CD4 counts during early human immunodeficiency virus (HIV) infection do not predict HIV transmission or disease progression, and hinder efforts to expand antiretroviral therapy (ART) coverage. Country-level HIV policies should follow World Health Organization guidelines and remove CD4 counts as a criterion for initiating ART.
Keywords: HIV, ART, CD4 cell count, universal test and treat, care continuum
Abstract
Antiretroviral therapy (ART) policy for people living with human immunodeficiency virus (HIV) has historically been based on clinical indications, such as opportunistic infections and CD4 cell counts. Studies suggest that CD4 counts early in HIV infection do not predict relevant public health outcomes such as disease progression, mortality, and HIV transmission in people living with HIV. CD4 counts also vary widely within individuals and among populations, leading to imprecise measurements and arbitrary ART initiation. To capture the clinical and preventive benefits of treatment, the global HIV response now focuses on increasing HIV diagnosis and ART coverage. CD4 counts for ART initiation were necessary when medications were expensive and had severe side effects, and when the impact of early ART initiation was unclear. However, current evidence suggests that although CD4 counts may still play a role in guiding clinical care to start prophylaxis for opportunistic infections, CD4 counts should cease to be required for ART initiation.
Triple-drug therapy was shown to be effective for treating people living with human immunodeficiency virus (HIV) in 1996 [1, 2], and it has been suggested that antiretroviral therapy (ART) can halt the HIV epidemic by preventing HIV illness, transmission, and death [3–6]. To optimize resource allocation and improve health, international health organizations have published guidelines that recommend which individuals should be eligible to initiate ART. Criteria for clinically driven ART initiation have been consistent over time, with World Health Organization (WHO) clinical stages III and IV being indicated for ART initiation in both the 2002 [7] and 2013 [8] WHO guidelines. However, for individuals in WHO clinical stages I and II, ART initiation is based on CD4+ T-cell count thresholds, which have been the subject of considerable debate, with different views being expressed at different times.
When the WHO published its first ART guidelines in 2002, 2 other institutions—the International AIDS Society (IAS) and the US Department of Health and Human Services (DHHS)—had published guidelines 2 years earlier. Despite having access to the same results, the 3 scientific committees reached different conclusions for when adults should start ART. All agreed that people with AIDS-defining illnesses (WHO clinical stages III and IV) should start ART, but in the absence of AIDS-defining illnesses, the WHO recommended ART for persons with a CD4 count ≤200 cells/µL [7], the IAS recommended ART for persons with a CD4 count ≤350 cells/µL or a viral load >30 000 copies/mL while considering ART for persons with a CD4 count 350–500 cells/µL [9], and the DHHS recommended ART for persons with a CD4 count ≤500 cells/µL or a viral load >10 000 copies/mL [10].
Over time, the WHO CD4 count–based ART eligibility criterion has increased, and in 2013 the WHO increased the CD4 count threshold for starting ART to ≤500 cells/µL, closer to the DHHS recommendation 13 years earlier. Despite the WHO's 2013 and now more recent recommendation for “test and treat” [8, 11], global ART guidelines among countries still display marked differences (Table 1), which might be explained by the local context of capacity and resource availability, but begs the question of whether recommendations should represent a higher but potentially unachievable standard of care, or a lesser but potentially achievable standard. In their 2000 guidelines, the WHO, explaining their decision to recommend ART only to those with a CD4 count ≤200 cells/µL, noted that “beginning therapy before the CD4 cell count falls below 200/mm3 clearly provides clinical benefits,” but that treatment should be limited to those with CD4 count <200 cells/mm3 because “the actual point above 200/mm3 at which to start therapy has not been definitively determined” [7]. The IAS also conceded that “treatment effects on survival at higher CD4+ cell counts is not documented,” but they nevertheless stated that the concerns “should not obscure the dramatic changes in HIV-related morbidity and mortality resulting from therapy in advanced disease” [9]. In the most inclusive guidelines, the DHHS states that their “aggressive approach is heavily based on … the principle that one should begin treatment before the development of significant immunosuppression” [10]. Even after considering the potential toxicity and costs of the early regimens, the disparity in CD4 count criteria over time demonstrates the lack of consensus over CD4 count–based ART initiation. We explore this issue by examining the value of CD4 counts as a reliable marker for ART initiation and prioritization given current scientific evidence.
Table 1.
