Abstract
Polyomaviruses (PyV) are potentially tumorigenic in humans. However, limited data exist on the population seroprevalence of PyVs and individual characteristics that relate to seropositivity. Using multiplex serology, we determined the seroprevalence of 10 human PyVs (BK, JC, KI, WU, MCV, HPyV6, HPyV7, TSV, HPyV9, and HPyV10) among controls from a population-based skin cancer case-control study (n = 460) conducted in New Hampshire between 1993 and 1995. On a subset of participants (n = 194), methylation at CpG dinucleotides across the genome was measured in peripheral blood using the Illumina Infinium HumanMethylation27 BeadChip array (Illumina Inc., San Diego, California), from which lymphocyte subtype proportions were inferred. All participants were seropositive for at least 1 PyV, with seroprevalences ranging from 17.6% (HPyV9) to 99.1% (HPyV10). Seropositivity to JC, MCV, and HPyV7 increased with age. JC and TSV seropositivity were more common among men than among women. Smokers were more likely to be HPyV9-seropositive but MCV-seronegative, and HPyV7 seropositivity was associated with prolonged glucocorticoid use. Based on DNA methylation profiles, differences were observed in CD8-positive T- and B-cell proportions by BK, JC, and HPyV9 seropositivity. Our findings suggest that PyV seropositivity is common in the United States and varies by sociodemographic and biological characteristics, including those related to immune function.
Keywords: antibodies, immunity, polyomavirus, prevalence
Polyomaviruses (PyV) are nonenveloped viruses with an icosahedral capsid approximately 45 nm in diameter, containing a circular double-stranded DNA genome (1, 2). The PyV genome contains genes encoding at least 2 capsid proteins (VP1, VP2, and VP3 for most viruses (3)), as well as small and large T antigens (TAg) (1). Members of the Polyomaviridae family have been discovered in humans, nonhuman primates, mice, birds, bats, and a host of other species (4). Urine and other body fluids have been implicated as the main vehicle for transmission within families or intimate contacts (5). However, the diversity of the human polyomavirome and its impact on human health has not yet been fully elucidated, as reflected in the continued discovery of new PyVs.
Although findings are largely based on studies of European populations and blood donors, PyVs appear to be ubiquitous within human populations (reviewed by DeCaprio and Garcea (6)). While often asymptomatic, under conditions of immunosuppression, BK virus (7) has been associated with PyV-associated nephropathy and cystitis (8, 9), JC virus (10) with progressive multifocal leukoencephalopathy (8, 9), and Trichodysplasia spinulosa-associated polyomavirus (TSV) with the rare skin disease Trichodysplasia spinulosa (11). Furthermore, Merkel cell polyomavirus (MCV) with mutations in the large TAg (12) has been implicated as a causal factor in Merkel cell carcinoma (13), regardless of immune status (14, 15). Although prevalent in various tissues across multiple populations (16), Karolinska Institute polyomavirus (KI) (17), Washington University polyomavirus (WU) (18), human polyomaviruses 6 and 7 (HPyV6 and HPyV7) (19), human polyomavirus 9 (HPyV9) (20), and Malawi/human polyomavirus 10 (HPyV10) (21) have not yet been associated with any specific disease phenotype (22).
To determine the seroprevalence of these viruses and the individual sociodemographic and biological characteristics associated with seropositivity, we measured the frequency of serum antibodies against 10 PyVs among controls from a US population-based case-control study.
METHODS
Study population
The study participants and methods have been described in detail elsewhere (23, 24). Briefly, our study included controls from a population-based case-control study of basal cell and squamous cell skin cancers. Controls were frequency-matched to the age (25–74 years) and sex distribution of skin cancer patients whose cases were diagnosed from July 1993 through June 1995. Residents of New Hampshire were selected from the New Hampshire Department of Transportation (ages <65 years) and Center for Medicaid and Medicare Services enrollment lists (ages ≥65 years), and were required to speak English and to have a listed telephone number. Data on sociodemographic factors (e.g., age, sex, educational level), lifestyle factors (e.g., cigarette smoking), sunlight-related characteristics (e.g., response to sunlight, number of severe sunburns), and medical history (e.g., use of glucocorticoids for ≥1 month, organ transplant recipient) were collected through personal interviews. All participants provided informed consent in accordance with the Committee for the Protection of Human Subjects at Dartmouth College (Hanover, New Hampshire).
Blood sample collection
Venous blood samples of 20–30 mL were collected in heparinized tubes for serological analysis (as described by Karagas et al. (23)). Blood was separated by centrifugation at 2,500 × g for 20 minutes at 4°C. Each component (plasma, red blood cells, buffy coat) was labeled and stored separately at −80°C until analysis. DNA was also extracted from the buffy coat for DNA methylation analysis. Plasma samples, masked with regard to individual identity and characteristics, were shipped to the German Cancer Research Center (Deutsches Krebsforschungszentrum; Heidelberg, Germany) on dry ice for serological analysis.
Human PyV serology
Antigen preparation and techniques used for human PyVs (25–27) closely follow methods previously described for human papillomaviruses (28, 29). Briefly, plasma samples were tested for antibodies against 10 human PyVs (capsid protein VP1 for BK, JC, KI, WU, MCV isolate 344, HPyV6, HPyV7, TSV, HPyV9, HPyV10; large TAg for BK, JC, MCV, HPyV6, HPyV7, TSV, HPyV10; and small TAg for MCV). The multiplex antibody detection approach was based on a glutathione S-transferase capture enzyme-linked immunosorbent assay method in combination with fluorescent bead technology (Luminex Corporation, Austin, Texas) (28, 30, 31).
Seroreactivity against PyV proteins was expressed as the median fluorescence intensity (MFI) of ≥100 beads of the same internal color. MFI values reflect antibody affinity, titer, and reactivity determined by dilution series (32). Standard cutpoints for seropositivity were chosen for the MFI of each PyV tested by visual inspection of frequency distribution curves (percentile plots), as done previously (15, 23, 27, 29); stringent criteria were chosen to increase specificity. For VP1, the cutoff value was 250 MFI units for all 10 PyVs (as done by Teras et al. (33)).
