Abstract
BACKGROUND
As a global measure of ventricular systolic and diastolic function, the myocardial performance index (MPI) can be an early indicator of hypertensive cardiomyopathy in children with essential hypertension (EH).
METHODS
Children with untreated newly diagnosed EH and white coat hypertension (WCH) by a 24-hour ambulatory blood pressure monitoring (ABPM), both groups without any identifiable etiology for the hypertension, were enrolled for the study. Echocardiograms and vascular ultrasounds for carotid artery intimal medial thickness were performed on all children prior to therapy. Diastolic function (peak E and A velocities, E/A ratio, isovolumic relaxation time, and deceleration times) and MPI were evaluated by simultaneous transmitral and transaortic spectral Doppler flow velocities. Systolic function was evaluated by shortening fraction and ejection fraction.
RESULTS
A cohort of 66 children (24 with EH, 42 with WCH, males 61%, median age of 13 years, range 10–17 years) were enrolled in the study. The demographic, anthropometric, laboratory tests, vascular ultrasound, and conventional echocardiographic parameters were similar between the 2 groups. There was a very small difference in MPI between the EH and WCH children (0.28 SD: 0.07 vs. 0.31 SD: 0.08, P = 0.045). However, in EH children, MPI increased by 0.14 units for every 10 unit increase in mean ABPM systolic BP (95% confidence interval: 0.03–0.25).
CONCLUSIONS
We found the increasing MPI was associated with increasing 24-hour mean systolic BP in children with EH. Therefore, MPI may have utility as a single, quick, noninvasive method of detection and tracking of subclinical hypertensive heart disease.
Keywords: blood pressure, carotid intimal medial thickness, diastolic function, echocardiogram, hypertension, left ventricular hypertrophy, systolic function, Tei index.
Essential hypertension (EH) plays an important role in the cardiovascular morbidity and mortality among humans. Among children with EH, early recognition and management of endorgan damage has the potential in altering adverse cardiovascular outcomes in adult life. Similar to adults, we have shown that children with EH have abnormal cardiovascular function with aortopathy,1 left ventricular hypertrophy (LVH),2 and early diastolic dysfunction3 at an early stage in their disease process. Although evaluation of childhood hypertension is more laborious than adult hypertension, monitoring blood pressure (BP) in clinic as well as by an ambulatory BP monitoring (ABPM) is important for both populations in order to distinguish those with ambulatory hypertension vs. those with white coat hypertension (WCH).4 This differentiation is important since endorgan damage and therapeutic approach differ between the 2 groups.5–10
The myocardial performance index (MPI) has been found to be among the most useful echocardiographic parameters to detect early hypertensive cardiomyopathy in various populations, including pregnant women,11 children with renal transplant,12 and adults with EH.13,14 Using tissue Doppler, the MPI has been shown to precede LVH in adults with EH15 and has been shown to correlate with ventricular hemodynamic measurements in both adult human16 and animal hearts.17 However, there is a paucity of data regarding MPI among hypertensive children. We hypothesized that the MPI, which is a noninvasive and global measure of ventricular systolic and diastolic function,18,19 may be an early indicator of hypertensive cardiomyopathy11,15,20,21 among children with EH. Therefore, the primary objective of this study was to determine MPI among children with EH and compare it to those in children with WCH as determined by 24-hour ABPM. Secondary objectives were to compare conventional echocardiographic parameters of both systolic and diastolic function between the 2 groups.
METHODS
Institutional approval
The study was approved by the institutional Committee for the Protection of Human Subjects at the University of Texas Health Science Center and Children’s Memorial Hermann Hospital, Texas Medical Center in Houston, Texas. All participants and parents gave informed assent and consent, respectively, for this study. We were careful in maintaining full patient confidentiality, safeguarding the rights and welfare of human subjects, and informing subjects, in a confidential manner, of the results obtained from the study.
Patient population
This was a single center, cross-sectional study of children who were diagnosed with elevated BP prior to antihypertensive therapy. We prospectively enrolled participants from those referred to our tertiary pediatric hypertension clinic. The children were seen in our clinic from 2 sources: (i) Referral Study Population: These patients were referred to the clinic after detection of elevated BP from either an ambulatory setting by a primary care provider or an inpatient setting. (ii) Recruited Study Population: Children who were identified by systematic school-based screening for hypertension in urban Houston public schools. These children recruited by screening comprised a small proportion of patients in our clinic and were students aged 11–18 years in Houston area public schools. Parents are notified in advance by a letter sent from each school regarding the screening program. Forms were provided for parents to sign and return if they did not wish their child to participate. At each screening, 3 seated BP measurements were made at least 1-minute apart using oscillometric monitors. Students found to have an average BP above the gender, age, and height-percentile specific 95th percentile per the Fourth Report4 underwent a second set of BP measurements 1–2 weeks later. Students found to have BP above the 95th percentile at the 2nd screening underwent a 3rd set of BP measurements an additional 1–2 weeks later. Students with elevated BP on all 3 occasions were considered to be hypertensive. Families of hypertensive children were informed of the persistent BP elevation and invited for a clinic-based evaluation, either to our clinic or with their primary care physician. Patients recruited by these 2 methods, i.e., school screening or referral as described above, have been reported to be similar in a prior publication22; this was confirmed in our dataset during our analysis. All enrolled children underwent further evaluation including ambulatory BP monitoring (ABPM) as described further below. Control population included those children who had a normal measures by ABPM and they underwent laboratory testing, vascular ultrasound, and a transthoracic echocardiogram in the same manner as study children. Demographic and anthropometric data were collected on all subjects at study entry.
