Disappointing results of pivotal trials evaluating shorter fluoroquinolone-containing regimens have fuelled debate over the predictive value of murine models and sputum-based surrogate endpoints. This analysis demonstrates consistency between the results of phase 3 trials and preceding preclinical and clinical studies.
Keywords: tuberculosis, fluoroquinolones, shortening regimen, REMox-TB, OFLOTUB
Abstract
The disappointing recent failure of fluoroquinolone-containing regimens to shorten the duration of tuberculosis treatment in costly phase 3 trials has raised serious questions about the reliability of preclinical tuberculosis models, especially mice, and the current paradigm of regimen development. Therefore we re-examined data from murine models and early-stage clinical trials on which the pivotal trials were based, concluding that phase 3 trial results were in line with preceding studies. Finally, we offer suggestions for a more efficient and integrated preclinical and clinical regimen development program where quantitative pharmacokinetic and pharmacodynamic models more predictive of curative treatment durations are set forth.
Tuberculosis exacts a massive toll on humanity. And yet there has been no substantial innovation in the regimens used to treat drug-susceptible pulmonary tuberculosis for over 3 decades. The composition and duration of the current first-line regimen was defined by dozens of randomized clinical trials conducted by the British Medical Research Council (BMRC), its partners, and other trial groups over a 30-year period beginning with the first randomized clinical trial in medicine demonstrating the efficacy of streptomycin [1, 2]. Key advances over this period included the use of combination therapy to reduce the risk of drug resistance, incorporation of rifampin and pyrazinamide to shorten the duration of treatment from at least 18 months to 6 months, and the substitution of ethambutol for streptomycin to provide a fully oral regimen. These trials also established prevention of relapse after treatment as the gold standard measure of treatment success.
The so-called short-course regimen for drug-susceptible tuberculosis consists of a 2-month intensive phase of rifampin (R), isoniazid (H), pyrazinamide (Z), and ethambutol (E), followed by a 4-month continuation phase of rifampin and isoniazid (abbreviated as 2RHZE/4RH) [3]. Although it is considered highly effective, implementing this lengthy and complex regimen consumes substantial health system resources and still results in high rates of initial default (diagnosed patients who never initiate treatment) and a further 5%–10% default rate during treatment [3]. Although further shortening and simplification of this regimen has been a major objective of tuberculosis drug development efforts, the process is long and costly and suffers from a profound lack of investment [4]. In particular, phase 3 clinical trials relying on relapse after treatment completion as a primary outcome measure require large sample sizes and up to 10 years to advance from inception to publication of results. Given these obstacles, phase 3 trial designs rely heavily on results from preclinical models and phase 2 trials studying surrogate endpoints. Until recently, preclinical studies evaluating novel regimens have been limited largely to mice. Despite having forecast the treatment-shortening potential of rifampin and pyrazinamide, murine models draw legitimate criticism for their pathological dissimilarity to human tuberculosis and interspecies differences in drug pharmacokinetics [5]. Likewise, whereas the results of clinical trials introducing rifampin and pyrazinamide into regimens suggested a relationship between the proportion of patients achieving negative sputum cultures at 2 months and proportions relapsing after treatment [6], the ability of this binary endpoint to forecast the duration of treatment needed to prevent relapse in a sufficiently high proportion of patients remains uncertain [7–9].
The evaluation of new experimental regimens in phase 3 trials offers a heretofore rare and important opportunity to re-examine the utility of the tools and analyses employed during preclinical and clinical regimen development. The highly anticipated results of phase 3 trials evaluating 4-month treatment regimens incorporating moxifloxacin and gatifloxacin into the first-line regimen were recently published [10–12]. Although these trials uniformly failed to achieve their primary objective of demonstrating the noninferiority of the 4-month fluoroquinolone-containing regimens compared to the standard 6-month regimen and rightfully cast doubt on the current state-of-the-art of tuberculosis regimen development, the opportunity to critically re-examine the results and interpretations of the preclinical and early clinical studies on which these trials were based should not be lost.
Interest in using fluoroquinolones to treat tuberculosis emerged after demonstrations of the in vitro and in vivo activity of ciprofloxacin and ofloxacin against Mycobacterium tuberculosis and their clinical efficacy against MDR-TB [13, 14]. Excitement intensified with the development of more potent fluoroquinolones in the 1990s: levofloxacin (L) and two 8-methoxyquinolones, moxifloxacin (M) and gatifloxacin (G) [15]. On the basis of in vitro activity data and limited murine model data on the antituberculosis activity of these agents [16], three phase 2 trials were launched to evaluate whether replacing the largely bacteriostatic agent ethambutol with moxifloxacin or gatifloxacin during the 2-month initial phase of treatment would increase the rate of sputum culture conversion. Investigators were soon encouraged by a report of few relapses after treatment with 4-month regimens incorporating ofloxacin into the first-line regimen (from a trial notably lacking a control group on standard therapy) [17]. By 2004, moxifloxacin had demonstrated early bactericidal activity (EBA) comparable to isoniazid in tuberculosis patients, and similar results soon followed for gatifloxacin and high-dose (ie, 1 g daily) levofloxacin [18–20]. After a concomitant series of experiments in mice indicated that replacing isoniazid with moxifloxacin increased the bactericidal activity of the first-line regimen and shortened the duration of treatment required to prevent relapse after treatment completion [21, 22], a fourth phase 2 trial was launched to evaluate this substitution [23].