Antiretroviral Therapy Policies in 99 Countries
| CD4 Count Policy, Cells/µL | 2004–2005 | 2006–2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 |
|---|---|---|---|---|---|---|---|---|
| Irrespective of CD4 count | Netherlands, DHHS | Australia, Brazil, France, South Korea | Spain, Thailand | |||||
| ≤500 (consider for ≥500) | DHHS | Italy | Argentina | Hong Kong | ||||
| ≤500 | Algeria | WHO, Bolivia, Ecuador, Ethiopia, Honduras, Madagascar, Mali, Oman, Rwanda, Tunisia, Uganda, Zambia, Zimbabwe | WHO, Bangladesh, El Salvador, Gabon, Kenya, Lesotho, Malawi, Mauritania, Myanmar, Namibia, Nepal, South Africa, South Sudan, Sri Lanka, Sudan, Venezuela, | |||||
| ≤350 (consider for ≤500) | Guyanaa | Uruguay, Guinea | Austriaa, Belize, Germanya, Mexico | Costa Rica | ||||
| ≤350 | Djibouti, Sierra Leone | Burkina Faso, Canada, Moldova, Niger, Papua New Guinea, Nicaragua, Sweden | WHO, Burundi, Chile, Democratic Republic of Congo, Ghana, Morocco, Nigeria, Swaziland | WHO, Angola, Haiti, Indonesia, Jamaica, Kazakhstan, Malaysia, Panama, Paraguay, Switzerland, Vietnam | WHO, Botswana, Benin, Cambodia, China, Guatemala, Peru, Mozambique, Tanzania | Great Britain, Dominican Republic, India | ||
| ≤250 (consider for ≤350) | Colombia | |||||||
| ≤200 (consider for ≤350) | Cape Verde | WHO, Afghanistan, Russia, Ukraine | WHO, Cuba | |||||
| ≤200 | WHO, Cote d'Ivoire, Pakistan | Bhutan, Comoros, Lao People's Democratic Republic, Liberia | Philippines | Cameroon |
The list is updated as of April 2015 and is contingent upon publication of national guidelines. Countries listed in italics are consistent with WHO guidelines in a given year; countries listed in bold recommend early antiretroviral therapy (ART) compared with the WHO recommendation.
Abbreviations: DHHS, US Department of Health and Human Services; WHO, World Health Organization.
a Austria, Germany, and Guyana additionally recommend considering ART at CD4 count ≥500 cells/µL.
Suitability of CD4 Cell Counts for Determining Eligibility to Start ART
A surrogate laboratory marker to be used as the primary eligibility criterion to begin ART must satisfy several clinical and public health criteria. From the clinical perspective, the marker must predict disease progression and the risk of transmitting the virus. From the public health perspective, the marker must produce consistent and reliable measurements, and be feasible to scale up as ART access increases (Table 2). Although many possible markers exist, most guidelines were and continue to be based on CD4 counts. Therefore, we will examine how well CD4 count fulfills each criterion for ART initiation, particularly soon after HIV infection, which is the period for which ART guidelines are being debated.
Table 2.
Criteria for Clinical Surrogate Marker for Antiretroviral Therapy Guidelinesa
| Criterion | Specific Outcomes | CD4 Count Suitability | Ideal? |
|---|---|---|---|
| Clinical outcomes |
|
|
No |
|
|
No | |
| Consistent measurements |
|
|
No |
|
|
No | |
|
|
No | |
| Feasible to implement |
|
|
No |
|
|
No |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus.
a The ideal clinical surrogate marker would have the following characteristics, none of which are satisfied by CD4 counts and existing CD4 count policy.
Predict Disease Progression, Response to ART, and HIV Transmission
A useful clinical surrogate marker for disease progression must indicate to healthcare providers and policy makers the current and expected future health states of the patient, as well as provide information regarding the public health and community consequences of clinical decisions. Although low CD4 counts provide a simple and direct measure of a person's prognosis in late-stage HIV, at high CD4 cell counts, the measure has little prognostic value. Furthermore, CD4 cell counts are not associated with a person's infectiousness, so they provide no important information in relation to HIV prevention.
Disease Progression
People living with HIV progress to AIDS an average of 7 years after infection, but there is considerable heterogeneity in time to AIDS [12]. The heterogeneity in HIV progression requires a surrogate marker that predicts one's expected rate of disease progression toward mortality. Studies demonstrate that low CD4 counts predict risk of mortality and opportunistic infection [12]. However, the correlation is weak early in HIV infection when ART initiation has been questioned. A quantitative review of data from 30 studies by Korenromp et al found that early in HIV infection, CD4 counts are poor predictors of clinical progression due to their high variability, even in perfectly healthy HIV-uninfected people, and that when the CD4 count is >625 cells/µL, CD4 counts provide zero prognostic value [13]. In a study of seroconverters in Uganda, Eller et al found that neither activation nor exhaustion of CD4 T cells was correlated with disease progression [14]. Furthermore, an analysis of the Multicenter AIDS Cohort Study found that median CD4 count explained only 29% and 35% of the variability in the probabilities of AIDS and death, respectively, whereas viral load explained 51% and 58%, respectively [15].
Overall, survival after seroconversion appears to be independent of CD4 cell counts [16]. Consequently, CD4 measurements indicate neither one's prognosis nor when retesting should occur, thus providing little information as to when ART should be initiated for those with high CD4 counts. At low CD4 cell counts, people are likely to have shown symptoms of AIDS-related opportunistic infections that are the reason for their presenting to a clinic, and this should always be followed by an HIV test. If a clinician feels it necessary to know how serious the person's condition is, then there may be marginal value for measuring the CD4 count, but as regular HIV testing and immediate treatment, following new WHO guidelines, are made available, the value of CD4 counts is likely to decrease even further.