Blood pressure protocol
Children with untreated newly diagnosed EH and WCH, both groups without any identifiable etiology for the hypertension, were enrolled for the study. The BP was reported as the patients BP factored by their age, gender, height-specific 95th percentile (BP index). The hypertension status was evaluated by both clinic and ABPM for 24 hours in all children as follows: Clinic hypertensive status was confirmed in all subjects at the first visit to the hypertension clinic by averaging the last 3 of 4 BP measurements performed by Critikon oscillometric monitor (Tampa, FL) after 5 minutes of rest and confirmed by manual auscultation with a mercury sphygmomanometer by trained personnel using methods recommended by the Fourth Report.4 Hypertension was diagnosed when 3 separate measurements of systolic and/or diastolic BP were recorded >95th percentile for post-conceptual age, adjusted for height, age, and gender per Fourth Report4 were documented in the medical record. All children who were above the age of 5 years except those admitted with a hypertensive emergency underwent an ambulatory BP monitoring (ABPM) using Spacelabs oscillometric monitors (Spacelabs, Redmond, WA). The children along with their families were instructed on avoidance of caffeinated beverages or supplements, any medications, herbal or over the counter products, smoking and alcohol for 24 hours prior to and during the ABPM. While on ABPM, the BP was automatically measured every 20 minutes for 24 hours. Subjects with 24-hour systolic BP or diastolic BP greater than the pediatric 95th percentile or BP load (percentage of BP values exceeding the 95th percentile for the 24-hour period) greater than 25% were considered to have ambulatory hypertension.23 Both BP and BP load will be used to define the severity of ambulatory hypertension. Specifically, more severe ambulatory hypertension was defined as mean systolic or diastolic BP greater than the 95th percentile and BP load greater than 50%. Subjects with casual hypertension but with 24-hour systolic BP and diastolic BP less than the pediatric 95th percentile and BP load less than 25% were considered to have WCH. Control population for this study comprised of children who were referred for elevated BP by their primary care providers but on further evaluation in the hypertension clinic had WCH.
Diagnosis of essential hypertension
Once hypertension was confirmed to have ambulatory hypertension using ABPM, all children underwent further evaluation for secondary hypertension per recommendations by the Fourth Working Group.4 The diagnosis of primary hypertension or EH was made by extensive evaluation per recommendations by the Fourth Working Group4 including a urinary evaluation, blood tests, renal ultrasound, and echocardiogram in all children. Thus, the criteria for the diagnosis of EH were: (i) clinic BP elevation above the 95th percentile on 3 previous occasions, (ii) positive 24-hour ABPM, (iii) absence of secondary causes of hypertension, and (iv) no concurrent medication with the potential to raise BP (e.g., steroids, central stimulants).
Echocardiography and vascular ultrasound protocol
All children underwent a transthoracic echocardiogram and a vascular ultrasound. The reproducibility of measurements for both echocardiogram and vascular ultrasound were determined for repeat assessments for 10 values by the same sonographer and by 2 sonographers independently yielding kappa values of greater than 0.8. The echocardiographic studies were performed on all participants using an Acuson Sequoia 512 ultrasound machine (Siemens, PA). The heart was imaged by trained pediatric sonographers via a complete transthoracic echocardiographic examination for cross-sectional two-dimensional grayscale images, Doppler, and M-mode imaging using a standard protocol to evaluate congenital cardiac disease24 and hypertensive cardiomyopathy.25,26 Those children with congenital cardiac disease were excluded from this study. The echocardiograms measurements were made in a manner blinded the BP status of the child. Estimates of left ventricular pump function included rate-corrected velocity of circumferential fiber shortening, shortening fraction, and ejection fraction. Estimates of left ventricular afterload was determined by end systolic wall stress, both meridional along long axis from apex to the base of the heart and circumferential along short axis. Estimates of left ventricular relaxation included mitral valve inflow velocities and time intervals. Quantification of left ventricular mass (LVM) was made from 2-dimensionally guided M-mode measurements made during diastole of the left ventricular internal dimension, interventricular septal thickness, and posterior wall thickness according to methods established by the American Society of Echocardiography. 25,26 The LVM was calculated using the equation reported by Devereux et al. 27 The LVM index (LVMI2.7) was calculated by dividing LVM by height in meters to the 2.7th power to minimize the effect of age, gender, ethnicity, and overweight status. 28,29 The LVH was defined as LVMI 38.6g/m2.7, a value reported to represent the pediatric 95th percentile of LVMI2.7in normotensive healthy children. 28,29 The measurements of diastolic function (peak E and A velocities, E/A ratio, isovolumic relaxation time, and deceleration times) were made by simultaneous transmitral and transaortic spectral Doppler flow velocities.25 The left ventricular endocardial shortening fraction, ejection fraction,24–26 velocity of circumferential fiber shortening corrected for the heart rate, circumferential end systolic wall stress,30,31 and meridional end systolic wall stress31,32 were evaluated in the standard manner using M-Mode. The MPI or the Tei index was assessed in the standard manner described by Tei et al. 19 by sequential recording of the mitral valve inflow at the tip of the mitral leaflets in diastole and the left ventricular outflow just below the aortic valve in systole via spectral pulse wave Doppler in the apical 4 chambered and 5-chambered view respectively. The MPI was defined as the sum of isovolumic contraction time and isovolumic relaxation time divided by the left ventricular ejection time. 18 In normal children aged 3 years and older, the average MPI value for left ventricular global function was determined at 0.33 in 1 study 33 and 0.35 in another study. 34 In adults and children, an MPI value of less than 0.40 is considered normal for the left ventricular global function with higher values indicating left ventricular dysfunction and we used this value to define normal vs. abnormal. Simultaneous electrocardiogram tracings were obtained along with the Doppler tracing and an average of 5 consecutive heart beats or cardiac cycles were used to determine the mean values.
