Summary
Jumonji domain-containing protein 6 (JMJD6) is positively associated with oral carcinogenesis and enriched in oral cancer stem cells (CSCs). JMJD6 is a novel regulator of oral CSC phenotype and promotes self-renewal in part via upregulating the expression of interleukin 4.
Abstract
Cancer stem cells (CSCs) are defined as a small subpopulation of cancer cells within a tumor and responsible for initiation and maintenance of tumor growth. Thus, understanding of molecular regulators of CSCs is of paramount importance for the development of effective cancer therapies. Here, we identified jumonji domain-containing protein 6 (JMJD6) as a novel molecular regulator of oral CSCs. JMJD6 is highly expressed in CSC-enriched populations of human oral squamous cell carcinoma (OSCC) cell lines. Moreover, immunohistochemical staining revealed significantly high level of JMJD6 in OSCC tissues compared to normal human oral epithelia, suggesting that expression of JMJD6 positively correlates with oral carcinogenesis. Subsequent functional analysis showed that knockdown of endogenous JMJD6 in OSCC strongly suppressed self-renewal capacity, a key characteristic of CSCs, and anchorage-independent growth. Conversely, ectopic expression of JMJD6 enhanced CSC characteristics including self-renewal, ALDH1 activity, migration/invasion and drug resistance. Expression of CSC-related genes was also markedly affected by modulating JMJD6 expression. Mechanistically, JMJD6 induces interleukin 4 (IL4) transcription by binding to its promoter region. IL4 rescues self-renewal capacity in JMJD6- knocked down OSCC cells, suggesting the importance of JMJD6-IL4 axis in oral CSCs. Our studies identify JMJD6 as a molecular determinant of CSC phenotype, suggesting that inhibition of JMJD6 may offer an effective therapeutic modality against oral cancer.
Introduction
Recent studies have uncovered and validated the pathophysiologic role of cancer stem cells (CSCs; also known as cancer initiating cells) in long-term sustenance of cancers (1). CSCs have been successfully isolated from various primary tumors and established cancer cell lines, including human oral squamous cell carcinoma (OSCC) (2). CSCs play a crucial role in tumor progression, in vivo tumorigenic potential, metastasis and recurrence and thus are considered as the root of the cancer. Therefore, advancing our understanding of the molecular properties and signaling pathways unique to oral CSCs is crucial for developing a new generation of targeted and effective therapies for oral cancer.
A group of histone demethylases epigenetically modulate gene transcription by removing histone methylation marks (3). As such, histone demethylases play a crucial role in governing gene transcription by altering chromatin accessibility and transcriptional machineries. Compelling evidence indicates that histone demethylases are implicated in various cellular processes, including carcinogenesis, cell fate choices and cell differentiation (4–6). Although substantial progress has been made in understanding the molecular regulation of CSCs, roles of histone demethylases in the regulation of oral CSCs have not been well investigated.
Jumonji domain-containing protein 6 (JMJD6) is a histone arginine demethylase that preferentially removes methyl groups from dimethylated arginine 2 of histone 3 (H3R2me2) and arginine 3 of histone 4 (H4R3me2) (7), thereby enabling the dynamic regulating of transcription. Interestingly, JMJD6 was originally identified as a phosphatidylserine receptor involved in clearance of apoptotic cells (8). JMJD6 also regulates gene expression by modulating RNA splicing (9), suggesting that JMJD6 is a multifaceted regulator of gene expression. Clinically, JMJD6 overexpression strongly linked to poor prognosis in human cancers, including breast and lung (10,11). JMJD6 promoted cancer cell migration/invasion (10) and angiogenic sprouting (12), two phenotypes of which are well known for CSC characteristics (13). JMJD6 is also known to be essential for the differentiation of multiple tissues and cells during embryogenesis (14). Recent studies revealed that histone methylation played a critical role in governing stemness of normal stem cells (15,16). Although CSCs share molecular and phenotypic characteristics with normal stem cells, a considerable knowledge gap remains in our understanding of the regulation of oral CSCs particularly by histone demethylases.
Our study demonstrates for the first time that JMJD6 is positively associated with oral carcinogenesis and enriched in oral CSCs. JMJD6 is a novel regulator of oral CSC phenotype and promotes self-renewal capacity, a key characteristic of CSCs, via upregulating the expression of interleukin 4 (IL4).
Materials and methods
Cells and cell culture
Four human OSCC cell lines, SCC4 (17), SCC9/TNF (18), YD38 (19), UM17b (20) and HOK-16B-BapT (21), were cultured in DMEM/Ham’s F12 (Invitrogen) supplemented with 10% fetal bovine serum (Gemini Bioproducts) and 0.4 µg/ml hydrocortisone (Sigma–Aldrich). Three non-malignant, immortalized oral epithelial cell lines, POE9n, OKF6/tert and HOK-16B, were cultured in Keratinocyte Growth Medium (KGM) (Lonza) as described previously (22,23). All cell lines were routinely tested and authenticated using cell morphology, proliferation rate, a panel of genetic markers and contamination checks. All cell lines were also tested for mycoplasma, using the MycoAlert Detection Kit (Cambrex). Human interleukin 4 (IL4) and neutralizing IL4 antibody were purchased from Cell Signaling.
Tumor sphere formation assay
Three thousand cells were grown in 3ml of serum-free DMEM/F12 media supplemented with 1:50 B27 (Invitrogen), 20ng/ml EGF, 20ng/ml, 10μg/ml insulin, penicillin, streptomycin and amphotericin B in Ultra-Low Attachment six-well plates (Corning) for 6–10 days. The assay was performed in triplicate, and the number of tumor spheres formed were observed and counted under a microscope.
Quantitative real-time PCR (qPCR)
Complementary DNAs was synthesized from 5 μg of total RNA using SuperScript first-strand synthesis system (Invitrogen). We used 1 μl complementary DNAs for qPCR amplification using SYBR Green I Master mix (Roche) and LightCycler 480 II (Roche). The primer sequences were obtained from the Universal Probe Library (Roche), and the sequences can be available upon request. Second derivative Cq value determination method was used to compare fold-differences according to the manufacturer’s instructions.
Western blotting
Western blotting was performed as described previously (18). We used the following primary antibodies for this study: JMJD6 (H-7; Santa Cruz Biotech), KDM7A (ab36044; Abcam), KDM8 (ab36104; Abcam), FGF4 (c-18; Santa Cruz Biotech), FLAG (F7425; Sigma–Aldrich) and GAPDH (FL-335; Santa Cruz Biotech).
