CNS involvement is common in systemic lupus erythematosus (SLE). Using diffusion-weighted imaging, Ercan et al. explore the link between cell-specific microstructural changes and neuropsychiatric symptoms. Increased diffusivity of predominantly glial metabolites is observed in SLE patients with previous CNS symptoms, and may be a marker for inflammation-related glial reactivity.
Keywords: neuropsychiatry, gliosis, biomarkers, inflammation, astrocytes
CNS involvement is common in systemic lupus erythematosus (SLE). Using diffusion-weighted imaging, Ercan et al. explore the link between cell-specific microstructural changes and neuropsychiatric symptoms. Increased diffusivity of predominantly glial metabolites is observed in SLE patients with previous CNS symptoms, and may be a marker for inflammation-related glial reactivity.
Abstract
Systemic lupus erythematosus is an inflammatory autoimmune disease with multi-organ involvement. Central nervous system involvement in systemic lupus erythematosus is common and results in several neurological and psychiatric symptoms that are poorly linked to standard magnetic resonance imaging outcome. Magnetic resonance imaging methods sensitive to tissue microstructural changes, such as diffusion tensor imaging and magnetization transfer imaging, show some correlation with neuropsychiatric systemic lupus erythematosus (NPSLE) symptoms. Histological examination of NPSLE brains reveals presence of cerebral oedema, loss of neurons and myelinated axons, microglial proliferation and reactive astrocytosis, microinfacrts and diffuse ischaemic changes, all of which can affect both diffusion tensor imaging and magnetization transfer imaging in a non-specific manner. Here we investigated the underlying cell-type specific microstructural alterations in the brain of patients with systemic lupus erythematosus with and without a history of central nervous system involvement. We did so combining diffusion tensor imaging with diffusion-weighted magnetic resonance spectroscopy, a powerful tool capable of characterizing cell-specific cytomorphological changes based on diffusion of intracellular metabolites. We used a 7 T magnetic resonance imaging scanner to acquire T1-weighted images, diffusion tensor imaging datasets, and single volume diffusion-weighted magnetic resonance spectroscopy data from the anterior body of the corpus callosum of 13 patients with systemic lupus erythematosus with past NPSLE, 16 patients with systemic lupus erythematosus without past NPSLE, and 19 healthy control subjects. Group comparisons were made between patients with systemic lupus erythematosus with/without past NPSLE and healthy controls on diffusion tensor imaging metrics and on diffusion coefficients of three brain metabolites: the exclusively neuronal/axonal N-acetylaspartate, and the predominantly glial creatine + phosphocreatine and choline compounds. In patients with systemic lupus erythematosus with past NPSLE, significantly higher diffusion tensor imaging mean and radial diffusivities were accompanied by a significantly higher intracellular diffusion of total creatine (0.202 ± 0.032 μm2/ms, P = 0.018) and total choline (0.142 ± 0.031 μm2/ms, P = 0.044) compared to healthy controls (0.171 ± 0.024 μm2/ms, 0.124 ± 0.018 μm2/ms, respectively). Total N-acetylaspartate, total creatine and total choline diffusion values from all patients with systemic lupus erythematosus correlated positively with systemic lupus erythematosus disease activity index score (P = 0.033, P = 0.040, P = 0.008, respectively). Our results indicate that intracellular alterations, and in particular changes in glia, as evidenced by increase in the average diffusivities of total choline and total creatine, correlate with systemic lupus erythematosus activity. The higher diffusivity of total creatine and total choline in patients with NPSLE, as well as the positive correlation of these diffusivities with the systemic lupus erythematosus disease activity index are in line with cytomorphological changes in reactive glia, suggesting that the diffusivities of choline compounds and of total creatine are potentially unique markers for glial reactivity in response to inflammation.
Introduction
Systemic lupus erythematosus (SLE) is a female predominant autoimmune disease that affects multiple organs (Jeltsch-David and Muller, 2014). CNS involvement in SLE is common and results in several neurological and psychiatric symptoms. These symptoms are poorly characterized by standard MRI, which appears normal in ∼50% of patients with neuropsychiatric systemic lupus erythematosus (NPSLE). Focal lesions and vascular infarcts, visible on MRI of patients with NPSLE, are non-specific and often do not correlate with clinical outcome and with symptom severity (Luyendijk et al., 2011).
MRI methods sensitive to tissue microstructural changes, such as diffusion tensor imaging (DTI) and magnetization transfer imaging (MTI), show diffuse white matter changes that correlate with the clinical status of patients with NPSLE (Bosma et al., 2000; Emmer et al., 2008; Jung et al., 2010, 2012; Ercan et al., 2015). Histological examination of NPSLE brains has revealed the presence of cerebral oedema, loss of neurons and myelinated axons, microglial proliferation and reactive astrocytosis, microinfarcts and diffuse ischaemic changes, all of which can affect the image contrast in DTI and MTI (Sibbitt et al., 2010). Therefore, although clinically informative, due to the lack of specificity, these imaging modalities provide limited insight into the microstructural deficit in NPSLE.
Magnetic resonance spectroscopy (MRS) reports on concentrations of cell-specific metabolites, and MRS studies have shown differences in the concentrations (relative to total creatine) of several brain metabolites, including significantly lower N-acetylaspartate (NAA) and significantly higher choline and myo-inositol levels in patients with SLE and NPSLE compared to healthy controls (Sibbitt et al., 1997; Axford, 2001). In addition, one study reported significantly lower NAA in SLE patients with high disease activity compared to those with low disease activity (Appenzeller et al., 2005). Although MRS provides cell-type specific information, it does not provide any structural information.
Diffusion-weighted magnetic resonance spectroscopy (DW-MRS) combines the cell-type specificity of MRS with the microstructural sensitivity of diffusion-weighted imaging (DWI), and allows studying of cell- and compartment-specific properties of tissue microstructure by probing the diffusion of intracellular brain metabolites (Nicolay et al., 2001; Kan et al., 2012; Ronen et al., 2013). Of these metabolites, N-acetylaspartate typically co-measured with N-acetylaspartylglutamate (NAAG) (NAA + NAAG = tNAA) resides almost exclusively in neurons/axons; creatine and phosphocreatine (Cr + PCr = tCr), pivotal in aerobic cell energetics, are found in all neural cells, but their astrocytic concentration is twice their neuronal one, and soluble choline-containing compounds (tCho) are predominantly glial, with a glial/neuronal concentration ratio of 3:1 (Urenjak et al., 1993; Choi et al., 2007). The diffusion properties of these metabolites are strongly dictated by the structural and physiological features of their respective intracellular space, and thus provide a unique in vivo probe for pathology affecting intracellular structures, such as ischaemia (Harada et al., 2002; Zheng et al., 2012), tumours (Harada et al., 2002; Colvin et al., 2008), and axonopathy in multiple sclerosis (Wood et al., 2012), as well as making accurate in vivo cell-specific characterization of tissue microstructure possible (Ronen et al., 2013, 2014).
In this study we use, for the first time, the sensitivity of DW-MRS to selectively report on axonal and glial microstructure (i) to investigate the underlying microstructural alterations in a normal appearing portion of the corpus callosum in the brain of SLE patients with and without history of NPSLE; and (ii) to assess the relationship between DW-MRS indices and SLE activity in the patient population in this study. These studies were performed at ultrahigh field (7 T) to obtain the sensitivity required for robust DW-MRS measurements.