Carotid artery duplex ultrasound was performed by protocol to measure carotid intimal medial thickness by experienced vascular sonographers in a standard manner35 who were unaware of the ABPM or echocardiography results. The thickest carotid intimal medial thickness complex of the far wall of the distal common carotid artery was measured in longitudinal B-mode section using a high-resolution 8 MHz transducer. The right and left carotid intimal medial thicknesses were averaged for the purpose of this study.
Statistical analysis
The cases with ambulatory hypertension (EH) and WCH were compared to one another. Data from the medical records was abstracted and tabulated. Continuous variables were compared between groups using parametric (t-tests, analysis of variance with post hoc Tukey) and nonparametric (Mann–Whitney, Kruskal Wallis) tests depending on the distribution of the variable. Chi-square tests were used to compare categorical variables across groups. Multivariable linear regression analyses were performed separately to assess the independent effects of EH (compared to WCH) and the independent effect of ABPM parameters. Both of these involved regression models that adjusted for various demographic, anthropometric, BP, laboratory, vascular ultrasound, and echocardiographic parameters. Regression models were run with and without inclusion of interaction terms to assess independent and interaction effects. All analyses were performed in STATA (v.10, College Station, TX). Statistical significance was assumed at a type I error rate of 0.05.
RESULTS
A total of 66 children (24 with EH, 42 with WCH, males 61%, median age of 13 years, range 10–17 years) were enrolled in the study. The demographic, anthropometric, vascular ultrasound, and laboratory parameters were similar between the EH and WCH groups (Table 1). The average systolic BP in the clinic were elevated in both groups (Table 2). However, the systolic BP was significantly higher among those with EH vs. WCH (mean: 139mm Hg, SD: 12mm Hg vs. 131mm Hg, SD 10mm Hg, respectively; P = 0.01). Table 3 compares the echocardiogram parameters between the 2 groups. Overall, LVH was seen in 36% of children whereby the left ventricular geometry was as follows: eccentric hypertrophy in 11 (26%) of the WCH children and in 8 (33%) of the EH children and concentric hypertrophy in 1 (2%) WCH children and in 4 (17%) EH children.
Table 1.
Demographic, anthropometric, laboratory, and vascular ultrasonography profiles of children with white coat hypertension and essential hypertension
| Parameter | White coat hypertension | Essential hypertension | P-value |
|---|---|---|---|
| Number (n) | 42 | 24 | |
| Age, years | 13.5 (1.7) | 13.5 (1.8) | 0.84 |
| Male, n (%) | 27 (64) | 13 (54) | 0.42 |
| Ethnicity, n (%) | |||
| NHW | 13 (31) | 8 (33.3) | 0.89 |
| Black | 15 (36) | 8 (33.3) | |
| Hispanic | 13 (31) | 8 (33.3) | |
| Asian | 1 (2) | 0 (0) | |
| Weight, kg | 72.7 (19.6) | 71 (21.1) | 0.74 |
| Height, m | 1.6 (0.1) | 1.6 (0.1) | 0.61 |
| BMI, kg/m2 | 27 (6.5) | 26.9 (8.7) | 0.99 |
| BMI z-score | 1.4 (0.9) | 1.30 (0.9) | 0.56 |
| Obese, n (%) | 13 (28) | 5 (19) | 0.35 |
| Total cholesterol, mg/dl | 171.7 (37.2) | 167.5 (30.9) | 0.70 |
| High density lipoprotein, mg/dl | 36.1 (8.3) | 39.4 (10) | 0.27 |
| Low density lipoprotein, mg/dl | 109.7 (30) | 105.3 (37.6) | 0.68 |
| Triglycerides, mg/dl | 117.7 (84.1) | 110.8 (97.2) | 0.80 |
| Fasting glucose, mg/dl | 89.2 (8.5) | 86.7 (12.8) | 0.42 |
| Urine albumin/creatinine, median (IQR) | 0.03 (0.02–0.05) | 0.04 (0.02–0.06) | 0.60 |
| Carotid intimal medial thickness, mm | 0.60 (0.11) | 0.56 (0.08) | 0.19 |
All values shown are mean (with SD) unless specified.
Abbreviations: BMI, body mass index; IQR, interquartile range.
Table 2.
Blood pressure profile of children with white coat hypertension and essential hypertension
| Parameter | Systolic | Diastolic | ||||
|---|---|---|---|---|---|---|
| White coat hypertension | Essential hypertension | P-value | White coat hypertension | Essential hypertension | P-value | |
| Clinic BPa (mm Hg) | 131 (10) | 139 (12) | 0.01 | 76 (10) | 78 (12) | 0.47 |
| Clinic BP Index | 1.0 (0.1) | 1.1 (0.1) | <0.01 | 0.9 (0.1) | 0.9 (0.1) | 0.40 |
| Ambulatory BP (mm Hg) | ||||||
| Total | 117 (5) | 131 (6) | <0.01 | 67 (5) | 72 (8) | <0.01 |
| Waking | 124 (5) | 137 (6) | <0.01 | 72 (6) | 77 (10) | 0.01 |
| Sleeping | 107 (7) | 119 (9) | <0.01 | 58 (6) | 62 (7) | 0.02 |
| Ambulatory BP index | ||||||
| Total | 0.93 (0.04) | 1.05 (0.04) | <0.01 | 0.87 (0.11) | 0.94 (0.11) | <0.01 |
| Waking | 0.93 (0.04) | 1.04 (0.04) | <0.01 | 0.85 (0.07) | 0.91 (0.12) | 0.01 |
| Sleeping | 0.92 (0.06) | 1.03 (0.08) | <<0.01 | 0.86 (0.09) | 0.92 (0.11) | 0.02 |
| Ambulatory BP load (%) | ||||||
| Total | 21 (12) | 65 (17) | <0.01 | 15 (12) | 30 (24) | <0.01 |
| Waking | 22 (15) | 64 (18) | <0.01 | 14 (13) | 30 (24) | <0.001 |
| Sleeping | 17 (16) | 57 (29) | <0.01 | 14 (14) | 30 (29) | <0.01 |
| Ambulatory BP dip (%) | 13 (5) | 13 (6) | 0.90 | 20 (9) | 20 (8) | 0.90 |
All values shown are mean (SD).
