Abstract
Objectives:
Autism spectrum disorder is a neurodevelopmental condition that typically displays socio-communicative impairment as well as restricted stereotyped interests and activities, in which gastrointestinal disturbances are commonly reported. We report the case of a boy with Autism Spectrum Disorder (ASD) diagnosis, severe cognitive disability and celiac disease in which an unexpected improvement of autistic core symptoms was observed after four months of probiotic treatment.
Method:
The case study refers to a 12 years old boy with ASD and severe cognitive disability attending the Villa Santa Maria Institute in resident care since 2009. Diagnosis of ASDs according to DSM-V criteria was confirmed by ADOS-2 assessment (Autism Diagnostic Observation Schedule).
The medication used was VSL#3, a multi-strain mixture of ten probiotics. The treatment lasted 4 weeks followed by a four month follow-up.
The rehabilitation program and the diet was maintained stable in the treatment period and in the follow up. ADOS-2 was assessed six times: two times before starting treatment; two times during the treatment and two times after interruption of the treatment.
Results:
The probiotic treatment reduced the severity of abdominal symptoms as expected but an improvement in Autistic core symptoms was unexpectedly clinically evident already after few weeks from probiotic treatment start. The score of Social Affect domain of ADOS improved changing from 20 to 18 after two months treatment with a further reduction of 1 point in the following two months. The level 17 of severity remained stable in the follow up period. It is well known that ADOS score does not fluctuate spontaneously along time in ASD and is absolutely stable.
Conclusions:
The appropriate use of probiotics deserves further research, which hopefully will open new avenues in the fight against ASD.
Keywords: Autism spectrum disorder, autism, probiotics
Introduction
Autism spectrum disorder (ASD) is a specific neurodevelopmental condition arising during the first 2 years of life that typically displays qualitative socio-communicative impairment and restricted, repetitive and stereotyped interests and activities. Variable degrees of intellectual disability and expressive/receptive language insufficiency are also reported.1
The etiopathogenesis of ASD is not yet clearly understood. Many family and twin studies point out the importance of both genetic and inherited predispositions even though epidemiological studies suggest a strong contribution of perinatal environmental factors. Genetic factors alone account for not more than 20%–30% of all cases, whereas other 70%–80% are the results of a complex interaction between environmental factors and inherited or de novo genetic susceptibility.2
The literature on the role played by derangement of physiological profile of gut bacteria, commonly called intestinal dysbiosis in ASD, is increasing and a number of studies have been published over the last 10 years.3 In particular, a review by Ianiro et al.4 has described the role of the microbiota in several diseases and the related treatment options that are currently available, such as the use of antibiotics targeting harmful bacteria like Clostridia genus or the use of probiotics enhancing the gut mucosal barrier.4
Case report
The case study refers to a 12-year-old boy with ASD and severe cognitive disability attending the Villa Santa Maria Institute in resident care since 2009. The boy was first diagnosed with pervasive developmental disorder (PDD) at the age of 2 years. He was admitted to the institute with a double diagnosis: PDD and celiac disease. The celiac disease diagnosis was made at the age of 6 years, based on chronic gastrointestinal (GI) symptoms, family history of a sister with celiac disease, positive genetic human leukocyte antigen (HLA) typing and a slight elevation of transglutaminase antibodies. IgA antiendomysial antibodies and histological examinations of duodenal biopsy were negative and a “latent” celiac disease diagnosis was made. A therapeutic trial based on a gluten-free diet was prescribed, however, without clinical benefits. The periodic blood-specific tests for celiac disease always came out negative. For this reason, in June 2013, we definitively suspended the gluten-free diet. The diagnosis of ASD according to the Diagnostic and Statistical Manual of Mental Disorders’ (5th ed.; DSM-5) criteria was confirmed by an Autism Diagnostic Observation Schedule-2 (ADOS-2) assessment in October 2013. Chronic GI symptoms (prevalent chronic constipation) remained unchanged and a diagnosis of irritable bowel disease was made. For this reason, in late February 2014, a probiotic treatment was prescribed.
