Twenty years ago McCarthy et al. described frontotemporal dementia parkinsonism linked to chromosome 17 (FTDP-17). They later identified a tau mutation (+14) in the exon/intron 10 splice site stem loop. Eleven of 12 predicted stem loop mutations were subsequently discovered. The authors now describe another Irish family with FTDP-17 due to missing stem loop mutation (+15).
Keywords: frontotemporal, dementia, MAPT, stem loop, FTDP-17
Twenty years ago McCarthy et al. described frontotemporal dementia parkinsonism linked to chromosome 17 (FTDP-17). They later identified a tau mutation (+14) in the exon/intron 10 splice site stem loop. Eleven of 12 predicted stem loop mutations were subsequently discovered. The authors now describe another Irish family with FTDP-17 due to missing stem loop mutation (+15).
Abstract
Frontotemporal lobar degeneration comprises a group of disorders characterized by behavioural, executive, language impairment and sometimes features of parkinsonism and motor neuron disease. In 1994 we described an Irish-American family with frontotemporal dementia linked to chromosome 17 associated with extensive tau pathology. We named this disinhibition-dementia-parkinsonism-amyotrophy complex. We subsequently identified mutations in the MAPT gene. Eleven MAPT gene splice site stem loop mutations were identified over time except for 5’ splice site of exon 10. We recently identified another Irish family with autosomal dominant early amnesia and behavioural change or parkinsonism associated with the ‘missing’ +15 mutation at the intronic boundary of exon 10. We performed a clinical, neuropsychological and neuroimaging study on the proband and four siblings, including two affected siblings. We sequenced MAPT and performed segregation analysis. We looked for a biological effect of the tau variant by performing real-time polymerase chain reaction analysis of RNA extracted from human embryonic kidney cells transfected with exon trapping constructs. We found a c.915+15A>C exon 10/intron 10 stem loop mutation in all affected subjects but not in the unaffected. The c.915+15A>C variant caused a shift in tau splicing pattern to a predominantly exon 10+ pattern presumably resulting in predominant 4 repeat tau and little 3 repeat tau. This strongly suggests that the c.915+15A>C variant is a mutation and that it causes frontotemporal dementia linked to chromosome 17 in this pedigree by shifting tau transcription and translation to +4 repeat tau. Tau (MAPT) screening should be considered in families where amnesia or atypical parkinsonism coexists with behavioural disturbance early in the disease process. We describe the final missing stem loop tau mutation predicted 15 years ago. Mutations have now been identified at all predicted sites within the ‘stem’ when the stem-loop model was first proposed and no mutations have been found within the ‘loop’ region as expected. Therefore we ‘close the tau loop’ having ‘opened the loop’ 21 years ago.
Introduction
Frontotemporal lobar degeneration comprises a group of disorders characterized by behavioural, executive and language impairment, sometimes with features of parkinsonism or motor neuron disease. Twenty-one years ago we described an Irish-American family with frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP-17) associated with frontotemporal atrophy, superficial cortical spongiform change, neuronal loss, gliosis, destruction of nigra and amygdala and extensive tau pathology (Lynch et al., 1994; Wilhelmsen et al., 1994; Sima et al., 1996). We named this disorder disinhibition-dementia-parkinsonism-amyotrophy-complex (DDPAC). We, and others, subsequently identified mutations in the microtubule-associated protein tau (MAPT) gene on chromosome 17q21.31 (Hutton et al., 1998; Spillantini et al., 1998). It is now recognized that 25–50% of frontotemporal lobar degeneration cases are hereditary and 15–20% of these carry mutations in MAPT (Onyike et al., 2013), 20–25% carry progranulin (GRN) mutations (Baker et al., 2006; Cruts et al., 2006, Borroni et al., 2008) and 3–48% carry C9orf72 mutations (DeJesus-Hernandez et al., 2011; Renton et al., 2011; Porta et al., 2015). Tau is a natively unfolded microtubule-associated protein concentrated in the axons of maturing and formed neurons and is critical for neuronal function (Wang and Liu, 2008). Hyperphosphorylated tau forms a core component of the intracellular filamentous deposits that characterize a number of neurodegenerative diseases (Rizzu et al., 1999; Wang and Liu, 2008; Spillantini and Goedert, 2013). Tau is involved in promoting neurite outgrowth, organizing axonal microtubules and both dynein and kinesin-dependent axonal transport (Magnani et al., 2007; Wang and Liu, 2008; Morfini et al., 2009). Tau isoform ratios are not conserved across the species. In adult rodents only 4R isoforms are expressed whereas in chickens 3R, 4R and 5R isoforms are present. Six tau isoforms are expressed in the adult human brain. These are produced by the alternative splicing of mRNA of the MAPT gene (Goedert et al., 1989). These isoforms differ from each other via the inclusion or exclusion of a 29 or 58 amino acid insert in the amino-terminal half (encoded by exons 2 and 3), and by the inclusion or exclusion of a 31 amino acid sequence that encodes the extra-repeat (encoded by exon 10) in the carboxy-terminal half of the tau gene.
Inclusion of exon 10 (10+) results in 4R tau and exclusion of exon 10 (10−) results in 3R tau. In adults the ratio of 3R:4R is closely regulated at ∼1:1 (Goedert and Jakes, 1990; Kar et al., 2011). Tau binds to microtubules using these repeat domains and notably 4R tau binds more efficiently than 3R (Goedert et al., 1990).
