Abstract
An invaluable part of the plastic surgeon's technical arsenal for soft tissue contouring, fat grafting continues to be plagued by unpredictable outcomes, resulting in either reoperation and/or patient dissatisfaction. Thus, extensive research has been conducted into the effects of adipose tissue procurement, processing, and placement on fat graft quality at both the cellular level and in terms of overall volume retention. Herein, we present an overview of the vast body of literature in these areas, with additional discussion of cell-assisted lipotransfer as a therapy to improve volume retention, and on the controversial use of autologous fat in the setting of prior irradiation.
Fat grafting has existed for over a century and has long been used for correction of both large and small volume deficits. The German surgeon Gustav Neuber first described the technique in 1893, reporting successful outcomes after transplanting fat beneath atrophic scars.1 Not long after, Vincent Czerny pioneered the use of autologous fat in breast surgery, employing a patient's own lipoma for post-mastectomy reconstruction.2 By 1914, fat grafting had been used for a variety of indications, ranging from craniofacial and breast reconstruction, to improvement of joint mobility after surgery for ankylosis.3 However, as surgeons continued to expand their use of fat grafting in clinical practice, they also began to note its limitations, chiefly the unpredictability of final volume retention. In his 1956 paper, Lyndon Peer found original adipocyte survival to be approximately 50% among free fat grafts, noting that increased trauma/mechanical handling negatively influenced volume retention.4
Initially described in the early 1980s, the widespread adoption of Illouz's variation of suction-assisted lipectomy meant an increase in the availability of autologous fat for grafting, in spite of the still unresolved questions concerning outcomes.5 Coleman's description of “lipostructure” represented the first attempt to address the variability of final volume retention via a standardized protocol for the processing and placement of lipoaspirate.6 However, close to two decades later, surgeons still report a wide range of fat graft resorption rates − from 10% to 90% − inspiring a large body of research into innovations in fat graft procurement, processing, and placement for optimization of the procedure.7-9 In the following review, we discuss some of the advancements in scientific understanding that have been made in each of these areas, in addition to what is known about the influence of recipient site on autologous fat graft survival (Table 1).
Table 1.
Summary of Key Issues Regarding Fat Grafting, From Processing to Placement in Recipient Site
| Procurement |
|
| Processing |
|
| Placement |
|
| Recipient site |
|
PROCUREMENT
Tumescent Solution
Nearly every step of autologous fat grafting has the potential to influence graft outcomes. While patient donor site has not been shown to significantly impact ultimate fat volume retention, donor site preparation − namely, the use of lidocaine-containing tumescent solution − has been demonstrated to affect harvested fat if not sufficiently cleared.10,11 Lidocaine alone has been associated with decreased adipocyte function, with Moore et al finding transient changes to lipolysis and glucose transport in the presence of local anesthetic.12 Interestingly, removal of lidocaine through washing harvested lipoaspirate returned these levels to normal. The effects of local anesthetic containing tumescent solution on fat graft retention have been confirmed in xenograft models, with quality of lipografts greatly improved following multiple washes and centriguation.13,14 In fact, Livaoglu et al evaluated the long-term effects (maximum 180 days postoperatively) of the use of lidocaine plus epinephrine and prilocaine in a xenograft model of excisional fat grafting, finding increased fibrosis and necrosis in grafts that had received injection with, but no removal of, the anesthetic-containing solution.15
Type of Liposuction
Current literature describes a newly-placed fat graft as consisting of three zones: an outer, “surviving” zone, an intermediate, “regenerating” zone, and a central, necrotic zone.16 According to Eto et al, the overall volume of a fat graft retained depends on the degree of survival of the regenerating zone, which contains adipose derived stromal cells (ASCs) with the potential for differentiation and replacement of adipocytes lost in the necrotic zone.16 Using a mouse model of autologous fat transfer, Kato et al highlighted the importance of ASCs in this process, noting that, with the exception of those in the surviving zone, all graft adipocytes died and were replaced by differentiation of ASCs within the regenerating zone.17 The integral role of ASCs in fat graft survival has been further substantiated by Phillips et al, who found a strong correlation between fat graft survival in a xenograft model and the prevalence of endogenous CD34+ cells within the grafted lipoaspirate (ASCs).18 In addition to contributing to adipogenesis within transplanted adipose tissue, ASCs have also been implicated in encouraging graft revascularization via paracrine effects.18,19
Given these findings, it is not surprising that the ability of liposuction techniques to preserve both adipocyte and ASC viability within aspirated fat has been of concern. In addition to the traditionally-used suction-assisted liposuction (SAL), more complex methods of aspirating fat are increasingly being employed by surgeons. These include ultrasound-assisted liposuction (UAL), laser-assisted liposuction (LAL), and mechanically-assisted liposuction (MAL), among others.20 Such techniques are ostensibly designed to prioritize relatively easy removal of adipose tissue with minimization of patient complications, not preservation of cellular and tissue integrity. Thus, several studies have investigated the effects of liposuction technique on the quality of fat obtained, and the subsequent implications for fat grafting.