Knockdown of JMJD6
JMJD6 was transiently knocked down with duplex small interfering RNA (siRNA) targeting JMJD6 or the control, scrambled siRNA (Santa Cruz Biotech), which was introduced using Lipofectamine RNAiMAX (Invitrogen). OSCC cells (2×105) were plated in 60-mm dishes and transfected with 15 μg siRNA. The cultures were harvested after two days post-transfection for expression and functional analyses. JMJD6 was also stably knocked down with lentiviral vectors expressing JMJD6 small hairpin RNA (shRNA) (pRS JMJD6shRNA; OriGene). Detailed methods of lentivirus production and infection can be found in our previous publications (24). Infected cells were selected with 0.5μg/ml puromycin for 2 weeks and used for experiments.
Anchorage-independent growth
To determine colony-forming efficiency in semi-solid medium, 1×104 cells were plated in culture medium containing 0.3% agarose over a base layer of serum-free medium containing 0.5% agarose. The assay was performed as described previously (18).
Overexpression of exogenous JMJD6
Exogenous JMJD6 was overexpressed with retroviral vectors expressing JMJD6 (p6352 MSCV-CMV-Flag-HA-JMJD6; Addgene). Detailed methods of retrovirus production and infection can be found in our previous publications (25). Infected cells were selected with 0.5μg/ml puromycin for 2 weeks and used for experiments.
ALDH1 assay
Detection of ALDH enzymatic activity was made by using Aldehyde Dehydrogenase-Based Cell Detction Kit (STEMCELL). Total of 1×106 cells were re-suspended in the ALDEFLUOR Assay Buffer in the volume of 1ml. Fluorescent non-toxic ALDEFLUOR Reagent BODIPYTM (1.25 µl) was added as a substrate to measure ALDH enzymatic activity in intact cells. Immediately after adding the substrate reagent, 0.5ml of the cell suspension was transferred into the control tube which contains specific inhibitor for ALDH, diethylaminobenzaldehyde (DEAB) for calculating background fluorescence. Then, cells were incubated at 35°C for 30min and fluorescence data acquisition was made by using a BD FACScan flow cytometer (BD Biosciences).
Migration and invasion assays
Cell migration was measured using transwell chambers with polycarbonate membranes (Corning) as described in our previous publication (18). Cell invasion was measured using Matrigel Basement Membrane Matrix (BD Biosciences), according to the method as described in manufacture protocol.
Chemosensitivity assays
Chemosensitivity of cells was determined by measuring cell viability using the tetrazolium salt (MTT) cell proliferation assay kit (ATCC). The cells were plated at 2×103 cells per well into 96-well plate and incubated in culture medium containing 0.05 µM doxorubicin, 5 µM methotrexate or 10 µM etoposide (Sigma-Aldrich) for 3 days. Absorbance at 570nm was determined using a microplate reader.
Chromatin immunoprecipitation assays
Chromatin immunoprecipitation (ChIP) assay was performed using a MAGnify™ ChIP kit (Invitrogen) following the manufacturer’s protocol. Immune complexes from monolayer culture and spheres derived from SCC4were obtained using 5 µg of JMJD6 antibody (H-7; Santa Cruz Biotech). Then, genomic DNA was isolated from the complexes and subjected to qPCR using the following IL4 promoter primers: sense, 5′-GCCTGTTATTCTGCCTCTATGC-3′; antisense, 5′-TGGAAACTGTCCTGTCATGG-3′.
Immunohistochemistry
Oral specimens that were previously collected for diagnostic purposes were obtained from the Oral Pathology Diagnostic Laboratory at the UCLA School of Dentistry. All tissue specimens were collected and processed according to the guidelines of the University of California at Los Angeles Institutional Review Board. Immunohistochemical staining was performed as described previously (26). The optimal concentration (1:100) of JMJD6 antibody (H-7; Santa Cruz Biotech) was first established using serially diluted primary antibody along with IgG as a negative control. The immunohistochemical expression of JMJD6 protein was scored by two independent examiners. The level of JMJD6 staining pattern was scored into three subgroups: (i) weak (+); (ii) moderate (++) and (iii) strong (+++).
Results
JMJD6 expression is enriched in OSCC tumor spheres
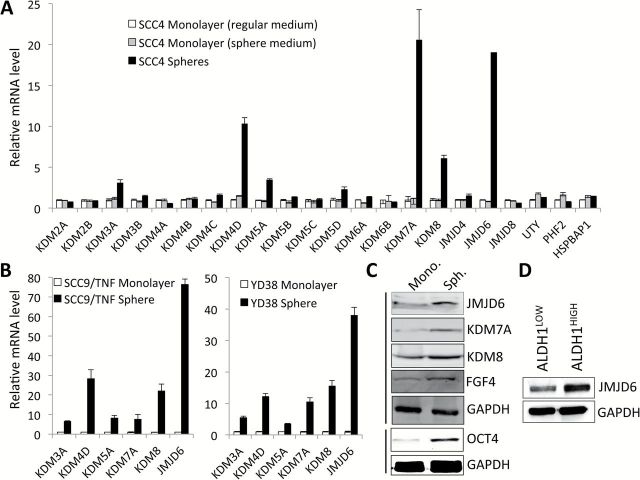
CSCs can be enriched in non-adherent tumor spheres cultured in ultra-low attachment plates (27). Thus, abundance and the growth kinetics of tumor spheres are indicative of CSC content and self-renewal capacity in a given culture of heterogeneous cancer cells. To investigate potential involvement of histone demethylases in promoting/maintaining oral CSCs, we profiled expression of 22 histone demethylases in tumor spheres (CSC-enriched population) and adherent monolayer culture (non-CSC population) derived from SCC4, a human tongue squamous cell carcinoma line (Figure 1A). Quantitative real-time PCR (qPCR) revealed that KDM3A, KDM4D, KDM5A, KDM7A, KDM8 and JMJD6 were highly expressed in the tumor spheres compared to the monolayer culture (Figure 1A). SCC4 monolayer culture grown in the sphere medium was used as a control to rule out specific sphere medium effect on histone demethylases expression. We found that their enrichment in tumor sphere is not artifact of different medium condition (Figure 1A). The enrichment of demethylases in tumor spheres was further confirmed in other OSCC cell lines, SCC9/TNFα and YD38 (Figure 1B). Among those histone demethylases, JMJD6 is greatly enriched in all tested OSCC tumor spheres (Figure 1A–C). Furthermore, we sorted the ALDH1HIGH and ALDH1LOW cell populations from SCC4 according to ALDH1 activity and examined JMJD6 expression in these two cell populations (Figure 1D). ALDH1 activity is one of CSC markers and known to enrich CSCs in solid malignancies, including head and neck cancer (28). The ALDH1HIGH population expressed higher JMJD6 protein than the ALDH1LOW population (Figure 1D). Overall, our data clearly indicate that JMJD6 is enriched in CSC populations.