Abbreviation: BP, blood pressure.
aClinic BP are average of 3 measurements.
Table 3.
Echocardiographic profile of children with white coat hypertension and essential hypertension
| Parameter | White coat hypertension | Essential hypertension | P-value |
|---|---|---|---|
| Heart rate, beats/min | 73.2 (12.7) | 73.6 (13.4) | 0.90 |
| Left ventricular mass, g | 136.4 (39.5) | 138.4 (42.5) | 0.85 |
| Left ventricular mass index, g/BSA | 74.7 (14.8) | 77.9 (19.3) | 0.45 |
| Left ventricular mass index 2.7, g/cm2.7 | 35.6 (8) | 36.7 (8.2) | 0.62 |
| Relative wall thickness | 0.34 (0.04) | 0.37 (0.05) | 0.05 |
| Left ventricular hypertrophy, n (%) | 12 (29) | 12 (50) | 0.08 |
| Shortening fraction, % | 43.5 (13) | 43.4 (9) | 0.97 |
| Ejection fraction, % | 0.80 (0.1) | 0.80 (0.1) | 0.63 |
| Meridional end systolic wall stress, g/cm2 | 30.7 (3.6) | 30.7 (3.1) | 0.96 |
| Circumferential end systolic wall stress, g/cm2 | 75.4 (27.9) | 78.1 (33.8) | 0.73 |
| Mitral valve E wave, cm/s | 101 (18.3) | 95.3 (20) | 0.24 |
| Mitral valve A wave, cm/s | 53.3 (13.1) | 52.9 (14.6) | 0.90 |
| Velocity of circumferential fiber shortening, circ/s | 1.06 (0.34) | 1.06 (0.24) | 0.95 |
| Mitral valve E/A | 1.97 (0.46) | 1.88 (0.48) | 0.46 |
| Mitral valve deceleration time, ms | 182.8 (30.6) | 170.6 (37.1) | 0.16 |
| Mitral valve acceleration time, ms | 91.6 (20.5) | 94.1 (16.4) | 0.61 |
| Isovolumic relaxation time, ms | 73.0 (14.2) | 74.5 (11.6) | 0.67 |
| Left ventricular ejection time, m | 294.8 (21.6) | 293 (24.1) | 0.76 |
| Myocardial performance index (MPI) | 0.28 (0.07) | 0.31 (0.08) | 0.04 |
Values are mean (with SD) for all except as noted.
The echocardiographic left ventricular diastolic parameters and systolic parameters were similar between the 2 groups (Table 3). However, MPI was higher among EH children compared to WCH (0.28 SD: 0.07 vs. 0.31 SD: 0.08, P = 0.04). Overall, 5 (20%) children with EH had MPI values greater than 0.4. Three of these children were overweight, while 2 of them had eccentric LVH. Comparatively, only 3 (7%) of the children with WCH had MPI values greater than 0.4; 2 of these 3 children in the WCH group were overweight and 1 of them also had eccentric LVH. Although a significant difference was observed in MPI between the EH and WCH patients, this was not apparent upon multivariable linear regression analysis when adjusted for gender, ethnicity, heart rate, carotid intimal medial thickness, and LVH. MPI correlated with the mean systolic (r = 0.63, P < 0.01) and diastolic (r = 0.50, P = 0.01) BP obtained from 24-hour ABPM in EH children (Figure 1A,B, respectively). This correlation was not observed in those with normal ABPM or WCH.
Figure 1.
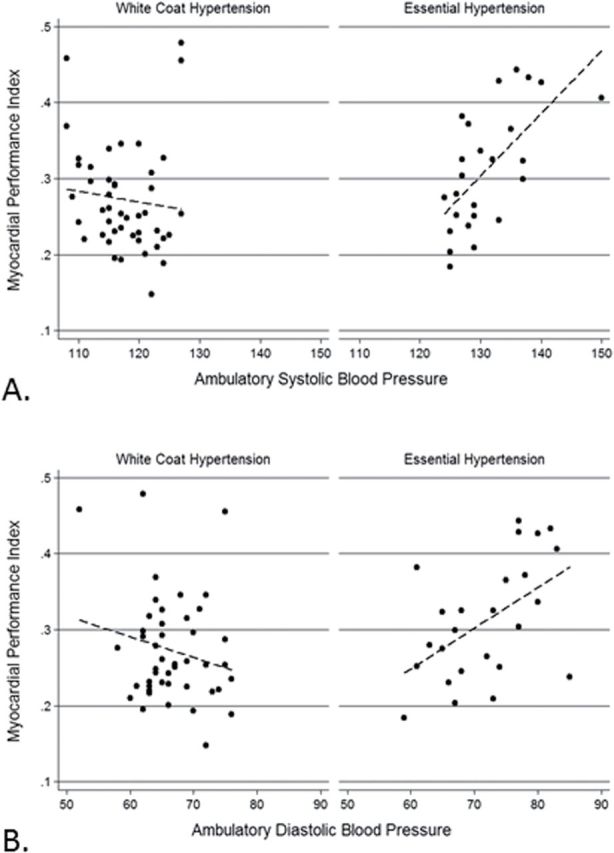
Correlation of the myocardial performance index with 24-hour ambulatory systolic (A) and diastolic (B) blood pressure among children with white coat hypertension and essential hypertension.