Our own laboratory has investigated biological properties and differentiation capacity of ASCs obtained from SAL or UAL lipoaspirate.21 As UAL utilizes high-frequency sound waves for targeted ablation of adipocytes prior to aspiration of fat, the potential benefits of localized, less traumatic removal of adipose tissue are counterbalanced by reports of the potential for thermal injury and seroma formation with UAL.22-24 Interestingly, we found that exposure to ultrasound during liposuction did not affect the in vitro osteogenic differentiation potential of ASCs.21 Unpublished findings from our laboratory have also similarly found no significant differences in adipogenic potential of ASCs from SAL and UAL lipoaspirate. This has been further corroborated by preliminary in vivo studies, with ASCs obtained from various lipoaspiration techniques equivalently enhancing cutaneous healing in a murine wound model. All of these findings parallel investigations by Fisher et al, who have shown no significant differences in the resorption rates of SAL- vs UAL-derived fat grafts in a xenograft model.25
These results are in contrast to what we have observed with LAL, which was associated with decreased ASC yield, viability, and proliferation in vitro in comparison to ASCs derived from SAL. Furthermore, though the in vitro differentiation potential of LAL-ASCs was not impaired, the cells demonstrated significantly reduced capacity to heal calvarial defects in vivo.26
Studies investigating the effects of MAL on adipose tissue have focused less on the functional consequences of the liposuction method, instead highlighting effects on the cellular composition of SVF. Bajek et al recently compared ASC surface marker expression between cells obtained from SAL and MAL; while they concluded that there were no significant differences in marker expression between the two cell populations, it is interesting to note that they observed significantly higher CD34 expression in UAL-ASCs (approximately 70% vs 25% in MAL-ASCs).27 While these findings were limited to cultured ASCs, which exhibit in vitro phenotypic drift over time in terms of surface marker expression, it would be interesting to determine whether those differences in prevalence of CD34+ cells are also present in freshly-isolated stromal vascular fraction (SVF) cells. A newer liposuction technique, water-assisted liposuction (WAL), has not only been found to maintain the viability of aspirated adipose tissue, but also facilitate the isolation of SVF with a higher proportion of CD34+ cells compared to traditional liposuction, making it a particularly promising method for fat grafting.28,29
Cannula Size and Harvesting Pressure
Independent of the overall size of a fat graft, the volume of the individual pieces, or “particles,” of adipose tissue within the graft, has been shown to impact overall retention. Gause et al defined a fat particle as an intact piece of adipose tissue consisting of undisturbed adipocytes and stromal cells.30 Though optimal particle dimensions have yet to be determined, the consensus thus far is that the size must be large enough to preserve necessary cellular components (ie, adipocytes and stromal cells) in some anatomical relationship, but small enough so as to not limit diffusion of nutrients.17,30 Intimately related to particle size is cannula size: larger harvesting cannulas facilitate the collection of larger fat particles and have been shown to facilitate better adipocyte viability and overall volume retention.31,32 For example, Erdim et al found that fat harvested with a 6 mm cannula was more viable than tissue harvested using 2 and 4 mm cannulas.33 Similarly, Kirkham et al found that fat grafts harvested with a 5 mm cannula underwent less resorption than those harvested with a 3 mm cannula.31
Mechanistically, this may be explained by the fact that larger diameter cannulas result in less shear stress, and more laminar flow of fat, leading to decreased tissue disruption during procurement.34 Indeed, the biomechanics of fat grafting play a critical role in outcomes: the amount of negative pressure applied during fat procurement has also been shown to play a role in affecting long-term retention. While some studies have found that negative pressure during liposuction begins to adversely affect adipose tissue after a threshold level of −700 mmHg is reached, other histological studies suggest that adipocyte deformation begins at pressures as low as −200 mmHg.35,36 Supporting this, Cheriyan et al demonstrated enhanced adipocyte viability when using a low pressure (−250 mmHg) as opposed to high pressure (−760 mmHg) procurement technique.37
PROCESSING
Preparation of Fat for Grafting
After collection, lipoaspirate is typically processed for removal of the oil and aqueous portions in order to isolate the adipose stroma for grafting. Various strategies for this exist, including centrifugation, decantation, filtration, and mesh/gauze rolling, with multiple studies having been conducted to determine the most appropriate processing technique. Centrifugation remains the most popular methodology for separation of these components, with the Coleman technique recommending centrifugation for 3 minutes at 1200 relative centrifugal force (rcf).6 However, though undoubtedly effective in concentrating the adipose stroma for grafting, concern exists regarding the potential detrimental effects of centrifugal force on adipocyte and ASC viability.38,39 For this reason, some research groups have investigated the use of low centrifuge speeds in lipoaspirate processing, though their findings are conflicting, with direct comparisons between studies complicated by the reporting of centrifuge speeds in rpm instead of rcf.39-41 And while controversial, some investigations of high centrifuge speeds suggests that there may be a threshold effect, with speeds >3000 rcf decreasing adipocyte viability and the number of ASCs present within fat grafts.42,43
Less strenuous methods such as gauze rolling and filtration have also been investigated for lipoaspirate processing. Fisher et al found gauze rolling to be superior to centrifugation (1200 rcf as prescribed by the Coleman technique) in terms of SVF cell yield and fat graft retention, though they recommended filtration and centrifugation as equally viable alternatives when processing large volumes of tissue.25 In contrast, Salinas et al found mesh/gauze filtration to be equivalent to centrifugation at 1200 rcf with respect to both ASC yield and fat graft retention. Interestingly, it is worth noting that they analyzed retention of 1 mL grafts at 4 weeks as opposed to Fisher et al, who studied 2 mL grafts at 6 weeks.44 Zhu et al evaluated the in vitro viability of fat processed using multiple methodologies, including an automated system for washing and filtration of adipose tissue. They found that system (Puregraft; Cytori Therapeutics, Inc., San Diego, CA) to provide superior removal of extraneous blood and oil, along with improved adipose tissue viability.45 The conflicting results of these various studies on which methodology is best appears to be representative of the larger body of literature: a recent systematic review collated the findings from 13 studies comparing fat processing methods, and determined that no general recommendation could be made as to which technique was superior.46
