Abstract
Background
In a standard two-stage SMART design, the intermediate response to the first-stage intervention is measured at a fixed time point for all participants. Subsequently, responders and non-responders are re-randomized and the final outcome of interest is measured at the end of the study. To reduce the side effects and costs associated with first-stage interventions in a SMART design, we proposed a novel time-varying SMART design in which individuals are re-randomized to the second-stage interventions as soon as a pre-fixed intermediate response is observed. With this strategy, the duration of the first-stage intervention will vary.
Methods
We developed a time-varying mixed effects model and a joint model that allows for modeling the outcomes of interest (intermediate and final) and the random durations of the first-stage interventions simultaneously. The joint model borrows strength from the survival sub-model in which the duration of the first-stage intervention (i.e., time to response to the first-stage intervention) is modeled. We performed a simulation study to evaluate the statistical properties of these models.
Results
Our simulation results showed that the two modeling approaches were both able to provide good estimations of the means of the final outcomes of all the embedded interventions in a SMART. However, the joint modeling approach was more accurate for estimating the coefficients of first-stage interventions and time of the intervention.
Conclusion
We conclude that the joint modeling approach provides more accurate parameter estimates and a higher estimated coverage probability than the single time-varying mixed effects model, and we recommend the joint model for analyzing data generated from time-varying SMART designs. In addition, we showed that the proposed time-varying SMART design is cost-efficient and equally effective in selecting the optimal embedded adaptive intervention as the standard SMART design.
Electronic supplementary material
The online version of this article (doi:10.1186/s12874-016-0202-7) contains supplementary material, which is available to authorized users.
Keywords: Adaptive interventions, Sequential multiple assignment randomized trial (SMART), Time-varying mixed effects model (TVMEM), Longitudinal model, Joint model
Background
Sequential, multiple assignment, randomized trial (SMART) designs and their analysis are being used to construct high-quality adaptive interventions that can be individualized by repeatedly adjusting the intervention(s) over time on the basis of individual progress [1–5]. The SMART design was pioneered by Murphy, building on the work of Lavori and Dawson [6, 7]. SMART designs involve an initial randomization of individuals to different intervention options, followed by re-randomization of some or all of the individuals to another set of available interventions at the second stage. At subsequent stages, the probability and type of intervention to which individuals are re-randomized may depend on the information collected from the previous stage(s) (e.g., how well the patient responded to the previous treatment; adherence to treatment protocol). Thus, there can be several adaptive interventions embedded within each SMART design. This allows for testing the tailoring variables and the efficacy of the interventions in the same trial. There are several practical examples of SMART studies that have been conducted (e.g., the CATIE trial [8] for antipsychotic medications in patients with schizophrenia, STAR*D for the treatment of depression, [9, 10] and phase II trials at MD Anderson for treating cancer [11]). The goal of these studies is to optimize the long-term outcomes by incorporating the participant’s characteristics and intermediate outcomes evaluated during the intervention [12, 13].
An example of a two-stage SMART design is a study that characterized cognition in nonverbal children with autism [14]. To improve verbal capacity, participants were initially randomized to receive either a combination of behavioral interventions (Joint Attention Symbolic Play Engagement and Regulation (JASPER) + Enhanced Milieu Training (EMT)) or an augmented intervention (JASPER + EMT+ speech-generating device [SGD]). Children were assessed for early response versus slow response to the first-stage treatment at the end of 12 weeks. The second-stage interventions, administered for an additional 12 weeks, were chosen on the basis of the response status (only slow responders to JASPER + EMT were re-randomized to intensified JASPER + EMT or received the augmented JASPER + EMT + SGD; slow responders to JASPER + EMT + SGD received intensified treatment; all early responders continued on the same intervention). There were three pre-fixed assessment time points: at 12 weeks, 24 weeks and 36 weeks (follow-up), which were the same for all participants in the study. Compared to multiple, one-stage-at-a-time, randomized trials, SMART designs provide better ability to compare the impact of a sequence of treatments, rather than examining each piece individually. For example, a SMART allows us to detect possible delayed effects in which an intervention at a previous stage has an effect that is less likely to occur unless it is followed by a particular subsequent intervention option. The typical modeling approach for the SMART design as described by Nahum-Shani et al. includes the indicators of intervention at each stage as covariates and thus accounts for the delayed effects on the final response. In order to develop a sequence of best decision rules for each individual, various statistical learning methods of estimating the optimal dynamic treatment regimens have been proposed, among which Q-learning has been developed for assessing the relative quality of the intervention options and estimating the optimal (i.e., most effective) sequence of decision rules with linear regression. For a two-stage SMART, the Q-learning approach controls for the optimal second-stage intervention option when assessing the effect of the first-stage intervention, and reduces the potential bias resulting from unmeasured causes of both the tailored variables and the primary outcome. A similar approach for deriving the optimal decision rules for SMART is A-learning, which is more robust to model misspecification than Q-learning for consistent estimation of the optimal treatment regime [15]. Zhao et al. introduced the two learning methods of BOWL and SOWL, [16] which are based on directly maximizing over all dynamic treatment regimens (DTRs) a nonparametric estimator of the expected long-term outcome. As an alternative to the above learning approaches, Zhang et al. [17] proposed a robust estimation of the optimal dynamic treatment regimens for sequential treatment decisions, which maximizes a doubly robust augmented inverse probability weighted estimator for the population mean outcome over a restricted class of regimes. All these approaches model the outcomes of interest as dependent variables, and for the predictor variables, they model the main and interacting effects of the intervention options at each stage and the baseline individual characteristics. The amount of time an intervention is administered, however, is not explicitly modeled, although it can be used as a covariate in these regressions.
There are examples of SMART designs in which a participant is assessed at several pre-fixed time points during the first-stage treatment and once he/she meets an assigned criterion for response status, he/she is re-randomized to the second stage of treatment. Such a SMART design has been applied to develop a dynamic treatment regime for individuals with alcohol dependence using the medication naltrexone [2, 18, 19]. At the beginning of the study, patients were randomized to either a stringent or a lenient criterion for early non-response. Initially, all patients received naltrexone. Starting at the end of the second week, patients who showed early response were assessed weekly for eight weeks, and those who met the assigned criterion for non-response were assigned to the second stage randomization in that week; whereas the responders were re-randomized at week eight. Another example of using a SMART design to evaluate multiple, fixed time points is the study of pharmacological and behavioral treatments for children with ADHD, where children were assessed monthly for response or non-response [19–23]. In addition, Lu et al. [19] developed repeated-measures piecewise marginal models for comparing embedded treatments in such SMART designs with multiple evaluations at fixed time points. In these studies, subjects were assessed at fixed time points; thus, the time of treatment takes values along a finite set of time points.
Although, SMART designs with outcome assessments at fixed time points exist, there are advantages to administering a drug as soon as an individual achieves an intermediate response. For example, the smoking cessation drugs varenicline and bupropion can increase the risk of psychological side effects such as unusual changes in behavior, hostility, agitation, depressed mood and suicidal thoughts [24–26]. In addition, varenicline costs approximately $300 per month. Therefore, allowing the duration of treatment to vary among participants for one or more stages of the study may reduce the side effects and costs associated with the interventions. For such time-varying SMART designs, the duration of treatment plays an important role in decision making, and including it in the analysis may increase the power of the study and better serve our goal of analysis. To further extend the assignment strategies discussed in the above examples and utilize the information contained in the treatment duration, in this paper, we proposed a novel time-varying SMART design, which enables us to more efficiently assign different intervention options as soon as an individual achieves a set of intermediate response goals. Therefore, the time of treatment is a continuous random variable for each individual that can take any value on a subset of the positive real line, and is treated as an endogenous variable. The existing statistical methods are inappropriate for analyzing data obtained from such a time-varying SMART design. Therefore, to fully utilize the potential of this type of time-varying SMART design in making more efficient decisions, we also proposed two analytic approaches that can be used to analyze data from such a time-varying SMART design. The first approach is a linear mixed model with time-varying fixed effects [27, 28], which is in fact a piecewise linear model. The second approach incorporates a joint modeling method in which a survival model is fitted jointly with the linear mixed model [29]. We performed simulations to evaluate the statistical properties of both methods. Our simulation results showed that both methods estimated the expected final outcome for each embedded adaptive intervention in such design accurately, but the joint-modeling method provided better estimates for certain parameters in the model.