Materials and methods
Human subjects
Twenty-nine patients with SLE (one male, 28 females, age: 43 ± 10 years) and 19 age- and sex-matched healthy volunteers (one male, 18 females, age: 41 ± 11 years) were included in the study. The study adhered to the Declaration of Helsinki and was approved by the institutional review board of our institution. Written informed consent was obtained from all subjects prior to the study. Of 29 patients with SLE, 13 had a history of NPSLE and 16 had no history. For convenience, patients with past NPSLE incidence are hereafter referred to as ‘patients with NPSLE’. All patients with SLE were diagnosed according to the 1982 revised American College of Rheumatology criteria (Tan et al., 1982; Hochberg, 1997). All patients with NPSLE were diagnosed at the Leiden NPSLE clinic after a standardized multidisciplinary medical examination (Zirkzee et al., 2012). Neuropsychiatric diagnoses were classified according to the 1999 American College of Rheumatology case definitions for NPSLE syndromes (ACR, 1999). In the NPSLE group, only patients with at least one CNS NPSLE syndrome were included: cerebrovascular disease (five patients), seizures (three patients), cognitive disorder (three patients), movement disorder (one patient), headache (two patients), acute confusional state (three patients), psychosis (one patient), transverse myelitis (one patient), and anxiety (one patient). To categorize the patients according to SLE disease activity, we calculated the systemic lupus erythematosus disease activity index 2000 (SLEDAI-2K) for each patient (Gladman et al., 2002). Permanent and irreversible damage due to SLE was assessed with the systemic lupus international collaborating clinics (SLICC)/American College of Rheumatology damage index (SDI) (Gladman et al., 1997, 2000). SLE patients with a SLEDAI-2K ≥ 8 were considered to have high SLE activity and were categorized as SLE-active (Appenzeller et al., 2005), while the remaining SLE patients were categorized as SLE-inactive. The demographics of the study and the clinical characteristics of the SLE patients are shown in Table 1.
Table 1.
Patient characteristics
| Patients with NPSLE n = 13 | Patients with SLE n = 16 | P | |
|---|---|---|---|
| Age, years (mean ± SD) | 43 ± 8 | 42 ± 11 | 0.721 |
| SLE disease duration, years) (mean ± SD) | 12 ± 9 | 8 ± 5 | 0.178 |
| SLEDAI-2K**, median (range) | 5 (2 to 22) | 2.5 (0 to 8) | 0.006 |
| SDI, median (range) | 1 (0 to 5) | 1 (0 to 4) | 0.241 |
| Antiphospholipid syndrome | 3/13 (23%) | 1/16 (6%) | 0.223 |
| Presence of auto-antibodies | |||
| Antinuclear antibody | 12/13 (92%) | 14/16 (88%) | 0.580 |
| Anti-ENA | 9/13 (70%) | 7/16 (44%) | 0.160 |
| Anti-DNA | 1/13 (8%) | 6/16 (38%) | 0.074 |
| Anti-RNP | 3/13 (23%) | 3/16 (19%) | 0.565 |
| Anti-SSA | 4/13 (31%) | 7/16 (44%) | 0.372 |
| Anti-SSB | 1/13 (8%) | 2/16 (13%) | 0.580 |
| Anti-Smith | 2/13 (15%) | 2/16 (13%) | 0.617 |
| Anticardiolipine auto-antibodies | 3/13 (23%) | 1/16 (6%) | 0.223 |
| Lupus anticoagulant | 6/13 (46%) | 2/16 (13%) | 0.055 |
| Anti-β2 glycoprotein IgG | 2/13 (15%) | 0/16 (0%) | 0.192 |
| Presence of ACR criteria ever in disease course | |||
| Malar rash | 5/13 (38%) | 8/16 (50%) | 0.404 |
| Discoid lupus | 2/13 (15%) | 0/16 (0%) | 0.192 |
| Photosensitivity | 5/13 (38%) | 7/16 (44%) | 0.537 |
| Ulcers | 7/13 (54%) | 7/16 (44%) | 0.434 |
| Arthritis* | 12/13 (92%) | 9/16 (56%) | 0.038 |
| Serositis | 4/13 (31%) | 6/16 (38%) | 0.507 |
| Lupus nephritis | 4/13 (31%) | 5/16 (31%) | 0.647 |
| Neurological disorder | 4/13 (31%) | 0/16 (0%) | 0.448 |
| Haematological disorder | 6/13 (46%) | 8/16 (50%) | 0.566 |
| Immunological disorder | 9/13 (70%) | 13/16 (81%) | 0.374 |
| Antinuclear antibodies | 13/13 (100%) | 16/16 (100%) | 0.374 |
| Current medication | |||
| Prednisone | 9/13 (70%) | 9/16 (56%) | 0.372 |
| Azathioprine | 3/13 (23%) | 6/16 (38%) | 0.336 |
| Methotrexate | 1/13 (8%) | 1/16 (6%) | 0.704 |
| Hydroxychloroquine | 11/13 (85%) | 12/16 (75%) | 0.435 |
| Mycophenolate mofetil | 3/13 (23%) | 2/16 (13%) | 0.396 |
ACR = American College of Rheumatology; IgG = immunoglobulin G; SDI = systemic lupus international collaborating clinics (SLICC)/American College of Rheumatology damage index.
*P < 0.05; **P < 0.01.
Data acquisition
All subjects were scanned on a 7 T Philips Achieva MRI scanner (Philips Healthcare) equipped with a 32-channel receive head coil (Nova Medical Inc.). The scan protocol consisted of a short survey scan and a sensitivity encoding reference scan followed by: (i) sagittal 3D T1-weighted images (field of view = 246 × 246 × 174 mm3, resolution: 0.85 × 0.85 × 1 mm3, repetition time/echo time = 4.00/1.84 ms, total scan time = 1.59 min); (ii) axial multislice diffusion tensor images (field of view = 224 × 224 × 150 mm3, resolution: 1.75 × 1.75 × 2.20 mm3, repetition time/echo time = 10 000/65 ms, 15 diffusion-weighting directions with b = 1000 s/mm2, total scan time: 3 min); and (iii) single-volume diffusion-weighted spectroscopy scans (detailed protocol below).
