Abstract
Anti-synthetase syndrome is an autoimmune condition, characterized by antibodies directed against an aminoacycl transfer RNA synthetase along with clinical features that can include interstitial lung disease, myositis, Raynaud’s phenomenon, and arthritis. There is a higher prevalence and increased severity of interstitial lung disease in patients with anti-synthetase syndrome, as compared to dermatomyositis and polymyositis, inflammatory myopathies with which it may overlap phenotypically. Diagnosis is made by a multidisciplinary approach, synthesizing rheumatology and pulmonary evaluations, along with serologic, radiographic, and occasionally muscle and/or lung biopsy results. Patients with anti-synthetase syndrome often require multi-modality immunosuppressive therapy in order to control the muscle and/or pulmonary manifestations of their disease. The long-term care of these patients mandates careful attention to the adverse effects and complications of chronic immunosuppressive therapy, as well as disease-related sequelae that can include progressive interstitial lung disease necessitating lung transplantation, pulmonary hypertension, malignancy and decreased survival. It is hoped that greater awareness of the clinical features of this syndrome will allow for earlier diagnosis and appropriate treatment to improve outcomes in patients with anti-synthetase syndrome.
I. Introduction
Anti-synthetase syndrome is an autoimmune disease characterized by autoantibodies against one of many aminoacyl transfer RNA (tRNA) synthetases with clinical features that may include interstitial lung disease (ILD), non-erosive arthritis, myositis, Raynaud’s phenomenon, unexplained fever and/or mechanic’s hands (1). Anti-synthetase syndrome is an idiopathic inflammatory myopathy (IIM), with a higher prevalence of ILD compared to dermatomyositis (DM) and polymyositis (PM), IIMs with which it shares many features. The ILD in anti-synthetase syndrome patients is often severe and rapidly progressive, causing much of the increased morbidity and mortality associated with anti-synthetase syndrome as compared to the other IIMs (2).
Since Bohan and Peter’s 1975 classification of DM and PM, additional research has added to our understanding of the heterogeneous phenotypes of patients with IIMs (3, 4). In the 1980s, aminoacyl tRNA synthetase autoantibodies were identified and linked to the IIMs (5, 6). In the early 1990s, several groups recognized that patients with these antibodies had distinct clinical features, leading to the formal recognition of anti-synthetase syndrome (7, 8). In addition, more subtle forms have been identified, including lung-dominant disease. Patients with anti-synthetase syndrome have a higher incidence of pulmonary involvement and symptoms considered to be more characteristic of other connective tissue diseases (CTDs), such as Raynaud’s phenomenon or gastroesophageal reflux. Patients with anti-synthetase syndrome may have corticosteroid-resistant myositis or ILD, frequently requiring additional immunosuppressive medications.
Evidence guiding management of anti-synthetase syndrome is primarily based on case series and reports. Historically, anti-synthetase syndrome was not considered a separate entity, and these patients were diagnosed with either DM or PM depending on their clinical presentation. Therefore, management decisions in anti-synthetase syndrome are frequently extrapolated from studies of patients with IIM that included patients with anti-synthetase syndrome as well. Given the lack of prospective data to guide treatments decisions, management choices are typically made based on practitioner experience and patient response to therapy.
This review will summarize the clinical, serologic, and radiographic features of anti-synthetase syndrome, with particular attention to anti-synthetase syndrome-associated ILD. We will also review the existing evidence guiding the use of immunosuppressive therapy and the long-term clinical management of anti-synthetase syndrome.
II. Diagnosis
Clinical Features
Peter and Bohan’s 1975 criteria for diagnosing DM and PM served for decades as the framework for the classification of the IIMs (3, 4). Patients were grouped into one of five classification categories using muscle biopsy, electromyography and serologic data along with physical examination findings. Since that time, Dalakas and Holhfeld proposed revised diagnostic criteria in 2003, with a greater emphasis on histologic and immunologic pathology to distinguish between the IIM subtypes (9).
While Dalakas and Holhfeld’s revised criteria provided clarity for distinguishing between the IIMs, it was not until 2010 that formal criteria for the diagnosis of anti-synthetase syndrome were introduced by Connors et al (1). These criteria proposed that all patients with anti-synthetase syndrome must have evidence for a tRNA synthetase autoantibody, in addition to one or more of the following clinical features: mechanic’s hands, Raynaud’s phenomenon, myositis, ILD, arthritis, and/or unexplained fever (table 1). In 2011, Solomon et al proposed alternative, stricter criteria, requiring two major or one major and two minor criteria, in addition to the presence of an aminoacyl-tRNA synthetase autoantibody (10). Historically, patients with these antibodies were diagnosed with an IIM or were unrecognized given their atypical clinical features. In fact, in a recent retrospective analysis of 198 patients with idiopathic interstitial pneumonia, 13 patients were later noted to have an anti-synthetase antibody (11).
Table 1.
Proposed Diagnostic Criteria for Anti-synthetase Syndrome
| Connors et al. (2010) (1) | Solomon et al (2011) (10) |
|---|---|
|
| |
| Required: Presence of an anti-aminoacyl tRNA synthetase antibody | Required: Presence of anti-aminoacyl tRNA synthetase antibody |
PLUS
one or more of the following clinical features:
|
PLUS
two major or one major and two minor criteria: Major:
Minor:
|
The diagnostic criteria proposed by Connors et al and Solomon et al permit recognition of the anti-synthetase syndrome as a distinct entity, which may be useful in both clinical and research settings. However, we caution that the absence of an anti-synthetase antibody does not preclude the presence of the anti-synthetase syndrome because autoantibody levels fluctuate depending on disease activity (12), centers and laboratories vary in which autoantibodies they routinely test, and there may be undiscovered autoantibodies not yet available for laboratory detection. In our experience, we have encountered patients with clinical features of the anti-synthetase syndrome phenotype along with rapidly progressive and severe ILD who do not have evidence of anti-synthetase antibodies. While a formal diagnosis of anti-synthetase syndrome is not possible if an anti-synthetase antibody is not present, these patients may benefit from pharmacologic therapy as is recommended for patients formally diagnosed with anti-synthetase syndrome.
Phenotypically, patients with anti-synthetase syndrome often have signs and symptoms that overlap with other CTDs, such as Raynaud’s phenomenon, severe gastroesophageal reflux disease, and non-erosive arthritis. ILD frequently predominates at presentation and contributes to the high morbidity and mortality in patients with anti-synthetase syndrome. The prevalence of ILD in anti-synthetase syndrome varies widely and is difficult to determine as these patients have only recently been studied as a distinct entity from the other IIMs. In a 2013 study of 203 anti-synthetase syndrome patients, the prevalence of ILD was 86%, more common than the prevalence of myositis (73%) or arthralgia/arthritis (60%) (13). In fact, anti-synthetase syndrome patients may not have classic myopathic symptoms as is disease-defining in PM (14), or the myositis may present later in the disease course. We carefully examine the hands in patients with ILD as the hyperkeratosis and scaling characteristic of mechanic’s hands are often subtle findings whose presence should prompt further testing for anti-synthetase antibodies and elevated muscle enzymes.
Depending on the clinical presentation, additional laboratory testing may help guide the clinical evaluation of disease extent and organ involvement (15). Myositis activity is typically assessed via elevations in creatinine kinase (CK) and aldolase. Monitoring these muscle enzymes in clinically amyopathic patients may uncover subclinical myositis and can track with muscle disease activity.