Upon multivariable linear regression analysis of only the children with EH, for every 10 unit increase in 24-hour mean systolic BP, the MPI value increased by 0.14 units (P < 0.01) (Table 4). Other than the mean systolic BP as obtained via ABPM, the multivariable linear regression analysis did not yield any other significant associations with MPI.
Table 4.
Change in myocardial performance index with changes in selected parameters among children with essential hypertension
| Parameter | Crude | Adjusted | ||
|---|---|---|---|---|
| Coefficient, β | 95% CI | Coefficient, β | 95% CI | |
| Mean SBPa | 0.082 | 0.040 to 0.125 | 0.137 | 0.028 to 0.246 |
| Mean DBPa | 0.054 | 0.015 to 0.092 | −0.012 | −0.091 to 0.066 |
| Age | −0.003 | −0.021 to 0.016 | 0.012 | −0.029 to 0.053 |
| Male | −0.016 | −0.083 to 0.050 | −0.019 | −0.108 to 0.07 |
| Ethnicity | ||||
| White | Referent | Referent | ||
| Black | −0.005 | −0.09 to 0.080 | 0.037 | −0.004 to 0.006 |
| Hispanic | −0.024 | −0.109 to 0.061 | −0.040 | −0.003 to 0.004 |
| BMI | 0.001 | −0.003 to 0.005 | 0.001 | −0.004 to 0.006 |
| Heart rate | 0.001 | −0.001 to 0.004 | 0.001 | −0.003 to 0.004 |
| CIMT | −0.028 | −0.517 to 0.460 | −0.182 | −0.981 to 0.616 |
| LVH | −0.015 | −0.081 to 0.051 | −0.008 | −0.089 to 0.073 |
Abbreviations: BMI, body mass index; CI, confidence interval; CIMT, carotid intimal medial thickness; DBP, diastolic blood pressure; LVH, left ventricular hypertrophy; SBP, systolic blood pressure.
aMean blood pressures are in 10 unit increments.
DISCUSSION
Although the prevalence of EH among children is much lower than in adults, the recent trends amongst adolescents in The United States of America have shown a “disconcertingly high” abnormal cardiovascular health behaviors and risk factors, including hypertension.36 The status of childhood cardiovascular health is a strong predictor of cardiovascular health in adulthood37 and hence early detection and treatment of childhood onset EH has implications into later life.
MPI evaluates both systolic and diastolic dysfunction as a single measure and has been used extensively for longitudinal tracking and prognostication in various forms of cardiomyopathy in adults. Several studies have shown similar results with MPI being among the earliest and the strongest predictor of left ventricular disease18,38–41 and adverse cardiovascular outcomes in adults.42,43 The MPI can detect early left ventricular diastolic function and has been found to correlate with hemodynamic parameters of diastolic dysfunction or impaired relaxation.17 Furthermore, as the left ventricular systolic function reduces, the MPI increases. This increase in MPI is inversely correlated to a decreasing ejection fraction.17 Thus, the Doppler-derived MPI is a powerful quantitative measure of global left ventricular function. MPI has been found to be the strongest independent predictor of the development of congestive heart failure from left ventricular dysfunction after myocardial infarction in adults.41 Furthermore, MPI can also be utilized as a longitudinal marker to evaluate any improvement in left ventricular function in adults with ischemic heart disease during therapy with renin–angiotensin inhibitors.44
We evaluated a group of well-phenotyped, multiethnic, untreated childhood onset EH by utilizing several standard diastolic (transmitral Doppler flow velocities and intervals) and systolic (endocardial shortening fraction, ejection fraction, velocity of circumferential fiber shortening, end systolic wall stress) echocardiographic parameters. In our study, these conventional measurements did not reveal any difference in left ventricular systolic and/or diastolic function between children with EH compared to children with WCH. However, the global measure of left ventricular systolic and diastolic function, as assessed by the MPI19 showed detectable differences between the same 2 groups in our patient population upon univariable analysis. The average MPI values in both groups were mostly within normal limits and the difference in the MPI values between the WCH and EH groups was very small at 0.03. Additionally, we failed to identify a significant difference in an adjusted model. This lack of an association may be due to the fact that our control population comprised of higher-risk children with obesity and WCH. Furthermore, a higher number of children with EH had abnormal MPI values greater than 0.4 compared to the children with WCH (20% vs. 7%); 2 of these 3 children in the WCH group were overweight and 1 of them also had eccentric LVH. It is also important to note that the classification of WCH or EH was based on the continuous variables obtained from the ABPM. Stratification of a continuous variables (such as mean systolic BP) into a categorical variable (such as EH) results in loss of information, decreased power and underestimation of the magnitude of variability in the outcome.45 These issues are more problematic in smaller sample sizes. It should be noted that the children in our study, most probably represent patients in early stages of their hypertensive disease process, with relatively minor changes in their MPI values.
