Abstract
Introduction:
Tuberculosis (TB) is one of the leading causes of death and disease worldwide. Tobacco smoking has been linked as a risk factor for TB. This study was aimed to affirm the strength of association between smoking and pulmonary TB.
Materials and Methods:
Pulmonary TB patients aged between 18 and 65 years were enrolled and followed-up until treatment completion. Two consecutive sputum smears were examined from each patient for the presence of acid-fast bacilli (AFB) using Ziehl–Neelsen technique. Radiological severity of disease was assessed using guidelines of National TB Association of USA. Sputum smears for AFB were graded for positivity as per WHO Revised National TB Control Programme criteria. Response was determined in terms of sputum conversion at the end of intensive phase and final treatment outcomes.
Results:
Sputum smear grading of 3+ increased from 12.5% to 68.18% and 66.66% as smoking index increased from <100 to 100–299 and >300 (P < 0.05). In nonsmokers, 79.2% patients had minimal disease while only 4.2% had advanced disease as compared to smokers where 52.4% had moderate disease, 26.2% advanced disease, and 21.4% minimal disease (P < 0.01). Smokers had significantly lower treatment success rate (69%) as against nonsmokers and former smokers (93.8% and 90.9%, respectively, P = 0.001) owing to a higher default rate among smokers (28.5%) than nonsmokers (6.3%) and former smokers (9.1%).
Conclusion:
Smokers during initial presentation, as well as at end of the treatment demonstrate more radiological findings, cavitary disease, and worse sputum AFB smear grading. Smokers also have a poorer treatment success rate largely due to high percentage of default rate thus suggesting noncompliance as a main confounder to treatment success. Focus needs to be made to reduce defaulters which are more common among smokers.
Keywords: Smoking, smoking and tuberculosis, smoking cessation, tuberculosis
INTRODUCTION
Tuberculosis (TB) is one of the leading infectious causes of death and disease worldwide and remains a major public health problem, despite socioeconomic development and advances in medical science. The WHO Global TB Control Report 2013 based on surveillance and survey data estimates that 8.6 million new cases of TB occurred in 2012 of which India accounts for about 2.0–2.4 million.[1] Smoking has been consistently linked with TB in recent years, and the increased risk among smokers may be explained by the effect of smoking on pulmonary host defences.
Chronic exposure to tobacco, as well as to a number of environmental pollutants, impairs the normal clearance of secretion on the trachea-bronchial mucosal surface and may thus allow the causative organism Mycobacterium tuberculosis, to escape the first level of host defences which prevent bacilli from reaching the alveoli.[2] Smoke also impairs the function of pulmonary alveolar surfactants.[3] Recent work has suggested a novel mechanism. Nicotine is hypothesized to act directly on nicotine acetylcholine receptors on macrophages to decrease production of intracellular tumor necrosis factor and thus impair killing of M. tuberculosis.[4] These effects of smoking on pulmonary host defence support a causal link between smoke exposure and either an increased risk of acquiring TB or progression of TB. Several studies have shown an association between smoking and TB-related infection, disease and increased mortality.[5,6,7,8,9] A recent meta-analysis reported that those who had a close contact with a smoking household member were 9 times more likely to have TB as compared to those who were distant contacts.[10] Although the current approach to TB control focuses on case detection and treatment, recent studies suggest that this strategy alone might not be sufficient to achieve this goal and it may also be necessary to reduce risk factors that contribute to the occurrence of TB infection and/or disease. Such risk factors may act at one of several steps in the natural history of the disease.[5] Tobacco attributable deaths are projected to increase from 3 million in 1990 to 8·4 million in 2020.[11] This harmful socioeconomic factor is less thought as a contributor to the morbidity and mortality from TB.
Thus, this study was done to affirm the strength of association between smoking and pulmonary TB and also to see the consequences that smoking has on the treatment outcome of this disease.
MATERIALS AND METHODS
Between July 2010 and March 2011, an observational study was conducted among 101 successive patients aged 18 years or more who were registered cases of pulmonary TB with National Institute of TB and Respiratory Diseases directly observed treatment short course center under Revised National TB Control Programme (RNTCP). Drug resistant and extrapulmonary TB patients and pregnant females were excluded. Prior to enrolment, approval of the Institute Ethics Committee was obtained. Patients were included irrespective of their previous anti-tubercular treatment status. All patients had two consecutive sputum smears examined for the presence of acid-fast bacilli (AFB) using Ziehl–Neelsen technique at the initiation of treatment and repeated as per recommendations of the RNTCP.[12] Among TB patients; smokers and nonsmokers were separately evaluated. A predesigned proforma was used to capture the clinical and radiological findings. Sputum AFB smear grading was done as per WHO guideline.[12] The radiological severity of the disease was assessed based on the guidelines of National TB Association of USA at presentation and end of the treatment.[13] Treatment outcomes were recorded as per RNTCP guidelines.[12] The radiological severity and sputum bacillary load were also assessed in terms of smoking index. Smokers were further divided as current smokers and former smokers. Former smokers were defined as a person who had smoked ≥100 cigarettes/beedis in his lifetime but was not smoking at the time of interview.