The probiotic used was VSL#3 (VSL Pharmaceuticals Inc., USA), a mixture which contains 9 × 1010 colony forming units (cfu)/g of viable, lyophilized bifidobacteria (Bifidobacterium breve, B. longum, B. infantis), 8 × 1010 lactobacilli (Lactobacillus acidophilus, L. plantarum, L. paracasei, L. bulgaricus, L. delbrueckii subsp.) and 20 × 1010 Streptococci (S. thermophilus, S. salivarius subsp.).
VSL#3, as a powder diluted in a glass of water, was dispensed by mouth and was regularly recorded on the therapy sheet. The treatment compliance was ensured by careful retrospective examination of the sheet.
The probiotic treatment already after few weeks reduced the severity of abdominal symptoms as expected. For this reason, this therapy was continued from February to May 2014.
Since it is well known that gut microbiome profile is very sensitive to diet composition,5 a carefully recorded diet was maintained during the entire period throughout a food diary compiled by a caregiver. Indeed, paramedical staff ensured that no other dietary supplements containing probiotics, prebiotics or antibiotics were administered in the treatment period or in the follow up.
The rehabilitation program, which the patient followed for the last 6 years, was based on daily behavior and communication treatments, weekly psychomotor therapy, daily health care, individualized Education Plan/School Services and parent/guardian involvement. The rehabilitation program continued during the study period without any particular change. An apparent improvement in ASD symptoms and manifestations was already clinically evident a few weeks after probiotic treatment. For this reason, we specifically explored whether therapy with probiotics could also have effects on ASD core symptoms, namely, social interaction, communication and/or restricted, repetitive and stereotyped patterns of behavior and interests by means of ADOS-2.
ADOS-2 examines behaviors in two distinct domains. One takes into account the social affect (SA) and the other the restricted, repetitive behaviors (RRB). Both provide for the ADOS-2 comparison score, a metric for gauging overall autism severity.
A skilled psychologist, with certified training in ADOS assessment, repeated ADOS-2 assessments six times over a period ranging from October 2013 to March 2015. The dates of assessment were as follows:
Baseline assessments: October 2013 and January 2014 (Time 0 and Time 1);
During treatment assessments (1 and 3 months after treatment start): late March and early June 2014 (Time 2 and Time 3);
After treatment assessments (4 and 10 months after treatment end): October 2014 and March 2015 (Time 4 and Time 5).
Figure 1 shows the course of SA ADOS dominion score, a core component of ASD, with a relevant decrease in value during treatment, which was maintained after the probiotic treatment ceased.
Figure 1.
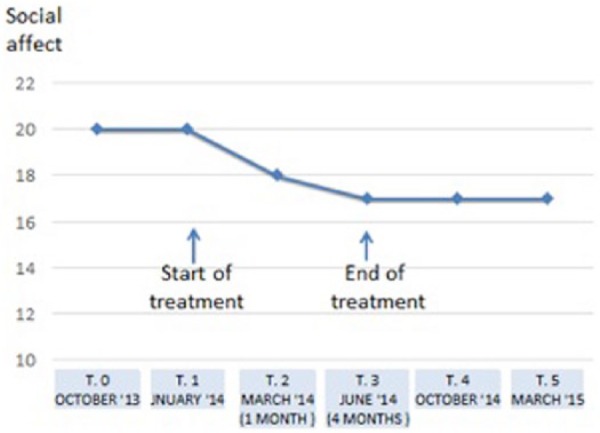
Effect of probiotic treatment on social affect, a core symptom of ASD.