In this study we demonstrated that sustained hypertension was associated with MPI elevation in children with EH. Although there are no trends of changes in the WCH children, higher MPI values were associated with increasing mean systolic BP in the EH children. MPI correlated with the mean systolic and diastolic BP obtained from 24-hour ABPM in EH children (Figure 1). We found the mean systolic BP as ascertained by 24-hour ABPM correlated significantly with MPI only in children with EH. Thus, in these children, for every 10 unit increase in 24-hour mean systolic BP, the MPI value increased by 0.14 units. In other studies, the MPI has been found to correlate with invasive measurements16 and has the advantage of being largely independent of heart rate and ventricular geometry.19,46,47 Our study had similar findings where MPI was independent of heart rate and ventricular geometry. Unlike LVH, MPI values were not found to be affected by body mass index in our study and hence MPI may be more reflective of endorgan damage in hypertensive cardiomyopathy. Thus the MPI can serve as a single, quick, noninvasive, reproducible, and easy method of detection of subclinical hypertensive heart disease18,19 even in the young. This simple echocardiographic parameter may be useful for tracking and determining the severity of the disease, although this needs to be established by future research. The MPI may be helpful in longitudinal tracking for progression of disease in the same child and thus help with therapeutic decision making.
In the current study, we performed a 24-hour ABPM on all children and differentiated those with EH and WCH. Due to barriers in obtaining ABPM on all children with elevated BP, the appropriate diagnosis may not be made in some children, thereby mislabeling children with WCH as having EH. Majority of pediatricians do no utilize an ABPM for the diagnosis of EH in children and hence their EH group includes children with WCH. We found that children with EH had a significantly higher systolic BP in the clinic in comparison to children with WCH, while their diastolic BP were similar. We also found that MPI had poor correlations with BP measurements in clinic but strong correlations ambulatory measurements on an ABPM. These findings in our study underscore the fact that children with WCH are not the same as those with EH. Therefore, appropriate diagnosis of EH with ABPM should be considered. Or else, MPI may serve as an alternative to an ABPM in evaluating a child with elevated BP in clinic, where the ABPM is unavailable to determine the severity of hypertension or exclude children with WCH. Where periodic ABPM measures are not possible in all children due to various reasons, MPI may serve as a surrogate measurement to follow-up prospectively on the cardiovascular changes due to sustained hypertension. Future studies should evaluate the prospective prognostic value of this measurement.
Limitations
The study evaluated a small number of children from a single tertiary care medical center, thus limiting the power and generalizability of the study. The lack of any observable significant association between demographic, BP, and echocardiographic parameters and MPI during multivariable analysis may be due to the relatively small sample size in our study. We recommend a larger study to confirm our findings. It is also possible that the MPI distribution in these children is independent on these parameters and is a function of unmeasured variables such as clinical outcomes. Finally, we did not evaluate the children by serum biomarkers such as brain natriuretic peptide levels, by imaging modalities such as cardiac magnetic resonance imaging, or by echocardiographic techniques such as tissue Doppler imaging, strain imaging, or speckle tracking.
We found the increasing MPI was associated with increasing 24-hour mean systolic BP as ascertained by an ABPM in children with EH. Therefore, MPI may have utility as a single, quick, noninvasive method of detection and tracking of subclinical hypertensive heart disease. The findings in this study also underscore the fact that children with WCH are not the same as those with EH.
DISCLOSURE
R.K.H., S.S.H., and T.P. declared no conflict of interest. K.M.-R. was supported by Arkansas Biosciences Institute, the major research component of the Tobacco Settlement Proceeds Act of 2000. M.G.-M. was supported by NIH.
ACKNOWLEDGMENTS
The project described was partially supported by Grant Number K23HL089391 (PI; M.G.-M.) from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health. A portion of the study was funded by Dr Monesha Gupta’s Faculty Development Grant from the University of Texas Health Science Center at Houston. A portion of the study was funded by Dr McNiece-Redwine’s Ruth L. Kirschstein National Research Service Individual Fellowship Award (F32 HL079813) and the University of Texas Health Science Center at Houston General Clinical Research Center (M01-RR 0255).
REFERENCES
- 1. Gupta-Malhotra M, Devereux RB, Dave A, Bell C, Portman R, Milewicz D. Aortic dilatation in children with systemic hypertension. J Am Soc Hypertens 2014; 8:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McNiece KL, Gupta-Malhotra M, Samuels J, Bell C, Garcia K, Poffenbarger T, Sorof JM, Portman RJ. Left ventricular hypertrophy in hypertensive adolescents: analysis of risk by 2004 National High Blood Pressure Education Program Working Group staging criteria. Hypertension 2007; 50:392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Agu NC, McNiece Redwine K, Bell C, Garcia KM, Martin DS, Poffenbarger TS, Bricker JT, Portman RJ, Gupta-Malhotra M. Detection of early diastolic alterations by tissue Doppler imaging in untreated childhood-onset essential hypertension. J Am Soc Hypertens 2014; 8:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004;114:555–576. [PubMed] [Google Scholar]