Autoantibodies
In patients with IIM, a variety of myositis-specific and associated autoantibodies have been described and are frequently tested in the evaluation of idiopathic ILD (16) (tables 2 and 3). The hallmark of anti-synthetase syndrome is the presence of myositis-specific anti-synthetase antibodies (table 2). Of the anti-synthetase antibodies, anti-Jo-1, an anti-histidyl-tRNA synthetase, is most commonly identified. Less common anti-synthetase antibodies include anti-threonyl (anti-PL7), anti-alanyl (anti-PL12), anti-isoleucyl (anti-OJ), and anti-gylcyl (anti-EJ) along with others infrequently tested in the clinical setting but reported in the literature.
Table 2.
Anti-synthetase Antibodies
| Anti-ARS antibodies | Auto-antigen | Clinical features |
|---|---|---|
| Anti-Jo1* | histidyl | Most common |
| Anti-PL7* | theronyl | Severe ILD |
| Anti-PL12* | alanyl | Severe ILD |
| Anti-OJ* | isoleucyl | |
| Anti-EJ* | glycyl | |
| Anti-KS | asparaginyl | |
| Anti-Zo | phenylalanyl | |
| Anti-SC | lysyl | |
| Anti-JS | glutaminyl | |
| Anti-YRS | tyrosyl |
ILD = Interstitial lung disease
available for testing at our institution
Table 3.
Myositis Specific and Associated Antibodies
| Myositis-specific |
| Anti-SRP |
| Anti-MDA-5 |
| Anti-Mi-2 |
| Myositis-associated |
| ANA |
| Anti-Ro/SSA |
| Anti-PM-Scl |
| Anti-KU |
| Anti-U2 snRNP |
Diagnostic testing for idiopathic ILD also involves other myositis-specific (table 3), including those against translational transport (anti-signal recognition particle autoantibodies), RNA helicase (anti-MDA5 autoantibodies) (17) and nuclear helicase (anti-Mi-2 autoantibodies (15). Myositis-associated autoantibodies often evaluated include, but are not limited to, anti-nuclear antibody (ANA), anti-Ro/SSA (18), anti-PM-Scl, anti-Ku, and anti-U1-RNP (15) (table 3). While anti-Ro/SSA and anti-La/SSB are traditionally associated with Sjogren’s disease and Sjogren’s-related ILD, anti-Ro/SSA has been independently associated with anti-synthetase syndrome and more severe and fibrotic ILD by HRCT (18, 19).
The reported prevalence of autoantibodies in patients with an IIM varies widely. In a 2006 study of 74 patients with IIM, 58% were positive for a myositis-specific autoantibody and 36% had a myositis-associated antibody (with overlap in 20% of patients) (20). In this case series and others, anti-Jo-1 predominated, and was found to be prevalent in 30% (over half of all patients with myositis-specific antibodies were Jo-1 positive). A 2010 Australian study of patients with anti-synthetase antibodies demonstrated a prevalence of the Jo-1 antibody in 88% of included patients (21). In this study, female patients were more common than male patients (30:12).
The immunologic pathogenesis of these autoantibodies has not been precisely determined. Aminoacyl-tRNA synthetases translate proteins from genetically transcribed mRNA. In 1999, Wakasugi and Schimmel identified that these synthetases may act as cytokines, assisting in cell death and recruitment of inflammatory cells (22). Further studies, summarized by Ascherman in 2015, have identified innate and adaptive immunity in the pathogenesis of anti-synthetase syndrome (23).
The clinical presentation and disease course differs depending on the anti-synthetase antibody present. Hamaguchi et al reviewed 166 patients with anti-synthetase antibodies, and demonstrated that patients with anti-Jo-1, anti-EJ, and anti-PL-7 were most often clinically diagnosed with DM or PM, while patients with anti-PL-12 were most often clinically diagnosed with amyopathic DM or ILD alone (24). Interstitial lung disease alone was common in patients with anti-KS and anti-OJ. A 2012 study of the long-term outcomes of patients with an anti-Jo-1 autoantibody (n=75) and anti-PL7/PL12 autoantibody (n=20) found that the prevalence of muscle symptoms (weakness and myalgia) was significantly lower in patients with anti-PL7/PL12 as compared to those with anti-Jo1 (25).
We recommend a high index of suspicion for anti-synthetase syndrome particularly in patients with idiopathic ILD and/or acute respiratory distress syndrome (ARDS) of unknown etiology. Anti-synthetase syndrome can present with fever, arthritis, or ILD in isolation. Therefore, we encourage physicians to consider this diagnosis in patients with unexplained acute or chronic interstitial pneumonia, and recommend laboratory evaluation for anti-synthetase antibodies in patients with otherwise unexplained ILD or acute lung injury.
Radiographic and Histopathologic Features of Anti-synthetase Syndrome
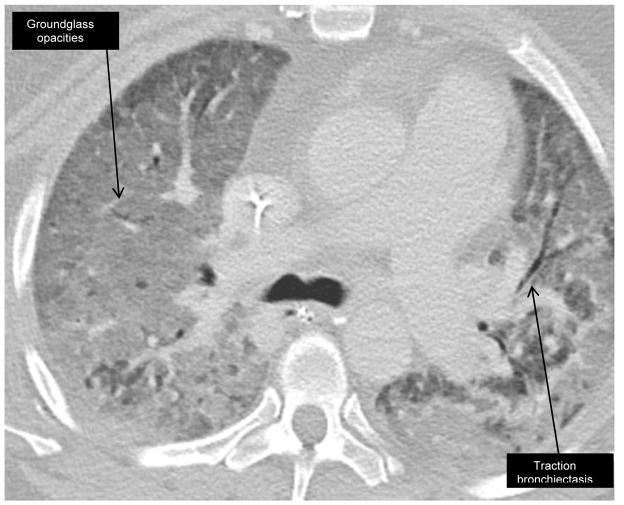
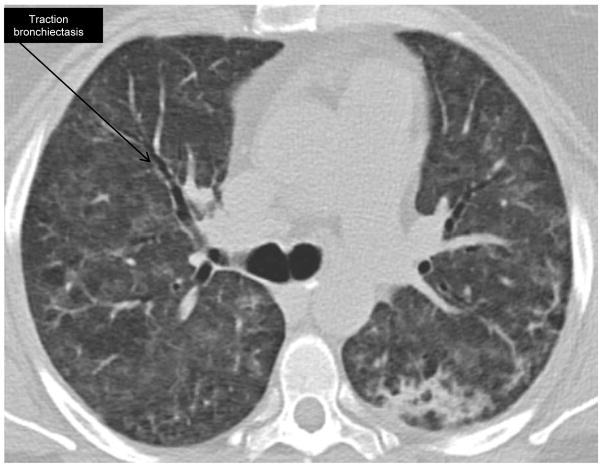
High resolution computed tomography (HRCT) imaging is routine in the work-up and management of ILD (figures 1 and 2). A 2015 study of the initial and follow-up CT images in 26 patients with anti-synthetase syndrome found that traction bronchiectasis, ground glass opacities and reticulation are the most common radiographic features at initial diagnosis (26). The CT patterns most often identified were non-specific interstitial pneumonia (NSIP), organizing pneumonia (OP) or a combination of the two. At median follow-up of 27 months, 38% of patients had evidence of honeycombing. The presence of NSIP and OP suggests an underlying autoimmune etiology and in our experience, is not uncommon in anti-synthetase syndrome (27). High-resolution computed tomography is used to monitor disease activity, along with history, physical examination, and serologic testing.
Figure 1. High-resolution CT in anti-synthetase syndrome at presentation.
48 year-old woman with Jo-1 positive anti-synthetase antibody syndrome, presented with acute respiratory distress syndrome. HRCT reveals bilateral, diffuse groundglass opacities.
Figure 2. High-resolution CT anti-synthetase syndrome after treatment.