The difficulty in determining the optimum processing technique is likely due to multiple factors, including technical differences in how each processing technique is performed. For example, gauze rolling as described by Pfaff et al consisted of gently rolling lipoaspirate on a nonstick gauze pad for approximately 30 seconds, until excess blood and oil had been “sufficiently removed.”41 In contrast, Fisher et al defined “gauze rolling” as using a scalpel handle to gently knead and roll fat on a nonstick dressing for a total of 5 minutes.25 Finally, when evaluating both ASC content and overall fat graft retention rates, it is important to consider patient specific differences. Our own laboratory has noted significant variability in ASC surface marker expression between patients, and patient age may be associated with some differences in both ASC and adipocyte viability.47-49 Furthermore, ASCs themselves are a significantly heterogenous cell population, and just as there exist pro-osteogenic and pro-adipogenic ASCs, so might there be differing subpopulations within adipose tissue with the potential to facilitate enhanced fat graft retention.50,51
ASCs and Cell Assisted Lipotransfer
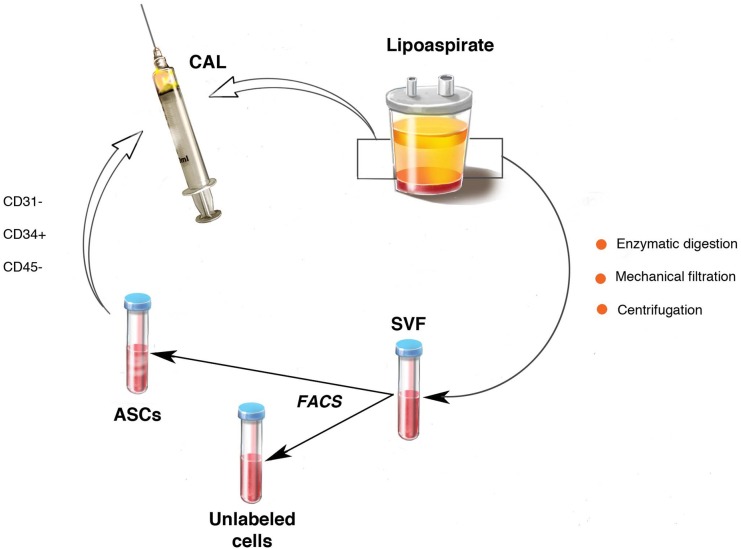
Recent reports have suggested supplementation of fat grafts with autologous cells to enhance graft volume retention. First described by Matsumoto et al in 2006, cell-assisted lipotransfer (CAL) is the process of enriching lipoaspirate with additional ASCs prior to fat grafting (Figure 1).52 This technique was borne out of the observation that collecting and processing lipoaspirate may deplete the native ASC population and contribute to unpredictable and often poor volume retention.53,54 Thus, it was reasoned that supplementation of lipoaspirate with additional ASCs would restore this deficiency and allow for improved fat graft survival.52 Clinical reports have supported this theory, with multiple studies demonstrating CAL's success in settings ranging from large volume fat grafting for breast reconstruction, to small volume reconstruction of craniofacial assymetries.54-57
Figure 1.
Adipose tissue can be prepared for cell-assisted lipotransfer by combination with ASCs isolated via flow cytometry from autologous SVF.
Though the precise role of ASCs in improving fat graft retention has yet to be determined, two hypotheses exist. The first is that, given their multipotent nature, ASCs are able to differentiate into adipocytes for replacement of those lost due to apoptosis or necrosis.16,17 The second is that, due to their ability to release pro-angiogenic growth factors such as vascular endothelial growth factor, ASCs promote angiogenesis and thus revascularization of free fat grafts. This second hypothesis has been supported by findings from our laboratory, which observed increased vascularity in fat grafts supplemented with SVF cells compared to unsupplemented grafts.58 Furthermore, re-isolation of supplemental cells following grafting revealed that the majority of ASCs did not persist in the grafts beyond 2 weeks, and that they expressed genes associated with angiogenesis rather than adipogenesis.19
In spite of these findings, there are also varied reports of CAL not being effective for improving fat graft retention, suggesting that, like any technique, CAL must be optimized before it is widely implemented into clinical use.59,60 One intrinsic parameter for optimization is the ratio of cells to fat that will lead to optimum fat graft retention. This has been investigated by our laboratory, which found that a concentration of 1 × 104 SVF cells per 200 µL fat graft improved retention by approximately 20% in a xenograft model of CAL.58 Similarly, Li et al found a concentration of 2 × 104 ASCs per 200 µL fat provided maximum graft retention when combined with platelet rich plasma, suggesting that, with further research, CAL may be optimized to serve as a strategy to ensure predictable retention rates.61
PLACEMENT
While we have thus far discussed the effects of liposuction procurement and lipoaspiration processing, the subsequent steps involved in fat graft placement have also been shown to significantly impact volume retention. As we have seen previously, the amount of shear stress and negative pressure present during adipose tissue procurement critically affect the overall quality of the fat graft. Similarly, shear stress is also applied during graft placement and may affect adipocyte viability. In light of this, larger injection cannulas may reduce shear stress during fat graft placement. Alternatively, Lee et al found that lowering of injection speed could be used to adjust flow rate, leading to improved volume retention.35 However, standardizing injection speeds amongst multiple surgeons is relatively impractical; thus, use of a low-shear automated injection device for fat grafting may improve outcomes. Studies evaluating such a device (Advanced Adipose Tissue Injector (ATI); Lifecell Corporation, Bridgewater, NJ) have shown that delivery of standardized volumes of fat with minimal shear stress during the injection process retained greater volume in vivo when compared to fat injected using a modified Coleman technique.62
In order to explain these findings, further analysis of the mechanical properties of fat grafts prepared using the ATI in comparison to the modified Coleman technique indicated that the standardized, low-shear stress conditions provided by the ATI allowed for preservation of the intrinsic structural properties of adipose tissue.63 This preservation of the structural characteristics of adipose tissue likely has larger implications for the preservation of resident adipocytes and ASCs. Whether considering hematopoietic stem cells or adipocytes, the niche, or cellular microenvironment, is integral to cell functioning.64 In fact, it has recently been shown that decellularized fat may serve as a scaffold and be repopulated and revascularized after implantation, demonstrating the importance of the mechanical aspects of the adipose-specific niche.65 The biomechanics of fat transfer therefore should not be ignored, and represent an appropriate lens through which to view the effects of fat procurement, processing, and placement.