Diffusion-weighted magnetic resonance spectroscopy
Protocol
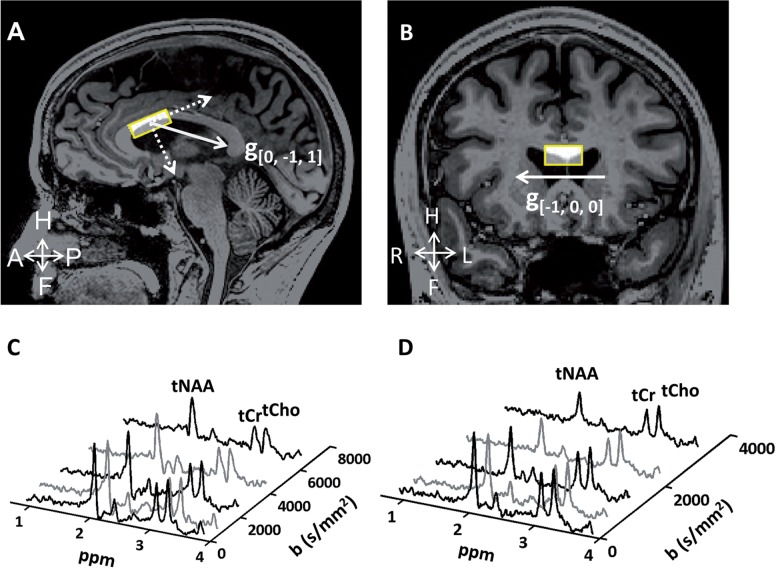
The DW-MRS sequence was based on the PRESS (Point Resolved Spectroscopy) sequence with bipolar diffusion-weighting gradients added on both sides of the 180° pulses. A 3 cm3 volume of interest [25 (AP) × 15 (RL) × 8 (FH) mm3] was positioned on the anterior body of the corpus callosum as shown in Fig. 1. The diffusion-weighting gradients were applied in two directions: a right-left direction in the volume of interest frame, mostly parallel to the direction of the callosal fibres [direction (1,0,0)], and a direction mostly perpendicular to the callosal fibres [direction (0,−1,1)], as shown in Fig. 1A and B. The centre frequency was set to the tNAA singlet peak at 2.0 ppm. Water suppression was performed using two frequency-selective excitation pulses, each followed by a dephasing gradient before metabolite excitation. Pencil beam second-order shimming was performed, resulting in a typical tNAA line width of 10 Hz. A peripheral pulse unit was used to gate data acquisition to the cardiac cycle, thereby minimizing signal fluctuations due to cardiac pulsation. The parameters for DW-MRS acquisitions were: echo time = 121 ms, repetition time = three cardiac cycles (∼3000 ms), cardiac trigger delay = 300 ms, number of time-domain points = 1024, spectral width = 3000 Hz, gradient duration (δ) = 37 ms, bipolar gap = 16 ms, diffusion time (Δ) = 60.5 ms with five different gradient amplitudes resulting in b-values of 212, 651, 1335, 2262, and 3462 s/mm2 in the (1,0,0) direction and 440, 1336, 2718, 4586, and 6945 s/mm2 in the (0,−1,1) direction. The total number of spectra per diffusion condition was 32, resulting in a total scan time of 10–15 min. Following this scan, a shorter scan (fewer signal averages) with identical volume of interest position and diffusion conditions was performed without water suppression and with the center frequency set at the water resonance frequency. These spectra were used for eddy-current correction in the post-processing stage.
Figure 1.
The position of the volume of interest in sagittal (A) and coronal (B) views. Gradients applied in directions approximately perpendicular (A) and parallel (B) to the callosal fibres are shown in solid lines. Typical spectra acquired with diffusion weighting in the (0,−1,1) and the (1,0,0) directions are shown as a function of b-value in C and D, respectively. Line broadening of 5 Hz was applied for display purposes. The attenuation of the tNAA peak is more pronounced in the direction approximately parallel to the axonal fibres, along which diffusion of intra-axonal molecules is less restricted than in the direction perpendicular to the fibres.
Processing
All spectral preprocessing was performed with custom codes in MATLAB® release R2014b (Mathworks, Natick, MA, USA). Spectral preprocessing consisted of correcting DW-MRS data for eddy currents, zero-order phasing, correction of frequency drift for individual acquisitions, and removal of the residual water peak: averaged spectra were generated for each condition (Wood et al., 2015). Figure 1C and D show typical sets of diffusion-weighted spectra obtained with diffusion-weighting in the (1, 0, 0) direction (Fig. 1C) and the (0, −1, 1) direction (Fig. 1D), respectively and from a healthy control subject. The resulting spectra were quantified with LCModel (Provencher 1993). Cramér–Rao lower bound (CRLB) values were used to evaluate the quality of the spectra for each diffusion condition, and the acceptance threshold for DW-MRS data inclusion was set at CRLB <20%. Based on this, datasets from one NPSLE subject and one SLE subject were excluded from the tNAA analysis.
The LCModel spectral estimates were used to calculate the diffusivity (Dpar) along the (1,0,0) direction (roughly parallel to the callosal fibres) and diffusivity (Dperp) along the (0,−1,1) direction (roughly perpendicular to the callosal fibres) for tNAA, tCr and tCho. These were calculated by performing a linear fit of the natural logarithm of the DW-MRS signal amplitudes as a function of the diffusion weighting value b, assuming a monoexpoential decay of the signal as a function of b in each direction:
| (1) |
where is the measured signal in direction i, is the signal without diffusion weighting, bi is the value of b in the direction i, and Di is the calculated diffusion coefficient for direction i. Even though it is possible that the metabolite diffusion-weighted signal decay displays non-monoexponential behaviour at very high values of b, our previous work has shown that in the range of b-values used in this study the assumption of mono-exponentiality is valid and diffusivity values are reproducible (Kan et al., 2012; Wood et al., 2015). An average of Dpar and Dperp was calculated to assess the average diffusivity (Davg) for tNAA, tCr and tCho (Ronen et al., 2013). The quality of the linear fittings was evaluated via calculation of the coefficient of determination and an acceptance threshold was set at 75%, leading to exclusion of Davg(tCr) values obtained from two patients with NPSLE and one patient with SLE.
Image processing
DTI volumes were motion-corrected with ExploreDTI (Leemans et al., 2009) and further processed with the DTI toolbox (Behrens et al., 2003) of the FMRIB Software Library (FSL release 5.0, http://www.fmrib.ox.ac.uk/fsl/) to obtain the following DTI measures for each subject: fractional anisotropy, mean diffusivity, axial diffusivity and radial diffusivity. These DTI metrics were further analysed with tract-based spatial statistics (TBSS) (Smith et al., 2006). Statistical differences between patients with NPSLE, patients with SLE and healthy control subjects were assessed on white matter tract voxels in FA-MNI152 standard space using 5000 permutations and ANOVA tests were performed with a significance threshold of P < 0.05 and were corrected for multiple comparisons based on threshold-free cluster enhancement (Winkler et al., 2014). One SLE patient dataset was excluded due to poor registration to FA-MNI152.
T1-weighted images were used for tissue segmentation within the volume of interest (Wood et al., 2015). Fractional anisotropy maps were registered to the T1-weighted image of the same subject first by affine transformation using FSL FLIRT (Jenkinson and Smith, 2001; Jenkinson et al., 2002) and subsequently by non-rigid transformation using FNIRT (Andersson et al., 2010). The inverse transformation matrices generated were used to register the DW-MRS volume of interest to the DTI space. Subsequently, the registration procedure in TBSS was applied to transform each volume of interest to MNI152 space.
T1-weighted volumes were further processed in FreeSurfer (http://surfer.nmr.mgh.harvard.edu/) and the intracranial volume, total brain volume, centre corpus callosum volume and mid-anterior corpus callosum volume were calculated for each subject. To evaluate whole brain and callosal atrophy due to SLE and NPSLE, total brain volume, centre corpus callosum volume and mid-anterior corpus callosum volume were normalized according to the intracranial volume of the same subject.
Statistical analyses
Patients with NPSLE and those with SLE were compared with respect to demographic characteristics, presence of autoantibodies and ACR criteria and current medication using chi-square tests and ANOVA or Mann-Whitney tests when appropriate. Primary dependent measures included in the statistics were Davg(tNAA), Davg(tCr), Davg(tCho). Shapiro-Wilk test was used to examine the distribution of variables, and resulted in all being normally distributed. Equality of variances of the different groups was assessed using Levene’s test. Between-group differences on all Davg values were evaluated using one-way-ANOVAs (pairwise comparisons). P < 0.05 was considered to represent statistically significant differences. Bonferroni’s correction was used to correct for multiple comparisons. Analysis of covariance was performed to analyse the influence of age and SLEDAI-2K on the differences of mean Davg values between groups. As SLEDAI-2K and SDI scores were not normally distributed, correlations between the metabolite Davg measurements and SLEDAI-2K score and SDI score were evaluated with Spearman’s rank correlation. Correlations between SLE duration and Davg values were assessed with Pearson’s correlation test. All statistical analyses were performed with SPSS version 20.0 for Windows (IBM SPSS statistics, Chicago, IL, USA). Scattered plots were generated using GraphPad Prism 5 for windows, version 5.01, GraphPad Software, USA.