Discussion
Whether patients with ASD have a higher rate of GI disorders has been long argued in international literature. While there are many cases of ASD where no GI symptoms are seen, a recent meta-analysis definitively confirms that children with ASD experience significantly more general GI symptoms, with a higher rate of diarrhea, constipation and abdominal pain.6 Furthermore, a higher risk of problem behaviors has been reported in individuals with ASD and GI symptoms.7 Vocal and motor behaviors, including problem behaviors such as self-injury and aggression, as well as overall changes in well-being state (e.g. sleep disturbance or irritability), may be behavioral manifestations of abdominal pain or GI discomfort in persons with ASD.8,9
Several studies have shown that in irritable bowel syndrome (IBS), probiotic treatment improves symptoms, visceral sensitivity and induces a significant change in the composition of the intestinal microbiota.10–14 The mechanism of action of probiotics in IBS is not merely correlated to the modulation of the microbiota composition. Probiotics affect the composition and functionality of microbiota, the interplay with visceral hypersensitivity, mucosal inflammation and motility, intestinal permeability and the gut–brain axis.11
VSL#3 administration in humans has been shown to increase Lactobacilli and Bifidobacteria count, whereas Enterococci, Coliforms, Bacteroides and Clostridia remained unchanged.15 An increase of Bifidobacteria in mice treated with VSL#3 has also been reported.16 Furthermore, VSL#3 has shown to have a direct effect on epithelial barrier function in interleukin-10 (IL-10) gene-deficient mice17 and to regulate the intestinal barrier, thereby increasing the tight junction protein expression in vivo and in vitro in rats.18 This probiotic mixture maintains tight junctions, prevents apoptosis in a murine model19 and regulates intestinal epithelial permeability in experimental ileitis by a tumor necrosis factor (TNF)-dependent mechanism in mice.20
From the clinical point of view, although there is insufficient evidence of efficacy for the use of various probiotics in the induction of ulcerative colitis remission, recent randomized controlled trial in adults and pediatric inflammatory bowel disease (IBD) patients shows that the use of VSL#3 therapy, added to conventional therapy, leads to higher rates of remission than standard therapy alone.21,22 For this reason, this product is a good candidate as therapeutic agent in ASD.
In our observation, while the RRB domain did not change, there was an unexpected improvement in autistic core symptoms. ADOS-2 SA domain score was reduced already after 2 months of treatment, going from 20 to 18 with a further reduction of 1 point in the following 2 months. The 17 severity levels remained stable during the 10-month follow-up period. This change was surprising since in our assessment records over several years, the patient status had remained steadily unchanged.
It is well known that ASD is caused by a complex interplay between genetic and environmental components among gut flora composition.1,2 An increasing body of knowledge underscores that gut flora influences a variety of social emotional and anxiety-like behaviors and contributes to brain development/function in animals,23,24 as well as in humans.25 In this context, the possibility that ASD is related to an impaired development of a physiological intestinal microbiota is not surprising and is supported by a number of scientific reports.3
A recent meta-analysis of the studies dealing with autism and microbiota reports that there is a significant difference in the intestinal bacteria in the gut of ASD patients compared with healthy children and that this might contribute to the disorder in a substantial number of individuals.26
After the intriguing hypothesis of a possible role of Clostridium tetani in the late-onset autism in 199827 and the first publication of a case report and the subsequent open-label trial in which oral Vancomycin treatment led to a short-term improvements of some autistic features,28 the literature about the role played by intestinal dysbiosis in autism is increasing,3,26 but only few studies explore the therapeutic role of probiotics in children with ASD.29,30 Unfortunately, these studies suffered from poor methodological quality due to the absence of control groups, the presence of etherogeneous concomitant treatments and/or small sample sizes.