Standardized delivery of small fat volumes may also enhance outcomes, as injection of larger parcels of fat, a particular concern with larger injection cannulas, may result in poor nutrient diffusion and greater ultimate resorption. Studies have confirmed small droplets of fat provide better take than larger ones.6,66,67 With this in mind, adherence to delivery of small aliquots of fat may still allow for reasonable retention of large total volumes seen with fat grafting for breast reconstruction.68,69
Fat Grafting the Recipient Site
Indications for fat grafting necessitate full evaluation of the recipient site, with consideration of how the soft tissue/skin envelope relates to anticipated volume placed and the local vascularity to support grafted fat.70 Realization of different recipient site demands have thus made the approach to fat grafting more complex. Particularly when the expected volume of fat grafting exceeds the capacity of the recipient site, multiple staged procedures may be necessary. Alternatively, pre-graft recipient site preparation may be considered, with negative pressure-induced pre-expansion of the skin envelope showing promise to facilitate large volume fat grafting of the breast.71,72
Many reconstructive indications for fat grafting also require working in damaged areas of the body, including those affected by prior radiation. The chronic skin changes resulting from irradiation can be broadly characterized by thickening and hypovascularity in the setting of an underlying inflammatory state.73,74 While hypovascularity is ostensibly the most concerning issue from the standpoint of a surgeon utilizing autologous fat grafting this, in combination with a fibrotic, dysfunctional dermis, predisposes patients to the development of wounds and abnormal healing.74 Interestingly, however, while fat grafts in irradiated recipient sites may demonstrate poor take, subcutaneous fat grafting has been demonstrated to restore the quality of overlying, irradiated skin.75 Fat grafting beneath skin showing the effects of chronic, radiation-induced fibrosis has been found to reverse some of the pathology seen, normalizing dermal thickness and significantly decreasing collagen content by 8 weeks post-grafting in a xenograft model. Additionally, the vascularity of irradiated skin overlying fat grafts was found to be significantly improved just 2 weeks after graft placement, likely due to pro-angiogenic paracrine signaling by resident ASCs within the adipose tissue.73 As fat graft volume retention is still an important consideration for breast reconstruction in irradiated, post-mastectomy patients, our laboratory has recently interrogated CAL as a technique by which to improve fat graft take in the setting of chronic radiation skin changes. Findings from our laboratory have determined that SVF-supplementation not only improves the restoration of quality of irradiated skin, but also improves volume retention.76
In the Setting of Prior Malignancy
Though highly promising as a technique to ameliorate the pathological state of irradiated skin, and undoubtedly useful in reconstruction of the contour deformities that accompany mastectomies or other cancer resections, the safety of fat grafting in irradiated sites remains somewhat unclear. This is due to the ASC content of grafted adipose tissue, which, though critical to the survival and integration of a fat graft, is concerning in the setting of prior malignancy, as varying reports have suggested a potential for ASCs to stimulate tumor recurrence.77,78 This controversy is further magnified in the setting of CAL, where supplemental ASCs are freely added to tissue prior to grafting.
A recent systematic review examining studies on the relationship between ASCs and malignancy over the past 14 years determined that ASCs are associated with promotion of tumor survival, particularly via pro-angiogenic effects that are particularly advantageous in hypoxic microenvironments.79 Other mechanisms by which ASCs may exert pro-tumorigenic effects include secretion of proliferative factors, as well as pro-metastatic factors.80-82 That said, there are also reports of anti-cancer effects of ASCs, most notably from in vitro experiments involving breast cancer, pancreatic cancer, and melanoma cell lines.83-85 Finally, though additional studies are certainly needed in order to precisely gauge tumor risk with use of ASCs in reconstructive surgery, there were no reports of breast cancer recurrence in either the RESTORE I or RESTORE II clinical trials, which evaluated the use of CAL in breast reconstruction over a follow-up period of one year.86
CONCLUSION
While theoretically a simple procedure, unpredictable retention rates have made fat grafting a challenge to surgeons for over a century. This has led to investigations into the effects of every step of the procedure, from procurement, to processing, to placement, and with regard to the recipient site, as well as both adipocytes and ASCs, the cells thought to ultimately facilitate graft survival. Several systematic reviews have attempted to determine the most critical factors in determining fat grafting outcomes.11,87,88 While comprehensive, these reviews stated similar limitations to those we have found when examining the fat grafting literature: namely, experimental studies outnumber clinical studies, with study results being difficult to compare across research groups due to the varied practices used in the many steps of fat grafting. There is a clear need for a greater number of comprehensive clinical studies, particularly as pertaining to the use of CAL, in order for the field to truly advance. That is not to say that we are not moving forward – while in 2009, the ASPS Fat Grafting Task Force recommended use of 3 or 4 mm blunt cannulas for fat harvesting, studies published in more recent years have found 5 and 6 mm cannulas to provide superior fat graft viability.11,31,33
Given the current literature, it is our opinion that the most critical of the steps involved in autologous fat grafting is fat placement, with special care to provide low shear stress conditions needed for optimum fat graft retention. The second most effective way to facilitate improved fat graft retention would appear to be the addition of supplemental ASCs. With continued advances in the development of automated ASC processing systems for efficient, intraoperative isolation of cells, along with further studies to elucidate the safety considerations when utilizing CAL in cancer reconstruction, we can expect increasing adoption of this technique in cosmetic and reconstructive settings. Overall, as we continue to improve our understanding of the science of fat grafting, we can continue to innovate and adjust our surgical techniques to see improved clinical outcomes.