The same patient eleven days after treatment with IV cyclophosphamide and high-dose corticosteroids. Tacrolimus was also initiated. The cyclophosphamide was subsequently switched to azathioprine after discharge from the hospital. Lung function normalized despite tapering off prednisone. Lung disease relapsed when patient self-discontinued tacrolimus. She is now back in remission on a prednisone taper, azathioprine and tacrolimus.
Lung biopsy, including transbronchial biopsy, video-assisted thorascopic surgical biopsy and surgical open lung biopsy, is infrequently performed in patients with anti-synthetase syndrome as diagnosis is typically made by synthesizing HRCT findings, serologic data, pulmonary function testing, physical examination and patient symptoms. Studies by Yousem et al examined the histopathologic findings of patients, using open surgical lung biopsies, autopsies and/or native lungs removed for transplantation, with anti-Jo-1 and anti-PL7 antibodies. In 20 patients with anti-Jo-1 antibody, 50% had diffuse alveolar damage and 35% usual interstitial pneumonia (UIP) on surgical lung biopsy (28) In eight patients with anti-PL7, lung biopsy showed UIP in half of the patients (29). A 2008 study of transbronchial biopsy findings in patients with DM/PM and interstitial pneumonia found that patients with bud-type fibrosis demonstrated increased response to immunosuppressive therapy as well as survival, as compared to patients with mural incorporation-type fibrosis (30). These studies taken together suggest that in anti-synthetase syndrome, ILD may be severe, fibrotic, and/or refractory to therapy.
Myositis is often absent or subclinical in anti-synthetase syndrome, with ILD the primary clinical manifestation, therefore muscle biopsy is not typically performed. While muscle biopsy is useful in the diagnosis of other IIMs (31), there is no evidence regarding the role of muscle biopsy in the diagnosis of anti-synthetase syndrome.
III. Treatment
Immunosuppressive agents treat the pulmonary and/or muscle manifestations of anti-synthetase syndrome. Muscle and lung disease may not track together and require separate monitoring by collaborating rheumatologists and pulmonologists. Corticosteroids have long been first-line in the treatment of IIMs, though when corticosteroids are used as monotherapy in anti-synthetase syndrome, there is frequent lung disease recurrence with corticosteroid tapering. Additional immunosuppressive agents are added for refractory muscle and/or lung disease and as corticosteroid-sparing agents. Frequently used adjunctive agents include azathioprine, mycophenolate mofetil, tacrolimus, rituximab, and cyclophosphamide, though there is little consensus about which agent is preferred, and the use of these agents to treat anti-synthetase syndrome related ILD is off-label (table 4). When muscle and/or lung disease symptoms stabilize, providers typically slowly taper corticosteroids, in order to spare patients the long-term side effects. There is no evidence to guide providers about the length of immunosuppressive therapy, rate of medication tapering or the consequences of withdrawal even after a patient has entered a clinical remission. In the experience of this review’s rheumatologist (JC), CK may never completely normalize in some African American patients. This is in line with previously published data that African-American patients often have higher mean CK levels (32).
Table 4.
Therapies for Anti-synthetase Syndrome-ILD
| Drug | Suggested Starting Dose | Clinical Use | Monitoring | Adverse effects* |
|---|---|---|---|---|
| Corticosteroids | 1 mg/kg/day | First-line therapy | Glucose, weight, blood pressure | Hypertension, weight gain, hyperglycemia |
| Azathioprine | 1 mg/kg/day | Most common second-line agent | CBC, renal/liver function | Leukopenia, hepatic injury, pancreatitis |
| Mycophenolate mofetil | 500 mg twice daily | Second-line agent in addition to corticosteroids | CBC | Diarrhea, cytopenias |
| Tacrolimus | 1 mg twice daily | Add-on therapy | Renal function, blood pressure, electrolytes, CBC, drug level | Renal failure, hypertension, hyperglycemia |
| Rituximab | ** | Rescue therapy, added to standard immunosuppression | CBC | Infection, neutropenia, infusion reaction |
| Cyclophosphamide | 1–2 mg/kg/day by mouth or 500–1000 mg IV every 4 weeks** | Rescue therapy (e.g. ARDS) | CBC, urinalysis, renal function | Malignancy, cytopenias, hemorrhagic cystitis, sterility |
| IVIG | ** | Add-on therapy | Immunoglobulin G levels | Infusion reaction, infection from donor |
ILD = Interstitial lung disease; ARDS = acute respiratory distress syndrome; IVIG = intravenous immunoglobulin G; mg = milligram; kg = kilogram
all except IVIG increase the risk of opportunistic infection
rheumatology consult strongly advised
Careful laboratory and clinical evaluation is imperative for patients treated with immunosuppressive agents. Prescribing providers should be familiar with evidence-based immunosuppressive clinical guidelines (33, 34). The aforementioned medications suppress normal immune function in an effort to combat aberrant pathogenic autoimmunity. An unintended consequence for patients treated with all of these immunosuppressive agents is the increased risk of opportunistic infections and malignancy, particularly skin cancer and lymphoma. If patients are to be treated with corticosteroids and an additional immunosuppressive agent, we recommend pneumocystis jiroveci prophylaxis (35).
Adherence to evidence-based vaccination recommendations is also necessary, though many immunosuppressive agents preclude administration of live vaccinations. Additionally, hepatitis B reactivation is common (up to 40%) in patients who are hepatitis B surface antigen positive and are treated with immunosuppressive agents (36). Hepatitis B surface antigen, anti-hepatitis B core antigen and hepatitis C antibody should be performed prior to administration of prednisone or other immunosuppressive therapy. Guidelines recommend prophylactic antiviral therapy in patients at risk for hepatitis B reactivation. Patients with positive hepatitis serologies should be treated in consultation with a liver specialist.
We recommend a multidisciplinary approach to care for these patients, with close collaboration between rheumatologists and pulmonologists. Careful titration of chosen agents, while monitoring disease status and organ function, allows the safe administration of these medications, by practitioners experienced with their use. As noted previously, most data supporting the use of the following agents in anti-synthetase syndrome is extrapolated from studies on DM and PM.
Corticosteroids
Since the first administration of “Compound E” in 1948, which would later be renamed “cortisone”, to treat rheumatoid arthritis, corticosteroids have become nearly ubiquitous in the management of autoimmune conditions (37). Corticosteroids have wide ranging mechanisms of action in their role as analogs of adrenal cortex innate functions. In this context, corticosteroids are prescribed for their anti-inflammatory, immunosuppressive, and anti-proliferative effects. They are the initial therapy given to treat active muscle and/or lung disease in patients with an IIM, with or without lung involvement (38).
Controlled trials have not been performed to establish the superiority of corticosteroids in the initial management of active disease, as compared to other immunosuppressive agents. A 1993 study of 113 patients with an IIM compared the efficacy of prednisone, methotrexate and azathioprine by clinical and autoantibody groups (38). Clinical response was mostly judged by myositis symptoms, not by lung disease symptoms. The first trial of prednisone alone for 4 weeks resulted in a partial improvement in most patients with anti-synthetase antibodies, but complete clinical response was rare. Given these findings, patients with anti-synthetase syndrome may require multimodality therapy at the outset, with frequent monitoring of lung function.
Adverse events in patients treated with corticosteroids long-term are frequent, and include hyperglycemia, hypertension, weight gain, adrenal suppression, osteoporosis and fractures, among many others (39). Patients should be monitored closely, and corticosteroids should be tapered when clinically indicated.