HIV Transmission
In addition to predicting the health of the patient, the optimal prognostic marker for staging should indicate the individual's risk of transmitting HIV. Studies have demonstrated the strong correlation between HIV RNA load and HIV transmission. Quinn et al found that each 1.0 log10 increase in plasma viral load was associated with a 2.45 rate ratio (95% confidence interval, 1.85–3.26) for sexual HIV transmission [17], similar to a study of mother-to-child transmission by Chuachoowong et al, which found a 6.1-fold increased odds of HIV transmission per 1.0 log10 increase in plasma viral load [18]. However, CD4 counts have low correlation with viral load and thus do not predict transmission [19]. Furthermore, people with high CD4 counts can also have high viral loads during acute and chronic infection. Kranzer et al found that in a South African township, only 13% of the population had a CD4 count ≤200 cells/µL, but 44% had a viral load >10 000 copies/mL [20]. In a rural Ugandan community, Jain et al found that only 17% of the population had a CD4 count ≤200 cells/µL, but 40% had a viral load >10 000 copies/mL [21]. In such settings, the poor correlation would result in the failure of a CD4 count–based ART criterion to substantially reduce HIV transmission.
Produce Consistent Measurements
To develop recommendations for guidelines, a marker used for ART initiation should produce consistent measurements and have low variability within individuals and among populations. International guidelines that rely on a surrogate marker would ideally be applicable at least in sub-Saharan Africa, which has >70% of the global population of people living with HIV [22]. However, CD4 counts vary greatly within individuals, across populations (Table 3), and among testing centers. As a result, a single CD4 measurement, or even repeated CD4 measurements, on the same day can be misleading because it indicates neither the individual's trajectory nor the individual's baseline CD4 count.
Table 3.
Factors Influencing CD4 Cell Count
| Factor | Trend |
|---|---|
| Time of day | Positive correlation [23, 24] |
| Body mass index | Positive correlation [25, 26] |
| Sex | Higher in females [25, 27–29] |
| Smoking | Higher in smokers [25, 27, 28] |
| Age | Positive correlation [29, 30] |
| Environment | Exposure to pathogens, acute illness [31, 32] |
CD4 counts exhibit significant variability depending on multiple factors that are not accounted for in existing antiretroviral therapy (ART) policies. The variability makes CD4 counts an unreliable marker for ART initiation.
Within Individuals
CD4 counts are highly variable within people living with HIV, and repeat measurements do not produce consistent results. CD4 counts have been shown to vary in people living with HIV by as much as 56 cells/µL (P = .038) [23] and 59 cells/µL (P = .018) [24] between morning and afternoon. Other factors such as body mass index [25, 26], sex [25, 27, 28], illness [31, 32], and smoking status [25] also significantly impact CD4 counts (Table 3). However, these factors associated with variations in CD4 counts are not accounted for in ART initiation criteria, and if patients are given different results on different days, the individual may receive ineffective clinical care. For example, a recent study of community-based HIV testing found that 65% of patients who were determined to be eligible for ART by point-of-care CD4 tests during home testing and counseling visits did not initiate ART at the local clinic because they were told they were not eligible when retested [33].
Among Populations
Among populations, CD4 measurements are also highly variable. A review of data from 12 observational studies in 8 countries in Africa found that in people without HIV, CD4 counts vary widely within populations (interquartile range, 169–603 cells/µL) and among populations (range, 699–1244 cells/µL) (Figure 1) [16]. This variability can lead both to healthy persons with low CD4 counts initiating ART and to sick persons with high CD4 counts being withheld ART. For example, the average CD4 count among those without HIV was found to be as high as 1150 cells/µL in Uganda and as low as 700 cells/µL in Botswana. CD4 counts also vary significantly by environmental factors, such as pathogen exposure [31, 32], that are country- and context-specific. Finally, Amornkul et al found a difference of 92 cells/µL (P = .02) between subtype C (503 cells/µL) and subtype A (595 cells/µL), 2 common HIV type 1 subtypes in sub-Saharan Africa, at 3 months after HIV infection [48]. With considerable variability across populations, using a global standard for CD4 count levels for initiation without adjusting for population and context does not make sense.
Figure 1.
