Abstract
As opposed to conscious, personally relevant (explicit) memories that we can recall at will, implicit (unconscious) memories are prototypical of ‘hidden’ memory; memories that exist, but that we do not know we possess. Nevertheless, our behaviour can be affected by these memories; in fact, these memories allow us to function in an ever-changing world. It is still unclear from behavioural studies whether similar memories can be formed during anaesthesia. Thus, a relevant question is whether implicit memory formation is a realistic possibility during anaesthesia, considering the underlying neurophysiology. A different conceptualization of memory taxonomy is presented, the serial parallel independent model of Tulving, which focuses on dynamic information processing with interactions among different memory systems rather than static classification of different types of memories. The neurophysiological basis for subliminal information processing is considered in the context of brain function as embodied in network interactions. Function of sensory cortices and thalamic activity during anaesthesia are reviewed. The role of sensory and perisensory cortices, in particular the auditory cortex, in support of memory function is discussed. Although improbable, with the current knowledge of neurophysiology one cannot rule out the possibility of memory formation during anaesthesia.
Keywords: anaesthesia, general; hypnosis, anaesthetic; memory; memory, episodic; memory, long-term; recognition (psychology)
Editor's key points.
Unconscious memory formation during anaesthesia after ablation of conscious memory is controversial.
The serial parallel independent model of memory formation provides a useful construct for conceptualizing anaesthetic effects.
Neurophysiological evidence for anatomically distributed memory formation provides a basis for formation and retrieval of sensate memories under anaesthesia.
The study of ‘unconscious memory during anaesthesia’ is rife with terminology, which reflects not only the uncertainty of observations attempted in these studies, but also vagueness about the underlying neurocognitive/psychological mechanisms that underlie these inconsistently documented phenomena. Implicit (unconscious) memory is frequently defined as an exclusionary phenomenon [i.e. that it is not explicit (conscious) memory]. This highlights the uncertainties present in understanding these memory processes.
Evidence of a memory is obtained by measuring a change in behaviour after formation of that memory, and in that sense, memory is a behaviour. A memory might be best defined as being embodied within a change in synaptic connections in the brain. These changes produce a brain different from what it was before, that difference being the memory.1 More accurately, the changes in synaptic connections embody a consolidated memory, which is the end result of a series of neurophysiological events associated with perturbations of the electrophysiological milieu in which the brain resides (e.g. hippocampal theta rhythms).2 In simpler terms, memory itself cannot be measured directly by a third party or monitor, as done with many physiological measures, such as blood pressure, temperature, or heart rhythm. The person in whom the memory is sought must play a role in providing evidence of whether a memory is present or not. This is difficult enough in the case of conscious memories, a relevant example being the determination of whether recognition memory is based on familiarity (i.e. the face is familiar, but I can′t remember the name) or on recollection of additional contextual details. Controversy abounds as to whether familiarity and recollection represent separate conscious memory processes, with associated unique electro- and neurophysiological signatures, or rather, are different expressions of a unitary system of memory.3–7 The ‘objective signatures’ are by necessity related to the behaviour of the individuals under study, which inherently inserts a significant degree of variability into these measurements. Additionally, the exact context in which the behaviour is measured can be critically important.8
These issues are compounded many-fold in the case of unconscious memory. Evidence of an unconscious memory is obtained when an associated behavioural change after exposure to study stimuli can be documented with a certain degree of statistical likelihood. This is the key problematic issue regarding unconscious memories. In theory, they can be embodied in the brain, but may not be able to be detected by changes in behaviour, these being highly dependent on methodology. Additional controversy arises regarding whether the person with the unconscious memory is aware of that memory.9 Evidence of awareness would classify the memory as a conscious one. Thus, unconscious memories must be detected by measures such as reaction time, preference for one stimulus and not another, etc., not under direct conscious control. Such measures may be considered analogous to how memory is detected in animals, which cannot report directly on what they remember.10
Thus, it is no surprise that the literature to support or refute the presence of an unconscious memory formed during anaesthesia is close to chance.11,12 Originally, it was thought that such variability was related to the specifics of the anaesthetic state. The most promising insight was to control the depth of anaesthesia as measured by Bispectral Index (BIS®) during presentation of material (usually isolated auditory word stimuli presented repeatedly). The likelihood of memory formation seemed to increase as BIS did.13–15 This was teleologically satisfying, and a ‘dose–response’ curve of unconscious memory was postulated, being more resistant to anaesthetics than conscious memories. The veracity of these observations was bolstered by methods to differentiate conscious from unconscious memory using cognitive manipulations such as the process dissociation procedure.15 Furthermore, modulation of the emotive background state (presence/absence of surgery, use of narcotics) also seemed a promising avenue to optimize conditions conducive to memory formation.14–16 Although a number of studies seemed to progress in a positive direction, ultimately no change in the ability to produce memories reliably during anaesthesia has occurred.17 Part of the issue may be that quantification of the anaesthetic state might require different methods and/or improved algorithms for signal analyses than offered in the current set of depth-of-anaesthesia monitors.18 In those studies that detect implicit memory, the observed effect is usually quite small. With the number of participants in a typical study, the error range is often close to including the null hypothesis, such that small changes in the data set, by a single observation in some instances, or in underlying assumptions (e.g. whether chance guessing is included in the process dissociation procedure model or not) would negate the observed effect.19