Azathioprine
Azathioprine decreases immune function by halting purine synthesis and incorporating metabolites into DNA, thus stopping replication particularly of immune cells. Azathioprine is frequently used as adjunctive therapy in patients with IIM-ILD, particularly in patients with corticosteroid-resistant disease. While there is little evidence demonstrating azathioprine’s superiority as the preferred corticosteroid-sparing immunosuppressive agent, azathioprine has been most frequently used as maintenance therapy often in conjunction with prednisone (40).
Evidence to support azathioprine’s efficacy in treatment of anti-synthetase syndrome-ILD is largely driven by studies in patients with IIM with myositis-predominant symptoms. A 1980 prospective, randomized controlled trial studied the use of azathioprine in PM in 16 patients, treated either with prednisone plus placebo or prednisone plus azathioprine (41). This study demonstrated no significant difference in muscle strength or CK level after three months. A follow-up study in this same group of patients demonstrated that at one year, the prednisone/azathioprine group required significantly less prednisone than the prednisone only group for myositis control and this group also had significant improvement in functional disability (42). A major limitation of this data is that it did not assess for the presence of ILD or ILD response to therapy.
Patients receiving azathioprine should have their complete blood count and renal/liver function monitored every 1–3 months (33). Adverse reactions to azathioprine include nausea, pancreatitis, hepatitis, and cytopenias. Rarely, an acute febrile hypersensitivity reaction can occur.
Mycophenolate mofetil
Mycophenolate mofetil inhibits proliferating lymphocytes by altering purine synthesis. It is used in post-transplant anti-rejection regimens and is also frequently used in the treatment of CTD. Swigris et al demonstrated its safety and efficacy in a heterogeneous group of patients with CTD-ILD in 2006. This case series assessed 28 patients treated with MMF for nearly 36 patient-years (43). All but two patients tolerated the medication and its use resulted in a significantly lower dose of corticosteroids. A 2013 study by Fischer et al demonstrated similar tolerability of mycophenolate mofetil in 125 patients with CTD-ILD (44). In this study, mycophenolate mofetil treatment was associated with stable or improved lung function over several years of follow-up. A subset of patients with IIM-ILD showed improved lung function with mycophenolate mofetil.
Common adverse effects include nausea, diarrhea/colitis, hypertension, hyperglycemia and cytopenias, among many more. Persistent or severe diarrhea may be a sign of serious colitis, and if it persists warrants further evaluation. Enteric-coated mycophenolic acid is an alternative to mycophenolate mofetil, as it has been demonstrated to have fewer gastrointestinal side effects with similar medication efficacy (45). Providers should monitor complete blood counts every 1–3 months and consider monitoring drug clearance if signs of intolerance occur (33).
Calcineurin Inhibitors: Tacrolimus and Cyclosporine
The calcineurin inhibitors tacrolimus and cyclosporine exert their anti-immune effects via inhibition of T cells. Tacrolimus has both greater potency and an improved safety profile when compared to cyclosporine (46), so may be the preferred calcineurin inhibitor in anti-synthetase syndrome-ILD. To the extent that the IIMs may be T-cell driven in their pathogenesis, these drugs are the logical choice as an adjunctive therapeutic agent.
A 2013 study of 18 patients with anti-Jo-1 antibodies (17 with ILD) who received cyclosporine after failing prednisone, revealed that these patients had a statistically significant improvement in forced vital capacity (FVC) and diffusing capacity for carbon monoxide (DLCO), without any life-threatening complications at one year of follow-up (47). Four patients stopped cyclosporine and experienced a relapse in ILD. Re-treatment with cyclosporine resulted in improvement in two patients.
The first successful use of tacrolimus was reported in 1999 by Chester Odis, in 8 patients with PM-ILD (48). Six of these patients had anti-Jo-1 and two had anti-SRP autoantibodies. Since that time, numerous case reports and studies have suggested the efficacy of tacrolimus in patients with CTD and IIM related ILD (49), even as add-on therapy in combination with corticosteroids and an additional immunosuppressive agent (50). A 2005 case series specifically studied tacrolimus efficacy in 15 patients with anti-synthetase syndrome-ILD. Most of these patients had Jo-1 autoantibodies, and experienced improvement in lung function and muscle symptoms, as well as a significant reduction in corticosteroid dose, with tacrolimus administration (51).
Providers should closely monitor calcineurin inhibitor serum trough levels, particularly with dosage changes, worsening myositis or ILD, or with fluctuating renal clearance. It is our practice to initiate tacrolimus at 1mg twice daily, and increase by 1–2 mg weekly until 12 hour target trough levels reach 5–8 ng/mL (50). Final doses are typically 1 mg to 5 mg, twice daily. Providers should closely follow blood pressure, renal function, electrolytes, lipids, and complete blood count (33). Adverse events in patients treated with tacrolimus include renal dysfunction, hyperkalemia, hyperylcemia, hypertension, tremor, and headache. If used as add-on therapy in addition to another immunosuppressive agent, we advocate that tacrolimus should be the first agent to stop after prednisone once a patient has entered a clinical remission, given the risk of renal dysfunction with the long-term use of tacrolimus.
Rituximab
Rituximab is a monoclonal antibody that targets the CD20 surface antigen on B-lymphocytes. Oddis et al performed the largest randomized placebo-controlled trial of rituximab treatment for refractory adult and juvenile DM and adult PM in 2012 (52). In this delayed-start study, 195 patients received rituximab plus non-standardized immunosuppression either at week 1 or at week 8 with total follow-up of 44 weeks. There was no difference in time to myositis disease control between the groups, though 83% of patients achieved the predefined clinical response by the end of the study. This study did not look at the presence of ILD or its response to this therapy.
Two small case series have suggested efficacy in patients with ILD due to either CTD or anti-synthetase syndrome. A 2009 case series described 11 patients with anti-synthetase syndrome-ILD and declining lung function who were treated with rituximab (53). At short-term follow-up (approximately 7 months) six of eleven patients had an improvement of over 10% in FVC. One patient died of pneumocystis jiroveci pneumonia, a complication of chronic immunosuppression.
In 2012, rituximab was proposed as “rescue therapy” in a case series of eight patients with severe ILD due to CTD (54). Four of these patients had an anti-Jo-1 antibody with HRCT suggestive of fibrotic NSIP. All of the patients included had a decline in lung function before rituximab initiation, and at follow-up 9–12 months later showed improvement in pulmonary symptoms and/or lung function. A 2014 follow-up study by the same authors of 50 patients with ILD (5% with IIM) treated with rituximab again suggested efficacy (55).
Common side effects of rituximab include headache, nausea and vomiting, diarrhea and infection (52). Other serious adverse events include cytopenias and fatal infusion reactions. Providers should ensure that patients do not have viral hepatitis before choosing rituximab, and should monitor complete blood counts before every rituximab infusion (33).
Cyclophosphamide
Cyclophosphamide is an anti-proliferative cytotoxic agent that alkylates DNA. Given the serious nature of many of the potential adverse effects associated with its use, it is typically reserved for the treatment of severe IIM-ILD, particularly the acute respiratory distress syndrome. Its efficacy in treating IIM with and without ILD has been demonstrated in several case series. A 2015 systematic review analyzed the findings of 12 studies performed between 1975 and 2014, in which 178 patients with an IIM and 141 patients with IIM-ILD were treated with cyclophosphamide (56). With the caveat that the dosages of cyclophosphamide varied as did the co-administered immunosuppressants, approximately 71% demonstrated improvement in vital capacity or FVC and 69% had improved DLCO. The most common side effects were nausea and vomiting. Several patients had evidence of opportunistic infections. Two of the most feared complications of cyclophosphamide, malignancy and hemorrhagic cystitis, were not observed.