Results
Diffusion-weighted magnetic resonance spectroscopy
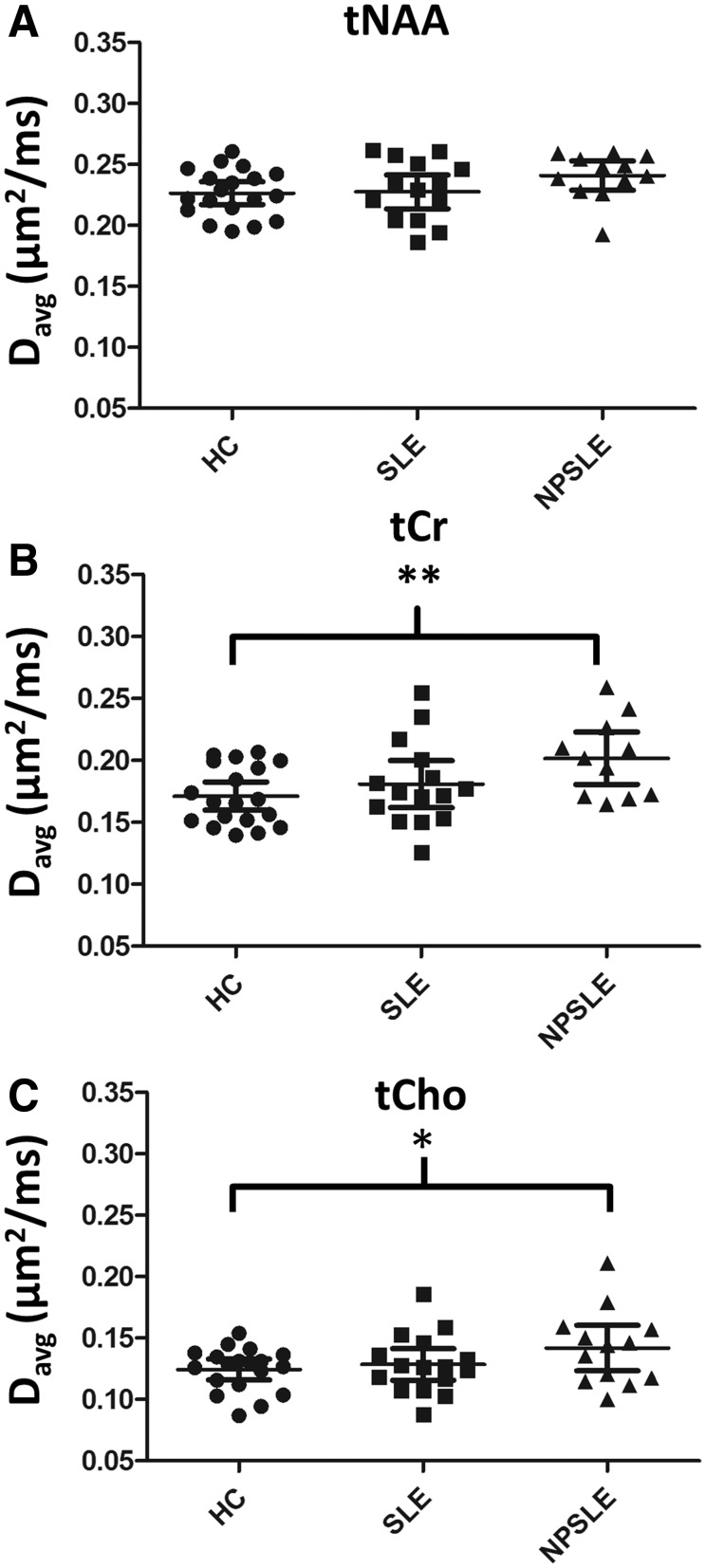
Average metabolite Davg values for the NPSLE, SLE and healthy control groups are shown in Table 2 and group Davg data for the three population groups are displayed in Fig. 2. When all three groups were compared with ANOVA with age included as a covariate, significant differences were found in Davg(tCho) (P = 0.006) and Davg(tCr) (P = 0.030). No significant differences were found in Davg(tNAA) among the groups. Pairwise comparisons showed that in patients with NPSLE, Davg(tCr) and Davg(tCho) were significantly higher than in healthy controls after correction for age (mean Davg(tCr) in NPSLE = 0.202 ± 0.032 μm2/ms, mean Davg(tCr) in healthy controls = 0.171 ± 0.024 μm2/ms, P = 0.018 and mean Davg(tCho) in NPSLE = 0.142 ± 0.031 μm2/ms, mean Davg(tCho) in healthy controls = 0.124 ± 0.018 μm2/ms, P = 0.044). No significant difference was found in Davg(tNAA) values between patients with NPSLE and healthy control subjects. No significant differences were observed in any metabolite Davg between NPSLE and SLE or between SLE and healthy control groups. SLEDAI-2K was used as a covariate when patients with SLE and those with NPSLE were compared. When all SLE patients, with and without past CNS involvement, were grouped together and compared to healthy control subjects, Davg(tCr) and Davg(tCho) remained significantly higher in patients with SLE than in healthy control subjects after correcting for age [mean Davg(tCr) in all SLE patients = 0.190 ± 0.034 μm2/ms, mean Davg(tCr) in healthy controls = 0.171 ± 0.024 μm2/ms, P = 0.060 and mean Davg(tCho) in all SLE patients = 0.135 ± 0.028 μm2/ms, mean Davg(tCho) in healthy controls = 0.124 ± 0.018 μm2/ms, P = 0.008].
Table 2.
Metabolite Davg values for patients with NPSLE, patients with SLE and healthy control subjects
| NPSLE n = 13 | SLE n = 16 | Healthy control n = 19 | % Difference NPSLE versus healthy control | |
|---|---|---|---|---|
| Davg(tNAA) μm2/ms | 0.241 ± 0.019 | 0.227 ± 0.025 | 0.226 ± 0.019 | 7% |
| Davg(tCr) μm2/ms | 0.202 ± 0.032* | 0.181 ± 0.034 | 0.171 ± 0.024 | 18% |
| Davg(tCho) μm2/ms | 0.142 ± 0.031* | 0.128 ± 0.024 | 0.124 ± 0.018 | 14% |
tCho = choline compounds; tCr = total creatine + phosphocreatine; tNAA = N-acetylaspartate.
*P < 0.05 versus healthy control subjects.