Disclosures
Dr Longaker has an equity in the TauTona Group (Menlo Park, CA), which developed and sold the Advanced Adipose Tissue Injector to the LifeCell Corporation (Branchburg, NJ). None of the other authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
REFERENCES
- 1.Mazzola RF, Mazzola IC. The fascinating history of fat grafting. J Craniofac Surg. 2013;244:1069-1071. [DOI] [PubMed] [Google Scholar]
- 2.Uroskie TW, Colen LB. History of breast reconstruction. Semin Plast Surg. 2004;182:65-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lexer E. Free Transplantation. Ann Surg. 1914;602:166-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peer LA. The neglected free fat graft. Plast Reconstr Surg (1946). 1956;184:233-250. [DOI] [PubMed] [Google Scholar]
- 5.Illouz YG. Body contouring by lipolysis: a 5-year experience with over 3000 cases. Plast Reconstr Surg. 1983;725:591-597. [DOI] [PubMed] [Google Scholar]
- 6.Coleman SR. Facial recontouring with lipostructure. Clin Plast Surg. 1997;242:347-367. [PubMed] [Google Scholar]
- 7.Herold C, Ueberreiter K, Busche MN, Vogt PM. Autologous fat transplantation: volumetric tools for estimation of volume survival. A systematic review. Aesthetic Plast Surg. 2013;372:380-387. [DOI] [PubMed] [Google Scholar]
- 8.Ross RJ, Shayan R, Mutimer KL, Ashton MW. Autologous fat grafting: current state of the art and critical review. Ann Plast Surg. 2014;733:352-357. [DOI] [PubMed] [Google Scholar]
- 9.Wetterau M, Szpalski C, Hazen A, Warren SM. Autologous fat grafting and facial reconstruction. J Craniofac Surg. 2012;231:315-318. [DOI] [PubMed] [Google Scholar]
- 10.Small K, Choi M, Petruolo O, Lee C, Karp N. Is there an ideal donor site of fat for secondary breast reconstruction? Aesthet Surg J. 2014;344:545-550. [DOI] [PubMed] [Google Scholar]
- 11.Gutowski KA, Force AFGT. Current applications and safety of autologous fat grafts: a report of the ASPS fat graft task force. Plast Reconstr Surg. 2009;1241:272-280. [DOI] [PubMed] [Google Scholar]
- 12.Moore JH, Jr., Kolaczynski JW, Morales LM, et al. Viability of fat obtained by syringe suction lipectomy: effects of local anesthesia with lidocaine. Aesthetic Plast Surg. 1995;194:335-339. [DOI] [PubMed] [Google Scholar]
- 13.Girard AC, Mirbeau S, Gence L, et al. Effect of Washes and Centrifugation on the Efficacy of Lipofilling With or Without Local Anesthetic. Plast Reconstr Surg Glob Open. 2015;38:e496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shoshani O, Berger J, Fodor L, et al. The effect of lidocaine and adrenaline on the viability of injected adipose tissue--an experimental study in nude mice. J Drugs Dermatol. 2005;43:311-316. [PubMed] [Google Scholar]
- 15.Livaoglu M, Buruk CK, Uraloglu M, et al. Effects of lidocaine plus epinephrine and prilocaine on autologous fat graft survival. J Craniofac Surg. 2012;234:1015-1018. [DOI] [PubMed] [Google Scholar]
- 16.Eto H, Kato H, Suga H, et al. The fate of adipocytes after nonvascularized fat grafting: evidence of early death and replacement of adipocytes. Plast Reconstr Surg. 2012;1295:1081-1092. [DOI] [PubMed] [Google Scholar]
- 17.Kato H, Mineda K, Eto H, et al. Degeneration, regeneration, and cicatrization after fat grafting: dynamic total tissue remodeling during the first 3 months. Plast Reconstr Surg. 2014;1333:303e-313e. [DOI] [PubMed] [Google Scholar]
- 18.Philips BJ, Grahovac TL, Valentin JE, et al. Prevalence of endogenous CD34+ adipose stem cells predicts human fat graft retention in a xenograft model. Plast Reconstr Surg. 2013;1324:845-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garza RM, Rennert RC, Paik KJ, et al. Studies in fat grafting: Part IV. Adipose-derived stromal cell gene expression in cell-assisted lipotransfer. Plast Reconstr Surg. 2015;1354:1045-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berry MG, Davies D. Liposuction: a review of principles and techniques. J Plast Reconstr Aesthet Surg. 2011;648:985-992. [DOI] [PubMed] [Google Scholar]
- 21.Panetta NJ, Gupta DM, Kwan MD, Wan DC, Commons GW, Longaker MT. Tissue harvest by means of suction-assisted or third-generation ultrasound-assisted lipoaspiration has no effect on osteogenic potential of human adipose-derived stromal cells. Plast Reconstr Surg. 2009;1241:65-73. [DOI] [PubMed] [Google Scholar]
- 22.Fodor PB. Personal experience with ultrasound-assisted lipoplasty: a pilot study comparing ultrasound-assisted lipoplasty with traditional lipoplasty. Plast Reconstr Surg. 2004;1136:1852-1854. [DOI] [PubMed] [Google Scholar]
- 23.Fodor PB, Watson J. Personal experience with ultrasound-assisted lipoplasty: a pilot study comparing ultrasound-assisted lipoplasty with traditional lipoplasty. Plast Reconstr Surg. 1998;1014:1103-1116; discussion 1117-1109. [DOI] [PubMed] [Google Scholar]