To evaluate the robustness of PyV VP1 seroprevalence estimates, we used a sliding cutpoint between 50 and 450 MFI units (see Web Figure 1, available at http://aje.oxfordjournals.org/). We also calculated cutpoints using a method adapted from van der Meijden et al. (34). This involved a frequency distribution analysis with a bin width of 250 MFI units, and the seronegative population defined by either all of the bins containing more than 10% of the study participants or the first bin. Cutoffs were then calculated as the mean seroresponse for persons who were seronegative plus 3 times the standard deviation. This resulted in similar seroprevalence estimates as the standard cutpoints, except for JC due to the use of 2 bins (as opposed to 1) in cutoff determination. Given the insensitivity of PyV seroprevalence to cutpoint definitions, we ultimately used the standard cutpoints in all analyses.
Statistical analyses
We first constructed a phylogenetic tree relating human PyVs from the complete genome sequences stored in the RefSeq database (http://www.ncbi.nlm.nih.gov/refseq/about/) using MUSCLE 3.8.31 to create a neighbor-joining tree without distance corrections (35). The accession numbers of the complete genome sequences for the 10 assayed human PyVs selected from RefSeq were: NC_001538.1 (BK), NC_001699.1 (JC), NC_009238.1 (KI), NC_009539.1 (WU), NC_010277.1 (MCV), NC_014406.1 (HPyV6), NC_014407.1 (HPyV7), NC_014361.1 (TSV), NC_015150.1 (HPyV9), and NC_018102.1 (HPyV10).
The frequency of PyV seropositivity for each virus was then examined both for PyV seropositivity overall (any PyV-positive vs. no PyV-positive) and by the number of PyV types to which an individual tested positive, using binary MFI cutpoints. In addition, we used the continuous MFI values to compute Spearman rank correlation coefficients (ρ) for correlations between the PyVs assayed.
We tested the relationships between various individual characteristics and PyV seropositivity using the χ2 test for categorical variables (i.e., age groups (25–44, 45–54, 55–64, 65–69, and 70–75 years), sex, education, smoking status, and glucocorticoid use), Fisher's exact test for categorical variables with small strata (i.e., <10 participants), and the Wilcoxon rank-sum test or Kruskal-Wallis test for continuous variables (i.e., age and mean number of PyVs seropositive). We further used logistic regression to assess the association between PyV seropositivity and each characteristic of interest while considering the potential confounding effects of other characteristics. Variables that altered associations between the characteristics of interest and PyV seropositivity (i.e., that changed our association estimates by more than 10% (36)) were included in our model. Only age group and sex were found to change the estimates, so we present P values adjusted for these variables. All statistical tests were 2-sided, and significance was assessed at the α = 0.05 level. All analyses of the serological data were performed in R, version 3.0.1 (R Foundation for Statistical Computing, Vienna, Austria).
Analysis of DNA methylation and immune cell proportions
DNA methylation data and inferred immune cell proportions have been previously reported by Marsit et al. (37) and Koestler et al. (38), respectively. Briefly, DNA was extracted from peripheral blood using the QIAmp DNA mini-kit (QIAGEN Inc., Valencia, California) and underwent sodium bisulfite modification with the EZ DNA Methylation kit (Zymo Research Corporation, Irvine, California) according to the manufacturers' protocols. DNA methylation data were obtained with the Illumina Infinium HumanMethylation27 BeadArray (Illumina Inc., San Diego, California), using methods described in detail elsewhere (37, 39–41). Array quality assurance was assessed, and poorly performing loci were removed (39, 40, 42). Sex-linked loci, CpG loci containing a single-nucleotide polymorphism, CpG loci assayed with a probe containing a single-nucleotide polymorphism, and autosomal probes reported to be cross-reactive or repetitive were removed (39, 43, 44). The proportion of immune cell subtypes was inferred from DNA methylation profiles using an algorithm described by Houseman et al. (45). We compared the median predicted proportions of adaptive lymphocytes for CD8-positive (CD8+) T cells, CD4-positive (CD4+) T cells, and B cells between persons who were PyV-seropositive and persons who were seronegative for each PyV type, except types with fewer than 10 seronegative participants (i.e., WU and HPyV10), using the Wilcoxon rank-sum test. All tests were 2-sided, and analyses for the methylation and immune cell proportions data were carried out in R, version 3.1.0.
RESULTS
Study population characteristics
Venous blood samples were available for serological analyses from 460 study participants (85.2% of the 540 interviewed; 1 participant was excluded due to an insufficient bead count during serological analysis). DNA was extracted from the buffy coat samples, and a subset of 194 study participants (42.2% of the 460 with blood samples) was randomly selected for DNA methylation analysis. No appreciable differences were noted in the characteristics of persons for whom we did not obtain serological data (data not shown). There were also no notable differences in the characteristics of the 460 persons with PyV serological data (Web Table 1) and the subset of 194 study participants with paired methylation data (Web Table 2).
The study population included a higher proportion of men than of women; almost half had completed at least high school or technical school, and most were former smokers. The mean age of participants was 61.2 (standard deviation (SD), 10.7) years. There was 1 organ transplant recipient (0.2%), and other participants (8.5%) reported the use of oral glucocorticoids for 1 month or longer (Table 1).
Table 1.