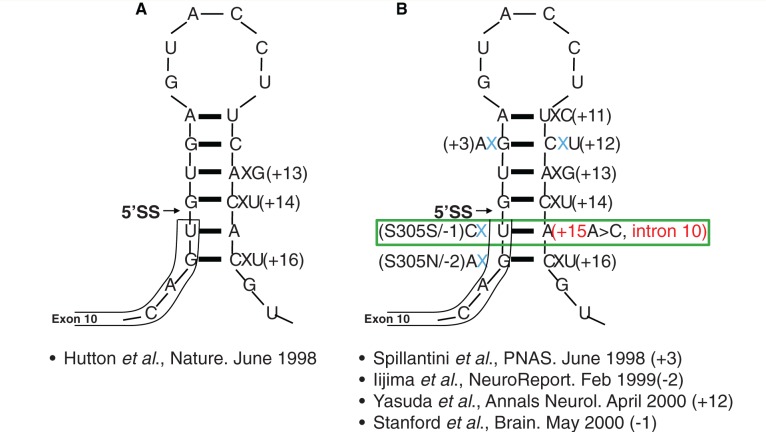
MAPT mutations may alter tau protein structure or disrupt the normal 3R:4R tau isoform ratio of tau isoforms (Goedert and Jakes, 2005). The original Irish-American family (DDPAC) (Lynch et al., 1994; Wilhelmsen et al., 1994) carried a +14 C>T exon 10/intron 10 boundary mutation. A cluster of mutations close to this 5’splice site of MAPT exon 10 were identified (−2, −1, +3, +12, +13, +14, +16) (Hutton et al., 1998; Spillantini et al., 1998; Grover et al., 1999; Iijima et al., 1999; Stanford et al., 2000; Yasuda et al., 2000). By 2000 structural studies had identified the residues in the stem loop where changes would have caused instability (Varani et al., 2000) and indeed Hutton (2000) predicted that further undiscovered stem-loop mutations would be found. Eleven splice site mutations were subsequently identified over time at each of the sites predicted to destabilize the stem loop, except for the elusive c.915+15A>C mutation (Qian and Liu, 2014).
We have recently identified another Irish family, with branches in the UK, in which the proband showed early amnesia and behavioural change associated with the ‘missing’ c.915+15A>C mutation at the intronic boundary downstream of exon 10.
Materials and methods
We identified a young adult male with FTDP-17 and recruited a number of his siblings as part of a prospective study looking at the earliest symptoms and biomarkers of frontotemporal lobar degeneration MAPT carriers.
We performed a large family study involving five siblings including three affected (Fig. 1). All Irish patients were prospectively assessed in Dublin Neurological Institute at the Mater Misericordiae University Hospital. The study was approved by the hospital Ethics Committee and informed consent was obtained from each participant. Medical records were reviewed and past medical history, family history, social history and medication use were gathered. We performed a full neurological examination on all patients. Brain neuroimaging [MRI, 18F-fluorodeoxyglucose (FDG) PET/CT], EEG, CSF analysis and basic laboratory blood tests were obtained from the proband.
Figure 1.
Patient’s family history of a young-onset behavioural, amnestic syndrome, suggesting an autosomal dominant pattern of inheritance.  proband;
proband;  male;
male;  male twins;
male twins;  affected male;
affected male;  deceased male;
deceased male;  female;
female;  affected female;
affected female;  deceased female;
deceased female;  carrier c.915+15A > C;
carrier c.915+15A > C;  non-carrier;
non-carrier;  not screened.
not screened.
We analysed CSF total tau content, phosphorylated tau and CSF amyloid-β. CSF was collected in sterile polypropylene tubes from the L3/L4 interspace, centrifuged at 2500g for 10 min at 4°C and the supernatant aliquoted, and stored at −80°C. Total tau, phospho-tau181 and amyloid-β1-42 concentrations were measured using the Innotest® hTau Ag, Innotest® Phospho-tau(181P) (Innogenetics) and MSD Multi-Array® Human Abeta 42 (MSD) ELISA kits (O’Dowd et al., 2013).
MRI brain and basic laboratory tests were also obtained from the two affected siblings. Blood samples for DNA extraction from affected and unaffected siblings were obtained. DNA was also extracted from archived post-mortem brain tissue from the proband’s deceased mother. Fluorescent Sanger sequence of exons 1–5, 7, 9–13 of the MAPT gene in the index case was analysed using Mutation Surveyor® software developed by SoftGenetics®, LLC. Mutations were named according to HGVS nomenclature and reference sequence NM_005910.5 where +1 is the A of the first coding ATG. Dosage analysis of the MAPT/GRN genes was performed using MRC Holland MLPA kit P275-C1.
The genetic variant identified in intron 10 was subsequently tested in other family members for segregation analysis within the pedigree.
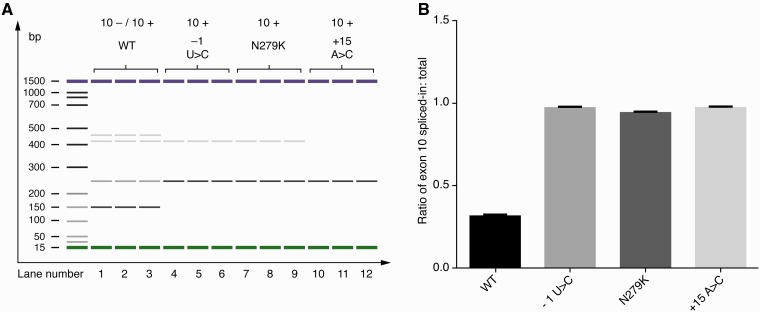
To demonstrate a possible biological effect of this new tau variant (c.915+15A>C) on MAPT transcription we performed real-time PCR analysis on RNA extracted from human embryonic kidney cells (HEK cells) transfected with exon trapping constructs containing mutant and wild-type exon 10 exonic and surrounding intronic sequence (this work was done under Prof. Michael Hutton’s supervision at Eli Lilly and Company). The real-time PCR products were separated on an Agilent DNA 1000 chip and quantified using the Agilent 2100 Expert software (Fig. 4).
Figure 4.