- 5. Cavallini MC, Roman MJ, Pickering TG, Schwartz JE, Pini R, Devereux RB. Is white coat hypertension associated with arterial disease or left ventricular hypertrophy? Hypertension 1995; 26:413–419. [DOI] [PubMed] [Google Scholar]
- 6. Khattar RS, Senior R, Lahiri A. Cardiovascular outcome in white-coat versus sustained mild hypertension: a 10-year follow-up study. Circulation 1998; 98:1892–1897. [DOI] [PubMed] [Google Scholar]
- 7. Cuspidi C, Rescaldani M, Tadic M, Sala C, Grassi G, Mancia G. White-coat hypertension, as defined by ambulatory blood pressure monitoring, and subclinical cardiac organ damage: a meta-analysis. J Hypertens 2015; 33:24–32. [DOI] [PubMed] [Google Scholar]
- 8. Stabouli S, Kotsis V, Toumanidis S, Papamichael C, Constantopoulos A, Zakopoulos N. White-coat and masked hypertension in children: association with target-organ damage. Pediatr Nephrol 2005; 20:1151–1155. [DOI] [PubMed] [Google Scholar]
- 9. Lande MB, Meagher CC, Fisher SG, Belani P, Wang H, Rashid M. Left ventricular mass index in children with white coat hypertension. J Pediatr 2008; 153:50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seeman T, Pohl M, Palyzova D, John U. Microalbuminuria in children with primary and white-coat hypertension. Pediatr Nephrol 2012; 27:461–467. [DOI] [PubMed] [Google Scholar]
- 11. Vázquez Blanco M, Roisinblit J, Grosso O, Rodriguez G, Robert S, Berensztein CS, Vega HR, Lerman J. Left ventricular function impairment in pregnancy-induced hypertension. Am J Hypertens 2001; 14:271–275. [DOI] [PubMed] [Google Scholar]
- 12. Kim GB, Kwon BS, Kang HG, Ha JW, Ha IS, Noh CI, Choi JY, Kim SJ, Yun YS, Bae EJ. Cardiac dysfunction after renal transplantation; incomplete resolution in pediatric population. Transplantation 2009; 87:1737–1743. [DOI] [PubMed] [Google Scholar]
- 13. Masugata H, Senda S, Okuyama H, Murao K, Inukai M, Hosomi N, Yukiiri K, Nishiyama A, Kohno M, Goda F. Comparison of central blood pressure and cardio-ankle vascular index for association with cardiac function in treated hypertensive patients. Hypertens Res 2009; 32:1136–1142. [DOI] [PubMed] [Google Scholar]
- 14. Yakabe K, Ikeda S, Naito T, Yamaguchi K, Iwasaki T, Nishimura E, Yoshinaga T, Furukawa K, Matsushita T, Shikuwa M, Miyahara Y, Kohno S. Left ventricular mass and global function in essential hypertension after antihypertensive therapy. J Int Med Res 2000; 28:9–19. [DOI] [PubMed] [Google Scholar]
- 15. Keser N, Yildiz S, Kurtoğ N, Dindar I. Modified TEI index: a promising parameter in essential hypertension? Echocardiography 2005; 22:296–304. [DOI] [PubMed] [Google Scholar]
- 16. Tei C, Nishimura RA, Seward JB, Tajik AJ. Noninvasive Doppler-derived myocardial performance index: correlation with simultaneous measurements of cardiac catheterization measurements. J Am Soc Echocardiogr 1997; 10:169–178. [DOI] [PubMed] [Google Scholar]
- 17. LaCorte JC, Cabreriza SE, Rabkin DG, Printz BF, Coku L, Weinberg A, Gersony WM, Spotnitz HM. Correlation of the Tei index with invasive measurements of ventricular function in a porcine model. J Am Soc Echocardiogr 2003; 16:442–447. [DOI] [PubMed] [Google Scholar]
- 18. Tei C, Dujardin KS, Hodge DO, Kyle RA, Tajik AJ, Seward JB. Doppler index combining systolic and diastolic myocardial performance: clinical value in cardiac amyloidosis. J Am Coll Cardiol 1996; 28:658–664. [DOI] [PubMed] [Google Scholar]
- 19. Tei C, Ling LH, Hodge DO, Bailey KR, Oh JK, Rodeheffer RJ, Tajik AJ, Seward JB. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function–a study in normals and dilated cardiomyopathy. J Cardiol 1995; 26:357–366. [PubMed] [Google Scholar]
- 20. Dujardin KS, Tei C, Yeo TC, Hodge DO, Rossi A, Seward JB. Prognostic value of a Doppler index combining systolic and diastolic performance in idiopathic-dilated cardiomyopathy. Am J Cardiol 1998; 82:1071–1076. [DOI] [PubMed] [Google Scholar]
- 21. Takasaki K, Miyata M, Imamura M, Yuasa T, Kuwahara E, Kubota K, Kono M, Ueya N, Horizoe Y, Chaen H, Mizukami N, Kisanuki A, Hamasaki S, Tei C. Left ventricular dysfunction assessed by cardiac time interval analysis among different geometric patterns in untreated hypertension. Circ J 2012; 76:1409–1414. [DOI] [PubMed] [Google Scholar]
- 22. Sorof JM, Turner J, Franco K, Portman RJ. Characteristics of hypertensive children identified by primary care referral compared with school-based screening. J Pediatr 2004; 144:485–489. [DOI] [PubMed] [Google Scholar]
- 23. Urbina E, Alpert B, Flynn J, Hayman L, Harshfield GA, Jacobson M, Mahoney L, McCrindle B, Mietus-Snyder M, Steinberger J, Daniels S. Ambulatory blood pressure monitoring in children and adolescents: recommendations for standard assessment: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the council on cardiovascular disease in the young and the council for high blood pressure research. Hypertension 2008; 52:433–451. [DOI] [PubMed] [Google Scholar]
- 24. Lai WW, Geva T, Shirali GS, Frommelt PC, Humes RA, Brook MM, Pignatelli RH, Rychik J. Guidelines and standards for performance of a pediatric echocardiogram: a report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr 2006; 19:1413–1430. [DOI] [PubMed] [Google Scholar]
- 25. Gottdiener JS, Bednarz J, Devereux R, Gardin J, Klein A, Manning WJ, Morehead A, Kitzman D, Oh J, Quinones M, Schiller NB, Stein JH, Weissman NJ. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr 2004; 17:1086–1119. [DOI] [PubMed] [Google Scholar]