Sociodemographic Characteristics of 460 Study Participants According to Seropositivity for 6 Types of Human Polyomaviruses, New Hampshire, 1993–1995a
| Variable | Totalb |
Mean No. (SD) of PyV Types for Which Participants Were Seropositive | PyV Type and Proportion Seropositivec,d |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | BK |
JC |
MCV |
HPyV7 |
TSV |
HPyV9 |
||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||||
| Overall | 460 | 100 | 7.3 (1.4) | 403 | 87.6 | 256 | 55.6e | 324 | 70.4f | 259 | 56.3g | 372 | 80.9 | 81 | 17.6 |
| Sex | |||||||||||||||
| Male | 280 | 100 | 7.5 (1.5)h | 246 | 87.8 | 168 | 60.0i | 204 | 72.8 | 164 | 58.6 | 239 | 85.4h | 54 | 19.3 |
| Female | 180 | 100 | 7.0 (1.3) | 157 | 87.2 | 88 | 48.9 | 120 | 66.7 | 95 | 52.8 | 133 | 73.9 | 27 | 15.0 |
| Education | |||||||||||||||
| Elementary to high school or technical school | 227 | 100 | 7.4 (1.4) | 201 | 88.5 | 126 | 55.5 | 163 | 71.8 | 131 | 57.7 | 193 | 85.0 | 41 | 18.1 |
| Any college | 144 | 100 | 7.1 (1.4) | 123 | 85.4 | 78 | 54.2 | 99 | 68.8 | 77 | 53.5 | 110 | 76.4 | 18 | 12.5 |
| College graduate or professional school | 89 | 100 | 7.4 (1.6) | 79 | 88.8 | 52 | 58.4 | 62 | 69.7 | 51 | 57.3 | 69 | 77.5 | 22 | 24.7 |
| Smoking status | |||||||||||||||
| Never smoker | 146 | 100 | 7.3 (1.3) | 130 | 89.0 | 81 | 55.5 | 111 | 76.0i,j | 79 | 54.1 | 119 | 81.5 | 15 | 10.3i,j |
| Former smoker | 230 | 100 | 7.4 (1.5) | 205 | 89.1 | 128 | 55.6 | 159 | 69.1 | 138 | 60.0 | 191 | 83.0 | 49 | 21.3 |
| Current smoker | 84 | 100 | 7.0 (1.6) | 68 | 81.1 | 47 | 56.0 | 54 | 64.3 | 42 | 50.0 | 62 | 73.8 | 17 | 20.2 |
| Glucocorticoid usek | |||||||||||||||
| Yes | 39 | 100 | 7.4 (1.3) | 35 | 89.7 | 22 | 56.4 | 23 | 59.0 | 30 | 76.9h,j | 32 | 82.0 | 5 | 12.8 |
| No | 415 | 100 | 7.3 (1.4) | 363 | 87.5 | 231 | 55.7 | 296 | 71.3 | 226 | 54.4 | 334 | 80.5 | 74 | 17.8 |
Abbreviations: PyV, polyomavirus; SD, standard deviation.
a Numbers may not sum to the overall totals because of missing data. Full data, including all tested PyVs, are shown in Web Table 1.
b All participants were seropositive for at least 1 of the 10 PyVs assayed.
c PyV infection was determined using seropositivity for the VP1 protein.
d Percentage of participants who were PyV-seropositive versus PyV-seronegative in each given stratum.
e P < 0.05 in a test for trend across age groups (25–44 years (n = 46), 45–54 years (n = 70), 55–64 years (n = 106), 65–69 years (n = 131), and 70–75 years (n = 107)).
f P < 0.005 in a test for trend across age groups.
g P < 0.01 in a test for trend across age groups.
h P < 0.005 for the difference in proportions between groups, as determined by χ2 or Fisher's exact tests for categorical variables and by Kruskal-Wallis or Wilcoxon rank-sum tests for continuous variables.
i P < 0.05 for the difference in proportions between groups.
j Age group- and sex-adjusted P value, as determined by logistic regression.
k Having used oral steroid or corticosteroid medications (e.g., cortisone or prednisone) for ≥1 month.
Seropositivity to PyVs
We did not find correlations or evidence of cross-reactivity between the VP1 capsid proteins of most PyV types. The exceptions were positive correlations between the closely related HPyV6 and HPyV7 (ρ = 0.59, P = 1.1e−67) and between MCV and HPyV9 (ρ = 0.43, P = 1.0e−33) (Web Figure 2). However, when analyses were restricted to participants who were seropositive for each PyV pair, we did not see any strong correlations between the VP1 capsid proteins (data not shown). In contrast, many of the PyV TAgs were positively correlated (data not shown). Because of the high correlation between TAgs, the immunodominance of VP1 (25), and the use of VP1 antibodies as cumulative exposure markers to PyVs, we focused our analyses on VP1 serostatus.
All participants were seropositive for at least 1 type of PyV (Figure 1). Seroprevalences ranged from 17.6% for HPyV9 to 99.1% for HPyV10 (Table 1; see Web Table 1 for comprehensive results). The mean number of PyV VP1s to which individuals tested positive was 7.3 (SD, 1.4), and the number increased with age group (P for trend = 0.002). Men were seropositive for a higher average number of PyVs (mean = 7.5 (SD, 1.5)) than women (mean = 7.0 (SD, 1.3); continuous P = 0.0007, χ2 P = 0.02) (Table 1).
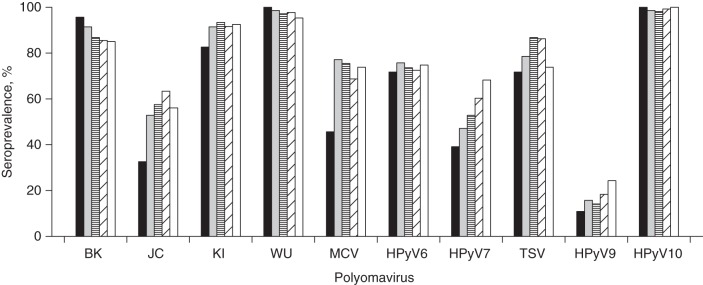
Figure 1.
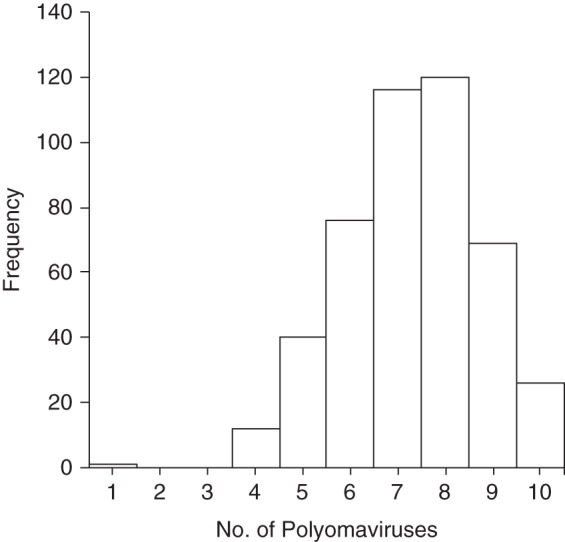
Number of different human polyomaviruses (PyV) against which an individual tested positive for the VP1 antigen among 460 study participants, New Hampshire, 1993–1995. Out of 10 types of human PyV assayed, the mean number of different types for which a participant was seropositive was 7.3 (standard deviation, 1.4), and the median was 7.0.
