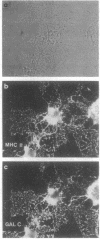
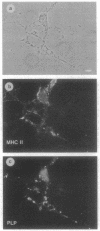
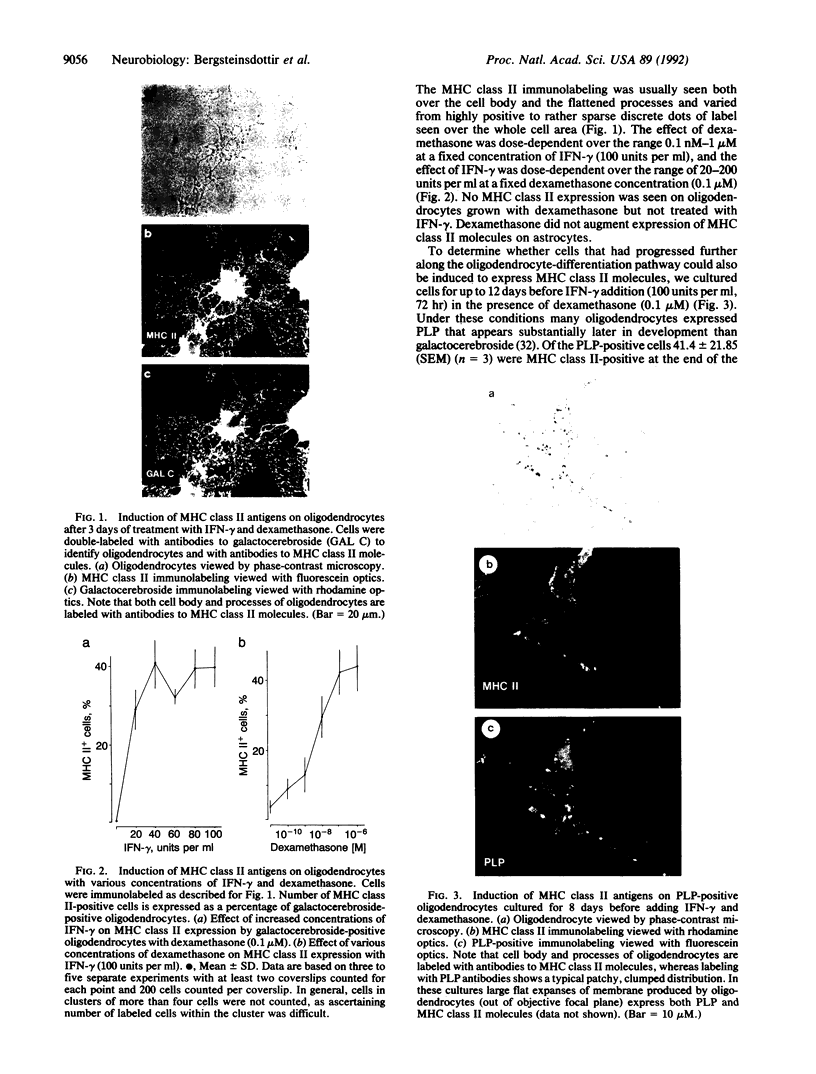
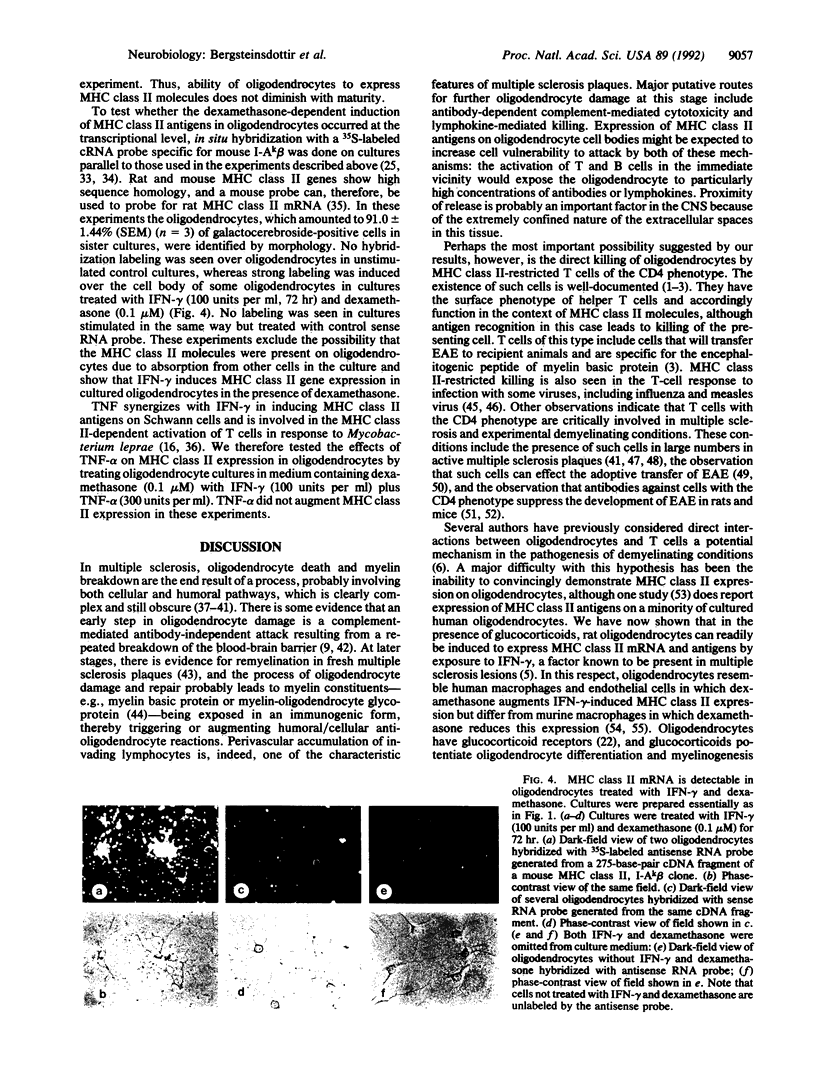
Abstract
Cells that express major histocompatibility complex (MHC) class II molecules can interact directly with CD4 T lymphocytes and either activate immune reactions or become the targets of T-cell-mediated cytotoxic attack. Using rat optic nerve cultures combined with immunocytochemistry and in situ hybridization, we have shown that oligodendrocytes, the major myelin-forming cells of the central nervous system and the main casualty of the immune attacks associated with multiple sclerosis and experimental allergic encephalomyelitis, can be readily induced to express MHC class II mRNA and surface antigens in vitro by exposure to gamma interferon, provided the glucocorticoid dexamethasone is included in the culture medium. Oligodendrocytes exposed to gamma interferon without dexamethasone fail to express MHC class II molecules, which may account for the failure of previous attempts to induce expression in these cells. In the experiments reported here MHC class II expression can be demonstrated both on galactocerebroside-positive cells and on mature oligodendrocytes that express proteolipid protein. These findings expand possibilities for understanding immune-related oligodendrocyte killing and demyelination in human and experimental demyelinating diseases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergsteinsdóttir K., Kingston A., Jessen K. R. Rat Schwann cells can be induced to express major histocompatibility complex class II molecules in vivo. J Neurocytol. 1992 May;21(5):382–390. doi: 10.1007/BF01191706. [DOI] [PubMed] [Google Scholar]
- Birmingham M. K., Sar M., Stumpf W. E. Localization of aldosterone and corticosterone in the central nervous system, assessed by quantitative autoradiography. Neurochem Res. 1984 Mar;9(3):333–350. doi: 10.1007/BF00963982. [DOI] [PubMed] [Google Scholar]
- Bloom B. R. Pathogenesis of a Third World disease: lessons from leprosy. Harvey Lect. 1988;84:41–63. [PubMed] [Google Scholar]
- Bohn M. C., Friedrich V. L., Jr Recovery of myelination in rat optic nerve after developmental retardation by cortisol. J Neurosci. 1982 Sep;2(9):1292–1298. doi: 10.1523/JNEUROSCI.02-09-01292.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdette D. N., Vandenbark A. A., Meshul C., Whitham R., Offner H. Basic protein-specific T-cell lines that induce experimental autoimmune encephalomyelitis in SJL/J mice: comparison with Lewis rat lines. Cell Immunol. 1988 Apr 1;112(2):351–363. doi: 10.1016/0008-8749(88)90304-8. [DOI] [PubMed] [Google Scholar]