- 26. Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, Lai WW, Geva T. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr 2010; 23:465–495; quiz 576. [DOI] [PubMed] [Google Scholar]
- 27. Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986; 57:450–458. [DOI] [PubMed] [Google Scholar]
- 28. Daniels SR, Kimball TR, Morrison JA, Khoury P, Meyer RA. Indexing left ventricular mass to account for differences in body size in children and adolescents without cardiovascular disease. Am J Cardiol 1995; 76:699–701. [DOI] [PubMed] [Google Scholar]
- 29. de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, Alderman MH. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol 1992; 20:1251–1260. [DOI] [PubMed] [Google Scholar]
- 30. Gaasch WH, Zile MR, Hoshino PK, Apstein CS, Blaustein AS. Stress-shortening relations and myocardial blood flow in compensated and failing canine hearts with pressure-overload hypertrophy. Circulation 1989; 79:872–883. [DOI] [PubMed] [Google Scholar]
- 31. Yuda S, Khoury V, Marwick TH. Influence of wall stress and left ventricular geometry on the accuracy of dobutamine stress echocardiography. J Am Coll Cardiol 2002; 40:1311–1319. [DOI] [PubMed] [Google Scholar]
- 32. Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest 1975; 56:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eto G, Ishii M, Tei C, Tsutsumi T, Akagi T, Kato H. Assessment of global left ventricular function in normal children and in children with dilated cardiomyopathy. J Am Soc Echocardiogr 1999; 12:1058–1064. [DOI] [PubMed] [Google Scholar]
- 34. Eidem BW, Tei C, O’Leary PW, Cetta F, Seward JB. Nongeometric quantitative assessment of right and left ventricular function: myocardial performance index in normal children and patients with Ebstein anomaly. J Am Soc Echocardiogr 1998; 11:849–856. [DOI] [PubMed] [Google Scholar]
- 35. Roman MJ, Naqvi TZ, Gardin JM, Gerhard-Herman M, Jaff M, Mohler E. Clinical application of noninvasive vascular ultrasound in cardiovascular risk stratification: a report from the American Society of Echocardiography and the Society of Vascular Medicine and Biology. J Am Soc Echocardiogr 2006; 19:943–954. [DOI] [PubMed] [Google Scholar]
- 36. Laitinen TT, Pahkala K, Venn A, Woo JG, Oikonen M, Dwyer T, Mikkilä V, Hutri-Kähönen N, Smith KJ, Gall SL, Morrison JA, Viikari JS, Raitakari OT, Magnussen CG, Juonala M. Childhood lifestyle and clinical determinants of adult ideal cardiovascular health: the Cardiovascular Risk in Young Finns Study, the Childhood Determinants of Adult Health Study, the Princeton Follow-Up Study. Int J Cardiol 2013; 169:126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Laitinen TT, Pahkala K, Magnussen CG, Viikari JS, Oikonen M, Taittonen L, Mikkilä V, Jokinen E, Hutri-Kähönen N, Laitinen T, Kähönen M, Lehtimäki T, Raitakari OT, Juonala M. Ideal cardiovascular health in childhood and cardiometabolic outcomes in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation 2012; 125:1971–1978. [DOI] [PubMed] [Google Scholar]
- 38. Poulsen SH, Jensen SE, Tei C, Seward JB, Egstrup K. Value of the Doppler index of myocardial performance in the early phase of acute myocardial infarction. J Am Soc Echocardiogr 2000; 13:723–730. [DOI] [PubMed] [Google Scholar]
- 39. Møller JE, Søndergaard E, Poulsen SH, Egstrup K. The Doppler echocardiographic myocardial performance index predicts left-ventricular dilation and cardiac death after myocardial infarction. Cardiology 2001; 95:105–111. [DOI] [PubMed] [Google Scholar]
- 40. Poulsen SH, Jensen SE, Nielsen JC, Møller JE, Egstrup K. Serial changes and prognostic implications of a Doppler-derived index of combined left ventricular systolic and diastolic myocardial performance in acute myocardial infarction. Am J Cardiol 2000; 85:19–25. [DOI] [PubMed] [Google Scholar]
- 41. Harjai KJ, Scott L, Vivekananthan K, Nunez E, Edupuganti R. The Tei index: a new prognostic index for patients with symptomatic heart failure. J Am Soc Echocardiogr 2002; 15:864–868. [DOI] [PubMed] [Google Scholar]
- 42. Ascione L, De Michele M, Accadia M, Rumolo S, Damiano L, D’Andrea A, Guarini P, Tuccillo B. Myocardial global performance index as a predictor of in-hospital cardiac events in patients with first myocardial infarction. J Am Soc Echocardiogr 2003; 16:1019–1023. [DOI] [PubMed] [Google Scholar]
- 43. Yuasa T, Otsuji Y, Kuwahara E, Takasaki K, Yoshifuku S, Yuge K, Kisanuki A, Toyonaga K, Lee S, Toda H, Kumanohoso T, Hamasaki S, Matsuoka T, Biro S, Minagoe S, Tei C. Noninvasive prediction of complications with anteroseptal acute myocardial infarction by left ventricular Tei index. J Am Soc Echocardiogr 2005; 18:20–25. [DOI] [PubMed] [Google Scholar]
- 44. Møller JE, Dahlström U, Gøtzsche O, Lahiri A, Skagen K, Andersen GS, Egstrup K. Effects of losartan and captopril on left ventricular systolic and diastolic function after acute myocardial infarction: results of the Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan (OPTIMAAL) echocardiographic substudy. Am Heart J 2004; 147:494–501. [DOI] [PubMed] [Google Scholar]
- 45. Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ 2006; 332:1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Poulsen SH, Nielsen JC, Andersen HR. The influence of heart rate on the Doppler-derived myocardial performance index. J Am Soc Echocardiogr 2000; 13:379–384. [DOI] [PubMed] [Google Scholar]
- 47. Møller JE, Poulsen SH, Egstrup K. Effect of preload alternations on a new Doppler echocardiographic index of combined systolic and diastolic performance. J Am Soc Echocardiogr 1999; 12:1065–1072. [DOI] [PubMed] [Google Scholar]