The results of these phase 2 clinical trials were mixed (Table 1). In the multicenter trial replacing ethambutol with moxifloxacin, the RHZM arms had a higher proportion of subjects with negative sputum cultures after 4 and 6 weeks, but not after 8 weeks, of treatment compared to the RHZE arms [24]. In the single-site trial conducted in Brazil, subjects receiving RHZM were more likely to have negative weekly sputum cultures at weeks 2 through 5, as well as week 8 of treatment and had a significantly shorter median time to sputum culture conversion [25]. In the OFLOTUB phase 2 trial, the use of moxifloxacin or gatifloxacin in place of ethambutol resulted in a more rapid decline of colony-forming unit counts in sputum but the use of ofloxacin did not [26]. However, in the nonlinear mixed effects model, the RHZM and RHZG arms reached the lower limit of detection in sputum culture only 1 week earlier than the RHZE control arm. Moreover, in the secondary analysis comparing the proportions of subjects with negative sputum cultures after 8 weeks of treatment, no fluoroquinolone-containing arm was statistically superior to the control arm. The multicenter trial evaluating the substitution of moxifloxacin for isoniazid found a nonsignificant 6% difference in the proportion with negative sputum cultures at 8 weeks [23]. A similar modest but statistically significant difference in sputum culture conversion at 2 months was observed in a later trial [27]. Despite the inconsistent and altogether modest benefit of the fluoroquinolones in phase 2 trials, large phase 3 trials were organized and launched, even before all phase 2 results were available.
Table 1.
Phase 2 Clinical Trial Results With Fluoroquinolone-containing Regimens
| Reference of Clinical Trial and Study Period |
Number and Location of Clinical Sites | Number of Subjects Enrolled/Analyzed | Proportion of HIV Infected Subjects | Regimen | Outcome | P (vs Control) | |
|---|---|---|---|---|---|---|---|
| Phase 2a | Gosling et al [18] 2003 | 1 site in Tanzania | 43 enrolled/32 analyzed for EBA | 12% (5 patients) | H 300 mg 5 d | Mean EBA (in log10 CFU/mL/d) = 0.77 | NS |
| R 600 mg 5 d | 0.28 | ||||||
| M 400 mg 5 d | 0.53 | ||||||
| Pletz et al [19] 2004 | 1 site in Germany | 17 enrolled/17 analyzed | 0% (excluded) | H 6 mg/kg 5 d | Mean EBA (in log10 CFU/mL/d) = 0.21 | NS | |
| M 400 mg 5 d | 0.27 | ||||||
| Johnson et al [20] 2006 | ≥3 sites in Brazil | 40 enrolled/38 analyzed in extended EBA | 0% (excluded) | H 300 mg 7 d | Mean EBA between d2–7 (in log10 CFU/mL) = 0.08 | NS | |
| L 1000 mg 7 d | 0.18 | ||||||
| M 400 mg 7 d | 0.17 | ||||||
| G 400 mg 7 d | 0.17 | ||||||
| Reference of Clinical Trial and Study Period | Number and Location of Clinical Sites | Number of Subjects Enrolled/Analyzed | Proportion of HIV Infected Patients | Regimen | Negative Sputum at 8 wks (Solid Medium) | P (vs Control) | |
| Phase 2b | Burman et al [24] July 2003–March 2005 | 22 sites in 4 countries in North America and Africa | 336 enrolled/277 analyzed | 22% (60 patients) | 2RHZE | 71%a | NS |
| 2RHZM | 71%a | ||||||
| Conde et al [25] October 2004–March 2007 | 1 site in Brazil | 170 enrolled/146 analyzed in mITT | 3% (5 patients) | 2RHZE | 63% | .03 (mITT) | |
| 2RHZM | 80% | ||||||
| Rustomjee et al [26] June 2004–June 2005 | 4 sites in South Africa | 217 enrolled/ 205 analyzed | 59% (127 patients) | 2RHZE | 64% | NS | |
| 2RHZM | 82% | ||||||
| 2RHZG | 77% | ||||||
| Dorman et al [23] February 2006–March 2007 | 22 sites in 5 countries in the Americas, Africa and Europe | 443 enrolled/381 analyzed in mITT | 11% (35 patients) | 2RHZE | 87% | .19 (mITT) | |
| 2RMZE | 91% | ||||||
Abbreviations: CFU, colony-forming unit; EBA, early bactericidal activity; HIV, human immunodeficiency virus; mITT, modified intention to treat; NS, not significant.
a Combined results on solid and liquid media.