To compare the power and cost efficiency of the time-varying SMART design to those of an analogous standard SMART design, we simulated two trials with identical sample sizes and intervention effects using (a) the time-varying SMART design and (b) the standard SMART design. These simulations showed that the time-varying SMART design is cost-efficient and has power similar to that of the standard SMART design in selecting the optimal embedded adaptive intervention.
Methods
Proposed time-varying SMART design
Figures 1 and 2 illustrate the proposed time-varying designs. Both two-stage time-varying SMARTs were designed to provide data regarding how the intensity and combination of two types of interventions might be adapted to a subject’s progress in a cost- and time-efficient manner.
Fig. 1.
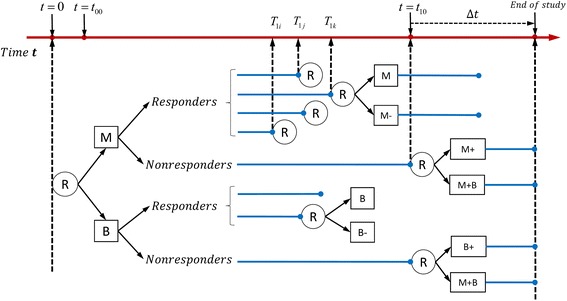
Example of time-varying two-stage SMART design with equal probability allocation: each participant is randomized twice
Fig. 2.
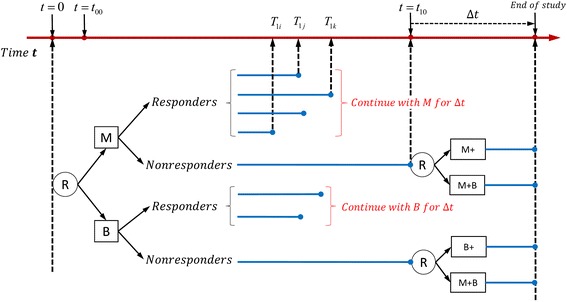
Example of time-varying two-stage SMART design with unequal probability allocation: only non-responders are re-randomized in the second stage
In the first example (see Fig. 1), suppose medication (M) and behavioral intervention (B) are two initial intervention options for individuals who are heavy smokers (e.g., those who smoke more than or equal to 25 cigarettes per day). The number of cigarettes a subject smokes per day is the outcome of interest and is measured at the beginning of the study, at several intermediate time points and at the end of the study. Let Y0 denote the number of cigarettes a subject smoked per day at the beginning of the study (t = 0). Subjects are randomly assigned to the medication or the behavioral interventions at the beginning of the study. Monitoring the outcome of interest begins at a pre-fixed time point (e.g. one week after the initial randomization and is denoted as t00) after the initial intervention is implemented, and t10 denotes the time point at which those who did not respond to a first-stage intervention are re-randomized. A subject is considered a responder to the first-stage intervention if there is a significant decrease in the number of cigarettes he/she smoked per day (e.g., the decrease in the number of cigarettes smoked per day is above a pre-fixed threshold, C) at an intermediate time point T1, before t10. Thus, T1 is a random variable of time and varies among responders. A subject is classified as a non-responder if the decrease in the number of cigarettes he or she smoked per day by t10 is below C. Therefore, all the non-responders are given the first-stage intervention for a fixed time period of t10 (e.g., the first month of initial interventions), which can be seen as the right-censored time point. Let Y1 denote the number of cigarettes smoked per day at the end of the first-stage intervention.
An indicator variable δ is defined as δ = I(T1 < t10), where I(⋅) is the indicator function that takes the value 1 if T1 < t10 (i.e., if the subject is a responder) and the value 0 if T1 ≥ t10 (i.e., if the subject is a non-responder). A responder is re-randomized either to continue with the first-stage intervention (M or B) or to receive the first-stage intervention at a reduced intensity (M- or B-); whereas a non-responder is re-randomized to receive the first-stage option at an increased intensity (M+ or B+) or augmented with the other type of intervention (i.e., adding a behavioral intervention for those who started with medication or adding medication for those who started with a behavioral intervention). We let all the subjects in this design stay on their second-stage interventions for a fixed time period, Δt (e.g., one month). Therefore, for a subject whose first-stage intervention time is T1, the total study time is T1 + Δt, which we denote as T2. For each participant, Y2 is the final measurement of the number of cigarettes smoked per day at T2; see Fig. 1).
The design illustrated in Fig. 2 is similar to that in Fig. 1 except that all the responders continue with their first-stage intervention options (i.e., each responder receives the same intervention after the response time point T1) in the second stage (see Fig. 2).
The adaptive interventions that are embedded within the two SMART designs in Figs. 1 and 2 are listed in Additional file 1: Tables S1 and S2.
Analytic approach
Let A1 and A2 be the indicators of the first- and second-stage intervention options, respectively. For each individual, we observe the data (Y0, A1, T1, Y1, A2, Y2, T2, δ). The outcomes of interest are the longitudinal measurements Y0, Y1, and Y2, which are fitted with a linear mixed model, assuming they share the same random intercepts at the subject level. Because the intervention options and their durations change over time in this design, we first proposed a straightforward time-varying mixed effects model (TVMEM) to analyze the outcomes. In this approach, the duration of time a treatment is administered is used as a covariate in the model. Such an approach is better than the approaches that ignore the time component of the intervention (i.e., the duration of the intervention influences its effect). However, the time duration is a random variable and one may gain statistical efficiency by treating it as a dependent variable in the modeling. Therefore, we also proposed a joint-modeling approach that simultaneously postulates a linear mixed effects model for the longitudinal measurements Y = (Y0, Y1, Y2) and a Cox model for the survival time T1. In particular, we fit a survival submodel for T1 jointly with the previously mentioned TVMEM that will efficiently extract the information contained in T1.
Analytic models
Time-varying mixed effects model of Y = (Y0, Y1, Y2)
A linear TVMEM is fitted to the longitudinal outcomes, with interventions and their interactions and durations included as predictors. For each individual i in the study, we have
| 1 |
where mi(t) is the unobserved true value of the longitudinal outcome at time point t, and bi is the subject-level random effects and is assumed to be normally distributed with a mean of zero and variance of σ2b; Zi is a vector of the baseline covariates (e.g., age, sex, comorbidities, etc.) with a corresponding vector of the regression coefficients η(t); Xi(t) is the vector of the first-stage and second-stage intervention options, their interactions, and duration of intervention with a corresponding vector of the regression coefficients β(t). Finally, εi(t) is the error term at time t and is assumed to be normally distributed and independent of bi.
In our study design, we consider three time points at which the outcomes of interest are measured: t = 0, T1i and T2i, where T1i and T2i are the respective time points at which individual i completes the first- and second-stage interventions. Therefore, A1i(t) takes the value of A1i at times T1i and T2i and is equal to 0 at t = 0, and A2i(t) takes the value of A2i at T2i and is equal to 0 at time points 0, and T1i. In this way, η(t) and β(t) are piecewise linear fixed coefficients; therefore, model (1) at the three time points is equivalent to the following three linear mixed-effects submodels:
| 2 |
| 3 |
and
| 4 |
where in equations (2) through (4), Y0i, Y1i and Y2i are the outcome values at time 0, T1i and T2i, respectively; A1i is the indicator of the first-stage intervention options (−1 for M and +1 for B), A2i = (A2Ri, A2NRi) is the indicator vector for the second-stage intervention options, where A2Ri is the indicator for the second-stage intervention options for the responders to the first-stage intervention (1 = continue the initial intervention; −1 = reduce the intensity of the initial intervention) and A2NRi is the indicator for the second-stage intervention options for the non-responders (1 = increase the initial intervention; −1 = augment the initial intervention with the other type of intervention), with A2Ri =0 for non-responders and A2NRi =0 for responders. A1i ⋅ A2Ri and A1i ⋅ A2NRi are the interaction effects of the first-stage intervention and second-stage intervention among responders and non-responders, respectively, in the submodel of Y2i (i.e., submodel (4)).
Parameters η0, η1, η2 and β00, β01, β02 are the coefficients of the baseline covariates and intercepts at time points 0, T1i and T2i, respectively; submodel (2) includes only baseline covariates as predictors for the outcomes at the beginning of the study (i.e., Y0i at t = 0); submodel (3) models the outcome of interest at the intermediate time point of the study (i.e., Y1i at t= T1i) and includes covariates A1i and T1i, for which the corresponding coefficients β11 and β31 account for the direct effect of A1i and indirect effects through T1i on Y1i; submodel (4) includes all the main and interacting effects of the intervention options at each stage and the duration T2i (T2i = T1i + Δt) as predictors, for which the coefficients β12 and β32 account for the delayed effect of A1i and delayed indirect effects of A1i through T2i. The coefficients β2 = (β22, β23) and β4 = (β41, β42) account for the effects of the second-stage interventions and the effects of their interactions with the first-stage interventions on the final outcome Y2i (measured at the end of the study, T2i).
