google Scholar]
- Kreis R, Kyathanally S. eds. (2015) Don’t use relative Cramer Rao lower bounds for elimination of low quality data! MRS Processing and Quantification. Poster 1976, 23rd ed. Toronto: Available at: http://www.ismrm.org/15/program_files/WedTP0 2.htm. [Google Scholar]
- Kuloglu M, Ustundag B, Atmaca M, Canatan H, Tezcan AE, Cinkilinc N. (2002) Lipid peroxidation and antioxidant enzyme levels in patients with schizophrenia and bipolar disorder. Cell Biochem Funct 20:171–175. [DOI] [PubMed] [Google Scholar]
- Lagopoulos J, Hermens DF, Tobias-Webb J, Duffy S, Naismith SL, White D, Scott E, Hickie IB. (2013) In vivo glutathione levels in young persons with bipolar disorder: a magnetic resonance spectroscopy study. J Psychiatr Res 47:412–417. [DOI] [PubMed] [Google Scholar]
- Li M, Metzger CD, Li W, Safron A, van Tol M-J, Lord A, Krause AL, Borchardt V, Dou W, Genz A, Heinze H-J, He H, Walter M. (2014) Dissociation of glutamate and cortical thickness is restricted to regions subserving trait but not state markers in major depressive disorder. J Affect Disord 169: 91–100. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Sung YH, Dager SR, Friedman SD, Lee J-Y, Kim SJ, Kim N, Dunner DL, Renshaw PF. (2006) Regional cerebral cortical thinning in bipolar disorder. Bipolar Disord 8:65–74. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Andreazza AC, Viale CI, Zanatto V, Cereser V, da Silva Vargas R, Kapczinski F, Portela LV, Souza DO, Salvador M, Gentil V. (2007) Oxidative stress parameters in unmedicated and treated bipolar subjects during initial manic episode: a possible role for lithium antioxidant effects. Neurosci Lett 421:33–36. [DOI] [PubMed] [Google Scholar]
- Michels L, Schulte-Vels T, Schick M, O’Gorman RL, Zeffiro T, Hasler G, Mueller-Pfeiffer C. (2014) Prefrontal GABA and glutathione imbalance in posttraumatic stress disorder: preliminary findings. Psychiatry Res 224:288–295. [DOI] [PubMed] [Google Scholar]
- Mlynárik V, Gruber S, Moser E. (2001) Proton T (1) and T (2) relaxation times of human brain metabolites at 3 Tesla. NMR Biomed 14:325–331. [DOI] [PubMed] [Google Scholar]
- Moore GJ, Galloway MP. (2002) Magnetic resonance spectroscopy: neurochemistry and treatment effects in affective disorders. Psychopharmacol Bull 36:5–23. [PubMed] [Google Scholar]
- Moreira MT, Smith LA, Foxcroft D. (2009) Social norms interventions to reduce alcohol misuse in university or college students. Cochrane Database Syst Rev:CD006748. [DOI] [PubMed] [Google Scholar]
- Orth M, Schapira AH. (2001) Mitochondria and degenerative disorders. Am J Med Genet 106:27–36. [DOI] [PubMed] [Google Scholar]
- Provencher SWS. (1993) Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30:672–679. [DOI] [PubMed] [Google Scholar]
- Raffa M, Barhoumi S, Atig F, Fendri C, Kerkeni A, Mechri A. (2012) Reduced antioxidant defense systems in schizophrenia and bipolar I disorder. Prog Neuropsychopharmacol Biol Psychiatry 39:371–375. [DOI] [PubMed] [Google Scholar]
- Regenold WT, Phatak P, Marano CM, Sassan A, Conley RR, Kling MA. (2009) Elevated cerebrospinal fluid lactate concentrations in patients with bipolar disorder and schizophrenia: implications for the mitochondrial dysfunction hypothesis. Biol Psychiatry 65:489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudkin TM, Arnold DL. (1999) Proton magnetic resonance spectroscopy for the diagnosis and management of cerebral disorders. Arch Neurol 56:919–926. [DOI] [PubMed] [Google Scholar]
- Schulte RF, Boesiger P. (2006) ProFit: two-dimensional prior-knowledge fitting of J-resolved spectra. NMR Biomed 19:255–263. [DOI] [PubMed] [Google Scholar]