- 24.Perez JA, van Tetering JP. Ultrasound-assisted lipoplasty: a review of over 350 consecutive cases using a two-stage technique. Aesthetic Plast Surg. 2003;271:68-76. [DOI] [PubMed] [Google Scholar]
- 25.Fisher C, Grahovac TL, Schafer ME, Shippert RD, Marra KG, Rubin JP. Comparison of harvest and processing techniques for fat grafting and adipose stem cell isolation. Plast Reconstr Surg. 2013;1322:351-361. [DOI] [PubMed] [Google Scholar]
- 26.Chung MT, Zimmermann AS, Paik KJ, et al. Isolation of human adipose-derived stromal cells using laser-assisted liposuction and their therapeutic potential in regenerative medicine. Stem Cells Transl Med. 2013;210:808-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bajek A, Gurtowska N, Gackowska L, et al. Does the liposuction method influence the phenotypic characteristic of human adipose-derived stem cells? Biosci Rep. 2015;353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer J, Salamon A, Herzmann N, et al. Isolation and Differentiation Potential of Human Mesenchymal Stem Cells From Adipose Tissue Harvested by Water Jet-Assisted Liposuction. Aesthet Surg J. 2015;358:1030-1039. [DOI] [PubMed] [Google Scholar]
- 29.Yin S, Luan J, Fu S, Wang Q, Zhuang Q. Does water-jet force make a difference in fat grafting? In vitro and in vivo evidence of improved lipoaspirate viability and fat graft survival. Plast Reconstr Surg. 2015;1351:127-138. [DOI] [PubMed] [Google Scholar]
- 30.Gause TM, II, Kling RE, Sivak WN, Marra KG, Rubin JP, Kokai LE. Particle size in fat graft retention: A review on the impact of harvesting technique in lipofilling surgical outcomes. Adipocyte. 2014;34:273-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirkham JC, Lee JH, Medina MA, III, McCormack MC, Randolph MA, Austen WG., Jr The impact of liposuction cannula size on adipocyte viability. Ann Plast Surg. 2012;694:479-481. [DOI] [PubMed] [Google Scholar]
- 32.Tambasco D, Arena V, Finocchi V, Grussu F, Cervelli D. The impact of liposuction cannula size on adipocyte viability. Ann Plast Surg. 2014;732:249-251. [DOI] [PubMed] [Google Scholar]
- 33.Erdim M, Tezel E, Numanoglu A, Sav A. The effects of the size of liposuction cannula on adipocyte survival and the optimum temperature for fat graft storage: an experimental study. J Plast Reconstr Aesthet Surg. 2009;629:1210-1214. [DOI] [PubMed] [Google Scholar]
- 34.Kirkham JC, Lee JH, Austen WG. Fat graft survival: physics matters: invited commentary to “The impact of liposuction cannula size on adipocyte viability”. Ann Plast Surg. 2014;733:359. [DOI] [PubMed] [Google Scholar]
- 35.Lee JH, Kirkham JC, McCormack MC, Nicholls AM, Randolph MA, Austen WG., Jr The effect of pressure and shear on autologous fat grafting. Plast Reconstr Surg. 2013;1315:1125-1136. [DOI] [PubMed] [Google Scholar]
- 36.Mojallal A, Auxenfans C, Lequeux C, Braye F, Damour O. Influence of negative pressure when harvesting adipose tissue on cell yield of the stromal-vascular fraction. Biomed Mater Eng. 2008;18(4-5):193-197. [PubMed] [Google Scholar]
- 37.Cheriyan T, Kao HK, Qiao X, Guo L. Low harvest pressure enhances autologous fat graft viability. Plast Reconstr Surg. 2014;1336:1365-1368. [DOI] [PubMed] [Google Scholar]
- 38.Conde-Green A, Wu I, Graham I, et al. Comparison of 3 techniques of fat grafting and cell-supplemented lipotransfer in athymic rats: a pilot study. Aesthet Surg J. 2013;335:713-721. [DOI] [PubMed] [Google Scholar]
- 39.Palumbo P, Miconi G, Cinque B, et al. In vitro evaluation of different methods of handling human liposuction aspirate and their effect on adipocytes and adipose derived stem cells. J Cell Physiol. 2015;2308:1974-1981. [DOI] [PubMed] [Google Scholar]
- 40.Ozkaya O, Egemen O, Barutca SA, Akan M. Long-term clinical outcomes of fat grafting by low-pressure aspiration and slow centrifugation (Lopasce technique) for different indications. J Plast Surg Hand Surg. 2013;475:394-398. [DOI] [PubMed] [Google Scholar]
- 41.Pfaff M, Wu W, Zellner E, Steinbacher DM. Processing technique for lipofilling influences adipose-derived stem cell concentration and cell viability in lipoaspirate. Aesthetic Plast Surg. 2014;381:224-229. [DOI] [PubMed] [Google Scholar]
- 42.Kurita M, Matsumoto D, Shigeura T, et al. Influences of centrifugation on cells and tissues in liposuction aspirates: optimized centrifugation for lipotransfer and cell isolation. Plast Reconstr Surg. 2008;1213:1033-1041; discussion 1042-1033. [DOI] [PubMed] [Google Scholar]
- 43.Son D, Choi T, Yeo H, Kim J, Han K. The effect of centrifugation condition on mature adipocytes and adipose stem cell viability. Ann Plast Surg. 2014;725:589-593. [DOI] [PubMed] [Google Scholar]