The conditional expectations for models (1)-(4) are provided in Additional file 2. We also provided conditional expectations of the final outcomes for each of the eight embedded adaptive interventions in the SMART design of Fig. 1 and four embedded adaptive interventions in the SMART design of Fig. 2 [see Additional file 2].
Joint model
In addition to the TVMEM, we postulate a relative risk model for T1i (time to the event of interest) as
| 5 |
where Wi is a vector of the baseline covariates, which could be different from vector Zi in model (1), and h0(⋅) is the baseline risk function. The underlying longitudinal measurement mi(0) at baseline (i.e., at time point t = 0), as approximated by the TVMEM, and at the first-stage intervention A1i are included as predictors in model (5) because the time point at which an individual responds to the first-stage intervention (i.e., T1i) depends only on the type of first-stage intervention the subject received and the baseline characteristics.
We jointly estimate the coefficients in models (1) and (5) by using the maximum likelihood estimation method. To define the joint distribution of the time-to-event and longitudinal outcomes, we assume that the random effect bi underlies both the longitudinal and survival processes for each subject. This means that the random effect accounts for both the association between the longitudinal and event outcomes and the correlation between the repeated measurements in the longitudinal process. We also assume that the longitudinal outcomes {Y0i, Y1i, Y2i} are independent of the time T1i conditional on the random effect bi. Therefore, the joint likelihood contribution for the ith subject can be formulated as p(T1i, δi, Yi; θ) =
, where p{Yi(tij)|bi; β, η} is the univariate normal density for the longitudinal responses at time point tij, which is the element from the vector ti = {tsi}2s = 0 = {0, T1i, T2i}; p(bi; σb) is the normal density with standard deviation σb for the random effects bi; and p(T1i, δi|bi; β, γ, α, η) is the likelihood for the time to the intermediate outcome and can be written as p(T1i, δi|bi; β, γ, α, η) = Si(T1i|mi(0), A1i; β, γ, α, η) = , where δi = I(T1i < t10). Parameters in the model are estimated by maximizing the corresponding log-likelihood function with respect to (β, γ, α, η). We obtained the maximum likelihood estimates using the R package “JM” [30].
The parameters (β12, β22, β23, β32, β41, β42) in submodel (4) (i.e., the model of final outcome Y2) are of primary interest and were estimated using the two approaches described above.
The data organization and implementation of these methods is presented in Additional file 3.
Simulations
For the example illustrated in Fig. 1, we considered two simulation scenarios in which Y0 and Y1 were simulated using submodels (2) and (3), respectively, and Y2 was simulated with and without the interaction terms (A1i ⋅ A2Ri and A1i ⋅ A2NRi) in submodel (4). In both scenarios, we simulated 500 replicates of n = 1000 individuals, and randomly assigned subjects (with probability .5) to one of the two first-stage interventions (i.e., A1 to be equal to 1 [behavioral intervention] or −1 [medication]). Responders and non-responders to the initial interventions were then re-randomized (with probability .5) to one of the corresponding second-stage intervention options (i.e., A2R and A2NR were randomly assigned to be 1 or −1 and A2R =0 for non-responders and A2NR =0 for responders; see Fig. 1). In both scenarios, the random effects {bi}ni = 1 for subjects i = 1, 2, …, n were generated from the normal distribution with a mean of 0 and a standard deviation of 5, and baseline outcomes {Y0i}ni = 1 were simulated using submodel (2) with parameters β00 = 10 and ε0i ~ N(0, 42). The intermediate outcomes {Y1i}ni = 1 were simulated using submodel (3) with parameters β01 = 1, β11 = 0.2, and β31 = 0.1 in the first scenario; whereas outcomes {Y1i}ni = 1 in the second scenario were simulated with β01 = 1, β11 = 0.6, and β31 = 0.1, with a standard deviation of 5 (i.e., ε1i ~ N(0, 52) in both scenarios and satisfying the conditions Y0i − Y1i ≥ 9 (C = 9) if subject i is a responder and Y0i − Y1i < 9 if subject i(i = 1, 2, … n) is a non-responder.
The time points T1i were generated from a left-truncated Weibull distribution (truncated from t00 =0.1, the start time for monitoring), with shape = 1 and scale= exp{γ0 + γ1A1i + αmi(0)}, where γ0 = − 1.5, γ1 = 0.4, and α = 0.25, and those for whom T1i was greater than 1 (non-responders), were assigned T1i = t10 = 1 (the maximum time the first-stage intervention is administered [t10]). The indicator of response status was then defined by δi = I(T1i < 1). The final outcomes Y2i(i = 1, …, n) were generated using submodel (4), with ε2i ~ N(0, 52). The values of the other parameters in submodel (4) are reported in Table 1 (without interactions) and Table 3 (with interactions).
Table 1.
Simulation results for the design in Fig. 1: the estimated means, based on 500 replicates, are reported for coefficients in model (4)
| Parameter estimation | ||||||||
| β 12 | β 22 | β 23 | β 32 | |||||
| (first-stage interventions A 1) | (second-stage interventions for responders A 2R) | (second-stage interventions for non-responders A 2NR) | (time of intervention T 2) | |||||
| True value | 0.4 | 0.5 | 0.5 | 2 | ||||
| Joint Model | Estimate | 0.407 | 0.503 | 0.502 | 1.790 | |||
| MSE | 0.011 | 0.029 | 0.016 | 0.147 | ||||
| CI% | 97.8 % | 95.0 % | 96.8 % | 94.2 % | ||||
| Length of CI | 0.478 | 0.674 | 0.538 | 1.447 | ||||
| TVMEM | Estimate | 0.275 | 0.503 | 0.501 | 4.073 | |||
| MSE | 0.026 | 0.030 | 0.017 | 4.400 | ||||
| CI% | 88.0 % | 95.6 % | 97.0 % | 0.0 % | ||||
| Length of CI | 0.484 | 0.695 | 0.549 | 1.436 | ||||
CI%: Coverage probability of the 95 % confidence interval
MSE mean squared error
Table 3.
Simulation results for the design in Fig. 1: the estimated means, based on 500 replicates, are reported for coefficients in model (4) with interactions
| Parameter estimation | ||||||||
| β 12 | β 22 | β 23 | β 32 | β 41 | β 42 | |||
| (first-stage interventions A 1) | (second-stage interventions for responders A 2R) | (second-stage interventions for non-responders A 2NR) | (time of intervention T 2) | (interaction term A 1. A 2R) | (interaction term A 1. A 2NR) | |||
| True value | −0.4 | 0.5 | 0.4 | 2.0 | 0.55 | −0.40 | ||
| Joint model | Estimate | −0.381 | 0.490 | 0.389 | 1.626 | 0.542 | −0.397 | |
| MSE | 0.014 | 0.038 | 0.019 | 0.305 | 0.037 | 0.019 | ||
| CI% | 97.4 % | 95.6 % | 96.8 % | 87.0 % | 96.8 % | 98.0 % | ||
| Length of CI | 0.521 | 0.789 | 0.593 | 1.671 | 0.789 | 0.593 | ||
| TVMEM | Estimate | −0.530 | 0.489 | 0.390 | 4.122 | 0.542 | −0.396 | |
| MSE | 0.029 | 0.039 | 0.020 | 4.697 | 0.037 | 0.020 | ||
| CI% | 88.2 % | 95.8 % | 96.4 % | 0.0 % | 97.0 % | 97.8 % | ||
| Length of CI | 0.525 | 0.809 | 0.605 | 1.618 | 0.809 | 0.605 | ||
CI%: Coverage probability of the 95 % confidence interval
MSE mean squared error
For the intervention strategy depicted in Fig. 1, there are eight adaptive interventions imbedded in the design and represented by the three indicators A1, A2R, and A2NR. For example, in adaptive intervention (A1, A2R, A2NR) = (−1, 1, 1), participants are initially randomized to the medication (A1 = − 1); those who respond are re-randomized to continue on the medication (A2R = 1) and those who do not respond are re-randomized to increased medication (A2NR = 1). Another example of an adaptive intervention is (A1, A2R, A2NR) = (1, 1, −1), in which participants are initially randomized to a behavioral intervention (A1 = 1); those who respond are re-randomized to continue on the behavioral intervention (A2R = 1), and those who do not respond are re-randomized to an augmented arm (M + B, A2NR = − 1).