Figure 1.
JMJD6 is highly expressed in oral CSCs. (A) Expression of 22 histone demethylases was assessed in tumor spheres (CSC-enriched population) and adherent monolayer culture (non-CSC population) derived from SCC4 by qPCR and normalized to GAPDH. SCC4 monolayer culture grown in the sphere medium was used as a control to rule out specific sphere medium effect on histone demethylases expression. (B) Overexpression of KDM3A, KDM4D, KDM5A, KDM7A, KDM8 and JMJD6 were further confirmed in CSC-enriched populations derived from other OSCC cell lines, SCC9/TNF and YD38. (C) Increased expression of selected histone demethylases in CSC-enriched population derived from SCC4 was confirmed by Western blot analysis. Expressions of CSC markers (OCT4 and FGF4) were also assessed in spheres and corresponding monolayer cultured cells derived from SCC4. GAPDH was used as a loading control. Mono., monolayer cultured cells and Sph., spheres (D) Level of JMJD6 protein was determined in ALDH1HIGH and ALDH1LOW cell population by Western blot analysis. The ALDH1HIGH (top 30%) and ALDH1LOW (bottom 20%) cell populations from SCC4 were sorted according to ALDH1 activity by flow cytometry.
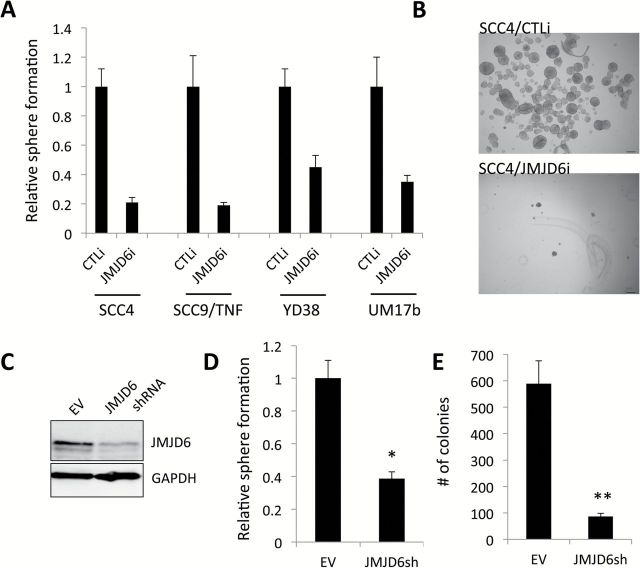
Knockdown of JMJD6 inhibits self-renewal capacity of OSCC cells
To assess the functional role of JMJD6 in self-renewal of OSCC, a key feature of CSCs, we knocked down JMJD6 in four OSCC cell lines using siRNA. JMJD6 siRNA showed significant suppressive effect on tumor sphere forming ability of all tested cell lines (Figure 2A and B). To further confirm this finding, we generated stable JMJD6-knockdown SCC4 cells using lentivirus-based shRNA targeting JMJD6. We confirmed the successful suppression of JMJD6 expression in SCC4 infected with the viruses expressing JMJD6 shRNA (Figure 2C). Consistently, the shRNA-mediated JMJD6 knockdown reduced the tumor sphere formation in SCC4 (Figure 2D), indicating that silencing JMJD6 suppresses the stem-like property of OSCC. Stable JMJD6 knockdown also suppressed anchorage-independent growth ability of the cells (Figure 2E). The ability to form clones in soft agar is reported as a characteristic of CSC in human cancer cells (29,30). We observed no difference in cell proliferation rates between SCC4/EV and SCC4/JMJD6shRNA cells over 10 days when grown in monolayer cell cultures (data not shown). Because the sphere formation and soft agar assays were performed for 6–10 days, it was unlikely that the inhibitory effects of JMJD6 knockdown were artifacts of slower cell proliferation.
Figure 2.
Knockdown of JMJD6 suppresses self-renewal capacity of OSCC cells. (A) Endogenous JMJD6 was knocked down in multiple human OSCC cell lines using siRNA against JMJD6 (JMJD6i). The cells transfected with control siRNA (CTLi) were included for comparison. We found 30–70% reduction of JMJD6 in the transfection assay (data not shown). JMJD6 knockdown effect on self-renewal capacity of OSCC cell lines was determined by tumor sphere formation assay. Single cells were plated in ultralow attachment plates at a density of 3000 cells/ml in serum-free tumor sphere medium. Tumor spheres were counted on day 7. Data are means ± SD of triplicate experiments. (B) Representative image of tumor spheres formed by SCC4 transfected with control siRNA (SCC4/CTLi) and JMJD6 siRNA (SCC4/JMJD6i). (C) JMJD6 was stably knocked down in SCC4 by JMJD6 shRNA. SCC4 cells transfected with control empty vector (EV) were included for comparison. JMJD6 knockdown by JMJD6 shRNA was confirmed by Western blot. (D) Effect of stable JMJD6 knockdown on self-renewal capacity of SCC4 was determined by tumor sphere formation assay. Data are means ± SD of triplicate experiments. *P < 0.05, unpaired two-tailed Student’s t test. (E) Effect of stable JMJD6 knockdown on anchorage independent growth of SCC4 was determined by soft agar assay. Ten thousand cells were plated in semi-solid agar, and colonies were counted for 2 weeks. The assay was performed in triplicate with 60-mm dishes. Data are means ± SD of triplicate experiments. **P < 0.01, unpaired two-tailed Student’s t test.
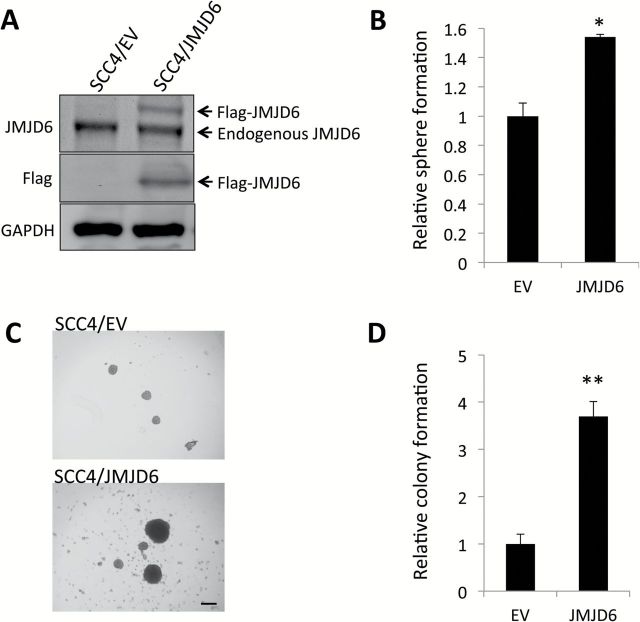
Ectopic overexpression of JMJD6 promotes self-renewal capacity and CSC properties in OSCC cells
Conversely, we overexpressed JMJD6 in SCC4 (Figure 3A) to further confirm the functional role of JMJD6 in self-renewal capacity. Using this system, we performed sphere formation assay and found that ectopic JMJD6 expression increased self-renewal capacity of SCC4 (Figure 3B). Interestingly, tumor spheres formed from SCC4/JMJD6 were conspicuously larger than those from the control SCC4/EV cells (Figure 3C). JMJD6 also increased anchorage-independent growth of SCC4 (Figure 3D).