A 2013 review of 42 patients with PM/DM-ILD compared outcomes in patients treated with either cyclophosphamide, azathioprine or mycophenolate mofetil (57). No significant difference in lung function was demonstrated, though six patients were switched to another agent from cyclophosphamide after six months in an attempt to avoid toxic side effects.
Concerns over the cytotoxic effects of cyclophosphamide often limit its administration to severe and refractory anti-synthetase syndrome-ILD. The use of cyclophosphamide has been linked to many different malignancies. It has also been associated with sterility, hemorrhagic cystitis, congestive heart failure, cytopenias, and opportunistic infections. Cyclophosphamide should be prescribed only by providers familiar with its adverse effects and need for frequent monitoring. Patients should have complete blood counts, renal function, and urinalysis prior to every administration of cyclophosphamide (usually every 1–2 weeks) (33).
Other therapies
Intravenous immunoglobulin (IVIG), a blood product containing the pooled immunoglobulin G antibodies from donors, is used in the treatment of many immune deficiency and autoimmune disorders, though its exact mechanism of action is unknown. In a randomized, placebo controlled trial of IVIG treatment in 15 patients with dermatomyositis, patients randomized to receive IVIG had significant improvement in muscle strength and symptoms (58). A recent case report suggested IVIG may be effective in ILD related to IIM as well (59). It is typically used as adjunctive therapy.
Finally, despite methotrexate’s demonstrated efficacy in IIM, methotrexate is typically avoided in patients with ILD due to its link to drug-induced pneumonitis (60). Methotrexate has demonstrated utility in active myositis and is considered first line therapy in IIM patients without ILD, therefore methotrexate may be required in refractory muscle disease. Its use in patients with ILD necessitates careful pulmonary monitoring.
IV. Co-morbidities and Survival
Pulmonary Hypertension
Patients with ILD are at additional risk for developing pulmonary hypertension (PH), as compared to IIM patients without ILD, which causes increased morbidity and mortality. In a 2013 study of 203 anti-synthetase syndrome patients with ILD and/or myositis/arthritis symptoms, 23.2% had a transthoracic echocardiogram suggestive of pulmonary hypertension (13). Of the 47 patients suspected of PH, 45% had a right heart catheterization performed following the echocardiogram, which confirmed pulmonary hypertension in 7.9% of the total study population. All 16 patients with confirmed PH had ILD. Survival in patients with PH and ILD was significantly lower than in patients with ILD who did not have PH.
Malignancy
Dermatomyositis has long been associated with increased risk of malignancy, however there is little evidence to guide the evaluation for malignancy in patients with anti-synthetase syndrome. The first case report of cancer-associated anti-synthetase syndrome was in 2008, in a 59 year old patients with colon adenocarcinoma (61). Several case reports and series have followed, with widely varying prevalence rates, up to approximately 14% (19, 62, 63)
In a larger retrospective analysis of 233 patients with anti-synthetase syndrome, the frequency of cancer was only 1.7% within three years from anti-synthetase syndrome diagnosis, which the authors assert was not much different than the general population in France (64). These studies suggest that care providers of these patients should follow age-appropriate cancer screening, but there is no evidence to guide additional or non-guideline based evaluation. Interestingly, treatment of the underlying malignancy has been demonstrated in case reports to improve myositis and ILD (65), further suggesting that IIM can be a paraneoplastic syndrome.
Survival
Several studies suggest that patients with anti-PL7/PL12 autoantibodies have aggressive ILD and decreased survival as compared to those with anti-Jo-1 autoantibodies. Additionally, patients with other anti-synthetase antibodies may not be appropriately diagnosed, which may slow the institution of therapy. One retrospective review found a 0.6 year median delay to diagnosis in patients with a non-Jo-1 anti-synthetase antibodies compared to patients with a Jo-1 antibody (66). In an analysis of patients with anti-synthetase syndrome, decreased survival was seen in patients without muscle weakness and with severe dyspnea (64). Patients with anti-PL7 or anti-PL12 often have severe and difficult to treat ILD, frequently without myositis (67), suggesting that the pulmonary manifestations of anti-synthetase syndrome are responsible for the difference in mortality. These findings were reproduced in a 2014 meta-analysis of 27 studies on anti-synthetase syndrome, in which the authors found that arthralgia and ILD predominate in anti-synthetase syndrome as compared to the other IIMs, and patients with Jo-1 have more myositis and a better prognosis compared to PL7 and PL12 (68).
In a 2015 survival analysis of 45 patients with anti-synthetase syndrome-ILD, 14% of patients had died at 5 years (69). Survivors more frequently had Jo-1 positivity and arthritis. Patients who died had a significantly lower baseline FVC and more patients that could not perform DLCO due to severity of disease. A 2013 retrospective evaluation of 202 patients, with an anti-synthetase antibody from the University of Pittsburgh CTD Registry during a 24 year time period, found a 5-year cumulative survival of 90% for Jo-1 patients and 75% for non-Jo-1 patients (66). The 10-year survival was 70% for Jo-1 patients and 49% for non-Jo-1 patients. The most common causes of death were pulmonary fibrosis and pulmonary hypertension. Of this cohort, 6% underwent lung transplantation. As patients with ILD due to anti-synthetase syndrome often have severe and aggressive ILD requiring multi-modality therapy, physicians should consider early referral to centers capable of lung transplantation when disease progresses or fails to adequately respond to treatment.
V. Conclusion
Anti-synthetase syndrome, characterized by the presence of an anti-tRNA synthetase antibody along with a distinct phenotype that includes a high burden of ILD, is an increasingly recognized clinical entity within the IIMs. Interstitial lung disease associated with anti-synthetase syndrome is often more severe and rapidly progressive when compared to other IIMs, which contributes to the increased morbidity and mortality seen in anti-synthetase syndrome (table 5).
Table 5.
Clinical Pearls in Anti-synthetase Syndrome
|
NSIP = non-specific interstitial pneumonia; OP = organizing pneumonia; ILD = interstitial lung disease; ANA = anti-nuclear antibody
Diagnosis is undertaken via a comprehensive history and physical examination focused on CTD signs and symptoms that include Raynaud’s phenomenon, mechanic’s hands, unexplained fever, myositis and non-erosive arthritis. HRCT imaging along with pulmonary function testing is crucial in screening for and characterizing the extent of pulmonary involvement. Muscle and lung biopsies are infrequently required for diagnosis, but can aid in understanding severity of disease and the likelihood of response to therapy.
Immunosuppressive agents, such as azathioprine, mycophenolate mofetil, and/or tacrolimus, are usually required in addition to corticosteroids for management of the myositis and pulmonary manifestations of anti-synthetase syndrome. No study has demonstrated the superiority of a particular agent, so the choice of therapy is guided by practitioner comfort and a careful assessment of potential risks of therapy in an individual patient. Our practice is to initiate prednisone and azathioprine or mycophenolate mofetil in patients with anti-synthetase syndrome related ILD, with the addition of tacrolimus for rapid disease control in more severe ILD (50). In the setting of anti-synthetase syndrome, we use rituximab for patients with severe progressive and/or refractory ILD and have used cyclophosphamide in ARDS.
In addition to monitoring ILD and myositis, providers should carefully attend to other co-morbidities associated with anti-synthetase syndrome, including pulmonary hypertension and screen for malignancy. Survival is decreased in patients with anti-synthetase syndrome-ILD as compared to patients with an IIM in general. Survival also appears to vary in anti-synthetase syndrome depending on the autoantibody present, with Jo-1 having the best prognosis. Referral to tertiary care centers capable of lung transplantation should be considered in patients with severe and/or rapidly progressive ILD.