A major breakthrough in the microbiota hypothesis of ASD comes from a recent study in which the authors demonstrated that on a particular model of autistic mice displaying behavioral and neurodevelopmental symptoms relevant to ASD (defective integrity of the mucosal intestinal barrier, dysbiosis of the commensal microbiota and alterations in serum metabolites), the administration of a particular commensal (B. fragilis) is able to reverse autistic symptoms and metabolic derangement.31
Probiotics can be useful to restore the microbial balance in the intestine, to relieve GI problems and to attenuate immunological abnormalities.32
The gut–brain axis, a complex communication network interfacing the gut and the brain of a single individual is almost certainly the comprehension key of the favorable effect exerted by probiotics in ASD. In addition to the well-known communication between the central and enteric nervous systems, gut–brain axis concept involves other pathways, among which are immune activation, intestinal barrier function and entero-endocrine signaling. All these communication lines are bidirectional and involve neuro-immuno-endocrine mediators. The reason for the development of such a complex network is to maintain GI homeostasis, keeping in mind its links with cognitive and affective functions. Recently, the role of enteric flora, or microbiota, has been recognized as a part of the gut–brain axis.33 The gut microbiota can modulate brain function, forming a crucial link in the bidirectional interactions between the intestine and the nervous system. The brain can influence commensal organisms either indirectly, via changes in GI motility and secretion, and intestinal permeability, or directly, via signaling molecules released into the gut lumen from cells in the lamina propria (enterochromaffin cells, neurons, immune cells). However, communication from enteric microbiota to the host can occur via epithelial-cell, receptor-mediated signaling and, when intestinal permeability is increased, through direct stimulation of host cells in the lamina propria.23,24,33
Conclusion
Our findings suggest a possible role of probiotics in the improvement of behavioral abnormalities associated with ASD. These results need to be obviously confirmed in well-controlled trials with sufficient group sizes. Of great importance to these trials will be the choice of the probiotic bacterial strains since the biologic actions of probiotic bacteria are highly strain specific.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Informed consent: On admission, we have obtained a written consent from the legal representatives for authorization to publish anonymously any information for scientific purposes.
References
- 1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Publications, 2013. [Google Scholar]
- 2. Lai MC, Lombardo MV, Baron-Cohen S. Autism. Lancet 2014; 383: 896–910. [DOI] [PubMed] [Google Scholar]
- 3. Grossi E, Terruzzi V. The role of intestinal dysbiosis in the pathogenesis of autism: minireview. Intern J Microbiol Adv Immunol 2014; 2: 201–204. [Google Scholar]
- 4. Ianiro G, Bibbò S, Gasbarrini A, et al. Therapeutic modulation of gut microbiota: current clinical applications and future perspectives. Curr Drug Targets 2014; 15: 762–770. [DOI] [PubMed] [Google Scholar]
- 5. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014; 505: 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McElhanon BO, McCracken C, Karpen S, et al. Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis. Pediatrics 2014; 133: 872–883. [DOI] [PubMed] [Google Scholar]
- 7. Committee on Educational Interventions for Children with Autism, Division of Behavioral and Social Sciences and Education, National Research Council. Educating children with autism (ed Lord C, McGee JP.). Washington, DC: National Academy Press, 2001. [Google Scholar]
- 8. McAtee M, Carr EG, Schulte C, et al. A contextual assessment inventory for problem behavior: initial development. J Posit Behav Interv 2004; 6(3): 148–165. [Google Scholar]
- 9. Carr EG, Owen-DeSchryver JS. Physical illness, pain, and problem behavior in minimally verbal people with developmental disabilities. J Autism Dev Disord 2007; 37(3): 413–424. [DOI] [PubMed] [Google Scholar]