Anaesthesia most commonly ablates conscious memory formation on the basis of sedation/hypnosis, with the maximal effect occurring when sufficient medication induces a loss of response to verbal or physical stimulation. This is the anaesthetic state considered in this manuscript. Many studies regarding mechanisms of anaesthesia have been carried out in volunteers who are unresponsive to different degrees of stimulation; however, it should be noted that in the clinical setting, this ‘anaesthetic’ state could be regarded as ‘sedation’ rather than ‘anaesthesia’. As propofol is such an easy drug to use in volunteer studies because of its ease of titration, many investigations use this amnesic drug. It should be pointed out that in the setting of unresponsiveness, the lack of conscious memory formation is entirely the result of sedation/hypnosis and not from the amnesic effect of propofol. The amnesic effects of propofol occur at lower concentrations than those that produce significant sedation and seem to inhibit memory processes subsequent to transfer of information from working to episodic (explicit) memory.20,21
This review focuses on whether unconscious memory formation during anaesthesia could have a plausible neurophysiological basis. If no rational neurophysiological basis exists for memory formation during anaesthesia, then the body of negative studies is more likely to be true. Positive results could be attributable to factors that plague psychology/psychiatry research, recently of topical interest.22,23 Alternatively, if a plausible basis can be put forward for memory formation, then more weight might be given to positive results, and indeed, it might be fruitful to refine methodology further in order to allow a predictable setting in which memory formation during anaesthesia could be studied.
Unconscious memory formation in the absence of anaesthetics will be considered first, in order to illustrate the brain processes necessary to form a memory without conscious awareness. But before that, it is most useful to consider anaesthetic effects on memory using conceptualizations of human memory systems. One such conceptualization that is not well known, but is useful in the present context, is the serial parallel independent (SPI) model proposed by Tulving.
Serial parallel independent model of human memory
In the last few decades, conceptualizations of human memory have evolved from considering memory as a unitary phenomenon to one of multiple systems that subserve specific memory functions. Initially, distinctions between short- and long-term memory were proposed, and this explained a number of experimental observations.24 Subsequently, short-term (working) memory was better modelled as a set of interacting subsystems, including the visuospatial scratch pad, phonologic loop, central executive, and more recently, the episodic buffer.25,26 Likewise, long-term memory was conceptualized as consisting of semantic vs episodic memory. The latter relates to personal memories, which occur not only in the context of a time and place, but also incorporate ‘autonoetic’ awareness of a subjective sense of self and time for past events, and the ability to ‘travel’ into the future in thought.27 The autonoetic quality is likely to differentiate human episodic memory from ‘episodic-like’ behaviour in animals, ultimately relegating the understanding of episodic memory in humans, and anaesthetic effects thereon, to studying humans themselves.28 Semantic memory concerns facts, concepts, and beliefs, and is considered a more ‘durable’ and ‘primitive’ memory system. There are many more lesion patients in whom episodic memory is impaired with intact semantic memory than the reverse, including what one might consider drug-induced temporary ‘lesions’ produced by propofol and benzodiazepines.29 In many ways, episodic memory can be considered a ‘slave’ system dependent on semantic memory. Thus, episodic memory is formed not only from factual information but also from one's beliefs contained in semantic memory.30 This relationship is useful to conceptualize false memory, where the highest level memory processes (i.e. episodic) do not differentiate between factual and non-factual information input.10
Conceptualizations of human memory are still evolving over time, as is the understanding of the underlying neurophysiological basis of memory. Although there is no doubt that the hippocampus is needed to encode new conscious (episodic) memories, its role in retrieval of those memories, and the possibility of reconsolidation of retrieved memories, are active areas of research.31,32 Additionally, it now appears that the hippocampus participates in processes independent of conscious awareness.33,34 There is always a chasm between cognitive concepts of memory, used to model behavioural results, and the underlying neuroanatomical and functional correlates of those behaviours. One needs to examine the impact of anaesthesia using both lenses. The SPI model of human memory was proposed by Tulving27 to tackle a number of issues and observations that other models could not address. Most of these relate to interactions between semantic and episodic memory systems, but the greatest benefit is obtained from conceptualiztion of the lowest ‘rung’ of the model, the perceptual representation system (PRS).35
The SPI model considers three parallel systems of memory: the PRS, semantic, and episodic systems (Fig. 1). The relationship between systems is defined by which process is operational, namely encoding, storage, or retrieval of memories. These are summarized in the SPI acronym, where encoding is Serial, storage is Parallel, and retrieval is Independent. During encoding, information is passed in a serial fashion from one system to another in hierarchical fashion. The model does not incorporate (at this stage) any ability to pass information directly from PRS to episodic memory, which fits well with recent observations.36 Once information has been processed and encoded, the perceptual, semantic, and episodic memories are stored separately in each system, in parallel, with the nature of memory being specific for each system. Thus the perceptual qualities of a memory are stored in PRS, whereas recollective details of the same memory, those that are personally relevant, are stored in episodic memory. Tulving uses the terminology of ‘perceptual’ memory in the SPI model; however, this terminology is potentially confusing when one considers the literature of anaesthetic effects on consciousness, where the concept of a ‘percept’ relates to the binding of information in disparate regions of the brain. To clarify, I will use the term ‘sensate’ to indicate the perceptual memory in Tulving's model. The serial nature of processing of information from sensation to perception and potential conscious memory formation is a common theme in other conceptualizations of memory, and the independence of sensory input (sensation) from awareness or perception can help to contextualize the interactions of anaesthetics on consciousness.37 Memories can be retrieved independently from each memory store, and on this basis, the SPI model is useful to characterize concepts such as the ‘remember–know’ distinction for recognition performance and production of false memories. The fact that retrieval of a memory can occur independently from a given system does not mean it has to occur from a single system; it can involve different levels of retrieval from the PRS, semantic, or episodic memory systems. Thus, ‘remembering’ an event from episodic memory is different from ‘knowing’ one from semantic memory, but both may contribute to recognition of a previous event. Thus, it can be seen that memory is a highly distributed process, and in fact, some authorities argue that the concept of ‘a memory’ is too simplistic.38
Fig 1.