- Brosnan C. F., Selmaj K., Raine C. S. Hypothesis: a role for tumor necrosis factor in immune-mediated demyelination and its relevance to multiple sclerosis. J Neuroimmunol. 1988 Apr;18(1):87–94. doi: 10.1016/0165-5728(88)90137-3. [DOI] [PubMed] [Google Scholar]
- Calder V. L., Wolswijk G., Noble M. The differentiation of O-2A progenitor cells into oligodendrocytes is associated with a loss of inducibility of Ia antigens. Eur J Immunol. 1988 Aug;18(8):1195–1201. doi: 10.1002/eji.1830180808. [DOI] [PubMed] [Google Scholar]
- Calder V., Owen S., Watson C., Feldmann M., Davison A. MS: a localized immune disease of the central nervous system. Immunol Today. 1989 Mar;10(3):99–103. doi: 10.1016/0167-5699(89)90235-1. [DOI] [PubMed] [Google Scholar]
- Compston A. Lymphocyte subpopulations in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 1983 Feb;46(2):105–114. doi: 10.1136/jnnp.46.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston A., Scolding N., Wren D., Noble M. The pathogenesis of demyelinating disease: insights from cell biology. Trends Neurosci. 1991 May;14(5):175–182. doi: 10.1016/0166-2236(91)90099-g. [DOI] [PubMed] [Google Scholar]
- Diamond A. G., Hood L. E., Howard J. C., Windle M., Winoto A. The class II genes of the rat MHC. J Immunol. 1989 May 1;142(9):3268–3274. [PubMed] [Google Scholar]
- Dubois-Dalcq M., Behar T., Hudson L., Lazzarini R. A. Emergence of three myelin proteins in oligodendrocytes cultured without neurons. J Cell Biol. 1986 Feb;102(2):384–392. doi: 10.1083/jcb.102.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles S. J., McMaster W. R. DNA sequence analysis of a rat RT1 class II A beta gene. Immunogenetics. 1985;22(6):653–663. doi: 10.1007/BF00430314. [DOI] [PubMed] [Google Scholar]
- Eisenbarth G. S., Walsh F. S., Nirenberg M. Monoclonal antibody to a plasma membrane antigen of neurons. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4913–4917. doi: 10.1073/pnas.76.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estess P., Begovich A. B., Koo M., Jones P. P., McDevitt H. O. Sequence analysis and structure-function correlations of murine q, k, u, s, and f haplotype I-A beta cDNA clones. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3594–3598. doi: 10.1073/pnas.83.11.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertsch-Ruggio D., Schoenberg D. R., Vogel S. N. Induction of macrophage Ia antigen expression by rIFN-gamma and down-regulation by IFN-alpha/beta and dexamethasone are regulated transcriptionally. J Immunol. 1988 Sep 1;141(5):1582–1589. [PubMed] [Google Scholar]
