See Sarazin et al. (doi:10.1093/brain/aww041) for a scientific commentary on this article.
The PET tracer [18F]-AV-1451 allows visualization of tau pathology in living subjects. Ossenkoppele et al. employ the tracer in patients with distinct Alzheimer's disease variants to investigate correlates of tau deposition. Pathological aggregation of tau, but not amyloid-β, is linked to patterns of neurodegeneration and clinical manifestations of Alzheimer’s disease.
Keywords: Alzheimer’s disease, tau, AV1451 PET, cognition, APOE
See Sarazin et al. (doi:10.1093/brain/aww041) for a scientific commentary on this article.
The PET tracer [18F]-AV-1451 allows visualization of tau pathology in living subjects. Ossenkoppele et al. employ the tracer in patients with distinct Alzheimer's disease variants to investigate correlates of tau deposition. Pathological aggregation of tau, but not amyloid-β, is linked to patterns of neurodegeneration and clinical manifestations of Alzheimer’s disease.
Abstract
See Sarazin et al. (doi:10.1093/brain/aww041) for a scientific commentary on this article.
The advent of the positron emission tomography tracer 18F-AV1451 provides the unique opportunity to visualize the regional distribution of tau pathology in the living human brain. In this study, we tested the hypothesis that tau pathology is closely linked to symptomatology and patterns of glucose hypometabolism in Alzheimer’s disease, in contrast to the more diffuse distribution of amyloid-β pathology. We included 20 patients meeting criteria for probable Alzheimer’s disease dementia or mild cognitive impairment due to Alzheimer’s disease, presenting with a variety of clinical phenotypes, and 15 amyloid-β-negative cognitively normal individuals, who underwent 18F-AV1451 (tau), 11C-PiB (amyloid-β) and 18F-FDG (glucose metabolism) positron emission tomography, apolipoprotein E (APOE) genotyping and neuropsychological testing. Voxel-wise contrasts against controls (at P < 0.05 family-wise error corrected) showed that 18F-AV1451 and 18F-FDG patterns in patients with posterior cortical atrophy (‘visual variant of Alzheimer’s disease’, n = 7) specifically targeted the clinically affected posterior brain regions, while 11C-PiB bound diffusely throughout the neocortex. Patients with an amnestic-predominant presentation (n = 5) showed highest 18F-AV1451 retention in medial temporal and lateral temporoparietal regions. Patients with logopenic variant primary progressive aphasia (‘language variant of Alzheimer’s disease’, n = 5) demonstrated asymmetric left greater than right hemisphere 18F-AV1451 uptake in three of five patients. Across 30 FreeSurfer-defined regions of interest in 16 Alzheimer’s disease patients with all three positron emission tomography scans available, there was a strong negative association between 18F-AV1451 and 18F-FDG uptake (Pearson’s r = −0.49 ± 0.07, P < 0.001) and less pronounced positive associations between 11C-PiB and 18F-FDG (Pearson’s r = 0.16 ± 0.09, P < 0.001) and 18F-AV1451 and 11C-PiB (Pearson’s r = 0.18 ± 0.09, P < 0.001). Voxel-wise linear regressions thresholded at P < 0.05 (uncorrected) showed that, across all patients, younger age was associated with greater 18F-AV1451 uptake in wide regions of the neocortex, while older age was associated with increased 18F-AV1451 in the medial temporal lobe. APOE ϵ4 carriers showed greater temporal and parietal 18F-AV1451 uptake than non-carriers. Finally, worse performance on domain-specific neuropsychological tests was associated with greater 18F-AV1451 uptake in key regions implicated in memory (medial temporal lobes), visuospatial function (occipital, right temporoparietal cortex) and language (left > right temporoparietal cortex). In conclusion, tau imaging—contrary to amyloid-β imaging—shows a strong regional association with clinical and anatomical heterogeneity in Alzheimer’s disease. Although preliminary, these results are consistent with and expand upon findings from post-mortem, animal and cerebrospinal fluid studies, and suggest that the pathological aggregation of tau is closely linked to patterns of neurodegeneration and clinical manifestations of Alzheimer’s disease.
Introduction
The ability to detect fibrillar amyloid-β plaque depositions using 11C-Pittsburgh compound B (PiB; Klunk et al., 2004) or fluorinated amyloid PET tracers (Vandenberghe et al., 2010; Barthel et al., 2011; Clark et al., 2011) has had a major impact on the field of Alzheimer’s disease. Among many other applications, amyloid-β imaging has been incorporated into diagnostic criteria for various stages of the disease (Albert et al., 2011; McKhann et al., 2011; Sperling et al., 2011; Dubois et al., 2014), has substantial impact on clinical decision-making (Ossenkoppele et al., 2013a; Sanchez-Juan et al., 2014) and patient management plans (Schipke et al., 2012; Grundman et al., 2013), and has shown potential as a surrogate outcome measure in clinical trials tailored to reduce cerebral amyloid-β plaque burden (Salloway et al., 2014; Liu et al., 2015). A yet unresolved issue after a decade of amyloid-β imaging research, however, is the disconnection between the diffuse distribution of amyloid-β pathology throughout the neocortex (Rabinovici et al., 2010; Wolk et al., 2012; Lehmann et al., 2013; Jung et al., 2015) and the selective patterns of brain atrophy and glucose hypometabolism that strongly correlate with clinical symptoms (Rabinovici et al., 2010; Ridgway et al., 2012; Lehmann et al., 2013; Madhavan et al., 2013). Thus, although amyloid-β deposition may be a prerequisite to developing Alzheimer’s disease dementia, at some point during the disease course additional factors are likely involved in determining regional neurodegeneration and symptomatology.
Tau, a microtubule-associated protein that aggregates into intracellular neurofibrillary tangles in Alzheimer’s disease (Weingarten et al., 1975; Braak and Braak, 1991), has often been suggested as a facilitator of the downstream effects of amyloid-β (Desikan et al., 2012; Jack and Holtzman, 2013). Tau pathology has a devastating effect on synaptic function (Gomez-Isla et al., 1997; Beharry et al., 2014; Spires-Jones and Hyman, 2014) and relates more strongly to cognitive functions during life than does amyloid-β (Arriagada et al., 1992; Nelson et al., 2012; van Rossum et al., 2012; Rolstad et al., 2013). However, current knowledge about relationships between tau pathology and other disease measures is mainly derived from animal, neuropathological and CSF studies. The recent advent of a PET tracer (i.e. 18F-AV1451, formerly called 18F-T807), which shows high affinity and selectivity for paired helical filament tau pathology in vitro (Chien et al., 2013; Xia et al., 2013; Marquie et al., 2015), now allows in vivo assessment of regional tau load.
Mapping the distribution of tau pathology may further our understanding of disease mechanisms in distinct clinical variants of Alzheimer’s disease. These clinical variants include posterior cortical atrophy (PCA, ‘visual variant of Alzheimer’s disease’; Crutch et al., 2012), logopenic variant primary progressive aphasia (logopenic variant PPA, ‘language variant of Alzheimer’s disease’; Gorno-Tempini et al., 2011), corticobasal syndrome (Alzheimer’s disease pathology is the causative pathology in up to 25% of cases; Dickson et al., 2002; Lee et al., 2011), and memory-predominant and behavioural/dysexecutive variants of Alzheimer’s disease (Ossenkoppele et al., 2015c). Age at onset has also been associated with clinical and anatomical variability; while patients with late-onset Alzheimer’s disease (≥65 years of age) typically present with amnestic symptoms and medial temporal lobe neurodegeneration, patients with early-onset (<65 years) tend to exhibit greater non-amnestic deficits and neocortical-predominant atrophy (Rabinovici et al., 2010; Mendez et al., 2012; Ossenkoppele et al., 2012; Smits et al., 2012; Moller et al., 2013; Cavedo et al., 2014). Furthermore, patients with Alzheimer’s disease who carry the apolipoprotein E (APOE) ϵ4 allele have shown greater medial temporal lobe vulnerability (Pievani et al., 2009; van der Flier et al., 2011; Ossenkoppele et al., 2013b; Lehmann et al., 2014) and post-mortem tau pathology (Beffert and Poirier, 1996; Tiraboschi et al., 2004) compared to non-carriers.
In a recent case study of a patient with PCA, 18F-AV1451 was found to be more closely linked to hypometabolism and cognitive deficits than was amyloid-β (Ossenkoppele et al., 2015d). Here we extend these findings in a larger sample and across multiple phenotypes, studying associations between tau pathology and factors that underlie heterogeneity in the manifestation of Alzheimer’s disease. We hypothesized that: (i) 18F-AV1451 would show distinct patterns in clinical variants of Alzheimer’s disease, in early-onset versus late-onset Alzheimer’s disease and in APOE ϵ4 carriers versus non-carriers; and (ii) the amount and distribution of 18F-AV1451 retention would correlate with neuropsychological performance.
Materials and methods
Participants
Twenty patients were recruited from the University of California San Francisco (UCSF) Alzheimer’s Disease Research Center. This represents a consecutive series of recruited research participants who met clinical criteria for probable Alzheimer’s disease dementia (McKhann et al., 2011) or mild cognitive impairment (Albert et al., 2011) due to Alzheimer’s disease, who underwent 18F-AV1451 PET scans between June 2014 and June 2015. All patients underwent a medical history and physical examination, a structured caregiver interview, brain MRI and neuropsychological testing. Seven patients met specific diagnostic criteria for PCA (Mendez et al., 2002), five for logopenic variant PPA (Gorno-Tempini et al., 2011), and one for corticobasal syndrome (Armstrong, 2014). Based on chart reviews and evaluation of the neuropsychological reports, the remaining patients were classified as amnestic (n = 5) and behavioural/dysexecutive variant Alzheimer’s disease (n = 1; Ossenkoppele et al., 2015c). One patient (59-year-old male) presented with a history of focal cortical deficits (i.e. agnosia, aphasia, apraxia) with relative preservation of memory function. This patient did not meet formal criteria for any of the Alzheimer’s disease variants, and will be referred to as ‘non-amnestic Alzheimer’s disease’. Nineteen (95%) patients had a visually positive PiB-PET scan as assessed according to previously published procedures (Rabinovici et al., 2010). Amyloid-β status was unknown for one patient with a clinical diagnosis of logopenic variant PPA. Exclusion criteria were: (i) meeting core clinical criteria for another dementia; (ii) clinically significant cerebrovascular disease; (iii) major systemic disease; (iv) history of a neurological disorder causing dementia; or (v) recent history of substance abuse.