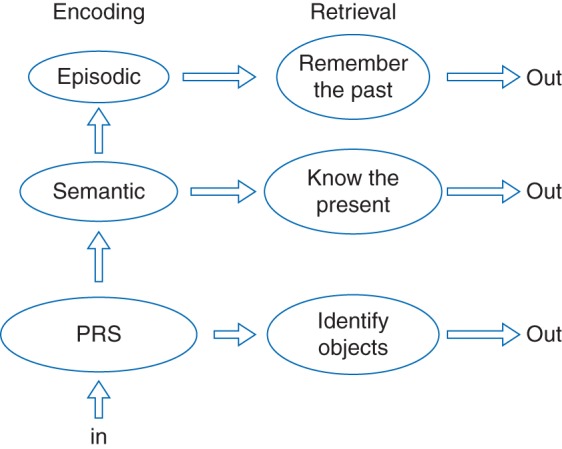
This is Tulving's conceptualization of human memory in the serial parallel independent (SPI) model. Three memory systems representing the perceptual representation system (PRS), semantic, and episodic memory are related in a hierarchical fashion during encoding, where information processing is Serial. Storage of memories is Parallel in each system, and these can be retrieved Independently. Of interest during anaesthesia is the PRS, the lowest ‘rung’ of the model (sensation). If any memory function operates during anaesthesia, it would be the PRS. It is likely that any perceptual (or sensate) memories would be stored in secondary sensory association cortices.
During anaesthesia, memory function, if present, is likely to be very basic. Of interest is the encoding of memories into the PRS during anaesthesia and the recognition of these memories (retrieval) directly from the PRS after anaesthesia. To ensure that retrieval is directly from the PRS and not other memory systems, procedures such as the process dissociation procedure can be used to determine contributions from the PRS and non-PRS memory systems.39 Most conceptualizations of memory involve a hierarchy of memory processing. Distinctions between processes can be made on behavioural and/or neuroanatomical grounds. For example, familiarity is frequently considered to be a dissociable component of recollection, because neurological lesions spare familiarity in the absence of recollection, whereas the opposite does not seem to occur.5 Some of these distinctions might not be mutually exclusive, another example being the role of the hippocampus in unconscious memory processes.40 The blurriness of traditional divisions might account for some of the difficulty in detecting unconscious or implicit memories, assuming mutual exclusion from conscious memory. Novel concepts regarding brain states during anaesthesia are emerging that also blur the traditional binary distinction between ‘awake’ and ‘anaesthetized’.41,42 In these conceptualizations, multiple brain states can exist within the same behavioural observations (e.g. unresponsiveness to voice or unremembered movement to command), and sensate memory formation might be possible in only a few of these.
Encoding and retrieval of memories in the perceptual representation system
In the SPI model, all information encoded into any type of memory must first pass through the PRS system. The PRS system by necessity involves sensory regions of the brain, the gateway for information from the outside world. It seems likely that physiological principles at play in one sensory system are relevant to others, as seems to be true for auditory, olfactory, and visual sensations.43 A necessary condition for memory formation during anaesthesia is that there is some functionality of the sensory system during anaesthesia. There is overwhelming evidence that auditory sensation is functional, albeit at a reduced level, during anaesthesia.44–49
In order to retrieve memories directly from the PRS, they must have been stored previously. The neurophysiology underlying memories that are unconscious (i.e. those that do not involve the semantic/episodic memory systems) is therefore relevant. In the absence of anaesthesia, this type of learning could be termed learning without awareness, or in more popular terminology, subliminal learning. What are the physiological underpinnings of learning without awareness, and if possible, where might these memories be stored? The latter question is difficult to answer, because it is still not known ‘where’ conscious memories are stored, which might vary through time. The perceptual system in the SPI model can be considered to be more primitive than other systems, and thus, might be characterized by simpler neurophysiology. Thus, conditions necessary for memory formation during anaesthesia include enough functionality of subliminal memory systems to allow encoding of sensate memories that can then be retrieved by processes independent of conscious effort.