+15-splice-site mutations increase incorporation of tau exon 10 into artificial mRNAs. (A) The effect of the c.915+15A > C mutation: a clear increase in exon 10+ RNA (10+/10− RNA ratio) induced by the c.915+15A > C mutation. The c.915+15A > C mutation (lanes 10–12) causes the tau splicing pattern to shift from the predominantly exon 10− (153bp product) pattern seen with the wild-type (WT) stem-loop (lanes 1–3) to predominantly exon 10+ (246 bp product). A second stem-loop destabilizing mutation, −1 U > C (lanes 4–6) and the exonic N279K mutation (lanes 7–9) both caused a shift in splicing pattern to one of predominantly exon 10+. The bands between 400–500 based pairs represent cryptic splice products linked to the use of an alternative 5’splice site in the construct (the alternative site is sometimes used when the main 5’site is blocked by the stem loop). When the stem loop is disrupted by a mutation the levels of these cryptic splice products are also reduced. Therefore these 400–500 bp products are not seen in the +15 mutation nor in the N279K mutation nor in the −1U>C mutation (Grover et al., 1999). Real-time PCR products from HEK cells transfected with exon trapping constructs were separated on an Agilent DNA 1000 chip and quantified using the Agilent 2100 Expert software. Bp = base pair; WT = wild-type; U = uracil; C = cytosine; A = adenine; N279K = exon 10 mutation associated with pallido-ponto-nigral degeneration. (B) The ratios of tau exon 10+ to total exon 10 (±SD) are shown indicating the predominance of exon 10+ in the splice site mutations −1 U>C and +15A>C and the exonic mutation N279K.
Results
Family study
The proband (Patient IV–1; Fig. 1) is a 44-year-old right-handed farmer with a 4-year history of a progressive amnestic syndrome, disinhibition, impulsivity, apathy, lack of motivation and Witzelsucht (altered sense of humour, a tendency to make puns, tell inappropriate jokes or pointless stories). The profound amnestic syndrome occurred in parallel with the behavioural change. The patient, described as a ‘workaholic’, could no longer work for a living because of apathy. Collateral history was obtained from his wife as the patient denied any illness.
Past medical history was unremarkable, and he had no history of mood disturbance. Alcohol intake was moderate, and he did not smoke tobacco. His wife described gradual onset of reduced initiative, becoming increasingly withdrawn with difficulty recalling names. He resorted to list-making for daily tasks.
He had disinhibited, jocular behaviour in public, was less cautious with money, and uninterested in his appearance and hygiene. His mood was labile, fluctuating between avolition and impulsivity, without insight. He played practical jokes; his wife described a ‘quirky’ sense of humour. On one particular occasion he put on a Halloween mask in a shop and ran around in a ‘childlike’ way. He became involved in a local lottery, which he avoided in the past.
Reading, counting and spatial orientation skills were intact; he was continent and continued to drive a car. He craved and hoarded sweet foodstuffs. He described no sudden jerks or difficulty walking and family reported he neither had hallucinations nor cognitive fluctuation.
Prosody was flat, but his language was normal. Flat affect improved partially on sertraline. Donepezil (10 mg once daily) was subsequently added, without effect. The patient’s family tree (Fig. 1) was consistent with autosomal dominant inheritance of young-onset dementia, with prominent behavioural changes in some family members in four generations. The patient’s deceased mother (Patient III–1; Fig. 1) had a behavioural change and memory loss in her early fifties; she developed a ‘sweet tooth’, a shuffling gait by the age of 54 and died of bronchopneumonia at the age of 57. At autopsy brain weight was 1150 g. Unfortunately brain histopathology was not performed.
The proband’s first cousin (Patient IV-8; Fig. 1), developed behavioural symptoms and memory problems by age 45. Short-term memory gradually deteriorated and she forgot names of good friends and had to make a list to shop. There was no language difficulty. She was diagnosed with frontotemporal dementia (FTD). She is currently 51 years old and wanders but lives at home with family support. Her sister (Patient IV-9; Fig. 1) developed behavioural problems by age 43. She began to wander, and was awkward and nervous in company (e.g. wandering off at a wedding to a hotel room without concern that others were worrying about her whereabouts). She developed a ‘sweet tooth’ with cravings for cream cakes. She was unconcerned and unsafe when crossing a busy road. She was unable to adjust to a change in her commute from a train to a bus and repeatedly was found walking beside the railway tracks. By age 49 she moved to sheltered accommodation because of safety concerns. Her mother (Patient III-2; Fig. 1) was diagnosed at age 50 with ‘Alzheimer disease’ (brain autopsy was not performed) and she died at age 55 years in a nursing home. The proband’s uncle (Patient III-5; Fig. 1) developed memory difficulties in his fifth decade and an ‘odd gait’. He was diagnosed with ‘Alzheimer disease’ in his early fifties and spent his last 4 years of life in a nursing home before dying in his sixth decade. Again no post-mortem was performed.
The uncle’s daughter (Patient IV-11; Fig. 1) developed behavioural change in her fifth decade and developed a ‘sweet tooth’ for cream cakes. She was treated for an obsessive-compulsive disorder and was diagnosed with FTD by age 49 years.
The proband’s other uncle (Patient III-6; Fig. 1) developed memory and behavioural problems without a diagnosis and died of a gastric cancer in his eighth decade.
At presentation the proband’s neurological examination revealed the presence of primitive reflexes (palmomental, grasp reflexes and glabellar tap), passivity, with occasional jocularity and disinhibition. There was no myoclonus, fasiculations or extrapyramidal signs.
A Montreal Cognitive Assessment of the proband (Patient IV-1; Fig. 1) revealed a score of 25/30 (missing four for delayed recall and one for abstract reasoning). The patient had two neuropsychological assessments performed, 5 months apart. On each occasion he was disinhibited, making numerous sexually inappropriate comments and had difficulty engaging with the assessment process. He had low average levels of performance on tests of vocabulary but impairments were noted on general knowledge testing from the Wechsler Adult Intelligence IV Test (Table 1).
Table 1.