Evidence guiding management decisions is limited, and is primarily based on case reports and series. Prospective studies are urgently needed to guide treatment choices and determine which immunosuppressive agents offer control of disease and improved survival. It is also imperative that studies examine the rate of relapse when these therapies are withdrawn, so as to guide the length of therapy.
Acknowledgments
Funding: Funding support is provided by the NIH funded Research Training in Respiratory Biology grant at the University of Chicago (T32 HL007605).
Footnotes
Conflict of Interest: no authors have conflicts of interest to disclose
References
- 1.Connors GR, Christopher-Stine L, Oddis CV, Danoff SK. Interstitial lung disease associated with the idiopathic inflammatory myopathies: What progress has been made in the past 35 years? Chest. 2010;138:1464–1474. doi: 10.1378/chest.10-0180. [DOI] [PubMed] [Google Scholar]
- 2.Kalluri M, Oddis CV. Pulmonary manifestations of the idiopathic inflammatory myopathies. Clinics in chest medicine. 2010;31:501–512. doi: 10.1016/j.ccm.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Bohan A, Peter JB. Polymyositis and Dermatomyositis. New England Journal of Medicine. 1975;292:344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 4.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) The New England journal of medicine. 1975;292:403–407. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 5.Mathews MB, Reichlin M, Hughes G, Bernstein R. Anti-threonyl-tRNA synthetase, a second myositis-related autoantibody. The Journal of experimental medicine. 1984;160:420–434. doi: 10.1084/jem.160.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathews MB, Bernstein RM. Myositis autoantibody inhibits histidyl-tRNA synthetase: a model for autoimmunity. 1983 doi: 10.1038/304177a0. [DOI] [PubMed] [Google Scholar]
- 7.Marguerie C, Bunn C, Beynon H, Bernstein R, Hughes J, So A, Walport M. Polymyositis, pulmonary fibrosis and autoantibodies to aminoacyl-tRNA synthetase enzymes. QJM. 1990;77:1019–1038. doi: 10.1093/qjmed/77.1.1019. [DOI] [PubMed] [Google Scholar]
- 8.Love LA, Leff RL, Fraser DD, Targoff IN, Dalakas M, Plotz PH, Miller FW. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine. 1991;70:360–374. doi: 10.1097/00005792-199111000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. The Lancet. 2003;362:971–982. doi: 10.1016/S0140-6736(03)14368-1. [DOI] [PubMed] [Google Scholar]
- 10.Solomon J, Swigris JJ, Brown KK. Myositis-related interstitial lung disease and antisynthetase syndrome. Jornal Brasileiro de Pneumologia. 2011;37:100–109. doi: 10.1590/s1806-37132011000100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe K, Handa T, Tanizawa K, Hosono Y, Taguchi Y, Noma S, Kobashi Y, Kubo T, Aihara K, Chin K. Detection of antisynthetase syndrome in patients with idiopathic interstitial pneumonias. Respiratory medicine. 2011;105:1238–1247. doi: 10.1016/j.rmed.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Stone KB, Oddis CV, Fertig N, Katsumata Y, Lucas M, Vogt M, Domsic R, Ascherman DP. Anti–Jo-1 antibody levels correlate with disease activity in idiopathic inflammatory myopathy. Arthritis & Rheumatism. 2007;56:3125–3131. doi: 10.1002/art.22865. [DOI] [PubMed] [Google Scholar]
- 13.Hervier B, Meyer A, Dieval C, Uzunhan Y, Devilliers H, Launay D, Canuet M, Têtu L, Agard C, Sibilia J. Pulmonary hypertension in antisynthetase syndrome: prevalence, etiology and survival. European Respiratory Journal. 2013 doi: 10.1183/09031936.00156312. erj01563-02012. [DOI] [PubMed] [Google Scholar]
- 14.Friedman AW, Targoff IN, Arnett FC. Seminars in arthritis and rheumatism. Elsevier; 1996. Interstitial lung disease with autoantibodies againstaminoacyl-tRNA synthetases in the absence of clinically apparent myositis; pp. 459–467. [DOI] [PubMed] [Google Scholar]
- 15.Targoff I. Laboratory testing in the diagnosis and management of idiopathic inflammatory myopathies. Rheumatic diseases clinics of North America. 2002;28:859. doi: 10.1016/s0889-857x(02)00032-7. [DOI] [PubMed] [Google Scholar]
- 16.Bahmer T, Romagnoli M, Girelli F, Claussen M, Rabe KF. The use of auto-antibody testing in the evaluation of interstitial lung disease (ILD)–A practical approach for the pulmonologist. Respiratory medicine. 2016;113:80–92. doi: 10.1016/j.rmed.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z, Cao M, Plana MN, Liang J, Cai H, Kuwana M, Sun L. Utility of Anti–Melanoma Differentiation–Associated Gene 5 Antibody Measurement in Identifying Patients With Dermatomyositis and a High Risk for Developing Rapidly Progressive Interstitial Lung Disease: A Review of the Literature and a Meta-Analysis. Arthritis care & research. 2013;65:1316–1324. doi: 10.1002/acr.21985. [DOI] [PubMed] [Google Scholar]
- 18.La Corte R, Lo Mo naco A, Locaputo A, Dolzani F, Trotta F. In patients with antisynthetase syndrome the occurrence of anti-Ro/SSA antibodies causes a more severe interstitial lung disease. Autoimmunity. 2006;39:249–253. doi: 10.1080/08916930600623791. [DOI] [PubMed] [Google Scholar]
- 19.Mileti LM, Strek ME, Niewold TB, Curran JJ, Sweiss NJ. Clinical characteristics of patients with anti-Jo-1 antibodies: a single center experience. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. 2009;15:254–255. doi: 10.1097/RHU.0b013e3181b0e910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghirardello A, Zampieri S, Tarricone E, Iaccarino L, Bendo R, Briani C, Rondinone R, Sarzi-Puttini P, Todesco S, Doria A. Clinical implications of autoantibody screening in patients with autoimmune myositis. Autoimmunity. 2006;39:217–221. doi: 10.1080/08916930600622645. [DOI] [PubMed] [Google Scholar]
- 21.Dugar M, Cox S, Limaye V, Blumbergs P, Roberts-Thomson P. Clinical heterogeneity and prognostic features of South Australian patients with anti-synthetase autoantibodies. Internal medicine journal. 2011;41:674–679. doi: 10.1111/j.1445-5994.2010.02164.x. [DOI] [PubMed] [Google Scholar]
- 22.Wakasugi K, Schimmel P. Two Distinct Cytokines Released from a Human Aminoacyl-tRNA Synthetase. Science. 1999;284:147–151. doi: 10.1126/science.284.5411.147. [DOI] [PubMed] [Google Scholar]
- 23.Ascherman D. Role of Jo-1 in the Immunopathogenesis of the Anti-synthetase Syndrome. Current rheumatology reports. 2015;17:1–7. doi: 10.1007/s11926-015-0532-1. [DOI] [PubMed] [Google Scholar]