- 10. Brenner DM, Moeller MJ, Chey WD, et al. The utility of probiotics in the treatment of irritable bowel syndrome: a systematic review. Am J Gastroenterol 2009; 104: 1033–1049. [DOI] [PubMed] [Google Scholar]
- 11. DuPont HL. Review article: evidence for the role of gut microbiota in irritable bowel syndrome and its potential influence on therapeutic targets. Aliment Pharmacol Ther 2014; 39: 1033–1042. [DOI] [PubMed] [Google Scholar]
- 12. O’Mahony L, McCarthy J, Kelly P, et al. A randomized, placebo-controlled, double-blind comparison of the probiotic bacteria lactobacillus and bifidobacterium in irritable bowel syndrome (IBS): symptom responses and relationship to cytokine profiles. Gastroenterology 2005; 128: 541–551. [DOI] [PubMed] [Google Scholar]
- 13. Guandalini S, Magazzu` G, Chiaro A, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr 2010; 51: 24–30. [DOI] [PubMed] [Google Scholar]
- 14. Yoon JS, Sohn W, Lee OY, et al. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol 2014; 29: 52–59. [DOI] [PubMed] [Google Scholar]
- 15. Brigidi P, Vitalia B, Swennena E, et al. Effects of probiotic administration upon the composition and enzymatic activity of human fecal microbiota in patients with irritable bowel syndrome or functional diarrhea. Res Microbiol 2001; 152: 735–741. [DOI] [PubMed] [Google Scholar]
- 16. Gaudier E, Michel C, Segain JP, et al. The VSL#3 probiotic mixture modifies microflora but does not heal chronic dextran-sodium sulfate-induced colitis or reinforce the mucus barrier in mice. J Nutr 2005; 135: 2753–2761. [DOI] [PubMed] [Google Scholar]
- 17. Madsen K, Cornish A, Soper P, et al. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 2001; 121: 580–591. [DOI] [PubMed] [Google Scholar]
- 18. Dai C, Zhao D, Jiang M. VSL#3 probiotics regulate the intestinal epithelial barrier in vivo and in vitro via the p38 and ERK signaling pathways. Int J Mol Med 2012; 29: 202–208. [DOI] [PubMed] [Google Scholar]
- 19. Mennigen R, Nolte K, Rijcken E, et al. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol 2009; 296: G1140–G1149. [DOI] [PubMed] [Google Scholar]
- 20. Corridoni D, Pastorelli L, Mattioli B, et al. Probiotic bacteria regulate intestinal epithelial permeability in experimental ileitis by a TNF dependent mechanism. PLoS ONE 2012; 7: e42067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cammarota G, Ianiro G, Cianci R, et al. The involvement of gut microbiota in inflammatory bowel disease pathogenesis: potential for therapy. Pharmacol Ther 2015; 149: 191–212. [DOI] [PubMed] [Google Scholar]
- 22. Dignass A, Lindsay JO, Sturm A, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis: current management. J Crohns Colitis 2012; 6: 991–1030. [DOI] [PubMed] [Google Scholar]
- 23. Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 2012; 10: 735–742. [DOI] [PubMed] [Google Scholar]
- 24. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012; 13: 701–712. [DOI] [PubMed] [Google Scholar]
- 25. Tillisch K, Labus J, Kilpatrick L, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013; 144: 1394–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cao X, Lin P, Jiang P, et al. Characteristics of the gastrointestinal microbiome in children with autism spectrum disorder: a systematic review. Shanghai Arch Psychiatry 2013; 25: 342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bolte ER. Autism and Clostridium tetani. Med Hypotheses 1998; 51: 133–144. [DOI] [PubMed] [Google Scholar]
- 28. Sandler RH, Finegold SM, Bolte ER, et al. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol 2000; 15: 429–435. [DOI] [PubMed] [Google Scholar]
- 29. Parracho HM, Bingham MO, Gibson GR, et al. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol 2005; 54: 987–991. [DOI] [PubMed] [Google Scholar]
- 30. Kaluzna-Czaplinska J, Blaszczyk S. The level of arabinitol in autistic children after probiotic therapy. Nutrition 28: 124–126. [DOI] [PubMed] [Google Scholar]
- 31. Hisiao EY, McBride SW, Hsien S, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013; 155: 1451–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mulle JG, Sharp WG, Cubells JF. The gut microbiome: a new frontier in autism research. Curr Psychiatry Rep 2013; 15: 337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grenham S, Clarke G, Cryan JF, et al. Brain-gut-microbe communication in health and disease. Front Physiol 2011; 2: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]