- 44.Salinas HM, Broelsch GF, Fernandes JR, et al. Comparative analysis of processing methods in fat grafting. Plast Reconstr Surg. 2014;1344:675-683. [DOI] [PubMed] [Google Scholar]
- 45.Zhu M, Cohen SR, Hicok KC, et al. Comparison of three different fat graft preparation methods: gravity separation, centrifugation, and simultaneous washing with filtration in a closed system. Plast Reconstr Surg. 2013;1314:873-880. [DOI] [PubMed] [Google Scholar]
- 46.Gupta R, Brace M, Taylor SM, Bezuhly M, Hong P. In search of the optimal processing technique for fat grafting. J Craniofac Surg. 2015;261:94-99. [DOI] [PubMed] [Google Scholar]
- 47.Choudhery MS, Badowski M, Muise A, Pierce J, Harris DT. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J Transl Med. 2014;12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geissler PJ, Davis K, Roostaeian J, Unger J, Huang J, Rohrich RJ. Improving fat transfer viability: the role of aging, body mass index, and harvest site. Plast Reconstr Surg. 2014;1342:227-232. [DOI] [PubMed] [Google Scholar]
- 49.Walmsley GG, Atashroo DA, Maan ZN, et al. High-Throughput Screening of Surface Marker Expression on Undifferentiated and Differentiated Human Adipose-Derived Stromal Cells. Tissue Eng Part A. 2015;21(15-16):2281-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chung MT, Liu C, Hyun JS, et al. CD90 (Thy-1)-positive selection enhances osteogenic capacity of human adipose-derived stromal cells. Tissue Eng Part A. 2013;19(7-8):989-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levi B, Wan DC, Glotzbach JP, et al. CD105 protein depletion enhances human adipose-derived stromal cell osteogenesis through reduction of transforming growth factor beta1 (TGF-beta1) signaling. J Biol Chem. 2011;28645:39497-39509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsumoto D, Sato K, Gonda K, et al. Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. Dec 2006;1212:3375-3382. [DOI] [PubMed] [Google Scholar]
- 53.Yoshimura K, Shigeura T, Matsumoto D, et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;2081:64-76. [DOI] [PubMed] [Google Scholar]
- 54.Yoshimura K, Sato K, Aoi N, Kurita M, Hirohi T, Harii K. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;321:48-55; discussion 56-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for facial lipoatrophy: efficacy of clinical use of adipose-derived stem cells. Dermatol Surg. 2008;349:1178-1185. [DOI] [PubMed] [Google Scholar]
- 56.Kolle SF, Fischer-Nielsen A, Mathiasen AB, et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. Lancet. 2013;3829898:1113-1120. [DOI] [PubMed] [Google Scholar]
- 57.Tanikawa DY, Aguena M, Bueno DF, Passos-Bueno MR, Alonso N. Fat grafts supplemented with adipose-derived stromal cells in the rehabilitation of patients with craniofacial microsomia. Plast Reconstr Surg. 2013;1321:141-152. [DOI] [PubMed] [Google Scholar]
- 58.Paik KJ, Zielins ER, Atashroo DA, et al. Studies in Fat Grafting: Part V. Cell-Assisted Lipotransfer to Enhance Fat Graft Retention Is Dose Dependent. Plast Reconstr Surg. 2015;1361:67-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peltoniemi HH, Salmi A, Miettinen S, et al. Stem cell enrichment does not warrant a higher graft survival in lipofilling of the breast: a prospective comparative study. J Plast Reconstr Aesthet Surg. 2013;6611:1494-1503. [DOI] [PubMed] [Google Scholar]
- 60.Wang L, Luo X, Lu Y, Fan ZH, Hu X. Is the Resorption of Grafted Fat Reduced in Cell-Assisted Lipotransfer for Breast Augmentation? Ann Plast Surg. 2015;752:128-134. [DOI] [PubMed] [Google Scholar]
- 61.Li K, Li F, Li J, et al. Increased survival of human free fat grafts with varying densities of human adipose-derived stem cells and platelet-rich plasma. J Tissue Eng Regen Med. 2014. doi:10.1002/term.1903. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 62.Chung MT, Paik KJ, Atashroo DA, et al. Studies in fat grafting: Part I.: Effects of injection technique on in vitro fat viability and in vivo volume retention. Plast Reconstr Surg. 2014;1341:29-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Atashroo D, Raphel J, Chung MT, et al. Studies in fat grafting: Part II. Effects of injection mechanics on material properties of fat. Plast Reconstr Surg. 2014;1341:39-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hyun JS, Montoro DT, Lo DD, et al. The seed and the soil: optimizing stem cells and their environment for tissue regeneration. Ann Plast Surg. 2013;702:235-239. [DOI] [PubMed] [Google Scholar]
- 65.Yuan Y, Zhang S, Gao J, Lu F. Spatial structural integrity is important for adipose regeneration after transplantation. Arch Dermatol Res. 2015;3078:693-704. [DOI] [PubMed] [Google Scholar]