Statistical analysis
Quantitative and qualitative data were expressed as median (interquartile range) and percentages, respectively. Quantitative data were compared using Mann–Whitney U-test while qualitative variables were compared using Pearson Chi-square test. A “P” value of <0.05 was considered significant.
RESULTS
Characteristics of enrolled patients
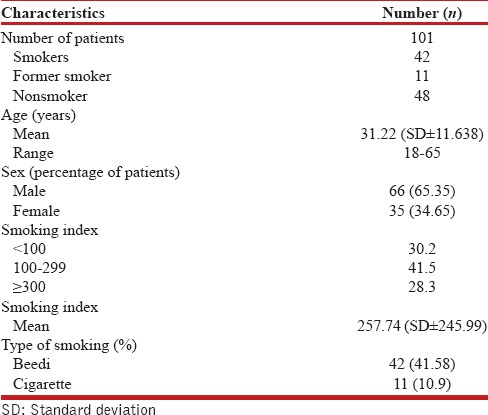
A total of 101 patients, aged between 18 and 65 years were enrolled over a period of 9 months. The male-female ratio was 1·8:1 with a mean age of 31·22 years (± standard deviation 11·638). Majority (40.6%) of the enrolled patients were in the age group of 21–30 years. Among male patients (n = 66), 62.12% were smokers, 13.63% former smokers while among females (n = 35) there were 2.86% smokers and 5.71% former smokers. Of the enrolled, 42 (41.58%) smoked beedi while 11 (10.9%) smoked cigarette and rest were nonsmokers [Table 1]. Beedi smokers comprised 79.24% of total smokers while cigarette smokers comprised 20.76%. Among current and former smokers, 30.2% had a smoking index of <100, 41.5% between 100 and 299 and 28.3% of >300.
Table 1.
Characteristics of the patients
Assessment at presentation
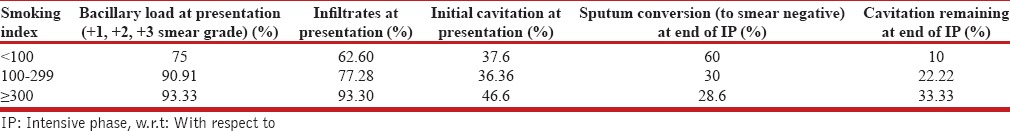
Assessment of bacillary load showed that out of total 47 patients who had 3+ smear grading, 49% were smokers, 40% nonsmokers, and 11% former smokers. As smoking index increased from <100 to 100–299 and >300, 3+ smear grade increased from 12.5% to 68.18% and 66.66%, respectively, (P < 0.05). The data indicates a positive correlation of sputum bacillary load among pulmonary TB patients with higher smoking index.
Among nonsmokers, 79.2% patients had minimal disease while only 4.2% had advanced disease as compared to smokers where 52.4% had moderate disease, 26.2% advanced disease and 21.4% minimal disease (P < 0.01). Moreover, among former smokers, 72.7% had moderate disease and 27.30% had minimal disease at presentation [Figure 1]. The extents of disease, as adjudged by infiltrates on chest X-ray, were much severe among smokers and former smokers as compared to nonsmokers. This was statistically significant for both the groups as compared to nonsmokers (P < 0.01).
Figure 1.