Whereas the phase 2 trial results were mixed, the phase 3 trials provided a definitive and disappointing answer. Three trials evaluated the replacement of ethambutol with moxifloxacin [10, 11] or gatifloxacin [10, 12] (Table 2). The REMox TB trial was a double-blind randomized trial comparing two 4-month moxifloxacin-containing regimens (2RHZM/2RHM and 2RMZE/2RM) to the 6-month control (2RHZE/4RH) [11]. Despite modestly faster time to sputum culture conversion, unfavorable outcomes (failure or relapse by 18 months after enrollment) occurred earlier and more frequently in the experimental arms compared to the control arm. The South Indian trial was an open label randomized trial comparing both 2RHZM/2RHM and 2RHZG/2RHG to 2RHZE/4RH but with each regimen administered thrice weekly instead of daily [10]. Initiation of the moxifloxacin arm was delayed for 1 year due to difficulty procuring the drug. Compared to the control arm, sputum culture conversion at 2 months was higher in the moxifloxacin arm but not the gatifloxacin arm. However, the trial was prematurely terminated due to higher tuberculosis recurrence rates in the gatifloxacin arm. The moxifloxacin arm was terminated 8 months later, having enrolled fewer subjects than the other 2 arms (118 vs 170 and 141). The difference in recurrence rates in the moxifloxacin and control arms was not statistically significant. However, the fewer subjects enrolled in the former arm may have further reduced the statistical power of the analyses. In the OFLOTUB open label randomized trial comparing 2RHZG/2RHG to 2RHZE/4RH [12], receipt of gatifloxacin was not associated with improved sputum culture conversion at 2 months. Moreover, the proportion of subjects with unfavorable outcomes (failure, relapse, or reinfection at 24 months post-enrollment, or death or withdrawal during treatment) was 21% in the experimental arm vs 17% in the control arm (modified intention to treat (mITT) analysis), a difference driven largely by a higher recurrence rate in gatifloxacin arm (14.6% vs 7.1%). The higher rates of unfavorable outcomes in the control arms in each trial compared to the oft-quoted efficacy rate of ≥95% derived from historical trials are likely multifactorial in nature. Contributing factors include analyses based on mITT rather than per protocol populations and enrollment of human immunodeficiency virus (HIV) coinfected subjects (who had more unfavorable outcomes in the REMox-TB and OFLOTUB trials but were excluded in the South Indian trial). One may also speculate about the negative impact of the reduction in pyrazinamide dose over time [28] and replacement of streptomycin with ethambutol in today's regimens [29] (especially among subjects infected with isoniazid-resistant isolates, who had numerically higher unfavorable outcomes in each trial). These results justify ongoing and future trials aimed at further optimization of the first-line regimen.
Table 2.
Phase 3 Clinical Trial Results With Fluoroquinolone-containing Regimens
| Reference of Clinical Trial and Study Period | Number and Location of Clinical Sites | Number of Subject Enrolled/Analyzed | Proportion of HIV Infected Subjects | Regimen | Unfavorable Outcomes | P (vs Control) mITT Analysis |
|---|---|---|---|---|---|---|
| REMox TB trial [11] January 2004–October 2013 | 9 countries in Africa, Asia, Central America (>15 sites) | 1931 enrolled/1674 included in mITT | 7% (110 patients) | 2RHZE/4RH | 16% | … |
| 2RHZM/2RHM | 23% | NS | ||||
| 2RMZE/2RM | 24% | NS | ||||
| South Indian trial [10] May 2004–October 2006 | 2 sites in South India | 429 enrolled/416 included in mITT | 0% (excluded) | 2RHZE/4RH | 9% | … |
| 2RHZM/2RHM | 11% | .38 | ||||
| 2RHZG/2RHG | 20% | .02 | ||||
| OFLOTUB trial [12] June 2005–October 2009 | 5 countries in Africa | 1836 enrolled/1585 included in mITT | 18% (304 patients) | 2RHZE/4RH | 17% | … |
| 2RHZG/2RHG | 21% | NS |
Abbreviations: HIV, human immunodeficiency virus; mITT, modified intention to treat; NS, not significant.