In addition, 15 cognitively normal controls were recruited through the Berkeley Aging Cohort. The eligibility criteria for controls include a minimum age of 60, preventing a more accurate age matching of controls to the relatively young patients in the present study. Further eligibility criteria included normal performance on cognitive tests, absence of neurological or psychiatric illness that potentially affects brain structure and function (including white matter abnormalities) and lack of major medical illnesses and medications that affect cognition. To enhance contrasts between Alzheimer’s disease and normal ageing, we excluded 12 additional control studies with 18F-AV1451 and 11C-PiB who met criteria for preclinical Alzheimer’s disease (Sperling et al., 2011) based on positive PiB-PET [distribution volume ratio (DVR) ≥ 1.08; Mormino et al., 2012]. Informed consent was obtained from all subjects or their assigned surrogate decision-makers, and UCSF, University of California Berkeley, and the Lawrence Berkeley National Laboratory (LBNL) institutional review boards for human research approved the study.
MRI
All patients and controls underwent MRI on a 3 T Siemens Tim Trio scanner or 1.5 T Magnetom Avanto scanner, respectively. Sequences included T1-weighted magnetization prepared rapid gradient echo (MP-RAGE) for structure and fluid attenuation inversion recovery (FLAIR) to assess cerebrovascular disease [(Villeneuve et al., 2014; Ossenkoppele et al., 2015a), see specifications in Supplementary material]. MP-RAGE sequences were processed using FreeSurfer 5.1 (Fischl et al., 2002) to define native-space reference regions and cortical regions of interest.
Positron emission tomography
PET scans were performed at LBNL on a Siemens Biograph 6 Truepoint PET/CT scanner in 3D acquisition mode, and on a ECAT EXACT HR scanner for a small subset of 11C-PiB (n = 3) and 18F-FDG (n = 3) PET scans in controls. A low-dose CT/transmission scan was performed for attenuation correction prior to all scans. 11C-PiB and 18F-FDG PET scans were performed as previously described (Lehmann et al., 2013). 18F-AV1451 was synthesized and radiolabelled at LBNL’s Biomedical Isotope Facility. Approximately 10 mCi of 18F-AV1451 was injected intravenously, and subjects participated in one of two acquisition schemes: 0–100-min post-injection full dynamic scans (4 × 15, 8 × 30, 9 × 60 s, and 2 × 3, 16 × 5 min frames) followed by 120–150 min (6 × 5 min frames: 13 controls, 15 patients), or 75–115 min (8 × 5 min frames: two control subjects, five patients). PET data were reconstructed using an ordered subset expectation maximization algorithm with weighted attenuation. Images were smoothed with a 4 mm Gaussian kernel with scatter correction and evaluated prior to analysis for patient motion and adequacy of statistical counts. All PET scans were acquired within an average of 68 ± 109 days (18F-AV1451 to 18F-FDG: 105 ± 122 days, 18F-AV1451 to 11C-PiB: 95 ± 121 days, 18F-FDG to 11C-PiB: 0 ± 2 days).
Image analyses
PET images were co-registered to the subjects’ MP-RAGE using Statistical Parametric Mapping (SPM) version 8 (Wellcome Trust Centre for Neuroimaging, Institute of Neurology at University College London). Dynamic 90 min 11C-PiB data were analysed using Logan graphical analysis with FreeSurfer-derived grey matter cerebellum as the reference region, yielding voxelwise DVR (Logan et al., 1996). One patient did not undergo 11C-PiB PET, and two patients had no 11C-PiB dynamic data available (but were positive on visual read of semiquantitative parametric images), yielding a total of 17/20 patients with 11C-PiB DVR data. 18F-FDG PET images were summed, and standardized uptake value ratios (SUVR) were calculated for the 30–60-min post-injection interval using mean activity in the pons (manually edited from FreeSurfer-derived brainstem) as the reference region (Minoshima et al., 1995). Sixteen patients had 18F-FDG PET data available. Consistent with the initial report on human 18F-AV1451/18F-T807 PET (Chien et al., 2013), SUVR images at t = 80–100 minutes post-injection were created by normalizing summed activity from the realigned frames to mean activity in cerebellar grey matter, an area relatively spared of neurofibrillary tangle pathology even in advanced Alzheimer’s disease (Braak and Braak, 1991; Marquie et al., 2015). SUVR80–100 showed the highest correlation across all time intervals with non-displaceable binding potentials derived from analyses with simplified reference tissue model 2 (SRTM2) in 23 controls and 15 patients with Alzheimer’s disease with dynamic data available (data not shown), and was therefore the interval of choice. We performed partial volume correction on all tracers using the Geometric Transfer Matrix approach (Rousset et al., 1998) and report both corrected and uncorrected data for region of interest analyses.
APOE status
Genotyping of the APOE allele (rs429358 and rs7412) was performed using a TaqMan® Allelic Discrimination Assay on an ABI 7900HT Fast Real-Time PCR system (Applied Biosystems) according to a previously described methodology (Coppola et al., 2012). APOE ϵ4 status was determined in 19/20 patients.
Neuropsychology
Within 1 year of the 18F-AV1451 PET scan (mean interval: 112 ± 83 days), 18/20 patients underwent a neuropsychological test battery that covered four major cognitive domains (Kramer et al., 2003; Lehmann et al., 2013; Ossenkoppele et al., 2015a): memory [immediate recall, delayed free recall, delayed cued recall and delayed recognition of the California Verbal Learning Test (9 items), and delayed recall of the Benson Figure], visuospatial or parietal function (copy of the Benson Figure, design fluency, calculations, face matching, and number location of the Visual Object and Space Perception battery), executive function [Modified Trail-making time and accuracy, Stroop interference (n correct and n errors), Digit Span backward, verbal fluency (D-words), and abstraction], and language [sentence repetition and comprehension, verbal agility, letter/word reading, picture vocabulary, category fluency (animals), and the Boston Naming Test]. Raw test scores were z-transformed using means and standard deviations (SD) of a group of 574 cognitively normal individuals [42.5% male, mean age: 66.1 ± 11.4, education: 17.2 ± 2.4, Mini-Mental State Examination (MMSE): 29.2 ± 1.1, all with a Clinical Dementia Rating (CDR) of 0 and a Geriatric Depression Scale (GDS) score ≤ 5] recruited at UCSF. Subsequently, these z-scores were averaged across all neuropsychological tests within a domain, resulting in composite scores for each cognitive domain.
Statistics
Baseline demographic and clinical features
Differences in baseline characteristics between groups were assessed using ANOVA with post hoc Bonferroni tests for continuous variables and χ2 and Kruskal-Wallis with post hoc Mann-Whitney U-tests for dichotomous or categorical data.
Voxelwise contrasts between patients and controls
For voxelwise statistical analyses in SPM8, parametric PET images were spatially normalized to Montreal Neurological Institute space, followed by smoothing using an 8 mm isotropic Gaussian kernel. Then, voxelwise contrasts were performed to examine differences in 18F-AV1451, 11C-PiB and 18F-FDG PET uptake in patients with PCA against healthy controls. Similar contrasts were performed between patients with logopenic variant PPA and amnestic Alzheimer’s disease against controls for 18F-AV1451 only, as 11C-PiB and 18F-FDG were not available in all patients. The contrasts were thresholded at P < 0.05 family-wise error corrected, without adjustment for nuisance variables.
Region of interest analyses for 18F-AV1451, 11C-PiB and 18F-FDG PET
Mean SUVR (18F-AV1451 and 18F-FDG) and DVR (11C-PiB) values were extracted for FreeSurfer-defined frontal (superior and middle gyri and orbitofrontal cortex), lateral temporal (superior, middle and inferior gyri), lateral parietal (inferior, superior and suprmarginal gyri), medial parietal (posterior cingulate and precuneus), occipital (calcarine, cuneus and lateral occipital cortex), medial temporal (hippocampus, entorhinal cortex and parahippocampus), and basal ganglia (putamen, caudate and pallidum). These regions were selected as we aimed to assess the full spectrum of Braak stages (Braak and Braak, 1991) as well as ‘off-target’ tracer uptake in basal ganglia structures (Marquie et al., 2015; Johnson et al., 2016). We also calculated a hemispheric asymmetry index [AI, similar to Rabinovici et al. (2008) and Frings et al. (2015)] for each region of interest using the formula AI [%] = 200 × (R − L)/(R + L) for 18F-AV1451 and 11C-PiB, or AI [%] = −200 × (R − L)/(R + L) for 18F-FDG. Thus, negative outcomes indicate more tau and amyloid-β, or more severe glucose hypometabolism in the left hemisphere, while positive values reflect right lateralized asymmetry. Differences in uptake values were assessed using ANOVA with post hoc Bonferroni tests.
Next, we performed Pearson correlations between the three PET tracers across 30 a priori selected left and right cortical [i.e. frontal, parietal, lateral temporal and occipital: based on Ossenkoppele et al. (2015d)] FreeSurfer regions of interest in native MRI space across 16 patients with Alzheimer’s disease with all tracers available. To control for interdependency resulting from multiple measurements (i.e. regions of interest) per subject, we additionally performed mixed effects models (i.e. a single model for each tracer comparison) assuming random intercepts and fixed slopes, using the maximum likelihood method for parameter estimation and likelihood ratio tests to assess significance.
Relationships between 18F-AV1451 with age, APOE and cognition
We conducted separate whole-brain voxelwise linear regressions between 18F-AV1451 uptake and age, APOE ϵ4 allele status (carrier or non-carrier), MMSE, and composite scores on four different cognitive domains (memory, visuospatial, executive and language functions) across all patients with Alzheimer’s disease. These analyses were thresholded at P < 0.05 uncorrected for multiple comparisons. In the model exploring effects of APOE ϵ4 allele status on voxelwise 18F-AV1451 uptake, analyses were performed with and without adjusting for global amyloid-β burden (measured as mean 11C-PiB retention in prefrontal, lateral temporal, parietal, and cingulate cortices; Ossenkoppele et al., 2014). Other analyses were performed without adjustment for nuisance variables. Finally, we performed region of interest analyses to test associations between regional 18F-AV1451 uptake patterns and age, memory, visuospatial performance and language ability. Rank correlations (Spearman’s rho or Kendall’s τ in case of ties) were calculated between age and the ratio of hippocampal to cortical 18F-AV1451, memory score and hippocampal 18F-AV1451, visuospatial score and occipital 18F-AV1451, and language and left temporoparietal 18F-AV1451. We examined the regional specificity of these correlations by relating composite cognitive scores to 18F-AV1451 uptake in control regions of interest: memory score and cortical (i.e. weighted average of frontal, temporal, parietal and occipital cortex) 18F-AV1451, visuospatial score and cortical 18F-AV1451, and language score and right temporoparietal/whole cortex 18F-AV1451. A schematic overview and visual representations of the FreeSurfer-defined regions of interest are provided in Supplementary Table 1 and Supplementary Fig. 1, respectively.
Results
Patients
Demographic and clinical characteristics are presented in Table 1. The Alzheimer’s disease patients were on average relatively young (64 ± 8 years on average across phenotypes) and in a mild stage of the disease (mean MMSE: 21 ± 6). As expected, patients with PCA showed more severe impairment on visuospatial tasks than patients with logopenic variant PPA or an amnestic-predominant presentation (P < 0.05), while patients with logopenic variant PPA performed worse on language tests than the other two groups (P < 0.05).