Figure 3.
JMJD6 increases self-renewal capacity and anchorage independent growth of SCC4. (A) JMJD6 expression was forced in SCC4 by infecting with retroviral vector expressing Flag-JMJD6, and its ectopic expression was confirmed by Western blot. (B) Effect of JMJD6 overexpression on self-renewal capacity of SCC4 was determined by tumor sphere formation assay. Data are means ± SD of triplicate experiments. *P < 0.05, unpaired two-tailed Student’s t test. (C) Representative image of tumor spheres formed by SCC4 infected with control empty virus (SCC4/EV) and virus expressing Flag-JMJD6 (SCC4/JMJD6). (D) Effect of JMJD6 overexpression on anchorage-independent growth of SCC4 was determined by soft agar assay. **P < 0.01, unpaired two-tailed Student’s t test.
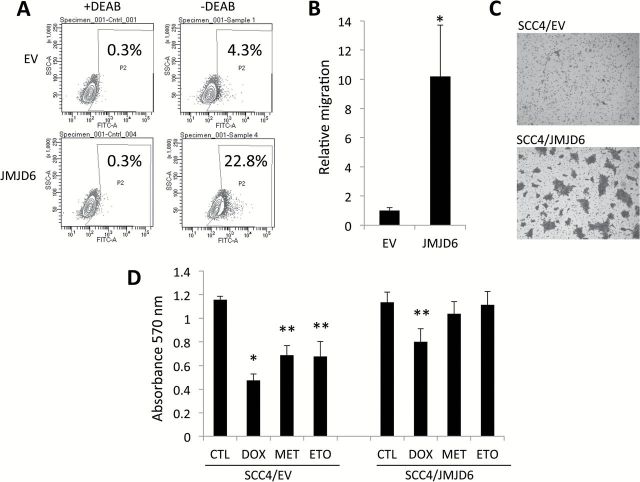
We next sought to determine whether increased JMJD6 expression is a critical driver for CSC phenotype in OSCC. Thus, we investigated whether JMJD6 could increase CSC population by performing the flow cytometric analysis of ALDH1 activity. The flow analysis revealed a significant increase in ALDH1+ cell population in the SCC4/JMJD6 cells compared to their control SCC4/EV (22.8 vs. 4.3%; Figure 4A). We examined the effect of JMJD6 on migration, another important characteristic of CSCs (13). As demonstrated by a transwell migration assay (Figure 4B), SCC4/JMJD6 migrated significantly faster than SCC4/EV. We also found a significant increase in invasion ability in SCC4/JMJD6 using Matrigel invasion assay (Figure 4C). Because another well-known characteristic of CSCs is their resistance to chemotherapy (18), we determined whether JMJD6 would enhance resistance to chemotherapeutic drugs. SCC4/JMJD6 displayed increased resistance to multiple cancer drugs, i.e. doxorubicin, methotrexate and etoposide, compared to SCC4/EV (Figure 4D). These findings clearly indicate that JMJD6 increases CSC population and properties in OSCC.
Figure 4.
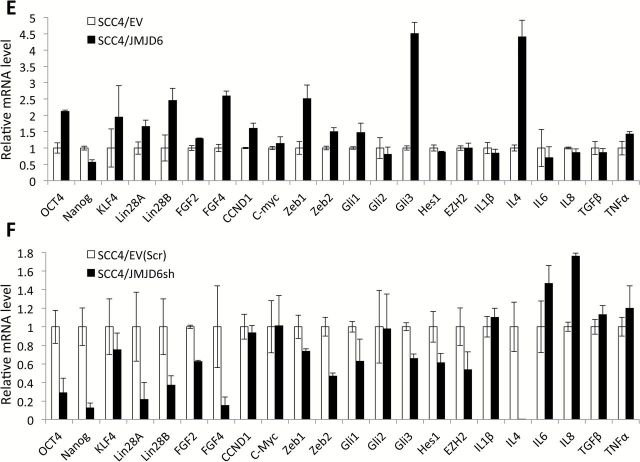
JMJD6 promotes CSC properties in SCC4. (A) Effect of JMJD6 overexpression on ALDH1 activity in SCC4 was determined by Aldefluor assay. Cells were labeled with Aldefluor with and without the ALDH inhibitor DEAB and analyzed by flow cytometry. The gate for ALDH + cells is determined in relation to the DEAB control (+DEAB) and shows the brightly fluorescent ALDH population versus the side scatter, a population that is absent/decreased in the presence of DEAB. The number shown in each panel reflects the percentage of ALDH + cells in each cell type. (B) Effect of JMJD6 overexpression on migration ability of SCC4 was determined by transwell chamber. Migration ability was described as number of migrated cells per field with data as mean ± SD for three randomly selected fields. *P < 0.01, unpaired two-tailed Student’s t test. (C) Effect of JMJD6 overexpression on invasion ability of SCC4 was determined by Matrigel assay. Representative image of Matrigel assay. (D) Effect of JMJD6 overexpression on chemosensitivity of SCC4 was determined by MTT assay. Cells were treated with no drug (CTL), 0.05 µM doxorubicin (DOX), 5 µM methotrexate (MET) or 10 µM etoposide (ETO) for 3 days, and their viability was measured using MTT assay. Absorbance at 570nm was determined using a microplate reader. Data are means ± SD of eight wells. *P < 0.01, unpaired two-tailed Student’s t test. **P < 0.05, unpaired two-tailed Student’s t test. (E) Effect of JMJD6 overexpression on CSC-related gene expression in SCC4 was validated by real-time qPCR. (F) Effect of JMJD6 knockdown on CSC-related gene expression in SCC4 was also validated by real-time qPCR.
JMJD6-regulated CSC phenotype was further confirmed by evaluating CSC-related gene expression. We found increased expression of CSC-related genes including Oct4, Lin28A, Lin28B, FGF4, Zeb1, Zeb2, Gli1, Gli3 and IL4 by JMJD6 (Figure 4E), whereas these CSC-related genes were decreased by JMJD6 knockdown in SCC4 cells (Figure 4F). Overall, our findings suggest that JMJD6 is associated with CSC phenotype in OSCC by regulating CSC-related genes.