For the design in Fig. 2, only the non-responders are re-randomized in the second stage. Therefore, there are four embedded adaptive interventions in this design, which are represented by the vector of two indicators (A1, A2NR). For example (−1,−1) represents the adaptive intervention in which participants are initially randomized to medication (A1 = − 1) and those who do not respond are re-randomized to the augmented arm (M + B, A2NR = − 1), whereas responders continue on the medication arm.
Using this design, we also simulated the treatment of 1000 subjects. However, instead of using equal probability allocations as in Fig. 1, we used unequal probability allocations at both stages. Specifically, each of the 1000 subjects were initially assigned to either A1 = − 1 (medication) or A1 =1 (behavioral intervention) with probabilities 0.4 and 0.6, respectively. Then, the non-responders were re-allocated into either A2NR = − 1 (augmented first-stage intervention, M + B) or A2NR =1 (intensified first-stage intervention, M+ or B+) with probabilities 0.55 and 0.45, respectively; whereas all responders were continued on their initial interventions (therefore, A2R =0) in their second stage. Random effects (bi), errors (εi), and longitudinal outcomes (Y0i, Y1i(i = 1, …, n)) were generated as described for Fig. 1. The final outcomes, Y2i(i = 1, …, n), were also generated using submodel (4), but without the variable A2Ri, with the parameter values reported in Tables 5 and 7 for the two scenarios, respectively. In the first scenario, outcomes Y2i(i = 1, …, n) were simulated without interaction terms and with the parameter values shown in Table 5; in the second scenario, outcomes Y2i(i = 1, …, n) were simulated with interaction terms and with the parameter values shown in Table 7.
Table 5.
Simulation results for the design in Fig. 2: the estimated means, based on 500 replicates, are reported for coefficients in model (4)
| Parameter estimation | ||||
| β 12 | β 23 | β 32 | ||
| (first-stage interventions A 1) | (second-stage interventions for non-responders A 2NR) | (time of intervention T 2) | ||
| True value | 0.450 | 0.40 | 2.0 | |
| Joint model | Estimate | 0.456 | 0.441 | 1.767 |
| MSE | 0.013 | 0.017 | 0.168 | |
| CI% | 95.6 % | 95.6 % | 93.6 % | |
| Length of CI | 0.482 | 0.536 | 1.452 | |
| TVMEM | Estimate | 0.388 | 0.439 | 4.046 |
| MSE | 0.016 | 0.017 | 4.297 | |
| CI% | 94.0 % | 96.0 % | 0.0 % | |
| Length of CI | 0.489 | 0.547 | 1.442 | |
CI%: Coverage probability of the 95 % confidence interval
MSE mean squared error
Table 7.
Simulation results for the design in Fig. 2: the estimated means, based on 500 replicates, are reported for coefficients in model (4) with interactions
| Parameter estimation | |||||
| β 12 | β 23 | β 32 | β 42 | ||
| (first-stage intervention A 1) | (second-stage interventions for responder A 2NR) | (time of intervention T 2) | (interaction termA 1 A 2NR)) | ||
| True value | 0.4 | 0.4 | 2.0 | −0.4 | |
| Joint model | Estimate | 0.408 | 0.422 | 1.784 | −0.400 |
| MSE | 0.012 | 0.020 | 0.157 | 0.019 | |
| CI% | 98.2 % | 97.2 % | 95.6 % | 97.2 % | |
| Length of CI | 0.513 | 0.594 | 1.542 | 0.593 | |
| TVMEM | Estimate | 0.298 | 0.419 | 4.308 | −0.399 |
| MSE | 0.021 | 0.021 | 5.439 | 0.020 | |
| CI% | 94.8 % | 97.4 % | 0.0 % | 97.4 % | |
| Length of CI | 0.520 | 0.608 | 1.532 | 0.607 | |
CI%: Coverage probability of the 95 % confidence interval; MSE: mean squared error
An alternate simulation approach
For the design illustrated in Fig. 1, we performed an alternate simulation approach that does not simulate values for T1i from the Weibull distribution. Instead, we considered a situation in which values of Y1i are monitored and T1i is the value for which the Y1i crosses the pre-specified boundary condition for the first time. In this simulation approach, random effects {bi}ni = 1 and error terms ε0, ε1 and ε2 were all simulated the same way as described above. Baseline outcomes {Y0i}ni = 1 are simulated using submodel (2) with β00 = 2 and ε0i ~ N (0, 22). Furthermore, we defined an individual i, as a responder if he/she had a certain percentage reduction in the intermediate outcome value, Y1i, compared to his/her baseline value Y0i. This may be a more appropriate definition of responders in some practical scenarios than a simple reduction by a fixed amount (e.g., C = 9) as was used in the previous simulations. In this simulation, those with a 40 % reduction from their baseline values were considered responders. The parameter values used for submodel (3) were β01 = −2, β11 = −0.5, and β31 = 5 . For an individual i, we first simulated ε1i ~N (0, 22), and calculated T*1i for which the β01 + β11A1i + β31T*1i + bi + ε1i equals the 40 % reduction from Y0i, the baseline value. Therefore, we define T1i = t00, if T*1i < t00; T1i = T*1i, if t00 ≤ T*1i ≤ t10; and T1i = t00, if T*1i ≥ t10. Then, T1i is substituted in the right side of equation (3) to obtain the value of Y1i for the individual i(i = 1, …, n). The final outcomes Y2i(i = 1, …, n) were generated using submodel (4), with ε2i ~ N (0, 22). As previously, we simulated 500 replicates of n = 1000 individuals in each trial, and randomly assigned subjects (with probability 0.5) to one of the two first-stage interventions (i.e., A1 to be equal to 1 [behavioral intervention] or −1 [medication]). Responders and non-responders to the initial interventions were then re-randomized (with probability 0.5) to one of the corresponding second-stage intervention options (i.e., A2R and A2NR were randomly assigned to be 1 or −1 and A2R =0 for non-responders and A2NR =0 for responders; see Fig. 1).
We evaluated the performance of our two proposed analytic approaches in these simulated data sets by measuring the (a) means of the estimates of each of the adaptive interventions embedded in the design, (b) parameter estimates in the model, (c) mean squared error (MSE), (d) estimated coverage probability of the 95 % confidence interval, and (e) length of the confidence interval.
Using these simulations parameters, we simulated two trials with identical sample sizes: (a) the time-varying SMART design and (b) the standard SMART design. We evaluated the performance of the time-varying SMART design and an analogous standard SMART design by measuring the (a) power to select the optimal embedded intervention, and (b) associated cost.
Results
Tables 1-4 show the results of the two simulation scenarios based on the design shown in Fig. 1. Similarly, Tables 5-8 show the results of the two simulation scenarios for the design in Fig. 2.
Table 4.
Simulation results for the design in Fig. 1: the estimated means, based on 500 replicates, are reported for the final outcomes of the eight adaptive interventions embedded in the design with interactions in model (4)
| Mean of the final outcomes | ||||||||
| (−1,-1,-1) | (−1,-1,1) | (1,-1,-1) | (1,-1,1) | (−1,1,-1) | (−1,1,1) | (1,1,-1) | (1,1,1) | |
| Simulated means | 5.456 | 6.306 | 5.009 | 5.000 | 5.400 | 6.249 | 5.577 | 5.565 |
| Estimated means by Joint model | 5.456 | 6.306 | 5.009 | 5.000 | 5.400 | 6.249 | 5.577 | 5.565 |
| Estimated means by TVMEM | 5.456 | 6.306 | 5.009 | 5.000 | 5.400 | 6.249 | 5.577 | 5.565 |
Table 8.