Table 1.
Demographic and clinical characteristics according to diagnostic group
| Posterior cortical atrophy | Logopenic variant PPA | Amnestic Alzheimer’s disease | Non-amnestic Alzheimer’s disease | Behavioural/dysexecutive variant Alzheimer’s disease | Corticobasal syndrome | Controls | |
|---|---|---|---|---|---|---|---|
| n | 7 | 5 | 5 | 1 | 1 | 1 | 15 |
| Age | 63 ± 10 | 65 ± 10 | 67 ± 8 | 59 | 59 | 60 | 79 ± 7a |
| Sex (male/female) | 4/3 | 1/4 | 3/2 | 1/0 | 1/0 | 0/1 | 6/9 |
| Education (years) | 15 ± 1 | 18 ± 2 | 17 ± 3 | 24 | 16 | 16 | 17 ± 1 |
| Handedness (R/L/A) | 6/1/0 | 3/2/0 | 4/0/0 (1 NA) | 1/0/0 | 0/0/1 | 1/0/0 | 14/0/1 |
| PiB status (+/−) | 7/0 | 4/0 (1 NA) | 5/0 | 1/0 | 1/0 | 1/0 | 0/15 |
| PiB global DVR | 1.86 ± 0.13 | 1.84 ± 0.38 | 1.79 ± 0.43 | 1.86 | 1.94 | 1.64 | 1.05 ± 0.03 |
| APOE ϵ4 (+/−) | 4/3 | 2/5 | 3/5 | 0/1 | 0/1 | − | 2/12 |
| CDR | 0.9 ± 0.2 | 0.6 ± 0.2 | 0.7 ± 0.3 | 0.5 | 1 | − | − |
| MMSE | 23 ± 4 | 18 ± 8 | 20 ± 5 | 27 | 21 | 16 | 29 ± 1a |
| Memory z-score | −2.5 ± 1.3 | −2.6 ± 1.5 | −3.4 ± 1.0 | −1.5 | −2.5 | − | − |
| Visuospatial z-score | −5.9 ± 2.9b | −1.9 ± 1.9 | −2.0 ± 1.4 | −0.8 | −1.4 | − | − |
| Executive z-score | −2.9 ± 2.0 | −3.7 ± 2.5 | −1.9 ± 1.9 | −0.8 | −2.4 | − | − |
| Language z-score | −2.1 ± 0.9 | −4.0 ± 1.6c | −1.7 ± 1.4 | −0.3 | −0.3 | − | − |
Differences in baseline characteristics between groups (n > 1) were assessed using ANOVA with post hoc Bonferroni tests for continuous variables and χ2 and Kruskal-Wallis with post hoc Mann-Whitney U-tests for dichotomous or categorical data.
aControls > patients, P < 0.05.
bPosterior cortical atrophy < Logopenic variant primary progressive aphasia and amnestic Alzheimer’s disease, P < 0.05.
cLogopenic variant primary progressive aphasia < posterior cortical atrophy and amnestic Alzheimer’s disease, P < 0.05.
A = ambidextrous; CDR = Clinical Dementia Rating; R = right; L = left; NA = not available.
Control subjects
The healthy controls were substantially older (79 ± 7 years) than the Alzheimer’s disease patients and 14% carried an APOE ϵ4 allele. In line with a recent study (Johnson et al., 2016), visual inspection and regional quantification of 18F-AV1451 images (Table 2 and Supplementary Fig. 2) showed some degree of uptake in medial temporal lobes (i.e. entorhinal cortex, parahippocampal gyrus and hippocampus) in all normal subjects. According to the staging proposed by Braak and Braak (1991), this pattern extended into fusiform gyrus and inferior temporal cortex in several subjects. In addition, most controls showed tracer uptake in striatum, substantia nigra and choroid plexus thought to represent ‘off-target’ tracer binding unrelated to tau (Marquie et al., 2015).
Table 2.
18F-AV1451, 18F-FDG and 11C-PiB retention values and asymmetry indices in regions of interest
| PCA |
Logopenic variant PPA |
Amnestic Alzheimer’s disease |
Controls |
|||||
|---|---|---|---|---|---|---|---|---|
| SUVR | AI (%) | SUVR | AI (%) | SUVR | AI (%) | SUVR | AI (%) | |
| 18F-AV1451 | ||||||||
| Frontal | 1.57 ± 0.33 | 8.8 ± 14.9 | 1.76 ± 0.59 | −11.8 ± 18.5 | 1.55 ± 0.39 | −2.5 ± 14.0 | 1.07 ± 0.06d | 0.4 ± 1.4 |
| Lateral temporal | 2.09 ± 0.47 | 8.8 ± 10.6 | 2.43 ± 0.47 | −4.2 ± 10.0 | 2.05 ± 0.28 | −8.4 ± 17.1 | 1.11 ± 0.05d | −0.1 ± 2.1 |
| Lateral parietal | 2.42 ± 0.50 | 3.6 ± 12.3 | 2.28 ± 0.74 | −13.5 ± 14.5 | 2.03 ± 0.47 | −5.8 ± 20.0 | 1.08 ± 0.08d | −0.4 ± 2.8 |
| Medial parietal | 2.47 ± 0.55a | 9.7 ± 13.3 | 2.11 ± 0.48 | −12.4 ± 15.5 | 1.93 ± 0.45 | −4.8 ± 18.6 | 1.12 ± 0.09d | 1.2 ± 2.1 |
| Occipital | 2.16 ± 0.43b | 11.3 ± 8.7 | 1.65 ± 0.17 | −7.5 ± 10.8 | 1.42 ± 0.31 | 1.3 ± 17.9 | 1.06 ± 0.06d | 1.3 ± 2.0 |
| Medial temporal | 1.43 ± 0.20 | 1.9 ± 5.5 | 1.41 ± 0.25 | −1.9 ± 7.1 | 1.70 ± 0.33c | −4.9 ± 7.3 | 1.18 ± 0.06d | −0.5 ± 4.6 |
| Basal ganglia | 1.33 ± 0.14 | 3.0 ± 4.2 | 1.21 ± 0.23 | −3.3 ± 7.3 | 1.44 ± 0.11 | −3.4 ± 7.9 | 1.47 ± 0.15e | −1.3 ± 2.6 |
| n = 7 | n = 5 | n = 5 | n = 15 | |||||
| 18F-FDG | ||||||||
| Frontal | 1.43 ± 0.06 | 3.0 ± 6.6 | 1.51 ± 0.03 | −10.8 ± 6.2 | 1.40 ± 0.18 | −6.6 ± 9.1 | 1.48 ± 0.08 | −1.1 ± 2.2 |
| Lateral temporal | 1.13 ± 0.09f | 11.3 ± 14.6 | 1.14 ± 0.20 | −12.3 ± 0.8 | 1.18 ± 0.04 | −9.0 ± 14.1 | 1.34 ± 0.12 | 0.3 ± 2.6h |
| Lateral parietal | 1.07 ± 0.08f | 16.3 ± 17.3 | 1.25 ± 0.15 | −14.9 ± 1.5 | 1.32 ± 0.08 | −6.4 ± 12.9 | 1.48 ± 0.14 | 0.2 ± 2.3h |
| Medial parietal | 1.34 ± 0.06f | 15.2 ± 13.4 | 1.35 ± 0.36 | −18.6 ± 6.1 | 1.51 ± 0.14 | −5.9 ± 10.0 | 1.65 ± 0.11 | −3.0 ± 2.2i |
| Occipital | 1.28 ± 0.23g | 11.7 ± 17.4 | 1.72 ± 0.24 | −6.5 ± 3.2 | 1.64 ± 0.10 | −1.6 ± 6.2 | 1.54 ± 0.11 | −2.0 ± 2.1j |
| Medial temporal | 1.02 ± 0.09 | 5.8 ± 8.6 | 1.10 ± 0.03 | −6.1 ± 4.3 | 1.03 ± 0.03 | −7.5 ± 7.9 | 1.10 ± 0.06 | −0.6 ± 2.9j |
| Basal ganglia | 1.43 ± 0.04 | 1.7 ± 8.2 | 1.60 ± 0.17 | −5.3 ± 2.5 | 1.46 ± 0.10 | −5.0 ± 4.6 | 1.51 ± 0.13 | 0.1 ± 2.9 |
| n = 7 | n = 2 | n = 4 | n = 13 | |||||
| DVR | AI (%) | DVR | AI (%) | DVR | AI (%) | DVR | AI (%) | |
| 11C-PiB | ||||||||
| Frontal | 1.84 ± 0.16 | 1.6 ± 4.7 | 1.91 ± 0.50 | −3.1 ± 6.8 | 1.61 ± 0.29 | 4.9 ± 5.6 | 1.00 ± 0.04k | 0.4 ± 1.3 |
| Lateral temporal | 1.65 ± 0.16 | −1.9 ± 6.1 | 1.73 ± 0.39 | −0.1 ± 2.5 | 1.51 ± 0.26 | 3.8 ± 3.4 | 0.99 ± 0.02k | −1.0 ± 3.0 |
| Lateral parietal | 1.73 ± 0.12 | −3.1 ± 5.2 | 1.85 ± 0.26 | 1.9 ± 3.8 | 1.61 ± 0.31 | 2.12 ± 4.5 | 1.04 ± 0.03k | −0.9 ± 2.4 |
| Medial parietal | 1.97 ± 0.15 | −1.4 ± 2.6 | 2.04 ± 0.25 | 0.6 ± 9.1 | 1.82 ± 0.33 | −0.1 ± 0.6 | 1.10 ± 0.04k | 1.7 ± 3.7 |
| Occipital | 1.50 ± 0.14 | 2.8 ± 5.6l | 1.58 ± 0.23 | −5.2 ± 2.2 | 1.31 ± 0.17 | 3.4 ± 3.5 | 1.04 ± 0.03k | 1.0 ± 3.4 |
| Medial temporal | 1.14 ± 0.08 | −1.9 ± 6.2 | 1.21 ± 0.12 | 2.8 ± 5.0 | 1.10 ± 0.08 | 7.5 ± 3.1 | 0.98 ± 0.05k | 0.8 ± 3.0 |
| Basal ganglia | 1.68 ± 0.16 | 1.3 ± 4.3 | 1.70 ± 0.31 | −0.7 ± 2.7 | 1.60 ± 0.18 | 1.6 ± 4.5 | 1.23 ± 0.06k | −0.8 ± 1.8 |
| n = 7 | n = 3 | n = 4 | n = 15 | |||||
Data are presented as group means ± SD; see Fig. 3 for individual data. AI for 18F-AV1451 and 11C-PiB were calculated using AI [%] = 200 × (R − L) / (R + L), and AI [%] = -200 × (R − L) / (R + L) for 18F-FDG. Negative values indicate greater AV1451 and PiB, and reduced FDG uptake in the left compared to the right hemisphere. Differences between groups for SUVR were assessed using ANOVA with post hoc Bonferroni tests at P < 0.05: aPCA > amnestic Alzheimer’s disease; bPCA > logopenic variant PPA and amnestic Alzheimer’s disease; cAmnestic Alzheimer’s disease > PCA and logopenic variant PPA; dControls < PCA, logopenic variant PPA and amnestic Alzheimer’s disease; eControls > logopenic variant PPA; fPCA < amnestic Alzheimer’s disease; gPCA < logopenic variant PPA and amnestic Alzheimer’s disease; hControls ≠ PCA, logopenic variant PPA and amnestic Alzheimer’s disease; iControls ≠ PCA, logopenic variant PPA; jControls ≠ PCA; kControls < PCA, logopenic variant PPA and amnestic Alzheimer’s disease; lPCA ≠ logopenic variant PPA.