Learning without awareness: the neurophysiology of subliminal learning
Unconscious memories are those we do not know we have; otherwise, these would be conscious memories. We are constantly barraged with sensory input, and we have to adapt to an ever-changing world. Some ability to process relevant incoming information and incorporate it into a ‘survival’ strategy must be present. In the initial stages after sensation, these processes by necessity need to be unconscious, to avoid what could be termed sensory overload.50–51 At a certain stage of processing, attention is then directed to incoming information, which is selected as being important, recruiting more brain networks that eventually result in a conscious percept.52,53
Thus, unconscious memory systems are very efficient not only at detecting relevant stimuli, as measured by heightened electrophysiological and neurobiological responses to a new stimulus (so-called orienting reflex), but also at learning complex information without taxing the deliberative conscious memory systems. For example, complex rules can be learned that result in performance improvement without any conscious knowledge of the underlying rule.54 Neural correlates of this learning reveal multiple brain regions involved in these processes. Information transfer between these regions is evidenced by synchrony, for example, during rule learning.55 Thus, to learn unconscious memories, it seems that some degree of network function is necessary to allow information flow between different brain regions. However, memory formation during anaesthesia might simply be a sensate memory of having experienced a stimulus previously and might not need more complex and distributed brain processes that underlie rule learning.
Conscious memory operations are traditionally typified by involvement of the hippocampus, which communicates with other cortical brain regions via the parahippocampus, entorhinal cortex, fornix–thalamic pathways, or a combination of these (there are few, if any, direct connections of the hippocampus to the cortex).56,57 Without the hippocampus, no conscious memory can be formed. In contrast, non-conscious memories are associated with other neural correlates, many of which may reside in other medial temporal lobe structures. Thus, hippocampal involvement, or lack thereof, can be a reasonable proxy of non-conscious memories in animal models. One type of memory in animals that demonstrates insensitivity to the presence/absence of the hippocampus is object recognition memory, which can be considered a model of a more ‘primitive’ memory system than the human episodic memory system.58 It was initially thought that object recognition memory required the hippocampus, but different studies provided conflicting results. More careful consideration of task design revealed that many studies were contaminated by the animal using visual/spatial environmental cues to identify previously experienced objects. When performed in an impoverished environment object recognition is unaffected by hippocampal lesions. It should be noted that object recognition memory is widely regarded as a model of declarative (conscious) memory in humans, but this is hard to reconcile with the lack of hippocampal involvement when spatial context is eliminated from the object recognition paradigm. A possibly analogous memory process is face recognition in humans, which has recently been shown to be independent of the hippocampus.59 Thus, non-episodic memories, in particular perceptual memory in the SPI model, are likely to be mediated by structures in the medial temporal lobe other than the hippocampus, such as the entorhinal cortex (the main input to the hippocampus) and the perirhinal and parahippocampal cortices.
It is becoming increasingly questionable whether a clear hippocampal–non-hippocampal divide genuinely exists, and various subregions of the hippocampus seem to be specialized for different functions.60–63 Evidence is emerging of hippocampal involvement in memory processes (e.g. working memory or contextual cueing) previously thought not to be dependent on the hippocampus.33,34,64,65 The hippocampus has connections with structures involved with very basic memory processing, such as the amygdala, and forebrain structures via the fornix.66 These in turn interact with various thalamic nuclei, which in turn support specific memory processes.67 Connections of anterior thalamic nuclei to parahippocampal and entorhinal cortices involve the retrosplenial and posterior cingulate cortices, and these structures form in part what is known as the default mode network. Likewise, many of these non-hippocampal structures are involved in object recognition memory.
As can be seen, a large and diverse set of neuroanatomical structures support memory functions, their roles being somewhat fluid dependent upon specific circumstances. Rather than dividing memory into static categories, it might be better to think of representations of information that support memory, or processing modes required for memory operations.33,38 Ultimately, object recognition and sensate memory seems to reside in secondary sensory association cortices, the location being determined by the modality of encoding. The time required to consolidate these memories is as yet unclear.43,68 Thus, if sensate memories can be formed during anaesthesia, it is likely that these are stored in secondary sensory cortices.