Simulation results for the design in Fig. 2: the estimated means, based on 500 replicates, are reported for the final outcomes of the four adaptive interventions embedded in the design with interactions in model (4)
| Mean of the final outcomes | |||||
| (−1,1) | (−1,-1) | (1,1) | (1,-1) | ||
| Simulated means | 5.487 | 4.622 | 6.032 | 6.059 | |
| Estimated means by joint model | 5.502 | 4.610 | 6.047 | 6.046 | |
| Estimated means by TVMEM | 5.500 | 4.611 | 6.045 | 6.048 | |
In Table 1 the true parameters were the coefficient of the first-stage interventions, β12 = 0.4; coefficient of the second-stage intervention for responders, β22 = 0.5; coefficient of the second-stage intervention for non-responders, β23 = 0.5; and coefficient of T2, the total time of the first- and second-stage interventions, β32 = 2. The estimates obtained using TVMEM were and , while the estimates obtained using the joint model were and . Both approaches estimated coefficients β22 and β23 accurately. The parameters β12 and β32 were estimated accurately using the joint model, but poorly using the TVMEM. Similarly, in terms of the MSE, the length of the 95 % confidence interval, and the estimated coverage probability of the 95 % confidence interval, both approaches performed similarly for estimating β22 and β23, but joint modeling performed better for estimating β12 and β32. For example, for β12, the estimated coverage probability obtained using the TVMEM was 88 %; whereas that obtained from the joint model was 97.8 %.
For each of the eight embedded adaptive interventions in the design, Table 2 shows that both approaches accurately estimated the means of the final outcome, E[Y2|(A1, A2R, A2NR)]. For example, the simulated means of the adaptive interventions (A1, A2R, A2NR) = (−1, −1, − 1), (−1, 1, 1), and (1, 1, 1) were 4.538, 5.536, and 6.564, respectively, and the estimated means were 4.543, 5.531, and 6.569, respectively, using the TVMEM and joint model.
Table 2.
Simulation results for the design in Fig. 1: the estimated means, based on 500 replicates, are reported for the final outcomes of the eight adaptive interventions embedded in the design
| Mean of the final outcomes | ||||||||
| (−1,-1,-1) | (−1,-1,1) | (1,-1,-1) | (1,-1,1) | (−1,1,-1) | (−1,1,1) | (1,1,-1) | (1,1,1) | |
| Simulated means | 4.538 | 5.087 | 5.554 | 6.275 | 4.988 | 5.536 | 5.842 | 6.564 |
| Estimated means by Joint model | 4.543 | 5.093 | 5.549 | 6.269 | 4.982 | 5.531 | 5.849 | 6.569 |
| Estimated means by TVMEM | 4.543 | 5.093 | 5.549 | 6.269 | 4.982 | 5.531 | 5.849 | 6.569 |
Tables 3 and 4 show results similar to those in Tables 1 and 2, respectively. In Table 3, the coefficient of interaction of the first-stage interventions and second-stage interventions among responders is denoted by β41, and the coefficient of interaction of the first-stage interventions and second-stage interventions among non-responders is denoted by β42. As shown in Table 3, both TVMEM and joint modeling accurately estimated parameters β22, β23, β41, and β42, with little difference in the MSE, estimated coverage probability, and length of the 95 % confidence interval. However, as in Table 1, the joint modeling approach estimated β12 and β32 more accurately than the TVMEM approach. For example, the true coefficient of T2 was β32 = 2.0, which was poorly estimated as 4.122 using the TVMEM and estimated as 1.626 using the joint model. Table 4 shows that the estimated means of the eight adaptive interventions obtained from both analytical approaches were identical and close to the simulated means up to the third decimal.
Similar trends were observed in Tables 5-8 for the two simulations of Fig. 2. β12 and β32 were better estimated using the joint modeling approach, whereas all the other parameters and the means of the final outcomes of the four adaptive interventions embedded in the design were accurately estimated using both approaches.
In Table 5, the true coefficient values of β12 =0.450 and β32 =2.0 were estimated as and using the TVMEM, and as and using the joint model. Coefficient β23 was accurately estimated using both models. As for the four adaptive interventions (i.e. (A1, A2NR) = (−1,1), (−1,-1), (1, 1) and (1, −1)) embedded in the design of Fig. 2, Table 6 shows that the simulated means were 5.213, 4.802, 6.330, and 5.805, respectively, and the estimated means were 5.228, 4.790, 6.344, and 5.793, respectively, using the TVMEM, and 5.230, 4.788, 6.345, and 5.792, respectively, using the joint model.
Table 6.
Simulation results for the design in Fig. 2: the estimated means, based on 500 replicates, are reported for the final outcomes of the four adaptive interventions embedded in the design
| Mean of the final outcomes | ||||
| (−1,1) | (−1,-1) | (1,1) | (1,-1) | |
| Simulated means | 5.213 | 4.802 | 6.330 | 5.805 |
| Estimated means by joint model | 5.230 | 4.788 | 6.345 | 5.792 |
| Estimated means by TVMEM | 5.228 | 4.790 | 6.344 | 5.793 |
In Table 7 shows that the true parameters β12 =0.40 and β32 =2.0 were respectively estimated as =0.298 and =4.308 using the TVMEM, and as =0.408 and =1.784 using the joint model. The other two parameters, β23 and β42, were accurately estimated using both approaches. Table 8 shows that the means were accurately estimated using both approaches.
Tables 9 and 10 show the results from the alternative simulation strategy. In Table 9, the true coefficient values of β12 = −0.6 and β32 = −1.5 were estimated as = −0.534 and = −2.367 using the TVMEM, and as = −0.608 and = −1.338 using the joint model. Coefficients β22, β23 and the means of the final outcomes of the eight adaptive interventions embedded in the design were accurately estimated using both approaches (Table 10).
Table 9.
Simulation results from the alternative simulation approach: the estimated means, based on 500 replicates, are reported for coefficients in model (4)
| Parameter estimation | ||||||||
| β 12 | β 23 | β 32 | β 42 | |||||
| (first-stage intervention A 1) | (second-stage interventions for responder A 2NR) | (time of intervention T 2) | (interaction term A 1 . A 2NR) | |||||
| True value | −0.6 | 0.5 | 0.4 | −1.5 | ||||
| Joint model | Estimate | −0.608 | 0.492 | 0.447 | −1.338 | |||
| MSE | 0.002 | 0.003 | 0.018 | 0.034 | ||||
| CI% | 97 % | 96 % | 87 % | 70 % | ||||
| Length of CI | 0.199 | 0.192 | 0.376 | 0.411 | ||||
| TVMEM | Estimate | −0.534 | 0.492 | 0.448 | −2.367 | |||
| MSE | 0.007 | 0.003 | 0.020 | 0.763 | ||||
| CI% | 74 % | 93 % | 84 % | 0 % | ||||
| Length of CI | 0.197 | 0.191 | 0.376 | 0.529 | ||||
CI%: Coverage probability of the 95 % confidence interval
MSE mean squared error
Table 10.
Simulation results from the alternative simulation approach: the estimated means, based on 500 replicates, are reported for the final outcomes of the eight adaptive interventions embedded in the design
| Mean of the final outcomes | ||||||||
| (−1,-1,-1) | (−1,-1,1) | (1,-1,-1) | (1,-1,1) | (−1,1,-1) | (−1,1,1) | (1,1,-1) | (1,1,1) | |
| Simulated means | −0.208 | −0.070 | −1.589 | −1.356 | 0.678 | 0.810 | −0.918 | −0.689 |
| Estimated means by Joint model | −0.203 | −0.066 | −1.595 | −1.359 | 0.675 | 0.804 | −0.915 | −0.683 |
| Estimated means by TVMEM | −0.203 | −0.063 | −1.594 | −1.363 | 0.672 | 0.804 | −0.912 | −0.684 |
Comparison of power between the time-varying SMART design and the standard SMART design
We analyzed the time-varying SMART design’s ability to select the most optimal embedded intervention and compared the associated power to that of the standard SMART design. We performed the comparison by conducing two trials with identical sample sizes and intervention effects using (a) the time-varying SMART design and (b) the standard SMART design. Figure 3 represents the standard SMART design that is analogous to the time-varying SMART design depicted in Fig. 1. The major difference between the two designs is that in the time-varying SMART design, a responder is re-randomized to the second-stage intervention at a random response time T1 (< t10); whereas in the standard SMART design, everyone is re-randomized at a fixed time point t10. Responders are defined similarly in both designs. In our example, a subject is considered a responder to the first-stage intervention if there is a significant decrease in the number of cigarettes the person smoked per day. The second-stage intervention is identical for both designs.
Fig. 3.