18F-AV1451 images of distinct Alzheimer’s disease variants
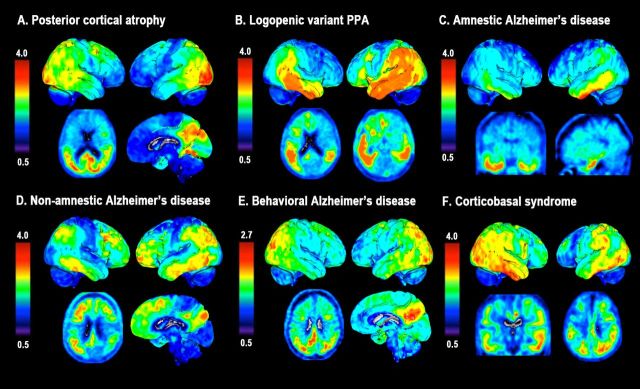
Figure 1 shows a selection of individuals with distinct phenotypes of Alzheimer’s disease illustrating the neuroanatomical correspondence between 18F-AV1451 uptake and the clinical presentation. 18F-AV1451 uptake was most prominent in clinically affected posterior regions in PCA (Fig. 1A), the language-dominant left hemisphere in logopenic variant PPA (Fig. 1B) and the basal temporal cortex and medial temporal lobe structures in amnestic-predominant Alzheimer’s disease (Fig. 1C). A patient presenting with dysexecutive, visuospatial and language complaints with preserved memory function showed 18F-AV1451 uptake in diffuse parts of the neocortical association cortex with relative sparing of the medial temporal lobes (Fig. 1D). A patient with the behavioural/dysexecutive variant of Alzheimer’s disease presenting with impaired organizational skills and judgement, inappropriate behaviour, diminished personal hygiene, hyperorality and newly acquired preference for sweet food, showed 18F-AV1451 uptake in classical temporoparietal regions and—in line with Ossenkoppele et al. (2015c)—relative sparing of the frontal cortex (Fig. 1E). Finally, a 11C-PiB-positive patient with corticobasal syndrome (mainly affecting the left hemibody) who initially presented with cognitive impairment (i.e. memory and executive dysfunction) showed an asymmetric 18F-AV1451 pattern with greater uptake in the right hemisphere and unique involvement of primary sensorimotor cortex (Fig. 1F), which was spared in other patients.
Figure 1.
18F-AV1451 uptake in individuals with distinct phenotypes of Alzheimer’s disease. SUVR 18F-AV1451 images (neurological orientation) in (A) a 59-year-old female (MMSE: 28) with posterior cortical atrophy [note that this a different patient than presented in Ossenkoppele et al. (2015d)]; (B) a 77-year-old female (MMSE: 17) with logopenic variant PPA; (C) a 71-year-old female (MMSE: 23) with amnestic Alzheimer’s disease; (D) a 59-year-old female (MMSE: 27) with non-amnestic Alzheimer’s disease; (E) a 59-year-old male (MMSE: 21) with a behavioural presentation of Alzheimer’s disease, and (F) a 60-year-old female (MMSE: 16) with a corticobasal syndrome affecting the left hemibody.
Voxelwise contrasts between patients and control subjects
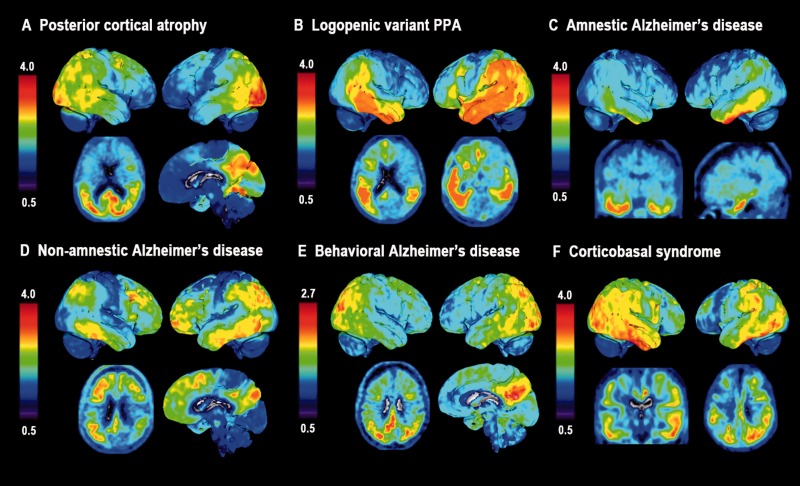
Next, we performed voxelwise contrasts for 18F-AV1451, 11C-PiB and 18F-FDG between patients with posterior cortical atrophy and controls. In line with our hypothesis and a previous case study (Ossenkoppele et al., 2015d), patients with PCA showed substantial inverse spatial overlap between greater 18F-AV1451 and reduced 18F-FDG uptake, specifically affecting brain regions implicated in higher-order visual processing, particularly in the right hemisphere (Fig. 2A). This is further illustrated by overlaying the binarized 18F-AV1451 and 18F-FDG significance maps (Fig. 2D). Additionally, 18F-AV1451 uptake was elevated in several brain regions where 18F-FDG uptake did not differ from controls, including primary visual cortex and posterior cortex in the left hemisphere. Contrary to the regional specificity of 18F-AV1451 and 18F-FDG, 11C-PiB retention was distributed throughout the neocortex and implicated both clinically affected and relatively spared regions (e.g. extensive binding in frontal cortex; Fig. 2A).
Figure 2.
Tracer uptake patterns in patients with different clinical Alzheimer’s disease variants. (A) Voxelwise contrasts, thresholded at P < 0.05 after family-wise error correction and without covariates, indicating regions in which patients with PCA had greater 18F-AV1451 (green), reduced 18F-FDG (red), and elevated 11C-PiB (blue) compared to healthy controls. (B and C) Contrasts for 18F-AV1451 uptake between amnestic Alzheimer’s disease (B) and logopenic variant PPA (C) patients against healthy controls at P < 0.05 after family-wise error correction and without covariates. In panel D, we binarized the 18F-AV1451 and 18F-FDG (P < 0.05 family-wise error corrected) contrasts between patients with PCA and controls, and show in cyan regions where both 18F-AV1451 and 18F-FDG differed from controls, and in blue brain regions where only the 18F-AV1451 contrast was significant. In E, individual 18F-AV1451 images of five logopenic variant PPA patients are displayed in order of their MMSE scores.
In line with the clinical presentation, the 18F-AV1451 pattern in patients with an amnestic-predominant presentation most prominently affected the medial temporal lobes and lateral temporoparietal cortex (Fig. 2B). When assessing the parametric images and AIs of the five logopenic variant PPA cases individually, three patients showed the predicted left > right asymmetry, one was fairly symmetric, and one showed right > left 18F-AV1451 uptake (Table 2 and Fig. 2E). Notably, the two patients with most advanced disease severity showed the greatest 18F-AV1451 uptake in the right hemisphere and in anterior brain regions. On group-level voxelwise analyses, the 18F-AV1451 pattern in patients with logopenic variant PPA was rather symmetric, with uptake mainly observed in bilateral temporoparietal regions that are typically involved in language function (Fig. 2C). Voxelwise 11C-PiB and 18F-FDG contrasts between amnestic Alzheimer’s disease and logopenic variant PPA patients against controls are shown in Supplementary Fig. 3. In general, the results are similar to those seen in patients with PCA (i.e. widespread neocortical 11C-PiB binding, while 18F-FDG uptake is more region-specific), but should be interpreted with caution given the smaller sample size due to missing scans.
Region of interest analyses between 18F-AV1451, 11C-PiB and 18F-FDG PET
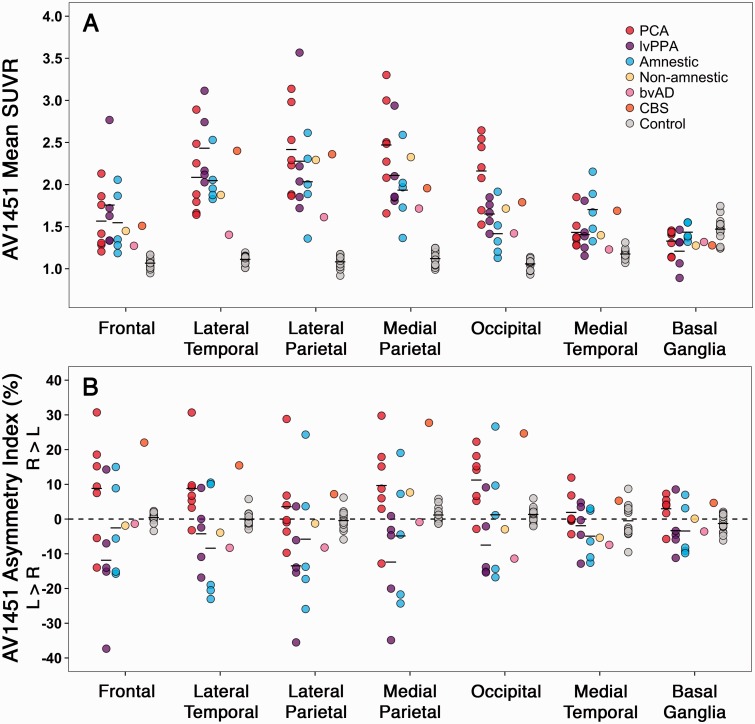
Mean uptake values and AIs of 18F-AV1451, 11C-PiB and 18F-FDG in eight regions of interest for each Alzheimer’s disease phenotype and the controls are shown in Table 2, Fig. 3, Supplementary Table 2 and Supplementary Fig. 4 (partial volume corrected data).
Figure 3.
18F-AV1451 uptake and hemispheric asymmetry in regions of interest. (A) 18F-AV1451 SUVR values for each Alzheimer’s disease patient and controls in seven bilateral regions of interest. (B) The degree of asymmetric tracer uptake within each region of interest as the percentage difference in SUVR value for left compared to right hemisphere: asymmetry index [%] = 200 × (R − L)/(R + L). Table 2 and Supplementary Table 2 show the group means and differences of 18F-AV1451 standardized uptake value ratios and asymmetry indices.