Neuropsychological test scores of the proband
| Test | Raw Score Time 1 | Standardized Score Time 1 | Raw Score Time 2 (6 months later) | Standardized Score Time 2 |
|---|---|---|---|---|
| RBANS List Learning Immediate recall | 22 | Scaled Score 6 | 22 | Scaled Score 6 |
| RBANS List Learning Delayed Recall | 0 | 0.04 percentile | 1 | 0.02 percentile |
| RBANS Recognition | 12/20 | <0.001 percentile | 13/20 | <0.0001 percentile |
| RBANS Story Immediate Recall | 16 | Scaled Score 9 | 18 | Scaled Score 11 |
| RBANS Story Delayed Recall | 3 | 0.05 percentile | 0 | <0.0001 percentile |
| RBANS naming | 5/10 | 0.003 percentile | 8 | 10th percentile |
| Category Fluency | 18 | Scaled Score 8 | - | - |
| Digit Span | 11 | Scaled Score 11 | - | - |
| FAS test | 36 | 9 | 37 | 9 |
| Hayling 1: Sensible Completion | 8 | 6 (average) | 6 | 6 |
| Hayling 2: Inhibition time | 15 | 6 (average) | 6 | 6 |
| Hayling 2: Overt Inhibition errors | 1 | Normal | 0 | Normal |
| Hayling B: Somewhat connected errors | 9 | Impaired | 10 | Impaired |
| Hayling Overall | 4 | Low average | 4 | Low Average |
| Brixton Test | 15 | 6 (average) | 23 | 4 (low average) |
| Frontal Assessment Battery | 18/18 | Normal | 15/18 (0 for inhibitory control) | |
| WAIS-IV: Vocabulary | 22 | 6 | - | - |
| WAIS-IV: Information | 5 | 4 | - | - |
FAS = F.A.S: phonemic fluency test; WAIS = Wechsler Adult Intelligence Scale®.
Raw scores are presented for specific subtests as performance was so low. The RBANS is a battery of neuropsychological screening tests where the scaled score average is 7–13. The FAS fluency test also has a scaled score range of 7–13. The Hayling test is a measure of behavioural inhibition and the scaling is 1–10 with 6 representing the average performance. Repetition, praxis and perception within normal limits on each test occasion.
On a list learning task, his immediate word list recall was weak but he showed a satisfactory learning curve over time. However, there was a rapid loss of information from memory with no recall of the list after a brief delay. This remained stable at the second assessment with adequate encoding but poor retention. A similar pattern was seen for prose passage recall with satisfactory immediate recall but little recollection after a delay period. The recognition testing for the list was performed and he was impaired at 12/20 and this pattern was maintained at Time 2. In our view this relates to a retention deficit rather than any frontally mediated retrieval deficit (although poor encoding could adversely affect later retrieval, but recognition should usually improve this too). Confrontational naming was impaired on both occasions but repetition and naming from auditory description were intact. Praxis was intact as was dot counting, fragmented number perception and functional reading with no evidence of dyslexia.
His initial score on the Frontal Assessment Battery at 46 years was 18/18 and 6 months later was 15/18. His overall score in the Haying Sentence Completion Test, a measure of behavioural inhibition, was within the low average range on both occasions. His error pattern revealed difficulty inhibiting lexical responses but these errors were not frankly disinhibited. On the Brixton Spatial Anticipation Test, a measure of mental flexibility and rule following, his initial score fell within the average range, and low average 5 months later. Performance on a phonemic fluency task (F, A, S test) lay within the average range at both testing sessions. Semantic fluency lay within the low average range. Digit Span lay within the average range. Overall his neuropsychological profile revealed deficits in retention of information with confrontational naming deficits and some mild inhibitory deficits on formal testing but all other cognitive skills, especially executive function, fell within normal limits except for a slight deterioration in executive tests over a 5-month period. These were in contrast to his marked behavioural disinhibition both socially and during the testing process.
Investigations
MRI brain of the proband at age 45 (Patient IV-1; Fig. 1) showed bilateral mild-to-moderate anterior temporal lobe atrophy, without marked frontal atrophy (Fig. 2A and B). FDG/PET brain at age 46 demonstrated low anterior temporal lobe metabolic activity with normal frontal activity (Fig. 2C). Follow-up FDG/PET at age 49 showed a subtle worsening of low anterior temporal lobe metabolic activity. Laboratory investigations were normal, including full blood count, erythrocyte sedimentation rate, blood film, auto-antibody, vasculitis and infectious screen [including Venereal Disease Research Laboratory Test (VDRL) and HIV], urinalysis, anti-neuronal antibodies [voltage-gated potassium channel antibodies (anti-VGKC) and N-methyl D-aspartate receptor antibodies (anti-NMDA)]. EEG was within normal limits.
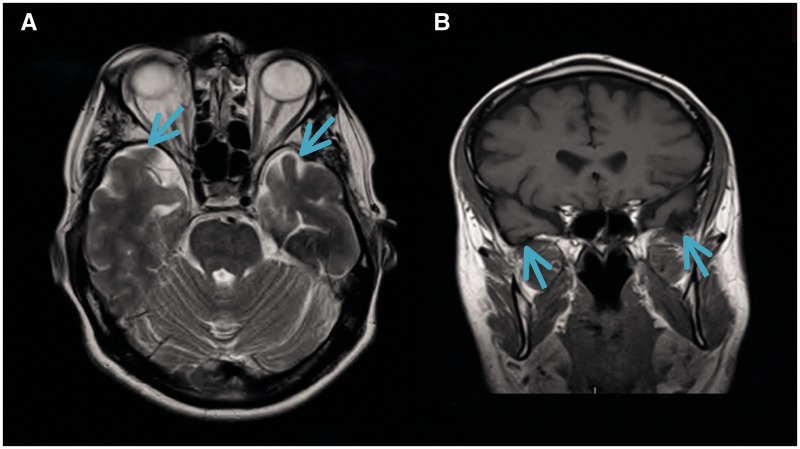
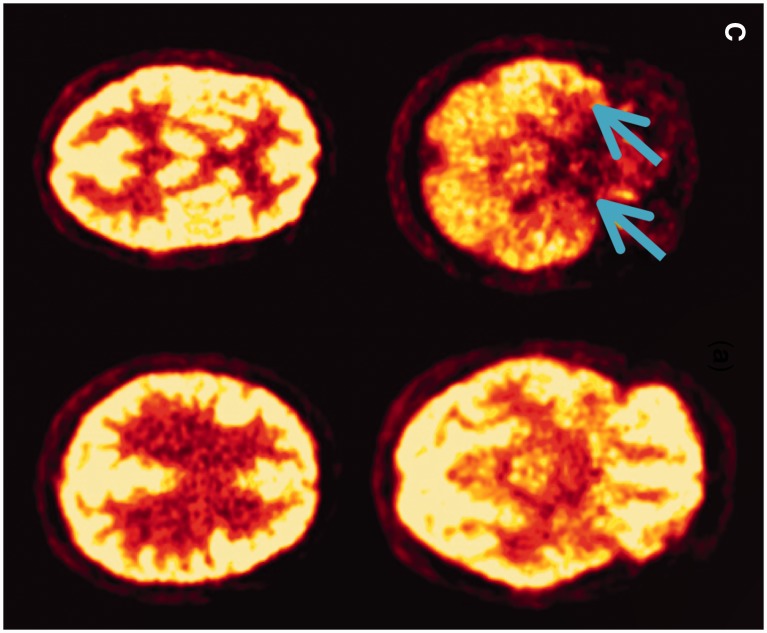
Figure 2.