JMJD6 regulates self-renewal capacity of OSCC cells via IL4 upregulation
Previous studies demonstrated the involvement of cytokines in CSC phenotypes (31–33). We also reported that inflammatory cytokine enhanced CSC phenotype of OSCC (18). Recently, to investigate the roles of cytokines in CSCs of OSCC, we profiled expression of 25 cytokines and found that cytokine IL4 was highly increased in SCC4 tumor spheres compared to monolayer culture (Figure 5A). IL4 was the most significantly affected gene by JMJD6 modulation (Figure 4E and F). Therefore, we sought to determine whether there was a functional link between JMJD6 and IL4 in regulating CSCs. To examine whether the JMJD6 effect on IL4 expression applies to multiple OSCC cell lines, we knocked down JMJD6 in two other OSCC cell lines, SCC9/TNF and UM17b. Knockdown of JMJD6 in the cell lines suppressed IL4 mRNA expression (Figure 5B). We further investigated whether JMJD6 could bind to the IL4 promoter. ChIP assay revealed that JMJD6 binds to and enriches in the promoter of IL4 in SCC4 tumor sphere compared to monolayer (Figure 5C). These results suggest that JMJD6 regulates IL4 expression by binding to IL4 promoter in CSCs. To determine the functional roles of IL4 in the JMJD6-regulated CSC phenotype, we performed tumor sphere formation assays using the JMJD6-knockdown OSCC cells both in the presence and absence of IL4 (Figure 5D). The assays revealed that addition of recombinant human IL4 rescued the stem-like property reduced by the JMJD6 knockdown as comparable to that of the control cells (Figure 5D). Conversely, IL4 neutralizing/blocking antibody suppressed the stem-like property of the JMJD6-overexpressing cells in a dose-dependent manner (Figure 5E). We also examined whether IL4 affected JMJD6 and CSC-related factors, and found no significant changes in their expression by IL4 (Figure 5F), indicating that JMJD6 lies upstream of IL4. Collectively, our data indicate that JMJD6 promotes oral CSC phenotype by upregulating IL4, suggesting a novel CSC regulatory mechanism by the JMJD6-IL4 axis.
Figure 5.
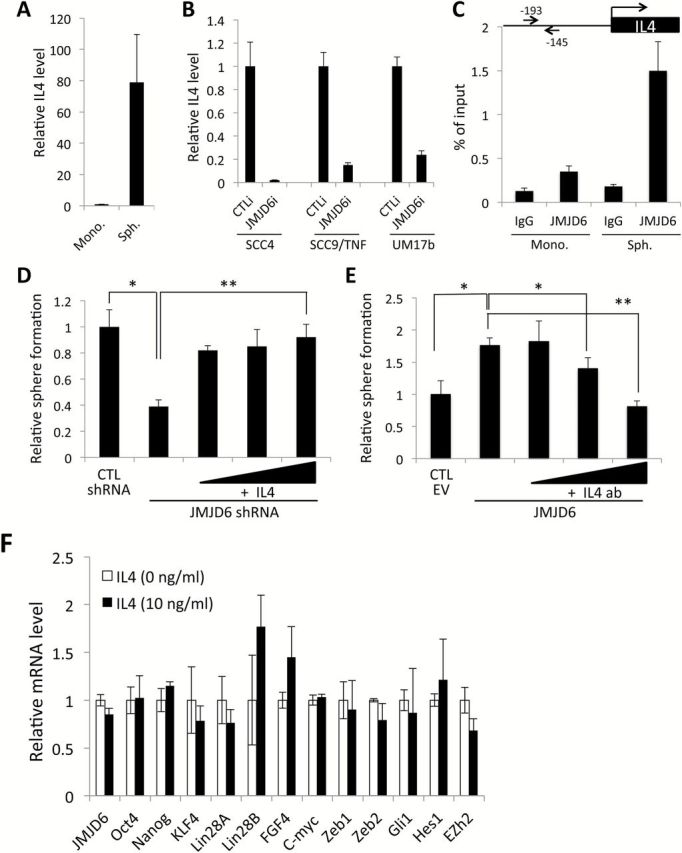
JMJD6 regulates self-renewal capacity of OSCC cells via IL4 upregulation. (A) IL4 is highly expressed in tumor spheres (Sph.) compared to monolayer culture (Mono.) derived from SCC4. (B) Effect of JMJD6 on IL4 expression was determined in multiple human OSCC cell lines by JMJD6 siRNA transfection followed by qPCR analysis of IL4. (C) Physical interaction of JMJD6 to promoter region of IL4 was examined by ChIP assay. The promoter region of IL4 was ChIP-ed with JMJD6 antibody or IgG control, and subsequently amplified with primer set (indicated in arrows). (D) Rescue effect of IL4 on self-renewal capacity reduced by JMJD6 knockdown was determined by tumor sphere formation assay. Tumor sphere assays were performed both in the presence or absence of IL4. IL4 was added at 0, 5, 10 or 20ng/ml concentrations in the sphere medium. Data are means ± SD of triplicate experiments. *P < 0.01, unpaired two-tailed Student’s t test. **P < 0.05, unpaired two-tailed Student’s t test. (E) Effect of IL4 neutralizing antibody on self-renewal capacity in JMJD6-overexpression OSCC cells was determined by tumor sphere formation assay. Tumor sphere assays were performed using SCC4/JMJD6 both in the presence or absence of IL4 antibody (IL4 ab). IL4 antibody was added at 0, 1, 2.5 or 5 ug/ml concentrations in the sphere medium. Data are means ± SD of triplicate experiments. *P < 0.05, unpaired two-tailed Student’s t test. **P < 0.01, unpaired two-tailed Student’s t test. (F) Effect of IL4 on JMJD6 and CSC-related gene expression was evaluated by qPCR analysis. SCC4 cells were treated with IL4 for 24h and subjected to qPCR analysis.