Bonferroni-corrected ANOVAs showed that patients with PCA had greater 18F-AV1451 retention in occipital and medial parietal (i.e. posterior cingulate and precuneus) regions of interest and reduced 18F-FDG uptake in occipital and lateral parietal regions compared to patients with amnestic and logopenic variant PPA (Table 2 and Fig. 3). Furthermore, patients with amnestic Alzheimer’s disease had increased 18F-AV1451 uptake in medial temporal lobe structures compared to the other Alzheimer’s disease groups. No differences between the Alzheimer’s disease phenotypes were found for 11C-PiB. 18F-AV1451 and 18F-FDG AIs showed R > L asymmetry for PCA, L > R asymmetry for logopenic variant PPA and subtle L > R asymmetry for amnestic Alzheimer’s disease patients across regions of interest (Table 2). No consistent patterns were observed for 11C-PiB. Partial volume correction of the PET data resulted in substantially greater tracer retention values but did not alter any of the differences described above.
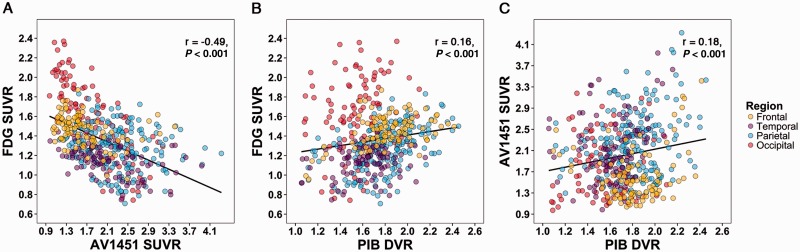
Pearson correlation across the different Alzheimer’s disease phenotypes (n = 16 with all tracers available) in 30 FreeSurfer-defined regions of interest showed a strong association between increased 18F-AV1451 and reduced 18F-FDG uptake (Fig. 4A, Pearson’s r = −0.49 ± 0.07, P < 0.001). In addition, there were modest positive associations between 11C-PiB DVR and 18F-FDG SUVR (Fig. 4B, Pearson’s r = 0.16 ± 0.09, P < 0.001) and between 18F-AV1451 SUVR and 11C-PiB DVR (Fig. 4C, Pearson’s r = 0.18 ± 0.09, P < 0.001). These results were supported by mixed effects models testing relationships between each of the three PET tracers, holding region of interest constant as a fixed effect and subject as a random effect. Mixed effects models showed a significant negative relationship between 18F-AV1451 and 18F-FDG [β = −0.23 ± 0.01, χ2(1) = 201.4, P < 0.001], and supported positive associations with smaller effect sizes between 11C-PiB and 18F-FDG [β = 0.29 ± 0.06, χ2(1) = 23.5, P < 0.001] and between 11C-PiB and 18F-AV1451 [β = 0.78 ± 0.15, χ2(1) = 26.5, P < 0.001]. The relationships between PET tracers in distinct cortical regions (i.e. frontal, parietal, lateral temporal and occipital) are reported in Supplementary Table 3.
Figure 4.
Direct region of interest comparisons between PET tracers. Across 16 Alzheimer’s disease patients, regions with greater 18F-AV1451 uptake were strongly associated with lower 18F-FDG metabolism (A). Modest, positive associations were also observed between 11C-PiB and 18F-FDG (B) and between 18F-AV1451 and 11C-PiB (C).
Repeating the Pearson correlation analyses using partial volume corrected data, we found a similarly strong association between increased 18F-AV1451 and reduced 18F-FDG uptake (Supplementary Fig. 5A, Pearson’s r = −0.41 ± 0.07, P < 0.001). In contrast to uncorrected data, the modest positive association between 11C-PiB DVR and 18F-FDG SUVR was no longer significant (Supplementary Fig. 5B, Pearson’s r = −0.01 ± 0.09, P = 0.88) and the association between 18F-AV1451 SUVR and 11C-PiB DVR was somewhat stronger after applying partial volume correction (Supplementary Fig. 5C, Pearson’s r = 0.37 ± 0.08, P < 0.001). Mixed effects models of partial volume corrected data showed a significant negative relationship between 18F-AV1451 and 18F-FDG [β = −0.11 ± 0.01, χ2(1) = 70.0, P < 0.001], no relationship between 11C-PiB and 18F-FDG [β = 0.02 ± 0.04, χ2(1) = 0.2, P = 0.642], and a significant positive relationship between 11C-PiB and 18F-AV1451 [β = 1.52 ± 0.11, χ2(1) = 163.8, P < 0.001]. The relationships between partial volume corrected PET tracers in distinct cortical regions (i.e. frontal, parietal, lateral temporal and occipital) are reported in Supplementary Table 3.
Associations of 18F-AV1451 with age and APOE ϵ4 status
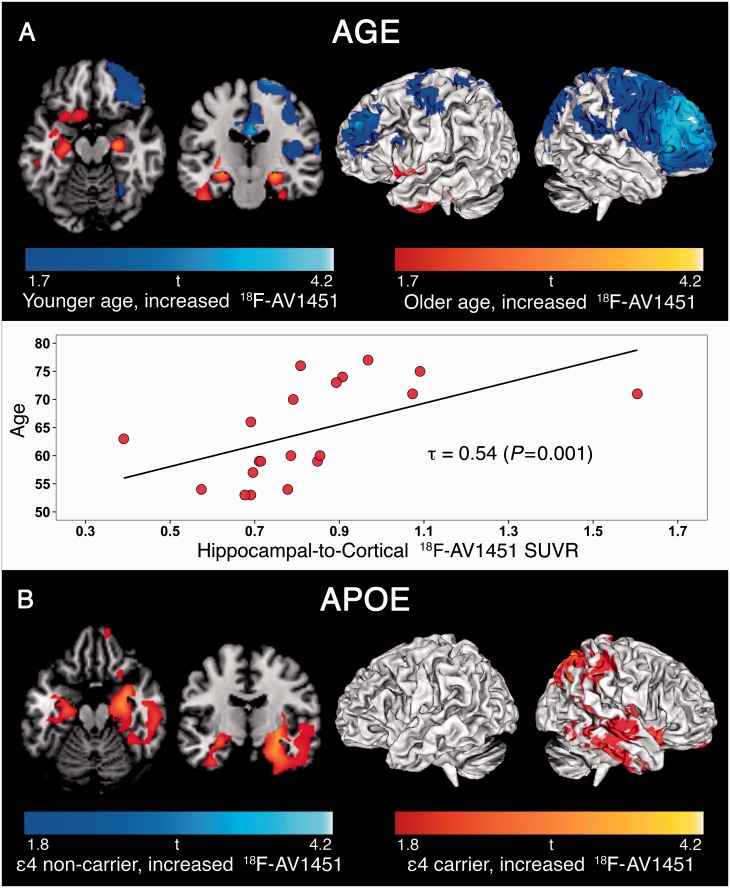
Whole-brain voxelwise linear regressions with 18F-AV1451 SUVR and age as continuous variables, thresholded at P < 0.05 uncorrected without covariates, showed that younger age was associated with greater 18F-AV1451 uptake in wide regions of the neocortex (Fig. 5A). Several frontal and medial parietal regions survived at P < 0.01 uncorrected (Supplementary Fig. 6A). The reverse contrast revealed that older age was associated with increased 18F-AV1451 uptake specifically in the medial temporal lobes (left > right). Consistent with our voxelwise results, region of interest analysis measuring the ratio of hippocampal-to-cortical 18F-AV1451 uptake showed a positive correlation with age [Fig. 5A, τ = 0.54, P = 0.001; Supplementary Fig. 7A (partial volume corrected), τ = 0.41, P = 0.012].
Figure 5.
Patterns of 18F-AV1451 retention in Alzheimer’s disease associated with age and APOE ϵ4 status. Result from voxelwise linear regression are displayed at P < 0.05 uncorrected for multiple comparisons, without covariates for age (A), and adjusted for global amyloid-β burden for APOE ϵ4 status (B).
Voxelwise analysis between APOE ϵ4 status (dichotomous: carriers, non-carriers) and 18F-AV1451 SUVR (continuous), controlling for global amyloid-β burden and thresholded at P < 0.05 uncorrected, showed that APOE ϵ4 carriers had greater 18F-AV1451 uptake in bilateral medial temporal and right temporoparietal cortex (Fig. 5B). Supplementary Fig. 6B shows that the right medial temporal lobe survived a slightly more stringent threshold of P < 0.01 uncorrected. There were no significant clusters with elevated 18F-AV1451 in APOE ϵ4 non-carriers. The results remained essentially the same when we did not adjust for global amyloid-β burden, and APOE ϵ4 carriers and non-carriers did not differ in age (63.6 ± 9.6 versus 65.2 ± 8.0 years, P = 0.35).