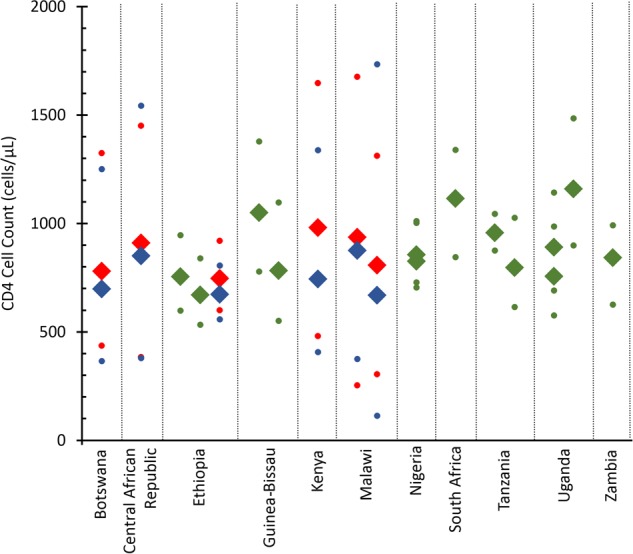
Median CD4 cell counts in African populations (Botswana [34], Central African Republic [35], Ethiopia [36, 37], Guinea-Bissau [38, 39], Kenya [40], Malawi [41], Nigeria [42], South Africa [6], Tanzania [43, 44], Uganda [45, 46], and Zambia [47]). Dots represent 95th percentiles in sample. Blue markers represent males, red markers represent females, and green markers represent both sexes.
Testing Variability
CD4 measurements conducted in laboratories display substantial variability as well. Among laboratories, Raboud et al estimated that 15% of the variability in CD4 measurements from the same blood sample could be attributed to laboratory factors [26]. Furthermore, Peeling et al reviewed 32 studies for 16 types of CD4 testing methods and found a variation of −35.2 to 13.1 cells/µL among the testing methods for those with a CD4 count ≤350 cells/µL, and, more important, a variation of −70.7 to 47 cells/µL for those with a CD4 count >350 cells/µL [49], demonstrating the unreliability of tests, particularly at high CD4 counts when staging for ART initiation is being contested.
Implementation for ART Scale-up
In the past, HIV staging for the initiation of ART was justified in relation to the lack of resources and concerns about ART toxicity. However, ART is now well tolerated [50], more potent [51, 52], easier to take [53], less costly [54], and proven to prevent illness, death, and transmission irrespective of CD4 cell count. Although the long-term effects of ART are unknown, it would be irresponsible for the possibility of long-term adverse effects to outweigh the substantial established immediate benefits of ART.
On the individual level, early ART has been found to significantly reduce the risks of AIDS [55], mortality [56], and HIV transmission in both heterosexual couples [57] and men who have sex with men [58], as well as to increase immune recovery [59]. The recent START (Strategic Timing of AntiRetroviral Treatment) [60] and TEMPRANO [61] trials further strengthened the evidence for initiating early ART by finding that individuals who initiated ART early—with CD4 counts >350 cells/µL or ahead of WHO guidelines, respectively—had a 57% and 44% lower probability of serious adverse events, respectively. These individual-level impacts translate into economic benefits [62, 63] and population-level improvements in life expectancy [64] and HIV morbidity [65]. The accumulation of evidence for earlier treatment has prompted shifts in HIV targets such as the Joint United Nations Programme on HIV/AIDS (UNAIDS) 90-90-90 targets of diagnosing 90% of all people living with HIV, starting ART for 90% of those diagnosed, and achieving viral suppression in 90% of those on ART [66]. The President's Emergency Plan for AIDS Relief (PEPFAR) goal to use the 90-90-90 targets and new WHO and International Association of Providers of AIDS Care (IAPAC) guidelines for test and start [67] as part of delivering the right interventions at the right time in the right place [68], and the IAPAC Fast Track Cities initiative that focuses on 90-90-90 in urban settings [69], are all examples of expanded HIV treatment targets that call for ART initiation irrespective of CD4 count. In addition to international ART targets, at the end of 2014, 8 high- and middle-income countries, including the United States, Brazil, and Australia, already offered ART irrespective of CD4 cell count (Table 1). However, they only represent 3% of the global HIV burden, whereas the majority of people living with HIV live in low- and middle-income countries that still have an ART eligibility criterion.
Large-scale implementation and access to achieve targets such as 90-90-90 would require integrating a laboratory marker such as CD4 count into primary care settings to decentralize HIV care. The test must not hinder other efforts for providing care and timely initiation of ART. CD4 counts do not fit these criteria, as they require reasonably complex and expensive laboratory equipment, and accompanying maintenance and supply chains. Although CD4 access has expanded, costs and access to functioning equipment have been problematic for people living with HIV and raise questions about the feasibility of providing regular CD4 counts for the nearly 37 million people who require lifelong ART.
Facilitate Decentralization
Decentralizing HIV care is critical to having a global impact on the HIV epidemic, but CD4 counts are a barrier to decentralization. Decentralization of care has been associated with reduced morbidity and mortality and increased linkage to ART [70, 71], with the presence of HIV staging in itself being a barrier to patient care. Although point-of-care CD4 tests have been developed and are consistent with laboratory measurements, the current recommendation by the WHO is to use laboratory CD4 cell counts when available [8]. Relying on laboratory CD4 tests, however, anchors ART initiation to large healthcare facilities to process the tests [72], preventing decentralization of HIV care.