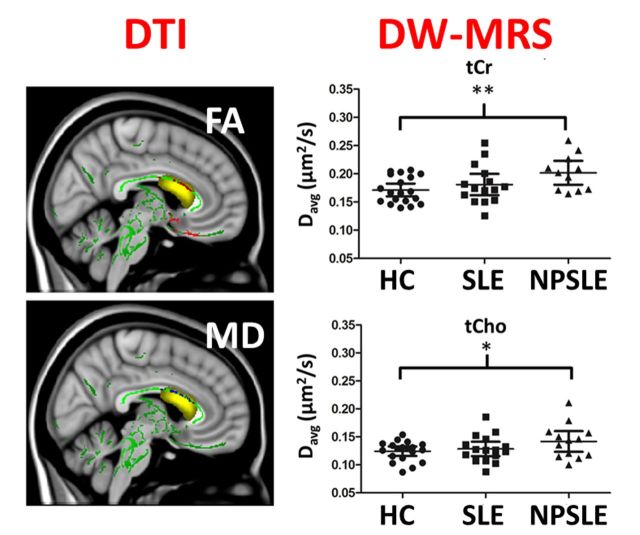
Figure 2.
Metabolite Davg values for healthy controls subjects and patients with SLE and patients with NPSLE. Davg (tNAA), Davg (tCr) and Davg (tCho) data are shown in A, B and C, respectively. Statistically significant differences are shown as *P < 0.05 and **P < 0.01. No significant differences were found between SLE and healthy control subjects, or between NPSLE and SLE in any of the metabolite Davg values. HC = healthy controls.
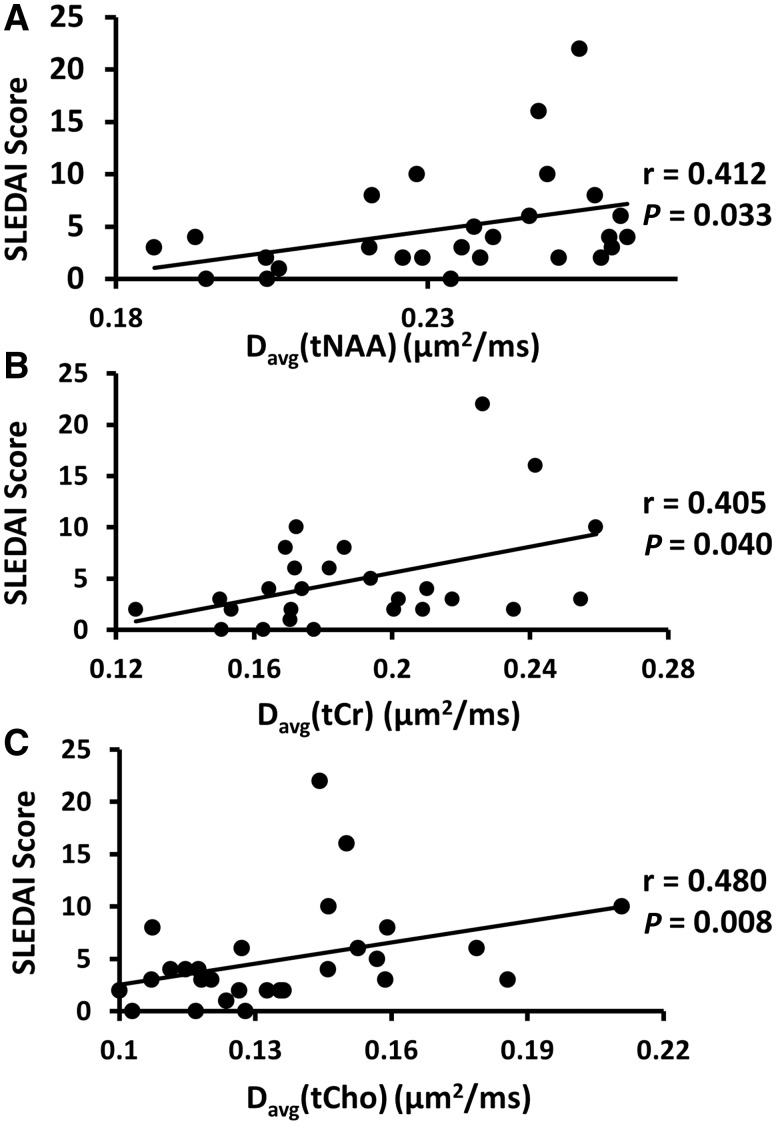
Average diffusion coefficients of all metabolites showed a link to disease activity in both the patients with SLE and those with NPSLE. When all patients with SLE were pooled together, regardless of their neuropsychiatric status, SLEDAI-2K scores correlated positively with Davg(tNAA) (r = 0.412, P = 0.033), Davg(tCr) (r = 0.405, P = 0.040) and Davg(tCho) (r = 0.480, P = 0.008). Scatter plots of the SLEDAI-2K scores of all patients as a function of metabolite Davg values are shown in Fig. 3. When patients with SLE and those with NPSLE categorized as SLE-active (SLEDAI-2K ≥ 8, n = 5) were compared to healthy control subjects, statistically significant differences in Davg(tCr) were found [Davg(tCr) in SLE-active = 0.216 ± 0.038 μm2/ms, Davg(tCr) in healthy controls = 0.171 ± 0.024 μm2/ms, P = 0.006], as well as in Davg(tCho) [Davg(tCho) in SLE active = 0.154 ± 0.037 μm2/ms, Davg(tCho) in healthy controls = 0.124 ± 0.018 μm2/ms, P = 0.006] after correcting for age. No correlation was found between metabolite Davg values and SLE duration or SDI scores.
Figure 3.
Correlation of metabolite Davg values with patient SLEDAI-2K scores. The resulting Spearman’s rank correlation r and significance of the correlations are shown for Davg (tNAA) (A), Davg(tCr) (B) and Davg(tCho) (C).
Diffusion tensor imaging and volumetric results
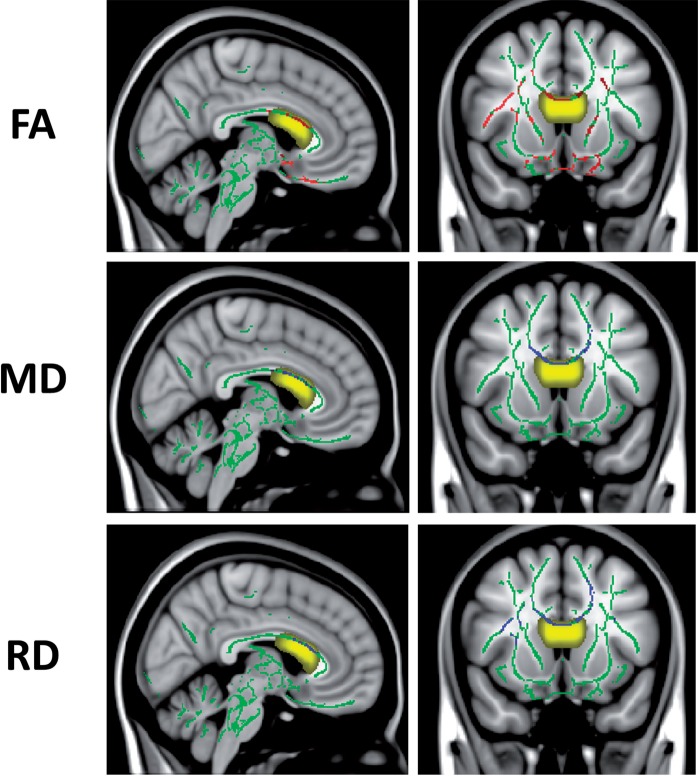
Significantly lower fractional anisotropy, higher mean diffusivity and higher radial diffusivity were found throughout white matter in the NPSLE patient group compared to the healthy control and SLE groups, including the callosal region within the DW-MRS volume of interest (P < 0.05). Figure 4 shows maps of statistically significant differences (P < 0.05) in DTI measures overlaid on the MNI152 T1-weighted image, and the cumulative DW-MRS volume of interest (i.e. the sum of all the individual volumes of interest following transformation from the individual subject coordinates to the FA-MNI152 coordinates). The voxels with significantly higher mean and radial diffusivity values in the patients with NPSLE compared to healthy controls are shown in blue and voxels with lower fractional anisotropy values in patients with NPSLE compared to healthy controls are shown in red. No significant differences were found in any DTI measure between SLE patients (with and without past CNS involvement) and healthy control subjects. No significant differences were found in corpus callosum volumes or total brain volumes between patients with SLE or those with NPSLE, and healthy control subjects.