Seroprevalence was consistent across age groups for KI, WU, HPyV6, TSV, HPyV9, and HPyV10 and increased with age group for JC (P for trend = 0.02), MCV (P = 0.002), and HPyV7 (P = 0.006) (Figure 2). A slight decrease by age group was observed for BK seroprevalence, albeit not of statistical significance (P = 0.4). Men had higher VP1 seropositivity for many of the PyVs than women, and this difference was statistically significant for JC (60.0% in men and 48.9% in women; P = 0.02) and TSV (85.4% in men and 73.9% in women; P = 0.003) (Table 1).
Figure 2.
Seroprevalences of human polyomaviruses (PyV) as determined by VP1 seroreactivity among 460 study participants, by age group, New Hampshire, 1993–1995. The black, gray, horizontally striped, diagonally striped, and white bars correspond to the age groups 24–44 years (n = 46), 45–54 years (n = 70), 55–64 years (n = 106), 65–69 years (n = 131), and 70–75 years (n = 107), respectively. The overall seroprevalence (%) and number of participants who were seropositive (n) for each PyV were as follows—BK: 87.6% (n = 403); JC: 55.6% (n = 256); KI: 91.3% (n =420); WU: 97.4% (n = 448); MCV: 70.4% (n = 324); HPyV6: 73.7% (n = 339); HPyV7: 56.3% (n = 259); TSV: 80.9% (n = 372); HPyV9: 17.6% (n =81); and HPyV10: 99.1% (n = 456).
Other individual characteristics were related to certain PyVs (Table 1). For HPyV9, a higher seroprevalence was observed among former or current smokers than among never smokers (age group- and sex-adjusted P = 0.03). MCV seropositivity was less common among participants who were current or former smokers than among those who had never smoked (adjusted P = 0.02). HPyV7 seropositivity was more common among persons who had used glucocorticoids for 1 month or longer (adjusted P = 0.003). The sole organ transplant recipient was seropositive for almost all PyV types, except HPyV9. PyV seropositivity did not vary appreciably by level of education.
PyVs and immune cell proportions
Differences in immune cell proportions by serostatus were observed for BK, JC, and HPyV9 seropositivity (Table 2). The median proportion of B cells was higher among persons who were BK-seropositive than among those seronegative (P =0.03). In contrast, the median proportion of B cells was lower among JC-seropositive persons than among seronegative persons (P = 0.02). CD8+ T cell proportions were lower for persons who were BK-seropositive (P = 0.02) and HPyV9-seropositive (P = 0.05) than for those seronegative. However, the extent of these differences was relatively small. Correlations of the continuous MFI values for all PyV VP1 capsid proteins with immune cell proportions were small and not statistically significant (Web Figure 3).
Table 2.
Median Proportions of Adaptive Lymphocytesa Among 194 Study Participants, According to Polyomavirus Seropositivity, New Hampshire, 1993–1995b
| PyV Type and Serostatus |
Total |
Median Proportion (IQR) of Adaptive Lymphocytes |
|||
|---|---|---|---|---|---|
| No. | %c | CD8+ T Cells | CD4+ T Cells | B Cells | |
| BK | |||||
| Seropositive | 164 | 84.5 | 0.15 (0.11–0.18)d | 0.13 (0.10–0.17) | 0.03 (0.02–0.04)d |
| Seronegative | 30 | 15.5 | 0.17 (0.13–0.21) | 0.14 (0.10–0.16) | 0.02 (0.00–0.03) |
| JC | |||||
| Seropositive | 105 | 54.1 | 0.15 (0.12–0.19) | 0.13 (0.11–0.17) | 0.02 (0.01–0.04)d |
| Seronegative | 89 | 45.9 | 0.15 (0.12–0.18) | 0.12 (0.10–0.16) | 0.03 (0.02–0.04) |
| KI | |||||
| Seropositive | 177 | 91.2 | 0.15 (0.12–0.18) | 0.13 (0.10–0.17) | 0.03 (0.01–0.04) |
| Seronegative | 17 | 8.8 | 0.13 (0.11–0.15) | 0.15 (0.12–0.17) | 0.03 (0.01–0.05) |
| MCV | |||||
| Seropositive | 130 | 67.0 | 0.14 (0.11–0.18) | 0.13 (0.10–0.17) | 0.03 (0.01–0.04) |
| Seronegative | 64 | 33.0 | 0.16 (0.12–0.18) | 0.13 (0.10–0.15) | 0.03 (0.01–0.05) |
| HPyV6 | |||||
| Seropositive | 144 | 74.2 | 0.15 (0.12–0.18) | 0.13 (0.11–0.17) | 0.03 (0.01–0.04) |
| Seronegative | 50 | 25.8 | 0.14 (0.10–0.18) | 0.12 (0.09–0.14) | 0.03 (0.01–0.04) |
| HPyV7 | |||||
| Seropositive | 120 | 61.8 | 0.15 (0.12–0.18) | 0.13 (0.10–0.16) | 0.02 (0.01–0.04) |
| Seronegative | 74 | 38.1 | 0.14 (0.12–0.20) | 0.13 (0.10–0.18) | 0.03 (0.01–0.05) |
| TSV | |||||
| Seropositive | 163 | 84.0 | 0.15 (0.12–0.19) | 0.13 (0.10–0.17) | 0.02 (0.01–0.04) |
| Seronegative | 31 | 16.0 | 0.14 (0.12–0.16) | 0.13 (0.10–0.17) | 0.03 (0.01–0.04) |
| HPyV9 | |||||
| Seropositive | 38 | 19.6 | 0.13 (0.12–0.16)d | 0.13 (0.11–0.16) | 0.02 (0.01–0.03) |
| Seronegative | 156 | 80.4 | 0.15 (0.12–0.19) | 0.13 (0.10–0.17) | 0.03 (0.01–0.04) |
Abbreviations: IQR, interquartile range; PyV, polyomavirus.
a Proportions of adaptive lymphocytes were determined using the Illumina Infinium HumanMethylation27 BeadChip array (Illumina Inc., San Diego, California) and an algorithm published by Houseman et al. (45).
b PyV infection was determined using seropositivity for the VP1 protein.
c Percentage of participants who were within each given stratum of PyV seroreactivity. Percentages may not sum to 100 because of rounding.
d P < 0.05 for difference in medians between groups, as determined by the Wilcoxon rank-sum test.