Associations of 18F-AV1451 with neuropsychological performance
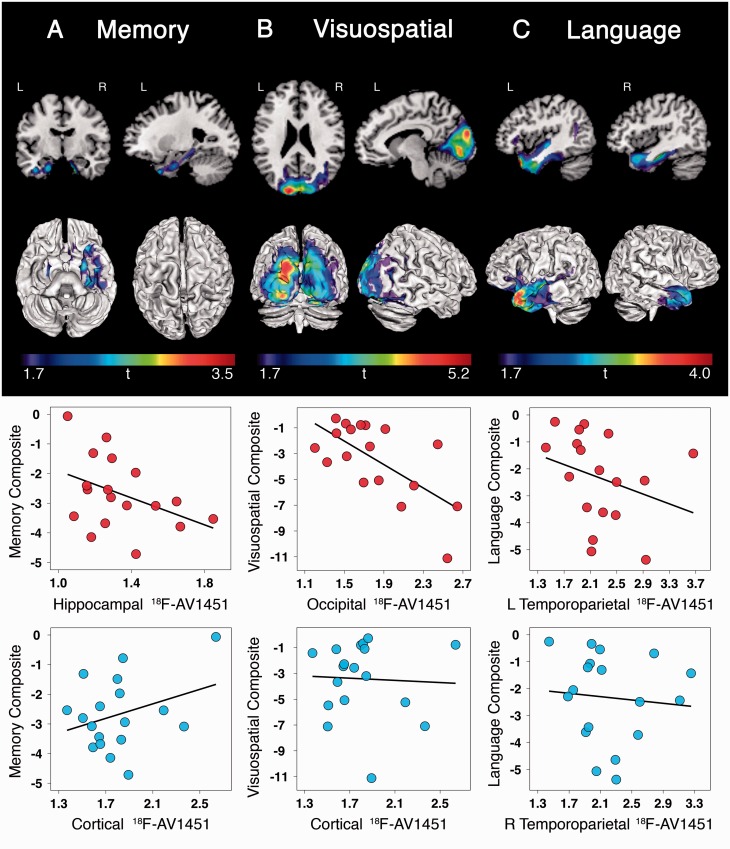
We performed separate voxelwise linear regressions of MMSE and cognitive domain composite scores on 18F-AV1451 SUVR at a statistical threshold of P < 0.05 uncorrected for multiple comparisons and without covariates. Worse MMSE scores were associated with higher 18F-AV1451 uptake in bilateral orbitofrontal cortex (left > right) and left anterior temporal cortex (data not shown). Region of interest analysis showed no relationship between MMSE and whole cortex (i.e. weighted average of frontal, temporal, parietal and occipital cortex) 18F-AV1451 (τ = 0.10, P = 0.568, partial volume corrected: τ = −0.03, P = 0.849). Patients with lower memory composite scores had higher tracer retention in hippocampus and neighbouring medial temporal regions, with some involvement of inferior temporal cortex (Fig. 6A). Region of interest analyses showed a trend toward worse memory performance with increased 18F-AV1451 uptake in hippocampus (Spearman’s rho = −0.40, P = 0.106; partial volume corrected Spearman’s rho = −0.45, P = 0.063), while no relationship was observed between memory and whole cortical 18F-AV1451 (Fig. 6A, Spearman’s rho = 0.05, P = 0.844; partial volume corrected Spearman’s rho = −0.19, P = 0.456). Worse visuospatial performance correlated with increased 18F-AV1451 in bilateral occipital lobe extending into right temporoparietal regions (Fig. 6B). This was confirmed in region of interest analyses showing an association between worse visuospatial performance and elevated 18F-AV1451 in occipital cortex (Spearman’s rho = −0.52, P = 0.028; partial volume corrected Spearman’s rho = −0.51, P = 0.032), but not in whole cortex (Fig. 6B, Spearman’s rho = 0.11, P = 0.674; partial volume corrected Spearman’s rho = −0.06, P = 0.818). We did not observe a relationship between worse executive function and greater 18F-AV1451 uptake in any region. Finally, lower language composite scores were associated with elevated 18F-AV1451 uptake in left temporoparietal regions typically implicated in language function, along with smaller clusters in right anterior temporal and inferior frontal cortices (Fig. 6C). In region of interest analyses, worse language performance was correlated with increased 18F-AV1451 uptake in left temporoparietal cortex (Spearman’s rho = −0.53, P = 0.026; partial volume corrected Spearman’s rho = −0.62, P = 0.007) but not in right temporoparietal cortex (Spearman’s rho = −0.23, P = 0.366; partial volume corrected Spearman’s rho = −0.37, P = 0.126) or whole cortex (Spearman’s rho = −0.16, P = 0.53; partial volume corrected Spearman’s rho = −0.33, P = 0.185). Supplementary Fig. 6 C–E shows the regions that survived voxelwise linear regression at a more conservative threshold of P < 0.01 uncorrected, and Supplementary Fig. 7B–D shows the partial volume corrected region of interest analyses.
Figure 6.
Associations between regional 18F-AV1451 and cognitive performance. Voxelwise contrasts (top; P < 0.05 uncorrected) show associations between increased 18F-AV1451 retention and worse performance on memory (A), visuospatial (B), and language testing (C). Region of interest analyses (bottom) demonstrate that cognitive impairment was associated with focal rather than global increases in 18F-AV1451 in regions underlying specific cognitive domains.
Discussion
In this study, we used the novel PET tracer 18F-AV1451 in distinct phenotypes of Alzheimer’s disease to examine associations between tau pathology and neuroanatomical and clinical heterogeneity in vivo. We found that tau pathology, as measured by 18F-AV1451 PET, preferentially occupied brain areas that are critical for cognitive functions uniquely affected in distinct variants of Alzheimer’s disease, and strongly co-localized with hypometabolic regions. In contrast, amyloid-β pathology, as measured by 11C-PiB PET, affected both clinically affected and unaffected regions and showed weak positive (without partial volume correction) or absent (with partial volume correction) associations with regional glucose metabolism. Older age was associated with greater tau pathology in medial temporal lobe structures, while younger patients had greater 18F-AV1451 uptake in the neocortical association cortex. APOE ϵ4 carriers had greater 18F-AV1451 uptake than non-carriers. Finally, increased 18F-AV1451 uptake was associated with worse performance on various cognitive domains in regionally specific patterns, in line with established brain-behaviour relationships. Taken together, these results are consistent with findings from post-mortem, animal and CSF studies, and provide preliminary evidence that the aggregation of tau is closely linked to patterns of neurodegeneration and the clinical manifestations of Alzheimer’s disease.
Distinct variants of Alzheimer’s disease
In previous studies of typical and atypical clinical presentations of Alzheimer’s disease, there has been a dissociation between the rather diffuse distribution of amyloid-β plaques and the highly specific patterns of neurodegeneration that strongly correlate with symptomatology (Rabinovici et al., 2010; Wolk et al., 2012; Lehmann et al., 2013; Jung et al., 2015). It has often been suggested that tau pathology, rather than amyloid-β, may be driving disease manifestation (Desikan et al., 2012; Jack and Holtzman, 2013). Tau imaging with 18F-AV1451 in conjunction with amyloid-β imaging now enables detailed characterization of the two hallmark Alzheimer’s disease pathologies in vivo. Visual inspection of individual images (Fig. 1) and formal group-level voxelwise analyses (Fig. 2) revealed that tau selectively targeted brain regions that are clinically affected, while amyloid-β was distributed throughout the association cortex (Table 2). For example, 18F-AV1451 was predominantly retained in occipital, parietal and occipitotemporal cortices in patients with PCA, clinically characterized by deficits in primary and higher-order visual processing. Furthermore, patients with an amnestic-predominant presentation showed prominent medial temporal lobe involvement, the patient with a behavioural/dysexecutive presentation had a classical temporoparietal pattern of 18F-AV1451 uptake with relative sparing of the frontal cortex, in line with the atrophy pattern shown in patients with this phenotype (Ossenkoppele et al., 2015c), and the patient with corticobasal syndrome uniquely showed involvement of the peri-rolandic cortex and had greater 18F-AV1451 contralateral to the affected body side. Given the positive 11C-PiB scan, the patient with corticobasal syndrome most likely has Alzheimer’s disease, rather than corticobasal degeneration (a 4-repeat tauopathy), as the causal aetiology. Age-related comorbid amyloid-β pathology is unusual at the patient’s age (16–18% across cognitively normal persons aged 60; Jack et al., 2014; Jansen et al., 2015) and Alzheimer’s disease pathology may be a more frequent cause of corticobasal syndrome in young patients (Ossenkoppele et al., 2015b). Furthermore, glucose hypometabolism was more prominent in temporoparietal than in frontal cortex, which has been associated with underlying Alzheimer’s disease pathology in patients with clinical corticobasal syndrome (Sha et al., 2015). Finally, 18F-AV1451 uptake in this patient fell within the Alzheimer’s disease range, which is well above the levels we have observed in other patients with suspected corticobasal degeneration (data not shown) and is in line with a recent post-mortem binding study showing that 18F-AV1451 binds to paired helical filaments of tau found in Alzheimer’s disease, but not to straight tau filaments found in corticobasal degeneration and other 4-repeat tauopathies (Marquie et al., 2015).
Based on previous neuroimaging studies (Rabinovici et al., 2008; Madhavan et al., 2013; Teichmann et al., 2013; Rogalski et al., 2014; Ossenkoppele et al., 2015a) and the specific language presentation, we hypothesized that patients with logopenic variant PPA would show 18F-AV1451 uptake in temporoparietal language regions in a clearly asymmetric fashion with greater tau in the left hemisphere compared to right hemisphere. Visual inspection of individual images and a quantitative laterality index indicated that three logopenic variant PPA showed the predicted left > right pattern, one was fairly symmetric and one patient had greater 18F-AV1451 uptake in right than in left hemisphere. This is in line with a neuropathological study of primary progressive aphasia due to underlying Alzheimer’s disease (Gefen et al., 2012) showing that, while most patients showed higher neurofibrillary pathology in the left hemisphere, a significant minority (14–29% depending on the brain region) had greater tangle pathology in the right hemisphere. At group-level voxelwise analyses, however, the 18F-AV1451 uptake pattern appeared to be fairly symmetrical, largely covering bilateral temporoparietal regions. This finding might be due to the small and heterogeneous sample comprising a wide range of demographic and clinical features. Notably, the two patients with lowest MMSE scores had greater involvement of the right hemisphere and frontal 18F-AV1451 uptake, potentially indicative of tau pathology selectively targeting the left hemisphere in early disease stages and subsequently spreading anteriorly in the left hemisphere and into inter-connected contralateral brain regions with advancing disease (Ridgway et al., 2012; Rohrer et al., 2013; Rogalski et al., 2014; Ossenkoppele et al., 2015a).
Age and APOE ϵ4
Consistent with previous studies showing selective vulnerability of the medial temporal lobes in late-onset Alzheimer’s disease and greater cortical neurodegeneration in early-onset Alzheimer’s disease (Rabinovici et al., 2010; Mendez et al., 2012; Smits et al., 2012; Moller et al., 2013; Cavedo et al., 2014), we found that older age was associated with greater 18F-AV1451 uptake in bilateral medial temporal structures, whereas younger age was associated with elevated 18F-AV1451 in occipital, parietal and frontal regions. These different uptake patterns as a function of age highly correspond to the greater overall and neocortical-predominant neurofibrillary tangle burden observed in patients with early-onset Alzheimer’s disease at autopsy (Nochlin et al., 1993; Bigio et al., 2002; Marshall et al., 2007). Moreover, we found that APOE ϵ4 carriers had greater 18F-AV1451 uptake in medial and lateral temporal and parietal lobes, even after accounting for global amyloid-β burden. This is consistent with some (Beffert and Poirier, 1996; Tiraboschi et al., 2004) but not all (Schmechel et al., 1993; Greenberg et al., 1995) post-mortem studies, and in line with a study showing that APOE ϵ4 directly facilitates phosphorylation of tau resulting in increased filamentous tau load (Harris et al., 2003). Taken together, the present study indicates that factors often linked with heterogeneity in the clinical manifestation of Alzheimer’s disease (i.e. age-at-onset and APOE ϵ4 status) are associated with distinct patterns of tau pathology. Our results are also consistent with the neuropathological observation of ‘hippocampal-sparing’ Alzheimer’s disease, where greater tangle burden in cortical versus medial temporal regions is observed in a subset of patients, and is correlated with early age-of-onset, non-amnestic clinical presentations, and lower prevalence of the APOE ϵ4 allele (Murray et al., 2011).