Figure 4.
Tract-based spatial statistics results showing regions with statistically significant differences in DTI measures in the white matter skeleton of patients with NPSLE and healthy control subjects (P < 0.05). Maps are shown for one sagittal (left) and one coronal (right) slice in MNI152 space. The mean fractional anisotropy skeleton is shown in green, regions with higher values in the patients with NPSLE compared to healthy control subjects are shown in blue and regions with lower values in patients with NPSLE compared to healthy control are shown in red. Cumulative volume of interests chosen for DW-MRS of patients with NPSLE and healthy controls are shown in yellow. FA = fractional anisotropy; MD = mean diffusivity; RD = radial diffusivity.
Discussion
This is the first study to address cell-specific microstructural alterations in the brain of SLE patients with DW-MRS at ultrahigh field. This study focused on measuring the diffusion properties of two predominantly glial metabolites, tCr and tCho, and one exclusively axonal/neuronal metabolite, tNAA. The most salient finding in this study is the strong and consistent link between both Davg(tCr) and Davg(tCho) and disease state, with respect to disease activity and to past CNS involvement, suggesting glial involvement in the brain of these patients. Two potential pathological mechanisms that can explain the significantly higher tCr and tCho diffusivities found in patients with NPSLE are inflammation-mediated morphological changes in microglia and astrocytes, and intracellular oedema, which would affect both glia and neurons/axons (Brooks et al., 2010; Graeber and Streit, 2010; Sofroniew and Vinters, 2010).
Astrocytic and microglial reactivity in response to inflammation and/or ischaemia are both highly consistent with an increase in intracellular diffusivity in glia. Reactivity-related cellular hypertrophy and thickening of the processes near the soma (especially in astrocytes) (Sofroniew, 2009) would result in an increase of the intracellular space, and a decrease in molecular crowding and intracellular tortuosity, leading to increased diffusivity in the cytosol. The pathogenesis of NPSLE is thought to involve various immune and inflammatory processes that can lead to neuronal injury and vasculopathy (Popescu and Kao, 2011). The inflammatory response to injury likely results in glial reactivity and cellular hypertrophy, especially in microglia and astrocytes (Graeber and Streit, 2010; Sofroniew and Vinters, 2010). Histolopathological investigations of brains of patients with NPSLE confirm the widespread presence of reactive microglia and astrocytes, as well as of lipid-laden macrophages among the heterogeneous pathological phenomena (Brooks et al., 2010). Furthermore, the correlation of in vivo MRS results with histological results from the same patients (Brooks et al., 2010) suggests: (i) an association between an increase in tCho concentrations and gliosis, vasculopathy and oedema; (ii) possible association of tCr with gliosis and reduced neuronal/axonal density; and (iii) an association between lower tNAA concentrations and a decrease in neuronal/axonal density (Brooks et al., 2010).
The higher Davg(tNAA) values found in patients with NPSLE compared to healthy controls may be attributed to changes in cytosolic viscosity in axons due either to neuronal/axonal damage or to cytotoxic oedema, both of which are seen in the histopathology of brains of patients with NPSLE (Sibbitt et al., 2010). Higher Davg(tNAA) has also been observed in a study of patients with schizophrenia where it was hypothesized that inflammatory processes may play a role (Du et al., 2013). On the other hand, lower tNAA parallel diffusivity values were found in patients with multiple sclerosis compared to healthy controls in a study focused on myelin and axonal changes in the corpus callosum (Wood et al., 2012). It is likely that the different behaviours in tNAA diffusivity seen in multiple sclerosis and in NPSLE reflect different intra-axonal pathological mechanisms associated with these two diseases. Central to multiple sclerosis are demyelination and axonopathy (Petzold et al., 2011; Stadelmann et al., 2011). As demyelination has no direct effect on diffusion in the intra-axonal space, it has been hypothesized that in multiple sclerosis the decrease in tNAA axosolic diffusivity stemmed from axonal damage that included unusual patterns of neurofilament phosphorylation and packing compared to normal tissue, and a less organized axoskeleton and/or problems with axonal transport (Petzold et al., 2008). In contrast to findings in multiple sclerosis, histology of patients with NPSLE have shown that cerebral oedema occurs much more frequently than axonal/neuronal loss (Sibbitt et al., 2010) and is thus more compatible with the increase in axosolic diffusivity, as evidenced by the higher levels of Davg(tNAA) observed in our study.
The high correlation in all patients with SLE or NPSLE between SLE disease activity, as quantified by the SLEDAI-2K score, and Davg(tCr) and Davg(tCho), suggests that the SLE-related peripheral inflammation and autoimmune response may have an effect on the brain, independent of overt clinical CNS involvement in SLE. Additionally, the correlation of Davg(tNAA) values with SLEDAI-2K scores suggests a permanent or continuous damage to axons correlated with high SLE activity. This is further corroborated by the finding that patients with higher disease activity (those we defined as SLE-active) have higher metabolite diffusivity levels and higher statistical significance in the difference in metabolite diffusivity compared to healthy controls. A previous MRS study in patients with NPSLE/SLE has shown a significantly lower tNAA/tCr level in SLE patients with a high SLEDAI-2K score, and that the level of tNAA/tCr was renormalized in follow-up for patients who were no longer SLE-active, regardless of their neuropsychiatric status (Appenzeller et al., 2005). This finding suggests a pathological mechanism, attributed by the authors to neuronal dysfunction that affects both neurons and axons, the degree of which depends on SLE disease activity, but which is essentially reversible in nature. In our view, and based on our corroborative findings, we attribute this finding to intracellular/intra-axonal oedema. It has been suggested that inflammation outside the brain can prime microglia and result in microglial activation for several weeks (Perry and Holmes, 2014). Our findings, as well as those described by Appenzeller et al. (2006), support the view that systemic inflammation affects the brain in NPSLE, and the underlying mechanism by which this occurs, e.g. potential disruption of the blood–brain barrier in SLE (Abbott et al., 2003), should be further investigated.