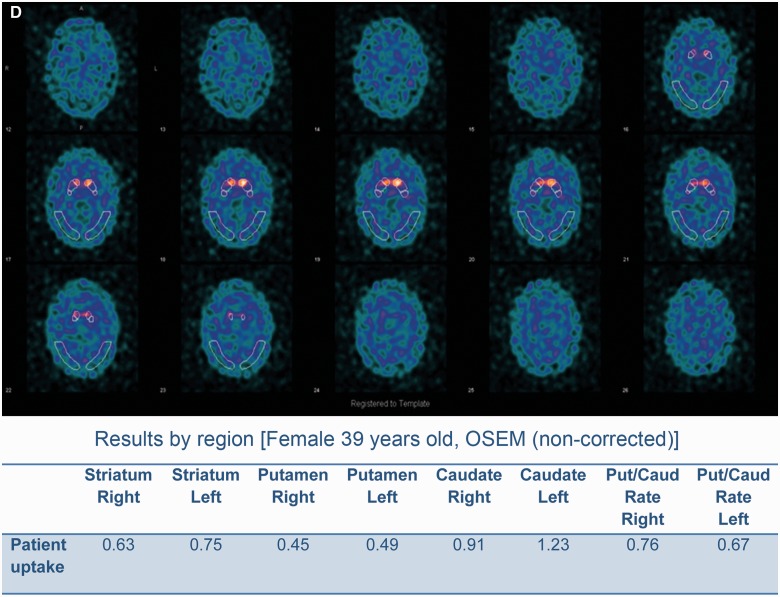
Morphological and functional brain imaging of proband (Patient IV-1) and proband's sister (Patient IV-2). (A) MRI brain (axial T2) showing bilateral mild-to-moderate temporal atrophy indicated by arrow (proband Patient IV-1). (B) MRI brain (coronal T1) showing bilateral mild temporal atrophy more pronounced on the left side, indicated by arrow (proband Patient IV-1). (C) FDG-PET scan displaying bilateral reduction in temporal tip activity indicated by arrow with normal frontal and parietal uptake (Proband Patient IV-1; Fig 1). (D) Nuclear medicine brain dopaminergic system imaging (123I-FP-CIT DAT scan) of the proband’s sister (Patient IV-2; Fig 1). Radiotracer uptake in the striatum was quantified and compared to background activity using automated GE software. Specific ratio values (basal ganglia nucleus compared to background activity) <2.0 may be considered abnormal.
CSF protein content was 51.7 mg/dl (reference range 15–45 mg/dl) and other routine CSF measures were normal. We found that CSF total tau content was 162.1 pg/ml (reference range 65–330), phosphorylated tau at 42.7 pg/ml (reference range 25–82), and CSF amyloid-β at 501 pg/ml (reference range 212–996) supporting a non-amyloid based process. Fluorescent sequencing and dosage analysis of exons 1–5, 7, 9–13 of the MAPT gene detected a novel unreported sequence variant, c.915+15A>C, at the exon10/intron10 boundary of MAPT.
The familial variant was identified in the patient’s affected female maternal first cousin (Patient IV-8; Fig. 1), the proband’s two sisters (Patients IV-4 and IV-2; Fig. 1) and in the proband’s deceased mother (Patient III-1; Fig. 1) DNA from extracted post-mortem tissue. This tau variant has not been previously reported.
Assessment of siblings
We subsequently tested four of the proband’s siblings for the familial MAPT variant and assessed them neurologically. Two asymptomatic sisters did not carry the variant, but two other sisters with neurological symptoms were found to carry the familial c.915+15A>C MAPT variant. Patient IV-4 (Fig. 1) had cognitive and behavioural change and Patient IV-2 (Fig. 1) had parkinsonism with eyelid opening apraxia.
The proband’s older sister (Patient IV-4; Fig. 1) had a history of postnatal depression treated with escitalopram (10 mg). She was slightly disinhibited and was not upset about the positive gene.
Test result
On assessment she had short term memory problems with Montreal Cognitive Assessment Test of 24/30 (missing five for delayed recall and one for naming) compared to an initial Montreal Cognitive Assessment Test of 26/30 (missing four for delayed recall) 18 months earlier. Her Frontal Assessment Battery was at 18/18. Brain MRI was normal. The proband’s younger sister (Patient IV-2; Fig. 1) also carrier of the c.915+15A>C MAPT familial variant and had a 1-year history of difficulty opening jars, left-sided clumsiness, difficulty opening her mouth, drooling of saliva, slowness, and 6 months of difficulty opening her eyes (eyelid opening apraxia). She had no hallucinations and no language problems. She scored 30/30 on Montreal Cognitive Assessment Test (initial score 18 months prior was 28/30). Her Frontal Assessment Battery was at 18/18. She takes citalopram (20 mg) for depression and anxiety associated with the gene test result. Neurological examination showed a masked facies, eyelid opening apraxia, brisk jaw jerk, brisk reflexes, spastic catch in the left wrist on pronation/supination and in lower limbs, clonus at the left ankle. She had left-sided bradykinesia and side-to-side neck movements were slow. MRI of the brain and cervical spine showed mild involutional change involving temporal lobes bilaterally. Her nuclear medicine brain dopaminergic system imaging (123I-FP-CIT DAT) scan showed a moderate-to-severe reduction in tracer uptake in the striatum bilaterally (Fig. 2C and D). Changes were more prominent on the right side (Fig. 2C). Basic blood test results were normal.