Neuropsychological performance
Expanding upon autopsy and CSF studies describing global associations between tau pathology and cognition (Arriagada et al., 1992; Nelson et al., 2012; van Rossum et al., 2012), we found regionally specific relationships between greater 18F-AV1451 and reduced memory, visuospatial and language functions. In addition, lower MMSE scores were associated with greater 18F-AV1451 uptake in orbitofrontal and anterior temporal cortices. While these may not be considered core Alzheimer’s disease regions, they could underscore an anterior spread of tau with advancing disease that coincides with cognitive decline. We did not observe any regional associations between 18F-AV1451 and poor executive function scores, which could be due to difficulty isolating executive function deficits from other cognitive deficits (i.e. visuospatial, language and memory) that may have affected performance on executive function tasks. Similarly, the bilateral 18F-AV1451 pattern associated with worse language performance could be associated with transneuronal spread of tau (Clavaguera et al., 2009; de Calignon et al., 2012; Raj et al., 2012; Zhou et al., 2012) from left hemisphere to right, or with a selective vulnerability of language regions (Rogalski et al., 2008; Miller et al., 2013) to the toxic effects of pathological tau.
Partial volume correction
To assess for confounding effects of atrophy on PET quantification we performed partial volume correction. This yielded substantially higher 18F-AV1451 uptake values in patients, while uptake values remained low by comparison in controls. Additionally, regional asymmetry of 18F-AV1451 uptake was increased after partial volume correction in PCA (right > left) and logopenic variant PPA (left > right) patients but remained equally symmetric in controls. In comparisons between PET tracers in patients, we observed a strong, negative relationship between 18F-AV1451 and 18F-FDG that survived partial volume correction. Furthermore, the association between 18F-AV1451 and 11C-PiB was strengthened after partial volume correction. In contrast, the mildly positive relationship that we initially observed between 11C-PiB and 18F-FDG disappeared after partial volume correction, suggesting this association was largely driven by partial volume effects. Correlations between 18F-AV1451 uptake patterns and neuropsychological performance in patients were similar with and without partial volume correction.
Strengths and limitations
A main strength of the present study is the inclusion of a variety of Alzheimer’s disease phenotypes, which allowed us to study relationships between tau pathology and neuroanatomical and clinical heterogeneity. There are also some limitations. First, this is a preliminary study with a relatively small sample size. Future studies including more patients and a better balance across Alzheimer’s disease variants are needed. Second, due to the small sample size, some voxelwise analyses (mainly the associations between tau pathology with age, APOE ϵ4 status and cognition) were conducted at fairly liberal thresholds and should be interpreted with caution, although the relative lack of noise (i.e. focal regional correlations opposed to scattered small clusters) and the convergence of region of interest analyses are encouraging. Third, some patients were missing 11C-PiB and 18F-FDG data (Table 2 and Supplementary Fig. 3), so we could only perform a fair comparison across tracers in patients with PCA (Fig. 2). Fourth, we did not assess relationships between molecular markers and brain atrophy because T1-weighted MRI was acquired at different scanners for patients and controls. Finally, as the healthy controls were significantly older than our relatively young Alzheimer’s disease patients, we did not adjust our voxelwise contrasts (Fig. 2) to avoid collinearity between age and 18F-AV1451 uptake.
Future directions
Tau imaging will help address fundamental questions about the mechanisms that drive clinical, anatomical and functional diversity in Alzheimer’s disease. Key topics for future research include the further study of cross-sectional and longitudinal relationships between tau, amyloid-β and neurodegeneration, and how each of these factors contributes to clinical progression. Understanding which protein is the major force driving neurodegeneration and cognitive decline is highly relevant for developing and testing effective therapies, and the advent of novel tau PET tracers (Villemagne et al., 2015) will for the first time allow in-depth testing of the entire amyloid cascade hypothesis (Hardy, 2009) in vivo.
Supplementary Material
Acknowledgements
We would like to thank Kristin Norton, Jamie Faria, Vyoma Shah, and Allison Fero for their contributions in the acquisition and analysis of PET data, and Giovanni Coppola and Anna Karydas for APOE genotyping.
Glossary
Abbreviations
- AI =
asymmetry index
- DVR =
distribution volume ratio
- MMSE =
Mini-Mental State Examination
- PCA =
posterior cortical atrophy
- PiB =
Pittsburgh compound B
- PPA =
primary progressive aphasia
- SUVR =
standardized uptake value ratio
Funding
This research was funded by Marie Curie FP7 International Outgoing Fellowship (628812; to R.O.); The donors of (Alzheimer’s Disease Research), a program of BrightFocus Foundation (to R.O.); Tau Consortium (to G.D.R. and W.J.J); National Institute on Aging grants (R01-AG045611; to G.D.R.), (R01-AG034570; to W.J.J.), (P50-AG023501; to B.L.M.), and (K23-AG038357; to K.A.V.); John Douglas French Alzheimer’s Foundation (to G.D.R. and B.L.M.); State of California Department of Health Services Alzheimer’s Disease Research Centre of California grant (04-33516; to B.L.M); Swedish Medical Association and the Swedish Foundation of Nuclear Medicine (to M.S.). Avid Radiopharmaceuticals enabled use of the 18F-AV1451 tracer, but did not provide direct funding and was not involved in data analysis or interpretation.
Supplementary material
Supplementary material is available at Brain online.
References
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011; 7: 270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong MJ. Diagnosis and treatment of corticobasal degeneration. Curr Treat Options Neurol 2014; 16: 282. [DOI] [PubMed] [Google Scholar]
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology 1992; 42: 631–9. [DOI] [PubMed] [Google Scholar]
- Barthel H, Gertz HJ, Dresel S, Peters O, Bartenstein P, Buerger K, et al. Cerebral amyloid-beta PET with florbetaben (18F) in patients with Alzheimer's disease and healthy controls: a multicentre phase 2 diagnostic study. Lancet Neurol 2011; 10: 424–35. [DOI] [PubMed] [Google Scholar]
- Beffert U, Poirier J. Apolipoprotein E, plaques, tangles and cholinergic dysfunction in Alzheimer's disease. Ann N Y Acad Sci 1996; 777: 166–74. [DOI] [PubMed] [Google Scholar]
- Beharry C, Cohen LS, Di J, Ibrahim K, Briffa-Mirabella S, Alonso Adel C. Tau-induced neurodegeneration: mechanisms and targets. Neurosci Bull 2014; 30: 346–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigio EH, Hynan LS, Sontag E, Satumtira S, White CL. Synapse loss is greater in presenile than senile onset Alzheimer disease: implications for the cognitive reserve hypothesis. Neuropathol Appl Neurobiol 2002; 28: 218–27. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991; 82: 239–59. [DOI] [PubMed] [Google Scholar]
- Cavedo E, Pievani M, Boccardi M, Galluzzi S, Bocchetta M, Bonetti M, et al. Medial temporal atrophy in early and late-onset Alzheimer's disease. Neurobiol Aging 2014; 35: 2004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien DT, Bahri S, Szardenings AK, Walsh JC, Mu F, Su MY, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J Alzheimer Dis 2013; 34: 457–68. [DOI] [PubMed] [Google Scholar]
- Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA, et al. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA 2011; 305: 275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol 2009; 11: 909–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola G, Chinnathambi S, Lee JJ, Dombroski BA, Baker MC, Soto-Ortolaza AI, et al. Evidence for a role of the rare p.A152T variant in MAPT in increasing the risk for FTD-spectrum and Alzheimer's diseases. Hum Mol Genet 2012; 21: 3500–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutch SJ, Lehmann M, Schott JM, Rabinovici GD, Rossor MN, Fox NC. Posterior cortical atrophy. Lancet Neurol 2012; 11: 170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Calignon A, Polydoro M, Suarez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, et al. Propagation of tau pathology in a model of early Alzheimer's disease. Neuron 2012; 73: 685–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, McEvoy LK, Thompson WK, Holland D, Brewer JB, Aisen PS, et al. Amyloid-beta–associated clinical decline occurs only in the presence of elevated P-tau. Arch Neurol 2012; 69: 709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Bergeron C, Chin SS, Duyckaerts C, Horoupian D, Ikeda K, et al. Office of rare diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol 2002; 61: 935–46. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol 2014; 13: 614–29. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33: 341–55. [DOI] [PubMed] [Google Scholar]
- Frings L, Hellwig S, Spehl TS, Bormann T, Buchert R, Vach W, et al. Asymmetries of amyloid-beta burden and neuronal dysfunction are positively correlated in Alzheimer's disease. Brain 2015; 138: 3089–99. [DOI] [PubMed] [Google Scholar]
- Gefen T, Gasho K, Rademaker A, Lalehzari M, Weintraub S, Rogalski E, et al. Clinically concordant variations of Alzheimer pathology in aphasic versus amnestic dementia. Brain 2012; 135: 1554–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, et al. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer's disease. Ann Neurol 1997; 41: 17–24. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology 2011; 76: 1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg SM, Rebeck GW, Vonsattel JP, Gomez-Isla T, Hyman BT. Apolipoprotein E epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann Neurol 1995; 38: 254–9. [DOI] [PubMed] [Google Scholar]
- Grundman M, Pontecorvo MJ, Salloway SP, Doraiswamy PM, Fleisher AS, Sadowsky CH, et al. Potential impact of amyloid imaging on diagnosis and intended management in patients with progressive cognitive decline. Alzheimer Dis Assoc Disord 2013; 27: 4–15. [DOI] [PubMed] [Google Scholar]
- Hardy J. The amyloid hypothesis for Alzheimer's disease: a critical reappraisal. J Neurochem 2009; 110: 1129–34. [DOI] [PubMed] [Google Scholar]
- Harris FM, Brecht WJ, Xu Q, Tesseur I, Kekonius L, Wyss-Coray T, et al. Carboxyl-terminal-truncated apolipoprotein E4 causes Alzheimer's disease-like neurodegeneration and behavioral deficits in transgenic mice. Proc Nat Acad Sci USA 2003; 100: 10966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Holtzman DM. Biomarker modeling of Alzheimer's disease. Neuron 2013; 80: 1347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Wiste HJ, Weigand SD, Rocca WA, Knopman DS, Mielke MM, et al. Age-specific population frequencies of cerebral beta-amyloidosis and neurodegeneration among people with normal cognitive function aged 50-89 years: a cross-sectional study. Lancet Neurol 2014; 13: 997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FR, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA 2015; 313: 1924–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, et al. Tau PET imaging in aging and early Alzheimer's disease. Ann Neurol 2016; 79: 110–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Whitwell JL, Duffy JR, Strand EA, Machulda MM, Senjem ML, et al. Regional beta-amyloid burden does not correlate with cognitive or language deficits in Alzheimer's disease presenting as aphasia. Eur J Neurol 2016; 23: 313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol 2004; 55: 306–19. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol 2003; 16: 211–18. [DOI] [PubMed] [Google Scholar]