- Fierz W., Endler B., Reske K., Wekerle H., Fontana A. Astrocytes as antigen-presenting cells. I. Induction of Ia antigen expression on astrocytes by T cells via immune interferon and its effect on antigen presentation. J Immunol. 1985 Jun;134(6):3785–3793. [PubMed] [Google Scholar]
- Fontana A., Fierz W., Wekerle H. Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature. 1984 Jan 19;307(5948):273–276. doi: 10.1038/307273a0. [DOI] [PubMed] [Google Scholar]
- Gilfillan S., Aiso S., Michie S. A., McDevitt H. O. The effect of excess beta-chain synthesis on cell-surface expression of allele-mismatched class II heterodimers in vivo. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7314–7318. doi: 10.1073/pnas.87.18.7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafler D. A., Weiner H. L. MS: a CNS and systemic autoimmune disease. Immunol Today. 1989 Mar;10(3):104–107. doi: 10.1016/0167-5699(89)90236-3. [DOI] [PubMed] [Google Scholar]
- Harding C. V., Unanue E. R. Quantitation of antigen-presenting cell MHC class II/peptide complexes necessary for T-cell stimulation. Nature. 1990 Aug 9;346(6284):574–576. doi: 10.1038/346574a0. [DOI] [PubMed] [Google Scholar]
- Hauser S. L., Bhan A. K., Gilles F., Kemp M., Kerr C., Weiner H. L. Immunohistochemical analysis of the cellular infiltrate in multiple sclerosis lesions. Ann Neurol. 1986 Jun;19(6):578–587. doi: 10.1002/ana.410190610. [DOI] [PubMed] [Google Scholar]
- Hirayama M., Eccleston P. A., Silberberg D. H. The mitotic history and radiosensitivity of developing oligodendrocytes in vitro. Dev Biol. 1984 Aug;104(2):413–420. doi: 10.1016/0012-1606(84)90096-4. [DOI] [PubMed] [Google Scholar]
- Hofman F. M., von Hanwehr R. I., Dinarello C. A., Mizel S. B., Hinton D., Merrill J. E. Immunoregulatory molecules and IL 2 receptors identified in multiple sclerosis brain. J Immunol. 1986 May 1;136(9):3239–3245. [PubMed] [Google Scholar]
- Jacobson S., Nepom G. T., Richert J. R., Biddison W. E., McFarland H. F. Identification of a specific HLA DR2 Ia molecule as a restriction element for measles virus-specific HLA class II-restricted cytotoxic T cell clones. J Exp Med. 1985 Jan 1;161(1):263–268. doi: 10.1084/jem.161.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai K., Zweiman B. Characteristics of in vitro cytotoxic effects of myelin basic protein-reactive T cell lines on syngeneic oligodendrocytes. J Neuroimmunol. 1990 Jan;26(1):57–67. doi: 10.1016/0165-5728(90)90120-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. U., Moretto G., Shin D. H. Expression of Ia antigens on the surface of human oligodendrocytes and astrocytes in culture. J Neuroimmunol. 1985 Dec;10(2):141–149. doi: 10.1016/0165-5728(85)90004-9. [DOI] [PubMed] [Google Scholar]