Learning during anaesthesia: neurophysiological considerations
Serial processing of sensation to conscious percept involves numerous processes, including those of attention. It is likely that anaesthetics will have differing influences on these processes, potentially allowing low-level processes to proceed whilst blocking others needed for conscious perception. For sensate memory formation to occur during anaesthesia, more than basic processing in primary sensory cortices is needed. It is now well established that sensation is preserved during anaesthesia, and local connectivity in sensory and perisensory regions is also well preserved.69 Sensation must become sensate memory in the secondary sensory cortices. It seems unlikely that direct encoding of memory from sensation in the primary sensory cortex to secondary association cortex occurs, and lesion studies in animals support this view.70 These studies demonstrated the importance of corticothalamocortical circuits in processing of sensation to association cortices. Additional support is provided by a re-analysis of data from the study by Boveroux and colleagues.69 The largest difference between the sedated, conscious state and the unresponsive state was characterized by negative corticocortical connections.71 Additionally, sensate memory resulting from a direct transfer of information from sensory cortex should have been reliably observed after decades of research searching for implicit memory formed during anaesthesia. Thus, it seems likely that the formation of sensate memory requires more diverse brain regions and functionality than solely present in primary and association sensory cortices. Other support is provided by the fact that even a very ‘basic’ form of memory, cross-modality associative memory, requires participation of the entorhinal cortex.72
The thalamocortical switch and sensory throughput
A key question is the differing roles of thalamic nuclei as consciousness ‘switches’ and in sensory transmission. The thalamus is a key target of anaesthesia, with the onset of unresponsiveness mediated by the midline intralaminar nuclei.73–75 With clear evidence of sensation in humans during unresponsiveness, other nuclei in the thalamus must still be able to transmit sensory information under anaesthesia. The multiplicity of effects of anaesthesia on different nuclei of the thalamus is becoming increasingly evident.73 Such functionality might not be static, and probably varies not only with the depth of anaesthesia, but also with other factors, such as oscillatory frequencies of inputs to the thalamus.76,77 The role of the thalamus in supporting memory function is being unravelled, with some nuclei (e.g. anterior thalamic) being almost as important as the hippocampus in conscious memory.67 As different thalamic nuclei are inhibited to different extents during anaesthesia, how anaesthesia affects specific memory processes could relate to differential effects on these thalamic structures.78,79 Even though connectivity of the primary auditory cortex to other brain regions is inhibited during anaesthesia, connectivity amongst other ‘higher’ brain regions is diminished but not eliminated.48,69 Different networks are affected to different extents, not always as one might expect. It is conceivable that ‘higher order’ networks subserving more complex cognitive functions (executive networks) are inhibited more than those with more basic functions, such as the default mode network, but this is not necessarily the case.69 A picture of somewhat unpredictable effects of anaesthetics on network connectivity and function is emerging.
Network function
Connectivity between different brain regions supporting consciousness is emerging as a key target of anaesthetic action.79–81 Thus, network function supporting information processing during anaesthesia is a major focus of current investigation, with comparisons before and after loss of responsiveness.82,83 Anaesthesia differentially inhibits but does not entirely eliminate network connectivity. It is important to relate these changes to those of functionality, for example information content and transfer. In order to understand learning during anaesthesia, it is important to identify those networks involved in learning without awareness and to determine their functionality during anaesthesia. The latter requires a robust method to detect behavioural changes associated with the formation of unconscious memory.
Conclusions
A myriad of neurophysiological factors, yet to be elucidated, are likely to be needed for memory formation during anaesthesia to occur, and this might explain why observations of memory formation during anaesthesia are seemingly random. Concepts of human memory are still evolving, and unconscious memory during anaesthesia should not necessarily be tied to a given conceptualization. As our understanding of anaesthetic mechanisms on consciousness and memory increases, further insights into human memory might be gained, resulting in novel conceptualizations.37 Translational knowledge of memory function from animal studies might be most relevant for more ‘primitive’ forms of memory. By contextualizing memory function in terms of cognitive models, some guidance for further research is hopefully obtained. As with the ‘hard problem’ of consciousness, and how anaesthetics interact with these processes to produce unresponsiveness, one should be able to refine methods to detect unconscious memories more reliably, and more importantly, define a state of anaesthesia in which such memory formation is not only plausible, but reproducible. A critical lack of information exists regarding functionality of various networks during anaesthesia. Indeed, the networks supporting memory function itself are not well defined, particularly with regard to memory other than conscious episodic memory. The anaesthetic state might provide a useful tool to identify lower level, more ‘resilient’ networks that support lower hierarchy memory processes. Once this not insignificant hurdle is overcome, then attention can be focused on the relevance of these memories to the well-being of our patients.
Authors' contribution
R.A.V. is the sole author of this paper, accountable for accuracy and integrity of the paper.
Declaration of interest
None declared.