Scaling up the HIV response also requires strategic allocation of resources. One important use of resources is monitoring people on ART—previously achieved with CD4 counts—to ensure response to medication, determine prophylaxis for opportunistic infections, and prevent the development of ART-resistant HIV strains. The WHO currently advises using viral load to monitor patients on ART, and a recent review [73] highlights the future of using viral load rather than CD4 counts for ART monitoring. Although CD4 counts play an important role in estimating risk of mortality and determining prophylaxis against some opportunistic infections such as cryptococcal meningitis late in HIV infection [74], their use for ART initiation detracts from other resources such as viral load for ART monitoring. In some settings where national programs recommend cotrimoxazole prophylaxis for all people living with HIV, CD4 counts may not be necessary for prophylaxis.
Improving the Care Continuum
People living with HIV have many stages to complete before being successfully treated with ART. These steps form the HIV care continuum, which tracks the stages of HIV testing, ART eligibility, ART initiation, and viral suppression [75]. Limiting ART eligibility further reduces the likelihood of viral suppression. To increase access to treatment for the estimated 37 million people who are infected with HIV, ART program design and care delivery will need to remove barriers to treatment such as pre-ART, clinic access, and complex regimens. The existence of such barriers has led to only 55% (range, 42%–95%) of people who test HIV positive receiving repeated CD4 counts and initiating ART [76]. In South Africa, the country with the highest burden of HIV, only an estimated 45% and 31% of people living with HIV have been tested or are on ART, respectively [77]. The UNAIDS 90-90-90 targets are designed with these challenges in mind and will, in most settings, require significant programmatic changes. Although CD4 staging is only one barrier to ART, removing CD4 staging and all ART prioritization would greatly enhance the global response to HIV.
CONCLUSIONS
Historically, there has been discordance in global ART initiation guidelines based on CD4 counts, suggesting that CD4 counts may not be a reliable surrogate marker for ART initiation. They do not predict disease progression or transmission, produce widely varying results within and among populations, and pose a barrier to scaling up HIV care and decentralization. CD4 counts should be removed for ART prioritization, and in moving forward, ART should be provided to people living with HIV irrespective of CD4 counts. CD4 counts played an important role early in the HIV epidemic as a concrete, biological clinical surrogate marker with which to rationally distribute scarce and expensive medications. If scarcity and cost were currently more severe, then perhaps a clinical marker such as viral load could prioritize those expected to transmit HIV and rapidly progress to AIDS. However, improvements in ART therapeutic profiles, dramatically reduced costs, and increasing evidence for the benefits of early treatment lead to the conclusion that ART prioritization is no longer necessary. Initiating more patients on ART and eliminating the costs of ART staging will create an environment conducive to HIV care decentralization and scale-up that will put the world on track not only to reach UNAIDS' 90-90-90 targets, but to exceed them and achieve an AIDS-free generation.
Notes
Author contributions. R. Y. was the lead author for this report, developed the outline, and wrote the first draft; all coauthors (R. M. G., S. G., B. G. W.) provided critical input on subsequent drafts. The corresponding author had final responsibility for the decision to submit for publication.
Disclaimer. The funding sources had no role in the conduct of the review, interpretation of the results, or writing of the manuscript.
Financial support. R. Y. was supported by the Whitaker Foundation.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Hammer SM, Squires KE, Hughes MD et al. . A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med 1997; 337:725–33. [DOI] [PubMed] [Google Scholar]
- 2. Gulick RM, Mellors JW, Havlir D et al. . Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med 1997; 337:734–9. [DOI] [PubMed] [Google Scholar]
- 3. Blower SM, Gershengorn HB, Grant RM. A tale of two futures: HIV and antiretroviral therapy in San Francisco. Science 2000; 287:650–4. [DOI] [PubMed] [Google Scholar]
- 4. Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet 2009; 373:48–57. [DOI] [PubMed] [Google Scholar]
- 5. Montaner JS, Hogg R, Wood E et al. . The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet 2006; 368:531–6. [DOI] [PubMed] [Google Scholar]
- 6. Auvert B, Males S, Puren A, Taljaard D, Caraël M, Williams B. Can highly active antiretroviral therapy reduce the spread of HIV? A study in a township of South Africa. J Acquir Immune Defic Syndr 2004; 36:613–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization. Scaling up antiretroviral therapy in resource-limited settings: guidelines for a public health approach. Geneva, Switzerland: WHO, 2002. [PubMed] [Google Scholar]