- 24.Hamaguchi Y, Fujimoto M, Matsushita T, Kaji K, Komura K, Hasegawa M, Kodera M, Muroi E, Fujikawa K, Seishima M. Common and distinct clinical features in adult patients with anti-aminoacyl-tRNA synthetase antibodies: heterogeneity within the syndrome. PloS one. 2013;8:e60442. doi: 10.1371/journal.pone.0060442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marie I, Josse S, Decaux O, Dominique S, Diot E, Landron C, Roblot P, Jouneau S, Hatron P, Tiev K. Comparison of long-term outcome between anti-Jo1-and anti-PL7/PL12 positive patients with antisynthetase syndrome. Autoimmunity reviews. 2012;11:739–745. doi: 10.1016/j.autrev.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Debray M-P, Borie R, Revel M-P, Naccache J-M, Khalil A, Toper C, Israel-Biet D, Estellat C, Brillet P-Y. Interstitial lung disease in anti-synthetase syndrome: Initial and follow-up CT findings. European journal of radiology. 2015;84:516–523. doi: 10.1016/j.ejrad.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 27.Travis WD, Costabel U, Hansell DM, King TE, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU, Behr J, Bouros D, Brown KK, Colby TV, Collard HR, Cordeiro CR, Cottin V, Crestani B, Drent M, Dudden RF, Egan J, Flaherty K, Hogaboam C, Inoue Y, Johkoh T, Kim DS, Kitaichi M, Loyd J, Martinez FJ, Myers J, Protzko S, Raghu G, Richeldi L, Sverzellati N, Swigris J, Valeyre D. An Official American Thoracic Society/European Respiratory Society Statement: Update of the International Multidisciplinary Classification of the Idiopathic Interstitial Pneumonias. American journal of respiratory and critical care medicine. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yousem SA, Gibson K, Kaminski N, Oddis CV, Ascherman DP. The pulmonary histopathologic manifestations of the anti-Jo-1 tRNA synthetase syndrome. Mod Pathol. 2010;23:874–880. doi: 10.1038/modpathol.2010.65. [DOI] [PubMed] [Google Scholar]
- 29.Yousem SA, Schneider F, Bi D, Oddis CV, Gibson K, Aggarwal R. The pulmonary histopathologic manifestations of the anti-PL7/antithreonyl transfer RNA synthetase syndrome. Human pathology. 2014;45:1199–1204. doi: 10.1016/j.humpath.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 30.Mochimaru H, Kawamoto M, Enomoto T, Saitoh Y, Abe S, Nei T, Fukuda Y, Kudoh S. Transbronchial biopsy is clinically useful in classifying patients with interstitial pneumonia associated with polymyositis and dermatomyositis. Respirology. 2008;13:863–870. doi: 10.1111/j.1440-1843.2008.01363.x. [DOI] [PubMed] [Google Scholar]
- 31.Dalakas MC. Muscle biopsy findings in inflammatory myopathies. Rheumatic Disease Clinics of North America. 2002;28:779–798. doi: 10.1016/s0889-857x(02)00030-3. [DOI] [PubMed] [Google Scholar]
- 32.Kenney K, Landau ME, Gonzalez RS, Hundertmark J, O’Brien K, Campbell WW. Serum creatine kinase after exercise: Drawing the line between physiological response and exertional rhabdomyolysis. Muscle & Nerve. 2012;45:356–362. doi: 10.1002/mus.22317. [DOI] [PubMed] [Google Scholar]
- 33.Baughman RP, Meyer KC, Nathanson I, Angel L, Bhorade SM, Chan KM, Culver D, Harrod CG, Hayney MS, Highland KB, Limper AH, Patrick H, Strange C, Whelan T. Monitoring of nonsteroidal immunosuppressive drugs in patients with lung disease and lung transplant recipients: American college of chest physicians evidence-based clinical practice guidelines. CHEST Journal. 2012;142:e1S–e111S. doi: 10.1378/chest.12-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer KC, Bierach J. Immunosuppressive therapy for autoimmune lung diseases. Immunology and allergy clinics of North America. 2012;32:633–669. doi: 10.1016/j.iac.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Vananuvat P, Suwannalai P, Sungkanuparph S, Limsuwan T, Ngamjanyaporn P, Janwityanujit S. Seminars in arthritis and rheumatism. Elsevier; 2011. Primary prophylaxis for Pneumocystis jirovecii pneumonia in patients with connective tissue diseases; pp. 497–502. [DOI] [PubMed] [Google Scholar]
- 36.Perrillo RP, Martin P, Lok AS. Preventing hepatitis B reactivation due to immunosuppressive drug treatments. Jama. 2015;313:1617–1618. doi: 10.1001/jama.2015.2571. [DOI] [PubMed] [Google Scholar]
- 37.Hench PS, Kendall EC, Slocumb CH, Polley HF. Effects of cortisone acetate and pituitary ACTH on rheumatoid arthritis, rheumatic fever and certain other conditions: A study in clinical physiology. Archives of internal medicine. 1950;85:545–666. doi: 10.1001/archinte.1950.00230100002001. [DOI] [PubMed] [Google Scholar]
- 38.Joffe MM, Love LA, Leff RL, Fraser DD, Targoff IN, Hicks JE, Plotz PH, Miller FW. Drug therapy of the idiopathic inflammatory myopathies: predictors of response to prednisone, azathioprine, and methotrexate and a comparison of their efficacy. The American journal of medicine. 1993;94:379–387. doi: 10.1016/0002-9343(93)90148-i. [DOI] [PubMed] [Google Scholar]
- 39.Liu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, Leigh R, Brown JP, Cohen A, Kim H. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013;9:30. doi: 10.1186/1710-1492-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marie I, Hatron P, Dominique S, Cherin P, Mouthon L, Menard JF. Short-term and long-term outcomes of interstitial lung disease in polymyositis and dermatomyositis: A series of 107 patients. Arthritis & Rheumatism. 2011;63:3439–3447. doi: 10.1002/art.30513. [DOI] [PubMed] [Google Scholar]
- 41.Bunch TW, Worthington JW, Combs JJ, Ilstrup DM, Engel AG. Azathioprine with Prednisone for PolymyositisA Controlled, Clinical Trial. Annals of internal medicine. 1980;92:365–369. doi: 10.7326/0003-4819-92-3-365. [DOI] [PubMed] [Google Scholar]
- 42.Bunch TW. Prednisone and azathioprine for polymyositis. Long-term followup Arthritis & Rheumatism. 1981;24:45–48. doi: 10.1002/art.1780240107. [DOI] [PubMed] [Google Scholar]
- 43.Swigris JJ, Olson AL, Fischer A, Lynch DA, Cosgrove GP, Frankel SK, Meehan RT, Brown KK. Mycophenolate mofetil is safe, well tolerated, and preserves lung function in patients with connective tissue disease-related interstitial lung disease. CHEST Journal. 2006;130:30–36. doi: 10.1378/chest.130.1.30. [DOI] [PubMed] [Google Scholar]
- 44.Fischer A, Brown KK, Du Bois RM, Frankel SK, Cosgrove GP, Fernandez-Perez ER, Huie TJ, Krishnamoorthy M, Meehan RT, Olson AL. Mycophenolate mofetil improves lung function in connective tissue disease-associated interstitial lung disease. The Journal of rheumatology. 2013;40:640–646. doi: 10.3899/jrheum.121043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robaeys G, Cassiman D, Verslype C, Monbaliu D, Aerts R, Pirenne J, Nevens F. Transplantation proceedings. Elsevier; 2009. Successful conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium (myfortic) in liver transplant patients with gastrointestinal side effects; pp. 610–613. [DOI] [PubMed] [Google Scholar]
- 46.Klein IH, Abrahams A, van Ede T, Hené RJ, Koomans HA, Ligtenberg G. Different effects of tacrolimus and cyclosporine on renal hemodynamics and blood pressure in healthy subjects. Transplantation. 2002;73:732–736. doi: 10.1097/00007890-200203150-00012. [DOI] [PubMed] [Google Scholar]