References
- 1.Mutso AA, Petre B, Huang L, et al. Reorganization of hippocampal functional connectivity with transition to chronic back pain. J Neurophysiol 2014; 111: 1065–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lega BC, Jacobs J, Kahana M. Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus 2012; 22: 748–61 [DOI] [PubMed] [Google Scholar]
- 3.Sauvage MM, Fortin NJ, Owens CB, Yonelinas AP, Eichenbaum H. Recognition memory: opposite effects of hippocampal damage on recollection and familiarity. Nat Neurosci 2008; 11: 16–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wixted JT, Squire LR. The role of the human hippocampus in familiarity-based and recollection-based recognition memory. Behav Brain Res 2010; 215: 197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vann SD, Tsivilis D, Denby CE, et al. Impaired recollection but spared familiarity in patients with extended hippocampal system damage revealed by 3 convergent methods. Proc Natl Acad Sci USA 2009; 106: 5442–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghetti S, Angelini L. The development of recollection and familiarity in childhood and adolescence: evidence from the dual-process signal detection model. Child Dev 2008; 79: 339–58 [DOI] [PubMed] [Google Scholar]
- 7.Wais PE, Mickes L, Wixted JT. Remember/know judgments probe degrees of recollection. J Cogn Neurosci 2008; 20: 400–5 [DOI] [PubMed] [Google Scholar]
- 8.Hanslmayr S, Staudigl T. How brain oscillations form memories—a processing based perspective on oscillatory subsequent memory effects. Neuroimage 2014; 85: 648–55 [DOI] [PubMed] [Google Scholar]
- 9.Newell BR, Shanks DR. Unconscious influences on decision making: a critical review. Behav Brain Sci 2014; 37: 1–19 [DOI] [PubMed] [Google Scholar]
- 10.Suthana N, Fried I. Percepts to recollections: insights from single neuron recordings in the human brain. Trends Cogn Sci 2012; 16: 427–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrade J. Learning during anaesthesia: a review. Br J Psychol 1995; 86: 479–506 [DOI] [PubMed] [Google Scholar]
- 12.Ghoneim MM, Block RI. Learning and memory during general anesthesia. Anesthesiology 1997; 87: 387–410 [DOI] [PubMed] [Google Scholar]
- 13.Iselin-Chaves IA, Willems SJ, Jermann FC, Forster A, Adam SR, Van der Linden M. Investigation of implicit memory during isoflurane anesthesia for elective surgery using the process dissociation procedure. Anesthesiology 2005; 103: 925–33 [DOI] [PubMed] [Google Scholar]
- 14.Deeprose C, Andrade J, Harrison D, Edwards N. Unconscious auditory priming during surgery with propofol and nitrous oxide anaesthesia: a replication. Br J Anaesth 2005; 94: 57–62 [DOI] [PubMed] [Google Scholar]
- 15.Lubke GH, Kerssens C, Phaf H, Sebel PS. Dependence of explicit and implicit memory on hypnotic state in trauma patients. Anesthesiology 1999; 90: 670–80 [DOI] [PubMed] [Google Scholar]
- 16.Deeprose C, Andrade J, Varma S, Edwards N. Unconscious learning during surgery with propofol anaesthesia. Br J Anaesth 2004; 92: 171–7 [DOI] [PubMed] [Google Scholar]
- 17.Lequeux P-Y, Hecquet F, Bredas P. Does anesthetic regimen influence implicit memory during general anesthesia? Anesth Analg 2014; 119: 1174–9 [DOI] [PubMed] [Google Scholar]
- 18.Blain-Moraes S, Tarnal V, Vanini G, et al. Neurophysiological correlates of sevoflurane-induced unconsciousness. Anesthesiology 2015; 122: 307–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hadzidiakos D, Horn N, Degener R, Buchner A, Rehberg B. Analysis of memory formation during general anesthesia (propofol/remifentanil) for elective surgery using the process-dissociation procedure. Anesthesiology 2009; 111: 293–301 [DOI] [PubMed] [Google Scholar]
- 20.Veselis RA, Reinsel RA, Feshchenko VA, Johnson R., Jr Information loss over time defines the memory defect of propofol: a comparative response with thiopental and dexmedetomidine. Anesthesiology 2004; 101: 831–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veselis RA, Reinsel RA, Feshchenko VA, Wronski M. The comparative amnestic effects of midazolam, propofol, thiopental, and fentanyl at equisedative concentrations. Anesthesiology 1997; 87: 749–64 [DOI] [PubMed] [Google Scholar]
- 22.Yong E. Replication studies: bad copy. Nature 2012; 485: 298–300 [DOI] [PubMed] [Google Scholar]
- 23.Shanks DR, Newell BR, Lee EH, et al. Priming intelligent behavior: an elusive phenomenon. PloS ONE 2013; 8: e56515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atkinson RC, Shiffrin RM. The control of short-term memory. Sci Am 1971; 225: 82–90 [DOI] [PubMed] [Google Scholar]
- 25.Baddeley AD. Working memory. Science 1992; 255: 556–9 [DOI] [PubMed] [Google Scholar]
- 26.Baddeley A. The episodic buffer: a new component of working memory? Trends Cogn Sci 2000; 4: 417–23 [DOI] [PubMed] [Google Scholar]
- 27.Tulving E. Episodic memory and common sense: how far apart? Philos Trans R Soc Lond B Biol Sci 2001; 356: 1505–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lega B, Burke J, Jacobs J, Kahana MJ. Slow-theta-to-gamma phase-amplitude coupling in human hippocampus supports the formation of new episodic memories. Cereb Cortex Advance Access published on October 14, 2014, doi:10.1093/cercor/bhu232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arndt J, Passannante A, Hirshman E. The effect of midazolam on implicit and explicit memory in category exemplar production and category cued recall. Memory 2004; 12: 158–73 [DOI] [PubMed] [Google Scholar]