DISCUSSION
In a large sample of the general adult US population, we examined the prevalence of antibodies against the VP1 capsid proteins from the first 10 discovered human PyVs: BK, JC, KI, WU, MCV, HPyV6, HPyV7, TSV, HPyV9, and HPyV10. The number of PyVs to which individuals tested positive ranged from at least 1 to all 10, with a mean of 7.3 PyVs. Thus, our findings support the notion that PyVs are widespread in the human population. We also found age, sex, smoking status, glucocorticoid use, and indicators of immunity to be associated with PyV seropositivity.
We observed seroprevalences for BK, JC, WU, MCV, HPyV6, HPyV7, and TSV that were within the range of what has been reported previously in adults (reviewed by DeCaprio and Garcea (6) and Moens et al. (46), and assessed by Nicol et al. (16)). For KI (91.3%), the seroprevalence was just above the highest seroprevalences reported in the literature (55%–90%) (2, 15, 46–51), with the highest seroprevalence (90.0%) being found in a US female population from Seattle, Washington (15). In Italian and German blood donors, the seroprevalence of HPyV9 ranged from 33% to 47% (16, 52, 53), and seroprevalence was 24% in an Australian population (34), whereas we found a seroprevalence of only 17.6%. For HPyV10, the seroprevalence was 42% in a large sample from Italy (54) and 66% in a sample from Colorado (55), but was almost 100% in our study. However, a different HPyV10 was isolated from a human sample and investigated, with the study from Colorado using an isolate roughly 95% identical to the isolate used in the Italian study (54, 55) and more than 99% identical to that used in our study (56), which may have affected antibody binding during serological analysis. Thus, there may be variations in the PyV prevalences resulting from the use of different assays, as well as by geographical region and characteristics of the study population. Techniques used for PyV antibody detection and antigen preparation, as well as definition of cutoff values, could also explain interstudy differences.
The trends we observed in seroprevalence by age were in agreement with the available literature. The increasing seroprevalence of JC and MCV with age was previously found in a large cohort from the Czech Republic (57) and among American blood donors (58). The increase in HPyV7 seroprevalence with age was reported in an Italian study (16) and may be explained by continuous transmission of HPyV7 throughout life (16). The slight fall in BK seroprevalence we observed was present in English cohorts (59, 60) and in a Czech Republic population survey (57), and could be related to diminished antibody reactivity against BK over time or to cohort effects in infection rates. For the PyVs that appeared stable during adulthood, primary infection probably occurred during childhood, and thus little change in seroprevalence for adults might have been expected. Indeed, there is evidence that primary infection occurs early in life for BK (59), KI (47, 49), WU (47, 49), TSV (16, 48), and HPyV10 (54).
Differences in PyV infection rates between men and women have generally not been observed in past studies (27, 49, 51, 54, 59). However, an English study found more men than women to have antibodies against JC (59), as was found in our study. Differences in TSV seroprevalence by sex have not been reported previously (16, 34, 48) and may be due to population differences.
Studies examining the role of PyVs in smoking-related cancers have not found associations between smoking status and BK, JC, or MCV seropositivity across multiple populations (61–63). We found seropositivity to MCV to be less common among smokers than among never smokers, as observed in both a Chinese study (63) and a Chilean study (62) of non-small-cell lung cancer samples assayed for MCV large TAg DNA, and in a Spanish study of bladder cancer and PyV seroprevalence (61)—although these differences were not statistically significant, possibly because those studies did not adjust for sex in their analyses.
We also found HPyV9 seropositivity to be higher among current and former smokers than among never smokers, which has not been reported previously. In theory, cigarette smoking could increase risk of a viral infection through various mechanisms, including 1) structural changes to the respiratory tract which may predispose a person to pathogen adherence; 2) altered composition of cellular immunity that in turn could impair one's ability to limit viral replication (i.e., decreased number of CD4+ T cells and B cell proliferation, and eventually lower serum immunoglobulin levels); and 3) enhanced cell infectivity (reviewed by Arcavi and Benowitz (64)). Interestingly, the prevalence of HPyV9 (first isolated from the blood and urine of a kidney transplant recipient (20)) appeared to increase following organ transplantation, probably due to the immunosuppression (65). Since smoking may also be immunosuppressive, HPyV9 could infect or reactivate under conditions of immune dysfunction.
Indeed, in previous studies, the prevalence of multiple PyVs has differed in immunodeficient persons versus immunocompetent persons. In our study, the single organ transplant recipient was seropositive for all PyVs but HPyV9, the least seroprevalent PyV (20, 65). In numerous prospective studies, BK (66, 67) and JC (68) DNA or viral shedding has been detected in renal transplant patients at a higher frequency following transplantation, and antibody reactivity against BK and JC was found to increase with time following transplantation (69). Additionally, BK replication has been associated with corticosteroid treatment in transplant recipients (66, 70). We also found HPyV7 seropositivity to be more common among persons who reported using glucocorticoids for at least 1 month, which have immunosuppressive properties (71). Furthermore, in a case series, Costa et al. (72) found high levels of JC viruria following long-term steroid use in an otherwise healthy adult; but additional data are needed. In a study on solid organ transplant recipients from Seattle, Washington, the reported seroprevalences for JC, KI, WU, MCV, HPyV6, and HPyV7 were lower than what we observed in our study population (73). Thus, the association of immunosuppression with PyV seropositivity will require additional study.
We further found individual PyVs to be associated with small but statistically significant changes in proportions of adaptive lymphocytes: Persons who were seropositive for BK had lower proportions of CD8+ T cells but higher proportions of B cells, which was consistent with the findings that anti-allograft rejection immunosuppressive drugs often suppress T cell activation (74) and are associated with PyV-associated nephropathy (75). HPyV9 seropositivity was also associated with lowered proportions of CD8+ T cells. Furthermore, JC seropositivity was associated with lowered proportions of B cells. Among immunocompromised (human immunodeficiency virus/acquired immunodeficiency syndrome) patients, immunoglobulin G responses against JC were higher among survivors of progressive multifocal leukoencephalopathy than among those who died of it (76), suggesting that B cells and the humoral immune response may be important in defense against JC. Impaired cellular immune responses and decreased immune surveillance could promote active PyV infection, as has been known for BK and JC (71). Smaller differences in immune cell proportions were observed in our study, and this might be expected among immunocompetent individuals. Thus, while our findings are speculative and multiple comparisons were made, they are consistent with the clinical and experimental evidence (6) suggesting that PyV infectivity may be influenced by conditions of immunosuppression and warrant further investigation of PyVs as potentially oncogenic viruses.