- Lee SE, Rabinovici GD, Mayo MC, Wilson SM, Seeley WW, DeArmond SJ, et al. Clinicopathological correlations in corticobasal degeneration. Ann Neurol 2011; 70: 327–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Ghosh PM, Madison C, Karydas A, Coppola G, O'Neil JP, et al. Greater medial temporal hypometabolism and lower cortical amyloid burden in ApoE4-positive AD patients. J Neurol Neurosurg Psychiatry 2014; 85: 266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Ghosh PM, Madison C, Laforce R, Jr, Corbetta-Rastelli C, Weiner MW, et al. Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer's disease. Brain 2013; 136: 844–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu E, Schmidt ME, Margolin R, Sperling R, Koeppe R, Mason NS, et al. Amyloid-beta 11C-PiB-PET imaging results from 2 randomized bapineuzumab phase 3 AD trials. Neurology 2015; 85: 692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 1996; 16: 834–40. [DOI] [PubMed] [Google Scholar]
- Madhavan A, Whitwell JL, Weigand SD, Duffy JR, Strand EA, Machulda MM, et al. FDG PET and MRI in logopenic primary progressive aphasia versus dementia of the Alzheimer's type. PloS One 2013; 8: e62471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall GA, Fairbanks LA, Tekin S, Vinters HV, Cummings JL. Early-onset Alzheimer's disease is associated with greater pathologic burden. J Geriatr Psychiatry Neurol 2007; 20: 29–33. [DOI] [PubMed] [Google Scholar]
- Marquie M, Normandin MD, Vanderburg CR, Costantino I, Bien EA, Rycyna LG, et al. Validating novel tau PET tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol 2015; 78: 787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers 2011; 7: 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MF, Ghajarania M, Perryman KM. Posterior cortical atrophy: clinical characteristics and differences compared to Alzheimer's disease. Dement Geriatr Cogn Disord 2002; 14: 33–40. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Lee AS, Joshi A, Shapira JS. Nonamnestic presentations of early-onset Alzheimer's disease. Am J Alzheimer Dis Other Dement 2012; 27: 413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ZA, Mandelli ML, Rankin KP, Henry ML, Babiak MC, Frazier DT, et al. Handedness and language learning disability differentially distribute in progressive aphasia variants. Brain 2013; 136: 3461–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoshima S, Frey KA, Foster NL, Kuhl DE. Preserved pontine glucose metabolism in Alzheimer disease: a reference region for functional brain image (PET) analysis. J Compu Assist Tomography 1995; 19: 541–7. [DOI] [PubMed] [Google Scholar]
- Moller C, Vrenken H, Jiskoot L, Versteeg A, Barkhof F, Scheltens P, et al. Different patterns of gray matter atrophy in early- and late-onset Alzheimer's disease. Neurobiol Aging 2013; 34: 2014–22. [DOI] [PubMed] [Google Scholar]
- Mormino EC, Brandel MG, Madison CM, Rabinovici GD, Marks S, Baker SL, et al. Not quite PIB-positive, not quite PIB-negative: slight PIB elevations in elderly normal control subjects are biologically relevant. NeuroImage 2012; 59: 1152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer's disease with distinct clinical characteristics: a retrospective study. Lancet Neurol 2011; 10: 785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol 2012; 71: 362–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nochlin D, van Belle G, Bird TD, Sumi SM. Comparison of the severity of neuropathologic changes in familial and sporadic Alzheimer's disease. Alzheimer Dis Assoc Disord 1993; 7: 212–22. [PubMed] [Google Scholar]
- Ossenkoppele R, Cohn-Sheehy BI, La Joie R, Vogel JW, Moller C, Lehmann M, et al. Atrophy patterns in early clinical stages across distinct phenotypes of Alzheimer's disease. Hum Brain Mapp 2015a; 36: 4421–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R, Jansen WJ, Rabinovici GD, Knol DL, van der Flier WM, van Berckel BN, et al. Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA 2015b; 313: 1939–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R, Madison C, Oh H, Wirth M, van Berckel BN, Jagust WJ. Is verbal episodic memory in elderly with amyloid deposits preserved through altered neuronal function? Cerebral Cortex 2014; 24: 2210–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R, Pijnenburg YA, Perry DC, Cohn-Sheehy BI, Scheltens NM, Vogel JW, et al. The behavioural/dysexecutive variant of Alzheimer's disease: clinical, neuroimaging and pathological features. Brain 2015c; 138: 2732–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R, Prins ND, Pijnenburg YA, Lemstra AW, van der Flier WM, Adriaanse SF, et al. Impact of molecular imaging on the diagnostic process in a memory clinic. Alzheimers Dement 2013a; 9: 414–21. [DOI] [PubMed] [Google Scholar]
- Ossenkoppele R, Schonhaut DR, Baker SL, O'Neil JP, Janabi M, Ghosh PM, et al. Tau, amyloid, and hypometabolism in a patient with posterior cortical atrophy. Ann Neurol 2015d; 77: 338–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R, van der Flier WM, Zwan MD, Adriaanse SF, Boellaard R, Windhorst AD, et al. Differential effect of APOE genotype on amyloid load and glucose metabolism in AD dementia. Neurology 2013b; 80: 359–65. [DOI] [PubMed] [Google Scholar]
- Ossenkoppele R, Zwan MD, Tolboom N, van Assema DM, Adriaanse SF, Kloet RW, et al. Amyloid burden and metabolic function in early-onset Alzheimer's disease: parietal lobe involvement. Brain 2012; 135: 2115–25. [DOI] [PubMed] [Google Scholar]
- Pievani M, Rasser PE, Galluzzi S, Benussi L, Ghidoni R, Sabattoli F, et al. Mapping the effect of APOE epsilon4 on gray matter loss in Alzheimer's disease in vivo. NeuroImage 2009; 45: 1090–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovici GD, Furst AJ, Alkalay A, Racine CA, O'Neil JP, Janabi M, et al. Increased metabolic vulnerability in early-onset Alzheimer's disease is not related to amyloid burden. Brain 2010; 133: 512–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC, et al. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol 2008; 64: 388–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A, Kuceyeski A, Weiner M. A network diffusion model of disease progression in dementia. Neuron 2012; 73: 1204–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway GR, Lehmann M, Barnes J, Rohrer JD, Warren JD, Crutch SJ, et al. Early-onset Alzheimer disease clinical variants: multivariate analyses of cortical thickness. Neurology 2012; 79: 80–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Martersteck A, Rademaker A, Wieneke C, Weintraub S, et al. Asymmetry of cortical decline in subtypes of primary progressive aphasia. Neurology 2014; 83: 1184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Johnson N, Weintraub S, Mesulam M. Increased frequency of learning disability in patients with primary progressive aphasia and their first-degree relatives. Arch Neurol 2008; 65: 244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Caso F, Mahoney C, Henry M, Rosen HJ, Rabinovici G, et al. Patterns of longitudinal brain atrophy in the logopenic variant of primary progressive aphasia. Brain Lang 2013; 127: 121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolstad S, Berg AI, Bjerke M, Johansson B, Zetterberg H, Wallin A. Cerebrospinal fluid biomarkers mirror rate of cognitive decline. J Alzheimers Dis 2013; 34: 949–56. [DOI] [PubMed] [Google Scholar]
- Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: principle and validation. J Nucl Med 1998; 39: 904–11. [PubMed] [Google Scholar]
- Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. New Engl J Med 2014; 370: 322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Juan P, Ghosh PM, Hagen J, Gesierich B, Henry M, Grinberg LT, et al. Practical utility of amyloid and FDG-PET in an academic dementia center. Neurology 2014; 82: 230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipke CG, Peters O, Heuser I, Grimmer T, Sabbagh MN, Sabri O, et al. Impact of beta-amyloid-specific florbetaben PET imaging on confidence in early diagnosis of Alzheimer's disease. Dement Geriatr Cogn Disord 2012; 33: 416–22. [DOI] [PubMed] [Google Scholar]
- Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, et al. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Nat Acad Sci USA 1993; 90: 9649–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha SJ, Ghosh PM, Lee SE, Corbetta-Rastelli C, Jagust WJ, Kornak J, et al. Predicting amyloid status in corticobasal syndrome using modified clinical criteria, magnetic resonance imaging and fluorodeoxyglucose positron emission tomography. Alzheimers Res Ther 2015; 7: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits LL, Pijnenburg YA, Koedam EL, van der Vlies AE, Reuling IE, Koene T, et al. Early onset Alzheimer's disease is associated with a distinct neuropsychological profile. J Alzheimers Dis 2012; 30: 101–8. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011; 7: 280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires-Jones TL, Hyman BT. The intersection of amyloid beta and tau at synapses in Alzheimer's disease. Neuron 2014; 82: 756–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichmann M, Kas A, Boutet C, Ferrieux S, Nogues M, Samri D, et al. Deciphering logopenic primary progressive aphasia: a clinical, imaging and biomarker investigation. Brain 2013; 136: 3474–88. [DOI] [PubMed] [Google Scholar]
- Tiraboschi P, Hansen LA, Masliah E, Alford M, Thal LJ, Corey-Bloom J. Impact of APOE genotype on neuropathologic and neurochemical markers of Alzheimer disease. Neurology 2004; 62: 1977–83. [DOI] [PubMed] [Google Scholar]
- van der Flier WM, Pijnenburg YA, Fox NC, Scheltens P. Early-onset versus late-onset Alzheimer's disease: the case of the missing APOE epsilon4 allele. Lancet Neurol 2011; 10: 280–8. [DOI] [PubMed] [Google Scholar]
- van Rossum IA, Visser PJ, Knol DL, van der Flier WM, Teunissen CE, Barkhof F, et al. Injury markers but not amyloid markers are associated with rapid progression from mild cognitive impairment to dementia in Alzheimer's disease. J Alzheimers Dis 2012; 29: 319–27. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Van Laere K, Ivanoiu A, Salmon E, Bastin C, Triau E, et al. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann Neurol 2010; 68: 319–29. [DOI] [PubMed] [Google Scholar]
- Villemagne VL, Fodero-Tavoletti MT, Masters CL, Rowe CC. Tau imaging: early progress and future directions. Lancet Neurol 2015; 14: 114–24. [DOI] [PubMed] [Google Scholar]
- Villeneuve S, Reed BR, Madison CM, Wirth M, Marchant NL, Kriger S, et al. Vascular risk and Abeta interact to reduce cortical thickness in AD vulnerable brain regions. Neurology 2014; 83: 40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Nat Acad Sci USA 1975; 72: 1858–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Price JC, Madeira C, Saxton JA, Snitz BE, Lopez OL, et al. Amyloid imaging in dementias with atypical presentation. Alzheimers Dement 2012; 8: 389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia CF, Arteaga J, Chen G, Gangadharmath U, Gomez LF, Kasi D, et al. [(18)F]T807, a novel tau positron emission tomography imaging agent for Alzheimer's disease. Alzheimers Dement 2013; 9: 666–76. [DOI] [PubMed] [Google Scholar]
- Zhou J, Gennatas ED, Kramer JH, Miller BL, Seeley WW. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron 2012; 73: 1216–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.