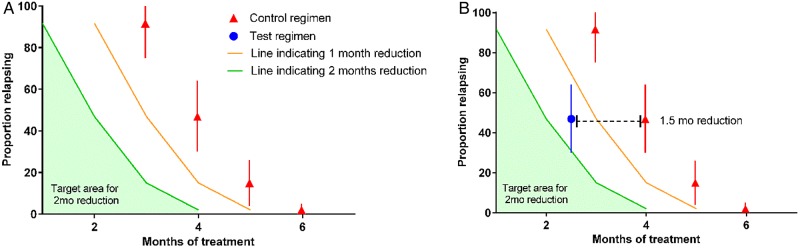
The failure of these phase 3 trials to demonstrate a treatment shortening effect of fluoroquinolones has amplified concerns about the reliability of the preclinical and early clinical approaches to regimen development that informed their design. The murine models used to evaluate moxifloxacin-containing regimens have acknowledged limitations [30], but did they provide inaccurate information for forecasting the treatment-shortening potential of these regimens? To answer this question, we compiled relapse data from all published murine model studies comparing regimens substituting moxifloxacin for either isoniazid or ethambutol with the standard of care and determined the magnitude of the treatment-shortening effect associated with moxifloxacin, based on relapse rates after treatment. Similar data for gatifloxacin do not exist in the published literature. Murine studies were grouped according to route of infection, incubation period, mouse strain, and experimental regimen tested. For each regimen in each group, the relapse data were combined to produce aggregate proportions of mice relapsing after receiving each regimen for various durations and the 95% confidence interval (95% CI). Figure 1 illustrates how the treatment shortening effect of a test regimen was estimated relative to a control regimen where the treatment shortening effect is approximately 1.5 months. If a test regimen is capable of shortening the treatment duration by a margin that is between 1 and 2 months, then the proportion of mice relapsing should fall between the yellow and the green lines (Figure 1B). And if a test regimen is not capable of shortening the treatment by at least 1 month, the proportion of mice relapsing should fall above the yellow line.
Figure 1.
Estimating treatment-shortening effects. Solid red triangles represent the proportion of mice relapsing after receiving the control regimen for various durations (error bars represent the 95% confidence interval) (see panel A). Yellow line indicates the hypothetical proportion of mice expected to relapse if a test regimen was capable of shortening the duration of treatment by 1 month without affecting the relapse rate. Likewise, the green line indicates the hypothetical proportion of mice expected to relapse if a test regimen was capable of shortening the duration of treatment by 2 months. Blue circle indicates the proportion of mice relapsing after treatment with the test regimen. If the test regimen is capable of shortening the treatment duration by 2 months or more, then the proportion of mice relapsing should fall within the green filled area under this curve.
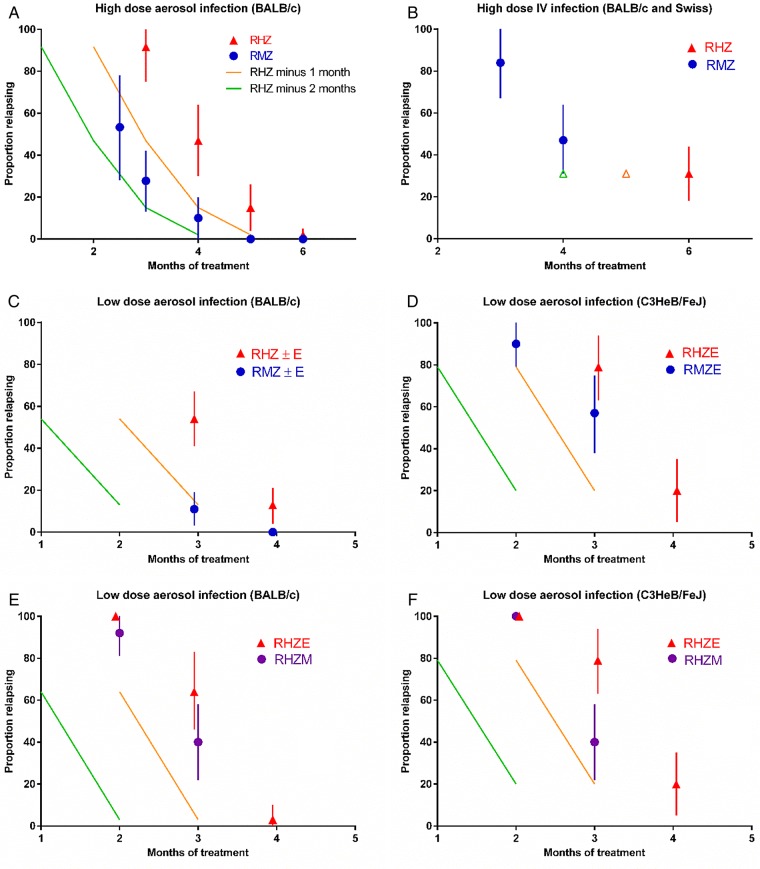
Prior to the completion of the phase 3 trials, the impact of replacing isoniazid with moxifloxacin was studied in 3 different murine models (subacute high-dose aerosol infection, subacute high-dose intravenous (IV) infection, and chronic low-dose aerosol infection) in experiments conducted by 3 independent groups of investigators using 3 different mouse strains and 2 different strains of M. tuberculosis. Based on the proportions of mice relapsing after treatment with various durations of each regimen (Figure 2), the overall size of the treatment-shortening effect of substituting moxifloxacin for isoniazid ranged from a maximum of between 1 and 2 months in the high-dose, subacute infection models (Figure 2A and 2B) to 1 month or less for the low-dose, chronic infection models (Figure 2C and 2D) but never reached 2 months [21, 30–32, 34]. Fewer murine data comparing 2RHZM/RHM with 2RHZ/RH, with or without E, are available. However, the effect size associated with use of moxifloxacin was clearly <1 month (Figure 2E and 2F) [35]. In all regimens, Z and E were discontinued after 2 months.