- Kingston A. E., Bergsteinsdottir K., Jessen K. R., Van der Meide P. H., Colston M. J., Mirsky R. Schwann cells co-cultured with stimulated T cells and antigen express major histocompatibility complex (MHC) class II determinants without interferon-gamma pretreatment: synergistic effects of interferon-gamma and tumor necrosis factor on MHC class II induction. Eur J Immunol. 1989 Jan;19(1):177–183. doi: 10.1002/eji.1830190128. [DOI] [PubMed] [Google Scholar]
- Kumar S., Cole R., Chiappelli F., de Vellis J. Differential regulation of oligodendrocyte markers by glucocorticoids: post-transcriptional regulation of both proteolipid protein and myelin basic protein and transcriptional regulation of glycerol phosphate dehydrogenase. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6807–6811. doi: 10.1073/pnas.86.17.6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C., Raine C. S. Multiple sclerosis: oligodendrocytes in active lesions do not express class II major histocompatibility complex molecules. J Neuroimmunol. 1989 Dec;25(2-3):261–266. doi: 10.1016/0165-5728(89)90145-8. [DOI] [PubMed] [Google Scholar]
- Lisak R. P., Hirayama M., Kuchmy D., Rosenzweig A., Kim S. U., Pleasure D. E., Silberberg D. H. Cultured human and rat oligodendrocytes and rat Schwann cells do not have immune response gene associated antigen (Ia) on their surface. Brain Res. 1983 Dec 19;289(1-2):285–292. doi: 10.1016/0006-8993(83)90029-x. [DOI] [PubMed] [Google Scholar]
- Lukacher A. E., Morrison L. A., Braciale V. L., Malissen B., Braciale T. J. Expression of specific cytolytic activity by H-2I region-restricted, influenza virus-specific T lymphocyte clones. J Exp Med. 1985 Jul 1;162(1):171–187. doi: 10.1084/jem.162.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manyak C. L., Tse H., Fischer P., Coker L., Sigal N. H., Koo G. C. Regulation of class II MHC molecules on human endothelial cells. Effects of IFN and dexamethasone. J Immunol. 1988 Jun 1;140(11):3817–3821. [PubMed] [Google Scholar]
- Mason D. Genetic variation in the stress response: susceptibility to experimental allergic encephalomyelitis and implications for human inflammatory disease. Immunol Today. 1991 Feb;12(2):57–60. doi: 10.1016/0167-5699(91)90158-P. [DOI] [PubMed] [Google Scholar]
- McMaster W. R., Williams A. F. Identification of Ia glycoproteins in rat thymus and purification from rat spleen. Eur J Immunol. 1979 Jun;9(6):426–433. doi: 10.1002/eji.1830090603. [DOI] [PubMed] [Google Scholar]
- Meyer J. S. Biochemical effects of corticosteroids on neural tissues. Physiol Rev. 1985 Oct;65(4):946–1020. doi: 10.1152/physrev.1985.65.4.946. [DOI] [PubMed] [Google Scholar]