Metabolite apparent diffusion coefficient values found in healthy control in this study are similar to those reported in previous DW-MRS studies performed on a similar region of the corpus callosum at 7 T and 3 T (Ronen et al., 2013; Wood et al., 2015). A recently published robustness and reproducibility study of DW-MRS in the anterior body of the corpus callosum, aimed to provide guidelines for DW-MRS acquisition for clinical studies such as the one presented here. Using power calculations based on actual data it was estimated that to observe a 10% difference in Davg values for tNAA, tCr and tCho in a case-control study, nine, four and 12 subjects, respectively, are sufficient (Wood et al., 2015). In this current study we observed a 7% increase in Davg(tNAA), 19% increase in Davg(tCr) and 14% in Davg(tCho) in patients with NPSLE compared to healthy control, with 13 and 19 subjects per group, respectively. This suggests that it is feasible to observe reliably the disease effect on metabolite Davg reported here. Moreover, based on our power estimation, the number of subjects required to observe reliably a difference in Davg(tCho) is higher than that required for Davg(tNAA). This further supports the notion that our findings indicate a larger effect size for glial than for axonal involvement in SLE/NPSLE. The DTI results are also consistent with previous studies on SLE and NPSLE (Emmer et al., 2010; Jung et al., 2010; Ercan et al., 2015). Significantly lower fractional anisotropy values in the anterior body of the corpus callosum of patients with NPSLE compared to healthy controls were previously reported in a DTI study on NPSLE where region of interest analysis was performed (Zimny et al., 2014). This study also included MRS measurements in normal appearing parietal white matter, and no differences in glial metabolite concentrations (tCr, tCho) between patients with NPSLE and healthy controls were reported. Although changes in concentrations of glial metabolites have been linked to glial reactivity and neuroinflammation (Chang et al., 2013) this may suggest that the diffusion properties of glial metabolites are more sensitive to glial reactivity than their cellular concentration. Atrophy measurements did not show any significant callosal or global atrophy in the patient population, possibly stemming from a type II error due to the small size of the cohort.
There were several challenges and limitations to the study. The main (unmet) challenge of this study was to scan patients with active neuropsychiatric symptoms at the time of the scan, as well as to include more patients with high SLE disease activity. Low incidence of active NPSLE was a factor, as well as significant potential discomfort to patients with clinically overt neuropsychiatric symptoms, preventing these patients from being involved in a research study that is not part of the routine clinical procedure. Another limitation of this study is the small number of patients per specific neuropsychiatric syndrome. This is a generally recognized problem in NPSLE studies, mainly related to the low prevalence and the high heterogeneity of NPSLE. Subsequently, definite conclusions concerning the relationship between DW-MRS findings and NPSLE syndromes cannot be drawn. In our patient cohort, the relative low number of patients with antiphospholipid syndrome made it difficult to evaluate the effect of ischaemic/vascular changes on metabolite diffusion. As a consequence, we did not separately analyse changes in ischaemic and inflammatory patients with NPSLE. Future studies will focus on separate evaluation of the effects of neuropsychiatric activity, ischaemic and inflammatory effects. The study would have benefitted from a separate MRS acquisition at short echo time, for an accurate evaluation of the metabolite concentrations in the same volume of interest, together with additional cell-specific metabolites such as glutamate, glutamine and myo-inositol.
In conclusion, the results presented in this study show for the first time that intracellular metabolite diffusion reflective of glial and neuronal/axonal involvement can be measured by DW-MRS in a complex autoimmune inflammatory disorder such as SLE/NPSLE. This technique has great potential for the study of the aetiology of disease-related changes in tissue microstructure of patients with SLE/NPSLE. We believe that if incorporated in a comprehensive diagnostic scanning protocol together with existing microstructural MRI tools, such as DTI, MTI and susceptibility weighted imaging, DW-MRS can contribute to the diagnostic process of these patients and may help unravel underlying pathogenic mechanisms in this complex disease.
Acknowledgements
We thank Gerda Labadie and Marjan van der Elst from the department of radiology and Liesbeth Beaart-van de Voorde and Diane van der Woude from the department of rheumatology at the Leiden University Medical Center for their help in patient recruitment, and the following researchers from the Gorter Center for High Field MRI Research: Sophie Schmid for patient intake and Wouter Teeuwisse for help regarding MRI safety.
Glossary
Abbreviations
- DTI =
diffusion tensor imaging
- MRS =
magnetic resonance spectroscopy
- NAA =
N-acetylaspartate
- (NP)SLE =
(neurospsychiatric) systemic lupus erythematosus
- SLEDAI-2K =
systemic lupus erythematosus disease activity index 2000
Funding
A.E.E. was supported by a Dutch Arthritis Association (Reumafonds) grant (# 2010_0000135). E.T.W. was supported by Intramural Research Program of the National Institute of Neurological Disorders and Stroke and is the recipient of an NINDS Competitive Intramural Graduate Fellowship award. The C.J. Gorter Center for High Field MRI is supported by grant number 176.010.2005 from the Nederlandse Organisatie voor Wetenschappelijk Onderzoek: Grootschalige Onderzoeksfaciliteiten (Dutch Organization for Scientific Research: Large-Scale Research Facilities).
References
- Abbott NJ, Mendonca LL, Dolman DE. The blood-brain barrier in systemic lupus erythematosus. Lupus 2003; 12: 908–15. [DOI] [PubMed] [Google Scholar]
- ACR. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum 1999; 42: 599–608. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. (2010). Non-linear registration, aka spatial normalisation. FMRIB technical report TR07JA2, from www.fmrib.ox.ac.uk/analysis/techrep.
- Appenzeller S, Costallat LT, Li LM, Cendes F. Magnetic resonance spectroscopy in the evaluation of central nervous system manifestations of systemic lupus erythematosus. Arthritis Rheum 2006; 55: 807–11. [DOI] [PubMed] [Google Scholar]
- Appenzeller S, Li LM, Costallat LT, Cendes F. Evidence of reversible axonal dysfunction in systemic lupus erythematosus: a proton MRS study. Brain 2005; 128: 2933–40. [DOI] [PubMed] [Google Scholar]
- Axford JS. Sensitivity of quantitative 1H magnetic resonance spectroscopy of the brain in detecting early neuronal damage in systemic lupus erythematosus. Ann Rheum Dis 2001; 60: 106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med 2003; 50: 1077–88. [DOI] [PubMed] [Google Scholar]
- Bosma GP, Rood MJ, Zwinderman AH, Huizinga TW, van Buchem MA. Evidence of central nervous system damage in patients with neuropsychiatric systemic lupus erythematosus, demonstrated by magnetization transfer imaging. Arthritis Rheum 2000; 43: 48–54. [DOI] [PubMed] [Google Scholar]
- Brooks WM, Sibbitt WL, Jr, Kornfeld M, Jung RE, Bankhurst AD, Roldan CA. The histopathologic associates of neurometabolite abnormalities in fatal neuropsychiatric systemic lupus erythematosus. Arthritis Rheum 2010; 62: 2055–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Munsaka SM, Kraft-Terry S, Ernst T. Magnetic resonance spectroscopy to assess neuroinflammation and neuropathic pain. J Neuroimmune Pharmacol 2013; 8: 576–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JK, Dedeoglu A, Jenkins BG. Application of MRS to mouse models of neurodegenerative illness. NMR Biomed 2007; 20: 216–37. [DOI] [PubMed] [Google Scholar]