Elevated expression of JMJD6 is associated with oral carcinogenesis
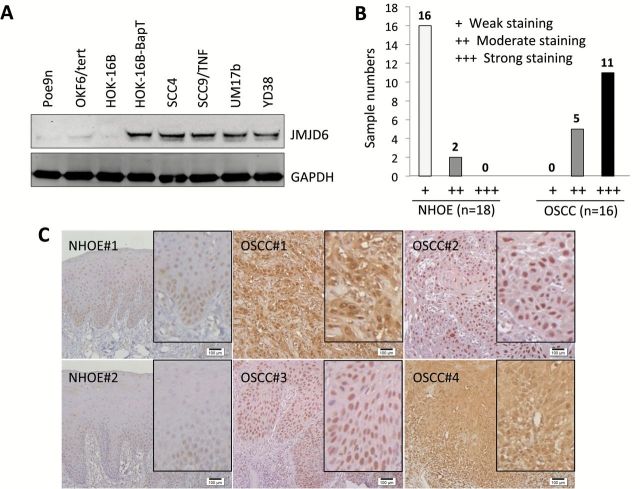
We then explored the role of JMJD6 in oral carcinogenesis. First, we compared the expression of JMJD6 in OSCC cell lines (HOK-16B-BapT, SCC4, SCC9/TNF, UM17b and YD38) to that in precancerous, immortalized oral epithelial cell lines (Poe9n, OKF6/tert and HOK-16B) by western blot analysis. All of the OSCC cell lines exhibited higher expression of JMJD6 protein than the precancerous cell lines (Figure 6A). To extend our findings, immunohistochemical staining for JMJD6 was performed using normal human oral epithelia (NHOE) and OSCC specimens. The results of in vivo JMJD6 staining are summarized in Figure 6B, and a typical JMJD6 staining observation in NHOE and OSCC tissue is shown in Figure 6C. In 18 NHOE, weak nuclear JMJD6 staining was detected in 16 cases (89%), and moderate nuclear staining detected in two cases (11%). Nuclear JMJD6 staining was prominent in basal cell layer of NHOE (Figure 6C). Of the 16 OSCC cases, 11 cases (69%) demonstrated strong staining and 5 cases (31%) with moderate staining. JMJD6 was also present predominantly in the nuclei of OSCC cells, with increased cytoplasmic staining in five cases. Taken together with the observation from cell lines, our findings indicate that expression of JMJD6 positively correlates with OSCC development.
Fig. 6.
Elevated expression of JMJD6 is associated with oral carcinogenesis. (A) Level of JMJD6 protein was determined in three precancerous, non-malignant oral epithelial cell lines (Poe9n, OKF6/tert and HOK-16B) and five OSCC cell lines (HOK-16B-BapT, SCC4, SCC9/TNF, UM17b and YD38) by performing Western blot. (B) In vivo JMJD6 expression was determined in normal human oral epithelia (NHOE) and OSCC tissues by immunohistochemical staining. NHOE and OSCC specimen were stained for JMJD6 expression using anti-JMJD6 antibody. (C) Representative examples of JMJD6 immunohistochemical staining in NHOE and OSCC tissues in vivo. Bar indicates 100 μm. Inserts showing magnified images.
Discussion
The aim of our study was to identify novel molecular determinant and regulation of oral CSCs. CSCs are viewed as the seed of cancer and hence as effective target of anticancer therapies. Therefore, findings from our studies are of paramount important for the development of more effective cancer therapies. In this study, we demonstrate, for the first time, that JMJD6 is a novel regulator of oral CSCs. Moreover, JMJD6 positively correlates with oral carcinogenesis and enriches in oral CSCs. We also provide evidence that JMJD6 maintains the CSC phenotype in part by regulating IL4, indicating the importance of JMJD6-IL4 axis in oral CSC. Therefore, JMJD6 could be an effective therapeutic target for oral cancer.
JMJD6 has been implicated in human cancer. Upregulation of JMJD6 expression was observed in various human cancers, including breast cancer (10), lung cancer (11) and colon cancer (34). A high expression of JMJD6 protein is also strongly linked to poor prognosis and aggressive behavior of human cancers. However, no information is available regarding JMJD6 expression during oral carcinogenesis. Consistently, our results showed that JMJD6 is highly expressed in OSCC compared to normal tissues in vivo (Figure 6). The level of JMJD6 in non-malignant oral epithelial cell lines was much lower than OSCC cell lines in vitro, indicating that JMJD6 plays an important role during oral carcinogenesis. Furthermore, JMJD6 consistently overexpressed in OSCC CSC populations compared to non-CSC populations. These observations support the hypothesis that JMJD6 is a novel molecular determinant of oral cancer progression. Interestingly, our immunohistochemistry study revealed that JMJD6 is a nuclear protein and presents dominantly in the basal cell layer of a normal tissue where self-renewing stem cells reside (35). Although the functional role of JMJD6 in the basal cell layer is unclear, our observation is consistent with previous observation (10,34).
By phenotypic and functional analysis, we demonstrate that JMJD6 is an important regulator of CSC phenotype in OSCC. In multiple OSCC cell lines, silencing of JMJD6 led to loss of self-renewal, migration (data not shown) and anchorage independent growth ability without a noticeable change in cell proliferation. Its minimal effect on cell proliferation was further demonstrated by our observation that modulation of JMJD6 expression failed to affect proliferating cell nuclear antigen, a well-known marker for cell proliferation (36). This finding is not consistent with the observation that JMJD6-depleted cancer cells display growth retardation (10). Currently we are not able to explain this discrepancy, but we speculate the possibility of cancer and cell type specificity of JMJD6 function. Conversely, JMJD6 overexpression promoted such CSC properties. There has been increasing evidence that reports the importance of JMJD6 in cell migration. Knockdown of JMJD6 in invasive breast cancer cell lines decreased cell migration, whereas its overexpression promoted cellular motility (10). In addition, JMJD6 interacts with splicing factor U2AF65 and modulates alternate splicing of VEGF receptor (9). Alternate splicing of VEGF receptor by U2AF65 promoted endothelial cell migration, and silencing of JMJD6 in endothelial cells led to decreased migration (12). Consistently, we demonstrated that JMJD6 increased motility and invasion ability of OSCC cells. However, underlying mechanism by which JMJD6 regulates OSCC migration has not been understood. Therefore, effects of JMJD6 on epithelial-to-mesenchymal transition and metastasis-related gene expression should be warranted to investigate.
Furthermore, JMJD6 was enriched in ALDH1HIGH cell population and increased ALDH1+ cell population. ALDH1 has been found to be a marker for stem cells in different types of cancer, including OSCC (37,38). ALDH1+ cancer cells displayed higher self-renewal, migration and tumorigenic potential than ALDH1− cells (37–39). Thus, we conclude that JMJD6 increases not only the number of CSCs, but also CSC properties.