Figure 2.
Shortening effects of moxifloxacin-containing regimens. See Figure 1 for explanation of the schematic. A, high-dose aerosol infection model in BALB/c mice, where substitution of M for H conferred a treatment-shortening effect that falls between 1 and 2 months (4 experiments) [21, 31–33]; (B) high-dose intravenous infection model in Swiss and BALB/c mice, where substitution of M for H conferred a treatment-shortening effect of <2 months (2 experiments) [31, 34]; (C) low-dose aerosol infection model in BALB/c mice, where substitution of M for H conferred a treatment-shortening effect of 1 month (3 experiments) [31, 35]; (D) low-dose aerosol infection model in C3HeB/FeJ mice, where substitution of M for H conferred a treatment-shortening effect of <1 month (2 experiments) [35]; (E) low-dose aerosol infection model in BALB/c mice, where substitution of M for E conferred a treatment-shortening effect of <1 month (2 experiments) [31, 35]; (F) low-dose aerosol infection model in C3HeB/FeJ mice, where substitution of M for E conferred a treatment-shortening effect of <1 month (2 experiments) [31, 35].
How does the size of the treatment-shortening effect in mice compare to human results? The REMox-TB and South Indian trials were designed to determine whether the moxifloxacin-containing regimens enable a 2-month reduction in treatment duration. Whether a smaller margin of benefit similar to that observed in the murine models exists in patients can only be inferred from the available data. Using a meta-regression model of the phase 2 trial results to estimate the duration of therapy necessary to prevent relapse, Wallis et al predicted that moxifloxacin may have “a role … to shorten treatment to 5 months; however, further shortening to 4 months was predicted to incur increased relapse risk” [8]. As the impact of moxifloxacin on sputum culture conversion in the phase 3 trials was similar to that observed in the phase 2 trials on which the model was based, the data from murine and human studies are consistent in finding that moxifloxacin-containing regimens may be superior to the current first-line regimen, but that the margin of benefit is not sufficiently large to enable shortening the duration of treatment by 2 months.
Post-mortem examinations of the decision making that led to the trials evaluating 4-month fluoroquinolone-containing regimens have emphasized the perceived inadequacies of commonly used murine models that do not develop caseating or cavitating lung lesions and surrogate markers based on sputum culture conversion to represent the sterilizing activity of regimens in the clinic. We share the views that further development and validation of more pathologically similar, yet reproducible, animal models such as C3HeB/FeJ mice [30], rabbits, and marmosets [36] is warranted, as each may develop cavitary disease. We also agree that more predictive biomarkers for phase 2 trials should be sought. However, the analyses of murine model data presented here and the predictions from the model of Wallis et al [8] suggest that the principal failure in the development of these regimens was not misplaced confidence in murine models and trials based on sputum culture-based surrogate endpoints but, rather, an overly optimistic translation of the output from these studies into expectations of a 2-month treatment-shortening effect.
Rather than discrediting highly tractable murine models and existing microbiological tools as uninformative [37], these late stage regimen “failures” should push the tuberculosis drug development community to critically examine how existing as well as emerging tools could be used more effectively to develop more accurate predictions of the treatment-shortening potential of new regimens. One key gap to fill is our inability to identify patients at highest risk of relapse, as such patients determine the necessary treatment duration and are therefore the most informative when evaluating the treatment-shortening potential of a new regimen, whether in phase 2 or phase 3. Subgroup analyses from the OFLOTUB trial revealed that the 4-month gatifloxacin-containing regimen met noninferiority criteria in patients without cavitary disease but not those with cavities [12]. This result, like other similar results [38], indicates that noncavitary disease may be among the criteria that could be used to identify a subset of patients that would benefit from use of a 4-month fluoroquinolone containing regimen. On the other hand, it indicates that in phase 2 trials subjects with noncavitary disease are less informative for predicting necessary treatment durations for the entire tuberculosis patient population. Future phase 2 trials may be more efficient and informative regarding such “one-size-fits-all” shortening regimens if they exclude subjects with noncavitary disease or otherwise enroll adequate numbers of participants with cavities to allow robust subgroup analysis.