- Schulte RF, Lange T, Beck J, Meier D, Boesiger P. (2006) Improved two-dimensional J-resolved spectroscopy. NMR Biomed 19:264–270. [DOI] [PubMed] [Google Scholar]
- Schurr A. (2006) Lactate: the ultimate cerebral oxidative energy substrate? J Cereb Blood Flow Metab 26:142–152. [DOI] [PubMed] [Google Scholar]
- Shanske AL, Shanske S, DiMauro S. (2001) The other human genome. Arch Pediatr Adolesc Med 155:1210–1216. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59:22–33; quiz34–quiz57. [PubMed] [Google Scholar]
- Smith SA, Levante TO, de Beer R, Luyten PR, van Ormondt D. (1994) Computer simulations in magnetic resonance. An object-oriented programming approach. J Magn Reson A:75–105. [Google Scholar]
- Soeiro de Souza MG, Salvadore G, Moreno RA, Otaduy MCG, Chaim KT, Gattaz WF, Zarate CA, Machado-Vieira R. (2013) Bcl-2 rs956572 Polymorphism is associated with increased anterior cingulate cortical glutamate in euthymic bipolar I disorder. Neuropsychopharmacology 38:468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeiro-de-Souza MG, Andreazza AC, Carvalho AF, Machado-Vieira R, Young LT, Moreno RA. (2013) Number of manic episodes is associated with elevated DNA oxidation in bipolar I disorder. Int J Neuropsychopharmacol 16:1505–1512. [DOI] [PubMed] [Google Scholar]
- Soeiro de Souza MG, Henning A, Machado-Vieira R, Moreno RA, Pastorello BF, da Costa Leite C, Vallada H, Otaduy MCG. (2015) Anterior cingulate Glutamate-Glutamine cycle metabolites are altered in euthymic bipolar I disorder. Eur Neuropsychopharmacol 25:2221–2229. [DOI] [PubMed] [Google Scholar]
- Stork C, Renshaw PF. (2005) Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Mol Psychiatry 10:900–919. [DOI] [PubMed] [Google Scholar]
- Sun X, Wang J-F, Tseng M, Young LT. (2006) Downregulation in components of the mitochondrial electron transport chain in the postmortem frontal cortex of subjects with bipolar disorder. J Psychiatry Neurosci 31:189–196. [PMC free article] [PubMed] [Google Scholar]
- Tkác I, Starcuk Z, Choi IY, Gruetter R. (1999) In vivo 1H NMR spectroscopy of rat brain at 1ms echo time. Magn Reson Med 41:649–656. [DOI] [PubMed] [Google Scholar]
- van der Veen JW, de Beer R, Luyten PR, van Ormondt D. (1988) Accurate quantification of in vivo 31P NMR signals using the variable projection method and prior knowledge. Magn Reson Med 6:92–98. [DOI] [PubMed] [Google Scholar]
- Wijtenburg SA, Yang S, Fischer BA, Rowland LM. (2015) In vivo assessment of neurotransmitters and modulators with magnetic resonance spectroscopy: application to schizophrenia. Neurosci Biobehav Rev 51:276–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Dydak U, Harezlak J, Nixon J, Dzemidzic M, Gunn AD, Karne HS, Anand A. (2013) Neurochemical abnormalities in unmedicated bipolar depression and mania: a 2D 1H MRS investigation. Psychiatry Res 213:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. (1978) A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 133:429–435. [DOI] [PubMed] [Google Scholar]
- Yüksel C, Ongur D. (2010) Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry 68:785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. (2001) Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging 20:45–57. [DOI] [PubMed] [Google Scholar]
- Zoelch N, Hock A, Scheidegger M, Hulka L, Quednow B, Henning A. eds. (2015) Necessity of tissue volume composition correction for internal referencing - MRS Processing and Quantification. Poster 1977, 23rd ed. Toronto: Available at: http://www.ismrm.org/15/program_files/WedTP02.htm. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.