We also showed that JMJD6 regulates the expression of several important CSC-related genes, i.e. Oct4, Lin28A, Lin28B, FGF4, Zeb1, Zeb2, Gli1 and Gli3. Studies have shown the important role of these genes in acquisition and maintenance of CSC phenotype. For instance, Oct4 has been detected in CSC of various cancers including melanoma, prostate cancer, lung cancer, and oral cancer (2,40,41). Oct4 promoted self-renewal and tumorigenic potential of melanoma cells (42). Lin28A is up-regulated in CSC-enriched OSCC population, and the overexpression of Lin28A in oral cancer cells increased their proliferation, colony formation and migration (43). Zeb1 and Zeb2 are significantly increased in head and neck CSCs compared to non-CSCs (44). Knockdown of Zeb1 and Zeb2 in head and neck cancer cells decreased their CSC properties such as self-renewal capacity, the expression of stemness markers, and drug resistance. Moreover, their suppression inhibited in vivo tumor growth and the rate of metastasis to distant site (44). Conversely, co-overexpression of Zeb1 and Zeb2 enhanced sphere-forming ability of head and neck cancer cells (44). These CSC-related genes were up-regulated by JMJD6 overexpression and down-regulated by JMJD6 knockdown in OSCC cells suggesting that JMJD6 may enhance cancer stemness by modulating various CSC factors.
A recent study has shown a critical role of various cytokines in the tumor microenvironment and in the regulation of CSC (45). We also reported that inflammatory cytokine enhanced CSC phenotype of OSCC (18). Indeed, we detected general upregulation of cytokines in OSCC CSC compared to non-CSC population (manuscript in preparation). IL4, a multifunctional cytokine, is known to promote tumor formation by inhibiting apoptosis and enhancing proliferation (46,47). IL4 is overexpressed in different types of cancer including breast, ovarian, colon, lung and thyroid (32). Autocrine production of IL4 by cancer cells conferred resistance to chemotherapy-induced cell death (48) and implicated in tumor growth and metastasis (46). Moreover, the production of IL4 in colon CSCs is responsible for their chemoresistance, which is an important CSC characteristic (32,49). We found that IL4 is highly increased in OSCC CSCs compared to non-CSCs. Our results indicate that IL4 upregulation is an important event in the JMJD6-induced self-renewal in OSCC CSC. We have extended these findings to show that IL4 treatment rescues self-renewal capacity suppressed by JMJD6 knockdown, and JMJD6 transactivates IL4 mRNA through binding to IL4 promoter region. Together our data identify, for the first time, the JMJD6-IL4 axis as a novel CSC regulatory mechanism in OSCC. Since the role of cytokines in CSCs has been widely studied, and the interaction between certain histone demethylases and cytokines has also been reported (50), we speculated that CSCs indeed interact with and are regulated by cells in tumor microenvironment, and involvement of cytokines in this interaction is probable and also crucial event for cancer progression and propagation.
In conclusion, we have identified that JMJD6 is a novel inducer of CSC properties in OSCC by upregulating IL4. Thus, the JMJD6-IL4 axis could be an important therapeutic target in OSCC. Because histone demethylases are readily inhibited by chemical inhibitors, targeting JMJD6 may be a plausible therapeutic modality against oral CSCs.
Funding
UCLA School of Dentistry faculty seed grant (to K.H.S.), (R01DE18295 to M.K.K.); (R01DE023348 to R.H.K.) from NIDCR/NIH and the grant from UCLA Chancellor’s Office (to N.H.P.).
Supplementary material
Supplementary Table 1 and Figures 1–4 can be found at http://carcin.oxfordjournals.org/
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- ALDH1
aldehyde dehydrogenase 1
- ChIP
chromatin immunoprecipitation
- CSCs
cancer stem cells
- IL4
interleukin 4
- JMJD6
Jumonji domain-containing protein 6
- NHOE
normal human oral epithelia
- OSCC
oral squamous cell carcinomas
- siRNA
small interfering RNA
- shRNA
small hairpin RNA
References
- 1. Clevers H. (2011) The cancer stem cell: premises, promises and challenges. Nat. Med., 17, 313–319. [DOI] [PubMed] [Google Scholar]
- 2. Chiou S.H., et al. (2008) Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin. Cancer Res., 14, 4085–4095. [DOI] [PubMed] [Google Scholar]
- 3. Kooistra S.M., et al. (2012) Molecular mechanisms and potential functions of histone demethylases. Nat. Rev. Mol. Cell Biol., 13, 297–311. [DOI] [PubMed] [Google Scholar]
- 4. Agger K., et al. (2009) The H3K27me3 demethylase JMJD3 contributes to the activation of the INK4A-ARF locus in response to oncogene- and stress-induced senescence. Genes Dev., 23, 1171–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hsia D.A., et al. (2010) KDM8, a H3K36me2 histone demethylase that acts in the cyclin A1 coding region to regulate cancer cell proliferation. Proc. Natl. Acad. Sci. USA, 107, 9671–9676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ye L., et al. (2012) Histone demethylases KDM4B and KDM6B promotes osteogenic differentiation of human MSCs. Cell Stem Cell, 11, 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chang B., et al. (2007) JMJD6 is a histone arginine demethylase. Science, 318, 444–447. [DOI] [PubMed] [Google Scholar]
- 8. Fadok V.A., et al. (2000) A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature, 405, 85–90. [DOI] [PubMed] [Google Scholar]
- 9. Webby C.J., et al. (2009) Jmjd6 catalyses lysyl-hydroxylation of U2AF65, a protein associated with RNA splicing. Science, 325, 90–93. [DOI] [PubMed] [Google Scholar]
- 10. Lee Y.F., et al. (2012) JMJD6 is a driver of cellular proliferation and motility and a marker of poor prognosis in breast cancer. Breast Cancer Res., 14, R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang J., et al. (2013) High expression of JMJD6 predicts unfavorable survival in lung adenocarcinoma. Tumour Biol., 34, 2397–2401. [DOI] [PubMed] [Google Scholar]
- 12. Boeckel J.N., et al. (2011) Jumonji domain-containing protein 6 (Jmjd6) is required for angiogenic sprouting and regulates splicing of VEGF-receptor 1. Proc. Natl. Acad. Sci. USA, 108, 3276–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beck B., et al. (2013) Unravelling cancer stem cell potential. Nat. Rev. Cancer, 13, 727–738. [DOI] [PubMed] [Google Scholar]
- 14. Hahn P., et al. (2010) Analysis of Jmjd6 cellular localization and testing for its involvement in histone demethylation. Plos One, 5, e13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang H., et al. (2011) Role for Dpy-30 in ES cell-fate specification by regulation of H3K4 methylation within bivalent domains (vol 144, pg 513, 2011). Cell, 144, 825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ang Y.S., et al. (2011) Wdr5 mediates self-renewal and reprogramming via the embryonic stem cell core transcriptional network. Cell, 145, 183–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Min B.M., et al. (1994) Inactivation of the P53 gene by either mutation or Hpv infection is extremely frequent in human oral squamous-cell carcinoma cell-lines. Oral Oncol. Eur. J. Cancer B, 30B, 338–345. [DOI] [PubMed] [Google Scholar]