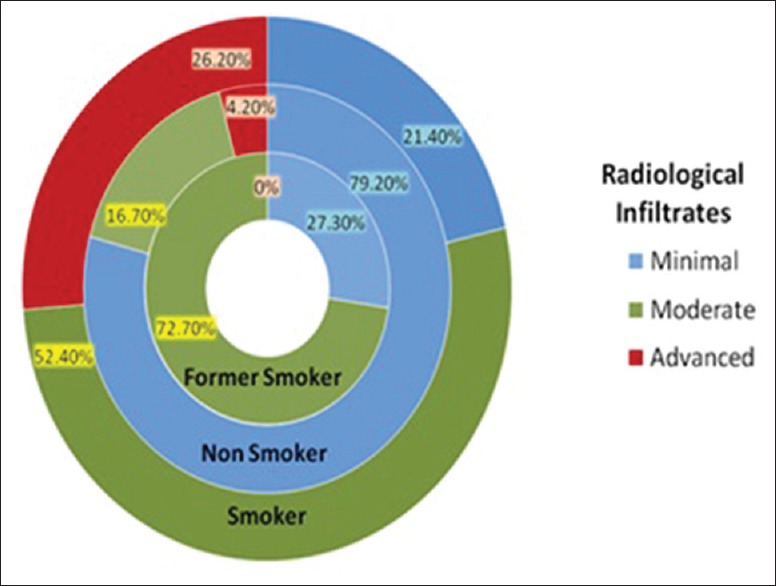
Radiological infiltrates among patients
Overall 31 (30.69%) subjects had the cavitary disease as evident on X-ray chest. Among nonsmokers, 18.8% had cavitation while among smokers and former smokers 40.4% and 45.5% respectively had the cavitary disease (P > 0.05).
Majority of patients had moderate degree of infiltrates irrespective of their smoking index (43.82–63.64%). However, number of patients presenting with moderate infiltrates were higher (≥60%) in subjects with a smoking index of >100 as against those with a smoking index of <100 (P > 0.05).
Assessment at end of intensive phase
By the end of intensive phase (IP), 11 patients were lost to follow-up (defaulters) which included 9 smokers and 2 nonsmokers. Maximum conversion rate at end of IP was found among nonsmokers (56.50%) while 36.40% each of smokers and former smokers could achieve negative sputum status by this time (P = 0.137) [Table 2].
Table 2.
Bacterio-radiological extent and response w.r.t smoking status
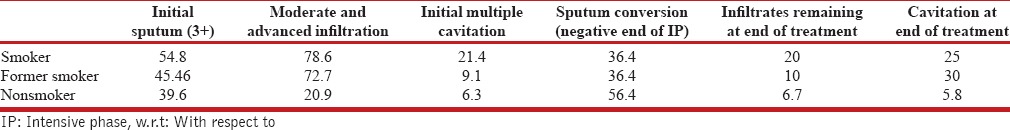
Until end of IP, 60% patients with smoking index <100 converted to negative while only 30% and 28.6% of patients with a SI of 100–299 and >300, respectively, could convert [Table 3]. As the intensity of smoking (as adjudged by SI) increased the conversion rate became poorer. However, this difference was also not statistically significant (P = 0.217).
Table 3.
Bacterio-radiological extent and response w.r.t smoking index
Assessment at end of treatment period
By the end of treatment period, five more patients were lost to follow-up in which three were smokers and one each of former smoker and nonsmoker. Final analysis at the end of treatment period was done on 88 patients (n = 88). At the end of treatment, 93.30% of nonsmokers showed complete clearance of infiltrates on chest X-ray while only 80% and 90% smokers and former smokers, respectively, showed complete clearance (P > 0.05). Among nonsmokers, 6.70% still had minimal infiltrates as compared to 13.30% of smokers and 10% of former smokers. At the end of treatment, 91.1% nonsmokers showed clearance of cavity as against 80% of the smokers and 70% of the former smokers. This data suggests that smokers and former smokers demonstrate more persistence of cavitation (as sequel) on X-ray at end of treatment as compared to nonsmokers (P < 0.05).
Treatment outcome based on smoking status
Cure rate among smokers, nonsmokers, and former smokers were 59.5%, 79.2%, and 81.8%, respectively. Treatment completion among smokers was 9.5%, whereas it was 14.6% and 9.1% among nonsmokers and former smokers, respectively. The overall treatment success rate thus at the end of treatment was 93.8% in nonsmokers, 69% in smokers, and 90.9% in former smokers. This difference was significant statistically (P = 0.000). Default rate among smokers was 28.5% as compared to nonsmokers (6.3%) and former smokers (9.1%), P = 0.0001 [Table 4]. Logistic regression was applied to evaluate the role of smoking on treatment default which demonstrated that smokers are six times more likely to default (odds ratio [OR] 6.207, 95% confidence interval [CI] 1.61–23.907) and former smokers are almost two times more likely to default (OR 1.5, 95% CI 0.141–15.96) compared to nonsmokers. The poor treatment success among smokers (69%) was largely contributed by higher defaulters in this group [Figure 2].
Table 4.