The factors contributing to the relapse diathesis associated with cavitary disease are multifactorial and include higher bacterial burdens, reduced drug penetration to the site of infection, and lack of adequate immune effector function at the cavity surface. The impact of initial bacterial burden, as measured by sputum smear grade or time-to-positivity in liquid culture systems, on relapse has recently been confirmed [39]. Not all predictors of relapse may be evident at treatment initiation. Other predictors may only emerge during therapy. For example, low systemic exposures to key sterilizing drugs (ie, rifamycins and pyrazinamide) due to pharmacokinetic variability among patient populations reduces the rate of sputum sterilization [40–43] and would be expected to interact with cavitation to amplify the risk of relapse. One simple hypothesis is that patients beginning treatment with the highest bacterial burdens and experiencing the slowest decline in bacterial burdens (eg, as measured by the β-slope in liquid culture systems [40]) over the initial 4–12 weeks of treatment constitute a subset most likely to relapse. Because a sizeable proportion of these patients remain culture-positive at 8 weeks, especially using more sensitive liquid culture systems, current phase 2 endpoints based on the proportion of subjects converting cultures at 8 weeks are inappropriately weighted toward outcomes in the patients least likely to relapse.
Although more detailed analyses of the phase 3 trial data are underway, we postulate that individual subjects' serial time-to-positivity data from liquid culture systems could be used to estimate the bacterial burden at initiation of treatment and over time on treatment in order to develop quantitative models of bacterial burden over time for individual subjects and that these curves could be extrapolated to a “sterilization” endpoint (or “cure boundary”) predictive of the duration of treatment needed to prevent relapse. Such an approach may have to be adjusted for important variables related to host immune status and for viable bacterial populations not present in, or not cultivable from, sputum. If successful, pharmacokinetic/pharmacodynamic (PK/PD) data could be incorporated to build integrative PK/PD models that could reveal further opportunities for regimen optimization and improve trial design. For example, it has been suggested that higher doses of moxifloxacin to counter metabolic induction by rifampin may have improved outcomes in the phase 3 trials. Rifapentine exposure was recently found to be strongly associated with sputum culture conversion [41] and the derived PK/PD model has been instrumental in planning an upcoming phase 3 trial. Similar quantitative analyses could be applied to, and refined in, preclinical models to provide a more predictive translational PK/PD-based framework for dose optimization, regimen selection, and clinical trial prediction. In this paradigm, hypotheses based on quantitative PK/PD relationships for component drugs developed in qualified preclinical models would be tested and refined in phase 2 trials to build greater confidence that a new regimen will perform as expected in phase 3. What preclinical models are qualified to provide data for this framework? To date, the hollow fiber model of tuberculosis is the only preclinical efficacy model that has been presented to a regulatory agency for (and received) a formal qualification decision for use in tuberculosis regimen development [44]. However, the Critical Path for TB Regimens initiative is currently evaluating a number of in vivo models, including traditional mouse strains as well as C3HeB/FeJ mice, marmosets, and other more pathologically similar models, for the qualification process. Rather than relying on any one preclinical model for the inputs needed for this PK/PD-based framework, it is likely that the use of different models with different qualifications (ie, some more tractable, some more closely representing human pathology, etc) in a coordinated and complementary fashion will constitute the most effective critical path. Importantly, the iterative approach to developing quantitative PK/PD models that seek to link preclinical and clinical outcomes is likely the most powerful and efficient way to determine which preclinical models are indeed qualified to inform tuberculosis regimen development. Although it will take time to perform gap-filling experiments and analyses, this ultimately may be the best way to understand what preclinical models and early-stage clinical trials can really tell us.
Note
Pontential conflicts of interest. R. E. C. has consulted for Merck and Otsuka industries and his spouse owns Merck stock. All other author report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Fox W, Ellard GA, Mitchison DA. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946–1986, with relevant subsequent publications. Int J Tuberc Lung Dis 1999; 3(10 suppl 2):S231–79. [PubMed] [Google Scholar]
- 2. Medical Research Council. Streptomycin treatment of pulmonary tuberculosis. Br Med J 1948; 2:769–82. [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Global tuberculosis report 2014. Geneva: World Health Organization, 2014. [Google Scholar]
- 4.Frick M. 2014 Report on Tuberculosis Research Funding Trends, 2005–2013. In: Treatment Action Group. Harrington M, Benzacar A, eds, 2014.