- 30.Windhorst C. The slave model of autobiographical memory. Cogn Process 2005; 6: 253–65 [DOI] [PubMed] [Google Scholar]
- 31.Eichenbaum H. A cortical–hippocampal system for declarative memory. Nat Rev Neurosci 2000; 1: 41–50 [DOI] [PubMed] [Google Scholar]
- 32.Eichenbaum H. To sleep, perchance to integrate. Proc Natl Acad Sci USA 2007; 104: 7317–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henke K. A model for memory systems based on processing modes rather than consciousness. Nat Rev Neurosci 2010; 11: 523–32 [DOI] [PubMed] [Google Scholar]
- 34.Park H, Quinlan J, Thornton E, Reder LM. The effect of midazolam on visual search: Implications for understanding amnesia. Proc Natl Acad Sci USA 2004; 101: 17879–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tulving E, Schacter DL. Priming and human memory systems. Science 1990; 247: 301–6 [DOI] [PubMed] [Google Scholar]
- 36.Saksida LM. Neuroscience. Remembering outside the box. Science 2009; 325: 40–1 [DOI] [PubMed] [Google Scholar]
- 37.Pandit JJ. Acceptably aware during general anaesthesia: ‘dysanaesthesia’ – the uncoupling of perception from sensory inputs. Conscious Cogn 2014; 27: 194–212 [DOI] [PubMed] [Google Scholar]
- 38.Nadel L, Hardt O. Update on memory systems and processes. Neuropsychopharmacology 2011; 36: 251–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacoby LL. A process dissociation framework: separating automatic from intentional uses of memory. J Mem Lang 1991; 33: 1–18 [Google Scholar]
- 40.Duss SB, Reber TP, Hänggi J, et al. Unconscious relational encoding depends on hippocampus. Brain 2014; 137: 3355–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pandit JJ. Isolated forearm – or isolated brain? Interpreting responses during anaesthesia – or ‘dysanaesthesia’. Anaesthesia 2013; 68: 995–1000 [DOI] [PubMed] [Google Scholar]
- 42.Sanders RD, Tononi G, Laureys S, Sleigh JW. Unresponsiveness ≠ unconsciousness. Anesthesiology 2012; 116: 946–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sacco T, Sacchetti B. Role of secondary sensory cortices in emotional memory storage and retrieval in rats. Science 2010; 329: 649–56 [DOI] [PubMed] [Google Scholar]
- 44.Veselis RA, Feshchenko VA, Reinsel RA, Beattie B, Akhurst TJ. Propofol and thiopental do not interfere with regional cerebral blood flow response at sedative concentrations. Anesthesiology 2005; 102: 26–34 [DOI] [PubMed] [Google Scholar]
- 45.Heinke W, Fiebach CJ, Schwarzbauer C, Meyer M, Olthoff D, Alter K. Sequential effects of propofol on functional brain activation induced by auditory language processing: an event-related functional magnetic resonance imaging study. Br J Anaesth 2004; 92: 641–50 [DOI] [PubMed] [Google Scholar]
- 46.Kerssens C, Hamann S, Peltier S, Hu XP, Byas-Smith MG, Sebel PS. Attenuated brain response to auditory word stimulation with sevoflurane: a functional magnetic resonance imaging study in humans. Anesthesiology 2005; 103: 11–9 [DOI] [PubMed] [Google Scholar]
- 47.Plourde G, Belin P, Chartrand D, et al. Cortical processing of complex auditory stimuli during alterations of consciousness with the general anesthetic propofol. Anesthesiology 2006; 104: 448–57 [DOI] [PubMed] [Google Scholar]
- 48.Liu X, Lauer KK, Ward BD, Rao SM, Li SJ, Hudetz AG. Propofol disrupts functional interactions between sensory and high-order processing of auditory verbal memory. Hum Brain Mapp 2012; 33: 2487–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DiFrancesco MW, Robertson SA, Karunanayaka P, Holland SK. BOLD fMRI in infants under sedation: comparing the impact of pentobarbital and propofol on auditory and language activation. J Magn Reson Imaging 2013; 38: 1184–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Näätänen R. Attention and Brain Function. Hillsdale, NJ: L. Erlbaum; 1992. 494 pp [Google Scholar]
- 51.Parker ES, Cahill L, McGaugh JL. A case of unusual autobiographical remembering. Neurocase 2006; 12: 35–49 [DOI] [PubMed] [Google Scholar]
- 52.Dehaene S, Changeux J-P, Naccache L, Sackur J, Sergent C. Conscious, preconscious, and subliminal processing: a testable taxonomy. Trends Cogn Sci 2006; 10: 204–11 [DOI] [PubMed] [Google Scholar]
- 53.Jacob H, Brück C, Domin M, Lotze M, Wildgruber D. I can't keep your face and voice out of my head: neural correlates of an attentional bias toward nonverbal emotional cues. Cereb Cortex 2014; 24: 1460–73 [DOI] [PubMed] [Google Scholar]
- 54.Berns GS, Cohen JD, Mintun MA. Brain regions responsive to novelty in the absence of awareness. Science 1997; 276: 1272–5 [DOI] [PubMed] [Google Scholar]
- 55.Rose M, Haider H, Buchel C. The emergence of explicit memory during learning. Cereb Cortex 2010; 20: 2787–97 [DOI] [PubMed] [Google Scholar]
- 56.Duvernoy HM, Bourgouin P. The Human Hippocampus: Functional Anatomy, Vascularization and Serial Sections with MRI. 2nd completely revised and expanded Edn Berlin, New York: Springer; 1998; viii, 213 pp [Google Scholar]