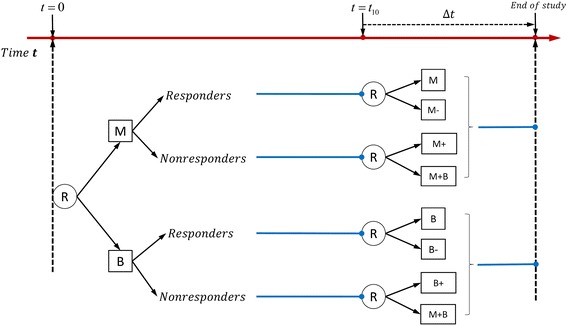
Example of standard SMART design with equal probability allocation: each participant is randomized twice
For both designs, we calculated the percentage of times that the best embedded intervention is selected (i.e., the power of the design). We simulated six parameter scenarios: the true parameters for the coefficient of the first-stage interventions, β21; coefficient of the second-stage intervention for responders, β22; coefficient of the second-stage intervention for non-responders, β23; coefficient of T2, the total time of the first- and second-stage interventions, β32; coefficient of interaction of the first-stage interventions and second-stage interventions among responders β41; and the coefficient of interaction of the first-stage interventions and second-stage interventions among non-responders β42. The simulated values of each of these parameters are reported in Tables 11 and 12. The simulation results are based on 500 replicates and are shown in Table 11 for comparing the two designs in Fig. 1 (time-varying SMART) and Fig. 3 (analogous standard SMART). Overall, both designs were equally effective in selecting the optimal embedded adaptive intervention. For example, when β21 = 0.4, β22 = 0.5, β23 = 0.5 and β32 = 2, using the joint model and implementing the time-varying SMART design showed 82.8 % power to select the optimal embedded adaptive intervention; whereas the power associated with the standard SMART design was 83.0 %. Similar results were obtained when comparing the time-varying SMART design in Fig. 2 and the standard SMART design in Fig. 4 (see Table 12).
Table 11.
| Comparison of designs in Figs. 1 and 3 | β 12 | β 22 | β 23 | β 32 | β 41 | β 42 | Power to select optimal embedded adaptive strategy | |
|---|---|---|---|---|---|---|---|---|
| Time-varying SMART | Standard SMART | |||||||
| Without interaction | 0.4 | 0.5 | 0.5 | 2 | 82.8 % | 83.0 % | ||
| 0.3 | −0.2 | 0.4 | 2 | 60.2 % | 59.2 % | |||
| 0.3 | −0.5 | 0.4 | 2 | 76.2 % | 75.0 % | |||
| With interaction | 0.4 | 0.5 | 0.5 | 2 | 0.5 | −0.3 | 99.2 % | 97.0 % |
| 0.6 | 0.5 | 0.4 | 2 | 0.2 | 0.2 | 63 % | 62.8 % | |
| 0.6 | −0.5 | −0.5 | 2 | 0.2 | −0.3 | 72.2 % | 73.4 % | |
Table 12.
| Comparison of designs in Figs. 2 and 4. | β 12 | β 23 | β 32 | β 42 | Power to select optimal embedded adaptive strategy | |
|---|---|---|---|---|---|---|
| Time-Varying SMART | Standard SMART | |||||
| Without interaction | 0.5 | 0.5 | 2 | 92.8 % | 90.6 % | |
| 0.45 | 0.4 | 2 | 86.6 % | 83.6 % | ||
| −0.2 | 0.2 | 2 | 68.4 % | 66.2 % | ||
| With interaction | 0.4 | 0.4 | 2 | −0.4 | 98.2 % | 97.4 % |
| 0.2 | 0.2 | 2 | −0.4 | 88.4 % | 87.6 % | |
| 0.4 | 0.1 | 2 | −0.25 | 77.4 % | 78.6 % | |
Fig. 4.
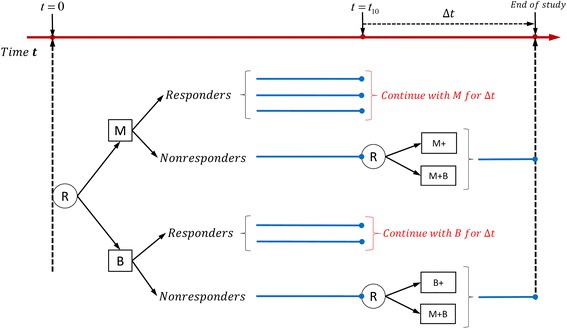
Example of standard SMART design: only non-responders are re-randomized in the second stage
Comparison of the cost associated with conducting the time-varying SMART design versus that associated with conducting the standard SMART design
To assess the cost associated with the conducting trials using these two competing designs, we considered a linear cost function for both SMART designs. Let c1 and c2 be the cost of the medication (M) and behavioral intervention (B), respectively. Additionally, we assumed that the reduced and increased intensity of the first-stage intervention are at half and twice the cost of the first-stage intervention, respectively, and that augmentation of the first-stage intervention in the second stage (M + B) has the cost c1 + c2. Using these parameters, the cost for the time-varying SMART design in Fig. 1 is
and the cost for the corresponding standard SMART in Fig. 3 is
Similarly, the cost for the time-varying SMART in Fig. 2 is
and the cost for the corresponding standard SMART in Fig. 4 is
Note that in the above equations, T1i = t10 for non-responders, and is the cost of the second stage for all the subjects assigned to the intervention M + B.
Figure 5 shows the cost as a function of c1 and c2, where red represents the cost of the time-varying SMART design and blue represents the cost of the standard SMART design. We can see that the cost of the time-varying SMART is less than the cost of the standard SMART in all scenarios. Table 13 shows the average costs and standard deviations calculated at select values of c1 and c2 based on 1000 replicates. For example, when the unit costs are c1 = 2 and c2 = 1 for medication and behavioral intervention, the average cost of the time-varying SMART in Fig. 1 is 3446.5, with standard deviation 49.87, while the average cost of the corresponding standard SMART is 3935.8, with standard deviation 41.47. Thus, the cost of the standard SMART is about 12 % higher than that of the time-varying SMART in this scenario.
Fig. 5.
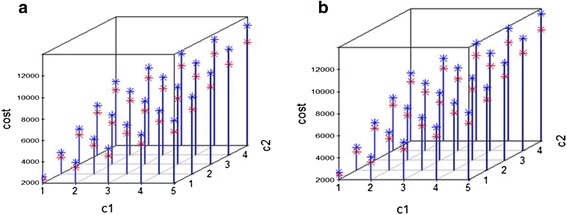
The cost associated with implementing a standard SMART (blue) and equivalent time-varying SMART (red)
Table 13.
Examples of the average cost for time-varying SMART and the standard SMART
| c 1 | c 2 | Average Cost(SD) | ||
|---|---|---|---|---|
| Time-varying SMART | Standard SMART | |||
| Design in Figs. 1 and 3: All the subjects are re-randomized | 2 | 1 | 3446.5(49.87) | 3935.8(41.47) |
| 1 | 1 | 2325.7(29.82) | 2631.4(19.15) | |
| 1 | 2 | 3526.5(57.00) | 3953.2(46.63) | |
| Design in Figs. 2 and 4: Only non-responders are re-randomized | 2 | 1 | 3593.4(45.40) | 4056.9(36.52) |
| 1 | 1 | 2416.1(24.12) | 2704.9(14.59) | |
| 1 | 2 | 3655.5(53.53) | 4056.7(42.77) | |
Discussion
In the standard SMART design, the timing of allocating the intervention is generally ignored, which leads to a model of regression without the predictor of a time variable. Therefore, in this article, we proposed a time-varying SMART design that allows the re-randomization to the second-stage interventions to occur at different time points for different individuals. The two modeling approaches we proposed for analyzing data using such time-varying SMART designs provided good estimations of the means of the final outcomes of all the embedded interventions. However, the joint modeling approach provided more accurate parameter estimates and higher estimated coverage probability than the TVMEM, and thus we recommend the joint model for analyzing data generated from time-varying SMART designs.
In the examples illustrated in Figs. 1 and 2, a participant was defined as a responder if there was a significant decrease in the number of cigarettes the participant smoked per day. One may question the validity of re-randomizing individuals who have a quick response to the first-stage intervention because such a response indicates the effectiveness of the intervention. However, if significant adverse effects are associated with the intervention (e.g., radiation therapy for many types of cancer is commonly associated with skin damage [31], fatigue [32, 33], diarrhea [34, 35], and rectal bleeding [36]), it is reasonable to shorten the duration of the intervention to avoid side effects. Therefore, the allocation strategy for the responders in the examples of the time-varying SMART design makes it more efficient than the standard SMART design.