- 5.Mitchison DA, Chang KC. Experimental models of tuberculosis: can we trust the mouse? Am J Respir Crit Care Med 2009; 180:201–2. [DOI] [PubMed] [Google Scholar]
- 6.Mitchison DA. Assessment of new sterilizing drugs for treating pulmonary tuberculosis by culture at 2 months. Am Rev Respir Dis 1993; 147:1062–3. [DOI] [PubMed] [Google Scholar]
- 7.Wallis RS, Pai M, Menzies D et al. Biomarkers and diagnostics for tuberculosis: progress, needs, and translation into practice. Lancet 2010; 375:1920–37. [DOI] [PubMed] [Google Scholar]
- 8.Wallis RS, Wang C, Meyer D, Thomas N. Month 2 culture status and treatment duration as predictors of tuberculosis relapse risk in a meta-regression model. PLoS One 2013; 8:e71116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phillips PP, Fielding K, Nunn AJ. An evaluation of culture results during treatment for tuberculosis as surrogate endpoints for treatment failure and relapse. PLoS One 2013; 8:e63840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jawahar MS, Banurekha VV, Paramasivan CN et al. Randomized clinical trial of thrice-weekly 4-month moxifloxacin or gatifloxacin containing regimens in the treatment of new sputum positive pulmonary tuberculosis patients. PLoS One 2013; 8:e67030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillespie SH, Crook AM, McHugh TD et al. Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med 2014; 371:1577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merle CS, Fielding K, Sow OB et al. A four-month gatifloxacin-containing regimen for treating tuberculosis. N Engl J Med 2014; 371:1588–98. [DOI] [PubMed] [Google Scholar]
- 13.Tahaoğlu K, Törün T, Sevim T et al. The treatment of multidrug-resistant tuberculosis in Turkey. N Engl J Med 2001; 345:170–4. [DOI] [PubMed] [Google Scholar]
- 14.Yew WW, Chan CK, Chau CH et al. Outcomes of patients with multidrug-resistant pulmonary tuberculosis treated with ofloxacin/levofloxacin-containing regimens. Chest 2000; 117:744–51. [DOI] [PubMed] [Google Scholar]
- 15.Bryskier A, Lowther J. Fluoroquinolones and tuberculosis. Expert Opin Investig Drugs 2002; 11:233–58. [DOI] [PubMed] [Google Scholar]
- 16.Miyazaki E, Miyazaki M, Chen JM, Chaisson RE, Bishai WR. Moxifloxacin (BAY12–8039), a new 8-methoxyquinolone, is active in a mouse model of tuberculosis. Antimicrob Agents Chemother 1999; 43:85–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narayanan P, Tuberculosis Research Centre. Shortening short course chemotherapy a randomised clinical trial for treatment of smear positive pulmonary tuberculosis with regimens using ofloxacin in the intensive phase. Indian J Tuberc 2002; 49:27–38. [Google Scholar]
- 18.Gosling RD, Uiso LO, Sam NE et al. The bactericidal activity of moxifloxacin in patients with pulmonary tuberculosis. Am J Respir Crit Care Med 2003; 168:1342–5. [DOI] [PubMed] [Google Scholar]
- 19.Pletz MW, De Roux A, Roth A, Neumann KH, Mauch H, Lode H. Early bactericidal activity of moxifloxacin in treatment of pulmonary tuberculosis: a prospective, randomized study. Antimicrob Agents Chemother 2004; 48:780–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson JL, Hadad DJ, Boom WH et al. Early and extended early bactericidal activity of levofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis 2006; 10:605–12. [PubMed] [Google Scholar]
- 21.Nuermberger EL, Yoshimatsu T, Tyagi S et al. Moxifloxacin-containing regimens of reduced duration produce a stable cure in murine tuberculosis. Am J Respir Crit Care Med 2004; 170:1131–4. [DOI] [PubMed] [Google Scholar]
- 22.Nuermberger EL, Yoshimatsu T, Tyagi S et al. Moxifloxacin-containing regimen greatly reduces time to culture conversion in murine tuberculosis. Am J Respir Crit Care Med 2004; 169:421–6. [DOI] [PubMed] [Google Scholar]
- 23.Dorman SE, Johnson JL, Goldberg S et al. Substitution of moxifloxacin for isoniazid during intensive phase treatment of pulmonary tuberculosis. Am J Respir Crit Care Med 2009; 180:273–80. [DOI] [PubMed] [Google Scholar]
- 24.Burman WJ, Goldberg S, Johnson JL et al. Moxifloxacin versus ethambutol in the first 2 months of treatment for pulmonary tuberculosis. Am J Respir Crit Care Med 2006; 174:331–8. [DOI] [PubMed] [Google Scholar]