- 57.Ward AM, Schultz AP, Huijbers W, Van Dijk KR, Hedden T, Sperling RA. The parahippocampal gyrus links the default-mode cortical network with the medial temporal lobe memory system. Hum Brain Mapp 2014; 35: 1061–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winters BD, Saksida LM, Bussey TJ. Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev 2008; 32: 1055–70 [DOI] [PubMed] [Google Scholar]
- 59.Smith CN, Jeneson A, Frascino JC, Kirwan CB, Hopkins RO, Squire LR. When recognition memory is independent of hippocampal function. Proc Natl Acad Sci USA 2014; 111: 9935–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strange B, Dolan R. Functional segregation within the human hippocampus. Mol Psychiatry 1999; 4: 508–11 [DOI] [PubMed] [Google Scholar]
- 61.Strange BA, Dolan RJ. Adaptive anterior hippocampal responses to oddball stimuli. Hippocampus 2001; 11: 690–8 [DOI] [PubMed] [Google Scholar]
- 62.Strange BA, Duggins A, Penny W, Dolan RJ, Friston KJ. Information theory, novelty and hippocampal responses: unpredicted or unpredictable? Neural Netw 2005; 18: 225–30 [DOI] [PubMed] [Google Scholar]
- 63.Reagh ZM, Watabe J, Ly M, Murray E, Yassa MA. Dissociated signals in human dentate gyrus and CA3 predict different facets of recognition memory. J Neurosci 2014; 34: 13301–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olson IR, Moore KS, Stark M, Chatterjee A. Visual working memory is impaired when the medial temporal lobe is damaged. J Cogn Neurosci 2006; 18: 1087–97 [DOI] [PubMed] [Google Scholar]
- 65.Olson IR, Page K, Moore KS, Chatterjee A, Verfaellie M. Working memory for conjunctions relies on the medial temporal lobe. J Neurosci 2006; 26: 4596–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fastenrath M, Coynel D, Spalek K, et al. Dynamic modulation of amygdala-hippocampal connectivity by emotional arousal. J Neurosci 2014; 34: 13935–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pergola G, Suchan B. Associative learning beyond the medial temporal lobe: many actors on the memory stage. Front Behav Neurosci 2013; 7: 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.López-Aranda MF, López-Téllez JF, Navarro-Lobato I, Masmudi-Martín M, Gutiérrez A, Khan ZU. Role of layer 6 of V2 visual cortex in object-recognition memory. Science 2009; 325: 87–9 [DOI] [PubMed] [Google Scholar]
- 69.Boveroux P, Vanhaudenhuyse A, Bruno M-A, et al. Breakdown of within- and between-network resting state functional magnetic resonance imaging connectivity during propofol-induced loss of consciousness. Anesthesiology 2010; 113: 1038–53 [DOI] [PubMed] [Google Scholar]
- 70.Theyel BB, Llano DA, Sherman SM. The corticothalamocortical circuit drives higher-order cortex in the mouse. Nat Neurosci 2010; 13: 84–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Monti MM, Lutkenhoff ES, Rubinov M, et al. Dynamic change of global and local information processing in propofol-induced loss and recovery of consciousness. PLoS Comput Biol 2013; 9: e1003271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen X, Guo Y, Feng J, et al. Encoding and retrieval of artificial visuoauditory memory traces in the auditory cortex requires the entorhinal cortex. J Neurosci 2013; 33: 9963–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baker R, Gent TC, Yang Q, et al. Altered activity in the central medial thalamus precedes changes in the neocortex during transitions into both sleep and propofol anesthesia. J Neurosci 2014; 34: 13326–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alkire MT, Asher CD, Franciscus AM, Hahn EL. Thalamic microinfusion of antibody to a voltage-gated potassium channel restores consciousness during anesthesia. Anesthesiology 2009; 110: 766–73 [DOI] [PubMed] [Google Scholar]
- 75.Alkire MT, McReynolds JR, Hahn EL, Trivedi AN. Thalamic microinjection of nicotine reverses sevoflurane-induced loss of righting reflex in the rat. Anesthesiology 2007; 107: 264–72 [DOI] [PubMed] [Google Scholar]
- 76.Saalmann YB. Intralaminar and medial thalamic influence on cortical synchrony, information transmission and cognition. Front Syst Neurosci 2014; 8: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S. The pulvinar regulates information transmission between cortical areas based on attention demands. Science 2012; 337: 753–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu X, Lauer KK, Ward BD, Li S-J, Hudetz AG. Differential effects of deep sedation with propofol on the specific and nonspecific thalamocortical systems: a functional magnetic resonance imaging study. Anesthesiology 2013; 118: 59–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hudetz AG. General anesthesia and human brain connectivity. Brain Connectivity 2012; 2: 291–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia. Science 2008; 322: 876–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mashour GA, Alkire MT. Consciousness, anesthesia, and the thalamocortical system. Anesthesiology 2013; 118: 13–5 [DOI] [PubMed] [Google Scholar]
- 82.Cimenser A, Purdon PL, Pierce ET, et al. Tracking brain states under general anesthesia by using global coherence analysis. Proc Natl Acad Sci USA 2011; 108: 8832–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lewis LD, Weiner VS, Mukamel EA, et al. Rapid fragmentation of neuronal networks at the onset of propofol-induced unconsciousness. Proc Natl Acad Sci USA 2012; 109: E3377–86 [DOI] [PMC free article] [PubMed] [Google Scholar]