We proposed two approaches for analyzing the longitudinal outcomes obtained from the time-varying SMART design: the TVMEM and the joint model. According to the simulation results, the joint modeling approach better estimated the effects of the duration of the intervention (i.e., T2) and the first-stage interventions (i.e., A1) in model (4). More specifically, the joint modeling approach had more accurate estimates, smaller MSEs, higher estimated coverage probabilities, and smaller 95 % confidence intervals (i.e., smaller estimated standard deviations) for the coefficients of the effects of the first-stage intervention and the time of intervention. Because we wanted to illustrate the cost efficiency of the proposed time-varying SMART design and its ability to select the optimal embedded adaptive intervention, we implemented a rather simplified linear mixed-effects submodels (2)-(4) of the more general TVMEM in model (1). We showed that the joint model performs better than the TVMEM in analyzing the data collected from such time-varying SMART designs. The joint modeling approach extracts part of the information contained in the time of the response, which is a function of the first-stage treatment assignment. Also, the association between the longitudinal and event outcomes is accounted for by the random effect that underlies both the longitudinal and survival processes for each subject. Therefore, although complex, time-varying SMART designs may require more complicated models for time and an extra layer of joint modeling, and as such one would expect a better performance from joint modeling in general. Nevertheless, both modeling approaches performed well in estimating the other parameters and the mean of the final outcomes for each adaptive intervention embedded in the corresponding designs. Furthermore, equation (1) is a general form of TVMEM, and in our study is equivalent to equations (2) ~ (4) at time points t = 0, T1i, T2i for each subject i. T1i is a subject-specific random variable, and coefficients in equation (3) can also be subject-specific. However, in practice, modeling coefficients to be subject-specific may lead to the estimation of too many parameters which, in some scenarios, may not be identifiable, particularly with small sample sizes. Therefore, as an initial attempt, we modeled T1i as a subject-specific random variable and the coefficients as fixed parameters. For example, coefficients β0(t), β1(t), β3(t) in equation (1) are fixed coefficients β01, β11, β31 in equation (3), as model (1) is equivalent to submodel (3) at time point T1i. More complicated models such as subject-specific and time-varying coefficients in submodels (2)-(4) can be considered, if the sample sizes are large.
We also illustrated the effectiveness of the joint modeling approach in accurately estimating the parameters even when no specific model was assumed for the duration of the first-stage intervention, T1i. The conclusions were qualitatively similar as that in the simulation where Weibull model was assumed for the duration of the first-stage intervention.
In the scenarios we considered here, the time at which individuals were re-randomized was assessed only for responders to the first-stage intervention. However, one may also consider varying times for the non-responders and for the second-stage interventions. For example, a non-responder showing severe side effects or no trend towards achieving intermediate goals may be re-randomized sooner than t10. The analytic approaches for such designs would be similar to the joint or time-varying mixed effects models proposed in this manuscript, for example, with an extra submodel for the duration of the second-stage interventions.
Instead of randomization with certain pre-defined probabilities (e.g., in the first two simulation scenarios, randomization with probability 0.5 was used for both stages; in the last two scenarios, unequal randomization with probabilities 0.4(0.6) and 0.55(0.45) was used for the two stages, respectively), information concerning potential moderators could be used to tailor and assign the interventions. For example, the choice of the first-stage intervention options could depend on the severity of the subject’s smoking habit at the beginning of the study; whereas the choice of the second-stage intervention option could depend on the subject’s adherence to the first-stage intervention. The analysis of such a randomization scheme would require assigning weights for each subject [37].
We also compared the cost and power associated with selecting the optimal embedded adaptive intervention for the proposed time-varying SMART design versus that for the analogous standard SMART design. Our simulation results showed similar power for the two designs. We used a linear cost function to assess the cost efficiency of the proposed design and found that it can have substantially lower cost than the standard design. Several other forms of cost functions can be used to assess cost efficiency. However, as long as the cost is an increasing function of time, the proposed time-varying SMART design will have lower cost than the standard SMART design. Therefore, the time-varying SMART design can be used to study how the intensity and combination of two types of interventions might be adapted to a subject’s progress in a cost- and time-efficient manner.
In our study, we assume that there is no unmeasured confounder. As suggested by Chakraborty and Murphy [38], the assumption of “no unmeasured confounders” holds in a SMART design if the randomization probabilities of A1 at most depend on the baseline covariates, and the randomization probabilities of A2 at most depend on the baseline covariates, the intermediate outcome, and A1. We performed additional simulations to investigate the role of unmeasured confounders on the parameter estimations. From these simulations, we see that when the unmeasured confounders affect only T1 and Y1, the parameter estimation is still accurate (Additional file 4: Table S4). However, when these unmeasured confounders affect Y2, there is bias in the estimation of T2 (Additional file 4: Tables S5-S6).
In the ADHD SMART study discussed by Nahum-Shani et al. [20], a weighted average was applied to the final outcomes when their primary goal of the study was to compare the imbedded adaptive intervention options in the SMART. In our Time-Varying SMART study, we used regression-based methods to identify more efficient adaptive decision rules for each subject along with their longitudinal outcomes. Similar to the analytic process of the standard SMART design by Q-learning in which a regression model for the outcome is postulated at each decision as a function of the patient’s information to that point, our TVMEM in equation (1) is equivalent to submodels (2)-(4) at three time points of longitudinal outcomes for each individual. Therefore, we did not include weights in this study of the time-varying SMART design. However, for increased complexity of time-varying SMART designs, weights may be incorporated into the analysis in a future study to develop more robust estimations and results.
Conclusion
The proposed time-varying two-stage SMART design can take into account the time associated with the first-stage interventions and thus could result in clinical trials with fewer side effects and lower cost. Additionally, the two modeling approaches we proposed are able to provide good estimations of the means of the final outcomes of all the embedded interventions. The joint modeling approach resulted in more accurate estimates and higher estimated coverage probabilities; therefore, we recommend using joint modeling to analyze data generated from the time-varying designs proposed in this manuscript.
Acknowledgements
We wish to thank the two reviewers for constructive comments on earlier version of the manuscript.
Funding
This work was supported in part by the National Institutes of Health (grants R01CA131324, R01DE022891, and R25DA026120 to S. Shete), and Cancer Prevention Research Institute of Texas grant RP130123 (SS). This research was supported, in part, by Barnhart Family Distinguished Professorship in Targeted Therapy (SS) and by the National Institutes of Health through Cancer Center Support Grant P30CA016672. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The data supporting the conclusions of this article are included within the article.
Authors’ contributions
Conception and design: TD, SS. Development of methodology: TD, SS. Simulation and methods implementation: TD. Writing, review, and/or revision of the manuscript: TD, SS. Study supervision: SS. Both authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Additional files
Embedded adaptive interventions in the SMART design of Figs. 1 and 2. Table S1. Eight embedded adaptive interventions in the SMART design of Fig. 1. Table S2. Four embedded adaptive interventions in the SMART design of Fig. 2, word document. (DOCX 14 kb)
Conditional expectation of TVMEM, word document. (DOCX 44 kb)
Data organization and Implementation. Table S3. Longitudinal data organization, word document. (DOCX 61 kb)
Table S4. The effect sizes associated with U1 and U2 influencing T1 and Y1 but not Y2. Table S5. The effect sizes associated with U1 and U2 influencing T1, Y1 and Y2. Table S6. The effect sizes associated with U1 and U2 influencing T1 and Y2 but not Y1, word document. (DOCX 49 kb)
Contributor Information
Tianjiao Dai, Email: tdai@mdanderson.org.
Sanjay Shete, Phone: (713) 745-2483, Email: sshete@mdanderson.org.