- Colvin DC, Yankeelov TE, Does MD, Yue Z, Quarles C, Gore JC. New insights into tumor microstructure using temporal diffusion spectroscopy. Cancer Res 2008; 68: 5941–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, Cooper AJ, Thida T, Shinn AK, Cohen BM, Ongur D. Myelin and axon abnormalities in schizophrenia measured with magnetic resonance imaging techniques. Biol Psychiatry 2013; 74: 451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmer BJ, Steup-Beekman GM, Steens SC, Huizinga TW, van Buchem MA, van der Grond J. Correlation of magnetization transfer ratio histogram parameters with neuropsychiatric systemic lupus erythematosus criteria and proton magnetic resonance spectroscopy: association of magnetization transfer ratio peak height with neuronal and cognitive dysfunction. Arthritis Rheum 2008; 58: 1451–7. [DOI] [PubMed] [Google Scholar]
- Emmer BJ, Veer IM, Steup-Beekman GM, Huizinga TW, van der Grond J, van Buchem MA. Tract-based spatial statistics on diffusion tensor imaging in systemic lupus erythematosus reveals localized involvement of white matter tracts. Arthritis Rheum 2010; 62: 3716–21. [DOI] [PubMed] [Google Scholar]
- Ercan E, Ingo C, Tritanon O, Magro-Checa C, Smith A, Smith S, et al. A multimodal MRI approach to identify and characterize microstructural brain changes in neuropsychiatric systemic lupus erythematosus. Neuroimage Clin 2015; 8: 337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladman DD, Goldsmith CH, Urowitz MB, Bacon P, Fortin P, Ginzler E, et al. The Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) Damage Index for Systemic Lupus Erythematosus International Comparison. J Rheumatol 2000; 27: 373–6. [PubMed] [Google Scholar]
- Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002; 29: 288–91. [PubMed] [Google Scholar]
- Gladman DD, Urowitz MB, Goldsmith CH, Fortin P, Ginzler E, Gordon C, et al. The reliability of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index in patients with systemic lupus erythematosus. Arthritis Rheum 1997; 40: 809–13. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ. Microglia: biology and pathology. Acta Neuropathol 2010; 119: 89–105. [DOI] [PubMed] [Google Scholar]
- Harada M, Uno M, Hong F, Hisaoka S, Nishitani H, Matsuda T. Diffusion-weighted in vivo localized proton MR spectroscopy of human cerebral ischemia and tumor. NMR Biomed 2002; 15: 69–74. [DOI] [PubMed] [Google Scholar]
- Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40: 1725. [DOI] [PubMed] [Google Scholar]
- Jeltsch-David H, Muller S. Neuropsychiatric systemic lupus erythematosus: pathogenesis and biomarkers. Nat Rev Neurol 2014; 10: 579–96. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002; 17: 825–41. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal 2001; 5: 143–56. [DOI] [PubMed] [Google Scholar]
- Jung RE, Caprihan A, Chavez RS, Flores RA, Sharrar J, Qualls CR, et al. Diffusion tensor imaging in neuropsychiatric systemic lupus erythematosus. BMC Neurol 2010; 10: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Chavez RS, Flores RA, Qualls C, Sibbitt WL, Jr, Roldan CA. White matter correlates of neuropsychological dysfunction in systemic lupus erythematosus. PLoS One 2012; 7: e28373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan HE, Techawiboonwong A, van Osch MJ, Versluis MJ, Deelchand DK, Henry PG, et al. Differences in apparent diffusion coefficients of brain metabolites between grey and white matter in the human brain measured at 7 T. Magn Reson Med 2012; 67: 1203–9. [DOI] [PubMed] [Google Scholar]
- Leemans A, Jeurissen B, Sijbers J, Jones DK. (2009). ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. In Proceeding of the 7th Annual Meeting of International Society for Magnetic Resonance in Medicine, Honolulu, HI, p. 3537.
- Luyendijk J, Steens SC, Ouwendijk WJ, Steup-Beekman GM, Bollen EL, van der Grond J, et al. Neuropsychiatric systemic lupus erythematosus: lessons learned from magnetic resonance imaging. Arthritis Rheum 2011; 63: 722–32. [DOI] [PubMed] [Google Scholar]
- Nicolay K, Braun KP, Graaf RA, Dijkhuizen RM, Kruiskamp MJ. Diffusion NMR spectroscopy. NMR Biomed 2001; 14: 94–111. [DOI] [PubMed] [Google Scholar]
- Perry VH, Holmes C. Microglial priming in neurodegenerative disease. Nat Rev Neurol 2014; 10: 217–24. [DOI] [PubMed] [Google Scholar]
- Petzold A, Gveric D, Groves M, Schmierer K, Grant D, Chapman M, et al. Phosphorylation and compactness of neurofilaments in multiple sclerosis: indicators of axonal pathology. Exp Neurol 2008; 213: 326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold A, Tozer DJ, Schmierer K. Axonal damage in the making: neurofilament phosphorylation, proton mobility and magnetisation transfer in multiple sclerosis normal appearing white matter. Exp Neurol 2011; 232: 234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu A, Kao AH. Neuropsychiatric systemic lupus erythematosus. Curr Neuropharmacol 2011; 9: 449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993; 30: 672–9. [DOI] [PubMed] [Google Scholar]
- Ronen I, Budde M, Ercan E, Annese J, Techawiboonwong A, Webb A. Microstructural organization of axons in the human corpus callosum quantified by diffusion-weighted magnetic resonance spectroscopy of N-acetylaspartate and post-mortem histology. Brain Struct Funct 2014; 219: 1773–85. [DOI] [PubMed] [Google Scholar]
- Ronen I, Ercan E, Webb A. Axonal and glial microstructural information obtained with diffusion-weighted magnetic resonance spectroscopy at 7T. Front Integr Neurosci 2013; 7: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibbitt WL, Brooks WM, Kornfeld M, Hart BL, Bankhurst AD, Roldan Ca. Magnetic resonance imaging and brain histopathology in neuropsychiatric systemic lupus erythematosus. Semin Arthritis Rheum 2010; 40: 32–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibbitt WL, Jr, Haseler LJ, Griffey RR, Friedman SD, Brooks WM. Neurometabolism of active neuropsychiatric lupus determined with proton MR spectroscopy. Am J Neuroradiol 1997; 18: 1271–7. [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006; 31: 1487–505. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 2009; 32: 638–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol 2010; 119: 7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadelmann C, Wegner C, Bruck W. Inflammation, demyelination, and degeneration - recent insights from MS pathology. Biochim Biophys Acta 2011; 1812: 275–82. [DOI] [PubMed] [Google Scholar]
- Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982; 25: 1271–7. [DOI] [PubMed] [Google Scholar]
- Urenjak J, Williams SR, Gadian DG, Noble M. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci 1993; 13: 981–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage 2014; 92: 381–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood ET, Ercan AE, Branzoli F, Webb A, Sati P, Reich DS, et al. Reproducibility and optimization of in vivo human diffusion-weighted MRS of the corpus callosum at 3T and 7T. NMR Biomed 2015; 28: 976–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood ET, Ronen I, Techawiboonwong A, Jones CK, Barker PB, Calabresi P, et al. Investigating axonal damage in multiple sclerosis by diffusion tensor spectroscopy. J Neurosci 2012; 32: 6665–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng DD, Liu ZH, Fang J, Wang XY, Zhang J. The effect of age and cerebral ischemia on diffusion-weighted proton MR spectroscopy of the human brain. Am J Neuroradiol 2012; 33: 563–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimny AM, Szmyrka-Kaczmarek P, Szewczyk J, Bladowska A, Pokryszko-Dragan E, Gruszka P, et al. In vivo evaluation of brain damage in the course of systemic lupus erythematosus using magnetic resonance spectroscopy, perfusion-weighted and diffusion-tensor imaging. Lupus 2014; 23: 10–19. [DOI] [PubMed] [Google Scholar]
- Zirkzee EJ, Steup-Beekman GM, van der Mast RC, Bollen EL, van der Wee NJ, Baptist E, et al. Prospective study of clinical phenotypes in neuropsychiatric systemic lupus erythematosus; multidisciplinary approach to diagnosis and therapy. J Rheumatol 2012; 39: 2118–26. [DOI] [PubMed] [Google Scholar]