The eldest sister (Patient IV-3; Fig. 1), who was 53 years old and a health-care worker, had behavioural and memory problems but was not formally assessed or genetically tested.
Real time PCR analysis of the products from HEK cells translated from exon trapping constructs showed a shift in the tau splicing pattern resulting in predominantly exon 10+ (246 bp product) pattern as compared to a predominantly exon 10− (153-bp product) pattern in cells transfected with the wild-type construct (Fig. 4A and B). This change is predicted to result in predominant 4 repeat tau (4R) (exon 10+) and little 3 repeat tau (3R) (exon 10−) being generated from the mutant Tau allele.
Discussion
Seventeen years ago we described multiple MAPT mutations associated with FTDP-17 including an exon 10/intron 10 boundary (C>T+14; G>A+3) stem loop mutation associated with FTDP-17, including the DDPAC family (Hutton et al., 1998; Spillantini et al., 1998). Thus we ‘opened the Tau loop’. Importantly, the identification of mutations in MAPT as the cause of FTDP-17 demonstrated that tau dysfunction alone is sufficient to cause neurodegeneration. Tau dysfunction has a central role in FTDP-17 and in other conditions including Alzheimer’s disease, progressive supranuclear palsy, Pick disease, corticobasal syndrome and other tauopathies (Murray et al., 2005). The intronic region bordering MAPT exon 10 forms a stem loop structure that partially controls the inclusion of exon 10 (leading to expression of 4R tau) or exclusion of exon 10 (leading to expression of 3R tau). Multiple mutations within the stem loop were found but one remained elusive (Hutton, 2000).
We now report the ‘missing’ c.915+15A>C mutation in a different Irish family, thus ‘closing the loop’ (Hutton, 2000) (Fig. 3). We confirm that the c.915+15A>C stem loop mutation alters MAPT splicing in HEK cells transfected with exon trapping constructs. This generates a shift from the normal 3R:4R pattern of tau isoforms to a predominance of 4R by exon 10+ inclusion as predicted by the reduced stability of the mutant stem-loop structure and similar to the effects observed previously with other stem loop mutations (e.g. −1 U>C) (Fig. 4). This strongly suggests that the c.915 +15 A>C variant is a pathogenic mutation and that it causes FTDP-17 in this pedigree by shifting tau transcription to overproduce 4R tau similar to other FTDP-17 mutations (Hutton et al., 1998; Spillantini et al., 1998).
Figure 3.
Stem loop structure at the exon 10/intron 10 boundary with the c.915+15A > C change highlighted. A = adenine; G = guanine; C = cytosine; U = uracil; 5’SS = 5 primer splicing site;+3 = familial multiple system tauopathy with presenile dementia (Spillantini et al., 1998); +11 = (Miyamoto et al., 2001; Kowalska et al., 2002); +12 = FTD-Kumamoto (Yasuda et al., 2000); +13 = (Pickering-Brown et al., 2002); +14 = DDPAC (Lynch et al., 1994); +16 = (Lantos et al., 2002). (A) The initial identification of stem loop mutations at exon 10/intron 10 boundary (Hutton et al., 1998) +14 is the original Irish-American DDPAC family. (B) The stem loop mutations identified, including −2, −1, +3 +11, +12, +13, +14, and +16. The c.915+15A > C mutation was not identified at the time but was predicted to exist (Hutton, 2000).
Most MAPT pathogenic mutations are located in exons 9–13 encoding the repeat domains and their adjacent introns. Exonic mutations are missense, silent or deletion in nature. The majority of these mutations reduce the ability of tau to interact with microtubules (Hasegawa et al., 1998) and increase the tendency for tau to assemble into filaments (Nacharaju et al., 1999). In contrast, mutations within exon 10 and the surrounding intronic sequences that alter splicing increase the splicing-in of exon 10 resulting in increased 4R tau. Intronic mutations found primarily at the 5’ splice site of the intron following exon 10, are located in the stem loop region and alter the correct functioning of this stem loop (Grover et al., 1999; Varani et al., 2000). The presence of the stem loop structure normally restricts access of the 5’ splice site to the spliceosome, thereby controlling the rate of splicing-in of exon 10. Intronic mutations in the stem region destabilize the stem loop structure thus promoting splicing-in of exon 10 with increased production of the 4R tau isoforms as a consequence (Kar et al., 2011). Mouse models of FTDP-17 including the MAPT N279K exon 10 splicing mutation and c.915+16 C>T exon 10/intron 10 stem loop mutations develop neurodegeneration as a result of aberrant splicing and recapitulate many of the disease hallmarks seen in patients with N279K or +16 mutations (Dawson et al., 2007; Umeda et al., 2013).
The precise mechanism by which the shift in exon 10 splicing leads to neurodegeneration is unclear but 4R tau aggregates more readily than 3R tau; the most parsimonious explanation is that the splicing mutations, like the exonic mutations that affect protein structure, cause an increase in tau aggregation (Umeda et al., 2013). Given that patients with these mutations appear normal into their fourth and fifth decades, there is little to suggest that this shift in splicing ratio alone has a major effect on microtubule function.
In this unique FTDP-17 family, the proband and other family members had a simultaneous behavioural syndrome typical for behavioural variant FTD and episodic memory deficits leading us to speculate whether the proband had atypical familial Alzheimer’s disease (Rippon et al., 2003). Clinically no parkinsonism or amyotrophy was evident in the proband. However, his younger sister (Patient IV-2; Fig. 1) subsequently presented with parkinsonism with eyelid opening apraxia, without cognition or behavioural change.