- 47.Cavagna L, Caporali R, Abdì-Alì L, Dore R, Meloni F, Montecucco C. Cyclosporine in anti-Jo1-positive patients with corticosteroid-refractory interstitial lung disease. The Journal of rheumatology. 2013;40:484–492. doi: 10.3899/jrheum.121026. [DOI] [PubMed] [Google Scholar]
- 48.Oddis CV, Sciurba FC, Elmagd KA, Starzl TE. Tacrolimus in refractory poly myositis with inter stitial lung disease. The Lancet. 1999;353:1762–1763. doi: 10.1016/S0140-6736(99)01927-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsubara S, Kondo K, Sugaya K, Miyamoto K. Effects of tacrolimus on dermatomyositis and polymyositis: a prospective, open, non-randomized study of nine patients and a review of the literature. Clinical rheumatology. 2012 doi: 10.1007/s10067-012-2044-y. [DOI] [PubMed] [Google Scholar]
- 50.Witt LJ, Demchuck C, Curran JJ, Strek ME. Benefit of adjunctive tacrolimus in connective tissue disease-interstitial lung disease. Pulmonary pharmacology & therapeutics. 2016 doi: 10.1016/j.pupt.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilkes MR, Sereika SM, Fertig N, Lucas MR, Oddis CV. Treatment of antisynthetase-associated interstitial lung disease with tacrolimus. Arthritis and rheumatism. 2005;52:2439–2446. doi: 10.1002/art.21240. [DOI] [PubMed] [Google Scholar]
- 52.Oddis CV, Reed AM, Aggarwal R, Rider LG, Ascherman DP, Levesque MC, Barohn RJ, Feldman BM, Harris-Love MO, Koontz DC, Fertig N, Kelley SS, Pryber SL, Miller FW, Rockette HE the RIMSG. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: A randomized, placebo-phase trial. Arthritis & Rheumatism. 2013;65:314–324. doi: 10.1002/art.37754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sem M, Molberg Ø, Lund MB, Gran JT. Rituximab treatment of the anti-synthetase syndrome—a retrospective case series. Rheumatology. 2009:kep157. doi: 10.1093/rheumatology/kep157. [DOI] [PubMed] [Google Scholar]
- 54.Keir GJ, Maher TM, Hansell DM, Denton CP, Ong VH, Singh S, Wells AU, Renzoni EA. Severe interstitial lung disease in connective tissue disease: rituximab as rescue therapy. European Respiratory Journal. 2012;40:641–648. doi: 10.1183/09031936.00163911. [DOI] [PubMed] [Google Scholar]
- 55.Keir GJ, Maher TM, Ming D, Abdullah R, de Lauretis A, Wickremasinghe M, Nicholson AG, Hansell DM, Wells AU, Renzoni EA. Rituximab in severe, treatment-refractory interstitial lung disease. Respirology. 2014;19:353–359. doi: 10.1111/resp.12214. [DOI] [PubMed] [Google Scholar]
- 56.Ge Y, Peng Q, Zhang S, Zhou H, Lu X, Wang G. Cyclophosphamide treatment for idiopathic inflammatory myopathies and related interstitial lung disease: a systematic review. Clinical rheumatology. 2015;34:99–105. doi: 10.1007/s10067-014-2803-z. [DOI] [PubMed] [Google Scholar]
- 57.Mira-Avendano IC, Parambil JG, Yadav R, Arrossi V, Xu M, Chapman JT, Culver DA. A retrospective review of clinical features and treatment outcomes in steroid-resistant interstitial lung disease from polymyositis/dermatomyositis. Respiratory medicine. 2013;107:890–896. doi: 10.1016/j.rmed.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 58.Dalakas MC, Illa I, Dambrosia JM, Soueidan SA, Stein DP, Otero C, Dinsmore ST, McCrosky S. A controlled trial of high-dose intravenous immune globulin infusions as treatment for dermatomyositis. New England Journal of Medicine. 1993;329:1993–2000. doi: 10.1056/NEJM199312303292704. [DOI] [PubMed] [Google Scholar]
- 59.Bakewell CJ, Raghu G. Polymyositis associated with severe interstitial lung disease: remission after three doses of IV immunoglobulin. CHEST Journal. 2011;139:441–443. doi: 10.1378/chest.10-0360. [DOI] [PubMed] [Google Scholar]
- 60.Imokawa S, Colby TV, Leslie KO, Helmers RA. Methotrexate pneumonitis: review of the literature and histopathological findings in nine patients. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2000;15:373–381. doi: 10.1034/j.1399-3003.2000.15b25.x. [DOI] [PubMed] [Google Scholar]
- 61.Rozelle A, Trieu S, Chung L. Malignancy in the setting of the anti-synthetase syndrome. JCR: Journal of Clinical Rheumatology. 2008;14:285–288. doi: 10.1097/RHU.0b013e31817d116f. [DOI] [PubMed] [Google Scholar]
- 62.Legault D, McDermott J, Crous-Tsanaclis AM, Boire G. Cancer-associated myositis in the presence of anti-Jo1 autoantibodies and the antisynthetase syndrome. The Journal of rheumatology. 2008;35:169–171. [PubMed] [Google Scholar]
- 63.Castañeda-Pomeda M, Prieto-González S, Grau JM. Antisynthetase syndrome and malignancy: our experience. JCR: Journal of Clinical Rheumatology. 2011;17:458. doi: 10.1097/RHU.0b013e31823b1878. [DOI] [PubMed] [Google Scholar]
- 64.Hervier B, Devilliers H, Stanciu R, Meyer A, Uzunhan Y, Masseau A, Dubucquoi S, Hatron P-Y, Musset L, Wallaert B. Hierarchical cluster and survival analyses of antisynthetase syndrome: phenotype and outcome are correlated with anti-tRNA synthetase antibody specificity. Autoimmunity reviews. 2012;12:210–217. doi: 10.1016/j.autrev.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 65.Zang Y-S, Xiu Q-Y, Fang Z, Li B, Xia T-B. Case Report: Dramatic Recovery of Lung Adenocarcinoma–Associated Dermatomyositis with Targeted Lung Cancer Therapy Alone. The oncologist. 2008;13:79–81. doi: 10.1634/theoncologist.2007-0172. [DOI] [PubMed] [Google Scholar]
- 66.Aggarwal R, Cassidy E, Fertig N, Koontz DC, Lucas M, Ascherman DP, Oddis CV. Patients with non-Jo-1 anti-tRNA-synthetase autoantibodies have worse survival than Jo-1 positive patients. Annals of the rheumatic diseases. 2013 doi: 10.1136/annrheumdis-2012-201800. annrheumdis-2012-201800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fischer A, Swigris JJ, du Bois RM, Lynch DA, Downey GP, Cosgrove GP, Frankel SK, Fernandez-Perez ER, Gillis JZ, Brown KK. Anti-synthetase syndrome in ANA and anti-Jo-1 negative patients presenting with idiopathic interstitial pneumonia. Respiratory medicine. 2009;103:1719–1724. doi: 10.1016/j.rmed.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lega J-C, Fabien N, Reynaud Q, Durieu I, Durupt S, Dutertre M, Cordier J-F, Cottin V. The clinical phenotype associated with myositis-specific and associated autoantibodies: a meta-analysis revisiting the so-called antisynthetase syndrome. Autoimmunity reviews. 2014;13:883–891. doi: 10.1016/j.autrev.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 69.Rojas-Serrano J, Herrera-Bringas D, Mejía M, Rivero H, Mateos-Toledo H, Figueroa J. Prognostic factors in a cohort of antisynthetase syndrome (ASS): serologic profile is associated with mortality in patients with interstitial lung disease (ILD) Clinical rheumatology. 2015;34:1563–1569. doi: 10.1007/s10067-015-3023-x. [DOI] [PubMed] [Google Scholar]