An advantage of our analysis was the availability of a large number of samples from participants in a population-based study. The comprehensive assessment of multiple types of human PyVs in a North American study population and the association of individual PyV seropositivity with sociodemographic characteristics, as well as measures of immune cell proportions derived from a novel algorithmic technique, were unique strengths of our research.
Limitations of our research included the use of PyV seroreactivity as a measure of PyV infection (27, 77). However, the glutathione S-transferase capture of recombinantly expressed VP1 capsid proteins was shown to be a reliable technique for assessing PyV seroreactivity and has been used as a marker of PyV infection in prior studies (25–27, 77). In addition, MCV viral load and antibody titer have been shown to have a strong positive monotonic correlation (78), although less is known about the other types of PyVs. The VP1 assay showed minimal signs of cross-reactivity in antibody detection between PyV types, although potential serological cross-reactivity between HPyV6 and HPyV7, and between MCV and HPyV9, may have limited our interpretation of findings for these PyV types. However, once we restricted the data to persons who were just PyV-seropositive, we did not see any VP1 cross-reactivity. Although it is possible that our seroprevalences could have been affected by our choice of cutpoint for converting a continuous MFI measure into a binary variable, our sensitivity analysis suggested that this did not appreciably impact our findings. The possibility of cross-reactivity with other, yet undiscovered, human PyVs cannot be excluded. For instance, we did not investigate the recently identified St. Louis polyomavirus (79), human polyomavirus 12 (80), or New Jersey polyomavirus (81). Finally, the immune cell proportions were derived from genome-wide DNA methylation profiles (45) rather than more labor-intensive methods such as flow cytometry, although cell lineage-specific DNA methylation patterns closely approximate leukocyte subsets purified from human blood (82). Since the same blood samples were used for both PyV antibody assays and DNA methylation assays, we cannot determine whether immune cell variability is a cause or a consequence of seroprevalence.
Our findings suggest that PyVs may be ubiquitous in the US population. The seroprevalence of specific PyV types may differ according to individual characteristics (such as age, sex, smoking status, glucocorticoid use) and immune profiles, which could affect disease risk.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Geisel School of Medicine at Dartmouth, Hanover, New Hampshire (Anala Gossai, Shohreh F. Farzan, Anne G. Hoen, Brock C. Christensen, Carmen J. Marsit, Margaret R. Karagas); German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (Tim Waterboer, Angelika Michel, Martina Willhauck-Fleckenstein, Michael Pawlita); Masonic Cancer Center, University of Minnesota, Minneapolis, Minnesota (Heather H. Nelson); Langone Medical Center, New York University, New York, New York (Shohreh F. Farzan); Department of Biomedical Data Science, Geisel School of Medicine at Dartmouth, Hanover, New Hampshire (Anne G. Hoen); Department of Pharmacology and Toxicology, Geisel School of Medicine at Dartmouth, Hanover, New Hampshire (Brock C. Christensen, Carmen J. Marsit); Department of Epidemiology, School of Public Health, Brown University, Providence, Rhode Island (Karl T. Kelsey); and Department of Community and Family Medicine, Geisel School of Medicine at Dartmouth, Hanover, New Hampshire (Brock C. Christensen, Margaret R. Karagas).
This work was supported by the National Cancer Institute (grants NCI R01CA057494, R01CA121147, and R01CA082354) and the National Institutes of Health (grants NIHGMS P20GM104416 and NIH/NLM K01LM011985).
Conflict of interest: none declared.
REFERENCES
- 1.Randhawa P, Vats A, Shapiro R. The pathobiology of polyomavirus infection in man. In: Ahsan N, ed. Polyomaviruses and Human Diseases. New York, NY: Springer Science+Business Media; 2006:148–159. [DOI] [PubMed] [Google Scholar]
- 2.Moens U, Ludvigsen M, Van Ghelue M. Human polyomaviruses in skin diseases. Patholog Res Int. 2011;2011123491:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schowalter RM, Buck CB. The Merkel cell polyomavirus minor capsid protein. PLoS Pathog. 2013;98:e1003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khalili K, Stoner GL. Human Polyomaviruses: Molecular and Clinical Perspectives. New York, NY: Wiley-Liss, Inc.; 2001. [Google Scholar]
- 5.Kitamura T, Kunitake T, Guo J et al. . Transmission of the human polyomavirus JC virus occurs both within the family and outside the family. J Clin Microbiol. 1994;3210:2359–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeCaprio JA, Garcea RL. A cornucopia of human polyomaviruses. Nat Rev Microbiol. 2013;114:264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardner SD, Field AM, Coleman DV et al. . New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971;2977712:1253–1257. [DOI] [PubMed] [Google Scholar]
- 8.Jiang M, Abend JR, Johnson SF et al. . The role of polyomaviruses in human disease. Virology. 2009;3842:266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imperiale MJ. The human polyomaviruses, BKV and JCV: molecular pathogenesis of acute disease and potential role in cancer. Virology. 2000;2671:1–7. [DOI] [PubMed] [Google Scholar]
- 10.Padgett BL, Zurhein GM, Walker DL et al. . Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971;2977712:1257–1260. [DOI] [PubMed] [Google Scholar]
- 11.van der Meijden E, Janssens RW, Lauber C et al. . Discovery of a new human polyomavirus associated with Trichodysplasia spinulosa in an immunocompromized patient. PLoS Pathog. 2010;67:e1001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shuda M, Feng H, Kwun HJ et al. . T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus. Proc Natl Acad Sci U S A. 2008;10542:16272–16277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng H, Shuda M, Chang Y et al. . Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;3195866:1096–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reisinger DM, Shiffer JD, Cognetta AB Jr et al. . Lack of evidence for basal or squamous cell carcinoma infection with Merkel cell polyomavirus in immunocompetent patients with Merkel cell carcinoma. J Am Acad Dermatol. 