- 18. Lee S.H., et al. (2012) TNFα enhances cancer stem cell-like phenotype via Notch-Hes1 activation in oral squamous cell carcinoma cells. Biochem. Biophys. Res. Commun., 424, 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee E.J., et al. (2005) Characterization of newly established oral cancer cell lines derived from six squamous cell carcinoma and two mucoepidermoid carcinoma cells. Exp. Mol. Med., 37, 379–390. [DOI] [PubMed] [Google Scholar]
- 20. Lin C.J., et al. (2007) Head and neck squamous cell carcinoma cell lines: Established models and rationale for selection. Head Neck J. Sci. Spec., 29, 163–188. [DOI] [PubMed] [Google Scholar]
- 21. Park N.H., et al. (1995) Combined oral carcinogenicity of HPV-16 and benzo(a)pyrene: an in vitro multistep carcinogenesis model. Oncogene, 10, 2145–2153. [PubMed] [Google Scholar]
- 22. Park N.H., et al. (1991) Immortalization of normal human oral keratinocytes with type 16 human papillomavirus. Carcinogenesis, 12, 1627–1631. [DOI] [PubMed] [Google Scholar]
- 23. Dickson M.A., et al. (2000) Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell. Biol., 20, 1436–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shin K.H., et al. (2007) p53 promotes the fidelity of DNA end-joining activity by, in part, enhancing the expression of heterogeneous nuclear ribonucleoprotein G. DNA Repair (Amst)., 6, 830–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shin K.H., et al. (2008) hnRNP G elicits tumor-suppressive activity in part by upregulating the expression of Txnip. Biochem. Biophys. Res. Commun., 372, 880–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shin K.H., et al. (2011) Expression and mutation analysis of heterogeneous nuclear ribonucleoprotein G in human oral cancer. Oral Oncol., 47, 1011–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y., et al. (2011) Transforming growth factor-β regulates the sphere-initiating stem cell-like feature in breast cancer through miRNA-181 and ATM. Oncogene, 30, 1470–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Monroe M.M., et al. (2011) Cancer stem cells in head and neck squamous cell carcinoma. J. Oncol., 2011, 762780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dou J., et al. (2007) Isolation and identification of cancer stem-like cells from murine melanoma cell lines. Cell. Mol. Immunol., 4, 467–472. [PubMed] [Google Scholar]
- 30. Han X.F., et al. (2013) A2780 human ovarian cancer cells with acquired paclitaxel resistance display cancer stem cell properties. Oncol. Lett., 6, 1295–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sansone P., et al. (2007) IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J. Clin. Invest., 117, 3988–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Todaro M., et al. (2007) Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell, 1, 389–402. [DOI] [PubMed] [Google Scholar]
- 33. Asiedu M.K., et al. (2011) TGFbeta/TNF(alpha)-mediated epithelial-mesenchymal transition generates breast cancer stem cells with a claudin-low phenotype. Cancer Res., 71, 4707–4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang F., et al. (2014) JMJD6 promotes colon carcinogenesis through negative regulation of p53 by hydroxylation. Plos Biol., 12, e1001819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Owens D.M., et al. (2003) Contribution of stem cells and differentiated cells to epidermal tumours. Nat. Rev. Cancer, 3, 444–451. [DOI] [PubMed] [Google Scholar]
- 36. Kang M.K., et al. (1998) Replicative senescence of normal human oral keratinocytes is associated with the loss of telomerase activity without shortening of telomeres. Cell Growth Differ., 9, 85–95. [PubMed] [Google Scholar]
- 37. Clay M.R., et al. (2010) Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck J. Sci. Spec., 32, 1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ota N., et al. (2014) In vitro and in vivo expression of aldehyde dehydrogenase 1 in oral squamous cell carcinoma. Int. J. Oncol., 44, 435–442. [DOI] [PubMed] [Google Scholar]
- 39. Richard V., et al. (2013) Multiple drug resistant, tumorigenic stem-like cells in oral cancer. Cancer Lett., 338, 300–316. [DOI] [PubMed] [Google Scholar]
- 40. Liu T., et al. (2010) Establishment and characterization of multi-drug resistant, prostate carcinoma-initiating stem-like cells from human prostate cancer cell lines 22RV1. Mol. Cell. Biochem., 340, 265–273. [DOI] [PubMed] [Google Scholar]
- 41. Zhang X., et al. (2010) Prognostic significance of OCT4 expression in adenocarcinoma of the lung. Jpn. J. Clin. Oncol., 40, 961–966. [DOI] [PubMed] [Google Scholar]
- 42. Kumar S.M., et al. (2012) Acquired cancer stem cell phenotypes through Oct4-mediated dedifferentiation. Oncogene, 31, 4898–4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hayashi S., et al. (2013) Lin28a is a putative factor in regulating cancer stem cell-like properties in side population cells of oral squamous cell carcinoma. Exp. Cell Res., 319, 1220–1228. [DOI] [PubMed] [Google Scholar]
- 44. Chu P.Y., et al. (2013) Epithelial-mesenchymal transition transcription factor ZEB1/ZEB2 co-expression predicts poor prognosis and maintains tumor-initiating properties in head and neck cancer. Oral Oncol., 49, 34–41. [DOI] [PubMed] [Google Scholar]
- 45. Chin A.R., et al. (2014) Cytokines driving breast cancer stemness. Mol. Cell. Endocrinol., 382, 598–602. [DOI] [PubMed] [Google Scholar]
- 46. Prokopchuk O., et al. (2005) Interleukin-4 enhances proliferation of human pancreatic cancer cells: evidence for autocrine and paracrine actions. Br. J. Cancer, 92, 921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li B.H., et al. (2008) IL-4/Stat6 activities correlate with apoptosis and metastasis in colon cancer cells. Biochem. Biophys. Res. Commun., 369, 554–560. [DOI] [PubMed] [Google Scholar]
- 48. Conticello C., et al. (2004) IL-4 protects tumor cells from anti-CD95 and chemotherapeutic agents via up-regulation of antiapoptotic proteins. J. Immunol., 172, 5467–5477. [DOI] [PubMed] [Google Scholar]
- 49. Di Stefano A.B., et al. (2010) Survivin is regulated by interleukin-4 in colon cancer stem cells. J. Cell. Physiol., 225, 555–561. [DOI] [PubMed] [Google Scholar]
- 50. Janzer A., et al. (2012) Lysine-specific demethylase 1 (LSD1) and histone deacetylase 1 (HDAC1) synergistically repress proinflammatory cytokines and classical complement pathway components. Biochem. Biophys. Res. Commun., 421, 665–670. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.