Treatment outcome based on smoking status
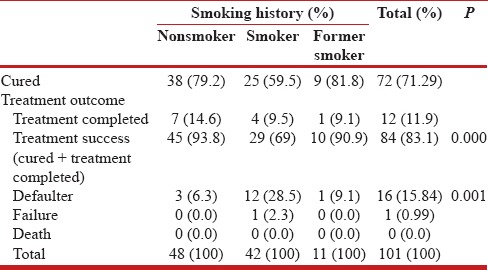
Figure 2.
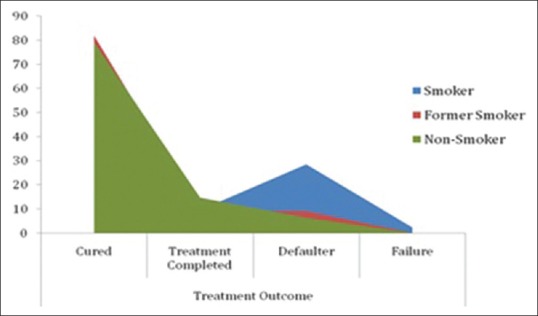
Treatment outcome based on smoking status
DISCUSSION
Of the 101 enrolled pulmonary TB patients, majority (40.6%), irrespective of history of smoking, were in the age group of 21–30 years. The Indian epidemiological data of TB prevalence also shows the age prevalence of 15–50 years.[14] Preponderance of male sex among pulmonary TB (PTB) cases, as found in our study, was consistent with findings of other studies.[6,7,8] Predominance of male patients may be due to high prevalence of PTB in general among males and secondly due to continuing unequal access to medical care facilities as compared to women and children, especially in developing countries like India.
Smoking prevalence among TB subjects was found to be between 33.3% and 62.5% in various studies[6,7,8,9] and this study also showed 52.5% smokers and 47.5% nonsmokers among total diagnosed TB subjects.
A higher bacillary load was found in patients with a history of smoking. However, due to smaller sample, this difference, when compared to nonsmokers, could not reach a statistical significance as found in other studies.[6,15] Sputum smear grade of 3+ was more common among moderate and heavy smokers as compared to those with a SI of <100 which was also observed by Agarwal and Agrawal.[7] The higher grades of sputum smear AFB positivity could possibly be explained by the fact that cavitation and advanced infiltrates are more common among smokers. This correlation was also demonstrated in a study done by Altet-Gomez et al.[15] At presentation, moderate and advanced degree of radiological infiltrates were more common among smokers (52.4% and 26.2%) as compared to nonsmokers (16.7% and 4.2%). A study by Abal et al.[16] also found far advanced radiographic abnormalities in smokers as compared to nonsmokers. Agarwal and Agarwal[7] also showed smoking led to more fibrosis and infiltrates (98.07% and 9.6%, respectively) as compared to nonsmokers.
In this study, it was observed that smokers (24/30, 80%) and former smokers (7/10, 70%) show decreased clearance of cavities on chest X-ray after treatment completion as compared to nonsmokers (41/45, 91%), which was statistically significant (P < 0.05). There is paucity of data on literature search on this aspect. However, severity score as adjudged by Racil et al.[17] showed that residual radiological severity score was more among smokers (13.8% vs. 0%, P < 0.05). The more radiological sequelae observed among smokers could be due to the late presentation and more pathophysiological damage. Pikas[18] had also suggested that there was enhanced moisture exuding function of lung among TB patients exposed to smoke. Moreover, it was observed that smoke modifies the normal response, thus the chronic inflammatory response may induce parenchymal tissue destruction and disrupt normal repair and defence mechanism leading to radiological sequelae.[19]
At the end of treatment, outcomes were assessed in terms of treatment success (cured and treatment completed), defaulters, and failures in all the three groups (nonsmoker, smoker, and former smoker). Smokers had significantly lower treatment success rate (69%) as against nonsmokers and former smokers (93.8% and 90.9%, respectively). This difference was statistically significant (P = 0.001). However, it was also observed that the default rate was higher among smokers (12/42, 28.5%) as compared to former smoker and nonsmokers (9.1% and 6.3%, respectively, P = 0.001). A higher default rate among smokers was largely responsible for the poor treatment success among them.