2010;633:400–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter JJ, Paulson KG, Wipf GC et al. . Association of Merkel cell polyomavirus-specific antibodies with Merkel cell carcinoma. J Natl Cancer Inst. 2009;10121:1510–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicol JT, Robinot R, Carpentier A et al. . Age-specific seroprevalences of Merkel cell polyomavirus, human polyomaviruses 6, 7, and 9, and Trichodysplasia spinulosa-associated polyomavirus. Clin Vaccine Immunol. 2013;203:363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allander T, Andreasson K, Gupta S et al. . Identification of a third human polyomavirus. J Virol. 2007;818:4130–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaynor AM, Nissen MD, Whiley DM et al. . Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathog. 2007;35:e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schowalter RM, Pastrana DV, Pumphrey KA et al. . Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe. 2010;76:509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scuda N, Hofmann J, Calvignac-Spencer S et al. . A novel human polyomavirus closely related to the African green monkey-derived lymphotropic polyomavirus. J Virol. 2011;859:4586–4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buck CB, Phan GQ, Raiji MT et al. . Complete genome sequence of a tenth human polyomavirus. J Virol. 2012;8619:10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehlers B, Wieland U. The novel human polyomaviruses HPyV6, 7, 9 and beyond. APMIS. 2013;1218:783–795. [DOI] [PubMed] [Google Scholar]
- 23.Karagas MR, Nelson HH, Sehr P et al. . Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst. 2006;986:389–395. [DOI] [PubMed] [Google Scholar]
- 24.Karagas MR, Greenberg ER, Spencer SK et al. . Increase in incidence rates of basal cell and squamous cell skin cancer in New Hampshire, USA. New Hampshire Skin Cancer Study Group. Int J Cancer. 1999;814:555–559. [DOI] [PubMed] [Google Scholar]
- 25.Kjærheim K, Røe OD, Waterboer T et al. . Absence of SV40 antibodies or DNA fragments in prediagnostic mesothelioma serum samples. Int J Cancer. 2007;12011:2459–2465. [DOI] [PubMed] [Google Scholar]
- 26.Rollison DE, Giuliano AR, Messina JL et al. . Case-control study of Merkel cell polyomavirus infection and cutaneous squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2012;211:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antonsson A, Green AC, Mallitt K-A et al. . Prevalence and stability of antibodies to the BK and JC polyomaviruses: a long-term longitudinal study of Australians. J Gen Virol. 2010;917:1849–1853. [DOI] [PubMed] [Google Scholar]
- 28.Sehr P, Müller M, Höpfl R et al. . HPV antibody detection by ELISA with capsid protein L1 fused to glutathione S-transferase. J Virol Methods. 2002;1061:61–70. [DOI] [PubMed] [Google Scholar]
- 29.Michael KM, Waterboer T, Sehr P et al. . Seroprevalence of 34 human papillomavirus types in the German general population. PLoS Pathog. 2008;46:e1000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sehr P, Zumbach K, Pawlita M. A generic capture ELISA for recombinant proteins fused to glutathione S-transferase: validation for HPV serology. J Immunol Methods. 2001;253(1-2):153–162. [DOI] [PubMed] [Google Scholar]
- 31.Waterboer T, Sehr P, Michael KM et al. . Multiplex human papillomavirus serology based on in situ-purified glutathione S-transferase fusion proteins. Clin Chem. 2005;5110:1845–1853. [DOI] [PubMed] [Google Scholar]
- 32.Waterboer T, Neale R, Michael KM et al. . Antibody responses to 26 skin human papillomavirus types in the Netherlands, Italy and Australia. J Gen Virol. 2009;908:1986–1998. [DOI] [PubMed] [Google Scholar]
- 33.Teras LR, Rollison DE, Pawlita M et al. . Prediagnostic circulating polyomavirus antibody levels and risk of non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2015;242:477–480. [DOI] [PubMed] [Google Scholar]
- 34.van der Meijden E, Bialasiewicz S, Rockett RJ et al. . Different serologic behavior of MCPyV, TSPyV, HPyV6, HPyV7 and HPyV9 polyomaviruses found on the skin. PLoS One. 2013;811:e81078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;13811:923–936. [DOI] [PubMed] [Google Scholar]
- 37.Marsit CJ, Koestler DC, Christensen BC et al. . DNA methylation array analysis identifies profiles of blood-derived DNA methylation associated with bladder cancer. J Clin Oncol. 2011;299:1133–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koestler DC, Marsit CJ, Christensen BC et al. . Peripheral blood immune cell methylation profiles are associated with nonhematopoietic cancers. Cancer Epidemiol Biomarkers Prev. 2012;218:1293–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koestler DC, Avissar-Whiting M, Houseman EA et al. . Differential DNA methylation in umbilical cord blood of infants exposed to low levels of arsenic in utero. Environ Health Perspect. 2013;1218:971–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bock C. Analysing and interpreting DNA methylation data. Nat Rev Genet. 2012;1310:705–719. [DOI] [PubMed] [Google Scholar]
- 41.Du P, Zhang X, Huang C-C et al. . Comparison of Beta-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinformatics. 2010;11:587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banister CE, Koestler DC, Maccani MA et al. . Infant growth restriction is associated with distinct patterns of DNA methylation in human placentas. Epigenetics. 2011;67:920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y-A, Choufani S, Ferreira JC et al. . Sequence overlap between autosomal and sex-linked probes on the Illumina HumanMethylation27 microarray. Genomics. 2011;974:214–222. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y-A, Choufani S, Grafodatskaya D et al. . Cross-reactive DNA microarray probes lead to false discovery of autosomal sex-associated DNA methylation. Am J Hum Genet. 2012;914:762–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Houseman EA, Accomando WP, Koestler DC et al. . DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics. 2012;13:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moens U, Van Ghelue M, Song X et al. . Serological cross-reactivity between human polyomaviruses. Rev Med Virol. 2013;234:250–264. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen NL, Le B-M, Wang D. Serologic evidence of frequent human infection with WU and KI polyomaviruses. Emerg Infect Dis. 2009;158:1199–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen T, Mattila PS, Jartti T et al. . Seroepidemiology of the newly found Trichodysplasia spinulosa-associated polyomavirus. J Infect Dis. 2011;20410:1523–1526. [DOI] [PubMed] [Google Scholar]
- 49.Neske F, Prifert C, Scheiner B et al. . High prevalence of antibodies against polyomavirus WU, polyomavirus KI, and human bocavirus in German blood donors. BMC Infect Dis. 2010;10:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moens U, Johannessen M, Bárcena-Panero A et al. . Emerging polyomaviruses in the human population. Rev Infect. 2010;12:59–93. [Google Scholar]