- Miller R. H., David S., Patel R., Abney E. R., Raff M. C. A quantitative immunohistochemical study of macroglial cell development in the rat optic nerve: in vivo evidence for two distinct astrocyte lineages. Dev Biol. 1985 Sep;111(1):35–41. doi: 10.1016/0012-1606(85)90432-4. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Ross D. T., Briner T. J., Gefter M. L. Cytolytic activity of antigen-specific T cells with helper phenotype. J Immunol. 1986 Jan;136(1):44–47. [PubMed] [Google Scholar]
- Poduslo S. E., Pak C. H., Miller K. Hydrocortisone induction during oligodendroglial differentiation. Neurosci Lett. 1990 May 18;113(1):84–88. doi: 10.1016/0304-3940(90)90499-y. [DOI] [PubMed] [Google Scholar]
- Prineas J. W., Kwon E. E., Goldenberg P. Z., Ilyas A. A., Quarles R. H., Benjamins J. A., Sprinkle T. J. Multiple sclerosis. Oligodendrocyte proliferation and differentiation in fresh lesions. Lab Invest. 1989 Nov;61(5):489–503. [PubMed] [Google Scholar]
- Samuel N. M., Jessen K. R., Grange J. M., Mirsky R. Gamma interferon, but not Mycobacterium leprae, induces major histocompatibility class II antigens on cultured rat Schwann cells. J Neurocytol. 1987 Apr;16(2):281–287. doi: 10.1007/BF01795311. [DOI] [PubMed] [Google Scholar]
- Sapolsky R. M., Krey L. C., McEwen B. S. Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J Neurosci. 1985 May;5(5):1222–1227. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skias D. D., Kim D. K., Reder A. T., Antel J. P., Lancki D. W., Fitch F. W. Susceptibility of astrocytes to class I MHC antigen-specific cytotoxicity. J Immunol. 1987 May 15;138(10):3254–3258. [PubMed] [Google Scholar]
- Sun D., Wekerle H. Ia-restricted encephalitogenic T lymphocytes mediating EAE lyse autoantigen-presenting astrocytes. Nature. 1986 Mar 6;320(6057):70–72. doi: 10.1038/320070a0. [DOI] [PubMed] [Google Scholar]
- Tite J. P., Powell M. B., Ruddle N. H. Protein-antigen specific Ia-restricted cytolytic T cells: analysis of frequency, target cell susceptibility, and mechanism of cytolysis. J Immunol. 1985 Jul;135(1):25–33. [PubMed] [Google Scholar]
- Traugott U., Lebon P. Interferon-gamma and Ia antigen are present on astrocytes in active chronic multiple sclerosis lesions. J Neurol Sci. 1988 Apr;84(2-3):257–264. doi: 10.1016/0022-510x(88)90130-x. [DOI] [PubMed] [Google Scholar]
- Traugott U., Reinherz E. L., Raine C. S. Multiple sclerosis: distribution of T cell subsets within active chronic lesions. Science. 1983 Jan 21;219(4582):308–310. doi: 10.1126/science.6217550. [DOI] [PubMed] [Google Scholar]
- Vielkind U., Walencewicz A., Levine J. M., Bohn M. C. Type II glucocorticoid receptors are expressed in oligodendrocytes and astrocytes. J Neurosci Res. 1990 Nov;27(3):360–373. doi: 10.1002/jnr.490270315. [DOI] [PubMed] [Google Scholar]