- 25.Conde MB, Efron A, Loredo C et al. Moxifloxacin versus ethambutol in the initial treatment of tuberculosis: a double-blind, randomised, controlled phase II trial. Lancet 2009; 373:1183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rustomjee R, Lienhardt C, Kanyok T et al. A phase II study of the sterilising activities of ofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis 2008; 12:128–38. [PubMed] [Google Scholar]
- 27.Jindani A, Harrison TS, Nunn AJ et al. High-dose rifapentine with moxifloxacin for pulmonary tuberculosis. N Engl J Med 2014; 371:1599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donald PR, Maritz JS, Diacon AH. Pyrazinamide pharmacokinetics and efficacy in adults and children. Tuberculosis (Edinb) 2012; 92:1–8. [DOI] [PubMed] [Google Scholar]
- 29.Jindani A, Doré CJ, Mitchison DA. Bactericidal and sterilizing activities of antituberculosis drugs during the first 14 days. Am J Respir Crit Care Med 2003; 167:1348–54. [DOI] [PubMed] [Google Scholar]
- 30.Gumbo T, Lenaerts AJ, Hanna D, Romero K, Nuermberger E. Nonclinical models for antituberculosis drug development: a landscape analysis. J Infect Dis 2015; 211(suppl 3):S83–95. [DOI] [PubMed] [Google Scholar]
- 31.De Groote MA, Gilliland JC, Wells CL et al. Comparative studies evaluating mouse models used for efficacy testing of experimental drugs against Mycobacterium tuberculosis. Antimicrob Agents Chemother 2011; 55:1237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenthal IM, Zhang M, Williams KN et al. Daily dosing of rifapentine cures tuberculosis in three months or less in the murine model. PLoS Med 2007; 4:e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nuermberger E, Tyagi S, Tasneen R et al. Powerful bactericidal and sterilizing activity of a regimen containing PA-824, moxifloxacin, and pyrazinamide in a murine model of tuberculosis. Antimicrob Agents Chemother 2008; 52:1522–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ibrahim M, Truffot-Pernot C, Andries K, Jarlier V, Veziris N. Sterilizing activity of R207910 (TMC207)-containing regimens in the murine model of tuberculosis. Am J Respir Crit Care Med 2009; 180:553–7. [DOI] [PubMed] [Google Scholar]
- 35.Li SY, Irwin SM, Converse PJ, Mdluli KE, Lenaerts AJ, Nuermberger EL. Evaluation of moxifloxacin-containing regimens in pathologically distinct murine tuberculosis models. Antimicrob Agents Chemother 2015; 59:4026–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Via LE, Weiner DM, Schimel D et al. Differential virulence and disease progression following Mycobacterium tuberculosis complex infection of the common marmoset (Callithrix jacchus). Infect Immun 2013; 81:2909–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warner DF, Mizrahi V. Shortening treatment for tuberculosis—to basics. N Engl J Med 2014; 371:1642–3. [DOI] [PubMed] [Google Scholar]
- 38.Benator D, Bhattacharya M, Bozeman L et al. Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet 2002; 360:528–34. [DOI] [PubMed] [Google Scholar]
- 39.Bark CM, Thiel BA, Johnson JL. Pretreatment time to detection of Mycobacterium tuberculosis in liquid culture is associated with relapse after therapy. J Clin Microbiol 2012; 50:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chigutsa E, Pasipanodya JG, Visser ME et al. Impact of nonlinear interactions of pharmacokinetics and MICs on sputum bacillary kill rates as a marker of sterilizing effect in tuberculosis. Antimicrob Agents Chemother 2015; 59:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dorman SE, Savic RM, Goldberg S et al. Daily rifapentine for treatment of pulmonary tuberculosis. A randomized, dose-ranging trial. Am J Respir Crit Care Med 2015; 191:333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Magis-Escurra C, van den Boogaard J, Ijdema D, Boeree M, Aarnoutse R. Therapeutic drug monitoring in the treatment of tuberculosis patients. Pulm Pharmacol Ther 2012; 25:83–6. [DOI] [PubMed] [Google Scholar]
- 43.Egelund EF, Barth AB, Peloquin CA. Population pharmacokinetics and its role in anti-tuberculosis drug development and optimization of treatment. Curr Pharm Des 2011; 17:2889–99. [DOI] [PubMed] [Google Scholar]
- 44.Chilukuri D, McMaster O, Bergman K, Colangelo P, Snow K, Toerner JG. The Hollow Fiber System Model in the nonclinical evaluation of antituberculosis drug regimens. Clin Infect Dis 2015; 61(suppl 1):S32–3. [DOI] [PubMed] [Google Scholar]