- 8. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva, Switzerland: WHO, 2013. [PubMed] [Google Scholar]
- 9. Carpenter CC, Cooper DA, Fischl MA et al. . Antiretroviral therapy in adults: updated recommendations of the International AIDS Society-USA Panel. JAMA 2000; 283:381–90. [DOI] [PubMed] [Google Scholar]
- 10. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents, January 28, 2000 by the Panel on Clinical Practices for Treatment of HIV Infection. HIV Clin Trials 2000; 1:60–110. [DOI] [PubMed] [Google Scholar]
- 11. World Health Organization . Treat all people living with HIV, offer antiretrovirals as additional prevention choice for people at “substantial” risk. Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 12. Pantaleo G, Fauci AS. Immunopathogenesis of HIV infection. Annu Rev Microbiol 1996; 50:825–54. [DOI] [PubMed] [Google Scholar]
- 13. Korenromp EL, Williams BG, Schmid GP, Dye C. Clinical prognostic value of RNA viral load and CD4 cell counts during untreated HIV-1 infection—a quantitative review. PLoS One 2009; 4:e5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eller MA, Opollo MS, Liu M et al. . HIV type 1 disease progression to AIDS and death in a rural Ugandan cohort is primarily dependent on viral load despite variable subtype and T-cell immune activation levels. J Infect Dis 2015; 211:1574–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mellors JW, Margolick JB, Phair JP et al. . Prognostic value of HIV-1 RNA, CD4 cell count, and CD4 cell count slope for progression to AIDS and death in untreated HIV-1 infection. JAMA 2007; 297:2349–50. [DOI] [PubMed] [Google Scholar]
- 16. Williams BG, Korenromp EL, Gouws E, Schmid GP, Auvert B, Dye C. HIV infection, antiretroviral therapy, and CD4+ cell count distributions in African populations. J Infect Dis 2006; 194:1450–8. [DOI] [PubMed] [Google Scholar]
- 17. Quinn TC, Wawer MJ, Sewankambo N et al. . Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med 2000; 342:921–9. [DOI] [PubMed] [Google Scholar]
- 18. Chuachoowong R, Shaffer N, Siriwasin W et al. . Short-course antenatal zidovudine reduces both cervicovaginal human immunodeficiency virus type 1 RNA levels and risk of perinatal transmission. Bangkok Collaborative Perinatal HIV Transmission Study Group. J Infect Dis 2000; 181:99–106. [DOI] [PubMed] [Google Scholar]
- 19. Anastos K, Gange SJ, Lau B et al. . Association of race and gender with HIV-1 RNA levels and immunologic progression. J Acquir Immune Defic Syndr 2000; 24:218–26. [DOI] [PubMed] [Google Scholar]
- 20. Kranzer K, Lawn SD, Johnson LF, Bekker LG, Wood R. Community viral load and CD4 count distribution among people living with HIV in a South African township: implications for treatment as prevention. J Acquir Immune Defic Syndr 2013; 63:498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jain V, Liegler T, Kabami J et al. . Assessment of population-based HIV RNA levels in a rural east African setting using a fingerprick-based blood collection method. Clin Infect Dis 2013; 56:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Joint United Nations Programme on HIV/AIDS. The Gap Report. Geneva, Switzerland: UNAIDS, 2014. [Google Scholar]
- 23. Bekele Y, Mengistu Y, de Wit TR, Wolday D. Timing of blood sampling for CD4 T-cell counting influences HAART decisions. Ethiop Med J 2011; 49:187–97. [PubMed] [Google Scholar]
- 24. Malone JL, Simms TE, Gray GC, Wagner KF, Burge JR, Burke DS. Sources of variability in repeated T-helper lymphocyte counts from human immunodeficiency virus type 1-infected patients: total lymphocyte count fluctuations and diurnal cycle are important. J Acquir Immune Defic Syndr 1990; 3:144–51. [PubMed] [Google Scholar]
- 25. Abuye C, Tsegaye A, West CE et al. . Determinants of CD4 counts among HIV-negative Ethiopians: role of body mass index, gender, cigarette smoking, khat (Catha edulis) chewing, and possibly altitude? J Clin Immunol 2005; 25:127–33. [DOI] [PubMed] [Google Scholar]
- 26. Raboud JM, Haley L, Montaner JS, Murphy C, Januszewska M, Schechter MT. Quantification of the variation due to laboratory and physiologic sources in CD4 lymphocyte counts of clinically stable HIV-infected individuals. J Acquir Immune Defic Syndr Hum Retrovirol 1995; 10(suppl 2):S67–73. [PubMed] [Google Scholar]
- 27. Mair C, Hawes SE, Agne HD et al. . Factors associated with CD4 lymphocyte counts in HIV-negative Senegalese individuals. Clin Exp Immunol 2008; 151:432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maini MK, Gilson RJ, Chavda N et al. . Reference ranges and sources of variability of CD4 counts in HIV-seronegative women and men. Genitourin Med 1996; 72:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malaza A, Mossong J, Bärnighausen T, Viljoen J, Newell ML. Population-based CD4 counts in a rural area in South Africa with high HIV prevalence and high antiretroviral treatment coverage. PLoS One 2013; 8:e70126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Van der Paal L, Shafer LA, Todd J, Mayanja BN, Whitworth JA, Grosskurth H. HIV-1 disease progression and mortality before the introduction of highly active antiretroviral therapy in rural Uganda. AIDS 2007; 21(suppl 6):S21–9. [DOI] [PubMed] [Google Scholar]