- Waksman B. H., Reynolds W. E. Multiple sclerosis as a disease of immune regulation. Proc Soc Exp Biol Med. 1984 Mar;175(3):282–294. doi: 10.3181/00379727-175-41798. [DOI] [PubMed] [Google Scholar]
- Waldor M. K., Sriram S., Hardy R., Herzenberg L. A., Herzenberg L. A., Lanier L., Lim M., Steinman L. Reversal of experimental allergic encephalomyelitis with monoclonal antibody to a T-cell subset marker. Science. 1985 Jan 25;227(4685):415–417. doi: 10.1126/science.3155574. [DOI] [PubMed] [Google Scholar]
- Walker W. S., Beelen R. H., Buckley P. J., Melvin S. L., Yen S. E. Some fixation reagents reduce or abolish the detectability of Ia-antigen and HLA-DR on cells. J Immunol Methods. 1984 Feb 24;67(1):89–99. doi: 10.1016/0022-1759(84)90088-7. [DOI] [PubMed] [Google Scholar]
- Watts T. H., McConnell H. M. High-affinity fluorescent peptide binding to I-Ad in lipid membranes. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9660–9664. doi: 10.1073/pnas.83.24.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekerle H., Schwab M., Linington C., Meyermann R. Antigen presentation in the peripheral nervous system: Schwann cells present endogenous myelin autoantigens to lymphocytes. Eur J Immunol. 1986 Dec;16(12):1551–1557. doi: 10.1002/eji.1830161214. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Bartlett P. F., Clark-Lewis I., Battye F., Schrader J. W. Inducible expression of H-2 and Ia antigens on brain cells. Nature. 1984 Aug 23;310(5979):688–691. doi: 10.1038/310688a0. [DOI] [PubMed] [Google Scholar]
- Wren D. R., Noble M. Oligodendrocytes and oligodendrocyte/type-2 astrocyte progenitor cells of adult rats are specifically susceptible to the lytic effects of complement in absence of antibody. Proc Natl Acad Sci U S A. 1989 Nov;86(22):9025–9029. doi: 10.1073/pnas.86.22.9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B. G., Linington C., Link H. Antibodies to myelin-oligodendrocyte glycoprotein in cerebrospinal fluid from patients with multiple sclerosis and controls. J Neuroimmunol. 1991 Feb;31(2):91–96. doi: 10.1016/0165-5728(91)90014-x. [DOI] [PubMed] [Google Scholar]
- Zamvil S. S., Mitchell D. J., Moore A. C., Kitamura K., Steinman L., Rothbard J. B. T-cell epitope of the autoantigen myelin basic protein that induces encephalomyelitis. Nature. 1986 Nov 20;324(6094):258–260. doi: 10.1038/324258a0. [DOI] [PubMed] [Google Scholar]
- van der Meide P. H., Dubbeld M., Vijverberg K., Kos T., Schellekens H. The purification and characterization of rat gamma interferon by use of two monoclonal antibodies. J Gen Virol. 1986 Jun;67(Pt 6):1059–1071. doi: 10.1099/0022-1317-67-6-1059. [DOI] [PubMed] [Google Scholar]