References
- 1.Almirall D, Nahum-Shani I, Sherwood NE, Murphy SA. Introduction to SMART designs for the development of adaptive interventions: with application to weight loss research. Transl Behav Med. 2014;4:260–74. doi: 10.1007/s13142-014-0265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dawson R, Lavori P.W. Placebo-free designs for evaluating new mental health treatments: the use of adaptive treatment strategies. Statistics in medicine. 2004;23:3249–3262. [DOI] [PubMed]
- 3.Murphy SA. An experimental design for the development of adaptive treatment strategies. Stat Med. 2005;24:1455–81. doi: 10.1002/sim.2022. [DOI] [PubMed] [Google Scholar]
- 4.Lavori PW, Dawson R. Introduction to dynamic treatment strategies and sequential multiple assignment randomization. Clin Trials. 2014;11:393–9. doi: 10.1177/1740774514527651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy SA, Collins LM, Rush AJ. Customizing treatment to the patient: adaptive treatment strategies. Drug Alcohol Depend. 2007;88(Suppl 2):S1–3. doi: 10.1016/j.drugalcdep.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lavori PW, Dawson R. Dynamic treatment regimes: practical design considerations. Clin Trials. 2004;1:9–20. doi: 10.1191/1740774S04cn002oa. [DOI] [PubMed] [Google Scholar]
- 7.Lavori PW, Dawson R, Rush AJ. Flexible treatment strategies in chronic disease: clinical and research implications. Biol Psychiatry. 2000;48:605–14. doi: 10.1016/S0006-3223(00)00946-X. [DOI] [PubMed] [Google Scholar]
- 8.Heinrichs RW. Cognitive improvement in response to antipsychotic drugs: neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64:631–2. doi: 10.1001/archpsyc.64.6.631. [DOI] [PubMed] [Google Scholar]
- 9.Rush AJ, Trivedi M, Fava M. Depression, IV: STAR*D treatment trial for depression. Am J Psychiatry. 2003;160:237. doi: 10.1176/appi.ajp.160.2.237. [DOI] [PubMed] [Google Scholar]
- 10.Lavori PW, Rush AJ, Wisniewski SR, Alpert J, Fava M, Kupfer DJ, et al. Strengthening clinical effectiveness trials: equipoise-stratified randomization. Biol Psychiatry. 2001;50:792–801. doi: 10.1016/S0006-3223(01)01223-9. [DOI] [PubMed] [Google Scholar]
- 11.Thall PF, Millikan RE, Sung HG. Evaluating multiple treatment courses in clinical trials. Stat Med. 2000;19:1011–28. doi: 10.1002/(SICI)1097-0258(20000430)19:8<1011::AID-SIM414>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 12.Collins LM, Murphy SA, Bierman KA. A conceptual framework for adaptive preventive interventions. Prevention Science. 2004;5:181-192. [DOI] [PMC free article] [PubMed]
- 13.Nahum-Shani I, Qian M, Almirall D, Pelham WE, Gnagy B, Fabiano GA, et al. Q-learning: a data analysis method for constructing adaptive interventions. Psychol Methods. 2012;17:478–94. doi: 10.1037/a0029373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasari C, Kaiser A, Goods K, Nietfeld J, Mathy P, Landa R, et al. Communication interventions for minimally verbal children with autism: a sequential multiple assignment randomized trial. J Am Acad Child Adolesc Psychiatry. 2014;53:635–46. doi: 10.1016/j.jaac.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulte PJ, Tsiatis AA, Laber EB, Davidian M. Q- and A-learning Methods for Estimating Optimal Dynamic Treatment Regimes. Stat Sci. 2014;29:640–61. doi: 10.1214/13-STS450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao YQ, Zeng D, Laber EB, Kosorok MR. New Statistical Learning Methods for Estimating Optimal Dynamic Treatment Regimes. J Am Stat Assoc. 2015;110:583–98. doi: 10.1080/01621459.2014.937488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang B, Tsiatis AA, Laber EB, Davidian M. Robust estimation of optimal dynamic treatment regimes for sequential treatment decisions. Biometrika. 2003;100:681–694. [DOI] [PMC free article] [PubMed]
- 18.Lu X, Lynch KG, Oslin DW, Murphy S. Comparing treatment policies with assistance from the structural nested mean model. Biometrics. 2016;72(1):10-19. [DOI] [PMC free article] [PubMed]
- 19.Lu X, Nahum-Shani I, Kasari C, Lynch KG, Oslin DW, Pelham WE et al. Comparing dynamic treatment regimes using repeated-measures outcomes: modeling considerations in SMART studies. Statistics in Medicine. 2016;35(10):1595-1615. [DOI] [PMC free article] [PubMed]
- 20.Nahum-Shani I, Qian M, Almirall D, Pelham WE, Gnagy B, Fabiano GA, et al. Experimental design and primary data analysis methods for comparing adaptive interventions. Psychol Methods. 2012;17:457–77. doi: 10.1037/a0029372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei H, Nahum-Shani I, Lynch K, Oslin D, Murphy SA. A “SMART” design for building individualized treatment sequences. Annu Rev Clin Psychol. 2012;8:21–48. doi: 10.1146/annurev-clinpsy-032511-143152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelham WE, Fabiano GA, Waxmonsky JG, Greiner AR, Gnagy EM, Pelham WE, et al. Treatment Sequencing for Childhood ADHD: A Multiple-Randomization Study of Adaptive Medication and Behavioral Interventions. Journal of Clinical Child and Adolescent Psychology. 45(4):396-415. doi:10.1080/15374416.2015.1105138. [DOI] [PMC free article] [PubMed]
- 23.Page TF, Pelham Iii WE, Fabiano GA, Greiner AR, Gnagy EM, Hart KC, et al. Comparative Cost Analysis of Sequential, Adaptive, Behavioral, Pharmacological, and Combined Treatments for Childhood ADHD. Journal of Clinical Child and Adolescent Psychology: the Official Journal For the Society of Clinical Child and Adolescent Psychology. American Psychological Association, Division 53. 1-12. PMID 26808137. doi:10.1080/15374416.2015.1055859. [DOI] [PMC free article] [PubMed]
- 24.Fagerstrom K, Hughes J. Varenicline in the treatment of tobacco dependence. Neuropsychiatr Dis Treat. 2008;4:353–63. doi: 10.2147/NDT.S927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ebbert JO, Wyatt KD, Hays JT, Klee EW, Hurt RD. Varenicline for smoking cessation: efficacy, safety, and treatment recommendations. Patient Prefer Adherence. 2010;4:355–62. doi: 10.2147/PPA.S10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cinciripini PM, Robinson JD, Karam-Hage M, Minnix JA, Lam C, Versace F, et al. Effects of varenicline and bupropion sustained-release use plus intensive smoking cessation counseling on prolonged abstinence from smoking and on depression, negative affect, and other symptoms of nicotine withdrawal. JAMA Psychiatry. 2013;70:522–33. doi: 10.1001/jamapsychiatry.2013.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan X, Shiyko MP, Li R, Li Y, Dierker L. A time-varying effect model for intensive longitudinal data. Psychol Methods. 2012;17:61–77. doi: 10.1037/a0025814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiyko MP, Lanza ST, Tan X, Li R, Shiffman S. Using the time-varying effect model (TVEM) to examine dynamic associations between negative affect and self confidence on smoking urges: differences between successful quitters and relapsers. Prev Sci. 2012;13:288–99. doi: 10.1007/s11121-011-0264-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henderson R, Diggle P, Dobson A. Joint modelling of longitudinal measurements and event time data. Biostatistics. 2000;1:465–80. doi: 10.1093/biostatistics/1.4.465. [DOI] [PubMed] [Google Scholar]
- 30.Rizopoulos D. JM: An R Package for the Joint Modelling of Longitudinal and Time-to-Event Data. J Stat Softw. 2015;35:1–33. [Google Scholar]
- 31.Collen EB, Mayer MN. Acute effects of radiation treatment: skin reactions. Can Vet J. 2006;47:931–5. [PMC free article] [PubMed] [Google Scholar]
- 32.Bower JE. Behavioral symptoms in patients with breast cancer and survivors. J Clin Oncol. 2008;26:768–77. doi: 10.1200/JCO.2007.14.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taunk NK, Haffty BG, Chen S, Khan AJ, Nelson C, Pierce D, et al. Comparison of radiation-induced fatigue across 3 different radiotherapeutic methods for early stage breast cancer. Cancer. 2011;117:4116–24. doi: 10.1002/cncr.26013. [DOI] [PubMed] [Google Scholar]
- 34.Hombrink J, Voss AC, Frohlich D, Glatzel M, Krauss A, Glaser FH. Therapy trends in the prevention of radiation-induced diarrhea after pelvic and abdominal irradiation. Results of a tricenter study. Strahlenther Onkol. 1995;171:49–53. [PubMed] [Google Scholar]
- 35.Harris K, Doyle M, Barnes EA, Sinclair E, Danjoux C, Barbera L, et al. Diarrhea as a radiation side effect “welcomed” by patients taking opioids. J Pain Symptom Manage. 2006;31:97–8. doi: 10.1016/j.jpainsymman.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Stacey R, Green JT. Radiation-induced small bowel disease: latest developments and clinical guidance. Ther Adv Chronic Dis. 2014;5:15–29. doi: 10.1177/2040622313510730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–60. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Chakraborty B, Murphy SA. Dynamic Treatment Regimes. Annu Rev Stat Appl. 2014;1:447–64. doi: 10.1146/annurev-statistics-022513-115553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the conclusions of this article are included within the article.