On imaging, the proband demonstrated bilateral anterior temporal lobe atrophy and hypometabolism on FTD/PET imaging. The clinical presentation of this family is consistent with the phenotype observed with other MAPT mutations which commonly present with symptoms of behavioural variant FTD and bilateral temporal atrophy (Rohrer and Warren, 2011; Whitwell et al., 2012). Parkinsonism may be present in patients with MAPT mutations and can rarely be the sole presenting feature. Episodic memory deficits, as observed in the current family, can be seen in patients with MAPT mutations, usually in conjunction with symptoms of behavioural variant FTD (Rohrer and Warren, 2011).
Early amnesia was an exclusion criterion in the 1998 clinical criteria for frontotemporal lobar degeneration (Neary et al., 1998), but is permitted within the more recently proposed diagnostic criteria (the International BvFTD Criteria Consortium) (Rascovsky et al., 2011). It is thought that the newer criteria are more sensitive (Costa et al., 2013). Tau screening should therefore be considered as part of the diagnostic work-up in families where amnesia or atypical parkinsonism co-exists with behavioural disturbance early in the disease process. The detection of the novel c.915+15A>C mutation 15 years after its prediction highlights the value of pursuing a complete family history with segregation analysis to guide appropriate molecular genetic testing. With the discovery of this c.915+15A>C MAPT pathogenic mutation all mutations have now been identified at all the predicted sites within the ‘stem’ when the stem-loop model was first proposed and no mutations have been found within the ‘loop’ region as expected. Moreover no mutations have been found in the intronic sequence beyond the predicted stem-loop structure (+17 onwards) that have been shown to be disease-causing. Thus having ‘opened the loop’ 21 years ago, we now ‘close the loop’.
Supplementary Material
Acknowledgements
We would like to express our sincere appreciation to the patients and families for their time, effort and participation. We would also like to express our appreciation to Ms Florence Grehan, Ms Deirdre Keaney and Mr Rafal Krol from the Biomedical Imaging and Media Services at the Mater Misericordiae University Hospital in Dublin for their time and effort devoted to the preparation of the thumbnail, cover proposal and our video abstract.
Glossary
Abbreviations
- FTD
frontotemporal dementia
- DDPAC
disinhibition-dementia-parkinsonism-amyotrophy complex
- FTDP-17
frontotemporal dementia with parkinsonism linked to chromosome 17
- RBANS
Repeatable Battery for the Assessment of Neuropsychological Status
Funding
US National Institutes of Health under the award number ROS NS076837-02 (E.F., D.O., S.O.D., E.H., S.C., B.K., T.L.). The Irish Institute of Clinical Neuroscience (SOD, TL) Biomedical Research Centre, Addenbrooke’s Hospital Cambridge and MRC (MGS).
Supplementary material
Supplementary material is available at Brain online.
References
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 2006; 442: 916–19. [DOI] [PubMed] [Google Scholar]
- Borroni B, Archetti S, Alberici A, Agosti C, Gennarelli M, Bigni B, et al. Progranulin genetic variations in frontotemporal lobar degeneration: evidence for low mutation frequency in an Italian clinical series. Neurogenetics 2008; 9: 197–205. [DOI] [PubMed] [Google Scholar]
- Costa S, Suárez-Calvet M, Antón S, Dols-Icardo O, Clarimón J, Alcolea D, et al. Comparison of 2 diagnostic criteria for the behavioral variant of frontotemporal dementia. Am J Alzheimers Dis Other Demen 2013; 28: 469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 2006; 442: 920–4. [DOI] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 2011; 72: 245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson HN, Cantillana V, Chen L, Vitek MP. The tau N279K exon 10 splicing mutation recapitulates frontotemporal dementia and parkinsonism linked to chromosome 17 tauopathy in a mouse model. J Neurosci 2007; 27: 9155–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 1989; 3: 519–26. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R. Expression of separate isoforms of human tau protein: correlation with the tau pattern in brain and effects on tubulin polymerization. EMBO J 1990; 9: 4225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Jakes R. Mutations causing neurodegenerative tauopathies. Biochem Biophys Acta 2005; 1739: 240–50. [DOI] [PubMed] [Google Scholar]
- Grover A, Houlden H, Baker M, Adamson J, Lewis J, Prihar G, et al. 5’ splice site mutations in tau associated with the inherited dementia FTDP-17 affect a stem-loop structure that regulates alternative splicing of exon 10. J Biol Chem 1999; 274: 15134–43. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Smith MJ, Goedert M. Tau proteins with FTDP-17 mutations have a reduced ability to promote microtubule assembly. FEBS Lett 1998; 437: 207–10. [DOI] [PubMed] [Google Scholar]
- Hutton M. ‘Missing’ tau mutation identified. Ann Neurol 2000; 47: 417–18. [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, et al. Association of missense and 5’-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 1998; 393: 702–5. [DOI] [PubMed] [Google Scholar]
- Iijima M, Tabira T, Poorkaj P, Schellenberg GD, Trojanowski JQ, Lee VM, et al. A distinct familial presenile dementia with a novel missense mutation in the tau gene. Neuroreport 1999; 10: 497–501. [DOI] [PubMed] [Google Scholar]
- Kar A, Fushimi K, Zhou X, Ray P, Shi C, Chen X, et al. RNA Helicase p68 (DDX5) Regulates tau Exon 10 Splicing by Modulating a Stem-Loop Structure at the 5' Splice Site. Mol Cell Biol 2011; 31: 1812–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalska A, Hasegawa M, Miyamoto K, Akiguchi I, Ikemoto A, Takahashi K, et al. A novel mutation at position +11 in the intron following exon 10 of the tau gene in FTDP-17. J Appl Genet 2002; 43: 535–43. [PubMed] [Google Scholar]