- 51.Kean JM, Rao S, Wang M et al. . Seroepidemiology of human polyomaviruses. PLoS Pathog. 2009;53:e1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trusch F, Klein M, Finsterbusch T et al. . Seroprevalence of human polyomavirus 9 and cross-reactivity to African green monkey-derived lymphotropic polyomavirus. J Gen Virol. 2012;934:698–705. [DOI] [PubMed] [Google Scholar]
- 53.Nicol JT, Touzé A, Robinot R et al. . Seroprevalence and cross-reactivity of human polyomavirus 9. Emerg Infect Dis. 2012;188:1329–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nicol JT, Leblond V, Arnold F et al. . Seroprevalence of human Malawi polyomavirus. J Clin Microbiol. 2014;521:321–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berrios C, Jung J, Primi B et al. . Malawi polyomavirus is a prevalent human virus that interacts with known tumor suppressors. J Virol. 2015;891:857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu G, Greninger AL, Isa P et al. . Discovery of a novel polyomavirus in acute diarrheal samples from children. PLoS One. 2012;711:e49449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Šroller V, Hamšíková E, Ludvíková V et al. . Seroprevalence rates of BKV, JCV, and MCPyV polyomaviruses in the general Czech Republic population. J Med Virol. 2014;869:1560–1568. [DOI] [PubMed] [Google Scholar]
- 58.Tolstov YL, Pastrana DV, Feng H et al. . Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays. Int J Cancer. 2009;1256:1250–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knowles WA, Pipkin P, Andrews N et al. . Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol. 2003;711:115–123. [DOI] [PubMed] [Google Scholar]
- 60.Gardner SD. Prevalence in England of antibody to human polyomavirus (B.K.). Br Med J. 1973;15845:77–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robles C, Viscidi R, Malats N et al. . Bladder cancer and seroreactivity to BK, JC and Merkel cell polyomaviruses: the Spanish Bladder Cancer Study. Int J Cancer. 2013;1333:597–603. [DOI] [PubMed] [Google Scholar]
- 62.Gheit T, Muñoz JP, Levican J et al. . Merkel cell polyomavirus in non-small cell lung carcinomas from Chile. Exp Mol Pathol. 2012;931:162–166. [DOI] [PubMed] [Google Scholar]
- 63.Xu S, Jiang J, Yu X et al. . Association of Merkel cell polyomavirus infection with EGFR mutation status in Chinese non-small cell lung cancer patients. Lung Cancer. 2014;833:341–346. [DOI] [PubMed] [Google Scholar]
- 64.Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med. 2004;16420:2206–2216. [DOI] [PubMed] [Google Scholar]
- 65.Van der Meijden E, Wunderink HF, Van der Blij-de Brouwer CS et al. . Human polyomavirus 9 infection in kidney transplant patients. Emerg Infect Dis. 2014;206:991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hirsch HH, Knowles W, Dickenmann M et al. . Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;3477:488–496. [DOI] [PubMed] [Google Scholar]
- 67.Hirsch HH, Vincenti F, Friman S et al. . Polyomavirus BK replication in de novo kidney transplant patients receiving tacrolimus or cyclosporine: a prospective, randomized, multicenter study. Am J Transplant. 2013;131:136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gardner SD, MacKenzie EF, Smith C et al. . Prospective study of the human polyomaviruses BK and JC and cytomegalovirus in renal transplant recipients. J Clin Pathol. 1984;375:578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Antonsson A, Pawlita M, Feltkamp MC et al. . Longitudinal study of seroprevalence and serostability of the human polyomaviruses JCV and BKV in organ transplant recipients. J Med Virol. 2013;852:327–335. [DOI] [PubMed] [Google Scholar]
- 70.Huang G, Chen LZ, Qiu J et al. . Prospective study of polyomavirus BK replication and nephropathy in renal transplant recipients in China: a single-center analysis of incidence, reduction in immunosuppression and clinical course. Clin Transplant. 2010;245:599–609. [DOI] [PubMed] [Google Scholar]
- 71.Wiedinger K, Bitsaktsis C, Chang S. Reactivation of human polyomaviruses in immunocompromised states. J Neurovirol. 2014;201:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Costal C, Bergallo M, Ferrara P et al. . High-level polyomavirus JC viruria following long-term steroid therapy. New Microbiol. 2010;334:405–407. [PubMed] [Google Scholar]
- 73.Madeleine MM, Carter JJ, Johnson LG et al. . Risk of squamous cell skin cancer after organ transplant associated with antibodies to cutaneous papillomaviruses, polyomaviruses, and TMC6/8 (EVER1/2) variants. Cancer Med. 2014;35:1440–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Costa C, Saldan A, Cavallo R. Evaluation of virus-specific cellular immune response in transplant patients. World J Virol. 2012;16:150–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Costa C, Cavallo R. Polyomavirus-associated nephropathy. World J Transplant. 2012;26:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khanna N, Wolbers M, Mueller NJ et al. . JC virus-specific immune responses in human immunodeficiency virus type 1 patients with progressive multifocal leukoencephalopathy. J Virol. 2009;839:4404–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Waterboer T, Sehr P, Pawlita M. Suppression of non-specific binding in serological Luminex assays. J Immunol Methods. 2006;309(1-2):200–204. [DOI] [PubMed] [Google Scholar]
- 78.Pastrana DV, Wieland U, Silling S et al. . Positive correlation between Merkel cell polyomavirus viral load and capsid-specific antibody titer. Med Microbiol Immunol. 2012;2011:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lim ES, Reyes A, Antonio M et al. . Discovery of STL polyomavirus, a polyomavirus of ancestral recombinant origin that encodes a unique T antigen by alternative splicing. Virology. 2013;4362:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Korup S, Rietscher J, Calvignac-Spencer S et al. . Identification of a novel human polyomavirus in organs of the gastrointestinal tract. PLoS One. 2013;83:e58021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mishra N, Pereira M, Rhodes RH et al. . Identification of a novel polyomavirus in a pancreatic transplant recipient with retinal blindness and vasculitic myopathy. J Infect Dis. 2014;21010:1595–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Accomando WP, Wiencke JK, Houseman EA et al. . Quantitative reconstruction of leukocyte subsets using DNA methylation. Genome Biol. 2014;153:R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.