- 66.Coleman WP., III Fat transplantation. Facial Plast Surg Clin North Am. 2008;164:451-458, vii. [DOI] [PubMed] [Google Scholar]
- 67.Holck DE, Lopez MA. Periocular autologous fat transfer. Facial Plast Surg Clin North Am. 2008;164:417-427, vi. [DOI] [PubMed] [Google Scholar]
- 68.Choi M, Small K, Levovitz C, Lee C, Fadl A, Karp NS. The volumetric analysis of fat graft survival in breast reconstruction. Plast Reconstr Surg. 2013;1312:185-191. [DOI] [PubMed] [Google Scholar]
- 69.Abboud MH, Dibo SA. Immediate Large-Volume Grafting of Autologous Fat to the Breast Following Implant Removal. Aesthet Surg J. 2015;357:819-829. [DOI] [PubMed] [Google Scholar]
- 70.Del Vecchio D, Rohrich RJ. A classification of clinical fat grafting: different problems, different solutions. Plast Reconstr Surg. 2012;1303:511-522. [DOI] [PubMed] [Google Scholar]
- 71.Khouri R, Del Vecchio D. Breast reconstruction and augmentation using pre-expansion and autologous fat transplantation. Clin Plast Surg. 2009;362:269-280, viii. [DOI] [PubMed] [Google Scholar]
- 72.Khouri RK, Eisenmann-Klein M, Cardoso E, et al. Brava(R) and Autologous Fat Transfer Is a Safe and Effective Breast Augmentation Alternative: Results of a Six-Year, Eighty-One Patients Prospective Multicenter Study. Plast Reconstr Surg. 2012;1295:1173-1187. [DOI] [PubMed] [Google Scholar]
- 73.Garza RM, Paik KJ, Chung MT, et al. Studies in fat grafting: Part III. Fat grafting irradiated tissue--improved skin quality and decreased fat graft retention. Plast Reconstr Surg. 2014;1342:249-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shukla L, Morrison WA, Shayan R. Adipose-derived stem cells in radiotherapy injury: a new frontier. Front Surg. 2015;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thanik VD, Chang CC, Zoumalan RA, et al. A novel mouse model of cutaneous radiation injury. Plast Reconstr Surg. 2011;1272:560-568. [DOI] [PubMed] [Google Scholar]
- 76.Luan A, Duscher D, Whittam AJ, et al. Cell-Assisted Lipotransfer Improves Volume Retention in Irradiated Recipient Sites and Rescues Radiation-Induced Skin Changes. Stem Cells. 2015. doi:10.1002/stem.2256. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rowan BG, Lacayo EA, Sheng M, et al. Human Adipose Tissue-Derived Stromal/Stem Cells Promote Migration and Early Metastasis of Head and Neck Cancer Xenografts. Aesthet Surg J. 2016;361:93-104. [DOI] [PubMed] [Google Scholar]
- 78.Rowan BG, Gimble JM, Sheng M, et al. Human adipose tissue-derived stromal/stem cells promote migration and early metastasis of triple negative breast cancer xenografts. PloS One. 2014;92:e89595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Freese KE, Kokai L, Edwards RP, et al. Adipose-derived stems cells and their role in human cancer development, growth, progression, and metastasis: a systematic review. Cancer Res. Apr 1 2015;757:1161-1168. [DOI] [PubMed] [Google Scholar]
- 80.Eterno V, Zambelli A, Pavesi L, et al. Adipose-derived Mesenchymal Stem Cells (ASCs) may favour breast cancer recurrence via HGF/c-Met signaling. Oncotarget. 2014;53:613-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;4497162:557-563. [DOI] [PubMed] [Google Scholar]
- 82.Kucerova L, Kovacovicova M, Polak S, et al. Interaction of human adipose tissue-derived mesenchymal stromal cells with breast cancer cells. Neoplasma. 2011;585:361-370. [DOI] [PubMed] [Google Scholar]
- 83.Ahn JO, Coh YR, Lee HW, Shin IS, Kang SK, Youn HY. Human adipose tissue-derived mesenchymal stem cells inhibit melanoma growth in vitro and in vivo. Anticancer Res. 2015;351:159-168. [PubMed] [Google Scholar]
- 84.Cousin B, Ravet E, Poglio S, et al. Adult stromal cells derived from human adipose tissue provoke pancreatic cancer cell death both in vitro and in vivo. PloS One. 2009;47:e6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ryu H, Oh JE, Rhee KJ, et al. Adipose tissue-derived mesenchymal stem cells cultured at high density express IFN-beta and suppress the growth of MCF-7 human breast cancer cells. Cancer Lett. 2014;3522:220-227. [DOI] [PubMed] [Google Scholar]
- 86.Alperovich M, Lee ZH, Friedlander PL, Rowan BG, Gimble JM, Chiu ES. Adipose stem cell therapy in cancer reconstruction: a critical review. Ann Plast Surg. 2014;73(suppl 1):S104-S107. [DOI] [PubMed] [Google Scholar]
- 87.Gir P, Brown SA, Oni G, Kashefi N, Mojallal A, Rohrich RJ. Fat grafting: evidence-based review on autologous fat harvesting, processing, reinjection, and storage. Plast Reconstr Surg. 2012;1301:249-258. [DOI] [PubMed] [Google Scholar]
- 88.Kakagia D, Pallua N. Autologous fat grafting: in search of the optimal technique. Surg Innov. 2014;213:327-336. [DOI] [PubMed] [Google Scholar]