- 31. Clerici M, Butto S, Lukwiya M et al. . Immune activation in Africa is environmentally-driven and is associated with upregulation of CCR5. Italian-Ugandan AIDS Project. AIDS 2000; 14:2083–92. [DOI] [PubMed] [Google Scholar]
- 32. Williams RC, Koster FT, Kilpatrick KA. Alterations in lymphocyte cell surface markers during various human infections. Am J Med 1983; 75:807–16. [DOI] [PubMed] [Google Scholar]
- 33. Barnabas RV, van Rooyen H, Tumwesigye E et al. . Initiation of antiretroviral therapy and viral suppression after home HIV testing and counselling in KwaZulu-Natal, South Africa, and Mbarara district, Uganda: a prospective, observational intervention study. Lancet HIV 2014; 1:e68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bussmann H, Wester CW, Masupu KV et al. . Low CD4+ T-lymphocyte values in human immunodeficiency virus-negative adults in Botswana. Clin Diagn Lab Immunol 2004; 11:930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Menard D, Mandeng MJ, Tothy MB, Kelembho EK, Gresenguet G, Talarmin A. Immunohematological reference ranges for adults from the Central African Republic. Clin Diagn Lab Immunol 2003; 10:443–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kassu A, Tsegaye A, Petros B et al. . Distribution of lymphocyte subsets in healthy human immunodeficiency virus-negative adult Ethiopians from two geographic locales. Clin Diagn Lab Immunol 2001; 8:1171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kassa E, Rinke de Wit TF, Hailu E et al. . Evaluation of the World Health Organization staging system for HIV infection and disease in Ethiopia: association between clinical stages and laboratory markers. AIDS 1999; 13:381–9. [DOI] [PubMed] [Google Scholar]
- 38. Ricard D, Wilkins A, N'Gum PT et al. . The effects of HIV-2 infection in a rural area of Guinea-Bissau. AIDS 1994; 8:977–82. [DOI] [PubMed] [Google Scholar]
- 39. Lisse IM, Poulsen AG, Aaby P, Knudsen K, Dias F. Serial CD4 and CD8 T-lymphocyte counts and associated mortality in an HIV-2-infected population in Guinea-Bissau. J Acquir Immune Defic Syndr Hum Retrovirol 1996; 13:355–62. [DOI] [PubMed] [Google Scholar]
- 40. Kibaya RS, Bautista CT, Sawe FK et al. . Reference ranges for the clinical laboratory derived from a rural population in Kericho, Kenya. PLoS One 2008; 3:e3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Crampin AC, Mwaungulu FD, Ambrose LR, Longwe H, French N. Normal range of CD4 cell counts and temporal changes in two HIV-negative Malawian populations. Open AIDS J 2011; 5:74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aina O, Dadik J, Charurat M et al. . Reference values of CD4T lymphocytes in human immunodeficiency virus-negative adult Nigerians. Clin Diagn Lab Immunol 2005; 12:525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Levin A, Brubaker G, Shao JS et al. . Determination of T-lymphocyte subsets on site in rural Tanzania: results in HIV-1 infected and non-infected individuals. Int J STD AIDS 1996; 7:288–91. [DOI] [PubMed] [Google Scholar]
- 44. Urassa W, Bakari M, Sandström E et al. . Rate of decline of absolute number and percentage of CD4 T lymphocytes among HIV-1-infected adults in Dar es Salaam, Tanzania. AIDS 2004; 18:433–8. [DOI] [PubMed] [Google Scholar]
- 45. Lugada ES, Mermin J, Kaharuza F et al. . Population-based hematologic and immunologic reference values for a healthy Ugandan population. Clin Diagn Lab Immunol 2004; 11:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tugume SB, Piwowar EM, Lutalo T et al. . Hematological reference ranges among healthy Ugandans. Clin Diagn Lab Immunol 1995; 2:233–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kelly P, Zulu I, Amadi B et al. . Morbidity and nutritional impairment in relation to CD4 count in a Zambian population with high HIV prevalence. Acta Trop 2002; 83:151–8. [DOI] [PubMed] [Google Scholar]
- 48. Amornkul PN, Karita E, Kamali A et al. . Disease progression by infecting HIV-1 subtype in a seroconverter cohort in sub-Saharan Africa. AIDS 2013; 27:2775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Peeling RW, Sollis KA, Glover S et al. . CD4 enumeration technologies: a systematic review of test performance for determining eligibility for antiretroviral therapy. PLoS One 2015; 10:e0115019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Patterson P, Socías E, Pryluka D, Lapadula P, Pérez H, Cahn P. Switching to nevirapine-based regimens after undetectable viral load is not associated with increased risk of discontinuation due to toxicity. J Int AIDS Soc 2014; 17(4 suppl 3):19794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Walmsley SL, Antela A, Clumeck N et al. . Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med 2013; 369:1807–18. [DOI] [PubMed] [Google Scholar]
- 52. Steigbigel RT, Cooper DA, Kumar PN et al. . Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med 2008; 359:339–54. [DOI] [PubMed] [Google Scholar]
- 53. Hanna DB, Hessol NA, Golub ET et al. . Increase in single-tablet regimen use and associated improvements in adherence-related outcomes in HIV-infected women. J Acquir Immune Defic Syndr 2014; 65:587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Clinton Health Access Initiative. Antiretroviral (ARV) ceiling price list. 2013. Available at: http://www.clintonhealthaccess.org/chai-arv-ceiling-price-list-2014/. Accessed 11 January 2016.