Smoking and TB both affects younger age group of population with male predominance. Smokers demonstrate extensive infiltrates as compared to nonsmokers also as the severity of smoking increased (smoking index) the bacillary load also increased (higher grades of sputum positivity). At end of treatment, smokers were left with more radiological sequelae in terms of cavitation. Smokers also had a poorer outcome in terms of treatment success rate as compared to nonsmokers. However, this was largely due to high percentage of default rate among smokers implying compliance or treatment adherence issues among smokers as a main confounder to treatment success. There is an aggressive need for default retrieval action among this subgroup of TB patients including motivation and counseling. TB control programs may also benefit from a focus on smoking cessation interventions. A further evaluation of psychosocial factors among these patients might help understand their behavior and improve treatment outcome.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.World Health Organization. Global Tuberculosis Report 2013. WHO/HTM/TB/2013. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 2.Pierson T, Learmonth-Pierson S, Pinto D, van Hoek ML. Cigarette smoke extract induces differential expression levels of beta-defensin peptides in human alveolar epithelial cells. Tob Induc Dis. 2013;11:10. doi: 10.1186/1617-9625-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Domagala-Kulawik J. Effects of cigarette smoke on the lung and systemic immunity. J Physiol Pharmacol. 2008;59(Suppl 6):19–34. [PubMed] [Google Scholar]
- 4.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–8. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 5.Lin HH, Ezzati M, Chang HY, Murray M. Association between tobacco smoking and active tuberculosis in Taiwan: Prospective cohort study. Am J Respir Crit Care Med. 2009;180:475–80. doi: 10.1164/rccm.200904-0549OC. [DOI] [PubMed] [Google Scholar]
- 6.Jee SH, Golub JE, Jo J, Park IS, Ohrr H, Samet JM. Smoking and risk of tuberculosis incidence, mortality, and recurrence in South Korean men and women. Am J Epidemiol. 2009;170:1478–85. doi: 10.1093/aje/kwp308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agarwal A, Agrawal VK. Impact of tobacco smoke on tuberculosis: A case control study. NJIRM. 2011;2:38–42. [Google Scholar]
- 8.Leung CC, Yew WW, Chan CK, Chang KC, Law WS, Lee SN, et al. Smoking adversely affects treatment response, outcome and relapse in tuberculosis. Eur Respir J. 2015;45:738–45. doi: 10.1183/09031936.00114214. [DOI] [PubMed] [Google Scholar]
- 9.Prasad R, Suryakant, Garg R, Singhal S, Dawar R, Agarwal GG. A case-control study of tobacco smoking and tuberculosis in India. Ann Thorac Med. 2009;4:208–10. doi: 10.4103/1817-1737.56007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin HH, Ezzati M, Murray M. Tobacco smoke, indoor air pollution and tuberculosis: A systematic review and meta-analysis. PLoS Med. 2007;4:e20. doi: 10.1371/journal.pmed.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349:1498–504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 12.Central TB Division, Directorate General Health Services, Ministry of Health and Family Welfare, Government of India. Revised National Tuberculosis Control Programme Training Manual for Mycobacterium tuberculosis Culture and Drug Susceptibility Testing. 2010. [Last accessed on 2010 Jun 06]. Available from: http://www.tbcindia.nic.in/pdfs/Training.manual.M.tuberculosis.CDST.pdf .
- 13.Falk A, O’Connor JB, Pratt PC. Diagnostic Standards and Classification of Tuberculosis. 12th ed. New York: National Tuberculosis and Respiratory Disease Association; 1969. Classification of pulmonary tuberculosis. [Google Scholar]
- 14.TB India, Govt. of India, Ministry of Health and Family Welfare. RNTPC Status Report: DOTS for all-all for DOTS. 2006. [Last accessed on 2015 Sep 14]. Available from: http://www.planningcommission.nic.in/reports/genrep/health/RNTCP_2011.pdf .
- 15.Altet-Gômez MN, Alcaide J, Godoy P, Romero MA, Hernández del Rey I. Clinical and epidemiological aspects of smoking and tuberculosis: A study of 13,038 cases. Int J Tuberc Lung Dis. 2005;9:430–6. [PubMed] [Google Scholar]
- 16.Abal AT, Jayakrishnan B, Parwer S, El Shamy A, Abahussain E, Sharma PN. Effect of cigarette smoking on sputum smear conversion in adults with active pulmonary tuberculosis. Respir Med. 2005;99:415–20. doi: 10.1016/j.rmed.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Racil H, Amar JB, Cheikrouhou S, Hassine E, Zarrouk M, Chaouch N, et al. Pulmonary tuberculosis in smokers. Presse Med. 2010;39:e25–8. doi: 10.1016/j.lpm.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 18.Pikas OB. Effect of smoking and alcohol drinking on the moisture-excreting lung function. Lik Sprava. 2000;6:65–7. [PubMed] [Google Scholar]
- 19.Global Initiative for Chronic Obstructive Lung Disease, Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. 2015. [Last accessed on 2015 Jul 12]. p. 5. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2011_Feb21.pdf .