- Lantos PL, Cairns NJ, Khan MN, King A, Revesz T, Janssen JC, et al. , Neuropathologic variation in frontotemporal dementia due to the intronic tau 10(+16) mutation. Neurology 2002; 58: 1169–75. [DOI] [PubMed] [Google Scholar]
- Lynch T, Sano M, Marder KS, Bell KL, Foster NL, Defendini RF, et al. Clinical characteristics of a family with chromosome 17-linked disinhibition-dementia- parkinsonism-amyotrophy complex. Neurology 1994; 44: 1878–84. [DOI] [PubMed] [Google Scholar]
- Magnani E, Fan J, Gasparini L, Golding M, Williams M, Schiavo G, et al. : Interaction between tau and the dynactin complex. EMBO J 2007; 26: 4546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K, Kowalska A, Hasegawa M, Tabira T, Takahashi K, Araki W, et al. , Familial frontotemporal dementia and parkinsonism with a novel mutation at an intron 10 +11-splice site in the tau gene. Ann Neurol 2001; 50: 117–120. [DOI] [PubMed] [Google Scholar]
- Morfini GA1, Burns M, Binder LI, Kanaan NM, LaPointe N, Bosco DA, et al. Axonal transport defects in neurodegenerative diseases. J Neurosci 2009; 29: 12776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B, Lynch T, Farrell M. Clinicopathological features of the tauopathies. Biochem Soc Trans 2005; 33(Pt 4): 595–9. [DOI] [PubMed] [Google Scholar]
- Nacharaju P, Lewis J, Easson C, Yen S, Hackett J, Hutton M, et al. Accelerated filament formation from tau protein with specific FTDP-17 missense mutations. FEBS Lett 1999; 447: 195–9. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998; 51: 1546–54. [DOI] [PubMed] [Google Scholar]
- O'Dowd ST, Ardah MT, Johansson P, Lomakin A, Benedek GB, Roberts KA, et al. The ELISA-measured increase in cerebrospinal fluid tau that discriminates Alzheimer's disease from other neurodegenerative disorders is not attributable to differential recognition of tau assembly forms. J Alzheimers Dis 2013; 33: 923–8. [DOI] [PubMed] [Google Scholar]
- Onyike CU, Diehl-Schmid J. The epidemiology of frontotemporal dementia. Int Rev Psychiatry Abingdon Engl 2013; 25: 130–7. [cited 2014 Jun 20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering-Brown SM, Richardson AM, Snowden JS, McDonagh AM, Burns A, Braude W, et al. , Inherited frontotemporal dementia in nine British families associated with intronic mutations in the tau gene. Brain 2002; 125(Pt 4): 732–51. [DOI] [PubMed] [Google Scholar]
- Porta S, Kwong LK, Trojanowski JQ, Lee VM. Drosha inclusions are new components of dipeptide-repeat protein aggregates in FTLD-TDP and ALS C9orf72 expansion cases. J Neuropathol Exp Neurol 2015; 74: 380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian W, Liu F. Regulation of alternative splicing of tau exon 10. Neurosci Bull 2014; 30: 367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain J Neurol 2011; 134(Pt 9): 2456–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS -FTD. Neuron 2011; 72: 257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippon GA, Crook R, Baker M, Halvorsen E, Chin S, Hutton M, et al. Presenilin 1 mutation in an african american family presenting with atypical Alzheimer dementia. Arch Neurol 2003; 60: 884–8. [DOI] [PubMed] [Google Scholar]
- Rizzu P, Van Swieten JC, Joosse M, Hasegawa M, Stevens M, Tibben A, et al. High prevalence of mutations in the microtubule-associated protein tau in a population study of frontotemporal dementia in the Netherlands. Am J Hum Genet 1999; 64: 414–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Warren JD. Phenotypic signatures of genetic frontotemporal dementia. Curr Opin Neurol 2011; 24: 542–9. [DOI] [PubMed] [Google Scholar]
- Sima AA, Defendini R, Keohane C, D'Amato C, Foster NL, Parchi P, et al. The neuropathology of chromosome 17-linked dementia. Ann Neurol 1996; 39: 734–43. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Goedert M, Tau pathology and neurodegeneration, Lancet Neurol 2013; 6: 609–22. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci USA 1998; 95: 7737–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford PM, Halliday GM, Brooks WS, Kwok JB, Storey CE, Creasey H, et al. Progressive supranuclear palsy pathology caused by a novel silent mutation in exon 10 of the tau gene: expansion of the disease phenotype caused by tau gene mutations. Brain J Neurol 2000; 123 (Pt 5): 880–93. [DOI] [PubMed] [Google Scholar]
- Umeda T, Yamashita T, Kimura T, Ohnishi K, Takuma H, Ozeki T, et al. Neurodegenerative disorder FTDP-17-related tau intron 10 +16C → T mutation increases tau exon 10 splicing and causes tauopathy in transgenic mice. Am J Pathol 2013; 183: 211–25. [DOI] [PubMed] [Google Scholar]
- Varani L, Spillantini MG, Goedert M, Varani G, Structural basis for recognition of the RNA major groove in the tau exon 10 splicing regulatory element by amynoglycoside antibiotics. Nucleic Acids Res 2000; 28: 710–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J-Z, Liu F. Microtubule-associated protein tau in development, degeneration and protection of neurons. Prog Neurobiol 2008; 85: 148–75. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen KC, Lynch T, Pavlou E, Higgins M, Nygaard TG. Localization of disinhibition-dementia-parkinsonism-amyotrophy complex to 17q21-22. Am J Hum Genet 1994; 55: 1159–65. [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Weigand SD, Boeve BF, Senjem ML, Gunter JL, DeJesus-Hernandez M, et al. Neuroimaging signatures of frontotemporal dementia genetics:C9ORF72, tau, progranulin and sporadics. Brain 2012; 135: 794–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda M, Takamatsu J, D'Souza I, Crowther RA, Kawamata T, Hasegawa M, et al. A novel mutation at position +12 in the intron following exon 10 of the tau gene in familial frontotemporal dementia (FTD-Kumamoto). Ann Neurol 2000; 47: 422–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.