- 55. Grinsztejn B, Hosseinipour MC, Ribaudo HJ et al. . Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis 2014; 14:281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kitahata MM, Gange SJ, Abraham AG et al. . Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med 2009; 360:1815–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cohen MS, Chen YQ, McCauley M et al. . Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rodger A, Bruun T, Cambiano V, Lundgren J. HIV transmission risk through condomless sex if HIV+ partner on suppressive ART: PARTNER Study. In: Conference on Retroviruses and Opportunistic Infections, Boston, MA, 2014. [Google Scholar]
- 59. Gras L, Kesselring AM, Griffin JT et al. . CD4 cell counts of 800 cells/mm3 or greater after 7 years of highly active antiretroviral therapy are feasible in most patients starting with 350 cells/mm3 or greater. J Acquir Immune Defic Syndr 2007; 45:183–92. [DOI] [PubMed] [Google Scholar]
- 60. INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. TEMPRANO ANRS 12136 Study Group. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015; 373:808–22. [DOI] [PubMed] [Google Scholar]
- 62. Thirumurthy H, Jafri A, Srinivas G et al. . Two-year impacts on employment and income among adults receiving antiretroviral therapy in Tamil Nadu, India: a cohort study. AIDS 2011; 25:239–46. [DOI] [PubMed] [Google Scholar]
- 63. Bor J, Tanser F, Newell ML, Bärnighausen T. In a study of a population cohort in South Africa, HIV patients on antiretrovirals had nearly full recovery of employment. Health Aff (Millwood) 2012; 31:1459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bor J, Herbst AJ, Newell ML, Bärnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science 2013; 339:961–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Montaner JS, Lima VD, Harrigan PR et al. . Expansion of HAART coverage is associated with sustained decreases in HIV/AIDS morbidity, mortality and HIV transmission: the “HIV Treatment as Prevention” experience in a Canadian setting. PLoS One 2014; 9:e87872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Joint United Nations Programme on HIV/AIDS. Ambitious treatment targets: writing the final chapter of the AIDS epidemic. Geneva, Switzerland: UNAIDS, 2014. [Google Scholar]
- 67. International Association of Providers of AIDS Care. Guidelines on optimizing the HIV care continuum recommend ‘test and start’ irrespective of CD4 count. Paris: IAPAC, 2015. [Google Scholar]
- 68. Birx D. PEPFAR's lessons learned: shared responsibility and accountability. In: 20th International AIDS Conference, Melbourne, Australia, 2014. [Google Scholar]
- 69. Joint United Nations Programme on HIV/AIDS. UNAIDS outlook: the cities report. Geneva, Switzerland: UNAIDS, 2014. [Google Scholar]
- 70. Mee P, Collinson MA, Madhavan S et al. . Determinants of the risk of dying of HIV/AIDS in a rural South African community over the period of the decentralised roll-out of antiretroviral therapy: a longitudinal study. Glob Health Action 2014; 7:24826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Auld AF. Decentralizing access to antiretroviral therapy services for adults in Swaziland. In: Conference on Retroviruses and Opportunistic Infections, Seattle, WA, 2015. [Google Scholar]
- 72. Alemnji G, Fonjungo P, Van Der Pol B, Peter T, Kantor R, Nkengasong J. The centrality of laboratory services in the HIV treatment and prevention cascade: the need for effective linkages and referrals in resource-limited settings. AIDS Patient Care STDS 2014; 28:268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ford N, Meintjes G, Pozniak A et al. . The future role of CD4 cell count for monitoring antiretroviral therapy. Lancet Infect Dis 2014; 15:241–7. [DOI] [PubMed] [Google Scholar]
- 74. Mugyenyi P, Walker AS, Hakim J et al. . Routine versus clinically driven laboratory monitoring of HIV antiretroviral therapy in Africa (DART): a randomised non-inferiority trial. Lancet 2010; 375:123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. McNairy ML, El-Sadr WM. The HIV care continuum: no partial credit given. AIDS 2012; 26:1735–8. [DOI] [PubMed] [Google Scholar]
- 76. Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med 2011; 8:e1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Shisana O, Rehle T, Simbayi L et al. . South African national HIV prevalence, incidence and behaviour survey, 2012. Cape Town: HSRC Press, 2014. [Google Scholar]


